Abstract
Patients with type 1 diabetes mellitus (T1DM) must make frequent decisions and lifestyle adjustments in order to manage their disorder. Automated treatment would reduce the need for these self-management decisions and reduce the risk for long-term complications. Investigators in the field of closed-loop glycemic control systems are now moving from inpatient to outpatient testing of such systems.
As outpatient systems are developed, the element of safety increases in importance. One such concern is the risk for hypoglycemia, due in part to the delayed onset and prolonged action duration of currently available subcutaneous insulin preparations. We found that, as compared to an insulin-only closed-loop system, a system that also delivers glucagon when needed led to substantially less hypoglycemia. Though the capability of glucagon delivery would mandate the need for a second hormone chamber, glucagon in small doses is tolerated very well.
People with T1DM often develop hyperglycemia from emotional stress or medical stress. Automated closed-loop systems should be able to detect such changes in insulin sensitivity and adapt insulin delivery accordingly. We recently verified the adaptability of a model-based closed-loop system in which the gain factors that govern a proportional-integral-derivative-like system are adjusted according to frequently measured insulin sensitivity. Automated systems can be tested by physical exercise to increase glucose uptake and insulin sensitivity or by administering corticosteroids to reduce insulin sensitivity.
Another source of risk in closed-loop systems is suboptimal performance of amperometric glucose sensors. Inaccuracy can result from calibration error, biofouling, and current drift. We found that concurrent use of more than one sensor typically leads to better sensor accuracy than use of a single sensor. For example, using the average of two sensors substantially reduces the proportion of large sensor errors. The use of more than two allows the use of voting algorithms, which can temporarily exclude a sensor whose signal is outlying.
Elements such as the use of glucagon to minimize hypoglycemia, adaptation to changes in insulin sensitivity, and sensor redundancy will likely increase safety during outpatient use of closed-loop glycemic control systems.
Keywords: artificial pancreas, glucagon, glucose sensors, insulin pump, type 1 diabetes mellitus
The Need for Insulin and Glucagon during Closed-Loop Control
Among patients with type 1 diabetes mellitus (T1DM), fear of hypoglycemia is a very common problem and often leads to impaired quality of life.1, 2 Such a fear often leads to relaxation of tight glycemic control, which in turn predisposes to long-term micro- and macrovascular complications. The issue of hypoglycemia must also be dealt with in the setting of the closed-loop artificial endocrine pancreas in which insulin is delivered via the subcutaneous route.
Insulin delivered subcutaneously has a delayed action and a prolonged effect. This pharmacodynamic profile is very different from that of endogenous insulin, which is rapidly secreted by the beta cell in response to a rising glucose and rapidly delivered into the portal venous system. The advent of insulin analogs in the 1980s greatly improved the treatment of diabetes by shortening delay and duration of action. Nonetheless, there remains a large gap between commercially available insulin and the ideal insulin preparation for closed-loop control. One method of compensating for these delays during closed-loop control is the provision of a pre-meal bolus to avoid marked postprandial hyperglycemia. Another is the often-used insulin-on-board (IOB) calculation designed to avoid hypoglycemia after large amounts of insulin have been given. Though shorter-acting than regular insulin, the effect of rapid insulin analogs may persist for 8–9 h after subcutaneous delivery.3,4 Due to this prolonged effect, cessation of insulin at times of impending hypoglycemia is often insufficient to prevent overt hypo-glycemia. The shortcomings of utilizing insulin alone in a closed-loop system have led our group5,6 and a group in Boston7,8 to study the addition of glucagon, an idea first proposed by Kadish in the 1960s.9
Glucagon, a hormone normally synthesized and secreted by the pancreatic alpha cell, is the first line of defense among many counter-regulatory hormones that prevent hypoglycemia. The major site of glucagon action is the liver. Glucagon is secreted into the portal circulation, exposing the liver to levels that are two to three times higher than other organs.10 In the liver, glucagon functions primarily to raise blood glucose via glycogenolysis. In diabetes, glucagon secretion is dysregulated. Over time, people with T1DM lose the ability to secrete glucagon and epinephrine in response to hypoglycemia, contributing to the problem of severe hypoglycemia and hypoglycemic unawareness.11 The cause of the glucagon secretory defect is likely multi-factorial, relating in large part to loss of insulin production, which regulates glucagon release by a paracrine effect. Neural factors and structural islet changes likely contribute to the dysregulation.12
The drug glucagon is currently U.S. Food and Drug Administration-approved as a parenteral injection for treatment of severe hypoglycemia. Glucagon’s appeal in a bihormonal, closed-loop system stems from its favorable pharmacodynamic profile. In particular, due to its rapid absorption, the onset of glucagon action is much quicker than the offset of insulin action.13,14 For this reason, the subcutaneous delivery of glucagon can prevent hypo-glycemia more quickly and more effectively than the discontinuation of subcutaneous insulin delivery.
