Abstract
Background
We have previously used insulin feedback (IFB) as a component of a closed-loop algorithm emulating the β cell. This was based on the observation that insulin secretion is inhibited by insulin concentration. We show here that the effect of IFB is to make a closed-loop system behave as if delays in the insulin pharmacokinetic (PK)/pharmacodynamic (PD) response are reduced. We examine whether the mechanism can be used to compensate for delays in the subcutaneous PK/PD insulin response.
Method
Closed-loop insulin delivery was performed in seven diabetic dogs using a proportional-integral-derivative model of the β cell modified by model-predicted IFB. The level of IFB was set using pole placement. Meal responses were obtained on three occasions: without IFB (NONE), reference IFB (REF), and 2xREF, with experiments performed in random order. The ability of the insulin model to predict insulin concentration was evaluated by correlation with the measured profile and results reported as R2. The ability of IFB to improve the meal response was evaluated by comparing peak and nadir postprandial glucose and area under the curve (AUC; repeated measures analysis of variance with post hoc test for linear trend).
Results
Insulin concentration was well predicted by the model (median R2 = 0.87, 0.79, and 0.90 for NONE, REF, and 2xREF, respectively). Peak postprandial glucose (294 ± 15, 243 ± 21, and 247 ± 16 mg/dl) and AUC (518.2 ± 36.13, 353.5 ± 45.04, and 280.3 ± 39.37 mg/dl·min) decreased with increasing IFB (p < .05, linear trend). Nadir glucose was not affected by IFB (76 ± 5.4, 68 ± 7.3, and 72 ± 4.3 mg/dl; p = .63).
Conclusions
Insulin feedback provides an effective mechanism to compensate for delay in the insulin PK/PD profile.
Keywords: artificial β cell, closed-loop insulin delivery, continuous glucose monitoring, continuous subcutaneous insulin infusion
Introduction
Closed-loop insulin delivery systems can potentially normalize blood glucose profiles in individuals with type 1 diabetes mellitus (T1DM). A system linking sub- cutaneous (SC) glucose sensing with SC insulin delivery is widely thought to be a viable means to achieve this goal, with numerous clinical studies having been performed to demonstrate the feasibility of the approach.1–7 Still, the use of SC site increases the complexity of the control problem,8 as delays in the pharmacokinetic (PK)/ pharmacodynamic (PD) insulin response,9–11 and to a lesser extent the SC glucose response,12 can lead to sub- optimal control. Medtronic has approached the problem through the use of digital filters to correct for the SC glucose delay12 and with a proportional-integral-derivative (PID) algorithm modified by insulin feedback (IFB)7,13 to compensate for delays in the insulin PK/PD profile. The PID algorithm was initially chosen based on the observation that its response to a step change in blood glucose was similar to the β cell’s.14–16 The algorithm was later modified to include a meal bolus emulating the β cell’s cephalic phase response17 and a feedback term emulating the effect of insulin concentration to inhibit insulin secretion.18,19 Clinical closed-loop studies showed improvement in the closed-loop response with each successive modification (References 5–7, respectively).
Although the algorithm was developed with the intent to emulate the β cell, the underlying PID control equations are well established in control theory,20 and the theoretical effect of adding a feedback term proportional to plasma insulin concentration can be derived from this same theory. Essentially, the expected effect is to make the combined system—controller plus subject—behave as if the insulin PK response is faster. The extent to which the PK response is made to appear as if it is faster can be set by adjusting the IFB gain using a technique known as pole placement,20 although practical limitations do exist. In the present work, we look to derive the theoretical framework underlying the pole-placement approach and to show in an animal model of T1DM that the modification produces the expected improvement in closed-loop performance.
Methods
Modification of Proportional-Integral-Derivative Insulin Delivery By Insulin Feedback
The PID equations describing insulin secretion16 used in previous clinical studies of closed-loop insulin delivery have been described in detail.5,6 Briefly, they are as
| (1) |
In Equation (1), n denotes the minute-to-minute intervals at which sensor glucose (SG) is available, dSGdt(n) denotes the rate of change of SG, KP defines the algorithm gain, and, TD and TI define the relative amounts of insulin delivered in what would be analogous to first- and second-phase β-cell insulin secretion.16 Gain (KP) was set in proportion to the animal’s daily insulin requirement (DIR; U/kg/day; KP = 0.01125 × DIR), and TI and TD were set to 450 and 90 min, respectively, as previously described.21 The underlying basal rate is determined by I(n), which was constrained to a maximum value, IMAX = KP[target - 60], to ensure insulin delivery would be suspended at SG ≤ 60 mg/dl and not increasing (dSGdt > 0). Target was set at 120 mg/dl.
