Abstract
Background
In Sweden, patients with diabetes mellitus frequently receive short-term (<3 months) continuous glucose monitoring (CGM) to study glucose patterns or long-term CGM to treat poor glycemic control or severe hypoglycemia. The effects of CGM on glycemic control in clinical practice in relation to indication and duration of use has not been completely studied.
Methods
Patients with diabetes, among which 99% were diagnosed as type 1, receiving CGM at 10 outpatient clinics in Sweden were studied retrospectively. Long-term use of CGM was defined as ≥3 months use of CGM and short-term as <3 months. A control group matched on start date and date of latest value 3 months after the start was selected for both long- and short-term groups.
Results
In 34 long-term users of CGM, over a mean follow-up of 1.1 years, the adjusted mean difference of hemoglobin A1c (HbA1c) compared with controls (n = 408) was -0.76 (95% confidence interval -1.17; -0.33, p < .001). Long-term users with indications for high HbA1c (n = 15) had a reduction of 1.2% in HbA1c from 10.1 to 8.9% (p = .003), whereas patients with hypoglycemia as their indication (n = 16) decreased by 0.3% (p = .17). Nonsevere hypoglycemic events decreased in long-term users within the same follow-up period (p = .004). Short-term users showed no statistically significant improvement in HbA1c compared with controls at 1.1 years (n = 41), p = .85 or at 2.6 years (n = 43), p = .19.
Conclusion
Long-term CGM use was associated with improved glycemic control in clinical practice and a reduction in nonsevere hypoglycemic events, whereas short-term use had no effect on HbA1c. The effect on glycemic control varied by indication.
Keywords: continuous glucose monitoring, hemoglobin A1c, hypoglycemia, indication, long, short
Introduction
Optimal glycemic control is associated with fewer disease-related complications in patients with diabetes mellitus.1,2 In both clinical practice and research, hemoglobin A1c (HbA1c), a measure of average blood glucose during the previous 2–3 months, is generally used to assess glycemic control.3 Patients with type 1 diabetes mellitus (T1DM) are generally treated with multiple daily injections (MDI) of insulin or insulin pump therapy, the latter of which is associated with a 0.3 percentage unit greater reduction in HbA1c compared with MDI.4–5 Despite intensive treatment, the majority of T1DM patients do not reach a goal of HbA1c < 7%,6 potentially because of concern over risk of hypoglycemia associated with intensive treatment.1
Continuous glucose monitoring (CGM) is a relatively new therapy in T1DM patients.7 In T1DM patients not achieving target HbA1c, Bergenstal and colleagues8 demonstrated that CGM combined with insulin pump therapy (sensor-augmented insulin pump therapy) reduced HbA1c by 0.6 percentage units without an increased risk of hypoglycemia. In Sweden, where the present study was carried out, long-term use of CGM is reimbursed in combination with Medtronic pump use if two or more severe hypoglycemic events have appeared in 1 year or if glycemic control is very poor (HbA1c > 9.8%).9 In children, sensors are also reimbursed if 10 or more blood glucose tests per day are medically indicated. Short-term use of CGM, generally <3 months, is sometimes used (depending on the clinics’ budgets) for studying trends of glycemic control.
To understand the effect of CGM on HbA1c in clinical practice, we studied two groups of patients: (1) using CGM for less than 3 months and (2) using CGM for 3 months or more.
Methods
Diab-Base (Journalia, Kungälv, Sweden), a patient-centered medical record system, was used for continuous evaluation of CGM.10,11 The interface includes coded phrases, dates, and laboratory data. For communication, the chosen input is integrated with free text and exported to the record as well as to informative letters to the patient. Diab-Base is used daily by physicians and nurses at 10 outpatient clinics in Sweden. Among other data, risk factors, therapies, and complications are tracked in the system. Since CGM is relatively new to clinical practice, the CGM data collection module was only recently implemented. Module validation with manual data collection was performed at Sahlgrenska University Hospital, Gothenburg, Sweden, before extending it to other clinics. Thus, the majority of patients in the present study were treated at the outpatient clinic at this center.