In our study of 13 adult subjects with T1DM, glucagon was very effective in reducing time spent in the hypo-glycemic range. When given in a brisk (front loaded or high gain) fashion, glucagon significantly reduced the time spent in the hypoglycemic range by 56% versus saline placebo (18 ± 11 vs 41 ± 13 min). In this study, both insulin and glucagon were delivered subcutaneously using a standard infusion set. The infusion rates were determined by glucose sensor values input into the fading memory proportional derivative algorithm (discussed in the next section). Glucagon prevented overt hypoglycemic events in approximately two-thirds of cases of incipient hypoglycemia. The probability of glucagon successfully preventing hypoglycemia was in part related to IOB and sensor accuracy. Higher IOB and delayed glucagon delivery (due to sensor overestimation of blood glucose) resulted in a higher probability of failure.5 Russell and colleagues7 found a similar rate of success as well as a correlation between higher insulin levels and glucagon failure. Rivera and colleagues15 made an important contribution by demonstrating that the ability of glucagon to stimulate glucose production is not only related to insulin level, but also to glucose level, with a threefold greater glucagon effect during hypoglycemia versus euglycemia.
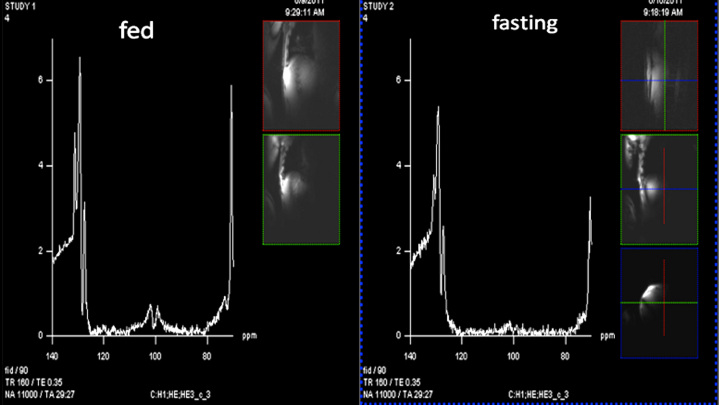
The short-term side effects of high-dose glucagon are well known, with the most prominent being nausea and vomiting. However, the dose of glucagon delivered at any one time by a closed-loop system is quite small, approximately 50–100 mcg, compared to the 1 mg dose of a glucagon emergency kit. During chronic secretion of large amounts by tumors, glucagon is known to cause serious skin rashes, but in lower intermittent doses, it has been very well tolerated in our experience. Haymond and colleagues16 found that one to two mini-doses of glucagon were effective in preventing and treating hypoglycemia in children. However, there exists a potential risk of glycogen depletion after glucagon is given repetitively; such depletion could explain the failures to prevent hypoglycemia during closed-loop studies. Studies are underway at Oregon Health and Science University to assess liver glycogen before and after repeated doses of glucagon by the use of an ultra high- resolution technique using a 7 Tesla magnet. This magnetic resonance spectroscopy (MRS) technique is based on previous reports in which less powerful magnets were utilized.17 Two magnetic resonance spectra obtained in one normal volunteer are shown in Figure 1; the glycogen signature (dual peak centered at ∼100 PPM) is substantially higher in the fed state (left panel) as compared to the fasting state (right panel).
Figure 1.
Two magnetic resonance spectra of hepatic glycogen obtained in a normal, nondiabetic volunteer with the use of a Siemens Magnetom 7 Tesla instrument. Development and programming were carried out by Dr. Mark Woods and Dr. Yu Cai from the Oregon Health and Science University Advanced Imaging Research Center. The y-axis is signal amplitude (unit-less) and the x-axis is frequency. The characteristic signature of glycogen is the double peak centered slightly above 100 PPM. Note that the peaks are substantially higher in the fed state (left panel, obtained 4 h after a mixed meal) than in the fasting state (right panel, obtained after 24 h fast). The small insets for each panel are scout magnetic resonance imaging scans to help localize the body-worn coils.
