Abstract
Background
Safe and effective glucose control in the intensive care unit (ICU) continues to be actively pursued. Large clinical trials have examined the safety and efficacy of insulin infusion protocols in medical and surgical ICUs. We report experiences of a single-center standardized nurse-driven insulin infusion protocol in three ICUs in an observational quality-improvement study.
Method
We analyzed the hourly point-of-care arterial blood glucose obtained during ICU insulin infusion protocol (protocol A) with a glucose target of 80–130 mg/dl in medical and surgical ICUs in February 2009. Following Normoglycemia in Intensive Care Evaluation and Survival Using Glucose Algorithm Regulation (NICE-SUGAR) study results, the protocol was amended (protocol B) to achieve target glucose of 110–150 mg/dl. The performance of protocol B was assessed in the ICUs in May 2010 and compared with protocol A with respect to glucose concentrations and rates of severe (<40 mg/dl) and moderate (40–60 mg/dl) hypoglycemia.
Results
With protocol A, in medical (n = 44) and surgical (n = 164) ICUs taken together, median glucose was 119 mg/dl, with severe and moderate hypoglycemia rates 1.4% (3/208) and 7.7% (16/208), respectively, which were significantly lower than those reported by the NICE-SUGAR and the Leuven studies. With protocol B, in medical (n = 44) and surgical (n = 167) ICUs taken together, median glucose was 132 mg/dl, with severe and moderate hypoglycemia of 0 % (0/211) and 0.5% (1/211), respectively.
Conclusion
The current ICU insulin infusion protocol (protocol B) reduces severe and moderate hypoglycemia without compromising glucose control when compared with protocol A. This could potentially impact patient-important outcomes.
Keywords: hypoglycemia, insulin infusion, intensive care unit, point-of-care glucose
Introduction
Hyperglycemia in the hospitalized patient with critical illness has been linked to increased morbidity and mortality.1,2 However, prospective interventional studies designed to examine the effects of intensive versus conventional glucose control in critically ill patients in the intensive care units (ICU) have provided conflicting results.3–6 As a result, a firm evidence-based consensus regarding optimal glycemic target in the critically ill continues to be elusive and controversial, at least in part related to risk of hypoglycemia. The meta-analyses by Griesdale and colleagues7 suggested that, although intensive insulin therapy significantly increased the risk of hypoglycemia and conferred no overall mortality benefit among critically ill patients (both medical and surgical ICU together), this approach was beneficial for those in the surgical ICU.6 The current guidelines by the American Association of Clinical Endocrinologists and the American Diabetes Association recommend a target glucose level of 140–180 mg/dl with treatment threshold of 180 mg/dl in ICU patients.8
Severe hypoglycemia (glucose <40 mg/dl) in the ICU patient has been shown to be an independent risk factor for increased morbidity and mortality.5,6,9 Single and multi-center clinical trials have examined the safety and efficacy of unique insulin infusion protocols in medical and surgical ICUs.3,5,6 Such trials, while achieving their desired glucose goals, have been fraught with increased frequency of severe hypoglycemia (<40 mg/dl). Furthermore, the role of moderate hypoglycemia (arbitrarily defined here as glucose values 40–60 mg/dl) on morbidity has been largely ignored, especially in the landmark Leuven studies.5,6 A report highlighting the relevance of glucose values <80 mg/dl in the critically ill underscores the importance of preventing hypoglycemia in this patient population.9
The purpose of our observational, retrospective, quality-improvement project was to determine the efficacy and safety of a nurse-driven, insulin infusion protocol before and after modification of the protocol following publication of the NICE-SUGAR (Normoglycemia in Intensive Care Evaluation and Survival Using Glucose Algorithm Regulation) study.3 Our hypothesis was that the modified Mayo ICU insulin infusion protocol (protocol B) would reduce the rates of severe hypo-glycemia without compromising glycemic control when compared with protocol A. As per institutional review board guidelines, because this was a quality-improvement project, this study was deemed exempt from Mayo institutional review board approval.