Insulin delivery with IFB (IDIFB(n)) was implemented without changing the algorithm’s overall gain by increasing the PID insulin delivery rate by a factor (1 + γ) equal to the amount needed to offset IFB (-γ × IFB). That is,
| (2) |
In Equation (2), γ is a scalar defining the IFB gain, and Îp(n) is a model estimate of insulin concentration normalized to insulin clearance such that, at steady state, IP(n) = IDIFB(n) and IDIFB = PID(n). A model estimate of insulin concentration was obtained as
| (3) |
where parameters K1 and K2 were obtained by fitting the PK insulin profile obtained by Mudaliar and colleagues9 to a two-compartment insulin model with the fit provided in the Appendix. A reference IFB gain (γREF = 0.5) was calculated to produce the fastest possible plasma insulin response to a step change in PID insulin delivery without generating appreciable overshoot of the predicted steady state plasma insulin response to that change (see Appendix for details).
Experimental Details
To assess the effect of IFB during meals, seven dogs previously made diabetic by surgical removal of the entire pancreas were used. Following surgery, animals were provided with a pancreatic enzyme concentrate with each meal (2.1–2.8 g/meal; PanaKare Plus Powder; Neogen Corporation), and glucose levels were managed with exogenous insulin. For at least 6 weeks prior to the study start, insulin was delivered by pump (Medtronic Paradigm pump or equivalent), with the pump record used to determine the animal’s DIR. During the study, each animal was studied on three occasions. On one occasion, a closed-loop meal response was obtained without IFB (NONE; γ = 0), with the other two occasions being used to evaluate the response at the reference IFB level (REF; γ = γREF) and twice the reference (2×REF; γ = 2 × γREF). Order of experiments was randomized.
On the day prior to experiments, animals were equipped with a new insulin infusion catheter. On the morning of experiments, a blood sampling catheter was placed in a cephalic vein. Blood samples were taken at 20 min intervals between 6:00 and 8:00 AM (open-loop fasting). At 8:00 AM, closed-loop control was initiated followed by the animal’s standard mixed meal at 9:00 AM. Control was continued until 3:00 PM. During the closed-loop period, blood samples were taken every 10–20 min and glucose concentration immediately assessed using a Bayer 865 automated analyzer (Bayer). The blood glucose values were extrapolated on a minute-to-minute interval. The minute-to-minute signal was then passed to the same digital filter routines used in prior closed-loop studies5–7,21 to obtain a smooth estimate of SG and its rate of change (dSGdt). This was done to recreate any signal delay associated with those signal-processing algorithms. Continuous glucose sensors were not used in the present study, thus eliminating the putative delay between plasma glucose and interstitial fluid glucose.12,22 At the time of transfer from open- to closed-loop control, I(n - 1) and the initial values of the predicted insulin concentration (IP(n - 1) and IP(n - 2)) were set to the animal’s overnight basal rate. Blood samples were centrifuged, and plasma was stored for later assay of insulin concentration (enzyme-linked immunosorbent assay; Mercodia, Uppsala, Sweden). The study was approved by the Veterans Affairs Animal Review Committee.
Statistical Analysis
Peak postprandial glucose level, incremental glucose, and insulin area under the curve (AUC) were compared using a one-way repeated measures analysis of variance with a post hoc test for linear trend (Bonferroni’s Multiple Comparison Test). Model-predicted plasma insulin concentration was compared to measured plasma insulin levels using correlation with results reported as R2. Data are presented with predicted plasma insulin levels, adjusted to insulin clearance, superimposed with measured values. Statistical calculations were performed using GraphPad Prism (Version 5.04; San Diego, CA). Data are reported as mean ± standard error of the mean.
Results
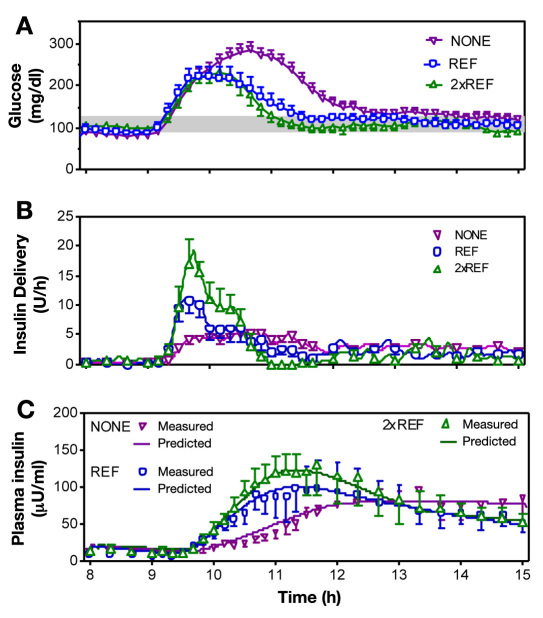
No differences were observed in blood glucose at the time closed-loop control was initiated (8:00 AM glucose; 89 ± 9 versus 97 ± 11 versus 106 ± 5 mg/dl; p = .43). Thereafter, peak postprandial glucose and glucose AUC decreased with increasing levels of IFB (Figure 1A; 294 ± 15, 243 ± 21, 247 ± 16 mg/dl, p = .026; and 518.2 ± 36.13, 353.5 ± 45.04, 280.3 ± 39.37 mg/dl min, p = .0116; NONE, REF, and 2xREF, respectively). Nadir glucose levels were unaffected by the increase in IFB (76 ± 5.4, 68 ± 7.3, and 72 ± 4.3 mg/dl for NONE, REF, and 2xREF, respectively; p = .63).
