Abstract
Background
Numerous tests have been developed to estimate insulin sensitivity (SI). However, most of the established tests are either too expensive for widespread application or do not yield reliable results. The dynamic insulin sensitivity and secretion test (DISST) uses assays of glucose, insulin, and C-peptide from nine samples to quantify SI and endogenous insulin secretion (UN) at a comparatively low cost. The quick dynamic insulin sensitivity test has shown that the DISST SI values are robust to significant assay omissions.
Methods
Eight DISST-based variations of the nine-sample assay regimen are proposed to investigate the effects of assay omission within the DISST-based framework. SI and UN were identified using the fully-sampled DISST and data from 218 nine-sample tests undertaken in 74 female individuals with elevated diabetes risk. This same data was then used with appropriate assay omissions to identify SI and UN with the eight DISST-based assay variations.
Results
Median intraprocedure proportional difference between SI values from fully-sampled DISST and the DISST-based variants was in the range of -17.9 to 7.8%. Correlations were in the range of r = 0.71 to 0.92 with the highest correlations between variants with the greatest commonality with the nine-sample DISST. Metrics of UN correlated relatively well between tests when C-peptide was assayed (r = 0.72 to 1) but were sometimes not well estimated when samples were not assayed for C-peptide (r = -0.14 to 0.75).
Conclusions
The DISST-based spectrum offers a series of tests with very distinct compromises of information yield, accuracy, assay cost, and clinical intensity. Thus, the spectrum of tests has the potential to enable researchers to better allocate funds by selecting an optimal test configuration for their particular application.
Keywords: dynamic insulin sensitivity tests, insulin resistance screening, insulin sensitivity, physiological modeling, prediabetes
Introduction
Numerous investigations have found that insulin sensitivity (SI) is an important metabolic marker of risk for cardiovascular disease1–4 and type 2 diabetes.5–8 However, use of SI to investigate the pathophysiology of these conditions has been limited by the lack of a widely accepted insulin sensitivity test that is both economical and accurate. Existing tests involve either intensive high-cost protocols that produce reliable SI values or lower intensity protocols that provide less accurate SI values at a lower cost.9,10 Surrogate measures of insulin sensitivity derived from oral glucose tolerance test data have exhibited reasonable compromises of cost and accuracy11–13 but have not become widely used.
The dynamic insulin sensitivity and secretion test (DISST) was originally developed to provide a means of assessing SI and endogenous insulin secretion (UN) with a favorable compromise of economy and accuracy.14–16 Subsequent investigation has shown that the cost of the original DISST could be significantly reduced by eliminating the insulin and C-peptide assays with only a moderate associated reduction in accuracy.17,18 Using only glucose measurements, the quick dynamic insulin sensitivity test (DISTq) is able to identify SI in real time.17,18 Although the DISTq can generate participant-specific SI values at a substantially lower cost, the standard DISST provides metrics of both SI and UN to provide a comprehensive observation of the participant’s metabolic health.
The DISST and DISTq use two very distinct assay protocols with different trade-offs on cost and accuracy of SI and UN identification. However, numerous variations of the standard DISST-based assay regimens could potentially provide tests with optimal compromises of information yield, assay cost, metric accuracy, and clinical intensity for a number of potential clinical applications. This article describes eight such variations that utilize the standard DISST protocol, and presents their accuracy in terms of repeating the findings of the fully sampled DISST15 in a moderately insulin-resistant cohort.19
Methods
Participants
Eighty-two female participants from the Otago region of New Zealand took part in a 10-week dietary intervention trial described by Te Morenga and colleagues.19 Inclusion criteria required that participants either had a body mass index greater than 25, or greater than 23 and a family history of type 2 diabetes, or ethnic disposition toward type 2 diabetes. Participants were excluded if they had a major illness, including established diabetes, at the time of testing. In total, 74 subjects provided 218 full DISST data sets. Participant characteristics are summarized in Table 1. Ethical approval for this study was granted by the University of Otago Ethics Committee.
Table 1.
Participant Characteristics and Insulin Sensitivity Results
| Status NGT/IFG/T2DMa | Body mass index Q1 Q2 Q3 | Sex M/F | Age Q1 Q2 Q3 | HOMA-IR Q1 Q2 Q3 | DISST-SI Q1 Q2 Q3 |
|---|---|---|---|---|---|
| 27.6 | 34.8 | 1.37 | 0.83 | ||
| 63/11/0 | 32.4 | 0/74 | 43 | 2.15 | 1.13 |
| 36.3 | 50.3 | 3.11 | 1.57 |
NGT, normal glucose tolerance; IFG, impaired fasting glucose; T2DM, type 2 diabetes mellitus.
DISST Protocol
The DISST was conducted at weeks 0, 4, and 10 of the intervention by a research nurse under medical super-vision. Participants attended the test after a 10–12 h fast and remained in a seated position for the test’s duration. Samples were drawn at t = 0, 5, 10, 15, 20, 25, 30, 35, and 45 min through a cannula that was inserted into the antecubital fossa. A 10 g bolus of intravenous glucose containing 50% dextrose and 50% normal saline was given via the same cannula within 1 min after the fasting sample. One unit of insulin (Actrapid®, Novo Nordisk, Copenhagen, Denmark) was given immediately after the 10 min sample. Immediately after drawing each blood sample, the cannula was flushed with 1–2 ml normal saline to prevent clotting. Approximately 3 ml of blood were withdrawn to remove the saline just prior to taking each blood sample. Samples were collected into separate vacutainers containing coagulant for measurement of insulin and C-peptide, and fluoride and oxalate anticogulants for measurement of glucose concentrations.