Patients and Methods
Study Design
We first performed a retrospective, descriptive analysis of our nurse-driven Mayo insulin infusion protocol with a glycemic target of 80–130 mg/dl (protocol A) in one medical and two surgical ICUs. In the light of published NICE-SUGAR data, an amended protocol was designed with a modified glycemic target of 110–150 mg/dl (protocol B). The insulin infusion protocol was activated if blood glucose was greater than 130 mg/dl in protocol A and greater than 150 mg/dl in protocol B at entry into the ICUs.
Glucose Measurement
Hourly blood glucose measurements were retrieved from the electronic database for the months of February 2009 (protocol A) and May 2010 (protocol B) for one medical and two surgical ICUs (general surgery and cardiothoracic surgery) on patients who were on the insulin infusion protocols. Hourly point-of-care arterial blood glucose was measured and recorded as per protocol using Roche Accu-Chek Inform® glucose meters. Interassay precision for the glucose meters across the institution was 4% to 6%.10 Daily quality controls were maintained, and testing was performed by laboratory personnel. All glucose values greater than 400 mg/dl or lower than 40 mg/dl obtained from glucose meters were verified with plasma glucose checks, wherever possible, performed in the laboratory. All meter glucose data were automatically downloaded into the laboratory information system. To avoid errors, all data were uploaded electronically, and manual data entry was not performed. The performance of protocol A was compared with protocol B in terms of glucose target achieved and hypoglycemia rates.
Hypoglycemia and Median Time to Goal
Severe hypoglycemia was defined as blood glucose <40 mg/dl and moderate hypoglycemia as blood glucose 40–60 mg/dl. Time to reach glycemic goal was calculated from the first glucose value outside the target range to the first glucose value within the target range. Median time to achieve glycemic goal, median glucose concentration, and moderate and severe hypoglycemic events were compared between protocols A and B and across medical and surgical ICUs in the two protocols. Each hypoglycemic event was analyzed for infusion protocol deviations, influence of concurrent medications, reason for hospitalization, and comorbidities. Protocol violation was defined as delayed or missed hourly glucose, delay in changing insulin infusion rate, and incorrect infusion rate.
Primary outcome measures were rates of moderate and severe hypoglycemia. Secondary outcome measures were glycemic control and causes of hypoglycemia that occurred.
Statistical Analysis
Median glucose concentrations and median times to achieve goal blood glucose in surgical and medical ICUs were compared in the same protocol as well as across the two protocols using the Wilcoxon test. Due to the skewed distribution of glucose values, glucose values of surgical and medical ICUs were also compared after log transformation using Student’s t-test. Severe and moderate hypoglycemic events were compared using two-tail Fisher test between two protocols in total cohort as well as across the medical and surgical ICUs. All statistical analyses were performed using JMP 8 software (SAS Institute, Carey, NC).
Results
Subject characteristics are shown in Table 1. Study cohorts in protocol A were comparable to that in protocol B in both medical and surgical ICUs in terms of age, gender, number of subjects, and type of diabetes. The study cohort in protocol A comprised 208 patients, of which 44 (male:female = 23:21) were from medical ICU and 164 were from combined surgical ICUs (general surgery 121, male:female = 84:37; cardiovascular surgery 43, male: female=29:14). In the medical ICU, 39% of patients had type 2 diabetes mellitus (T2DM) and 2% had type 1 diabetes mellitus (T1DM), while in surgical ICUs, 21% had T2DM and 2% had T1DM.
Table 1.