Figure 1.
(A) Plasma glucose profiles obtained with three different levels of IFB (NONE, REF, and 2xREF). (B) Closed-loop insulin delivery profiles. (C) Measured (open symbols) and model-predicted insulin concentration.
The time to reach peak insulin delivery during the meal was reduced (Figure 1B; 90 ± 15, 52 ± 9.5, and 53 ± 4.4 min; p = .0326), and the peak insulin delivery was increased (6.9 ± 0.52 versus 14.8 ± 2.0 versus 24.9 ± 3.3 U/h; p = .0004) as the level of IFB increased. Total insulin delivered during the meals was not different (19 ± 2.8 versus 19 ± 2.5 versus 20 ± 2.7 U; hour 9 to hour 15). Although the total insulin delivered was not different, the distribution was shifted to the earlier time points, increased in the initial 2 h (7.3 ± 0.74 versus 11 ± 2.4 versus 15 ± 2.6 U; p = .0058), and decreased in the third hour (3.5 ± 0.68 versus 1.8 ± 0.48 versus 0.28 ± 0.17 U; p = .0003).
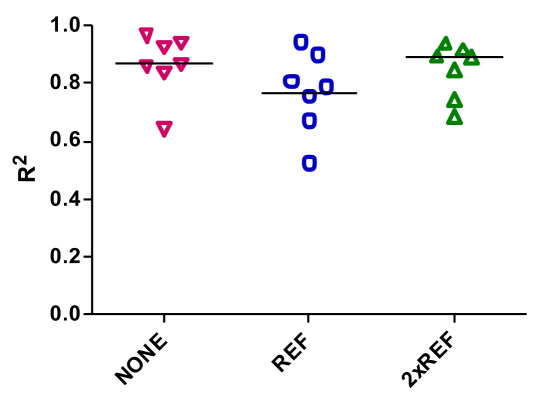
Model-predicted insulin concentration was well correlated with the average of the measured values in plasma at all levels of IFB (Figure 1C; R2 = 0.9619, 0.9734, and 0.9911, NONE, REF, and 2xREF, respectively). R2 estimated from the individual experiments varied (0.64 to 0.96 with no IFB; 0.52 to 0.94 with REF, and 0.68 to 0.94 in 2xREF), with median R2 values of 0.87, 0.79, and 0.90 (Figure 2).
Figure 2.
Correlation coefficients for the individual experiments that comprise the average profile shown in Figure 1C. Each point represents a single experiment.
Discussion
Extending the PID model of insulin secretion16 to include the putative effect of insulin concentration to suppress insulin secretion23 improves the meal response of an artificial β cell (Figure 1A). The use of IFB in this study increased insulin delivery during the first 1–2 h of a meal and decreased it at later time points with no net increase in the amount of insulin delivered (Figure 1B). Peak postprandial glucose level and the glucose AUC were reduced as the IFB gain was increased. Model estimates of plasma insulin concentrations were, on average, close to the average measured concentration (Figure 1C) but did not necessarily fit each individual experiment (Figure 2).
Although increasing the amount of IFB showed a linear trend to reduce the peak postprandial value, most of the benefit was achieved at the reference level (γ = 0.5; Figure 1A). Feedback of all the model states used to reconstruct the PK/PD profile (ISC, IP, and IEFF; Figure 3) or optimization of the other PID control parameters KP, TI, TD could potentially further improve the response. However, feedback of all the model states results in six tuning parameters, one gain for each of the three components in the PK/PD response and one gain for each of the P, D, and I terms. Generally, once the number of control parameters increases to more than three or four, choosing an optimal configuration may require a computer-simulation model.20 No model has been universally accepted for this purpose,24–26 although a simulator developed at the University of Virginia27 has been accepted by the Food and Drug Administration for replacement of animal studies. We have previously used low-order identifiable virtual patient model28,29 to assess how the IFB mechanism would have been expected to affect the peak and nadir meal concentration had it been in place for our first clinical PID study.5 With that model,28,29 we showed the IFB mechanism to effect similar improvements to those observed in a subsequent clinical study of closed-loop control (compare Figure 47 of Reference 13 and Figure 1 of Reference 7). Results from the present study show by direct comparison of different levels of IFB in the same animal that closed-loop control is improved by IFB.
