Abstract
Introduction
The effects of pancreatic polypeptide (PP) infusion were examined in patients on insulin pump therapy to determine whether PP administration can reduce insulin requirements in patients with type 1 diabetes mellitus (T1DM) or type 3c diabetes mellitus (T3cDM; pancreatogenic).
Methods
Ten subjects with long-standing T1DM (n = 7) or T3cDM (n = 3) on insulin pump treatment received a 72 h subcutaneous infusion of 2 pmol/kg/min bovine PP or saline by portable infusion pump in a single-blinded, randomized, crossover design.
Results
Pancreatic polypeptide infusion raised plasma PP levels to 450–700 pmol/liter. Daily insulin infusion requirements (I) fell from 48 ± 6.9 to 40 ± 7.5 U on day 2 (p < .05) and from 46 ± 7.7 to 37 ± 6.6 U on day 3 (p < .05) of PP infusion compared with saline. Corrected for average blood glucose concentration (G), I/G fell in 10/10 subjects during the second 24 h period and in 7/10 subjects during the third 24 h period; sensitivity to insulin, calculated as 1/(I/G), increased 45% ± 12% on day 2 (p < .01) and 34% ± 14% on day 3 (p < .05) of PP infusion. Pancreatic polypeptide responses to a test meal were compared with the change in insulin infusion requirements in 5 subjects; the reduction in insulin requirements seen during PP infusion correlated with the degree of baseline PP deficiency (p < .002).
Conclusions
A concurrent subcutaneous infusion of PP enhances insulin sensitivity and reduces insulin requirements in patients with long-standing T1DM and T3cDM on insulin pump therapy. The benefit of PP infusion correlated with the degree of PP deficiency.
Keywords: hepatic insulin sensitivity, insulin, insulin pump treatment, pancreatic polypeptide, type 1 diabetes, type 3c diabetes
Introduction
Pancreatic polypeptide (PP) is a 36-amino-acid straight- chain peptide and a member of the “PP family” of enteropancreatic hormones that also includes neuro-peptide Y and peptide YY.1,2 Pancreatic polypeptide is secreted predominantly from the islets in the ventral portion (uncinate process) of the head of the pancreas in response to nutrient ingestion and remains elevated in the plasma for up to 3 h after a meal.3–5 The physiologic role of PP remained uncertain for more than a decade after its original isolation and identification by Kimmel and colleagues6 and, separately, by Lin and Chance.7 Animal and clinical studies initially suggested a role in satiety and in regulation of pancreatic exocrine and biliary secretion.1,5,8 It has since been shown to play a role in glucose homeostasis through its mediation of hepatic sensitivity to insulin by means of its regulation of hepatic insulin receptor (IR) gene expression and hepatocyte IR availability.9–12
Pancreatogenic or apancreatic diabetes is classified as a form of secondary or type 3c diabetes mellitus (T3cDM) by the American Diabetes Association13 and the Centers for Disease Control.14 This category includes diabetes in association with, or as a consequence of, acute and chronic pancreatitis (CP), pancreatic neoplasms, pancreatic resection, pancreatic trauma, fibrocalculous pancreatopathy, cystic fibrosis, hemochromatosis, and pancreatic agenesis. In North America and Western Europe, the majority of patients with T3cDM have CP as the cause.15
A deficient PP response to nutrients has been shown in patients with T3cDM due to CP,16 pancreatic resection,17 and cystic fibrosis.18 Isolated hepatic resistance to insulin, despite normal or increased peripheral insulin sensitivity, has also been shown in T3cDM due to CP,19 pancreatic cancer,20 pancreatic resection,21 and cystic fibrosis,22 and an increase in hepatocyte PP receptors, now referred to as Y4 receptors,23 has been shown in CP.24 Administration of PP has been shown to improve hepatic sensitivity to insulin and glucose homeostasis in rats,10,25 dogs,9,26,27 and patients28,29 with T3cDM due to CP or pancreatic resection.
Clinical studies have demonstrated that hepatic sensitivity to insulin is increased, and glucose tolerance improved, after an 8 h infusion of PP in patients who are PP-deficient due to pancreatic resection28 or CP.29 These findings suggested that PP administration might have an insulin-sparing effect in patients with insulin-dependent diabetes. This hypothesis was therefore examined in this present clinical study of PP administration in patients with type 1 diabetes mellitus (T1DM) or T3cDM on stable insulin pump therapy. Our objective was to determine whether continuous subcutaneous infusion of PP would increase insulin sensitivity as assessed by reduction in daily insulin pump therapy requirements in a single-blinded, randomized, crossover study design.
Methods
Selection of Volunteers
Ten diabetic subjects [7 subjects with autoimmune T1DM of 8–35 years duration and 3 subjects with T3cDM of 1–13 years after proximal (2 subjects) or total (1 subject) pancreatectomy] were enrolled in the study (Table 1). All subjects had hemoglobin A1c levels ≤8.5% and were using continuous subcutaneous infusion of insulin by programmable pump. The subjects included 7 females and 3 males aged 45.2 ± 5.8 years [mean ± standard error of the mean (SE), range 22–69 years], with body mass index 26.3 ± 1.5 (range 20.0–34.5).