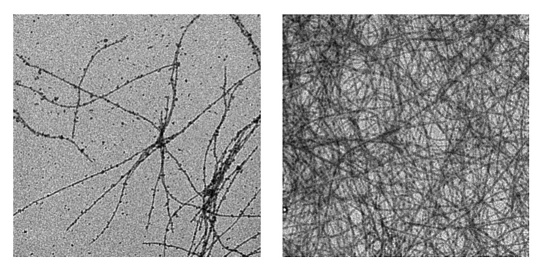
Stability of glucagon after reconstitution is also a major hurdle that must be overcome prior to its general appli- cation in a closed-loop glycemic control system. In standard commercially available systems, immediately after aqueous reconstitution at acid pH, glucagon begins to form fibrils. These fibrils, a form of beta sheet amyloid, are dependent on many factors, including glucagon concentration, heat, pH, and agitation. Figure 2 shows early minimal fibril formation soon after reconstitution of commercially available glucagon (left panel) and dense fibril formation after 7 days of aging the same preparation (right panel).
Figure 2.
Transmission electron micrographs (TEM) of native glucagon reconstituted in sterile water. The left panel shows freshly reconstituted glucagon (1 mg/ml, Novo Gluca-Gen) and the right panel shows the same preparation after being aged for 7 days at 37 °C. Both specimens were diluted to 0.25 mg/ml at time of TEM analysis. Samples were viewed on a Philips CM120/Biotwin TEM and photographed on a Gatan multiscan CCD camera in the Oregon Health and Science University Electron Microscopy facility. Note the greatly increased fibril density in the aged specimen. The width of each micrograph is 4.14 μm.
Our group has demonstrated that the use of an alkaline preparation of native glucagon (pH 10) greatly increases the stability of glucagon, and eliminates the cytotoxicity seen with commercially available glucagon preparations that are formulated with lactose and hydrogen chloride at pH 2–3.18 Steiner and colleagues19 have addressed this issue by increasing solubility at neutral pH by using a lysolecithin surfactant, an alcohol, and a sugar to prevent glucagon self-association. A group led by DiMarchi and colleagues20 has created multiple glucagon analogs with excellent stability and retention of physiologic action.
Fading Memory Proportional Derivative Algorithm
We have reviewed various algorithms utilized by different groups to control glycemia in the closed-loop setting.21 Our group uses a system that is based on a proportional gain, a derivative gain, and a fading memory of pro-portional and derivative errors. This algorithm was developed in animals22,23 in which T1DM was created by alloxan.24 The algorithm was also tested in humans with T1DM.6 The proportional and derivative gains are based on fixed gain factors, which are then adjusted by a simple estimate of tissue sensitivity to insulin: the total daily insulin requirement (TDIR) of insulin usually required by the subject. A small amount of the insulin infusion is a basal component given continuously. This component is fixed at normal and high glucose levels but linearly declines as glucose falls below the target level. When the glucose level reaches 60% of the target value, this basal rate is zero. The TDIR is adjusted for hemoglobin A1C because some persons with diabetes are chronically undertreated with insulin (which would falsely lower the gain).
To minimize development of hypoglycemia, insulin delivery is reduced as IOB rises. IOB is calculated every 5 min using a model that we developed, which is based on a pharmacokinetic/dynamic study of aspart insulin by Holmes and colleagues.3 In our system, meals are announced and a portion of the usual meal insulin is given as a pre-meal bolus. Using bihormonal control with this algorithm in subjects with T1DM, glucose averaged 135–140 mg/dl and hypoglycemia was rare, with a blood glucose less than 70 mg/dl occurring for an average of only 18 min per day.6
Compensation for Stress Hyperglycemia during Closed-Loop Treatment
During times of stress, glucose can be very erratic in patients with diabetes. Stress can include emotional upheaval, medical stress (e.g., infection, myocardial infarction, stroke), and surgical stress. Typically, stress creates a state of insulin resistance due to endogenous release of corticosteroids and catecholamines, with con-comitant activation of the sympathetic nervous system. Stress hyperglycemia is very frustrating to patients and physicians alike and constitutes a barrier to management in the hospital and at home during illness.