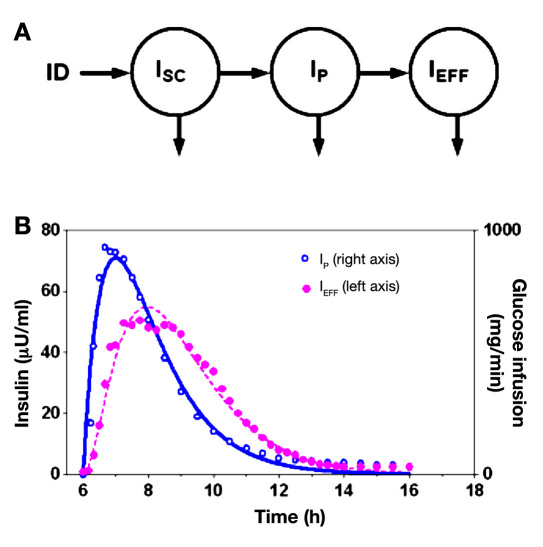
Figure 3.
(A) Model representation of insulin concentration in the SC depot (ISC) plasma (IP), and remote compartment used to characterize insulin’s effect (IEFF). (B) Insulin PK/PD data (symbols) taken from a study by Mudaliar and colleagues,9 with fitted lines indicting model prediction using Equations (A2) and (A3) in the Appendix.
The need or relative advantage of performing an animal study versus performing a computer simulation study is unclear. Each approach has advantages and disadvantages. Advantages to the computer simulation approach include the ability to simulate multiple meals over a 24 h period. This is difficult in an animal model such as the dog, as dogs may not be accustomed to consuming more than one meal per day. Computer simulation models can also be programmed with the exact insulin PK/PD observed in humans. The extent to which the dog, or other animal model, has a similar insulin PK/PD profile to that of a human is not known. However, the high correlation observed in this study between the measured canine plasma insulin response and the prediction obtained from model identified with human data9 suggests that the PK profiles in dog and human are not substantially different. Nonetheless, differences in the insulin PD response may still exist, as could differences in the shape of the glucose appearance rate after a meal. These limitations in the animal model can theoretically be addressed in a computer-simulation model. However, simulation models24–29can have substantial differences in their PK/PD profiles and in how they characterize glucose appearance rate following meals. Thus conclusions derived from the different models can be expected to vary substantially and to be different from that which might be obtained from an animal study such as that performed here. Until these differences are understood, animal studies such as the one performed here may still provide a substantial advantage when being used to guide closed-loop algorithm development.
The IFB mechanism assessed in the present study requires that the time delays in the SC insulin PK response be provided to the closed-loop algorithm. This is not a substantial obstacle in that PK/PD profiles are published for different types of insulin,30 different age groups,10 and different days of catheter implantation.31 The PK/PD profile obtained in the study by Mudaliar and colleagues9 was used here, as it is the curve used to define insulin-on-board curve in the Medtronic pump (see the Medtronic Pump user manual). The curve itself is well represented by a two-compartment insulin model describing the PK response with a third compartment used to describe the PD response (fit shown in Figure 3). Using a model allows for a computation efficient method [Equation (3)] of keeping track of the expected plasma insulin level during open- or closed-loop insulin delivery; however, the insulin concentration, effect, and more commonly used insulin-on-board (IOB) curve can all be obtained independent of a model by convolving the measured PK/PD response with past insulin delivery. This approach simply adds a series of measured PK/PD curves scaled by the bolus amount (linear sum; IOB calculated as percentage of the effect remaining). This is more computation demanding than applying Equation (3) but does not require model assumptions. In both cases, the response is assumed linear; for example, if 1 U lowers the glucose level 30 mg/dl, 2 U is assumed to lower the glucose level by 60 mg/dl; if there is 1 U of IOB and another unit is given, the amount of IOB is assumed to be 2 U.
