Abstract
Background
Glycated albumin (GA) is a medium-term glycemic control marker of diabetes and may be more sensitive to changes in plasma glucose than hemoglobin A1c. We studied where and how many fructosyl groups bind to albumin, and which glycation sites are measured by the enzymatic method for GA. We also studied the basic performance of the enzymatic method for GA.
Methods
Glycated albumin was measured using an enzymatic method (Lucica®GA-L, Asahi Kasei Pharma) on a biochemical autoanalyzer. Molecular weights of purified GA and nonglycated albumin were measured by a mass spectrometry system. Two hundred one healthy volunteers with normal results of oral glucose tolerance testing were recruited to determine the reference range in Americans.
Results
The present method measured only glycated amino acids from albumin in serum protein. We estimate that the number of glycated amino acids measured by this method was approximately two per molecule of albumin. The general performance (sensitivity, specificity, reproducibility, linearity, interference) of the method was good. The reference range of GA% in Americans with normal glucose tolerance was determined to be 11.9–15.8% (mean ± 2 standard deviations). Significant differences were not observed between the sexes; however, race differences were observed (higher levels in blacks relative to whites).
Conclusions
The method was specific for measuring glycated amino acids in albumin and had good basic performance characteristics. The reference range in Americans was 11.9–15.8%. This method may be a useful indicator for diabetes control.
Keywords: diabetes, enzymatic method, glycated albumin, reference range
Introduction
The levels of several glycated proteins are increased in patients with diabetes and it has been suggested that some of these proteins are involved in the development of chronic diabetic complications.1 Based on the results of the Diabetes Control and Complications Trial (DCCT),2 a hemoglobin A1c (HbA1c) level of <7% has been recommended to prevent the onset and progression of chronic diabetic complications. However, HbA1c may not be suitable for evaluating short-term variations in glycemic control due to the long lifespan of erythrocytes (120 days).3 Furthermore, HbA1c is influenced by hemolytic anemia and any other condition that affects the lifespan of erythrocytes, and by the presence of abnormal hemoglobin; therefore, it may be flawed as an indicator of blood glucose control.4–6
Glycated albumin (GA) is an important indicator of diabetes control7 as it is more sensitive to changes in blood glucose than HbA1c.8–10 Measuring the GA level should provide useful information on glycemic control when monitoring the effects of changes in therapy in patients with diabetes8–11 or gestational diabetes,8,9,12 when more rapid treatment responses are needed as HbA1c levels change more slowly. Moreover, measurement of GA levels provides accurate information for glycemic control in the case of variant hemoglobins and any other condition that affects the lifespan of the erythrocyte, such as hemolytic and renal anemia.4,9,10
It is known that there are many glycation sites on albumin,13,14 and so, measured GA levels differ depending on the glycation site measured. Historically, GA has been measured by several methods including boronate affinity chromatography,15,16 ion exchange chromatography,7,17 thiobarbituric acid (TBA) assay,7 immunoassay,18,19 enzyme-linked boronate-immunoassay,20 and high-performance liquid chromatography (HPLC).21,22 Table 1 summarizes the normal ranges and analytes measured by these GA measurement methods.
Table 1.