Adult men and nonpregnant women with T1DM and an HbA1c value ≥1 at both start and during use (after at least 3 months) of CGM therapy were included. Patients without an HbA1c value ≥1 at initial CGM use were excluded. Patients were divided into two groups based on duration of CGM use: (1) short-term: <3 months and (2) long-term: ≥3 months. The last measured HbA1c value during CGM therapy was used as the outcome variable of interest for long-term users. For short-term users, the last available HbA1c value ≥3 months after start of CGM and before any long-term CGM was the selected outcome variable. The date for a possible second short-term CGM was not used as a restriction for follow-up time in the short-term CGM group. Patients having used both short-term (as first therapy) and long-term CGM therapy were included in both study groups. Other variables recorded were age, sex, type of diabetes (1 or 2), diabetes therapy, body mass index, diabetes duration, insulin dose, and indication for CGM. Indications in the Diab-Base CGM module consisted of serious hypoglycemia, high HbA1c, and problematic fluctuations in glucose levels. Effects on HbA1c were also studied with respect to indication. Hypoglycemic events were self-rated by the patients in a protocol in which they classified their experience of nonsevere hypoglycemic events during the last month (recorded as 0 to <5, 5 to <10, 10 to <15, and ≥15 events) and number of severe hypoglycemic events during the last year. The hypoglycemia value closest in time to the follow-up HbA1c value was analyzed.
All hospitals in Sweden are associated with a quality assessment organization that regularly validates HbA1c laboratory methods. Since diabetes clinics in Sweden used HbA1c methods calibrated to the high-performance liquid chromatography Mono-S method until September 2010, all HbA1c values have been converted to National Glycohemoglobin Standardization Program (NGSP) levels (Diabetes Control and Complications Trial standard).12 This study was approved by the Ethics Committee of Gothenburg University, Gothenburg, Sweden.
Statistical Analysis
Continuous variables are described as mean ± standard deviation (SD) or median (range) and categorical variables as numbers (%). Changes in HbA1c and number of non-severe hypoglycemia episodes over time were analyzed with the Wilcoxon signed-rank test within the long- and short-term CGM groups. For intergroup comparisons, Fisher’s exact test was used for dichotomous variables and Mann-Whitney U-test for continuous variables.
The control groups were composed of T1DM patients who were not using CGM. They were selected matching the highest possible number of unique control patients to each patient from the long- and short-term CGM groups separately with respect to the CGM start date and the date for the last HbA1c value after 3 months. To obtain the highest possible number of control patients, t-statistics and the p value from the t-test were used to test the differences between groups with respect to the two dates. The time interval allowed around the calendar dates was ±182 days. The highest possible number was 12 for each long-term CGM patient and 28 for each short-term CGM patient. The mean difference between the long-term patients and their corresponding controls with respect to the two dates was approximately 1 month and was not statistically significant (p = .69 for difference in start date, p = .57 for difference in latest HbA1c date after 3 months). For short-term patients versus controls, mean difference in days was not greater than 2 weeks and not statistically significant (p = .97 for difference in start date, p = .88 for difference in latest HbA1c date after 3 months). When comparing the change in HbA1c from start to last value after 3 months between patients vs controls, an adjusted analysis was performed using analysis of covariance. Adjustments were done for those variables that significantly differed (p < .05) between the two groups at baseline. Boxplots were used to graphically present changes in HbA1c. All tests were two-tailed and conducted at the .05 significance level.
Results
Baseline Characteristics
Baseline characteristics and indications by duration of CGM use are given in Table 1. There were 34 long-term and 43 short-term CGM users included. Mean time of CGM usage was 1.1 years for long-term users and 33 days for short-term users. At baseline, mean HbA1c in long-term users was 8.8% and 8.4% in short-term users. All study subjects had T1DM except for one short-term user. The majority of patients (long-term: n = 30, 91%; short-term: n = 30, 71%) were treated with an insulin pump, with the remainder treated with MDI. In long-term users, most patients reported many nonsevere hypoglycemic events (>15 last month) or a few nonsevere hypoglycemic events (<5 last month). Short-term users mainly reported <5 or 10–15 nonsevere hypoglycemic events during the corresponding period. The indication for CGM use was high HbA1c or hypoglycemia in all but 3 long-term patients, whereas in short-term users, problems with fluctuating blood glucose were also a common indication.