Table 1.
Subject Characteristics and Primary Dataa
| Subject # | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Mean | SEM |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | 64 | 34 | 69 | 53 | 22 | 22 | 27 | 51 | 43 | 67 | 45.2 | 5.77 |
| Gender | F | F | M | F | F | F | F | M | F | M | ||
| BMI | 20.1 | 24.0 | 23.7 | 28.0 | 29.3 | 26.5 | 34.5 | 31.5 | 25.5 | 20.0 | 26.31 | 1.480 |
| DM type | T3 | T1 | T3 | T1 | T1 | T1 | T1 | T1 | T1 | T3 | ||
| Duration | 1 | 18 | 13 | 35 | 18 | 8 | 17 | 33 | 27 | 1 | ||
| HbA1c% | 8.1 | 6.2 | 8.4 | 7.3 | 7.4 | 7.6 | 6.0 | 7.1 | 6.5 | 8.5 | 7.31 | 0.340 |
| Day 2 Saline | ||||||||||||
| Avg Glucose | 141.7 | 164.8 | 96.9 | 122.6 | 143.5 | 182.3 | 109.3 | 171.7 | 136.4 | 211.0 | 148.03 | 11.021 |
| G Nadir | 65 | 62 | 54 | 58 | 73 | 40 | 75 | 101 | 71 | 175 | 77.4 | 11.34 |
| Tot Insulin | 25.0 | 53.1 | 47.1 | 27.1 | 50.2 | 71.4 | 87.2 | 53.2 | 52.9 | 14.2 | 48.13 | 6.894 |
| I/G | 0.18 | 0.32 | 0.49 | 0.22 | 0.35 | 0.39 | 0.80 | 0.31 | 0.39 | 0.07 | 0.351 | 0.0624 |
| 1/(I/G) | 5.68 | 3.10 | 2.06 | 4.52 | 2.85 | 2.55 | 1.25 | 3.23 | 2.58 | 14.90 | 4.27 | 1.244 |
| Day 3 Saline | ||||||||||||
| Avg Glucose | 196.5 | 110.6 | 166.4 | 125.2 | 144.7 | 281.2 | 98.8 | 122.0 | 120.6 | 303.4 | 166.91 | 22.800 |
| G Nadir | 62 | 40 | 50 | 56 | 61 | 135 | 19 | 45 | 45 | 230 | 74.3 | 18.74 |
| Tot Insulin | 24.0 | 49.6 | 52.7 | 23.3 | 63.7 | 53.0 | 99.5 | 48.5 | 29.0 | 19.5 | 46.28 | 7.658 |
| I/G | 0.12 | 0.45 | 0.32 | 0.19 | 0.44 | 0.19 | 1.01 | 0.40 | 0.24 | 0.06 | 0.34 | 0.0850 |
| 1/(I/G) | 8.18 | 2.23 | 3.16 | 5.37 | 2.27 | 5.30 | 0.99 | 2.52 | 4.16 | 15.52 | 4.97 | 1.343 |
| Day 2 PP | ||||||||||||
| Avg Glucose | 185.3 | 146.1 | 156.1 | 137.9 | 142.2 | 190.1 | 158.4 | 132.6 | 153.3 | 339.0 | 174.10 | 19.267 |
| G Nadir | 82 | 73 | 40 | 94 | 88 | 80 | 73 | 50 | 51 | 175 | 80.6 | 11.28 |
| Tot Insulin | 25.9 | 30.8 | 30.9 | 20.1 | 34.1 | 60.0 | 98.8 | 39.8 | 37.9 | 19.1 | 39.75 | 7.528 |
| I/G | 0.14 | 0.21 | 0.20 | 0.15 | 0.24 | 0.32 | 0.62 | 0.30 | 0.25 | 0.06 | 0.248 | 0.0485 |
| 1/(I/G) | 7.15 | 4.74 | 5.05 | 6.86 | 4.17 | 3.17 | 1.60 | 3.33 | 4.05 | 17.71 | 5.78 | 1.425 |
| Day 3 PP | ||||||||||||
| Avg Glucose | 254.0 | 111.6 | 145.9 | 120.6 | 110.8 | 178.0 | 138.3 | 207.3 | 122.3 | 316.0 | 170.48 | 21.866 |
| G Nadir | 53 | 61 | 34 | 72 | 87 | 78 | 59 | 49 | 52 | 240 | 78.5 | 17.63 |
| Tot Insulin | 19.9 | 35.4 | 24.6 | 19.8 | 37.1 | 53.1 | 88.2 | 41.5 | 32.5 | 20.2 | 37.23 | 6.628 |
| I/G | 0.08 | 0.32 | 0.17 | 0.16 | 0.34 | 0.30 | 0.64 | 0.20 | 0.27 | 0.06 | 0.253 | 0.0522 |
| 1/(I/G)) | 12.75 | 3.15 | 6.01 | 6.09 | 2.99 | 3.35 | 1.57 | 5.00 | 3.76 | 15.66 | 6.03 | 1.448 |
BMI = body mass index; DM =diabetes; T1 =type 1 DM; T3 =type 3c DM; Avg = average; Tot = Total; G nadir = lowest glucose value during 24 h period; Duration = duration of diabetes (yrs); I/G = Total 24 h insulin requirement/average 24 h glucose value; 1/(I/G) = insulin sensitivity index; SEM = standard error of the mean; F = female; M = male; HbA1c = hemoglobin A1c
We obtained an investigator-initiated new drug authori-zation from the Food and Drug Administration for PP infusion (FDA IND 71,216). All methods and procedures were approved by the University of Massachusetts and Johns Hopkins University Institutional Review Boards, and the study was registered as a clinical trial with the National Institutes of Health (NCT 00791076). All volunteers provided written informed consent in accordance with the Declaration of Helsinki II.