Whole blood samples were centrifuged at 1650 g for 15 min, and then the plasma was pipetted into poly-ethylene cryovials and stored at -80 °C for up to 12 months. At the completion of the data collection period, laboratory analyses of all assays were performed by batch to minimize laboratory measurement bias. Serum insulin and C-peptide were measured using a specific insulin electrochemiluminescence immunoassay (Roche, Catalog Number 12017547) for the Elecsys® analyzer (Roche Diagnostics, Mannheim, Germany) after polyethylene glycol precipitation of immunoglobulins, with a coefficient of variation of 1.5%. Plasma glucose was measured enzyma-tically with Roche kits and calibrators on a Cobas Mira® analyzer (Hexokinase Catalog Number 11447513216, Roche, Mannheim, Germany) with a coefficient of variation of 0.5%.
Design Strategy of the Various Proposed Protocols
Eight variations of the standard DISST sampling and assay protocol were evaluated by their ability to reproduce the SI and UN values identified by the fully sampled DISST (DISST-FS).15 Each variation uses different sampling and assay regimens to provide a distinct compromise of economy, accuracy, and information. Sample omissions effectively reduce clinical effort and intensity by skipping some DISST-FS scheduled blood samples. Assay omissions still require the same blood samples but only one or two species are assayed to minimize overall assay cost. Five of the sampling protocols were based on DISTq identification methods.17 Thus, these protocols could not provide estimates of patient-specific first and subsequent pass hepatic extraction (xL and nL, respectively) and UN values.
DISST-FS
DISST-FS is a low-dose, short-duration insulin modified intravenous glucose tolerance test.14–16,20 The DISST modeling approach has enabled high accuracy insulin sensitivity and insulin secretion metrics to be derived from a 45 min, nine-sample protocol that is less frequently sampled than established dynamic protocols. It utilizes C-peptide, insulin, and glucose assays from every available sample time.
Short
The Short protocol was designed to capture the major dynamics of C-peptide, insulin, and glucose responses with reduced overall test time and fewer samples.
DISST-E/SI
The DISST-E/SI identification method uses six glucose, six insulin, and three C-peptide assays from seven samples. Three significant metrics can be derived from typical UN profiles: the basal insulin production rate (UB), the first-phase secretion (U1), and the second-phase production (U2). Only three C-peptide assays are required to directly and uniquely identify these rates.
Sparse
The Sparse protocol was designed to reduce clinical intensity by taking only three samples to define the three major UN metrics and SI. The second sample taken 5 min after the glucose bolus in the DISST-FS is subject to incomplete mixing of the glucose bolus in the blood-stream14–21 and is thus not used to define the participant’s specific glucose distribution volume (VG). A proportion (29%) of the participant’s lean body mass (LBM)22 is used to estimate VG in this protocol.
DIST-SI
DIST-SI identifies SI but not UN, thus it does not require C-peptide assays. Population-based parameter estimations from the DISTq17 were used to estimate the UN profiles based on the participants’ SI values. The DISST nomenclature is reduced to DIST when insulin secretion values are not reported.
DIST-SI-2
DIST-SI-2 involves further simplification of the DIST-SI protocol by taking fewer samples and performing fewer assays than the DIST-SI protocol. The period of greatest importance to SI identification is the later part of the test protocol. Thus, only two samples, taken at the end of the test, were assayed for insulin, while the full glucose response was observed with four glucose assays.
DISTq-FS
DISTq-FS requires eight glucose assays to define SI in an iterative identification process.17,18 The method estimates the participant’s endogenous insulin production and insulin pharmacokinetics with a series of functions of the participant’s SI and anatomical data (height, weight, age, and sex).23 Thus, the method is a posteriori and iterative in nature.
DISTq-S
This protocol is a simplification of the Short DIST specifically and uses only four glucose assays to define a value for SI. The second sample (at t = 5) is not assayed for this identification method. However, this sample may allow for later assays of the insulin or C-peptide to increase resolution of the insulin concentration reconstruction or obtain metrics of first-phase insulin production.
DISTq30
DISTq30 identifies SI using very sparse data (two glucose assays only). Functions of the participant’s LBM are used to define the glucose distribution volume in the absence of measured data.
Table 2 shows the sampling and assay protocols used by each protocol. Table 3 summarizes the clinical burden of the tests in terms of protocol duration and assay cost as well as the potential outcomes. Assay costs in New Zealand are approximately $2.50 for glucose, $25 for insulin, and $35 for C-peptide (NZ$).
Table 2.