Table 1.
Baseline Characteristics of Short-Term CGM Patients, Long-Term CGM Patients, and Control Groups. Two Different Control Groups Were Used to Match the Two Different Groups at Each Point of Time
| Variablea,b | Long-term use CGM(≥3 months) (n = 34) | Controls (long-term)(n = 408) | Short-term use CGM(<3 months) (n = 43) | Controls (short-term)(n = 1204) |
|---|---|---|---|---|
| Age (years) at CGM start | 44.0 (10.2)42.4 (22.8; 66.2)n = 34 | 44.6 (16.2)44.4 (17.5; 85.6)n = 408 | 42.7 (10.4)41.6 (20.6; 66.0)n = 43 | 44.2 (15.5)43.5 (16.9; 86.8)n = 1204 |
| Gender | ||||
| Male | 19 (55.9%) | 194 (47.5%) | 15 (34.9%) | 631 (52.4%) |
| Female | 15 (44.1%) | 214 (52.5%) | 28 (65.1%) | 573 (47.6%) |
| Weight (kg) at CGM start | 76.4 (16.0)73.9 (55.0; 115.0)n = 30 | 74.2 (14.5)71.8 (44.9; 142.0)n = 398 | 74.5 (12.2)73.5 (56.0; 106.0)n = 37 | 74.0 (14.0)72.0 (42.7; 136.0)n = 1124 |
| Body mass index at CGM start | 24.7 (4.3)24.0 (19.3; 36.1)n = 23 | 24.6 (3.9)23.8 (16.6; 40.2)n = 375 | 25.4 (4.0)25.1 (18.6; 35.9)n = 34 | 24.5 (3.7)23.9 (14.8; 42.5)n = 1048 |
| Diabetes duration (years) | 26.4 (13.2)26.6 (0.9; 53.5)n = 33 | 25.8 (16.2)24.4 (0.0; 105.0)n = 388 | 26.8 (10.6)27.0 (9.2; 48.0)n = 41 | 23.9 (15.3)22.7 (0.0; 102.9)n = 1165 |
| Diabetes type | ||||
| Type 1 | 34 (100.0%) | 408 (100.0%) | 42 (97.7%) | 1204 (100.0%) |
| Type 2 | 0 | 1 (2.3%) | ||
| Insulin dose, units/kg at CGM start | 0.527 (0.183)0.494 (0.265; 1.096)n = 29 | 0.584 (0.203)0.561 (0.000; 1.583)n = 395 | 0.473 (0.192)0.470 (0.226; 1.149)n = 36 | 0.577 (0.218)0.557 (0.000; 2.205)n = 1109 |
| Insulin type at CGM start | ||||
| Pump | 30 (90.9%) | 68 (16.9%) | 30 (71.4%) | 162 (13.9%) |
| Injection | 3 (9.1%) | 335 (83.1%) | 12 (28.6%) | 1004 (86.1%) |
| Missing | 1 | 5 | 1 | 38 |
| Indication | ||||
| Serious hypoglycemiac | 16 (47.1%) | 14 (32.6%) | ||
| High blood sugar | 15 (44.1%) | 9 (20.9%) | ||
| Fluctuating blood sugar | 3 (8.8%) | 18 (41.9%) | ||
| Other | 0 | 2 (4.7%) | ||
| CGM duration (days) | 406.2 (376.4)231.0 (91.0; 1424.0)n = 34 | 33.2 (21.6)28.0 (4.0; 83.0)n = 43 | ||
| HbA1c (%, NGSP) at CGM start | 8.79 (1.85)8.50 (6.06; 13.23)n = 34 | 8.19 (1.35)7.97 (5.20; 14.57)n = 408 | 8.43 (1.27)8.26 (5.29; 12.08)n = 43 | 7.88 (1.36)7.68 (4.72; 14.57)n = 1204 |
| Hypoglycemiac events at CGM start | ||||
| 0 to <5 | 8 (30.8%) | 12 (29.3%) | ||
| 5 to <10 | 6 (23.1%) | 8 (19.5%) | ||
| 10 to <15 | 5 (19.2%) | 15 (36.6%) | ||
| ≥15 | 7 (26.9%) | 6 (14.6%) | ||
| Missing | 8 | 2 | ||
Forcategoricalvariables, n(%) ispresented.