Study Design
A standardized test meal (STM) of 475 ml of Ensure Plus® was ingested within 10 min at ∼8:00 am by each volunteer after a 12 h fast on the first day of each 72 h study period, and blood samples were obtained for 180 min. Then, in a single-blind, randomized, crossover design, a continuous fixed subcutaneous infusion of 2 pmol/kg/min synthetic PP (bPP) reconstituted in 3.0 ml normal saline or 3.0 ml normal saline alone was begun by miniature portable pump (Disetronic Panomat T-10, Disetronic Medical Systems AG, Burghdorf, Switzerland). During the last 3 h of the 72 h infusion period, a second STM was performed.
The two study periods (i.e., PP versus saline) were scheduled at least 4 weeks apart, during which the subjects were asked to maintain their normal activity and dietary habits. Subjects were instructed to follow their normal routine for adjustments of their insulin infusion pumps based on finger stick blood glucose measurements during each test period. At the conclusion of each 72 h infusion period, bolus and continuous insulin infusion rates were downloaded from each insulin pump to calculate daily insulin usage, and blood glucose levels were obtained from either the subjects’ finger stick glucose diary (n = 3) or from the data downloaded from a blinded continuous glucose monitor (Minimed Glucose Monitor, Medtronic, Minneapolis, MN; n = 7). Mean plasma glucose (G), calculated as the area under the curve (AUC) of all glucose measurements by the trapezoidal rule, and total insulin infusion (I) require-ments were assessed during the second and third 24 h interval of each 72 h infusion period.
Bovine PP was synthesized by the Peptide/Protein Core Facility, Endocrine Unit, Department of Medicine, Massachusetts General Hospital, Boston, MA. The peptide was >99% pure and displayed a single peak on high-performance liquid chromatography with a peptide content of 85%. The bPP was lyophilized in vials under sterile conditions for single use and was certified to be both pyrogen-free and sterile.
Analytical Techniques
During the STM, blood samples were collected with heparinized syringes. Plasma glucose was immediately analyzed by the glucose oxidase method (Beckman Glucose Analyzer II, Beckman Instruments, Fullerton, CA). The remaining blood samples were placed in prechilled test tubes containing ethylenediaminetetraacetic acid and protease inhibitors. Plasma PP levels during the preinfusion and postinfusion STMs were measured, as previously described,30,31 in five consecutive subjects. The antihuman PP antibody used (E1040, Millepore Corp, St. Charles, MO) cross-reacts similarly with bovine PP.32
Standard methods were used to compute means, SEs, and Pearson correlation coefficients. All statistical tests were two tailed. Data are shown as mean ± SE and p values of <0.05 were regarded as statistically significant.
Results
There were no adverse outcomes or side effects associated with the 72 h PP infusion in any subject. Subjects were queried regarding the incidence and severity of hypo-glycemic events during each 72 h infusion period. The number and severity of hypoglycemic events was similar in both PP and saline infusion periods (Table 1). None required physician intervention, and none was reported to be associated with severe or unusual symptoms or sequelae. Two subjects reported that they had discontinued insulin infusions entirely for 2–4 h during parts of the PP infusion period based on blood glucose measurements, according to their standard protocol.
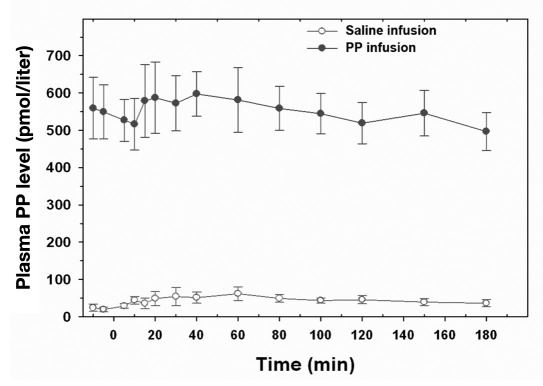
Basal and STM-stimulated peak PP levels before PP infusion were 24.2 ± 9.7 and 62.0 ± 18.6 pmol/liter, respectively, in five consecutive subjects. The 72 h PP infusion period resulted in a significant stable increase in the plasma PP levels in each subject tested, based on serum samples obtained from the STM performed during the last 3 h of the 72 h infusion period (Figure 1). Plasma PP levels ranged from 450–700 pmol/liter during PP infusion, which is within the range of endogenous PP levels seen after oral nutrients.1,2 Average blood glucose levels during the 72 h PP infusion were similar to those observed during the 72 h saline infusion (174 ± 19 versus 148 ± 11 mg/dl on day 2, p = not significant, and 170 ± 22 versus 167 ± 23 mg/dl on day 3, p = not significant).