Proposed Sample and Assay Schedules for Glucose (G), Insulin (I), and C-Peptide (C). S Denotes a Sample that is Drawn and Stored but Not Assayed
| Assay regimen | Assays | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 5 | 10 | 15 | 20 | 25 | 30 | 35 | 45 | G | I | C | |
| DISST-FS | GIC | IC | GIC | GC | GIC | GIC | GIC | GIC | GIC | 8 | 8 | 9 |
| Short | GIC | IC | GIC | - | GIC | - | GIC | - | - | 4 | 5 | 5 |
| DISST-E/SI | GIC | IC | GI | G | GIC | GI | GI | - | - | 6 | 6 | 3 |
| Sparse | GIC | IC | - | - | - | GIC | - | - | - | 2 | 3 | 3 |
| DIST-SI | GI | I | GI | G | GI | GI | GI | - | - | 6 | 6 | 0 |
| DIST-SI-2 | G | - | G | - | GI | - | GI | - | - | 4 | 2 | 0 |
| DISTq-FS | G | S | G | G | G | G | G | G | G | 8 | 0 | 0 |
| DISTq-S | G | S | G | - | G | - | G | - | - | 4 | 0 | 0 |
| DISTq30 | G | - | - | - | - | - | G | - | - | 2 | 0 | 0 |
| HOMA | GI | - | - | - | - | - | - | - | - | 1 | 1 | 0 |
Table 3.
Duration, Relative Assay Cost, and Outcomes of the Proposed Protocols
| Samples | Protocol duration (min) | Assay cost (NZ$) | Real-time results | Measured UN | |
|---|---|---|---|---|---|
| DISST-FS | 9 | 45 | 535 | Y | |
| Short | 5 | 30 | 310 | Y | |
| DISST-E/SI | 7 | 30 | 270 | Y | |
| Sparse | 3 | 25 | 185 | Y | |
| DIST-SI | 7 | 30 | 165 | ||
| DIST-SI-2 | 4 | 30 | 60 | ||
| DISTq-FS | 9 | 45 | 20 | Y | |
| DISTq-S | 5 | 30 | 10 | Y | |
| DISTq30 | 2 | 30 | 5 | Y | |
| HOMA | 1 | 2 | 27 |
The homeostasis model assessment (HOMA) metric is included to provide context and comparison to the DISST-based outcomes.
SI and UN Identification Methods
Insulin sensitivity and secretion metrics are defined by identifying parameters of a physiological model against DISST test data. The model is presented in detail by Lotz and colleagues.14–15
UN profiles are either defined using deconvolution (DC) when C-peptide assays are available or the population-based estimates of the DISTq method (EDISTq) when C-peptide assays are not available. The DC method was developed by Eaton and colleagues.24 and validated by Van Cauter and colleagues.23 It has been used with the DISST test data14,20,25 and proven robust to assay omissions.26 The DISTq identification methods and population-based estimates have been published elsewhere.17,18 The identi-fication of UN metrics from the DISST-E/SI data requires a slight variation on the stated DC approach as the final blood sample of the DISST-E/SI is not assayed for C-peptide. Thus, the UN rate is assumed constant after the final C-peptide assay.
Plasma and interstitium insulin concentrations are defined using either the iterative integral method (IIM)17,27 or the DISTq estimation methods (EDISTq).17,18 The IIM identifies participant-specific values of nL and xL to simulate the observed insulin pharmacokinetics and simulated profiles of insulin in the two compartments. The DISTq method uses the population-based parameter estimation equations to define nL as a function of SI and sets xL to a population constant (70%).28–30 These values are used to simulate the participant’s insulin concentration in the plasma and interstitium. Note that the DIST-SI-2 uses the DISTq parameter estimation for basal insulin (Ib) and IIM to identify nL with a fixed xL.
SI and VG are identified using the IIM. However, the Sparse and DISTq30 protocols do not have sufficient glucose data to identify VG. In these cases, VG is estimated as a proportion (29%) of LBM as calculated by Hume.22 The value of 29% was defined using linear regression of the VG value identified from DISST-FS data to LBM in an isolated cohort.
Table 4 summarizes the identification methods used by each sampling protocol.
Table 4.
Identification Methods for the Various Protocols
| UN | Insulin | Glucose | |
|---|---|---|---|
| DISST-FS | DC | IIM | IIM |
| Short | DC | IIM | IIM |
| DISST-E/SI | DCa | IIM | IIM |
| Sparse | DC | IIM | IIMa |
| DIST-SI | EDISTq | IIM | IIM |
| DIST-SI-2 | EDISTq | IIM-EDISTqa | IIM |
| DISTq-FS | EDISTq | EDISTq | IIM |
| DISTq-S | EDISTq | EDISTq | IIM |
| DISTq30 | EDISTq | EDISTq | IIMa |
Indicates that the identification method must be adjusted to account for sparse sampling.
Analysis
DISST-FS data (n = 218) with appropriate assay omissions will be used to directly identify SI and UN using the standard DISST-FS method15 and the eight DISST-based variants. The SI, UB, U1, and U2 values from the alternative protocols were compared to the corresponding values obtained by the DISST-FS using Pearson’s correlation coefficients and quartiles of the proportional differences (Δ). The proportional differences will be defined with Equation (1).
| (1) |
where VP is the value defined by the DISST-based variant and VFS is the DISST-FS value.
The simple HOMA insulin sensitivity index was also calculated from plasma insulin and glucose assays derived from the fasting blood samples taken during each DISST-FS. The correlation between HOMA and DISST-FS is presented for comparison.