Subject Characteristics
| Subject characteristic | ICU | Protocol A | Protocol B | P value |
|---|---|---|---|---|
| Combined | 67 (20–93; 23) | 68 (20–91;22) | 0.87 | |
| Median age (min–max; IQRa) | Medical | 70 (20–93; 13) | 67 (20–91; 22) | 0.34 |
| Surgical | 66 (22–91; 23) | 68 (21–91; 19) | 0.78 | |
| Combined | 136:72 | 128:83 | 0.31 | |
| Gender (male:female) | Medical | 23:21 | 28:16 | 0.38 |
| Surgical | 113:51 | 100:67 | 0.08 | |
| Combined | 208 | 211 | – | |
| Number of subjects (n) | Medical | 44 | 44 | 1.0 |
| Surgical | 164 | 167 | 1.0 | |
| T1DM = 4 | T1DM = 3 | 0.72 | ||
| Combined | T2DM = 52 | T2DM = 56 | 0.74 | |
| No diabetes = 152 | No diabetes = 152 | 1.0 | ||
| T1DM = 1 | T1DM = 1 | 1.0 | ||
| Type of diabetes | Medical | T2DM = 17 | T2DM = 22 | 0.39 |
| No diabetes = 26 | No diabetes = 21 | 0.67 | ||
| T1DM = 3 | T1DM = 2 | 0.68 | ||
| Surgical | T2DM = 35 | T2DM = 34 | 1.0 | |
| No diabetes = 126 | No diabetes = 131 | 0.89 |
IQR, interquartile range
The study cohort in protocol B comprised 211 patients, of which 44 patients (male:female = 28:16) were from medical ICU and 167 patients were from the combined surgical ICUs (general surgery 146, male:female = 86:60; cardiovascular surgery 21, male:female = 14:7). In the medical ICU, 50% of patients had T2DM and 2% had T1DM, while in surgical ICUs, 20% had T2DM and 1% had T1DM.
Protocol A
In medical and surgical ICUs combined, median glucose was 118.5 mg/dl (range: 23–600 mg/dl) and time to goal was 2 h 47 min. Severe hypoglycemia occurred in 1.4% (3/208; 4 episodes) of the patients, while moderate hypoglycemia occurred in 7.2% (15/208; 17 episodes) of the patients (Table 2).
Table 2.
Median Glucose, Time to Goal, and Hypoglycemia with Protocols A and B in Intensive Care Units
| Both Medical and Surgical ICUs | |||||
|---|---|---|---|---|---|
| Protocol | Type of diabetes | Median time to goal hh:mm (min–max; IQRa) | Median glucose mg/dl (min–max; IQRa) | Number of subjects with glucose <40 mg/dl | Number of subjects with glucose 40–60 mg/dl |
| A N = 208 | T1DM = 4 T2DM = 52 No diabetes = 152 | 2:47 (00:00–39:38; 2:45) | 118.5 (23–600; 35) | 3 | 15 |
| B N = 211 | T1DM = 3 T2DM = 56 No diabetes = 152 | 2:04 (00:00–11:20; 2:41) | 132 (53–590; 30) | 0 | 1 |
| P value | 0.001 | 0.001 | 0.12 | 0.0002 | |
| Medical ICU | |||||
| A N = 44 | T1DM = 1 T2DM = 17 No diabetes = 26 | 2:55 (0:52–32:13; 2:12) | 120 (36–600; 38) | 1 | 6 |
| B N = 44 | T1DM = 1 T2DM = 22 No diabetes = 21 | 1:22 (0:10–11:15; 3:54) | 136 (53–590; 42) | 0 | 1 |
| P value | 0.11 | 0.001 | 1 | 0.11 | |
| Surgical ICUs | |||||
| A N = 164 | T1DM = 3 T2DM = 35 No diabetes = 126 | 2:46 (00:00–39:38; 3:15) | 118.5 (23–550; 35) | 2 | 9 |
| B N = 167 | T1DM = 2 T2DM = 34 No diabetes = 131 | 2:05 (00:00–11:20; 2:11) | 131 (62–300; 28) | 0 | 0 |
| P value | – | 0.003 | 0.001 | 0.24 | 0.0015 |
IQR, interquartile range
Of importance, median time to hypoglycemia (<60 mg/dl) from the start of insulin infusion in the medical ICU was 23 h 24 min (range: 3 h 2 min to 174 h 1 min), in the general surgical ICU was 31 h 55 min (range: 2 h 18 min to 333 h 48 min), and in the cardiothoracic ICU was 4 h 30 min (range: 3 h 53 min to 24 h 52 min). Hence there was a wide variability in the onset of hypoglycemia among the ICUs, likely precluding analyses of causal issues related to the implementation of infusion protocols in specific ICUs.