Figure 1.
Plasma PP levels during a STM administered at time 0 during the last 3 h of the 72 h infusion of PP or saline in five subjects (mean ± SE).
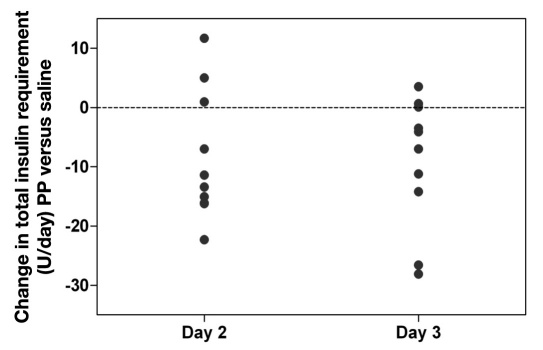
Total insulin requirements (I) were lower in 7 out of 10 subjects during the 72 h PP infusion than during the saline infusion and fell from 48.1 ± 6.9 U/day (day 2 saline) to 39.8 ± 7.5 U/day (day 2 PP infusion; p < .05) and from 46.3 ± 7.7 U/day (day 3 saline) to 37.2 ± 6.6 U/day (day 3 PP infusion; p < .05). This represented a 15.2% ± 7.8% and 16.4% ± 6.4% reduction in daily insulin usage for day 2 and day 3 of PP infusion, respectively (Figure 2). The change in total daily insulin requirements ranged from +11.7 to -22.3 U on day 2 of PP infusion (mean -8.4 ± 3.44 U, p < .05) and from +3.5 to -28.1 U on day 3 of PP infusion (mean -9.0 ± 3.49 U, p < .05).
Figure 2.
Change in total daily insulin infusion requirement (I) during PP infusion compared with saline infusion during day 2 and day 3 of 72 h infusion periods. The reduction in daily insulin requirements averaged 8.4 ± 3.44 U on day 2 (p < .05) and 9.0 ± 3.49 U on day 3 (p < .05).
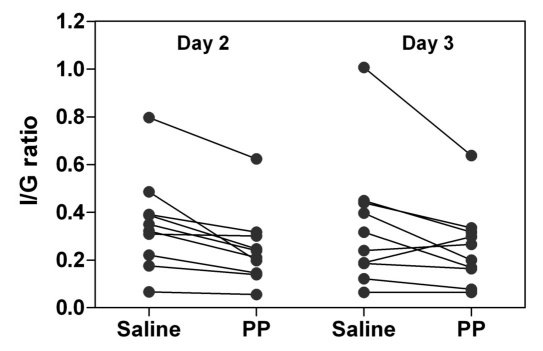
The total insulin requirement (I) for the second and third 24 h interval of each 72 h period was corrected for the mean blood glucose level (G), which was calculated using the trapezoidal rule from all glucose values obtained. The individual I/G values decreased during PP infusion on the second day (in 10 out of 10 subjects) and the third day (in 7 out of 10 subjects) of the study (Figure 3). The average I/G value decreased 28% ± 4.8% during the second day of the PP infusion compared with saline (p < .001) and 17% ± 10% during the third day of PP infusion (p < .05). Insulin sensitivity, calculated as 1/(I/G), increased 45% ± 12% on day 2 (p < .01) and 34% ± 14% on day 3 (p < .05) of PP infusion, compared with saline infusion.
Figure 3.
Total daily insulin infusion requirement (I)/average daily blood glucose level (G) ratios during 72 h saline infusion or 72 h PP infusion in 10 subjects (7 with T1DM and 3 with T3cDM) on insulin pump treatment during day 2 and day 3 of infusions. The I/G ratios for PP infusion versus saline infusion were reduced by 28% ± 4.8% on day 2 (p < .001) and by 17% ± 10% on day 3 (p < .05).
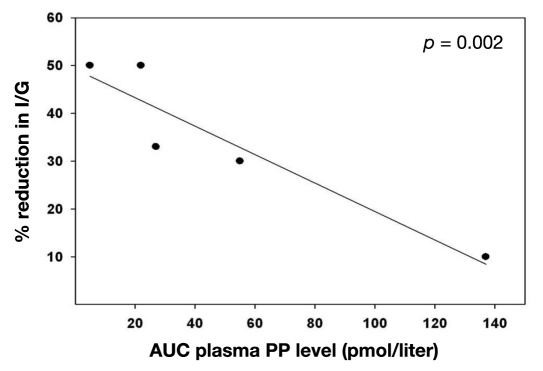
The AUC of plasma PP levels during the initial STM was measured in five subjects, and the results were correlated with the change in the I/G value during the PP infusion period compared with the saline infusion period. There was a significant negative correlation with the STM-stimulated PP levels and the reduction in the insulin/glucose ratio observed during PP infusion (p = .002; Figure 4). This indicated that the effectiveness of PP infusion in enhancing sensitivity to insulin and in reducing the daily insulin requirement was proportional to the degree of PP deficiency present before PP infusion.