Results
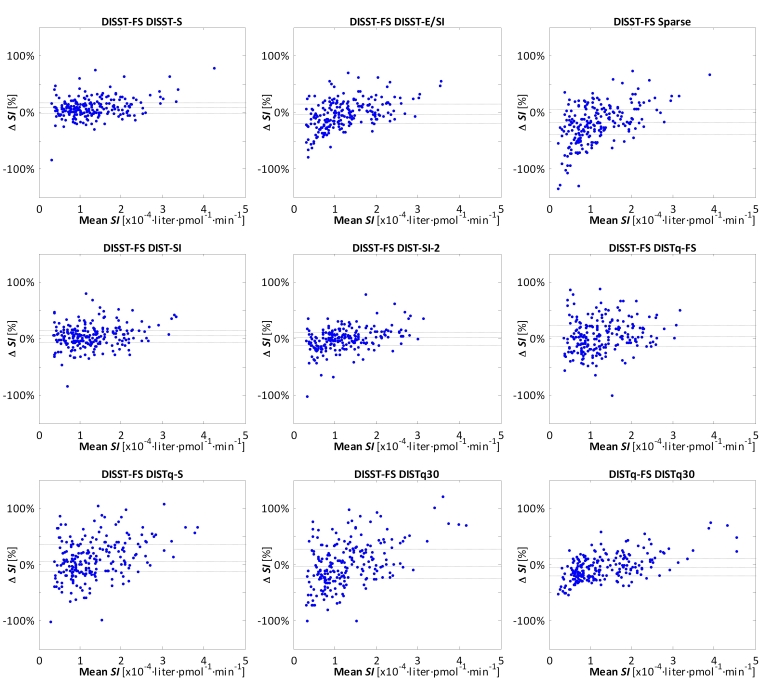
The accuracy and information produced by the tests were intrinsically linked to assay cost and clinical burden. Table 5 summarizes the performance of all proposed protocols with respect to their ability to replicate the SI and UN values identified using the DISST-FS. Figure 1 presents Bland-Altman representations of the equivalence between SI values from DISST-FS and the DISST-based variants.
Table 5.
Correlations and Quartiles of Proportional Differences of SI and UN Values Identified with the Proposed Protocols and the DISST-FS
| SIr(Q1, Q2, Q3 [%]) | UBr(Q1, Q2, Q3 [%]) | U1r(Q1, Q2, Q3 [%]) | U2r(Q1, Q2, Q3 [%]) | |
|---|---|---|---|---|
| DISST-FS | 1 (0, 0, 0) | 1 (0, 0, 0) | 1 (0, 0, 0) | 1 (0, 0, 0) |
| Short | 0.90 (-1.1, 7.8, 17.0) | 1 (0, 0, 0) | 1 (0, 0, 0) | 0.89 (-14.6, -4.44, 9.5) |
| DISST-E/SI | 0.90 (-19.3, -3.6, 15.6) | 1 (0, 0, 0) | 1 (0, 0, 0) | 0.72 (-11.0, 8.9, 33.0) |
| Sparse | 0.84 (-39.3, -17.9, 5.7) | 1 (0, 0, 0) | 1 (0, 0, 0) | 0.88 (-22.2, 2.5, 25.6) |
| DIST-SI | 0.91 (-7.0, 4.2, 15.5) | 0.62 (-20.0, -5.3, 15.0) | 0.07 (-27.3, -7.6, 23.9) | 0.75 (-31.8, -10.6, 14.5) |
| DIST-SI-2 | 0.92 (-10.9, 1.0, 11.2) | 0.68 (-17.4, -2.2, 17.1) | 0.09 (-28.4, -6.2, 24.7) | 0.74 (-27.9, -7.6, 23.5) |
| DISTq-FS | 0.83 (-13.8, 3.2, 22.4) | 0.56 (-19.9, -2.5, 16.7) | -0.07 (-28.2, -7.9, 24.7) | 0.70 (-33.0, -9.6, 15.7) |
| DISTq-S | 0.77 (-12.7, 6.4, 34.9) | 0.53 (-25.2, -6.7, 14.6) | -0.14 (-30.7, -8.6, 24.6) | 0.69 (-36.8, -15.3, 11.6) |
| DISTq30 | 0.71 (-25.1, -4.2, 26.4) | 0.53 (-22.7, -0.8, 20.9) | -0.14 (-30.7, -6.4, 25.7) | 0.71 (-30.4, -3.9, 21.5) |
| HOMA | -0.35 (–) | – | – | – |
Figure 1.
Bland-Altman representations of the proportional differences between SI values from DISST-FS and the DISST-based variations. DISTq30 is also compared to DISTq-FS. Median and interquartile ranges are shown as gray lines.
The sparser DIST-SI-2 method showed the greatest ability to replicate the SI metrics of the DISST-FS. It was closely followed by DIST-SI, Short protocol, DISST-E/SI, and Sparse protocol. DISTq-S and DISTq30 correlated highly to DISTq-FS at r = 0.94 and r = 0.89, respectively. Despite a substantial reduction in the number of assays, the DISTq30 estimates of insulin sensitivity were highly correlated with the DISTq-FS (r = 0.89) with limited bias (-4.2%), thus providing validation for the LBM-based estimation of VG.