Forcontinuousvariables, mean(SD) / median/ (minimum; maximum) / narepresented.
Strict definition for hypoglycemia: All patients that have follow-up hypoglycemia within 6 months around the latest HbA1c value 3 months after CGM.
Effect of CGM Use on HbA1c
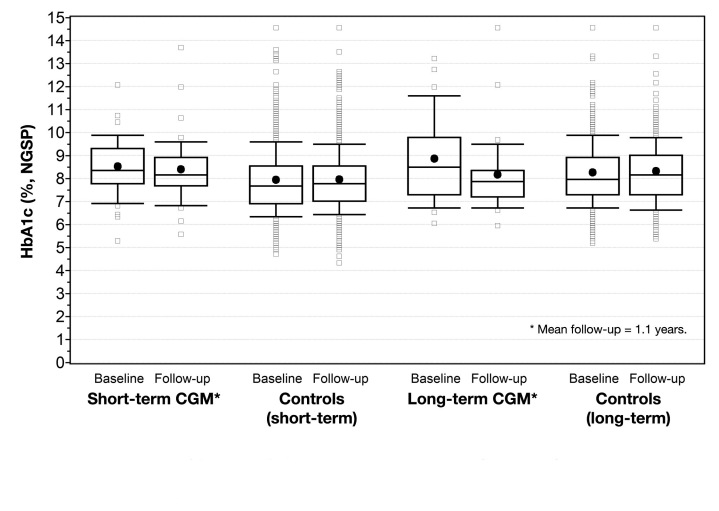
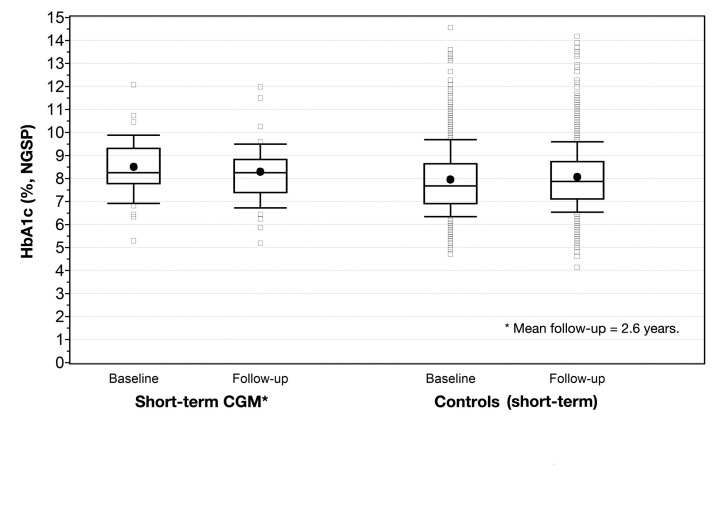
Figures 1 and 2 display the changes in HbA1c for long-term, short-term, and control patients.
Figure 1.
HbA1c at baseline and follow-up for short-term CGM users, long-term CGM users, and controls at 1.1 years. The dot represents mean value; horizontal line, median. The top portion of the box represents the 75th percentile; lower postion, 25th percentile. The upper whisker represents the 90th percentile; lower whisker, 10th percentile.
Figure 2.
HbA1c at baseline and follow-up for short-term CGM users and controls at 2.6 years. The dot represents mean value; horizontal line, median. The top portion of the box represents the 75th percentile; lower postion, 25th percentile. The upper whisker represents the 90th percentile; lower whisker, 10th percentile.