Figure 4.
Correlation of percentage reduction in daily insulin infusion requirement (I)/average daily blood glucose level (G) ratio observed during PP infusion versus saline infusion and AUC of plasma PP response to the baseline STM in five diabetes subjects on insulin pump therapy (p < .002).
Discussion
Alterations in circulating PP levels have been reported in several states of glucose intolerance, such as T1DM and type 2 diabetes mellitus,33 CP,16,34 pancreatectomy,17 obesity,35 and aging.36,37 Intravenous PP infusion in normal volunteers failed to produce any changes in glucose, insulin, or glucagon,38 however, so the role of PP as a glucoregulatory hormone remained unclear until PP administration was studied in the setting of PP deficiency. Although insulin resistance was understood to be a component of pancreatogenic diabetes mellitus or T3cDM,39 it was not until glucose clamp studies coupled with tracer methodologies were performed that the central role of hepatic insulin resistance in T3cDM was appreciated.9,28,29 The role of PP as a regulator of hepatic IR function and expression became clear based on in vitro studies in which isolated liver perfusion10 or dispersed hepatocytes11,12,25 were studied. Furthermore, when PP replacement was studied, no beneficial effects were observed until several hours after the initiation of PP replacement. This implied that PP did not act either as a secretagogue or as a mediator of cellular glucose metabolism per se but was affecting the transcription or translation of primary mediators of hepatic glucose production.
In one of our prior studies, five male patients with documented alcohol-induced CP and six healthy male control subjects matched for age and body mass index underwent a hyperinsulinemic–euglycemic glucose clamp study during which the rate of endogenous (hepatic) glucose production was quantified on separate occasions, before and after 8 h intravenous PP or saline infusions.29 The impaired suppression of hepatic glucose production by insulin in CP patients seen in the first study was completely reversed during the final 2 h of an 8 h 2 pmol/kg/min PP infusion in the second study. During oral glucose tolerance test (OGTT) studies performed the morning after the clamp studies, CP patients initially demonstrated impaired glucose tolerance or a diabetic glucose tolerance curve, but each patient with impaired or diabetic glucose responses demonstrated a lower mean plasma glucose during the second OGTT performed after the PP infusion. One month after PP infusion, a third study without PP infusion was repeated, and mean plasma glucose levels during the OGTT had returned to their initial levels. This study showed that PP administration was therapeutic in PP-deficient CP patients with glucose intolerance and/or T3cDM. Although laboratory studies have demonstrated a therapeutic effect of PP administration in rodent models of type 2 diabetes mellitus,40,41 clinical studies on the possible therapeutic role of PP in T1DM or type 2 diabetes mellitus have not been reported.
This study was therefore designed to examine the effect of prolonged PP administration in patients whose entire insulin requirement was exogenous and therefore measureable. It shows that a continuous subcutaneous infusion of PP infusion resulted in increased insulin sensitivity and a reduction in daily insulin requirements in a mixed group of T1DM and T3cDM patients on insulin pump therapy. The effectiveness of PP infusion in reducing insulin requirements appears to be related to the degree of PP deficiency present in these patients. Although PP deficiency is expected in patients with T3cDM due to proximal or total pancreatic resection and PP replacement was therefore expected to be beneficial in this group,19 the demonstration that PP infusion reduces the insulin requirements in more than half of the T1DM patients in our study suggests a possible therapeutic role for PP in T1DM as well as T3cDM patients.
Our study design was intended to assess whether a limited (72 h) infusion of PP would demonstrate potential benefit. Our prior studies with intravenous infusion of PP have shown that PP replacement does not induce a reversal of hepatic insulin resistance until several hours of PP administration have occurred. For this reason, we did not calculate insulin requirements during the first 24 h of each subcutaneous infusion period but rather examined the effects seen on day 2 and day 3 of each infusion period. Our results suggest that a longer period of PP administration should be investigated, as no adverse outcomes or side effects were observed during this study and the reduction in insulin requirement persisted throughout the period of PP infusion. In one laboratory study, CP with PP deficiency was induced in dogs by pancreatic duct ligation 3–4 months prior to study.9 Continuous subcutaneous infusion of PP for 14 days resulted in a progressive lowering of hyper-glycemic responses to oral glucose as well as improvements in hepatic insulin sensitivity that persisted for as long as 9 weeks after discontinuation of the infusion.
Limitations of our study include the small number of subjects who were enrolled, the limited duration of PP administration, and the absence of any measurements of insulin action during the infusion period. Our subject group included both T3cDM and T1DM patients, and a benefit of PP infusion was seen in at least five of seven T1DM subjects as well as in all three T3cDM subjects. This finding suggests that some long-standing T1DM patients may have a mixed form of diabetes, in which hepatic insulin resistance, which is characteristic of T3cDM, also prevails due to concomitant PP deficiency. Pancreatic polypeptide secretion has been shown to be increased early in the course of T1DM,33 presumably as a compensatory response to relative insulin deficiency. As the autoimmune process progressively destroys all islet tissue, however, PP deficiency may be a late consequence in long-standing T1DM.