Protocols that assayed C-peptide from the t = 0 and t = 5 min samples generated the same measures of UB and U1 to those generated by the DISST-FS, as expected. Reducing the number of C-peptide samples had a greater effect on U2. DISTq was not intended for estimation of UN metrics.
The correlations between HOMA and the insulin sensitivity metrics of DISST-FS were weaker than correlations between the DISST-FS and all alternative DISST-based protocols.
Discussion
Relatively high SI correlations (r ∼0.9) and a lack of bias (Q2 bias range -17.9 to 7.8%) between the protocols that assayed insulin and the DISST-FS test show that the limited sampling protocols could be used as low-cost alternatives to the fully sampled test without significantly diminishing test resolution. In particular, the Sparse protocol insulin sensitivity values identified using only three samples correlated well to the fully sampled test (r = 0.84) and also captured all major dynamics of the UN profile. This is despite the protocol requiring just over half the time and one third of the assay cost. DISTq provided accurate estimates of SI values that compared favorably with estimates generated by more intense and costly fully sampled methods. The DISTq-FS method performed in accordance with published findings. DISTq-S and DISTq30 also correlated relatively well to the DISST-FS, particularly in comparison with the well-accepted HOMA, which performed poorly.
Overall, the findings of the spectrum analysis show the considerable robustness of the DISST model-based SI metric to significant assay omissions. While the highest possible accuracy is achieved with the most frequently sampled tests and comprehensively assayed samples, reducing the sample resolution only has a mildly deleterious effect on the accuracy of the insulin sensitivity outcomes.
Sampling regimens that utilized C-peptide measurements produced insulin secretion metrics that were highly correlated to the DISST-FS despite reductions in sampling. Lotz and colleagues26 found a similar robustness of endogenous insulin production metrics to assay omission. In particular, the Sparse protocol has shown that most of the insulin secretion information obtained from nine C-peptide assays can be obtained with only three [r(UB) = 1, r(U1) = 1, r(U-2) = 0.88]. As C-peptide assays contribute to a significant portion of the standard DISST assay cost, considerable cost savings are enabled by the robustness of the model to assay omissions. Protocols that utilized DISTq estimation methods were not intended to accurately predict participant-specific values of insulin secretion. Thus, the poor correlation is not considered a negative result and the minimal bias indicates that the general magnitude of prediction was accurate at a cohort level.
The participant inclusion criteria led to selection of a cohort that tended toward the insulin-resistant range. As correlations measure variable spread as well as linearity,31 it is likely that the correlations reported for this targeted cohort are less than would be identified with the same analysis undertaken with a general cohort. Furthermore, the insulin secretion metrics of insulin-resistant participants are much more variable than those of sensitive individuals.17,18,32
This investigation used clinical data from the same test procedure to analyze each DISST-based test and HOMA. Thus, the effects of participant variation between tests, which reduces the equivalence measured by most intertest investigations, did not affect the outcomes of this analysis. Furthermore, assay error had a distinct and lesser impact on this analysis compared to normal intertest investigations. In particular, assay error may cause the over- and underestimation of subsequent test outcomes in typical intertest investigations, whereas assay error in this analysis only varied the outcomes between tests when there were distinct assay regimens within a species. Intraindividual repeatability was not assessed in this study. The original data was from a 10-week dietary intervention study in which significant changes in SI were expected for half the cohort and limited changes for the control group. Thus, true SI changes, or the lack thereof, across subjects would be arguable. Equally, the time between tests of 10 weeks would, even for the control group, also include other natural variations. A properly designed repeatability investigation would focus on multiple tests over 3–7 days to mitigate these issues, which was not the case here.
The tests of the DISST-based spectrum have wide potential for a number of applications. In particular, the lower-cost DISTq measures could potentially enable screening of insulin resistance where the cost or poor resolution of the established tests has been a deterrent in the past. DISTq provides a better compromise of accuracy and economy than that made available by established low-cost surrogate insulin sensitivity measures and is also capable of providing real-time results.
The higher cost tests that also quantify insulin secretion could be used in clinical investigations of metabolic conditions or changes over time. Although these tests require a greater clinical intensity and assay cost than the DISTq protocols, they are considerably less burdensome than established high-accuracy sensitivity measures. Thus, DISST-based tests could potentially enable greater numbers of participants to be tested. Furthermore, the established measures do not frequently measure UN, which is a key indicator of β-cell function and the pathogenesis of type 2 diabetes.9,33,34
Optimal numbers of test participants could be recruited for lower resolution investigations of insulin sensitivity and secretion by using the DISST-based tests that provide a compromise between cost and resolution. For example, investigations that aim to measure the effects of a particular dietary intervention on a number of physiological markers including insulin sensitivity, the DIST-SI-2 protocol may be appropriate. In contrast, cost-restrictive pathophysiological studies of type 2 diabetes with large insulin-resistant cohorts should perhaps utilize the low cost but informative Sparse protocol, which can also produce β-cell function estimates.
