Abstract
Background
This usability test investigated the overall preference and usability of the novel NovoTwist® insulin pen needle versus conventional screw-thread needles, when used with Next Generation FlexPen®, in children and adolescents with diabetes.
Methods
This was an open-label, randomized, crossover usability test in children and adolescents with type 1 diabetes who administered insulin with an insulin pen. Test needles were NovoTwist and the participant’s current screw-thread needle (or NovoFine® needle). Following instruction, participants attached the needle to Next Generation FlexPen, made an injection into a foam cushion, and detached the needle. This procedure was conducted three times with both needles in a random order. Responses to 13 questions on user experience with each needle (including overall preference, ease of attachment/detachment of needle/cap, handling, learning, confidence in attachment, and convenience of use) were subsequently recorded on a six-point rating scale (1 = very difficult; 6 = very easy).
Results
Fifteen children aged ≥6 to ≤12 years and 15 adolescents aged ≥13 to ≤17 years participated in the test. A significantly higher proportion of children and adolescents (77%) indicated that they would prefer to use NovoTwist compared with screw-thread needles (p = .005). NovoTwist was preferred by most children and adolescents for overall ease of use (77%; p = .005), for ease of attachment (87%; p < .001) and detachment (83%; p < .001), and as the most appropriate needle to handle for daily injections (73%; p = .016). The mean rating for confidence in correct needle attachment was not significantly different between the two needle types. Seven out of eight parents of children who required assistance for their daily insulin injections stated that they would be “very likely” to allow their child to attach NovoTwist.
Conclusions
These factors may promote confidence in this needle, and thus in self-injecting, among younger patients and their parents.
Keywords: attachment, diabetes, ease of use, insulin pens, NovoTwist
Introduction
Optimum outcomes for patients with type 1 diabetes mellitus require consistent long-term adherence to complex invasive management. Children and adolescents have been shown to have worse adherence, even in groups selected for greater motivation1 and, unsurprisingly, they also have worse glycemic outcomes. The reasons for the worse outcomes are complex but include concerns over needle pain and needle phobia, a loss of control over lifestyle choices, and the premature shift in responsibility from parents.2 Offering greater choices for diabetes care can improve adherence. For instance, there is evidence that the use of insulin pens can improve adherence to insulin treatment compared with the use of vials and syringes,3 but the results on adherence and cost vary in different studies.4–6 The high degree of patient satisfaction with new insulin pens for pediatric use may also potentially contribute to improved treatment adherence in children.7 The reduction in pain is also an advantage of insulin pens compared with vials and syringes.8
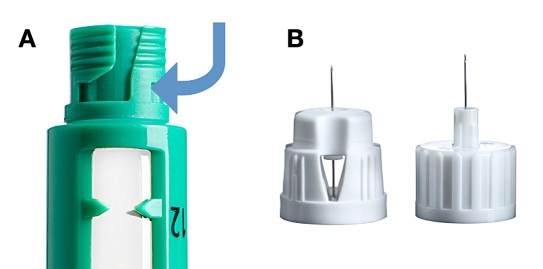
Insulin pens reduce the injection phobia, improve the confidence in dose delivery, and remove the inconvenience associated with vial and syringe systems.9 Consequently, insulin pens are the predominant devices for insulin delivery, especially in Europe (reviewed by Perfetti10). Importantly, insulin pens provide a more accurate and precise delivery of insulin, particularly at low doses (≤5 IU), which can be of particular benefit to children who usually require smaller doses.11 The Next Generation FlexPen® (Novo Nordisk A/S, Denmark) is a prefilled insulin pen that, like Victoza® Pen (Novo Nordisk A/S, Denmark), can be used with NovoTwist®(Novo Nordisk A/S, Denmark). The Next Generation FlexPen is also compatible with Penfill® cartridges for use with the NovoPen® family of durable devices (Novo Nordisk A/S, Denmark). NovoTwist is attached via a bayonet fitting, where the needle is pressed down and turned a quarter of a turn, compared with the conventional screw-thread needle, which requires several turns to attach and detach the needle correctly (Figure 1). Three studies showed that adult patients found NovoTwist easier to attach12,13 and detach12 than other needles, and patients preferred12–14 NovoTwist to conventional screw-thread needles. If preference is also improved in children and adolescents, this may also assist in greater adherence to diabetes management.
