Abstract
The Coalition for Clinical Research—Self-Monitoring of Blood Glucose Scientific Board convened a meeting in San Francisco, CA, July 20–21, 2011, to discuss the current practice of self-monitoring of blood glucose (SMBG) in non-insulin-treated (NIT) type 2 diabetes mellitus (T2DM). Twelve physician panel members from academia, practice, and government attended this meeting. These experts came from the United States, Brazil, Canada, France, Germany, Italy, and the United Kingdom. In addition, three consultants from Australia, Germany, and the United States contributed to the group’s final report. This coalition was organized by Diabetes Technology Society. Self-monitoring of blood glucose was studied from eight perspectives related to patients with NIT T2DM: (1) epidemiological studies; (2) randomized controlled trials (RCT)s and meta-analyses; (3) targets, timing, and frequency of SMBG use; (4) incidence and role of SMBG in preventing hypoglycemia with single-drug regimens and combination regimens consisting of antihyperglycemic agents other than secretagogues and insulin; (5) comparison of SMBG with continuous glucose monitoring; (6) technological capabilities and limitations of SMBG; (7) barriers to appropriate use of SMBG; and (8) methods and end points for appropriate future clinical trials. The panel emphasized recent studies, which reflect the current approach for applying this intervention. Among the participants there was consensus that:
SMBG is an established practice for patients with NIT T2DM, and to be most effective, it should be performed in a structured format where information obtained from this measurement is used to guide treatment;
New, high-quality efficacy data from RCTs have demonstrated efficacy of SMBG in NIT T2DM in trials reported since 2008;
Both patients and health care professionals require education on how to respond to the data for SMBG to be effective; and
Additional well-defined studies are needed to assess the benefits and costs of SMBG with end points not limited to hemoglobin A1c.
Keywords: hemoglobin A1c, non-insulin treated, self-monitored blood glucose, type 2 diabetes
Introduction
Self-monitoring of blood glucose (SMBG) is a widely practiced diagnostic measure in both patients who are using insulin and patients who are not using insulin.1 Self-monitoring of blood glucose has been conducted since the 1980s, and this practice continues to evolve. The benefits of this intervention for patients with type 1 diabetes mellitus (T1DM) and for insulin-treated patients with type 2 diabetes mellitus (T2DM) are well established.2,3 Purported benefits of SMBG in T2DM patients include (although are not limited to) the following:
Preventing, identifying, and treating hypoglycemia;
Providing feedback on the results of lifestyle and pharmacologic treatments;
Enhancing patient education on the impact of nutrition, activity, and medication choices;
Providing information for both patient and health care professionals (HCPs) to inform treatment modifications and titrations; and
Increasing patient empowerment and adherence to treatment.
The benefits of SMBG have been questioned, however, in non-insulin-treated (NIT) T2DM patients.4–6 Indeed, over the years since SMBG was developed, the medical literature have both supported and refuted the benefits of SMBG for these patients.4 Since 2008, a new paradigm has evolved in the practice of SMBG. The current approach is in a structured fashion. By using SMBG results to determine therapeutic decisions, this practice can take on greater value for patients and greater responsibility for HCPs. Patients must use the information to determine their self-care, and HCPs must develop and explain treatment plans that can incorporate SMBG information.7 In light of the rapidly evolving paradigm in SMBG practice, as well as the methodological limitations of many older studies that have assessed its benefits, it appears that this is an appropriate time to review the current status of this widely practiced intervention in NIT T2DM.
The Coalition for Clinical Research—Self-Monitoring of Blood Glucose (CCR-SMBG) convened a meeting in San Francisco, California, July 20–21, 2011, to discuss the current practice of SMBG in NIT T2DM. Twelve physician panel members who attended this meeting came from the United States, Canada, the United Kingdom, France, Germany, Italy, and Brazil. In addition, three expert consultants from Australia, Germany, and the United States contributed to the group’s final report. This meeting was organized by Diabetes Technology Society.
The purpose of the meeting was to review and discuss the medical literature and the patterns of medical practice to determine the current status of SMBG in NIT T2DM. The panel emphasized recent outcomes studies, which have been conducted since 2008 and reflect the current approach for applying this intervention.
Self-monitoring of blood glucose was studied from eight perspectives related to patients with NIT T2DM: (1) epidemiological studies of SMBG; (2) randomized controlled trials (RCTs) and meta-analyses; (3) targets, timing, and frequency of SMBG use; (4) incidence and role of SMBG in preventing hypoglycemia with single-drug regimens and combination regimens consisting of antihyperglycemic agents other than secretagogues and insulin; (5) comparison of SMBG with continuous glucose monitoring (CGM); (6) technological capabilities and limitations of SMBG; (7) barriers to appropriate use of SMBG; and (8) methods and end points for appropriate future clinical trials. This article represents the consensus findings of the CCR-SMBG panel members and its consultants.