The DISTq test could also be used to measure the efficacy of intervention provided the intervention was expected to have a minimal effect on the participant’s insulin clearance rates and insulin production response to the DISST stimulus. The DISTq SI identification process estimates the participant’s insulinemic response to test stimulus rather than observing it through insulin or C-peptide assays. Thus, changes in glucose decay as a result of shifts in the participant’s insulinemic response will be incorrectly attributed to SI by the DISTq identification process. However, the DISTq tests would be suitable for tracking SI changes provided that consistency in insulin clearance and insulin production can be reasonably assumed for the duration of the intervention.
Finally, the various sampling schedules could allow more assays from the samples taken to enable higher resolution analyses, or less assays to be performed to reduce overall cost. The DISST-based hierarchy does not require an additional clinical procedure to be undertaken to increase the SI resolution. For example, the DIST-SI protocol requires seven blood samples, which yield six glucose and six insulin assays. If cost savings were desired, only two glucose assays could be undertaken on the available samples and the DISTq30 could provide a comparatively low resolution SI result. However, if this result was close to a diagnostic threshold and a more accurate diagnosis were desired, stored samples could be reassayed. Further assays of insulin and/or C-peptide, as well as glucose when not done previously, could result in a higher resolution SI value using the DIST-SI method, or a DISST-S result that includes participant-specific UN metrics. This approach increases storage costs but minimizes cost for participants who can be diagnosed with a lower resolution test. Table 6 shows all potential sample schedules and subsequent possible assay and identification methods for each sampling protocol defined.
Table 6.
Potential for Different Sample Regimens to Allow Reassays and Reanalyses with Alternative Tests
| Analyses possible with samples | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Protocol completed | DISST-FS | Short | DISST-E/SI | Sparse | DIST-SI | DIST-SI-2 | DISTq-FS | DISTq-S | DISTq30 |
| DISST-FS | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Short | Y | Y | Y | Y | |||||
| DISST-E/SI | Y | Y | Y | Y | Y | Y | |||
| Sparse | Y | ||||||||
| DIST-SI | Y | Y | Y | Y | Y | Y | Y | ||
| DIST-SI-2 | Y | Y | Y | ||||||
| DISTq-FS | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| DISTq-S | Y | Y | Y | Y | |||||
| DISTq30 | Y | ||||||||
Conclusions
The DISST-based spectrum of tests utilizes the robustness of the model-based insulin sensitivity and secretion metrics to the omission of assays to provide a series of tests that encompass a wide range of compromises between economy and accuracy. Thus, researchers could potentially select the best DISST-based test for a wide range of clinical applications. In particular, the more intensely sampled and assayed tests could offer comprehensive metabolic information, while the low-cost tests could enable screening applications that were inhibited by the poor resolution of low-cost insulin sensitivity surrogates. The tests that compromise accuracy and cost could potentially allow an optimal number of participants to be tested in clinical trials, thus making the best use of available funds.
Glossary
Abbreviations
- (DC)
deconvolution
- (DISST)
dynamic insulin sensitivity and secretion test
- (DISTq)
quick dynamic insulin sensitivity test
- (EDISTq)
DISTq identification methods
- (HOMA)
homeostasis model assessment
- (IIM)
iterative integral method
- (LBM)
lean body mass
- (SI)
insulin sensitivity
- (U1)
first phase insulin production
- (U2)
second phase insulin production
- (UB)
basal insulin production
- (UN)
endogenous insulin production
- (VG)
glucose distribution volume
Appendix 1
The DISST physiological model is shown in Equations (2) to (6). Equations (2) and (3) define the C-peptide pharmacokinetics. Equations (4) and (5) define the plasma and interstitial insulin pharmacokinetics, respectively. Equation (6) describes the decay of glucose as a function of available interstitial insulin and glucose suppression of hepatic glucose production.
| (2) |
| (3) |
| (4) |
| (5) |
| (6) |
where C and Y are the plasma and interstitial C-peptide concentrations, respectively (pmol·liter-1); k1-3 are transport rate parameters (min-1); UN is the endogenous insulin production profile (pmol·min-1); VP and VQ are the volumes of distribution of insulin in the plasma and interstitium, respectively (liter); nI is the rate of transition of insulin between the plasma and interstitium (liter·min-1); nK is the renal insulin clearance rate (min-1); nL is the hepatic insulin clearance rate (min-1); aI is the saturation of clearance (liter·pmol-1); xL is the proportion of first pass hepatic insulin extraction (1); UX is the exogenous insulin bolus (pmol·min-1); nC is the rate of insulin clearance in the interstitium (min-1); Ġis the concentration of glucose (mg·dl-1); pG is the rate of glucose-dependent suppression of hepatic glucose release (min-1); PX is the exogenous glucose bolus (mg·min-1); SI is the insulin sensitivity (l·pmol-1·min-1); VG is the glucose distribution volume (dl); and the B subscript denotes the basal concentration of the respective species.
The basal rate of insulin production (UB) is defined as the UN value at t = 0, while the first phase production rate (U1) is defined as the average of UN between t = 1 and 6 min, and the second phase (U2) is defined as the average of UN between t = 6 and 36 min. SI measures the effect of interstitial insulin on the decay of available glucose.
Funding
Funding was provided by the Health Research Council of New Zealand, the Riddet Institute, and the New Zealand Foundation for Research, Science and Technology.