In addition to being more computationally efficient, introducing an insulin PK/PD model makes it easier to choose an appropriate IFB gain (γ in the present study). Here the IFB gain was set using a method known as “pole placement.”20 In the method, a model is used to identify all the delays in the PK/PD response. For the model used here, one delay was associated with the movement of insulin from the SC injection site to plasma, one was associated with the clearance of insulin from plasma, and one was associated with movement of insulin from plasma to interstitial fluid surrounding insulin-sensitive tissue.32 The reciprocal values (min-1) of these estimates are known in the engineering control literature as “poles.”20 Pole placement refers to a method of obtaining the relationship between the location of these poles and the location where they appear to be once the insulin delivery pattern is modified by IFB. Strictly speaking, IFB does not depend on a model or the method used to obtain the IFB gain. As is the case with IOB calculations performed in many of today’s pumps, convolution can be used to obtain a model-independent estimate of predicted insulin concentration, and the feedback gain (γ) can be adjusted empirically. Support for the use of a model for keeping track of the expected insulin concentration is provided in work by El-Khatib and associates,1 showing that an equation similar to that used here [Equation (3)] is able to predict plasma insulin levels, provided appropriate parameters are set.
In summary, the prospective randomized controlled study described here showed the PID closed-loop meal response to be substantially improved with the addition of the IFB component. Results and conclusions obtained in this study are consistent with model-simulation studies13 and clinical data.7 The pole-placement method provides insight into how IFB can compensate for delays in the insulin PK/PD response by making the system behave as if the PK/PD profile is faster. Further studies are needed to define how fast the profile can be made to appear and if other control parameters can be optimized.
Glossary
Abbreviations
- (AUC)
area under the curve
- (DIR)
daily insulin requirement
- (IFB)
insulin feedback
- (IOB)
insulin on board
- (PD)
pharmacodynamic
- (PID)
proportional integral derivative
- (PK)
pharmacokinetic
- (REF)
reference IFB
- (SC)
subcutaneous
- (SG)
sensor glucose
- (T1DM)
type 1 diabetes mellitus
Appendix
Derivation of the insulin PK/PD model and theoretical effect of IFB are presented in this appendix. Although IFB is applied here with PID control, the same principles can be applied to any control algorithm. Material presented in this appendix assumes that the reader is familiar with differential equations, Laplace transforms, Z-transforms, and nonlinear least squares parameter identification.
To obtain the model estimate of insulin concentration used in the present study, the PK/PD response published by Mudaliar and colleagues9 (symbols; Figure 3B) was fit to a three-compartment model (Figure 3A). The model includes compartments describing the concentration of insulin at the SC infusion site (ISC), in plasma (IP), and in a remote compartment assumed to be interstitial fluid surrounding insulin-sensitive tissues32 (IEFF). Equations describing the model are
| (A1) |
| (A2) |
| (A3) |
Parameters characterizing the PK/PD response (data)—insulin clearance KINS (ml/min) and delays 1/α1, 1/α2, and 1/α3 (min)—were identified using nonlinear least squares routines available in MLAB (Civilized Software; Bethesda, MD; see Figure 3B, lines). Of these, 1/α1 and 1/α2 (identified as 50 and 70 min) are required for the IFB used in this report, and 1/(identified as 55 min) is provided for future reference.
The theoretical effect of introducing IFB into the closed-loop insulin delivery system can be derived by transforming Equations (A1) and (A2) into the Laplace domain.20 This allows an algebraic relationship between ID and IP to be obtained:
| (A4) |
In this equation, insulin concentration is normalized to insulin clearance without loss of generalizability, as the clearance parameter can be absorbed into the IFB parameter (γ) defined later; dividing insulin concentration by a scalar and multiplying the IFB gain γ by the same scalar has no effect. Defining G(s) as
| (A5) |
allows insulin concentration IP(s) in the absence of IFB to be obtained as
| (A6) |
and insulin concentration with IFB to be obtained as
| (A7) |
where Equation (2) of the main text replaces the unmodified PID delivery [(note that γ = 0 results in the unmodified form given in Equation (6)]. Algebraic manipulation of Equation (A7) yields
| (A8) |
Substituting G(s) from Equation (A5) and performing additional algebraic simplifications yields
| (A9) |
where the denominator in Equation (9) can be factored to yield
| (A10) |
Comparison of the plasma insulin response to PID control with IFB [Equation (A10)] to that obtained without IFB [Equation (A5)] shows the effect of IFB is to replace the original PK time constants (1/α1, 1/α2) with new time constants (1/q1, 1/q2) defined by
| (A11) |
From this equation, a critical value of IFB can be defined as
| (A12) |
Insulin feedback gains above this value yield negative values for the term inside of square root leading to complex poles.
The expected plasma insulin profile for arbitrary ID profiles with different values of γ can be obtained by inverse Laplace transform. For the special case where insulin is delivered as a series of discrete boluses, as is common in commercially available insulin pumps, including the pump used in the present study, a more efficient method based on the Z-transform can be used to calculate the expected plasma insulin profile:
| (A13) |
In Equation (A13), T is the sample interval at which the control is performed (1 min for the present study). Functionally, z-1 shifts discrete samples back time, resulting in a discrete time equation for IP(n):
| (A14) |
from which K1 and K2 of text Equation (3) can be obtained by inspection and K0 = 1 – K1 + K2 can be derived using the steady state constraint that IP equal ID (compare to equation used by El-Khatib and associates1).
