Abstract
Background
The Paradigm®Veo™ System includes a low glucose suspend (LGS) feature which suspends insulin delivery when a prespecified glucose threshold setting is reached by the associated continuous glucose monitoring (CGM) sensor. The ASPIRE (Automation to Simulate Pancreatic Insulin REsponse) study is a multicenter, in-clinic, randomized, crossover study to examine the efficacy of LGS in exercise-induced hypoglycemia.
Methods
Insulin-pump users underwent two separate exercise sessions, one with the LGS feature set to suspend insulin (LGS-on) when the CGM-detected glucose concentration was ≤70 mg/dl and one with the LGS feature off. Exercise sessions were conducted after an overnight fast and with initial plasma glucose level as measured by the YSI 2300 STAT Plus glucose analyzer (YSI) of 100–140 mg/dl. Subjects exercised until their YSI value fell to ≤85 mg/dl; subsequent YSI values <70 mg/dl were recorded for up to 4 h to measure the duration and nadir of hypoglycemia. The protocol required that subjects with YSI values <50 or >300 mg/dl were rescued with carbohydrates or insulin, respectively, based on the provider’s recommendation. The primary end point was comparison of duration and severity of hypoglycemia between LGS-on and LGS-off sessions. Secondary end points included areas under the glucose concentration curve, CGM sensor accuracy, and last YSI glucose. Device- and procedure-related adverse events and serious adverse events were recorded.
Results
Fifty adults and teenagers (17–58 years) with type 1 diabetes were randomized. Study completion is expected in November 2011.
Keywords: exercise-induced hypoglycemia, low glucose suspend, semi-closed loop, sensor-augmented insulin pump, Veo insulin pump
Introduction
Prevention of exercise-induced hypoglycemia is an area of active investigation. Algorithms to guide additional carbohydrate intake have been proposed, either alone1 or in combination with real-time continuous glucose monitoring (CGM).2 Simple maneuvers such as raising the low glucose alarm threshold setting during exercise may assist in preventing exercise-induced hypoglycemia.3
The MiniMed Paradigm® Veo™ insulin pump (Medtronic MiniMed, Inc., Northridge, CA) has been commercially available in Europe since June 2009. It is the first pump that can automatically suspend insulin delivery in response to CGM data. Initial analysis of the clinical performance of its low glucose suspend (LGS) feature has been favorable.4 When the LGS feature is on, the pump will suspend insulin delivery if the CGM sensor reading drops below a prespecified threshold. Activation of the LGS is accompanied by an alarm. If the user responds to the alarm, then a choice is required to either resume basal delivery or allow the pump suspension to continue for up to 2 h. If no response is made to the LGS alarm, then the pump will automatically resume basal insulin delivery after 2 h. The LGS feature is intended to reduce the duration and severity of hypoglycemia, and is not intended to prevent hypoglycemia.
Retrospective data analysis from 7 months of real-world use of the system from 935 patients showed that (1) LGS-mediated pump suspensions occurred approximately 0.55 times per patient per day, (2) only 11% of pump suspensions lasted for >115 min, (3) 60% began in the afternoon or evening, and (4) median duration of pump suspensions was between 9 and 10 min.5 This observa-tional study suggested that use of the LGS feature was associated with reductions in both hyperglycemic and hypoglycemic exposure.5
The ASPIRE (Automation to Simulate Pancreatic Insulin Response) study is designed to assess efficacy of the LGS feature with respect to reducing the duration and severity of exercise-induced hypoglycemia. The ASPIRE study is the first randomized, controlled, crossover study of the LGS feature. Here we outline the design and methods of the ASPIRE study. Details of study conduct and experimental results will be made available separately.
Research Design and Methods
The protocol was finalized with guidance from the Food and Drug Administration and from the study’s principal investigators. Five clinical centers experienced in CGM and insulin pump therapy were involved in the study. The study was approved by the Institutional Review Boards (IRBs) of the participating investigational centers. California subjects reviewed the California Experimental Subject’s Bill of Rights, and all subjects signed the informed consent approved by their institution’s IRB. Subjects aged 16–17 years provided informed assents and a parent or guardian provided informed consent.