Reported Normal Range of Glycated Albumina
| Method | Normal range (%) | Analyte | Reference |
|---|---|---|---|
| Enzyme-linked immunoassay | 0.4–2.0 | Total or partial GAA | 19 |
| Radioimmunoassay | 2.1–4.9c | 18 | |
| Enzyme-linked boronate immunoassay | 3.4–7.2b | 20 | |
| Boronate affinity chromatography | 1.5–5.4 | ALB molecule | 15 |
| 6.8–10.3 | 16 | ||
| Carboxymethyl cellulose ion exchange method | 3.2–10.8b | 7 | |
| 6–15 | 17 | ||
| Boronate affinity (HPLC) | 13.9–18.3b | 21 | |
| TBA method | 3.9–12.7b | Total GAA | 7 |
| Enzymatic method | 12.3–16.9 | 29 |
TBA, thiobarbituric acid; GAA, glycated amino acids; ALB, albumin
Calculated normal range (mean ± 2 SD) from reported mean and SD
Calculated normal range (mean ± 2 SD) from 0.53 ± 0.05 nmol/mg human serum albumin (mean ± SD)
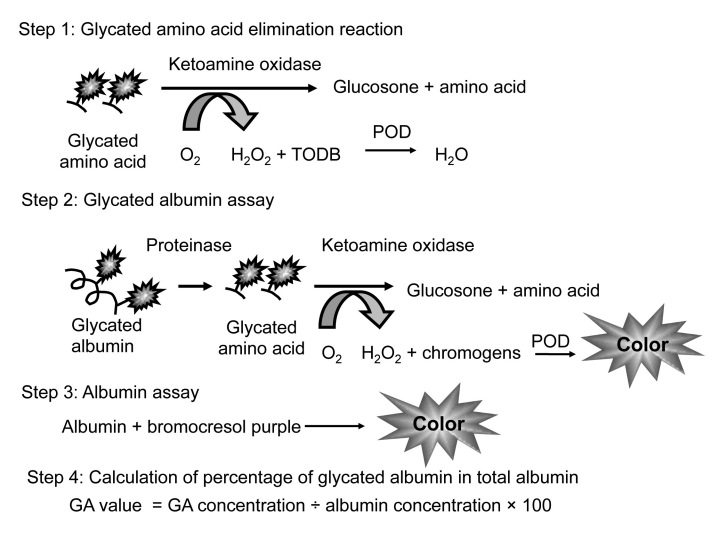
We developed an enzymatic method to measure GA more easily and accurately.23,24 In the enzymatic assay, endogenous glycated amino acids and peroxide are first eliminated by ketoamine oxidase and peroxidase reaction. Second, GA is hydrolyzed to amino acid or peptide by an albumin-specific proteinase, and then the glycated amino acid or peptide is oxidized by ketoamine oxidase producing hydrogen peroxide, which is measured quantitatively. Third, the albumin concentration is measured by the bromocresol purple (BCP) method.25 Finally, GA levels are calculated as a percentage of GA relative to total albumin (Figure 1).
Figure 1.
Assay procedure of an enzymatic method for glycated albumin. POD, peroxidase; TODB, N,N’-bis(4-sulfobutyl)-3-methylanilin, disodium salt.
If the enzymatic method were specific to albumin and could measure all of the glycation sites of albumin, it would be an ideal method for determining the GA. We studied the specificity of the present method and the number of glycated amino acids measured in one albumin molecule using this enzymatic method. We also studied the basic performance and determined the reference range for GA values in nondiabetic Americans using this method.
Materials and Methods
Materials
Common reagents and purified serum proteins were purchased from Sigma-Aldrich Co. (St. Louis, MO). Pooled serum was purchased from Veritas Co. (Tokyo, Japan). Purification of glycated albumin was performed as we reported in another study.23 The kit (Lucica®GA-L) for the enzymatic method was purchased from Asahi Kasei Pharma Co. (Tokyo, Japan).10 The glycated amino acid concentration of the calibrator was calculated as reported in another study.20
Performance Study of Enzymatic Method for Glycated Albumin
Automated enzymatic assay of GA was performed with a Model 7170s biological auto analyzer (Hitachi, Tokyo, Japan). Measurements were done according to the package insert of the Lucica GA-L. Automated HPLC assay of GA was performed as described in other studies.21,22 Molecular weights of purified GA and nonglycated albumin (NGA) were measured by a mass spectrometry system. Measurements were performed using QSTAR® Pulsar i mass spectrometer (Applied Biosystems, Carlsbad, CA). All analyses were performed in positive ion mode, and the measured range was 1000–2000 Da.
General performances (linearity, recovery, reproducibility, interference, stability of samples) of the enzymatic assay for glycated albumin were evaluated as described in another study.23 To study the reagent stability, reagents were stored at 4 and 10 °C for 1 year and measured for normal and abnormal concentrations of GA serums (-80 °C stored) at 0, 12, and 13 months.