For these reasons, we have added a component to our algorithm designed to quickly recognize changes in tissue sensitivity to insulin and to institute a compensatory change in the proportional gain factor, derivative gain factor, basal insulin delivery, and the handling of IOB. Our algorithm measures tissue sensitivity to insulin using a method based on the studies of Hovorka25 and Bergman26. Sensitivity to insulin in our model is conceptualized as a composite of three components: (1) sensitivity of fat and muscle to insulin, (2) ability of insulin to suppress endogenous glucose production, and (3) ability of insulin to promote movement of glucose from the measurable (mainly plasma) compartment to the unmeasurable (mainly interstitial) compartment. These sensitivity measurements are calculated every 30 min. The composite insulin sensitivity value then leads to a new TDIR based on an exponential relationship between sensitivity and TDIR.
The model assumes that each change in composite sensitivity is equally distributed to each of the three insulin action components. We recognize that this assumption may be an over-simplification and that changes in sensitivity may not affect these three components equally in every subject. In order to better understand the effect of changing insulin sensitivity on the insulin action components, we carried out model analyses during which we weighted the effect on each component differently. We found that a change in insulin sensitivity exclusive to hepatic glucose production (X3) led to glycemic effects that were similar to an exclusive effect on glucose distribution (X1), though the hepatic effect led to slightly greater changes in glycemia. The model’s response to a change of sensitivity exclusive to glucose disposal (X2) resulted in a slightly more prolonged rise in glucose levels that eventually reached a level similar to that resulting from a change in distribution. This modeling exercise showed that proportionate changes in each of the insulin action components affect glucose homeostasis similarly and thus supports our decision to distribute changes equally across the three insulin action components. Nonetheless, we acknowledge that the model almost certainly does not agree perfectly with the true human physiological state, but it has proven successful in determining the change in total insulin requirement, which in turn allows the controller to adapt to situations where more or less insulin is required. For a more detailed description of the algorithm and results of a corticosteroid administration study in persons with T1DM, see “A Controlled Study of the Effectiveness of an Adaptive Closed-Loop Algorithm to Minimize Cortico-steroid-Induced Stress Hyperglycemia in Type 1 Diabetes” by El Youssef and colleagues,27 also in this issue of Journal of Diabetes Science and Technology.
The equation given below provides the amount of insulin per day (TDIR) needed to maintain a steady-state target glucose at different estimated sensitivities. Coefficient A is based on the target glucose and is 21.24 for a target of 110 mg/dl.
After each update of the sensitivity composite, the new TDIR triggers a revision in the proportional and derivative gain factors, in pre-meal insulin bolus amount, and in the degree of IOB required to reduce insulin infusion rate.
We very recently tested this system of adapting to reduced insulin sensitivity by administering hydrocortisone during closed-loop control in persons with T1DM.28 Each subject received 40 mg of hydrocortisone by mouth as a loading dose, then 20 mg every 4 h for six additional doses. The measure of composite sensitivity declined substantially several hours after the first dose and stayed low until the last dose. TDIR rose as expected, leading to the expected rises in proportional and derivative gain factors and total insulin infusion rate. Hypoglycemia was rare. Hyperglycemia developed when sensitivity declined. The increased insulin infusion rate dealt effectively with the hyperglycemia though this adaptation required several hours. Toward the end of the experiment, in a postabsorptive state, the reference blood glucose had come down and, despite continued hydrocortisone administration, was very comparable to the postabsorptive glucose starting value upon initiating the experiment (on average, ∼ 200 mg/dl). It should be noted that by changing (tuning) the relationship between sensitivity and TDIR, we could create a more aggressive adaptation to the hydrocortisone with less hyperglycemia, though there might be an increased prevalence of hypoglycemia.