Over a mean follow-up of 1.1 years, there was a statistically significant 0.70% reduction in HbA1c in long-term CGM users (p < .001). Insulin regimen and insulin dose differed at baseline between long-term users and the control group. After adjustments for these variables, the significant effect remained [adjusted mean difference of HbA1c: -0.76 (95% CI: -1.17; -0.33, p < .001)]. When analyzing the 26 long-term users without any preceding short-term CGM use, the effect was slightly greater (reduction of 0.8 ± 1.1%, p = .001).
Over a mean follow-up of 2.6 years, short-term use (n = 43) showed no statistically significant effect on HbA1c (-0.20%, p = .11). Sex, HbA1c, insulin regimen, and insulin dose differed at baseline between the short-term users and the control group. After adjustments for these variables, there was not a significant effect on HbA1c [adjusted mean difference HbA1c: -0.22 (95% CI: -0.545; 0.101, p = .19)]. When studying the effect of short-term use at the same period of follow-up as long-term (by analyzing the latest HbA1c value between 3 months and 1.5 years), there were 41 patients having valid data of HbA1c. The decrease in HbA1c was not statistically significant at this point in time [-0.13% ± 0.77% (p = .056)]. Sex, HbA1c, insulin regimen, and insulin dose differed between patients and controls at baseline. After adjustments for these variables, there was no significant effect on the change in HbA1c to 1.1 years when comparing short-term CGM patients with controls [adjusted mean difference HbA1c: 0.028 (95% CI: -0.27; 0.33, p = .85)].
When studying the effects on HbA1c by groups divided by indication, long-term users with the indication of high HbA1c showed a 1.2 ± 1.1% reduction from 10.1 ± 1.6% to 8.9 ± 2.0% (p = .003). Long-term users with indications for serious hypoglycemic episodes had reductions in HbA1c of 0.3% from 7.7 ± 1.3% to 7.4 ± 1.0% (p = .17).
Hypoglycemic Events
Information on nonsevere hypoglycemic events was available for 26 (76%) long-term users and 41 (95%) short-term users. Detailed descriptive data on nonsevere hypo-glycemia and follow-up duration for the two groups are given in Table 2. In long-term users, the number of hypoglycemic events decreased for at least one five-step scale in 50% of patients; 38% remained within the same scale and 12% had an increase by one five-step scale. This change from start to follow-up value during long-term CGM use was statistically significant (p = .004). The short-term use of CGM did not show the same effect on decreasing nonsevere hypoglycemic events—22% decreased for at least one five-step scale, 51% remained in the same scale, and 27% had an increase of at least one five-step scale (p = .91).
Table 2.
Effects of Long- and Short-Term Use of CGM on Nonsevere Hypoglycemia
| Long-term CGM users(≥3 months)(n = 34) | Short-term CGM users(<3 months)(n = 43) | |||
|---|---|---|---|---|
| Hypoglycemia at CGM start | Follow-up hypoglycemia value | Hypoglycemia at CGM start | Follow-up hypoglycemia value | |
| Hypoglycemia at CGM start | ||||
| 0 to <5 | 8 (30.8%) | 11 (42.3%) | 12 (29.3%) | 12 (29.3%) |
| 5 to <10 | 6 (23.1%) | 12 (46.2%) | 8 (19.5%) | 10 (24.4%) |
| 10 to <15 | 5 (19.2%) | 1 (3.8%) | 15 (36.6%) | 11 (26.8%) |
| ≥15 | 7 (26.9%) | 2 (7.7%) | 6 (14.6%) | 8 (19.5%) |
| Missing | 8 | 8 | 2 | 2 |
| Change in hypoglycemia from CGM start to follow-up valuea,b | ||||
| Reduction in hypoglycemia (2 × 5-step scale) | 7 (26.9%) | 5 (12.2%) | ||
| Reduction in hypoglycemia (1 × 5-step scale) | 6 (23.1%) | 4 (9.8%) | ||
| Hypoglycemia cases within the same 5-step scale | 10 (38.5%) | 21 (51.2%) | ||
| Increase in hypoglycemia (1 × 5-step scale) | 3 (11.5%) | 8 (19.5%) | ||
| Increase in hypoglycemia (2 × 5-step scale) | 3 (7.3%) | |||
| Missing | 8 | 2 | ||
| Number of days between CGM start and hypoglycemia follow-up datec | 434.0 (449.0) | 1056 (431) | ||
| 199.5 | 1022 | |||
| (6.0; 1501.0) | (356; 1748) | |||
| n = 26 | n = 41 | |||
Wilcoxonsigned-ranktestforchangefromCGMstarttofollow-uphypoglycemiavalue: long-termCGM: p = .0042
Wilcoxonsigned-ranktestforchangefromCGMstarttofollow-uphypoglycemiavalue: short-termCGM: p = .9142
Forcontinuousvariables, mean(SD) / median/ (minimum; maximum) / narepresented.