We infer that the reduced insulin requirements observed are secondary to enhanced insulin sensitivity during PP infusion. As all our T1DM patients and one of our T3cDM patients were functionally apancreatic in terms of endogenous insulin secretion, the reduction in insulin requirement is likely due to enhanced insulin sensitivity. Although not measured in this study, all our prior laboratory and clinical studies have demonstrated an enhancement in hepatic insulin sensitivity when PP administration demonstrates a therapeutic benefit. The 72 h limit of administration of PP in our current study was a requirement of the FDA IND 71,216 authorization and therefore beyond our control.
Insulin therapy of diabetes is imperfect due to multiple factors. The inability to regulate insulin delivery precisely for physiologic glycemic control is one deficiency, but as pointed out in a review by Lebovitz,42 the lack of replacement of additional islet-cell products that normally contribute to glucose regulation or that mediate insulin’s effects is another. A reduction in insulin requirements in patients who require insulin therapy carries the potential benefits of reducing the risks of hypoglycemia and hyperinsulinemia or, in some patients, of eliminating the need for insulin completely. Many patients with T3cDM require small doses of insulin to maintain glycemic control, and our findings suggest that, in this group at least, PP replacement therapy may have significant benefit. A larger, long-term, clinical study seems indicated to determine if this benefit extends to a significant proportion of patients with T1DM.
Conclusions
A 72 h continuous subcutaneous infusion of 2.0 pmol/kg/min bPP significantly reduced the insulin requirements of 10 patients on insulin pump therapy of T1DM (n = 7) or T3cDM (n = 3) compared with saline infusion in a randomized, single-blinded, cross-over design. No adverse events or side effects occurred. A larger, longer-term study of PP administration in T1DM and T3cDM patients appears safe and potentially valuable.
Acknowledgments
The authors gratefully acknowledge the technical assistance of Yunfeng Cui, M.D., Ph.D., and Crystal Grant, M.D., Ph.D., and the research coordination of Ms. Melissa Scudder. Presented, in part, at the combined meeting of the American Pancreatic Association and the Japan Pancreas Society, Honolulu, HI, November 2009.
Glossary
Abbreviations
- (bPP)
bovine pancreatic polypeptide
- (CP)
chronic pancreatitis
- (IR)
insulin receptor
- (OGTT)
oral glucose tolerance test
- (PP)
pancreatic polypeptide
- (SE)
standard error of the mean
- (STM)
standardized test meal
- (T1DM)
type 1 diabetes mellitus
- (T3cDM)
type 3c diabetes mellitus
Funding
This work was supported by the Department of Surgery, Johns Hopkins University.
References
- 1.Taylor IL. Pancreatic polypeptide family: pancreatic polypeptide, neuropeptide Y and peptide YY. 2nd ed. Bethesda: American Physiological Society; 1989. [Google Scholar]
- 2.Kono T, Wang XP, Fisher WE, Andersen DK, Brunicardi FC. Pancreatic polypeptide. In: Martini L, editor. Encyclopedia of endocrine diseases. Vol. 3. San Diego: Elsevier; 2004. pp. 488–496. [Google Scholar]
- 3.Schwartz TW, Rehfeld JF, Stadil F, Larson LI, Chance RE, Moon N. Pancreatic-polypeptide response to food in duodenal-ulcer patients before and after vagotomy. Lancet. 1976;1(7969):1102–1105. doi: 10.1016/s0140-6736(76)90065-9. [DOI] [PubMed] [Google Scholar]
- 4.Orci L, Malaisse-Lagae F, Baetens D, Perrelet A. Pancreatic-poly-peptide-rich regions in human pancreas. Lancet. 1978;2(8101):1200–1201. doi: 10.1016/s0140-6736(78)92181-5. [DOI] [PubMed] [Google Scholar]
- 5.Hazelwood RL. The pancreatic polypeptide (PP-fold) family: gastro-intestinal, vascular, and feeding behavioral implications. Proc Soc Exp Biol Med. 1993;202(1):44–63. doi: 10.3181/00379727-202-43511g. [DOI] [PubMed] [Google Scholar]
- 6.Kimmel JR, Hayden LJ, Pollock HG. Isolation and characterization of a new pancreatic polypeptide hormone. J Biol Chem. 1975;250(24):9369–9376. [PubMed] [Google Scholar]
- 7.Lin TM, Chance RE. Gastrointestinal actions of a new bovine pancreatic polypeptide (BPP) In: Chey WY, Brooks FP, editors. Endocrinology of the Gut. Thorofare: Charles B. Slack; 1974. pp. 143–145. [Google Scholar]
- 8.Putnam WS, Liddle RA, Williams JA. Inhibitory regulation of rat exocrine pancreas by peptide YY and pancreatic polypeptide. Am J Physiol. 1989;256(4):G698–703. doi: 10.1152/ajpgi.1989.256.4.G698. Pt 1. [DOI] [PubMed] [Google Scholar]
- 9.Sun YS, Brunicardi FC, Druck P, Walfisch S, Berlin SA, Chance RE, Gingerich RL, Elahi D, Andersen DK. Reversal of abnormal glucose metabolism in chronic pancreatitis by administration of pancreatic polypeptide. Am J Surg. 1986;151(1):130–140. doi: 10.1016/0002-9610(86)90023-1. [DOI] [PubMed] [Google Scholar]
- 10.Goldstein JA, Kirwin JD, Seymour NE, Trachtenberg JE, Rademaker EA, Andersen DK. Reversal of in vitro hepatic insulin resistance in chronic pancreatitis by pancreatic polypeptide in the rat. Surgery. 1989;106(6):1128–1133. [PubMed] [Google Scholar]
- 11.Seymour NE, Volpert AR, Andersen DK. Regulation of hepatic insulin receptors by pancreatic polypeptide in fasting and feeding. J Surg Res. 1996;65(1):1–4. doi: 10.1006/jsre.1996.9999. [DOI] [PubMed] [Google Scholar]
- 12.Spector SA, Frattini JC, Zdankiewicz PD, Nathan JD, Wang JP, Andersen DK, Seymour NE. Insulin receptor gene expression in chronic pancreatitis: the effect of pancreatic polypeptide. Surg Forum. 1997;48:168–171. [Google Scholar]
- 13.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2011;34(Suppl 1):S62–S69. doi: 10.2337/dc11-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Centers for Disease Control. National diabetes fact sheet, 2007. http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2007.pdf.