Study Design
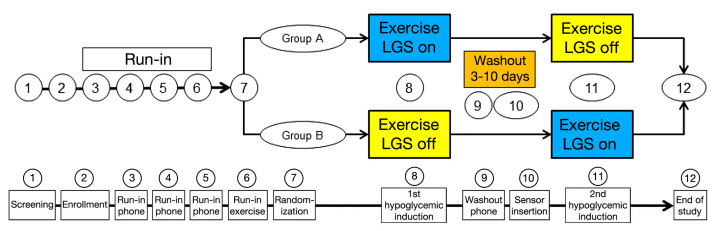
This study design is a multicenter, randomized, controlled, crossover with a run-in period. Subjects 16–60 years of age with type 1 diabetes for at least 1 year and at least 3 months of experience with a Medtronic insulin pump were recruited. Subjects were selected based on the investigator’s assessment that they would be able to tolerate the exercise experiments, but there was no formal assessment of physical fitness. The inclusion and exclusion criteria are listed in Table 1, and the overall visit schedule is presented in Figure 1. Enrollment proceeded in stages, with adults aged 22–60 years studied first. The study’s Clinical Events Committee (CEC) reviewed the hypoglycemic induction data on the first 10 enrolled adults, which included all adverse events and symptoms associated with exercise or exercise-induced hypoglycemia during the experiment. Upon determining that the protocol did not pose an unacceptable safety risk, the CEC allowed 4 young adults aged 18–21 years to enroll; a similar safety review then occurred before 4 pediatric subjects aged 16–17 years were enrolled. The remaining 32 enrolled subjects were adults.
Table 1.
ASPIRE study inclusion and exclusion criteria at time of enrollmenta
| Inclusion Criteria |
|---|
| Aged 16–60 years at signature of IC |
| Diagnosed with type 1 diabetes at least 12 months prior to IC |
| Weight >45 kg |
| Medtronic insulin pump user ≥3 months |
| Hemoglobin A1c ≥ 7.0% and ≤ 10.0% |
| Ability to exercise for an extended period of time |
| Documented stress treadmill test for diabetes of >20 years’ duration |
| Adequate treatment for celiac disease, if it exists |
| Willing to follow protocol and procedures for the study |
| Exclusion Criteria |
| Systolic blood pressure >140 mm Hg |
| Diastolic blood pressure >90 mm Hg |
| History of hypoglycemic seizure or hypoglycemic coma within the last 2 years |
| Unable to tolerate tape adhesive in the area of sensor placement |
| Active adverse skin condition in the area of sensor placement not resolved |
| Pregnant or planning to become pregnant during the course of the study |
| History of myocardial infarction, unstable angina, coronary artery bypass surgery, coronary artery stenting, transient ischemic attack, cerebrovascular accident, angina, congestive heart failure, ventricular rhythm disturbances or thromboembolic disease |
| Active Graves disease |
| Renal impairment |
| Creatinine, hematocrit, potassium, TSH, or T4 values outside of the normal reference range |
| History of smoking for >5 years |
| Electrocardiogram or stress treadmill findings indicating active ischemia or a condition that would compromise subject safety |
| Participation in another drug or device investigational study |
| On beta blocker medication |
| Oral or injectable steroids taken within past 30 days |
| Unwilling or unable to follow the protocol |
| History of diagnosed medical eating disorder, known illicit drug use, or known abuse of prescription medication |
| History of visual impairment |
| History of current alcohol abuse |
| Any other condition including abnormalities found on the screening tests which in the opinion of the Investigator, may preclude participation in the study |
IC, informed consent form.
Figure 1.
ASPIRE study design and visit plan.
After enrollment, all subjects were required to perform a 2-week run-in period that included three phone visits from the investigational center staff to verify and optimize each subject’s basal rates. The run-in period included an exercise visit wherein each subject’s glycemic response to exercise was quantified via finger stick or venous glucose readings (visit 6); the exercise regimen was reproduced in the exercise-induced hypoglycemia visits (visits 8 and 11). During the run-in period, subjects used the Paradigm Veo pump with the LGS feature off, and the OneTouch® UltraLink™ blood glucose (BG) meter for periodic CGM sensor calibration.
Following the run-in period, subjects were randomized into two groups. The first hypoglycemia induction visit for group A was conducted with the LGS feature set to suspend insulin delivery at CGM sensor readings of ≤70 mg/dl (LGS on); the first hypoglycemia induction visit for group B was with the LGS feature off. After a washout period lasting from 3–10 days, a subsequent hypoglycemia induction visit was conducted with the LGS feature off for group A and on for group B (Figure 1).