In the medical ICU, median glucose was 120 mg/dl (range: 36–600 mg/dl) and time to goal was 2 h 55 min (Table 2). In the combined surgical ICUs, median glucose was 118.5 mg/dl (range: 23–550 mg/dl) and median time to goal was 2 h 46 min (Table 2). There was no significant difference in median glucose values (p = .65) and time to achieve glycemic goal (p = .78) between medical ICU and combined surgical ICU.
In the medical ICU, 2.3% of patients (n = 1/44; 1 episode) had severe hypoglycemia and 13.6% (n = 6/44; 7 episodes) had moderate hypoglycemia (Table 2). In surgical ICUs, 1.2% of patients (n = 2/164; 3 episodes) had severe hypoglycemia and 5.4% (n = 9/164; 10 episodes) had moderate hypoglycemia (Table 2). Further analyses of all causes of hypoglycemia revealed protocol violations in 86% (18/21; 4/21 episodes were due to missed hourly glucose values and 14/21 episodes were due to incorrect insulin infusion rates that resulted from not changing the insulin infusion rates based on prevailing glucose concentrations) of the episodes. The remaining 14% (3/21) episodes occurred despite patients having hourly glucose values and adherence to protocol insulin infusion rates. Of 16 patients with hypoglycemia, 7 had T2DM (39%) and 1 had T1DM (6.3%).
Protocol B
In the combined medical and surgical ICUs, median glucose was 132 mg/dl (range: 53–590 mg/dl) and time to goal was 2 h 4 min. There was no severe hypoglycemia, while only one patient had a single episode of moderate hypo-glycemia (n = 1/211; one episode; Table 2).
In the medical ICU, median glucose was 136 mg/dl (range: 53–590 mg/dl) and time to goal was 1 h 22 min (Table 2). In the combined surgical ICUs, median glucose was 131 mg/dl (range: 62–300 mg/dl) and median time to goal was 2 h 5 min (Table 2). There was no significant difference in median glucose values (p = .7) and time to achieve glycemic goal (p = .87) between medical ICU and combined surgical ICUs.
There were no episodes of severe hypoglycemia in either medical or combined surgical ICUs, with one episode of moderate hypoglycemia (53 mg/dl) in the medical ICU. The patient with moderate hypoglycemia had T1DM, and hypoglycemia occurred despite having hourly glucose values and adherence to protocol insulin infusion rates.
In the medical ICU, no significant reduction in moderate (p = .06) as well as severe (p = 1.0) hypoglycemia was noted when switched from protocol A to B (Table 2). In contrast, in surgical ICUs, significant reduction in moderate hypoglycemia was observed (p = .001) without any change in severe hypoglycemia (p = .12) when switched from protocol A to B (Table 2). Similarly significant decrease in moderate hypoglycemia was noted in protocol B when compared with protocol A for combined medical and surgical ICUs (p < .001), but severe hypoglycemia was not different across the two protocols (p = .06; Table 2). However, it is likely that low rates of hypoglycemia during protocol B precludes reliable interpretation of differences in hypoglycemia rates between the two protocols.
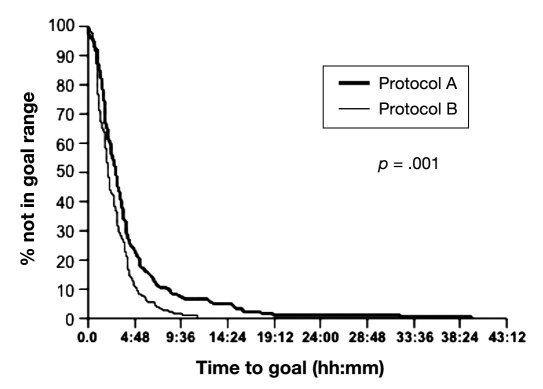
As shown in Figure 1, the median time to achieve glucose goal was slightly but significantly lower for protocol B than protocol A (p < .001).