Figure 1.
(A) Bayonet fitting on NovoTwist needle, and (B) NovoTwist 5 mm 32 G tip (left) and NovoFine 6 mm 32 G tip (right).
Here we report the results of a usability test of NovoTwist needles versus conventional screw-thread needles, when used with the Next Generation FlexPen, in children and adolescents with diabetes. The primary objective of the test was to evaluate the overall preference for NovoTwist versus screw-thread needles in children and adolescents. Secondary end points included the perception of ease of using the needle.
Methods
Materials
A Next Generation FlexPen with test medium was used in conjunction with either NovoTwist 32 G tip 5 mm needles (Novo Nordisk A/S, Denmark) or the patient’s own screw-thread needles (if the patient did not bring their own needles, NovoFine® 32 G tip 6 mm needles (Novo Nordisk A/S, Denmark) were used for the comparison).
Test Procedures
This was an open-label, randomized, crossover usability test in children aged 6 to 12 years and adolescents aged 13 to 17 years with type 1 diabetes who were already self-injecting with an insulin pen. Following instruction, participants attached the needle to a Next Generation FlexPen, made an injection into a foam cushion, and detached the needle—this procedure was conducted three times using a new needle each time.
This process was conducted with NovoTwist and the participant’s current screw-thread needle (or NovoFine needle) in a random order.
Participants
The test was carried out at nine centers in the United Kingdom. Male or female children/adolescents ≥6 to ≤17 years of age with type 1 diabetes being treated with insulin and who were using an insulin pen were included in the test. Participants were accompanied by a parent to the interview. Parents accompanying young children were allowed to moderate the interview questions and reply so as to help the understanding for the child and to facilitate the process for a more valid result of the test. Exclusion criteria included language barriers that precluded an adequate understanding of the procedures or cooperation of the test, visual impairment requiring assistance when injecting, and any personal or family ties to a pharmaceutical company or marketing research agency. In addition, Penfine® (Ypsomed AG, Burgdorf, Switzerland) users were excluded from the test, as this needle and NovoTwist both have different attachment features and this test aimed to evaluate NovoTwist among patients who were using conventional screw-thread needles. Informed consent and confidentiality agreement regarding the test products were obtained before any test-related activities, in line with local ethics committee requirements.
Questionnaire and Statistical Analysis
The questionnaire (Appendix) used in the test contained two types of questions:
Preference questions, which were asked following handling of both needles, and
Rating questions (including ease of attachment/detachment of needle/cap, handling, and learning; confidence in attachment; and convenience of use) in which each needle was evaluated immediately following handling.
Responses to preference questions were recorded as a direct preference for needle A or needle B or no preference. However, for the primary end point of overall preference, only two options were provided, a preference for needle A or needle B. The preference for NovoTwist was tested against a value of 50% (null hypothesis), with a two-tailed, one-sample binomial test using a 95% confidence interval. Responses to questions on user rating were recorded on a six-point rating scale (1 = very difficult; 6 = very easy). Parents of children who required assistance when injecting rated the likelihood of allowing their children to attach NovoTwist on a scale of 1 (unlikely) to 6 (very likely).
The primary objective of the test was to evaluate the overall preference for NovoTwist versus screw-thread needles in children and adolescents. Secondary end points included perceptions of overall ease of handling, overall ease of learning how to use, ease of attachment and detachment of the needle, and ease of disposal of the needle, as assessed by patients answering an overall rating question related to the process they had just carried out.