Compensation for Sensor Inaccuracy by Redundancy
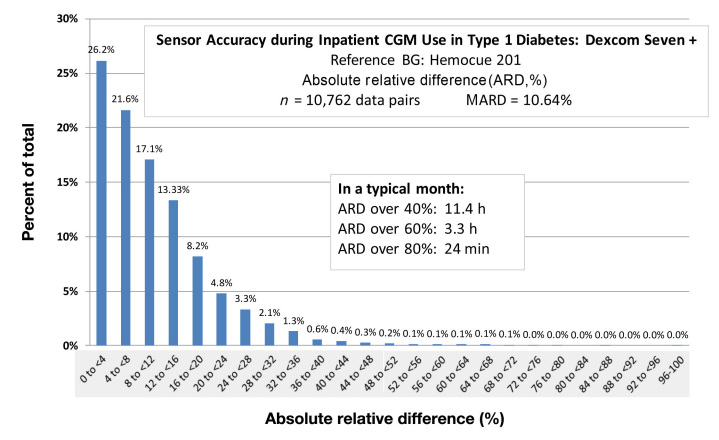
Modern amperometric glucose sensors are quite accurate. However, as shown in Figure 3, there are times when sensors drift out of calibration, leading to clinically significant error. Figure 3 is a histogram showing accuracy data from 10,762 data pairs (reference glucose and sensor glucose) collected in persons with T1DM participating in a recent artificial pancreas study. Sensors were calibrated by a very accurate instrument, the Hemocue 201. The absolute relative difference (ARD) is plotted with error magnitude increasing from left to right. The average differences are excellent with a mean ARD of 10.6%. However, it is important to note that if normalized to a 1 month period of time, there would be 11.4 h wherein the ARD would exceed 40% (and 3.3 h wherein it would exceed 60%). Such errors might lead to clinical errors in the setting of an artificial pancreas, especially if the errors are overestimates. With the use of standard outpatient glucose meters as the calibrating instrument, there would undoubtedly be a greater number of large sensor errors.
Figure 3.
A frequency histogram of sensor accuracy (ARD) obtained in subcutaneous amperometric sensors (Dexcom® SEVEN® PLUS) in persons with T1DM during closed-loop glycemic control studies. Sensors were calibrated every 6 h with arterialized venous blood using a very accurate glucose measurement device (Hemocue 201). Although mean ARD indicates very good accuracy, it should be noted that a small percentage of readings indicate poor sensor accuracy that may lead to insulin delivery errors during closed-loop treatment. CGM, continuous glucose monitoring; BG, blood glucose.
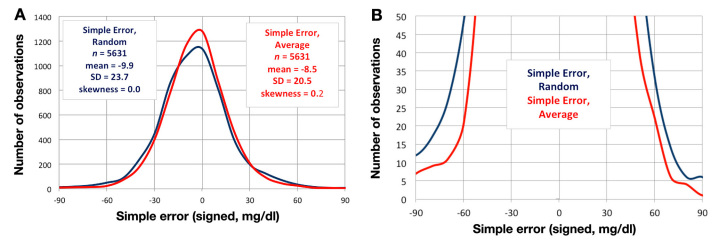
Using the same data set, Figure 4A shows frequency histo-grams that illustrate simple (signed) error magnitudes for a single (random) sensor and for the average of the two sensors’ simple errors. Both curves are predominantly Gaussian and have very low skewness coefficients, which suggests a high degree of symmetry. Figure 4B is a plot of the same data shown in Figure 4A but has an expanded y-axis to better show the distribution of error in the tails. The tail regions show that there were substantially fewer large-negative and large-positive errors with the use of averaging. In fact, for averaged values, there were only 1.2% of values that were either less than -60 mg/dl or greater than 60 mg/dl (as compared to 2.7% of the randomly chosen values). This difference represents a 56% reduction in large errors obtained with averaging.
Figure 4.
(A) A frequency histogram obtained in subcutaneous amperometric sensors (Dexcom® SEVEN® PLUS) in 14 persons with T1DM, each during 66 hours of closed-loop monitoring and treatment. Each subject had two subcutaneous Dexcom SEVEN PLUS sensors. At each time point, simple sensor error was measured (sensor glucose – venous reference Hemocue glucose) in a random sensor (blue) and the average of the two sensors (red). Note Gaussian distribution of both data sets with very low skewness coefficients, indicating symmetry. There was a slight negative bias of –8.5–10 mg/dl for both data sets. (B) The same frequency histogram as in Figure 4A, now plotted on an expanded y-axis in order to discriminate better between the distribution of random versus average data values. Especially at the tails, the average values have a substantially lower error than the random sensor values. For averaged values, there were only 1.2% of values that were either less than -60 mg/dl or greater than 60 mg/dl (as compared to 2.7% of the randomly chosen values).