Reference Range Study
Healthy subjects without a known history of diabetes and residing in North Carolina underwent a 75 g oral glucose tolerance test (OGTT). Those with normal OGTT results [n = 201, mean ± standard deviation (SD) fasting blood glucose; 85.6 ± 6.6 mg/dl, 2-hour blood glucose; 86.9 ± 21.3 mg/dl] were selected (male/female = 95/106, white/black = 107/94). The study was performed in accordance with the principles of the Declaration of Helsinki as revised in 2000. The Institutional Review Board at the Wake Forest School of Medicine approved the study protocol and all participants provided written informed consent.
Glycated albumin was measured using a Lucica GA-L kit on frozen serum samples and GA analysis was performed using an automated ADVIA 1650 instrument (Siemens Medical Solutions Diagnostics, Tarrytown, NY). Next, GA (%) was computed according to the manufacturer’s instructions as [(GA ÷ modified BCP serum albumin) 100 ÷ 1.14 + 2.9]. The reference range was shown as mean ± 2 SD.
Statistics
All statistical analyses were performed using StatFlex (Artec Inc., Osaka, Japan). A p value of less than .05 was considered statistically significant. Data are shown as mean ± SD. Comparisons between groups were carried out by the Mann-Whitney U test. Spearman’s rank correlation coefficients were produced using the entire sample set.
Results
1. Performance of Enzymatic Method for Glycated Albumin
Specificity for Albumin
Table 2 shows the specificity of the present method for albumin; the method does not measure glycated amino acids in serum proteins other than albumin.
Table 2.
Specificity of Enzymatic Method for Glycated Albumin
| Sample | Concentration in vivo | GA [ ] | Influence rate | ALB [ ] | Influence rate |
|---|---|---|---|---|---|
| g/dl | g/dl | % | g/dl | % | |
| Human serum albumin | 4.4 | 3.12 | 99.9 | 3.69 | 100.1 |
| γ-globulin | 1.2 | 0.01 | 0.3 | 0.00 | 0.12 |
| Transferrin | 0.3 | 0.00 | -0.1 | 0.00 | 0.09 |
| α2-macroglobulin | 0.2 | 0.00 | -0.1 | 0.02 | 0.58 |
| Haptoglobulin | 0.2 | 0.00 | 0.0 | 0.00 | 0.04 |
| α1-antitrypsin | 0.3 | 0.01 | 0.3 | 0.01 | 0.14 |
| α1-acid glycoprotein | 0.1 | 0.00 | 0.0 | 0.00 | -0.03 |
Number of Glycated Amino Acids Measured by the Enzymatic Method
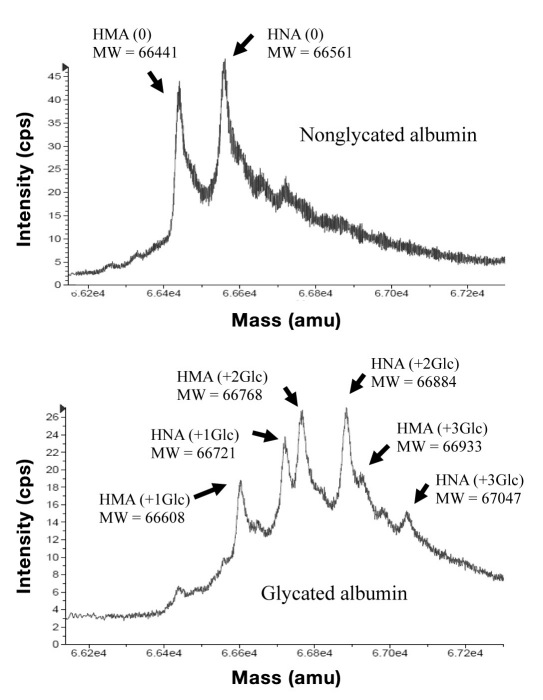
Figure 2 shows mass spectrograms of GA and NGA purified from pooled serum obtained from normal subjects. In the case of NGA, human mercaptalbumin [HMA, molecular weight (MW) = 66441], which has one sulfhydryl group of Cys-34 for each albumin molecule, and human nonmercaptalbumin (HNA, MW = 66561), which has an oxidized sulfhydryl group binding cysteine molecule, were observed.
Figure 2.