In addition to the primary and secondary end points, further evaluation and safety objectives were assessed in the questionnaire, including improvement of ease of daily injection and preference for appropriateness for everyday use and safety.
Results
Fifteen children aged ≥6 to ≤12 years and fifteen adolescents aged ≥13 to ≤17 years participated in the test (Table 1). Mean duration since diagnosis with type 1 diabetes was 5.4 ± 2.75 years. Mean duration of injecting insulin was 4.8 years (range 6 months to 13.6 years).
Table 1.
Baseline Patient and Disease Characteristics
| Characteristic | Patients (n = 30)a |
|---|---|
| Mean age, years ± standard deviation | 13.2 ± 2.75 |
| Male | 11 |
| Left-handed | 2 |
| Duration of insulin self-injection | |
| <1 year | 5 |
| 1–3 years | 6 |
| >3–6 years | 9 |
| >6–9 years | 5 |
| >9 years | 5 |
| Injection device usedb | |
| Autopen® | 5 |
| FlexPen® | 5 |
| Humapen® | 2 |
| NovoPen® | 22 |
| OptiClik® | 1 |
| SoloSTAR® | 4 |
| Other | 4 |
| Needle used | |
| MicroFine® | 19 |
| NovoFine® | 11 |
Number, unless stated otherwise in left-hand column.
More than one pen type may have been used.
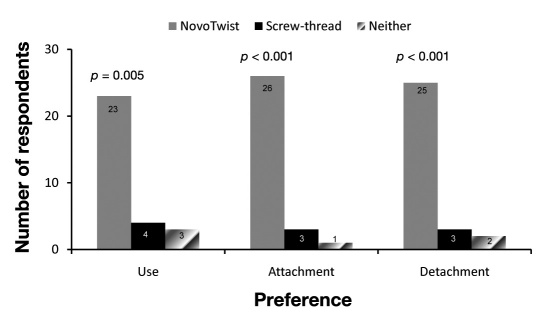
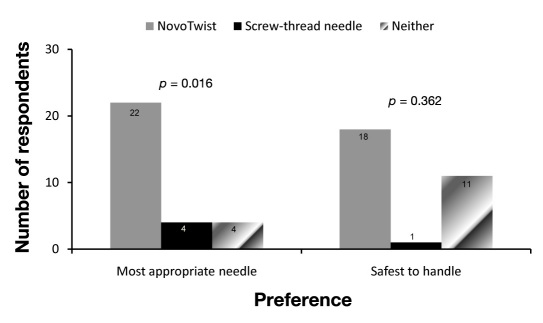
Of the 30 children and adolescents, 23 (77%) indicated that they would prefer to use NovoTwist, the primary end point of the study, and 7 (23%) indicated a preference for screw-thread needles (p = .005). Most participants found NovoTwist easier to use (23/30, 77%; p = .005), easier to attach (26/30, 87%; p < .001), and easier to detach (25/30, 83%; p < .001) than conventional screw-thread needles (Figure 2). Most children and adolescents also rated Novo-Twist as the safest needle to handle (18/30, 60%; p = .362) and the most appropriate needle for everyday use (22/30, 73%; p = .016) compared with conventional screw-thread needles (Figure 3).
Figure 2.
Overall preference for ease of use, attachment, and detachment of NovoTwist compared with a traditional screw-thread needle (n = 30).
Figure 3.
Child and adolescent preference for NovoTwist as the safest and most appropriate needle to use compared with traditional screw-thread needle (n = 30).