The standard deviations (SDs) of simple errors and their change with averaging provide useful information regarding potential benefits from averaging. The SD for simple (signed) error values for Sensor 1 (the randomly chosen sensor) was 23.7 mg/dl and for Sensor 2 was 24.2 mg/dl (mean of the two: 23.9 mg/dl). The SD for the simple error of the average of the two sensors was 20.5 mg/dl. Thus, using an average yielded an approximate 15% reduction in SD as compared to single sensors. In addition, there was a significant correlation between the simple error of Sensor 1 and Sensor 2: r = 0.46, r2 = 0.21. The significant correlation and the finding of only a 15% reduction in SD might suggest that the benefit from averaging is minimal. However, in the tail regions of the error distribution, where the negative and positive errors are large, there was a substantial benefit in terms of the large errors as discussed above and as shown in Figure 4B.
In unpublished data, we have evaluated the spatial effect of two or more sensors on the accuracy benefit from redundancy. We found that the two sensors can be very close to one another (within 7 mm) and still maintain the benefit of redundancy.29
To better understand benefits of sensor redundancy, we also examined larger numbers of sensors in each individual. For example, in animals, we found that the use of four sensing electrodes within an array led to an improvement of sensor accuracy as compared to a single electrode.30–32 In addition to averaging, we also examined the use of two sensors in order to allow switching of the sensor used to control the main algorithm. Switching was used when a sensor had declining accuracy and was found to lead to better sensor accuracy as compared to a randomly chosen sensor.6
We acknowledge that, in addition to accuracy, user convenience is important. It may be technically difficult to develop small devices that maintain the benefit of redundancy from multiple sensing units. Alternatively, more accurate and stable sensor outputs might be achieved with the use of a single sensor to the extent that novel use of materials and sensing methodologies are exploited.
Conclusion
Hypoglycemia is a feared consequence of tight glycemic control. The addition of automated subcutaneous glucagon delivery to an artificial pancreas system safely reduces the risk of hypoglycemia. Though aqueous preparations of glucagon are currently unstable and not suitable for prolonged pump use, several programs are underway in an effort to stabilize native glucagon or to create a stable analog of glucagon. Though sensor accuracy is usually quite good, drift can sometimes lead to errors of a magnitude sufficient to cause clinical errors. Such a degree of inaccuracy can be greatly reduced by using more than one sensor concurrently.
Glossary
Abbreviations
- (ARD)
absolute relative difference
- (IOB)
insulin on board
- (MRS)
magnetic resonance spectroscopy
- (SD)
standard deviation
- (T1DM)
type 1 diabetes mellitus
- (TDIR)
total daily insulin requirement
- (TEM)
transmission electron microscopy
Funding
Juvenile Diabetes Research Foundation, Legacy Good Samaritan Foundation, Murdock Trust, Leona M. and Harry B. Helmsley Charitable Trust, HEDCO Trust, and National Institutes of Health (Dr. Castle’s salary).
Disclosures
Dr. Ward holds stock in iSense Corporation, now part of Bayer HealthCare Diabetes Care Division. All authors receive research support from Biodel, Inc and Thermalin, Inc.