Mass spectrograms of purified GA and NGA. HMA = human mercaptalbumin, HNA = human nonmercaptalbumin, HMA (+1 Glc) = one glucose-bonded HMA, HMA (+2 Glc) = two glucose-bonded HMA, HMA (+3 Glc) = three glucose-bonded HMA.
In the case of GA, a major peak of one glucose-bonded HMA (MW = 66608) and HNA (MW = 66721), a major peak of two glucose-bonded HMA (MW = 66768) and HNA (MW = 66884), and a minor peak of three glucose-bonded HMA (MW = 66933) and HNA (MW = 67047) were observed.
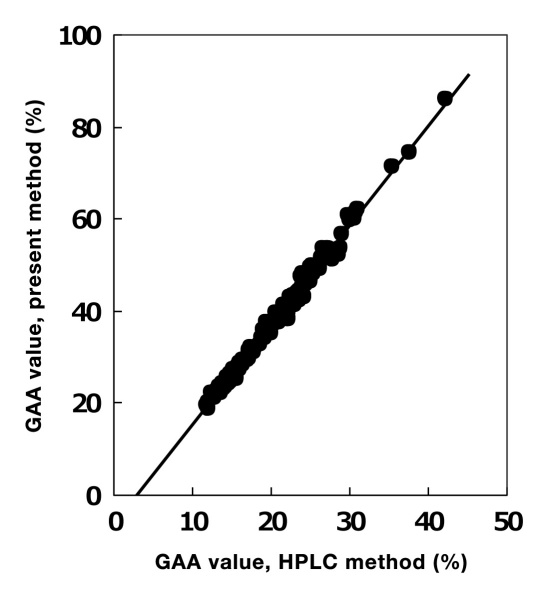
Figure 3 shows the correlation curve between the present method and HPLC method, with glycated amino acid concentration plotted on the y-axis. The correlation curve is linear: [r = 0.997, y = 2.16(x + 2.99)].
Figure 3.
Correlation between glycated amino acid (GAA) ratio (%; present method, glycated amino acid concentration/albumin concentration × 100) and HPLC. The GAA ratio measured by the present method was significantly correlated with GA value measured by HPLC22) (r = 0.997).
General Performance of Enzymatic Assay for Glycated Albumin
General performance of the present method was good. The linearity curve was linear (r = 1.00) between 3.2 and 68.1%. Dilution curves of albumin and glycated albumin were linear between 0.0–80.0 and 0.0–40.9 g/liter, respectively. Analytical recoveries of exogenous GA added to serum were 95.7–99.7%. Within-run (n = 20) and between-run coefficients of variation (CVs) were 0.63–0.93% and 0.68–0.75%, respectively. The addition of up to 146 and 152 mg/dl (final concentration) of free and conjugated bilirubin, respectively, a turbidity index of 1930 chyle per 10.0 g/liter of glucose and 1.0 g/liter ascorbic acid, 4 mmol/liter of glycated amino acid, and 18 g/liter of human serum globulin and g-globulin did not affect the results. Hemoglobin at a concentration of 1.96 g/liter caused a 5% negative bias in the assay for GA. There were no differences between serum and plasma (ethylene-diaminetetraacetic acid, heparin, and sodium fluoride as an inhibitor of glycolysis).
Samples were stable for 1 day at 25 °C, 1 week at 4 and 10 °C, 2 months at -20 °C, and 4 years at -80 °C (data not shown). The reagent was stable at 4 and 10 °C for 12 months (data not shown).
2. Reference Range of GA in the American Population
Table 3 shows the results of the reference range study. The reference range of GA in the American population ranged from 11.9–15.8% (n = 201, mean ± 2 SD). No significant gender differences were observed (male: 13.74 ± 0.91 vs female 13.92% ± 1.00, p = .17). Significant racial differences were seen (white: 13.52 ± 0.92 vs black 14.19 ± 0.88%, p < .05).
Table 3.