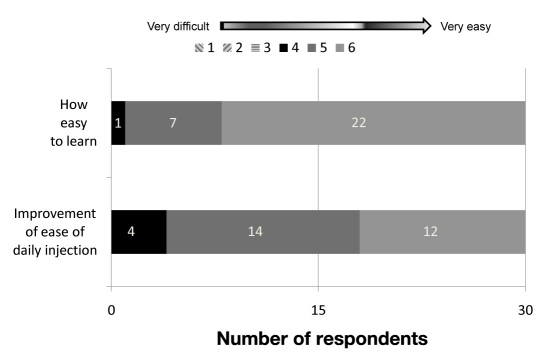
Higher ratings were given for NovoTwist than for screw-thread needles for overall ease of use (mean score 5.7 and 5.2, respectively; p = .029), ease of attaching the needle (mean score 5.6 and 4.9, respectively; p = .003), ease of detaching the needle (mean score 5.7 and 5.0, respectively; p = .004), ease of removing the needle cap (mean score 5.7 and 5.0, respectively; p = .011), ease of handling needle when disposing of it (mean score 5.8 and 5,4 respectively; p = .014), and convenience (in making life simpler; mean score 5.4 and 4.6, respectively; p = .007). A similar rating was given for NovoTwist and screw-thread needles for confidence in correct needle attach-ment (mean score 5.3 and 5.4, respectively; p = .45). Seventy-three percent of respondents rated NovoTwist as very easy to learn to use (mean overall score 5.7 out of 6.0). Intuitiveness of learning how to use the needle was also highly rated, with a mean score of 5.1, and 87% (26/30) scored NovoTwist as either 5 or 6 out of 6 as a great deal of improvement for ease of daily injections of insulin (mean overall score 5.3 out of 6.0; Figure 4). All respondents also rated NovoTwist as very easy to handle when disposing of the needle, with a mean score of 5.8 out of 6.0 versus 25/30 who rated conventional screw-thread needles easy to handle when disposing of (mean score 5.4 out of 6.0). As individual verbatim answers were given in response to “please tell us why you prefer this needle,” the results to this question are not reported here.
Figure 4.
Ratings for ease of learning and improvement in ease of daily injection of NovoTwist among children and adolescents (n = 30).
However, seven of the eight parents of children who required assistance in attaching needles/injecting insulin stated that they would be very likely to allow their children to attach NovoTwist themselves (p = .004).
Discussion
This usability test showed that significantly more children and adolescents had an overall preference for NovoTwist needles compared with screw-thread needles. The findings of this study are in agreement with three other studies12–14 of NovoTwist in adults, which showed that adults with diabetes preferred NovoTwist for ease of use,12 ease of attachment,12,13 and ease of detachment12 compared with conventional screw-thread needles. Similarly, in this study, most children and adolescents also found NovoTwist the easiest needle to use, easier to attach, and easier to detach than screw-thread needles. Most participants in this study also rated NovoTwist the safest needle to handle, very easy to learn, and appropriate for daily injections over conventional screw-thread needles, which may assist in reducing the time spent on daily insulin injections and improve adherence to treatment. In a previous study, NovoTwist was evaluated as being the least time consuming and most user-friendly needle for attachment and detachment.14 Furthermore, in a test of the usability of NovoTwist in adults with manual dexterity impairment, significantly more respondents preferred NovoTwist and most respondents found NovoTwist the most appropriate needle for performing everyday injections compared with conventional screw-thread needles.12
The participants’ response to NovoTwist in this study is encouraging, as it is a novel needle attachment system that the patients had no previous experience with, compared with screw-thread needles that the patients may have used since initiating their insulin regimens. Seven out of eight parents of children who required assistance in their daily treatment stated that they would be very likely to allow their children to attach NovoTwist. Although speculative, this finding suggests that parents have confidence in the handling features of NovoTwist and may be more willing to hand over more responsibility to the child, if this is relevant. In many situations, of course, what is desired is improved parent/child/health care professional teamwork in managing the child’s diabetes, but any improvements in the needle-pen systems that enhance confidence will improve this team effort. This is a significant finding, given the major concerns that parents have over the self-management of diabetes in their children.15 It may also provide an important stepping stone to overcoming a number of barriers to self-management of diabetes in adolescents.16 However, this is likely to be just one of many factors that may enhance self-management in adolescents or children.