References
- 1.Nixon R, Pickup JC. Fear of hypoglycemia in type 1 diabetes managed by continuous subcutaneous insulin infusion: is it associated with poor glycemic control? Diabetes Technol Ther. 2011;13:93–98. doi: 10.1089/dia.2010.0192. [DOI] [PubMed] [Google Scholar]
- 2.Anderbro T, Amsberg S, Adamson U, Bolinder J, Lins PE, Wredling R, Moberg E, Lisspers J, Johansson UB. Fear of hypoglycaemia in adults with Type 1 diabetes. Diabet Med. 2010;27(10):1151–1158. doi: 10.1111/j.1464-5491.2010.03078.x. doi: 10.1111/j.1464-5491.2010.03078.x. [DOI] [PubMed] [Google Scholar]
- 3.Holmes G, Galitz L, Hu P, Lyness W. Pharmacokinetics of insulin aspart in obesity, renal impairment, or hepatic impairment. Br J Clin Pharmacol. 2005;60(5):469–476. doi: 10.1111/j.1365-2125.2005.02476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Howey DC, Bowsher RR, Brunelle RL, Woodworth JR. [Lys(B28), Pro(B29)]-human insulin. A rapidly absorbed analogue of human insulin. Diabetes. 1994;43(3):396–402. doi: 10.2337/diab.43.3.396. [DOI] [PubMed] [Google Scholar]
- 5.Castle JR, Engle JM, El Youssef J, Massoud RG, Ward WK. Factors influencing the effectiveness of glucagon for preventing hypoglycemia. J Diabetes Sci Technol. 2010;4:1305–1310. doi: 10.1177/193229681000400603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castle JR, Engle JM, El Youssef J, Massoud RG, Yuen KC, Kagan R, Ward WK. Novel use of glucagon in a closed-loop system for prevention of hypoglycemia in type 1 diabetes. Diabetes Care. 2010;33(6):1282–1287. doi: 10.2337/dc09-2254. Epub 2010 Mar 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Russell SJ, El-Khatib FH, Nathan DM, Damiano ER. Efficacy determinants of subcutaneous microdose glucagon during closed-loop control. J Diabetes Sci Technol. 2010;4(6):1288–1304. doi: 10.1177/193229681000400602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El-Khatib FH, Russell SJ, Nathan DM, Sutherlin RG, Damiano ER. A bihormonal closed-loop artificial pancreas for type 1 diabetes. Sci Transl Med. 2010;2(27):27ra27. doi: 10.1126/scitranslmed.3000619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kadish A. Automation control of blood sugar a servomechanism for glucose monitoring and control. Trans Am Soc Artif Intern Organs. 1963;9:363–367. [PubMed] [Google Scholar]
- 10.Taborsky GJ., Jr The physiology of glucagon. J Diabetes Sci Technol. 2010;4(6):1338–1344. doi: 10.1177/193229681000400607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyle PJ, Shah SD, Cryer PE. Insulin, glucagon, and catecholamines in prevention of hypoglycemia during fasting. Am J Physiol. 1989;256(5):E651–61. doi: 10.1152/ajpendo.1989.256.5.E651. (Pt 1) [DOI] [PubMed] [Google Scholar]
- 12.Farhy LS, McCall AL. Models of glucagon secretion, their application to the analysis of the defects in glucagon counter-regulation and potential extension to approximate glucagon action. J Diabetes Sci Technol. 2010;4(6):1345–1356. doi: 10.1177/193229681000400608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ward WK, Engle JM, Duman HM, Bergstrom C, Kim S, Federiuk IF. The benefit of subcutaneous glucagon during closed-loop glycemic control in rats with type 1 diabetes. IEEE Sensors Journal. 2008;8:89–95. [Google Scholar]
- 14.El-Khatib FH, Jiang J, Gerrity RG, Damiano ER. Pharmacodynamics and stability of subcutaneously infused glucagon in a type 1 diabetic Swine model in vivo. Diabetes Technol Ther. 2007;9:135–144. doi: 10.1089/dia.2006.0006. [DOI] [PubMed] [Google Scholar]
- 15.Rivera N, Ramnanan CJ, An Z, Farmer T, Smith M, Farmer B, Irimia JM, Snead W, Lautz M, Roach PJ, Cherrington AD. Insulin-induced hypoglycemia increases hepatic sensitivity to glucagon in dogs. J Clin Invest. 2010;120(12):4425–4435. doi: 10.1172/JCI40919. doi: 10.1172/JCI40919. Epub 2010 Nov 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haymond MW, Schreiner B. Mini-dose glucagon rescue for hypoglycemia in children with type 1 diabetes. Diabetes Care. 2001;24(4):643–645. doi: 10.2337/diacare.24.4.643. [DOI] [PubMed] [Google Scholar]