Reference Range for Glycated Albumin in Americans
| Measure | All | Males | Females | Whites | Blacks | White Males | White Females | Black Males | Black Females |
|---|---|---|---|---|---|---|---|---|---|
| n | 201 | 95 | 106 | 107 | 94 | 45 | 62 | 50 | 44 |
| Mean | 13.83 | 13.74 | 13.92 | 13.52 | 14.19 | 13.26 | 13.70 | 14.16 | 14.23 |
| SD | 0.96 | 0.91 | 1.00 | 0.92 | 0.88 | 0.75 | 0.99 | 0.84 | 0.90 |
| Median | 13.78 | 13.72 | 13.88 | 13.45 | 14.17 | 13.15 | 13.73 | 14.09 | 14.26 |
| 90% | 15.13 | 14.94 | 15.34 | 14.61 | 15.52 | 14.47 | 14.86 | 15.40 | 15.64 |
| Q3 | 14.47 | 14.45 | 14.52 | 14.09 | 14.67 | 13.72 | 14.38 | 14.51 | 14.73 |
| Q1 | 13.19 | 13.06 | 13.27 | 12.85 | 13.56 | 12.82 | 13.02 | 13.70 | 13.45 |
| 10% | 12.71 | 12.71 | 11.06 | 12.46 | 13.21 | 12.38 | 12.48 | 13.19 | 13.21 |
Discussion
Several methods for measuring GA have been developed but their reference ranges differ (Table 1). Because the enzymatic method is ideal for measuring analytes quantitatively, we studied the (1) specificity, (2) target molecules of GA measurement, and (3) basic performance, including reference range in an American population, of this method.
As shown in Table 2, this method is specific for glycated amino acids in albumin. According to Figure 2, the distribution of the number of binding sugars to one glycated albumin molecule purified from normal individuals was from one to three, and most GA molecules have approximately two glucose molecules. As shown in Figure 3, the slope of the correlation curve between the present method and HPLC method was ∼2. The GA value measured by the HPLC method is the ratio of glycated albumin molecules to total albumin molecules, whereas the GA value measured by the present method is the ratio of glycated amino acid concentration to albumin concentration. Therefore, it is assumed that the present method can measure all of the glycated amino acids in albumin molecules.
The Committee on Diabetes Mellitus Indices and Japan Society of Clinical Chemistry (JSCC) recommended a reference procedure for measuring GA. Because albumin has multiple glycation sites,26 the Committee set out to determine a suitable site for measuring glycation. Based on these results, GA was defined as albumin containing lysine residues bound to glucose, and a method based on isotope dilution mass spectrometry (IDMS) was proposed.27
The IDMS method consists of (1) purification of albumin from serum, (2) addition of stable isotope of Nε-(1-deoxy-D-fructose-1-yl)-L-lysine (DOF-Lys); DOF-Lys and lysine (Lys) as internal standards, (3) hydrogenation of glycation site and hydrolysis, and (4) reduced pressure drying; the isotope ratios of DOF-Lys and Lys are determined by liquid chromatography-mass spectrometry. Precision of the reference procedure is good (within-run CV = 1.2% and day-to-day CV = 1.4%), and compatibility with the present method is favorable (r = 0.996). Because the IDMS method measures all of the glycated Lys in albumin, it is thought that the enzymatic method for GA also measures all of the glycated Lys in albumin.27
The difference between reference ranges among various methods for GA could be due to (1) definition of GA level and (2) target molecules of GA measurement (glycated amino acid or GA molecule, and if the method measured glycated amino acids, which glycation sites were measured).
Affinity chromatography,15,16 ion exchange chroma-tography,7,17 and HPLC methods21,22 define GA level as the ratio of GA peak area to total area of albumin peak, and the target molecule of GA measurement is the glycated albumin molecule. On the other hand, TBA7 and enzymatic assays23,24 define the GA level as the ratio of total glycated amino acid concentration to albumin concentration. The target molecule of GA measurement is total glycated amino acid in albumin.
In the case of immunoassay18,19 and enzyme-linked boronate affinity immunoassay,20 GA level is assumed to be defined as the ratio of glycated amino acid concentration to albumin concentration. However, the GA level might differ depending on the specificity of the antibody used. In addition, we should take into consideration the steric hindrance of antibody or enzyme-linked boronate. Therefore, the definitions of GA level of these methods are unclear. The enzymatic method is especially suitable for measuring GA given the Committee on Diabetes Mellitus Indices and JSCC’s stated importance of measuring all glycation sites.