Figure 1.
Time to achieve glucose goals during insulin protocols A and B in ICUs. Figure shows the percentage of subjects achieving glucose goal as a function of median time to reach target glucose for protocols A and B.
Discussion
Commonly observed stress hyperglycemia was considered as an adaptive beneficial response in critically ill hospitalized patients in the 20th century.11 There has been accumulating evidence demonstrating that even modest hyperglycemia is an important risk factor in terms of mortality and morbidity in critically ill patients with, e.g., myocardial infarction, stroke, traumatic brain injury, or sepsis.12 In 2001, Van Den Berghe and associates6 reported that intensive glucose control reduced morbidity and mortality in critically ill patients in the surgical ICU. However, this result may need to be considered in the context of possible overfeeding of these patients. When this study design was reproduced in the setting of a medical ICU, the same authors found that intensive glucose control significantly reduced morbidity but not mortality in selected patients only (i.e., those in ICU beyond 3 days).12 Subsequently, several large multicenter studies could not confirm the survival benefit of intensive glycemic control in both medical and surgical ICUs.3,4,13,14 Table 3 compiles the target glucose concentrations, achieved glucose concentrations, and rates of severe hypoglycemia obtained in several large clinical trials and compares those with our results obtained from both protocol A and protocol B.
Table 3.
Comparison of Frequency of Severe Hypoglycemia in Published Clinical Trials
| First author/study | ICU | Target glycemic range (mg/dl) | Median glucose (mg/dl) | Glucose <40 mg/dl |
|---|---|---|---|---|
| Van den Berghe6 | Surgical | 80–110 | 103 | 5.1% |
| Van den Berghe5 | Medical | 80–110 | 111 | 18.7% |
| Brunkhorst13 (VISEP) | Combined | 80–110 | 130 | 17% |
| Preiser4 (GLUCONTROL) | Combined | 110 | 9.8% | |
| Finfer3 (NICE-SUGAR) | Combined | 81–108 | 118 | 6.8% |
| Mayo ICU insulin infusion protocol A | Combined | 80–130 | 118.5 | 1.4% |
| Mayo ICU insulin infusion protocol B | Combined | 110–150 | 132 | 0% |
The VISEP (Volume Substitution and Insulin Therapy in Severe Sepsis) and GLUCONTROL (Glucose Control Regimens by Insulin in Intensive Care Unit Patients) studies were stopped prematurely, as intensive insulin therapy was associated with a significantly increased rate of severe hypoglycemic events4,13 (Table 3). The NICE-SUGAR results showed that a glucose target of 140–180 mg/dl resulted in lower mortality and hypoglycemic events than a target of 81–108 mg/dl. On the other hand, severe hypoglycemia was also shown to be an independent risk factor for mortality in the surgical ICU study by Van den Berghe and associates.6 Furthermore, studies report that even mild or moderate hypoglycemia is an independent predictor of hospital mortality.9
Considering the high rates of hypoglycemia reported with tight glycemic goals in critically ill patients in previous studies and lack of survival benefit, our initial insulin infusion protocol (protocol A) was designed to lower the rates of hypoglycemia while retaining the benefits of reasonable glucose control using the glucose target of 80–130 mg/dl (instead of 80–110 mg/dl, as chosen in the Leuven studies). With protocol A, median glucose value in the medical and surgical ICUs (120 and 118 mg/dl, respectively) were higher than reported by Van den Berghe and associates.5,6 Time taken to achieve glycemic goal was not different between medical and combined surgical ICUs. Following publication of the NICE-SUGAR study, we modified the target glucose goal in critically ill to 110–150 mg/dl and designed a modified protocol B. We then examined the glucose parameters in the same ICUs where the original data set was collected and compared the results obtained from protocol B with protocol A. As anticipated, we found that, with protocol B, median glucose achieved in the medical ICU was 136 mg/dl and in the surgical ICU was 131 mg/dl; median glucose for the combined ICUs was 132 mg/dl. The rate of severe hypoglycemia was abolished to 0%.