- 17.Shulman GI, Rossetti L, Rothman DL, Blair JB, Smith D. Quantitative analysis of glycogen repletion by nuclear magnetic resonance spectroscopy in the conscious rat. J Clin Invest. 1987;80(2):387–393. doi: 10.1172/JCI113084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ward WK, Massoud RG, Szybala CJ, Engle JM, El Youssef J, Carroll JM, Roberts CT, Jr, DiMarchi RD. In vitro and in vivo evaluation of native glucagon and glucagon analog (MAR-D28) during aging: lack of cytotoxicity and preservation of hyperglycemic effect. J Diabetes Sci Technol. 2010;4(6):1311–1321. doi: 10.1177/193229681000400604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steiner SS, Li M, Hauser R, Pohl R. Stabilized glucagon formulation for bihormonal pump use. J Diabetes Sci Technol. 2010;4(6):1332–1337. doi: 10.1177/193229681000400606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chabenne JR, DiMarchi MA, Gelfanov VM, DiMarchi RD. Optimization of the native glucagon sequence for medicinal purposes. J Diabetes Sci Technol. 2010;4(6):1322–1331. doi: 10.1177/193229681000400605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.El Youssef J, Castle J, Ward W. A Review of Closed-Loop Algorithms for Glycemic Control in the Treatment of Type 1 Diabetes. Algorithms. 2009;2(1):518–532. [Google Scholar]
- 22.Ward WK, Wood MD, Casey HM, Quinn MJ, Federiuk IF. An implantable subcutaneous glucose sensor array in ketosis-prone rats: closed loop glycemic control. Artif Organs. 2005;29(2):131–143. doi: 10.1111/j.1525-1594.2005.29024.x. [DOI] [PubMed] [Google Scholar]
- 23.Gopakumaran B, Duman HM, Overholser DP, Federiuk IF, Quinn MJ, Wood MD, Ward WK. A novel insulin delivery algorithm in rats with type 1 diabetes: the fading memory proportional-derivative method. Artif Organs. 2005;29(8):599–607. doi: 10.1111/j.1525-1594.2005.29096.x. [DOI] [PubMed] [Google Scholar]
- 24.Federiuk IF, Casey HM, Quinn MJ, Wood MD, Ward WK. Induction of type-1 diabetes mellitus in laboratory rats by use of alloxan: route of administration, pitfalls, and insulin treatment. Comp Med. 2004;54(3):252–257. [PubMed] [Google Scholar]
- 25.Hovorka R, Canonico V, Chassin LJ, Haueter U, Massi-Benedetti M, Orsini Federici M, Pieber TR, Schaller HC, Schaupp L, Vering T, Wilinska ME. Nonlinear model predictive control of glucose concentration in subjects with type 1 diabetes. Physiol Meas. 2004;25(4):905–920. doi: 10.1088/0967-3334/25/4/010. [DOI] [PubMed] [Google Scholar]
- 26.Bergman RN, Phillips LS, Cobelli C. Physiologic evaluation of factors controlling glucose tolerance in man: measurement of insulin sensitivity and beta-cell glucose sensitivity from the response to intravenous glucose. J Clin Invest. 1981;68(6):1456–1467. doi: 10.1172/JCI110398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.El Youssef J, Castle JR, Branigan DL, Massoud RG, Breen ME, Ward K. A controlled study of the effectiveness of an adaptive closed-loop algorithm to minimize corticosteriod-induced stress hypoglycemia in type 1 diabetes. J of Diabetes Sci Technol. 2011;5(6):1312–1326. doi: 10.1177/193229681100500602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.El Youssef J, Castle JR, Massoud RG, Branigan D, Ward WK. Automated Adaptive Closed Loop Insulin Delivery for Stress Hyperglycemia in Type 1 Diabetes. Proceedings of ADA Scientific Sessions; June 2011; San Diego. 2011. [Google Scholar]
- 29.Castle JR, El Youssef J, Massoud RG, Branigan D, Ward WK. The Benefit of Multiple Glucose Sensors in Type 1 Diabetes: Implications for Artificial Pancreas Design. Proceedings of ADA Scientific Sessions; June 2011; San Diego. 2011. [Google Scholar]
- 30.Ward WK, Troupe JE. Assessment of chronically implanted subcutaneous glucose sensors in dogs: the effect of surrounding fluid masses. ASAIO J. 1999;45(6):555–561. doi: 10.1097/00002480-199911000-00009. [DOI] [PubMed] [Google Scholar]
- 31.Ward WK, Wood MD, Troupe JE. Understanding spontaneous output fluctuations of an amperometric glucose sensor: effect of inhalation anesthesia and use of a nonenzyme containing electrode. ASAIO J. 2000;46(5):540–546. doi: 10.1097/00002480-200009000-00006. [DOI] [PubMed] [Google Scholar]
- 32.Ward WK, Wood MD, Troupe JE. Rise in background current over time in a subcutaneous glucose sensor in the rabbit: relevance to calibration and accuracy. Biosens Bioelectron. 2000;15(1-2):53–61. doi: 10.1016/s0956-5663(00)00051-8. [DOI] [PubMed] [Google Scholar]