Parent–child relationships are extremely important for the successful management of diabetes in young patients, particularly in relation to adherence to insulin treatment regimens.17–23 One of the major concerns of parents is their child’s ability to manage his or her own diabetes treatment.15,17,22 Indeed, a large study of adolescents reported parents as being too protective, worrying too much, or trying to control the diabetes regimen most or all of the time.15 Ability for self-management of diabetes in teenagers has been cited as one of the major reasons for conflict within families.18,22
Previous studies showed a positive significant correlation between injection pain and needle diameter.24 Needles with the thickest diameters caused the most pain on insertion into skin, while thin needles caused significantly less pain in both children and adults. Furthermore, use of NovoTwist 5 mm 32 G tip needles, which is one of the thinnest commercially available needles, further reduced the frequency and severity of injection pain in children compared with the NovoFine 6 mm 32 G tip.25
NovoTwist is available in 5 and 8 mm needle lengths, and the length of the needle is important for the depositing of insulin to the subcutaneous layer. If the needle is too long, there is a risk of intramuscular depositing of insulin, and if the needle is too short, then insulin may be deposited intradermally.26 Indeed, shorter needles (8 mm) have previously been shown to reduce the risk of intra-muscular injections in children with type 1 diabetes compared with longer needles (12.7 mm).26 Furthermore, NovoFine 5 mm 32 G tip needles have been shown to be safe for subcutaneous injections in children with low risk of intramuscular injections and limited back flow when an angled pinched skin-fold technique was used.25 These factors may have contributed to the preference in this study for NovoTwist as the safest needle to handle and the most appropriate needle for everyday use.
Limitations of the current test include the small sample size, and the results of this test should be ratified in larger randomized studies of child and adolescent preference for needle-pen systems. Larger studies should also investigate if there are any ethnic or gender differences in the preference for needle-pen systems and whether this needle would be preferred by certain groups with diabetes such as children and adolescents with manual dexterity problems. One factor that requires clarification is the link between child preference for a needle-pen system and adherence to insulin regimens; this could be the focus of future larger studies.
Previous studies have shown that, among users of insulin injection pens, Next Generation FlexPen was a popular choice of pen,13 and the introduction of NovoTwist needle is likely to enhance patient preference for this system. However, few preference studies include data from children, and the current test is therefore important in providing information on the preferences of children and adolescents. A further limitation of the current test was that the children/adolescents were accompanied by parents, and it is not known whether the responses of children were influenced by their parent’s perceptions of the two needles. However, it was considered necessary for a parent to accompany a young child in order to facilitate the child’s understanding of the process to produce more valid test results.
Acknowledgments
The test was carried out by Kate Seymour of Aequus Research, London, UK. Editorial assistance was supplied by John Clarke of ESP Bioscience, Crowthorne, UK, and was funded by Novo Nordisk A/S, Bagsvaerd, Denmark.
Appendix: Questionnaire
Complete for each needle following handling
1. How easy/difficult was it to attach the needle? (Please circle one number only.)
| Very difficult | Very easy | |||||
|---|---|---|---|---|---|---|
| Needle A | 1 | 2 | 3 | 4 | 5 | 6 |
| Needle B | 1 | 2 | 3 | 4 | 5 | 6 |
2. How easy or difficult was it to remove the needle cap? (Please circle one number only.)
| Very difficult | Very easy | |||||
|---|---|---|---|---|---|---|
| Needle A | 1 | 2 | 3 | 4 | 5 | 6 |
| Needle B | 1 | 2 | 3 | 4 | 5 | 6 |
3. How confident were you that the needle was correctly attached? (Please circle one number only.)
| Not at all confident | Very confident | |||||
|---|---|---|---|---|---|---|
| Needle A | 1 | 2 | 3 | 4 | 5 | 6 |
| Needle B | 1 | 2 | 3 | 4 | 5 | 6 |
4. How easy or difficult was it to detach/remove the needle from the pen? (Please circle one number only.)
| Very difficult | Very easy | |||||
|---|---|---|---|---|---|---|
| Needle A | 1 | 2 | 3 | 4 | 5 | 6 |
| Needle B | 1 | 2 | 3 | 4 | 5 | 6 |
5. After removing the needle from the pen, how easy or difficult was it to handle the needle when disposing of it? (Please circle one number only.)