General performance of the enzymatic assay for GA was good. In addition, the samples for the enzymatic assay for GA were stable for 4 years at -80 °C. Nathan and colleagues28 studied the -80 °C stability of the samples in the DCCT for GA assay and concluded that “samples stored for as long as 23 years are suitable for the GA assay.” The samples for GA would be stable at -80 °C.
The reference range of the present method in the American population was determined to be 11.9–15.8% (n = 201, mean ± 2 SD). Tominaga and colleagues29 selected a reference population by OGTT and reported that the reference range of GA in the Japanese population was 12.3–16.9% (n = 699, mean ± 2 SD), as given in a report of the Committee on Standardization of Laboratory Testing related to Diabetes Mellitus of the Japan Diabetes Society. On the other hand, Rita and colleagues30 reported that the normal control group of GA ranged from 11.7–16.9% (n = 32, 2.5th–97.5th percentile) in the Italian population. The reference range of GA levels in the American population corresponded well to that of the Japanese population and normal control range of the Italian population.
In our report, race but not gender impacted assay results. Therefore, we believe that GA may be a useful clinical indicator for diabetes control. Selvin and colleagues31 reported that “Differences between black and white persons in glycated albumin, fructosamine, and 1,5-anhydroglucitol levels parallel differences between these groups in HbA1c.” Our data corresponded well to their results. They also reported that “Racial differences in hemoglobin glycation and erythrocyte turnover cannot explain racial disparities in the serum markers.”31 Studies are needed that will determine whether black individuals have systematically higher levels of glycemia.
Conclusions
The present method specifically measures glycated amino acids in albumin in a reliable fashion and may be useful in diabetes management. The reference range for the American population is 11.9–15.8%. This method may be a useful indicator for managing diabetes.
Glossary
Abbreviations
- (BCP)
bromocresol purple
- (CV)
coefficient of variation
- (DCCT)
Diabetes Control and Complications Trial
- (DOF-Lys)
Nε-(1-deoxy-D-fructose-1-yl)-L-lysine
- (GA)
glycated albumin
- (HbA1c)
hemoglobin A1c
- (HMA)
human mercaptalbumin
- (HNA)
human nonmercaptalbumin
- (HPLC)
high-performance liquid chromatography
- (IDMS)
isotope dilution mass spectrometry
- (JSCC)
Japan Society of Clinical Chemistry
- (MW)
molecular weight
- (NGA)
nonglycated albumin
- (OGTT)
oral glucose tolerance test
- (SD)
standard deviation
- (TBA)
thiobarbituric acid
Disclosure
Dr. Takuji Kohzuma is an employee of Asahi Kasei Pharma Co., which has developed and distributed the glycated albumin kits used in the current study. Asahi Kasei Pharma Co. provided partial support for this study.