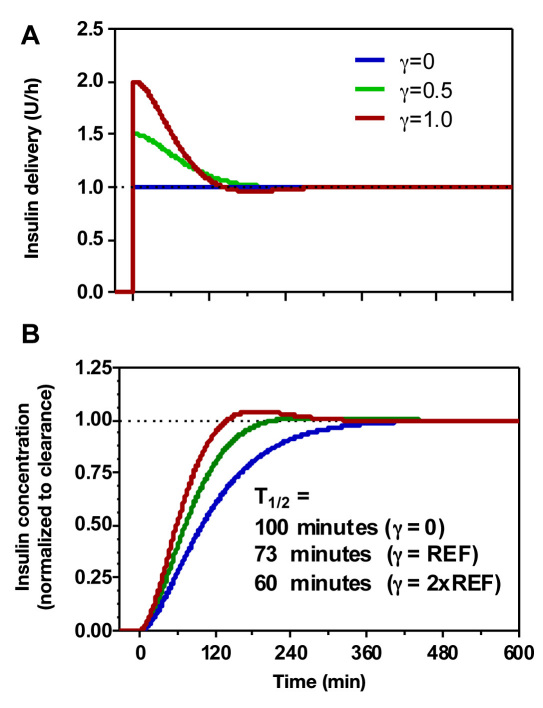
The effect of IFB to make the system behave “as if” the undesirable PK delays defined by 1/α1 and 1/α2, replaced with the more desirable values 1/q1 and 1/q2 in Equation (A10), can be visualized by simulating the plasma insulin response to stepwise increase in PID insulin delivery. With γ = 0, the rise in plasma insulin following a step change in PID(n) is biexponential with time constants 1/and 1/α2 (Figure 4B, blue line). With IFB, the initial insulin delivery is increased, and the plasma response is faster (T1/2 decreases from ∼100 to 73 and 60 min for the two IFB levels used in the present study), but there is no change in the steady state delivery rate (Figure 4A). For the reference value used in this study (γ = 0.5), there is little overshoot in expected plasma concentration (Figure 4B, green curve), although the values of q1 and q2 are complex [γREF> γCR; Equation (A12)]; increasing the value to 2xREF results in a more noticeable overshoot.
Figure 4.
(A) Simulated insulin delivery and (B) concentration with increasing levels of IFB (NONE, REF, and 2xREF).
An arbitrary pole placement that avoids the overshoot and reduces the apparent delay between insulin concentration and insulin effect (1/α3 = 55 min in Figure 4) can be achieved by feedback of all three PK/PD states identified in Figure 3 [see Equations (18)–(20) of Reference 13 for equations relating feedback gains to pole location]. While it is theoretically possible to specify the delays to be of any value, attempts to make the delays arbitrarily small can lead to excess gain [(1 + γ) term in Equation (3)]. This can increase the system’s sensitivity to noise and is more likely to produce negative insulin delivery rates needing to be set to zero. For example, a mirror image of the curves shown in Figure 4 can be shown to exist for a step down in basal rate from 1 to 0.5 U/h, but if the step down goes from 1 to 0, “negative” insulin delivery rates will result. These would necessarily be set to zero. Generally, the benefit of having more desirable pole locations (i.e., shorter delays) needs to be weighed against the potential impact of generating negative insulin delivery requests that are set to zero. An accurate simulation model can potentially guide the choice of IFB gain, provided the simulation model’s PK/PD profile is not substantially different from that observed in the patient population.
Funding
This work was supported by National Institutes of Health Grant RO1-DK64701 to Garry M. Steil.
Disclosures
Garry M. Steil was employed by Medtronic MiniMed during the conduct of these studies. Mikhail Loutseiko, Gayane Voskanyan, and Barry Keenan hold stock in Medtronic.