Treatment Methods
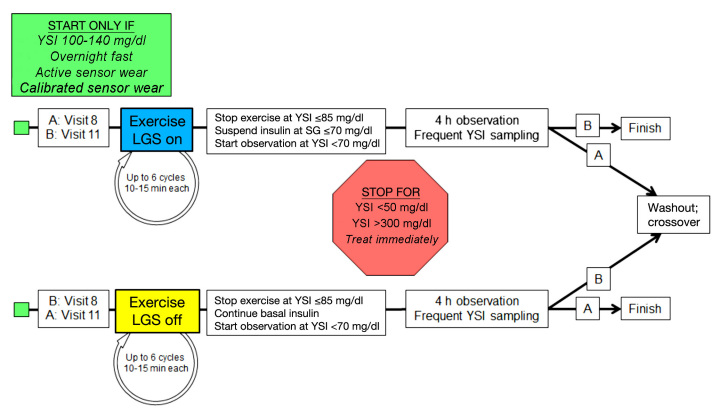
Conduct of the hypoglycemia induction visits is outlined in Figure 2. Visits were to start between 7:00 a.m. and 9:00 a.m. after an overnight fast. During the in-clinic procedure, the UltraLink meter was used to calibrate the CGM sensor. The reference instrument (YSI 2300 STAT Plus, YSI Life Sciences, Yellow Springs, OH) was also set up. Venous access was established for frequent blood sample collection. Plasma glucose values were collected at 5–30 min intervals; the time between each blood sample was determined by the YSI values. Once subjects reached a YSI glucose level 100–140 mg/dl, the experiment was initiated. The exercise session consisted of up to six cycles of mild- to moderate-intensity exercise on a stationary bicycle or treadmill lasting 15–30 min each.
Figure 2.
Exercise-induced hypoglycemia visits. If a stopping condition was met, the visit was repeated. SG, interstitial fluid glucose concentration as measured by the glucose sensor.
Each cycle was followed by a rest period of 5–15 min. There was no attempt to standardize the duration or intensity of exercise. Subjects exercised until they reached a YSI value of ≤85 mg/dl. If the subject did not reach this YSI value with exercise, the experiment was repeated. In addition, subjects who were prematurely terminated from the experiment because of low or high BG values or who did not reach target range (<70 mg/dl by YSI) could repeat the experiment up to three times following a washout period of 3–10 days.
The observation period started once a YSI level <70 mg/dl was observed. All hypoglycemic induction visits were supervised by a registered nurse and a physician trained in diabetes management. If, during the experiment a subject’s BG concentration remained >250 mg/dl, then a recheck (finger stick or YSI) was required. If the BG concentration was repeatedly >250 mg/dl, a check of ketones was required. Additional insulin was to be given for BG concentrations >250 mg/dl in the presence of large urine ketones, a blood ketone level of >1.5 mM, or BG concentration >300 mg/dl. If insulin was needed at any time for the critically elevated glucose levels mentioned above, the experiment was to be terminated.
Upon completion of the experiment, the investigational center uploaded the YSI values and pump and sensor data into Medtronic CareLink Clinical. After a washout period of 3–10 days following the first hypoglycemia induction visit, the subject then repeated the experiment. At the end of the second hypoglycemia induction visit, all study device data were uploaded and returned. Staff at the investigational center performed final follow-up and an end-of-study phone call visit.
The primary end point of this study is a comparison of the duration and severity of hypoglycemia (as measured in venous plasma with the YSI reference instrument) with LGS feature turned on versus LGS feature turned off. The study will be considered successful if use of the LGS feature results in significant reductions in either the duration or severity of exercise-induced hypoglycemia. Secondary end points include area under the curve of time spent with YSI glucose values <60 mg/dl, <70 mg/dl, and >180 mg/dl. Continuous glucose monitoring sensor values will be compared to YSI glucose values <90 mg/dl to estimate sensor accuracy. The YSI value 4 h after reaching <70 mg/dl and the number of times the experiment was terminated for YSI glucose values <50 mg/dl or >300 mg/dl were recorded. Device- and procedure-related adverse events were recorded. Unanticipated device effects were also to be collected for this study.
Sample size estimation
Fifty subjects were projected to provide 80% power to detect a treatment group difference as small as 15% using a paired t-test with a one-sided 2.5% criterion significance level and a standard deviation (SD) of 49.5 min (a 102 min duration for LGS-on experiments and a 120 min duration for LGS-off experiments). The target sample size provided a 99% power to detect a treatment group difference as small as 15% or 3 mg/dl in severity of hypoglycemia, using a paired t-test with a one-sided 2.5% criterion significance level and a SD of 5 mg/dl (20 mg/dl severity for LGS-off experiments and a 17 mg/dl severity for LGS-on experiments).