In long-term users with an indication for serious hypo-glycemia (n = 14), 6 patients (43%) had a decrease in the number of nonsevere hypoglycemic events by two five-step scales. Two patients (14%) had a decrease by one five-step scale, 5 patients (36%) remained within the same scale, and 1 patient (7%) had an increase by one five-step scale (p = .0156). No statistically significant changes in the number of nonsevere hypoglycemic events were evident for short-term users with serious hypoglycemia as an indication, nor for any of the groups with high HbA1c as an indication.
Discussion
In this cohort of patients with diabetes (99% with T1DM), we demonstrated a statistically significant improvement in HbA1c in patients using CGM long term. In contrast, patients using CGM short term (<3 months) showed no statistically significant reduction in HbA1c. The effect of long-term CGM use on glycemic control was greater in patients with a primary indication to lower HbA1c than those receiving CGM to prevent hypoglycemia. In a subgroup of patients with valid data on nonsevere hypoglycemia, a preventive effect was also shown by long-term CGM therapy and was greater in patients with indications for serious hypoglycemia.
The magnitude of effect found on HbA1c by long-term use of CGM is of interest. Patients receiving CGM had a 0.8% reduction in HbA1c. In T1DM patients, reductions at this level are greater than those generally seen with use of other novel therapies such as insulin analogs and insulin pump therapy.4,5,11,13–15 Reductions of this magnitude are also associated with large reductions in risk for diabetes complications.16,17 In a large clinical trial of sensor-augmented insulin pump therapy, HbA1c was reduced by 0.6% compared with a control group receiving MDI.8 Because therapy between groups in that study differed by both insulin pump and CGM, it is difficult to determine how much of the difference was attributable to CGM therapy per se. In another study of patients in everyday clinical practice,18 the addition of CGM showed a reduction of 0.4% in HbA1c and was also associated with a statistically significant decrease in severe hypoglycemia.
In Sweden, it is common for patients to receive CGM for approximately 2–8 weeks from a pedagogic point of view to study glucose patterns at different activities in an attempt to improve HbA1c or hypoglycemia after the treatment period. The finding that there was no effect on glycemic control in short-term users indicates that a greater focus on long-term use of CGM may be relevant. The likely reason for the greater effect on HbA1c by long-term use of CGM is continuous feedback of glucose levels, which are disrupted for short-term users. Further, it is noteworthy that the long-term CGM users were treated for a mean follow-up period of 1.1 years; the beneficial effects on HbA1c need to be confirmed for a longer time perspective.