- 15.Hardt PD, Brendel MD, Kloer HU, Bretzel RG. Is pancreatic diabetes (type 3c diabetes) underdiagnosed and misdiagnosed? Diabetes Care. 2008;31(Suppl 2):S165–9. doi: 10.2337/dc08-s244. [DOI] [PubMed] [Google Scholar]
- 16.Sive A, Vinik AI, Van Tonder S, Lund A. Impaired pancreatic poly-peptide secretion in chronic pancreatitis. J Clin Endocrinol Metab. 1978;47(3):556–559. doi: 10.1210/jcem-47-3-556. [DOI] [PubMed] [Google Scholar]
- 17.Inoue K, Tobe T, Suzuki T, Hosotani R, Kogire M, Fuchigami A, Miyashita T, Tsuda K, Seino Y. Plasma cholecystokinin and pancreatic polypeptide response after radical pancreatoduodenectomy with Billroth I and Billroth II type of reconstruction. Ann Surg. 1987;206(2):148–154. doi: 10.1097/00000658-198708000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adrian TE, McKiernan J, Johnstone DI, Hiller EJ, Vyas H, Sarson DL, Bloom SR. Hormonal abnormalities of the pancreas and gut in cystic fibrosis. Gastroenterology. 1980;79(3):460–465. [PubMed] [Google Scholar]
- 19.Andersen DK. Mechanisms and emerging treatments of the metabolic complications of chronic pancreatitis. Pancreas. 2007;35(1):1–15. doi: 10.1097/mpa.0b013e31805d01b0. [DOI] [PubMed] [Google Scholar]
- 20.Cersosimo E, Pisters PW, Pesola G, McDermott K, Bajorunas D, Brennan MF. Insulin secretion and action in patients with pancreatic cancer. Cancer. 1991;67(2):486–493. doi: 10.1002/1097-0142(19910115)67:2<486::aid-cncr2820670228>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 21.Slezak LA, Andersen DK. Pancreatic resection: effects on glucose metabolism. World J Surg. 2001;25(4):452–460. doi: 10.1007/s002680020337. [DOI] [PubMed] [Google Scholar]
- 22.Kien CL, Horswill CA, Zipf WB, McCoy KS, O’Dorisio T. Elevated hepatic glucose production in children with cystic fibrosis. Pediatr Res. 1995;37(5):600–605. doi: 10.1203/00006450-199505000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Berglund MM, Hipskind PA, Gehlert DR. Recent developments in our understanding of the physiological role of PP-fold peptide receptor subtypes. Exp Biol Med (Maywood) 2003;228(3):217–244. doi: 10.1177/153537020322800301. [DOI] [PubMed] [Google Scholar]
- 24.Seymour NE, Spector SA, Andersen DK, Elm MS, Whitcomb DC. Overexpression of hepatic pancreatic polypeptide receptors in chronic pancreatitis. J Surg Res. 1998;76(1):47–52. doi: 10.1006/jsre.1998.5284. [DOI] [PubMed] [Google Scholar]
- 25.Seymour NE, Volpert AR, Lee EL, Andersen DK, Hernandez C. Alterations in hepatocyte insulin binding in chronic pancreatitis: effects of pancreatic polypeptide. Am J Surg. 1995;169(1):105–110. doi: 10.1016/s0002-9610(99)80117-2. [DOI] [PubMed] [Google Scholar]
- 26.Prillaman HM, Cox SB, Freedlender AE, Cornett GE, Jones HA, Flanagan TL, Chance RE, Hoffmann JA, Andersen DK, Elahi D, Hanks JB. The effect of pancreatic polypeptide on glucose disposal after surgical alterations of the pancreas. Ann Surg. 1992;216(5):574–582. doi: 10.1097/00000658-199211000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanazaki K, Nosé Y, Brunicardi FC. Artificial endocrine pancreas. J Am Coll Surg. 2001;193(3):310–322. doi: 10.1016/s1072-7515(01)01014-6. [DOI] [PubMed] [Google Scholar]
- 28.Seymour NE, Brunicardi FC, Chaiken RL, Lebovitz HE, Chance RE, Gingerich RL, Elahi D, Andersen DK. Reversal of abnormal glucose production after pancreatic resection by pancreatic polypeptide administration in man. Surgery. 1988;104(2):119–129. [PubMed] [Google Scholar]