| Very difficult | Very easy | |||||
|---|---|---|---|---|---|---|
| Needle A | 1 | 2 | 3 | 4 | 5 | 6 |
| Needle B | 1 | 2 | 3 | 4 | 5 | 6 |
6. Overall, how easy or difficult was it to learn how to use this needle? (Not applicable for Needle B. Please circle one number only.)
| Very difficult | Very easy | |||||
|---|---|---|---|---|---|---|
| Needle A | 1 | 2 | 3 | 4 | 5 | 6 |
7. Overall, how intuitive was it to learn how to use this needle? (Not applicable for Needle B. Please circle one number only.)
| Not at all intuitive | Very intuitive | |||||
|---|---|---|---|---|---|---|
| Needle A | 1 | 2 | 3 | 4 | 5 | 6 |
8. Overall, how easy or difficult was it to use this needle? (Please circle one number only.)
| Very difficult | Very easy | |||||
|---|---|---|---|---|---|---|
| Needle A | 1 | 2 | 3 | 4 | 5 | 6 |
| Needle B | 1 | 2 | 3 | 4 | 5 | 6 |
9. Overall, how convenient (in terms of making your life simpler) do you find this needle to be? (Please circle one number only.)
| Very inconvenientt | Very convenient | |||||
|---|---|---|---|---|---|---|
| Needle A | 1 | 2 | 3 | 4 | 5 | 6 |
| Needle B | 1 | 2 | 3 | 4 | 5 | 6 |
10. To what extent would this new needle make it easier for you to inject on a daily basis? (Not applicable if Needle B is respondent's own needle. Please circle one number only.)
| Not at all | A great deal | |||||
|---|---|---|---|---|---|---|
| Needle A | 1 | 2 | 3 | 4 | 5 | 6 |
Interviewer, when both needles are assessed individually ask following questions:
Comparing the needles:
| In your opinion, which needle… | Needle A | Needle B | Both the same |
|---|---|---|---|
| is the easiest to attach? | 1 | 2 | 3 |
| is the easiest to detach? | 1 | 2 | 3 |
| is easiest to use overall? | 1 | 2 | 3 |
| is safest to handle? | 1 | 2 | 3 |
| is most appropriate for performing everyday injections overall? | 1 | 2 | 3 |
11. Overall, which needle would you prefer to use?
| Needle A: twist needle | Needle B: own needle/screw-thread needle |
|---|---|
| 1 | 2 |
12. Please tell me why you prefer this needle?
13. If your current needle was Needle A, how likely would you be to allow your child to attach this needle themselves? (Please circle one number only.)
| Not at all likely | Very likely | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 |
Funding
The test was sponsored by Novo Nordisk A/S, Bagsvaerd, Denmark.
Disclosures
Søren Kruse Lilleøre and Gitte Ter-Borch are employees of Novo Nordisk.