One limitation of this study is the relatively small sample size. Another is that valid data on hypoglycemia were not present in all participants and that we did not evaluate the effect on severe hypoglycemic events, since the number of events during the last year was recorded and several participants had not enough follow-up time. Furthermore, the number of nonsevere hypoglycemic events were self-rated by the patients in a questionnaire at clinical visits and not objectively verified by physicians or diabetes educators. It should also be noted that patients selected for long-term use of CGM might be more suitable or interested in the therapy, thus our results may not be generalizable to all patients with impaired glycemic control. However, this study illustrates that CGM is an efficient practical therapy at least in certain subgroups of patients with impaired glycemic control, which is important because these patients are generally difficult to treat. It should be noted that 30 of the 34 patients with long-term use of CGM were on insulin pump therapy, potentially because of the criteria for reimbursement in Sweden. When it comes to short-term use of CGM, a minor effect on HbA1c cannot be excluded but a larger sample size is needed to confirm this hypothesis. Finally, it should be noted that a strength of the present study is the large control group, which is commonly absent in studies of clinical practice patterns.19
In conclusion, this study shows that CGM therapy is associated with improvements in HbA1c in patients with T1DM and impaired glycemic control, which may potentially lower risk of diabetes complications. Long-term use of CGM also probably reduces nonsevere hypoglycemic events but effects on severe hypoglycemic events remain to be studied. The effect on glycemic control and hypoglycemic events seems to vary substantially depending on the indication of CGM. Short-term use of CGM in clinical practice may be of interest from a pedagogic perspective but shows no large long-term beneficial effect on glycemic control or nonsevere hypoglycemic events. Continuous glucose monitoring should be considered as a treatment option for patients with poor glycemic control and continuously monitored to see if there is a beneficial effect on HbA1c or hypoglycemia but discontinued if no effects are demonstrated.
Acknowledgments
We would like to thank all staff working with Diab-Base and Joseph Murphy for editorial assistance.
Glossary
Abbreviations
- (CGM)
continuous glucose monitoring
- (CI)
confidence interval
- (HbA1c)
hemoglobin A1c
- (MDI)
multiple daily injections
- (NGSP)
National Glycohemoglobin Standardization Program
- (SD)
standard deviation
- (T1DM)
type 1 diabetes mellitus
Funding
This study was supported by unrestricted grants from Abbott Scandinavia, the John and Asta Falkman Foundation, and the Therese Sandwall Foundation.
Disclosures
Marcus Lind has received a grant from Abbott Scandinavia and served as a consultant or received honoraria from Abbott Scandinavia and Medtronic. Ragnar Hanås has received honoraria or served as a consultant for Abbott Scandinavia, Dexcom, and Medtronic.
References
- 1.Diabetes Control and Complications Trial Study Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 2.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352(9131):837–853. [PubMed] [Google Scholar]
- 3.Jeffcoate SL. Diabetes control and complications: the role of glycated haemoglobin, 25 years on. Diabet Med. 2004;21(7):657–665. doi: 10.1046/j.1464-5491.2003.01065.x. [DOI] [PubMed] [Google Scholar]
- 4.Misso ML, Egberts KJ, Page M, O’Connor D, Shaw J. Continuous subcutaneous insulin infusion (CSII) versus multiple insulin injections for type 1 diabetes mellitus. Cochrane Database Syst Rev. 2010;(1):CD005103. doi: 10.1002/14651858.CD005103.pub2. [DOI] [PubMed] [Google Scholar]
- 5.Monami M, Lamanna C, Marchionni N, Mannucci E. Continuous subcutaneous insulin infusion versus multiple daily insulin injections in type 1 diabetes: a meta-analysis. Acta Diabetol. 2010;47(Suppl 1):77–81. doi: 10.1007/s00592-009-0132-5. [DOI] [PubMed] [Google Scholar]