Epidemiological Studies of Self-Monitoring of Blood Glucose in the Literature
Key Points
Epidemiological studies are hypothesis-generating with regard to the design of RCTs.
Epidemiological data are consistent with benefit but not conclusive.
Because there are multiple components required to perform SMBG, studies should be designed to clarify which component or components will be important in achieving glycemic goals.
Epidemiological studies are a cornerstone method of public health research of diabetes. For assessing the potential benefits of SMBG in NIT T2DM, these studies help inform policy decisions, provide insights into the use of test strips, and contribute to the creation of studies of this practice.8
Large-scale epidemiological studies have generated conflicting outcomes. Some of them do not show that the use of SMBG is associated with improved glycemic control or survival in NIT T2DM.9–11 However, a clear benefit of SMBG in NIT T2DM has been observed in two major epidemiological studies.
The first of these two studies was a longitudinal study of new and ongoing use of SMBG that included more than 30,000 patients of the Kaiser Permanente Northern California integrated health plan.12 Self-monitoring of blood glucose frequency was based on the number of blood glucose (BG) test strips dispensed. In NIT T2DM, greater SMBG practice frequency in new users was associated with lower hemoglobin A1c (A1C) levels. The frequency of performing SMBG among partakers of this practice was inversely associated with their A1C levels.
The second such study was the ROSSO study, a retro-spective, epidemiological cohort study. This study reported that fatal and nonfatal event rates were lower in T2DM patients who performed SMBG compared with those who did not perform SMBG.13 The study identified SMBG as an independent predictor of morbidity and mortality in T2DM patients as well as in the subgroup of NIT T2DM patients. It was hypothesized that SMBG catalyzed the effects via healthier lifestyle and/or better disease management.
Further epidemiological research on SMBG is also of importance with regard to the assessment of the potential benefits of each of the various components of SMBG in NIT T2DM to determine which part of this intervention is the most beneficial. The intervention comprises multiple components, including time spent with an educator for education about self-management, performance of the SMBG test, interpretation of test results, and action taken based on the test results.
Randomized Controlled Trials and Meta-Analyses in the Literature
Key Points
Several recently reported well-designed studies that have incorporated an educational and a therapeutic intervention in response to BG values have demonstrated efficacy in lowering A1C.
Many SMBG RCTs are of little value because of small size, confounding factors, low baseline A1C, or the lack of an educational and therapeutic intervention in response to BG values obtained.
Benefits of SMBG cannot be isolated, because SMBG is a measurement and, fundamentally, the value of a measurement is dependent on how it is used.
Several systematic reviews and meta-analyses of RCTs have been published since 2008, all suggesting that SMBG is associated with a statistically significant, clinically modest reduction in A1C levels (Table 1).14–17 Many of these trials are of limited value, because they did not include an educational and therapeutic intervention in response to BG values and their designs incorporated small size, confounding factors, or low baseline A1C (Table 2).
Table 1.
Results of Meta-Analyses Since 2008
| Poolsup (2008)14 SMBG versus no SMBG SMBG versus no SMBG—SMBG results used to modify therapy SMBG versus no SMBG—SMBG results not used to modify therapy | A1C (95% confidence interval) -0.24% (-0.37 to -0.12; p = .0002; seven trials) -0.27% (-0.41 to -0.14; p = .0001; six trials) -0.12% (-0.32 to 0.08; p = not significant; six trials) |
|---|---|
| Towfigh (2008)15 SMBG versus no SMBG ≥ 1 year SMBG versus no SMBG 6 months | A1C (95% confidence interval) -0.16% (-0.38 to 0.05; p = not significant; five trials) -0.21% (-0.38 to -0.04; p < .05; six trials) |
| St. John (2010)16 SMBG versus no SMBG SMBG versus no SMBG < 1 year SMBG versus no SMBG ≥ 1 year | A1C (95% confidence interval) -0.22% (-0.34 to -0.11; p < .05; seven trials) -0.26% (-0.40 to -0.11; p = .001; five trials) -0.17% (-0.36 to 0.02; p = .072; two trials) |
| Clar (2010)17 SMBG versus no SMBG Enhanced SMBG versus no SMBG | A1C (95% confidence interval) -0.21% (-0.31 to -0.10; p < .0001; ten trials) -0.52% (-0.98 to -0.06; p = .03; four trials) |
Table 2.
Common Design Flaws in Self-Monitoring of Blood Glucose Trials
| Subject selection: Low baseline A1C |
|---|
| Subjects not using SMBG data |
| • Not instructed on interpreting meaning of SMBG |
| • Not permitted to respond to SMBG results |
| HCPs not using SMBG data |
| • Lack of medication titration algorithms |
| Design issues |
| • Small sample size |
| • Crossover effect: same HCP caring for both groups |
An expert panel recognized this deficiency in the data in 2008.7 Subsequently, most recent well-designed RCTs of SMBG interventions have incorporated an educational and a therapeutic component in response to BG values, and have demonstrated reductions in A1C.18–20 These studies have focused on instructing patients on (1) how and when to perform SMBG, (2) the meaning of various BG levels (which can be important given the common problem of low numeracy),21 and (3) how behavior and actions affect SMBG results. Therefore, SMBG has the potential to empower patients in their own care to gain a greater understanding of which factors affect their glycemic levels. Equally important to providing instructions to patients is ensuring that data are available to the patients’ care team along with guidance on how to use this information. This means that algorithms should also be made available, if necessary, to guide adjustment of diabetes medications.