References
- 1.Cohen MP. Nonenzymatic glycation: a central mechanism in diabetic microvasculopathy? J Diabet Complications. 1988;2(4):214–217. doi: 10.1016/s0891-6632(88)80012-6. [DOI] [PubMed] [Google Scholar]
- 2.Diabetes Control Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1933;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 3.Tahara Y, Shima K. Kinetics of HbA1c, glycated albumin, and fructosamine and analysis of their weight functions against preceding plasma glucose level. Diabetes Care. 1995;18(4):440–447. doi: 10.2337/diacare.18.4.440. [DOI] [PubMed] [Google Scholar]
- 4.Panzer S, Kronik G, Lechner K, Bettelheim P, Neumann E, Dudczak R. Glycosylated hemoglobins (GHb): an index of red cell survival. Blood. 1982;59(6):1348–1350. [PubMed] [Google Scholar]
- 5.Jeffcoate SL. Diabetes control and complications: the role of glycated haemoglobin, 25 years on. Diabet Med. 2004;21(7):657–665. doi: 10.1046/j.1464-5491.2003.01065.x. [DOI] [PubMed] [Google Scholar]
- 6.Bry L, Chen PC, Sacks DB. Effects of hemoglobin variants and chemically modified derivatives on assays for glycohemoglobin. Clin Chem. 2001;47(2):153–163. [PubMed] [Google Scholar]
- 7.Guthrow CE, Morris MA, Day JF, Thorpe SR, Baynes JW. Enhanced nonenzymatic glucosylation of human serum albumin in diabetes mellitus. Proc Natl Acad Sci USA. 1979;76(9):4258–4261. doi: 10.1073/pnas.76.9.4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roohk HV, Zaidi AR. A review of glycated albumin as an intermediate glycation index for controlling diabetes. J Diabetes Sci Technol. 2008;2(6):1114–1121. doi: 10.1177/193229680800200620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koga M, Kasayama S. Clinical impact of glycated albumin as another glycemic control marker. Endocr J. 2010;57(9):751–762. doi: 10.1507/endocrj.k10e-138. [DOI] [PubMed] [Google Scholar]
- 10.Kohzuma T, Koga M. Lucica GA-L glycated albumin assay kit: a new diagnostic test for diabetes mellitus. Mol Diagn Ther. 2010;14(1):49–51. doi: 10.1007/BF03256353. [DOI] [PubMed] [Google Scholar]
- 11.Takahashi S, Uchino H, Shimizu T, Kanazawa A, Tamura Y, Sakai K, Watada H, Hirose T, Kawamori R, Tanaka Y. Comparison of glycated albumin (GA) and glycated hemoglobin (HbA1C) in type 2 diabetic patients: usefulness of GA for evaluation of short-term changes in glycemic control. Endocr J. 2007;54(1):139–144. doi: 10.1507/endocrj.k06-103. [DOI] [PubMed] [Google Scholar]
- 12.Kashimoto K, Noguchi S, Morimoto Y, Hamada S, Wasada K, Imai S, Murata Y, Kasayama S, Koga M. A1C nut not serum glycated albumin in elevated in late pregnancy owing to iron deficiency. Diabetes Care. 2008;31:1945–1948. doi: 10.2337/dc08-0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iberg N, Flückiger R. Nonenzymatic glycosylation of albumin in vivo. Identification of multiple glycosylated sites. J Biol Chem. 1986;1261(29):13542–13545. [PubMed] [Google Scholar]
- 14.Garlick RL, Mazer JS. The principal site of nonenzymatic glyco-sylation of human serum albumin in vivo. J Biol Chem, 1983;258(10):6142–6146. [PubMed] [Google Scholar]
- 15.Reed P, Bhatnager D, Dhar H, Winocour PH. Precise measurement of glycated serum albumin by column affinity chromatography and immunoturbidmetry. Clin Chim Acta. 1986;161(2):191–199. doi: 10.1016/0009-8981(86)90212-3. [DOI] [PubMed] [Google Scholar]
- 16.Yatscoff RW, Tevaarwerk GJM, MacDonald JC. Quantification of nonenzymically glycated albumin and total serum protein by affinity chromatography. Clin Chem. 1984;30(3):446–449. [PubMed] [Google Scholar]
- 17.Day JF, Thorpe SR, Baynes JW. Nonenzymatically glucosylated albumin. In vitro preparation and isolation from normal human serum. J Biol Chem. 1979;254(3):595–597. [PubMed] [Google Scholar]
- 18.Ohe Y, Matsuura M, Nakajima Y, Shin S, Hashimoto F, Hirota M, Shima K. Radioimmunoassay of glycated albumin with monoclonal antibody to glucitol-lysine. Clin Chim Acta. 1987;169(2-3):229–238. doi: 10.1016/0009-8981(87)90323-8. [DOI] [PubMed] [Google Scholar]