References
- 1.El-Khatib FH, Russell SJ, Nathan DM, Sutherlin RG, Damiano ER. A bihormonal closed-loop artificial pancreas for type 1 diabetes. Sci Transl Med. 2010;2(27):27ra27. doi: 10.1126/scitranslmed.3000619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hovorka R, Allen JM, Elleri D, Chassin LJ, Harris J, Xing D, Kollman C, Hovorka T, Larsen AM, Nodale M, De Palma A, Wilinska ME, Acerini CL, Dunger DB. Manual closed-loop insulin delivery in children and adolescents with type 1 diabetes: a phase 2 randomised crossover trial. Lancet. 2010;375(9716):743–751. doi: 10.1016/S0140-6736(09)61998-X. [DOI] [PubMed] [Google Scholar]
- 3.Clarke WL, Anderson S, Breton M, Patek S, Kashmer L, Kovatchev B. Closed-loop artificial pancreas using subcutaneous glucose sensing and insulin delivery and a model predictive control algorithm: the Virginia Experience. J Diabetes Sci Technol. 2009;3(5):1031–1038. doi: 10.1177/193229680900300506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruttomesso D, Farret A, Costa S, Marescotti MC, Vettore M, Avogaro A, Tiengo A, Dalla Man C, Place J, Facchinetti A, Guerra S, Magni L, De Nicolao G, Cobelli C, Renard E, Maran A. Closed-loop artificial pancreas using subcutaneous glucose sensing and insulin delivery and a model predictive control algorithm: preliminary studies in Padova and Montpellier. J Diabetes Sci Technol. 2009;3(5):1014–1021. doi: 10.1177/193229680900300504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steil GM, Rebrin K, Darwin C, Hariri F, Saad MF. Feasibility of automating insulin delivery for the treatment of type 1 diabetes. Diabetes. 2006;55(12):3344–3350. doi: 10.2337/db06-0419. [DOI] [PubMed] [Google Scholar]
- 6.Weinzimer SA, Steil GM, Swan KL, Dziura J, Kurtz N, Tamborlane WV. Fully automated closed-loop insulin delivery versus semiautomated hybrid control in pediatric patients with type 1 diabetes using an artificial pancreas. Diabetes Care. 2008;31(5):934–939. doi: 10.2337/dc07-1967. [DOI] [PubMed] [Google Scholar]
- 7.Steil GM, Palerm CC, Kurtz N, Voskanyan G, Roy A, Paz S, Kandeel FR. The effect of insulin feedback on closed loop glucose control. J Clin Endocrinol Metab. 2011;96(5):1402–1408. doi: 10.1210/jc.2010-2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bequette BW. A critical assessment of algorithms and challenges in the development of a closed-loop artificial pancreas. Diabetes Technol Ther. 2005;7(1):28–47. doi: 10.1089/dia.2005.7.28. [DOI] [PubMed] [Google Scholar]
- 9.Mudaliar SR, Lindberg FA, Joyce M, Beerdsen P, Strange P, Lin A, Henry RR. Insulin aspart (B28 asp-insulin): a fast-acting analog of human insulabsorption kinetics and action profile compared with regular human insulin in healthy nondiabetic subjects. Diabetes Care. 1999;22(9):1501–1506. doi: 10.2337/diacare.22.9.1501. [DOI] [PubMed] [Google Scholar]
- 10.Swan KL, Weinzimer SA, Dziura JD, Steil GM, Voskanyan GR, Steffen AT, Martin ML, Tamborlane WV. Effect of puberty on the pharmacodynamic and pharmacokinetic properties of insulin pump therapy in youth with type 1 diabetes. Diabetes Care. 2008;31(1):44–46. doi: 10.2337/dc07-0737. [DOI] [PubMed] [Google Scholar]
- 11.Weinzimer SA, Ternand C, Howard C, Chang CT, Becker DJ, Laffel LM, Insulin Aspart Pediatric Pump Study Group A randomized trial comparing continuous subcutaneous insulin infusion of insulin aspart versus insulin lispro in children and adolescents with type 1 diabetes. Diabetes Care. 2008;31(2):210–215. doi: 10.2337/dc07-1378. [DOI] [PubMed] [Google Scholar]
- 12.Rebrin K, Sheppard NF, Jr, Steil GM. Use of subcutaneous interstitial fluid glucose to estimate blood glucose: revisiting delay and sensor offset. J Diabetes Sci Technol. 2010;4(5):1087–1098. doi: 10.1177/193229681000400507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanderian SS, Jr, Steil GM. Apparatus and method for controlling insulin infusion with state variable feedback. U.S. Patent 7,806,886, October 5, 2010.