References
- 1.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 2.Hood KK, Peterson CM, Rohan JM, Drotar D. Association between adherence and glycemic control in pediatric type 1 diabetes: a meta-analysis. Pediatrics. 2009;124(6):e1171–9. doi: 10.1542/peds.2009-0207. [DOI] [PubMed] [Google Scholar]
- 3.Baser O, Bouchard J, DeLuzio T, Henk H, Aagren M. Assessment of adherence and healthcare costs of insulin device (FlexPen) versus conventional vial/syringe. Adv Ther. 2010;27(2):94–104. doi: 10.1007/s12325-010-0009-6. [DOI] [PubMed] [Google Scholar]
- 4.Cobden D, Lee WC, Balu S, Joshi AV, Pashos CL. Health outcomes and economic impact of therapy conversion to a biphasic insulin analog pen among privately insured patients with type 2 diabetes mellitus. Pharmacotherapy. 2007;27(7):948–962. doi: 10.1592/phco.27.7.948. [DOI] [PubMed] [Google Scholar]
- 5.Lee WC, Balu S, Cobden D, Joshi AV, Pashos CL. Medication adherence and the associated health-economic impact among patients with type 2 diabetes mellitus converting to insulin pen therapy: an analysis of third-party managed care claims data. Clin Ther. 2006;28(10):1712–1725. doi: 10.1016/j.clinthera.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Pawaskar MD, Camacho FT, Anderson RT, Cobden D, Joshi AV, Balkrishnan R. Health care costs and medication adherence associated with initiation of insulin pen therapy in medicaid-enrolled patients with type 2 diabetes: a retrospective database analysis. Clin Ther. 2007;29 doi: 10.1016/j.clinthera.2007.07.007. Spec No:1294-305. [DOI] [PubMed] [Google Scholar]
- 7.Olsen BS, Lilleøre SK, Korsholm CN, Kracht T. Novopen Echo® for the delivery of insula comparison of usability, functionality and preference among pediatric subjects, their parents, and health care professionals. J Diabetes Sci Technol. 2010;4(6):1468–1475. doi: 10.1177/193229681000400622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanas R, Ludvigsson J. Experience of pain from insulin injections and needle phobia in young patients with IDDM. Pract Diabetes Int. 1997;14(4):95–99. [Google Scholar]
- 9.Korytkowski M, Niskanen L, Asakura T. FlexPen: addressing issues of confidence and convenience in insulin delivery. Clin Ther. 2005;27(Suppl B):S89–100. doi: 10.1016/j.clinthera.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 10.Perfetti R. Reusable and disposable insulin pens for the treatment of diabetes: understanding the global differences in user preference and an evaluation of inpatient insulin pen use. Diabetes Technol Ther. 2010;12(Suppl 1):S79–85. doi: 10.1089/dia.2009.0179. [DOI] [PubMed] [Google Scholar]
- 11.Lteif AN, Schwenk WF. Accuracy of pen injectors versus insulin syringes in children with type 1 diabetes. Diabetes Care. 1999;22(1):137–140. doi: 10.2337/diacare.22.1.137. [DOI] [PubMed] [Google Scholar]
- 12.Hansen B, Lilleøre SK, Ter-Borch G. Needle with a novel attachment versus conventional screw-thread needles: a preference and usability test among adults with diabetes and impaired manual dexterity. Diabetes Technol Ther. 2011;13(5):579–585. doi: 10.1089/dia.2010.0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sommavilla B, Jørgensen C, Jensen KH. Safety, simplicity and convenience of a modified prefilled insulin pen. Expert Opin Pharmacother. 2008;9(13):2223–2232. doi: 10.1517/14656566.9.13.2223. [DOI] [PubMed] [Google Scholar]
- 14.Lytzen L, Ostfeldt L. Comparative assessment of NovoTwist™, a novel insulin pen needle system. 1985-PO. American Diabetes Association 69th Scientific Sessions; 2009. [Google Scholar]