- 19.Cohen MP, Hud E. Measurement of plasma glycoalbumin levels with a monoclonal antibody based ELISA. J Immunol Meth. 1989;122(2):279–283. doi: 10.1016/0022-1759(89)90275-5. [DOI] [PubMed] [Google Scholar]
- 20.Ikeda K, Sakamoto Y, Kawasaki Y, Miyake T, Tanaka K, Urata T, Katayama Y, Ueda S, Horiuchi S. Determination of glycated albumin by enzyme-linked boronate immunoassay (ELBIA) Clin Chem. 1998;44(2):256–263. [PubMed] [Google Scholar]
- 21.Yasukawa K, Abe F, Shida N, Koizumi Y, Uchida T, Noguchi K, Shima K. High-performance affinity chromatography system for rapid, efficient assay of glycated albumin. J Chromatogr. 1992;597(1-2):271–275. doi: 10.1016/0021-9673(92)80120-j. [DOI] [PubMed] [Google Scholar]
- 22.Uchida T, Kozuma T, Yasukawa K, Shima K. Glycated albumin analyzer. In: Hanai T, Hatano H, editors. Advances in liquid chromatography. Singapore: World Scientific; 1996. pp. 33–41. [Google Scholar]
- 23.Kouzuma T, Usami T, Yamakoshi M, Takahashi M, Imamura S. An enzymatic method for the measurement of glycated albumin in biological samples. Clin Chim Acta, 2002;324(1-2):61–71. doi: 10.1016/s0009-8981(02)00207-3. [DOI] [PubMed] [Google Scholar]
- 24.Kouzuma T, Uemastu Y, Usami T, Imamura S. Study of glycated amino acid elimination reaction for an improved enzymatic glycated albumin measurement method. Clin Chim Acta. 2004;346(2):135–143. doi: 10.1016/j.cccn.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 25.Muramoto Y, Matsushita M, Irino T. Reduction of reaction differences between human mercaptalbumin and human non-mercaptalbumin measured by the bromocresol purple method. Clin Chim Acta. 1999;289:69–78. doi: 10.1016/s0009-8981(99)00158-8. [DOI] [PubMed] [Google Scholar]
- 26.Kisugi R, Kouzuma T, Yamamoto T, Akizuki S, Miyamoto H, Someya Y, Yokoyama J, Abe I, Hirai N, Ohnishi A. Structural and glycation site changes of albumin in diabetic patients with very high glycated albumin. Clin Chim Acta. 2007;382(1-2):59–64. doi: 10.1016/j.cca.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 27.Committee on Diabetes Mellitus Indices Japan Society of Clinical Chemistry. Takei I, Hoshino T, Tominaga M, Nakayama T, Kuwa K, Matsumoto MUY, Ishibashi M, Yasukawa K, Tani W, Okahashi M, Watanabe N, Ono K, Kohzuma T, Taniguchi Y. JSCC recommended method for glycated albumin measurement in serum. Jpn J Clin Chem. 2008;37(2):178–191. [Google Scholar]
- 28.Nathan DM, Steffes MW, Sun W, Rynders GP, Lachin JM. Determining stability of stored samples retrospectively: the validation of glycated albumin. Clin Chem. 2011;57(2):286–290. doi: 10.1373/clinchem.2010.150250. [DOI] [PubMed] [Google Scholar]
- 29.Tominaga M, Makino H, Yoshino G, Kuwa K, Takei I, Aono Y, Hoshino T, Umemoto M, Shimatsu A, Sanke T, Kuwashima M, Taminato T, Ono J. Report of the Committee on Standardization of Laboratory Testing Related to Diabetes Mellitus of the Japan Diabetes Society: determination of reference intervals of hemoglobin A1c (IFCC) and glycoalbumin in the Japanese population. J Japan Diab Soc. 2006;49(10):825–833. [Google Scholar]
- 30.Paroni R, Ceriotti F, Galanello R, Leoni GB, Panico A, Scurati E, Paleari R, Chemello L, Quaino V, Scaldaferri L, Lapolla A, Mosca A. Performance characteristics and clinical utility of an enzymatic method for the measurement of glycated albumin in plasma. Clin Biochem. 2007;40(18):1398–1405. doi: 10.1016/j.clinbiochem.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 31.Selvin E, Steffes MW, Ballantyne CM, Hoogeveen RC, Coresh J, Brancati FL. Racial differences in glycemic markers: a cross-sectional analysis of community-based data. Ann Intern Med. 2011;154(5):303–309. doi: 10.1059/0003-4819-154-5-201103010-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]