Severe hypoglycemic events in combined medical and surgical ICUs using protocol A was much lower than previous reports.3,6,12 Similarly, with protocol A, severe hypoglycemic events in medical and surgical ICUs were lower than previous similar reports when compared separately as per the type of ICU.4,5 We also report here rates of moderate hypoglycemia (7.7% in combined medical and surgical ICUs during protocol A that have been hitherto unreported in prior large clinical trials. With the use of protocol B, while severe hypoglycemic events were reduced to zero, moderate hypoglycemia was reduced to a single episode. This patient had T1DM with multiple comorbidities (hypothyroidism, stroke, and pneumonia) and was admitted to the medical ICU. Since there is evidence that even mild to moderate hypoglycemia is associated with increased hospital mortality, it is important to keep track of these episodes and take measures to mitigate both moderate and severe hypoglycemia without compromising glycemic control.9
The current study represents part of a quality-improve-ment project within our institution. Hence we report here a purely descriptive analysis and attempt to explore the frequency and possible reasons that may have led to hypoglycemia in our cohort. Patient-important outcome data from a larger cohort will need to be analyzed to determine the short- and long-term clinical impact of our achieved level of glycemic control.
Limitations of the Study
In this study, we have defined target glucose for moderate hypoglycemia as less than 60 mg/dl. However, the concerning evidence of adverse outcomes at blood glucose below 80 mg/dl suggests that it would be more meaningful to also include these patients.9 Unavailability of Acute Physiology and Chronic Health Evaluation or Simplified Acute Physiology Score predictive data in every patient precludes us to comment on risk factors associated with hypoglycemia. We do not have any data for nursing time required to utilize the two protocols. Severe hypoglycemia was occasionally not confirmed with plasma glucose confirmatory checks prior to initiating measures to treat the patient, as the delay required to do so would have been unsafe for these critically ill patients. Furthermore, although reflectance meters utilized to measure arterial blood glucose values in our ICUs were quality checked, these meters are likely less accurate than laboratory-derived values (the latter are time-consuming). Clearly, more precise real-time methods to measure bedside glucose values in the ICU are critical in our quest to implement safe and effective glucose control in the critically ill.
The study data were obtained as a part of a quality-improvement project, and due to limited resources, the data analyzed were limited and adequate sample size and power calculations were not feasible. We are in the process of acquiring further resources to expand the scope of the study and obtain data regarding patient outcomes.
Conclusions
We conclude that (1) glucose control can be achieved effectively and safely with a nurse-driven insulin infusion protocol in all ICU units and (2) hourly blood glucose checks are important while critically ill patients are maintained on an intravenous insulin infusion to minimize risk of hypoglycemia.