- 15.Cameron FJ, Skinner TC, de Beaufort CE, Hoey H, Swift PG, Aanstoot H, Aman J, Martul P, Chiarelli F, Daneman D, Danne T, Dorchy H, Kaprio EA, Kaufman F, Kocova M, Mortensen HB, Njølstad PR, Phillip M, Robertson KJ, Schoenle EJ, Urakami T, Vanelli M, Ackermann RW, Skovlund SE, Hvidoere Study Group on Childhood Diabetes Are family factors universally related to metabolic outcomes in adolescents with Type 1 diabetes? Diabet Med. 2008;25(4):463–468. doi: 10.1111/j.1464-5491.2008.02399.x. [DOI] [PubMed] [Google Scholar]
- 16.Mulvaney SA, Mudasiru E, Schlundt DG, Baughman CL, Fleming M, VanderWoude A, Russell WE, Elasy TA, Rothman R. Self-management in type 2 diabetes: the adolescent perspective. Diabetes Educ. 2008;34(4):674–682. doi: 10.1177/0145721708320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anderson BJ, Brackett J, Ho J, Laffel LM. An office-based inter-vention to maintain parent-adolescent teamwork in diabetes management. Impact on parent involvement, family conflict, and subsequent glycemic control. Diabetes Care. 1999;22(25):713–721. doi: 10.2337/diacare.22.5.713. [DOI] [PubMed] [Google Scholar]
- 18.Anderson BJ, Vangsness L, Connell A, Butler D, Goebel-Fabbri A, Laffel LM. Family conflict, adherence, and glycaemic control in youth with short duration type 1 diabetes. Diabet Med. 2002;19(8):635–642. doi: 10.1046/j.1464-5491.2002.00752.x. [DOI] [PubMed] [Google Scholar]
- 19.Berg CA, Butler JM, Osborn P, King G, Palmer DL, Butner J, Murray M, Lindsay R, Donaldson D, Foster C, Swinyard M, Wiebe DJ. Role of parental monitoring in understanding the benefits of parental acceptance on adolescent adherence and metabolic control of type 1 diabetes. Diabetes Care. 2008;31(4):678–683. doi: 10.2337/dc07-1678. [DOI] [PubMed] [Google Scholar]
- 20.Duke DC, Geffken GR, Lewin AB, Williams LB, Storch EA, Silverstein JH. Glycemic control in youth with type 1 diabetes: family predictors and mediators. J Pediatr Psychol. 2008;33(7):719–727. doi: 10.1093/jpepsy/jsn012. [DOI] [PubMed] [Google Scholar]
- 21.Helgeson VS, Reynolds KA, Siminerio L, Escobar O, Becker D. Parent and adolescent distribution of responsibility for diabetes self-care: links to health outcomes. J Pediatr Psychol. 2008;33(5):497–508. doi: 10.1093/jpepsy/jsm081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leonard BJ, Garwick A, Adwan JZ. Adolescents’ perceptions of parental roles and involvement in diabetes management. J Pediatr Nurs. 2005;20(6):405–414. doi: 10.1016/j.pedn.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 23.Miller-Johnson S, Emery RE, Marvin RS, Clarke W, Lovinger R, Martin M. Parent-child relationships and the management of insulin-dependent diabetes mellitus. J Consult Clin Psychol. 1994;62(3):603–610. doi: 10.1037//0022-006x.62.3.603. [DOI] [PubMed] [Google Scholar]
- 24.Arendt-Nielsen L, Egekvist H, Bjerring P. Pain following controlled cutaneous insertion of needles with different diameters. Somatosens Mot Res. 2006;23(1-2):37–43. doi: 10.1080/08990220600700925. [DOI] [PubMed] [Google Scholar]
- 25.Hofman PL, Derraik JG, Pinto TE, Tregurtha S, Faherty A, Peart JM, Drury PL, Robinson E, Tehranchi R, Donsmark M, Cutfield WS. Defining the ideal injection techniques when using 5-mm needles in children and adults. Diabetes Care. 2010;33(9):1940–1944. doi: 10.2337/dc10-0871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tubiana-Rufi N, Belarbi N, Du Pasquier-Fediaevsky L, Polak M, Kakou B, Leridon L, Hassan M, Czernichow P. Short needles (8 mm) reduce the risk of intramuscular injections in children with ty1pe 1 diabetes. Diabetes Care. 1999;22(10):1621–1625. doi: 10.2337/diacare.22.10.1621. [DOI] [PubMed] [Google Scholar]