The analysis will include the intent-to-treat and per-protocol populations. The treatment difference will be calculated and tested for statistical significance using a linear model and will provide a 95% confidence interval for treatment effect contrast. The linear model will include carryover and treatment effects. The test for carryover effect will be set at a = 0.1. If the carryover effect is significant, then only period 1 data will be used to estimate treatment effect. Parameters in the model will include hypoglycemia duration and severity, group assignment, inter-subject variance, carryover effect, effect of the treatment, and random residual variation. An interim analysis will be performed on the first 30 subjects (15 in each arm) using the O’Brien-Fleming approach and used to provide direction for continuing enrollment. For this two-stage design, hypothesis testing will be conducted at p1 = 0.005 for the first stage or interim analysis, and p2= 0.048 for the second stage or final analysis. Descriptions of adverse events (ketonemia, diabetic keto-acidosis, and rebound hyperglycemia) will be presented for all randomized subjects. Secondary efficacy end point comparisons will be descriptive.
Discussion
Hypoglycemia is one of the biggest barriers in implementing and achieving tight glucose control in subjects with type 1 diabetes. Automatic suspension of insulin delivery with a semi closed-loop feedback system utilizing CGM may reduce the duration and severity of hypoglycemia without increasing the risk of severe hyperglycemia or ketoacidosis.
The ASPIRE study design did not include measurements of aerobic fitness or plasma insulin concentrations, and there was no attempt to standardize the intensity or duration of exercise among patients or between visits. Importantly, the time needed for full biochemical recovery from hypoglycemia may be more than the minimum washout period of 3 days, so subjects participating in second or subsequent visits may have decreased glycogen stores or other risk factors for prolonged hypoglycemia. The choice of 70 mg/dl for LGS activation represents a compromise between patient safety and hypothesis testing, as do the other glucose concentrations chosen for initiating exercise, stopping exercise, and terminating the experiment. The study’s use of exercise and basal insulin represents a common pathway to hypoglycemia and one where the LGS feature may be most beneficial; additional studies of nocturnal hypoglycemia and hypoglycemia resulting from overdelivery of bolus insulin are warranted. Once completed, the ASPIRE study will provide information on the efficacy of automatic pump suspension in exercise-induced hypoglycemia in subjects with type 1 diabetes.
Glossary
Abbreviations
- (ASPIRE)
Automation to Simulate Pancreatic Insulin Response
- (BG)
blood glucose
- (CEC)
Clinical Events Committee
- (CGM)
continuous glucose Monitoring
- (IRB)
Institutional Review Board
- (LGS)
low glucose suspend
- (SD)
standard deviation
- (YSI)
plasma glucose concentration as measured by the YSI glucose analyzer
Funding
Medtronic, Inc, has provided funding for this study.
Disclosures
Ronald L. Brazg, Timothy S. Bailey, Satish Garg, Bruce A. Buckingham, and Robert H. Slover have received research support from Medtronic, Inc. David C. Klonoff has consulted for Medtronic, Inc. Xuan Nguyen, Scott W. Lee, John Shin, and John B. Welsh are employees of Medtronic, Inc.
References
- 1.Francescato MP, Carrato S. Management of exercise-induced hypo-glycemic imbalances in type 1 diabetes. Curr Diabetes Rev. 2011;7(4):253–263. doi: 10.2174/157339911796397875. [DOI] [PubMed] [Google Scholar]
- 2.Riddell MC, Milliken J. Preventing exercise-induced hypoglycemia in type 1 diabetes using real-time continuous glucose monitoring and a new carbohydrate intake algorithm: an observational field study. Diabetes Technol Ther. 2011;13(8):819–825. doi: 10.1089/dia.2011.0052. [DOI] [PubMed] [Google Scholar]
- 3.Iscoe KE, Davey RJ, Fournier PA. Increasing the low-glucose alarm of a continuous glucose monitoring system prevents exercise-induced hypoglycemia without triggering any false alarms. Diabetes Care. 2011;34(6):e109. doi: 10.2337/dc10-2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pickup JC. Semi-closed loop insulin delivery systems: Early experience with low-glucose insulin suspend pumps. Diabetes Technol Ther. 2011;13(7):695–698. doi: 10.1089/dia.2011.0103. [DOI] [PubMed] [Google Scholar]
- 5.Agrawal P, Welsh JB, Kannard B, Askari S, Yang Q, Kaufman FR. Usage and effectiveness of the low glucose suspend feature of the Medtronic Paradigm Veo insulin pump. J Diabetes Sci Technol. 2011;5(5):1137–1141. doi: 10.1177/193229681100500514. [DOI] [PMC free article] [PubMed] [Google Scholar]