- 6.Eeg-Olofsson K, Cederholm J, Nilsson PM, Zethelius B, Svensson AM, Gudbjörnsdóttir S, Eliasson B. Glycemic control and cardiovascular disease in 7,454 patients with type 1 diabetes: an observational study from the Swedish National Diabetes Register (NDR) Diabetes Care. 2010;33(7):1640–1646. doi: 10.2337/dc10-0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirsch IB. Clinical review: realistic expectations and practical use of continuous glucose monitoring for the endocrinologist. J Clin Endocrinol Metab. 2009;94(7):2232–2238. doi: 10.1210/jc.2008-2625. [DOI] [PubMed] [Google Scholar]
- 8.Bergenstal RM, Tamborlane WV, Ahmann A, Buse JB, Dailey G, Davis SN, Joyce C, Peoples T, Perkins BA, Welsh JB, Willi SM, Wood MA, STAR 3 Study Group Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med. 2010;363(4):311–320. doi: 10.1056/NEJMoa1002853. [DOI] [PubMed] [Google Scholar]
- 9.Dental and Pharmaceutical Benefits Agency. [Decision on reimburse-ment for glucose]. [Document in Swedish] Dental and Pharmaceutical Benefits Agency: Stockholm; 2010. 2485/2010. Available from: http://www.tlv.se/Upload/Beslut_2010/bes101222-minilink.pdf. [Google Scholar]
- 10.Lind M, Odén A, Fahlén M, Eliasson B. The true value of HbA1c as a predictor of diabetic complications: simulations of HbA1c variables. PLoS One. 2009;4(2):e4412. doi: 10.1371/journal.pone.0004412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fahlén M, Eliasson B, Odén A. Optimization of basal insulin delivery in type 1 diabetes: a retrospective study on the use of continuous subcutaneous insulin infusion and insulin glargine. Diabet Med. 2005;22(4):382–386. doi: 10.1111/j.1464-5491.2004.01444.x. [DOI] [PubMed] [Google Scholar]
- 12.Hoelzel W, Weykamp C, Jeppsson JO, Miedema K, Barr JR, Goodall I, Hoshino T, John WG, Kobold U, Little R, Mosca A, Mauri P, Paroni R, Susanto F, Takei I, Thienpont L, Umemoto M, Wiedmeyer HM, IFCC Working Group on HbA1c Standardization IFCC reference system for measurement of hemoglobin A1c in human blood and the national standardization schemes in the United States, Japan, and Sweden: a method-comparison study. Clin Chem. 2004;50(1):166–174. doi: 10.1373/clinchem.2003.024802. [DOI] [PubMed] [Google Scholar]
- 13.Lind M, Fahlén M, Happich M, Odén A, Eliasson B. The effect of insulin lispro on glycemic control in a large patient cohort. Diabetes Technol Ther. 2009;11(1):51–56. doi: 10.1089/dia.2007.0297. [DOI] [PubMed] [Google Scholar]
- 14.Singh SR, Ahmad F, Lal A, Yu C, Bai Z, Bennett H. Efficacy and safety of insulin analogues for the management of diabetes mellitus: a meta-analysis. CMAJ. 2009;180(4):385–397. doi: 10.1503/cmaj.081041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Plank J, Siebenhofer A, Berghold A, Jeitler K, Horvath K, Mrak P, Pieber TR. Systematic review and meta-analysis of short-acting insulin analogues in patients with diabetes mellitus. Arch Intern Med. 2005;165(12):1337–1344. doi: 10.1001/archinte.165.12.1337. [DOI] [PubMed] [Google Scholar]
- 16.Diabetes Control Complications Trial Study Group. The relation-ship of glycemic exposure (HbA1c) to the risk of development and progression of retinopathy in the Diabetes Control and Complications Trial. Diabetes. 1995;44(8):968–983. [PubMed] [Google Scholar]
- 17.Lind M, Odén A, Fahlén M, Eliasson B. The shape of the metabolic memory of HbA1c: re-analysing the DCCT with respect to time-dependent effects. Diabetologia. 2010;53(6):1093–1098. doi: 10.1007/s00125-010-1706-z. [DOI] [PubMed] [Google Scholar]
- 18.Leinung M, Thompson S, Nardacci E. Benefits of continuous glucose monitor use in clinical practice. Endocr Pract. 2010;16(3):371–375. doi: 10.4158/EP09287.OR. [DOI] [PubMed] [Google Scholar]
- 19.Lind M. Glycaemic control: evaluation of HBA1c as a risk factor and the effect of modern insulins in clinical practice. Gothenburg (Sweden): Sahlgrenska Academy, University of Gothenburg; 2009. Available from: http://gupea.ub.gu.se/handle/2077/19641. [Google Scholar]