- 14.Elahi D. In praise of the hyperglycemic clamp. A method for assessment of beta-cell sensitivity and insulin resistance. Diabetes Care. 1996;19(3):278–286. doi: 10.2337/diacare.19.3.278. [DOI] [PubMed] [Google Scholar]
- 15.Steil GM, Panteleon AE, Rebrin K. Closed-loop insulin delivery-the path to physiological glucose control. Adv Drug Deliv Rev. 2004;56(2):125–144. doi: 10.1016/j.addr.2003.08.011. 2. [DOI] [PubMed] [Google Scholar]
- 16.Steil GM, Rebrin K, Janowski R, Darwin C, Saad MF. Modeling beta-cell insulin secretion–implications for closed-loop glucose homeostasis. Diabetes Technol Ther. 2003;5(6):953–964. doi: 10.1089/152091503322640999. [DOI] [PubMed] [Google Scholar]
- 17.Ahrén B, Holst JJ. The cephalic insulin response to meal ingestion in humans is dependent on both cholinergic and noncholinergic mechanisms and is important for postprandial glycemia. Diabetes. 2001;50(5):1030–1038. doi: 10.2337/diabetes.50.5.1030. [DOI] [PubMed] [Google Scholar]
- 18.Insulin inhibition of insulin secretion. N Engl J Med. 1982;307(17):1084–1085. doi: 10.1056/NEJM198210213071719. [DOI] [PubMed] [Google Scholar]
- 19.DeFronzo RA, Binder C, Wahren J, Felig P, Ferrannini E, Faber OK. Sensitivity of insulin secretion to feedback inhibition by hyper-insulinaemia. Acta Endocrinol (Copenh) 1981;98(1):81–86. doi: 10.1530/acta.0.0980081. [DOI] [PubMed] [Google Scholar]
- 20.Ogata K. Modern control engineering. 3rd ed. Upper Saddle River: Prentice-Hall; 1997. [Google Scholar]
- 21.Panteleon AE, Loutseiko M, Steil GM, Rebrin K. Evaluation of the effect of gain on the meal response of an automated closed-loop insulin delivery system. Diabetes. 2006;55(7):1995–2000. doi: 10.2337/db05-1346. [DOI] [PubMed] [Google Scholar]
- 22.Rebrin K, Steil GM. Can interstitial glucose assessment replace blood glucose measurements? Diabetes Technol Ther. 2000;2(3):461–472. doi: 10.1089/15209150050194332. [DOI] [PubMed] [Google Scholar]
- 23.Elahi D, Nagulesparan M, Hershcopf RJ, Muller DC, Tobin JD, Blix PM, Rubenstein AH, Unger RH, Andres R. Feedback inhibition of insulin secretion by insulrelation to the hyperinsulinemia of obesity. N Engl J Med. 1982;306(20):1196–1202. doi: 10.1056/NEJM198205203062002. [DOI] [PubMed] [Google Scholar]
- 24.Steil GM, Clark B, Kanderian S, Rebrin K. Modeling insulin action for development of a closed-loop artificial pancreas. Diabetes Technol Ther. 2005;7(1):94–108. doi: 10.1089/dia.2005.7.94. [DOI] [PubMed] [Google Scholar]
- 25.Steil GM, Reifman J. Mathematical modeling research to support the development of automated insulin-delivery systems. J Diabetes Sci Technol. 2009;3(2):388–395. doi: 10.1177/193229680900300223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steil GM, Hipszer B, Reifman J. Update on mathematical modeling research to support the development of automated insulin delivery systems. J Diabetes Sci Technol. 2010;4(3):759–769. doi: 10.1177/193229681000400334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kovatchev BP, Breton M, Man CD, Cobelli C. In silico preclinical trials: a proof of concept in closed-loop control of type 1 diabetes. J Diabetes Sci Technol. 2009;3(1):44–55. doi: 10.1177/193229680900300106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stocker D, Kanderian S, Jr, Cortina GJ, Nitzan T, Plummer J, Steil GM, Mastrototaro JJ. Virtual Patient Software System for educating and treating individuals with diabetes. U.S. Patent Publication WO/2006/132899, December 14, 2006.
- 29.Kanderian SS, Weinzimer S, Voskanyan G, Steil GM. Identification of intraday metabolic profiles during closed-loop glucose control in individuals with type 1 diabetes. J Diabetes Sci Technol. 2009;3(5):1047–1057. doi: 10.1177/193229680900300508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Homko C, Deluzio A, Jimenez C, Kolaczynski JW, Boden G. Comparison of insulin aspart and lispro: pharmacokinetic and metabolic effects. Diabetes Care. 2003;26(7):2027–2031. doi: 10.2337/diacare.26.7.2027. [DOI] [PubMed] [Google Scholar]
- 31.Swan KL, Dziura JD, Steil GM, Voskanyan GR, Sikes KA, Steffen AT, Martin ML, Tamborlane WV, Weinzimer SA. Effect of age of infusion site and type of rapid-acting analog on pharmacodynamic parameters of insulin boluses in youth with type 1 diabetes receiving insulin pump therapy. Diabetes Care. 2009;32(2):240–244. doi: 10.2337/dc08-0595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steil GM, Ader M, Moore DM, Rebrin K, Bergman RN. Trans-endothelial insulin transport is not saturable in vivo. No evidence for a receptor-mediated process. J Clin Invest. 1996;97(6):1497–1503. doi: 10.1172/JCI118572. [DOI] [PMC free article] [PubMed] [Google Scholar]