References
- 1.McLaughlin T, Abbasi F, Lamendola C, Reaven G. Heterogeneity in prevalence of risk factors for cardiovascular disease and type 2 diabetes in obese individuals: effect of differences in insulin sensitivity. Arch Intern Med. 2007;167(7):642–648. doi: 10.1001/archinte.167.7.642. [DOI] [PubMed] [Google Scholar]
- 2.Hanley AJ, Williams K, Festa A, Wagenknecht LE, D’Agostino RB, Jr, Haffner SM. Liver markers and development of the metabolic syndrome: the insulin resistance atherosclerosis study. Diabetes. 2005;54(11):3140–3147. doi: 10.2337/diabetes.54.11.3140. [DOI] [PubMed] [Google Scholar]
- 3.Zimmet P, Boyko EJ, Collier GR, de Courten M. Etiology of the metabolic syndrome: potential role of insulin resistance, leptin resistance, and other players. Ann N Y Acad Sci. 1999;892:25–44. doi: 10.1111/j.1749-6632.1999.tb07783.x. [DOI] [PubMed] [Google Scholar]
- 4.Santaguida PL, Balion C, Hunt D, Morrison K, Gerstein H, Raina P, Booker L, Yazdi H. Diagnosis, prognosis, and treatment of impaired glucose tolerance and impared fasting glucose. Evid Rep Technol Assess. 2005;128:1–11. [PMC free article] [PubMed] [Google Scholar]
- 5.DeFronzo RA, Ferrannini E. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipid-emia, and atherosclerotic cardiovascular disease. Diabetes Care. 1991;14(3):173–194. doi: 10.2337/diacare.14.3.173. [DOI] [PubMed] [Google Scholar]
- 6.Ferrannini E. Insulin resistance is central to the burden of diabetes. Diabetes Metab Rev. 1997;13(2):81–86. doi: 10.1002/(sici)1099-0895(199706)13:2<81::aid-dmr184>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 7.Martin BC, Warram JH, Krolewski AS, Bergman RN, Soeldner JS, Kahn CR. Role of glucose and insulin resistance in development of type 2 diabetes mellitus: results of a 25-year follow-up study. Lancet. 1992;340(8825):925–929. doi: 10.1016/0140-6736(92)92814-v. [DOI] [PubMed] [Google Scholar]
- 8.Harris RH, Donahue K, Rathore SS, Frame P, Woolf SH, Lohr KN. Screening adults for type 2 diabetes: a review of the evidence for the U.S. Preventative Services Task Force. Ann Intern Med. 2003;138(3):215–229. doi: 10.7326/0003-4819-138-3-200302040-00015. [DOI] [PubMed] [Google Scholar]
- 9.Pacini G, Mari A. Methods for clinical assessment of insulin sensitivity and beta-cell function. Best Pract Res Clin Endocrinol Metab. 2003;17(3):305–322. doi: 10.1016/s1521-690x(03)00042-3. [DOI] [PubMed] [Google Scholar]
- 10.Ferrannini E, Mari A. How to measure insulin sensitivity. J Hypertens. 1998;16(7):895–906. doi: 10.1097/00004872-199816070-00001. [DOI] [PubMed] [Google Scholar]
- 11.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 12.Cederholm J, Wibell L. Insulin release and peripheral sensitivity at the oral glucose tolerance test. Diabetes Res Clin Pract. 1990;10(2):167–175. doi: 10.1016/0168-8227(90)90040-z. [DOI] [PubMed] [Google Scholar]
- 13.Soonthornpun S, Setasuban W, Thamprasit A, Chayanunnukul W, Rattarasarn C, Geater A. Novel insulin sensitivity index derived from oral glucose tolerance test. J Clin Endocrinol Metab. 2003;88(3):1019–1023. doi: 10.1210/jc.2002-021127. [DOI] [PubMed] [Google Scholar]
- 14.Lotz TF. High resolution clinical model-based assessment of insulin sensitivity. Christchurch (New Zealand): Department of Mechanical Engineering, University of Canterbury; 2007. p. 247. Available from: http://hdl.handle.net/10092/1571. [Google Scholar]
- 15.Lotz TF, Chase JG, McAuley KA, Shaw GM, Docherty PD, Berkeley JE, Williams SM, Hann CE, Mann JI. Design and clinical pilot testing of the model-based dynamic insulin sensitivity and secretion test (DISST) J Diabetes Sci Technol. 2010;4(6):1408–1423. doi: 10.1177/193229681000400616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McAuley KA, Berkeley JE, Docherty PD, Lotz TF, Te Morenga LA, Shaw GM, Williams SM, Chase JG, Mann JI. The dynamic insulin sensitivity and secretion test: a novel measure of insulin sensitivity. Metabolism. 2011 doi: 10.1016/j.metabol.2011.05.009. Jun. Available online http://www.sciencedirect.com/science/article/pii/S0026049511001387. [DOI] [PubMed] [Google Scholar]