- 29.Brunicardi FC, Chaiken RL, Ryan AS, Seymour NE, Hoffmann JA, Lebovitz HE, Chance RE, Gingerich RL, Andersen DK, Elahi D. Pancreatic polypeptide administration improves abnormal glucose metabolism in patients with chronic pancreatitis. J Clin Endocrinol Metab. 1996;81(10):3566–3572. doi: 10.1210/jcem.81.10.8855802. [DOI] [PubMed] [Google Scholar]
- 30.Gingerich RL, Lacy PE, Chance RE, Johnson MG. Regional pancreatic concentration and in-vitro secretion of canine pancreatic polypeptide, insulin, and glucagon. Diabetes. 1978;27(2):96–101. doi: 10.2337/diab.27.2.96. [DOI] [PubMed] [Google Scholar]
- 31.Elahi D, Clark BA, McAloon-Dyke M, Wong GA, Brown RS, Shapiro ME, Minaker KL, Flanagan TL, Pruitt TL, Gingerich RL, Hanks JB, Andersen DK. Islet cell responses to glucose in human transplanted pancreas. Am J Physiol. 1991;261(6):E800–8. doi: 10.1152/ajpendo.1991.261.6.E800. Pt 1. [DOI] [PubMed] [Google Scholar]
- 32.Gehlert DR, Schober DA, Beavers L, Gadski R, Hoffman JA, Smiley DL, Chance RE, Lundell I, Larhammar D. Characterization of the peptide binding requirements for the cloned human pancreatic polypeptide-preferring receptor. Mol Pharmacol. 1996;50(1):112–118. [PubMed] [Google Scholar]
- 33.Floyd JC, Jr, Fajans SS, Pek S, Chance RE. A newly recognized pancreatic polypeptide: plasma levels in health and disease. Recent Prog Horm Res. 1976;33:519–570. doi: 10.1016/b978-0-12-571133-3.50019-2. [DOI] [PubMed] [Google Scholar]
- 34.Valenzuela JE, Taylor IL, Walsh JH. Pancreatic polypeptide response in patients with chronic pancreatitis. Dig Dis Sci. 1979;24(11):862–864. doi: 10.1007/BF01324903. [DOI] [PubMed] [Google Scholar]
- 35.Glaser B, Zoghlin G, Pienta K, Vinik AI. Pancreatic polypeptide response to secretin in obesity: effects of glucose intolerance. Horm Metab Res. 1988;20(5):288–292. doi: 10.1055/s-2007-1010817. [DOI] [PubMed] [Google Scholar]
- 36.Berger D, Crowther RC, Floyd JC, Jr, Pek S, Fajans SS. Effect of age on fasting plasma levels of pancreatic hormones in man. J Clin Endocrinol Metab. 1978;47(6):1183–1189. doi: 10.1210/jcem-47-6-1183. [DOI] [PubMed] [Google Scholar]
- 37.Brunicardi FC, Druck P, Sun YS, Elahi D, Gingerich RL, Andersen DK. Regulation of pancreatic polypeptide secretion in the isolated perfused human pancreas. Am J Surg. 1988;155(1):63–69. doi: 10.1016/s0002-9610(88)80259-9. [DOI] [PubMed] [Google Scholar]
- 38.Adrian TE, Greenberg GR, Bloom SR. Actions of pancreatic polypeptide in man. In: Bloom SR, Polak JM, editors. Gut hormones. 2nd ed. Edinburgh: Churchill Livingston; 1981. pp. 206–212. [Google Scholar]
- 39.Nosadini R, del Prato S, Tiengo A, Duner E, Toffolo G, Cobelli C, Faronato PP, Moghetti P, Muggeo M. Insulin sensitivity, binding, and kinetics in pancreatogenic and type I diabetes. Diabetes. 1982;31(4):346–355. doi: 10.2337/diab.31.4.346. Pt 1. [DOI] [PubMed] [Google Scholar]
- 40.Gates RJ, Lazarus NR. The ability of pancreatic polypeptides (APP and BPP) to return to normal the hyperglycaemia, hyper-insulinaemia and weight gain of New Zealand obese mice. Horm Res. 1977;8(4):189–202. doi: 10.1159/000178800. [DOI] [PubMed] [Google Scholar]
- 41.Gettys TW, Garcia R, Savage K, Whitcomb DC, Kanayama S, Taylor IL. Insulin-sparing effects of pancreatic polypeptide in congenitally obese rodents. Pancreas. 1991;6(1):46–53. doi: 10.1097/00006676-199101000-00007. [DOI] [PubMed] [Google Scholar]
- 42.Lebovitz HE. Adjunct therapy for type 1 diabetes mellitus. Nat Rev Endocrinol. 2010;6(6):326–334. doi: 10.1038/nrendo.2010.49. [DOI] [PubMed] [Google Scholar]