Glossary
Abbreviations
- (ICU)
intensive care unit
- (NICE-SUGAR)
Normoglycemia in Intensive Care Evaluation and Survival Using Glucose Algorithm Regulation
- (T1DM)
type 1 diabetes mellitus
- (T2DM)
type 2 diabetes mellitus
References
- 1.Faustino EV, Apkon M. Persistent hyperglycemia in critically ill children. J Pediatr. 2005;146(1):30–34. doi: 10.1016/j.jpeds.2004.08.076. [DOI] [PubMed] [Google Scholar]
- 2.Krinsley JS. Association between hyperglycemia and increased hospital mortality in a heterogeneous population of critically ill patients. Mayo Clin Proc. 2003;78(12):1471–1478. doi: 10.4065/78.12.1471. [DOI] [PubMed] [Google Scholar]
- 3.NICE-SUGAR Study Investigators. Finfer S, Chittock DR, Su SY, Blair D, Foster D, Dhingra V, Bellomo R, Cook D, Dodek P, Henderson WR, Hébert PC, Heritier S, Heyland DK, McArthur C, McDonald E, Mitchell I, Myburgh JA, Norton R, Potter J, Robinson BG, Ronco JJ. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360(13):1283–1297. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 4.Preiser JC, Devos P, Ruiz-Santana S, Mélot C, Annane D, Groeneveld J, Iapichino G, Leverve X, Nitenberg G, Singer P, Wernerman J, Joannidis M, Stecher A, Chioléro R. A prospective randomised multi-centre controlled trial on tight glucose control by intensive insulin therapy in adult intensive care units: the Glucontrol study. Intensive Care Med. 2009;35(10):1738–1748. doi: 10.1007/s00134-009-1585-2. [DOI] [PubMed] [Google Scholar]
- 5.Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, Van Wijngaerden E, Bobbaers H, Bouillon R. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354(5):449–461. doi: 10.1056/NEJMoa052521. [DOI] [PubMed] [Google Scholar]
- 6.Van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345(19):1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 7.Griesdale DE, de Souza RJ, van Dam RM, Heyland DK, Cook DJ, Malhotra A, Dhaliwal R, Henderson WR, Chittock DR, Finfer S, Talmor D. Intensive insulin therapy and mortality among critically ill patients: a meta-analysis including NICE-SUGAR study data. CMAJ. 2009;180(8):821–827. doi: 10.1503/cmaj.090206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moghissi ES, Korytkowski MT, DiNardo M, Einhorn D, Hellman R, Hirsch IB, Inzucchi SE, Ismail-Beigi F, Kirkman MS, Umpierrez GE, American Association of Clinical Endocrinologists; American Diabetes Association American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care. 2009;32(6):1119–1131. doi: 10.2337/dc09-9029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Egi M, Bellomo R, Stachowski E, French CJ, Hart GK, Taori G, Hegarty C, Bailey M. Hypoglycemia and outcome in critically ill patients. Mayo Clin Proc. 2010;85(3):217–224. doi: 10.4065/mcp.2009.0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karon BS, Gandhi GY, Nuttall GA, Bryant SC, Schaff HV, McMahon MM, Santrach PJ. Accuracy of roche accu-chek inform whole blood capillary, arterial, and venous glucose values in patients receiving intensive intravenous insulin therapy after cardiac surgery. Am J Clin Pathol. 2007;127(6):919–926. doi: 10.1309/6RFQCKAAJGKWB8M4. [DOI] [PubMed] [Google Scholar]
- 11.Vanhorebeek I, Langouche L, Van den Berghe G. Tight blood glucose control with insulin in the ICU: facts and controversies. Chest. 2007;132(1):268–278. doi: 10.1378/chest.06-3121. [DOI] [PubMed] [Google Scholar]
- 12.Vanhorebeek I, Ingels C, Van den Berghe G. Intensive insulin therapy in high-risk cardiac surgery patients: evidence from the Leuven randomized study. Semin Thorac Cardiovasc Surg. 2006;18(4):309–316. doi: 10.1053/j.semtcvs.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Brunkhorst FM, Engel C, Bloos F, Meier-Hellmann A, Ragaller M, Weiler N, Moerer O, Gruendling M, Oppert M, Grond S, Olthoff D, Jaschinski U, John S, Rossaint R, Welte T, Schaefer M, Kern P, Kuhnt E, Kiehntopf M, Hartog C, Natanson C, Loeffler M, Reinhart K, German Competence Network Sepsis (SepNet) Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358(2):125–139. doi: 10.1056/NEJMoa070716. [DOI] [PubMed] [Google Scholar]
- 14.Mesotten D, Van den Berghe G. Clinical benefits of tight glycaemic control: focus on the intensive care unit. Best Pract Res Clin Anaesthesiol. 2009;23(4):421–429. doi: 10.1016/j.bpa.2009.08.006. [DOI] [PubMed] [Google Scholar]