- 17.Docherty PD, Chase JG, Lotz T, Hann CE, Shaw GM, Berkeley JE, Mann JI, McAuley KA. DISTq: an iterative analysis of glucose data for low-cost real-time and accurate estimation of insulin sensitivity. Open Med Inform J. 2009;3:65–76. doi: 10.2174/1874431100903010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Docherty PD, Chase JG, Lotz TF, Hann CE, Shaw GM, Berkeley JE, Te Morenga L, Mann JI, McAuley KA. Independent cohort cross-validation of the real-time DISTq estimation of insulin sensitivity. Comput Methods Programs Biomed. 2011;102(2):94–104. doi: 10.1016/j.cmpb.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Te Morenga L, Williams SM, Brown R, Mann JI. Effect of a relatively high-protein, high-fiber diet on body composition and metabolic risk factors in overweight women. Eur J Clin Nutr. 2010;64(11):1323–1331. doi: 10.1038/ejcn.2010.163. [DOI] [PubMed] [Google Scholar]
- 20.Lotz TF, Chase JG, McAuley KA, Shaw GM, Wong XW, Lin J, LeCompte AJ, Hann CE, Mann JI. Monte Carlo analysis of a new model-based method for insulin sensitivity testing. Comput Methods Programs Biomed. 2008;89(3):215–225. doi: 10.1016/j.cmpb.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 21.Edsberg B, Herly D, Hildebrandt P, Kühl C. Insulin bolus given by a sprinkler needle: effect on absorption and glycaemic response to a meal. Br Med J (Clin Res Ed) 1987;294(6584):1373–1376. doi: 10.1136/bmj.294.6584.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hume R. Prediction of lean body mass from height and weight. J Clin Pathol. 1966;19(4):389–391. doi: 10.1136/jcp.19.4.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Cauter E, Mestrez F, Sturis J, Polonsky KS. Estimation of insulin secretion rates from C-peptide levels. Comparison of individual and standard kinetic parameters for C-peptide clearance. Diabetes. 1992;41(3):368–377. doi: 10.2337/diab.41.3.368. [DOI] [PubMed] [Google Scholar]
- 24.Eaton RP, Allen RC, Schade DS, Erickson KM, Standefer J. Prehepatic insulin production in man: kinetic analysis using peripheral connecting peptide behavior. J Clin Endocrinol Metab. 1980;51(3):520–528. doi: 10.1210/jcem-51-3-520. [DOI] [PubMed] [Google Scholar]
- 25.McAuley KA, Mann JI, Chase JG, Lotz TF, Shaw GM. Point: HOMA–satisfactory for the time being: HOMA: the best bet for the simple determination of insulin sensitivity, until something better comes along. Diabetes Care. 2007;30(9):2411–2413. doi: 10.2337/dc07-1067. [DOI] [PubMed] [Google Scholar]
- 26.Lotz TF, Göltenbott U, Chase JG, Docherty P, Hann CE. A minimal C-peptide sampling method to capture peak and total prehepatic insulin secretion in model-based experimental insulin sensitivity studies. J Diabetes Sci Technol. 2009;3(4):875–886. doi: 10.1177/193229680900300435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hann CE, Chase JG, Lin J, Lotz T, Doran CV, Shaw GM. Integral-based parameter identification for long-term dynamic verification of a glucose-insulin system model. Comput Methods Programs Biomed. 2005;77(3):259–270. doi: 10.1016/j.cmpb.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 28.Toffolo G, Campioni M, Basu R, Rizza RA, Cobelli C. A minimal model of insulin secretion and kinetics to assess hepatic insulin extraction. Am J Physiol Endocrinol Metab. 2006;290(1):E169–76. doi: 10.1152/ajpendo.00473.2004. [DOI] [PubMed] [Google Scholar]
- 29.Cobelli C, Bettini F, Caumo A, Quon MJ. Overestimation of minimal model glucose effectiveness in presence of insulin response is due to undermodeling. Am J Physiol. 1998;275(6) Pt 1:E1031–6. doi: 10.1152/ajpendo.1998.275.6.E1031. [DOI] [PubMed] [Google Scholar]
- 30.Meier JJ, Veldhuis JD, Butler PC. Pulsatile insulin secretion dictates systemic insulin delivery by regulating hepatic insulin extraction in humans. Diabetes. 2005;54(6):1649–1656. doi: 10.2337/diabetes.54.6.1649. [DOI] [PubMed] [Google Scholar]
- 31.Salkind NJ, Rasmussen K, editors. Encyclopedia of measurement and statistics. Thousand Oaks (CA): Sage Publications; 2007. [Google Scholar]
- 32.Cobelli C, Toffolo G, Dalla Man C, Campioni M, Denti P, Caumo A, Butler P, Rizza R. Assessment of beta-cell function in humans, simultaneously with insulin sensitivity and hepatic extraction, from intravenous and oral glucose tests. Am J Physiol Endocrinol Metab. 2007;293(1):E1–15. doi: 10.1152/ajpendo.00421.2006. [DOI] [PubMed] [Google Scholar]
- 33.Ferrannini E, Mari A. Beta cell function and its relation to insulin action in humans: a critical appraisal. Diabetologia. 2004;47(5):943–956. doi: 10.1007/s00125-004-1381-z. [DOI] [PubMed] [Google Scholar]
- 34.Ferrannini E, Gastaldelli A, Miyazaki Y, Matsuda M, Mari A, DeFronzo RA. beta-Cell function in subjects spanning the range from normal glucose tolerance to overt diabetes: a new analysis. J Clin Endocrinol Metab. 2005;90(1):493–500. doi: 10.1210/jc.2004-1133. [DOI] [PubMed] [Google Scholar]