In the STeP trial, 483 poorly controlled insulin-naïve subjects (mean A1C 8.9%) were randomized to an active control group (ACG) with enhanced usual care or to a structured testing group (STG) with enhanced usual care and at least quarterly use of structured SMBG.18 At 1 year, there was a significantly greater reduction in mean A1C in the STG compared with the ACG (Δ = -0.3%; p = .04). Significantly more STG subjects received a treatment change recommendation compared with ACG patients.
In the ROSES trial, subjects were randomly allocated to either a self-monitoring-based disease management strategy or to usual care.19 Education was centered on how to modify lifestyle according to self-monitoring readings. Results of SMBG were discussed during monthly telephone contact. After 6 months, significantly greater reductions in mean A1C (Δ = -0.5%; p = .04) and body weight (Δ = -4.0 kg; p = .02) were observed in the SMBG group compared with the control group.
In the St. Carlos trial, newly diagnosed T2DM patients were randomized to either an SMBG-based intervention or an A1C-based control group.20 The SMBG intervention cohort used this monitoring intervention as both an educational tool to adhere to lifestyle changes as well as a therapeutic tool to apply step-by-step pharmacological treatment. Treatment decisions for the A1C cohort were based strictly on A1C test results. After 1 year of follow-up, the median A1C level and body mass index were significantly reduced in patients in the intervention group (from 6.6% to 6.1% and from 29.6 to 27.9 kg/m2, respectively; p < .05 and p < .01, respectively). In the A1C control group, there was no change in median A1C level or body mass index.
Overall, these findings should be viewed in the context of prior attempts to study the effects of SMBG. There are numerous potential benefits to SMBG, including (1) reducing A1C, glycemic variability (GV), and hypo-glycemia; (2) improving lifestyle and medication adherence; and (3) accelerating medication titration. Of these benefits, studies have been primarily focused on A1C reduction. Fundamentally, however, assessing the role of SMBG in isolation may not be any more useful than studying whether checking A1C leads to better glucose control. Self-monitoring of blood glucose is a fundamental element to virtually all diabetes drug therapy trials, and this measurement is incapable of having an impact on outcomes unless either the patient or the HCP can utilize the information. In most negative studies described in meta-analyses of SMBG practice, the measurement was not permitted to influence either the patient or the HCP. Other trials of this practice have been undermined by small sample size, low baseline A1C levels (limiting potential impact), or poor study design. One problem with individual randomization in behavioral trials (such as performing SMBG) is that randomizing both intervention and control subjects to the same investigator can lead to contamination of controls, who are influenced by the cohort of intervention partakers to adhere to the intervention more faithfully than expected, and this alteration in behavior results in reduced observable efficacy. This problem can be avoided with cluster randomization in which investigators are randomized and each investi-gator provides all subjects with either control care or intervention care.
Future studies are needed to identify specific subgroups that may benefit particularly well or poorly from SMBG, optimal testing frequencies, and other potential outcomes that go beyond A1C.
Targets, Timing, and Frequency of Self-Monitoring of Blood Glucose Use in Non-Insulin-Treated Type 2 Diabetes Mellitus
Key Points
All people with diabetes should know how to perform SMBG in a way that is appropriate and useful to them and their clinicians.
Targets, timing, and frequency of SMBG should be individualized.
Self-monitoring of blood glucose can empower patients, inform and reinforce appropriate lifestyle interventions, and facilitate patient/clinician selection/titration of medications.
Self-monitoring of blood glucose is especially important for NIT T2DM patients at risk for hyperglycemic or hypoglycemic events.
Treatment of T2DM hyperglycemia requires diabetes self-management education, lifestyle interventions, and usually pharmacologic treatment, most commonly consisting of combinations of antihyperglycemic agents with complementary mechanisms of action. One important component of diabetes self-management education is learning how to perform SMBG.22
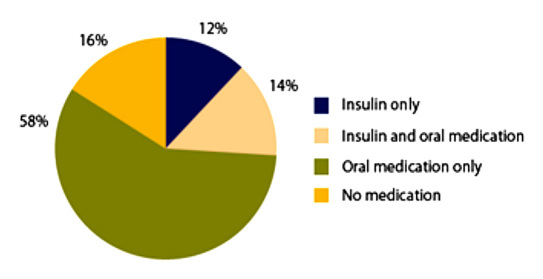
There are now at least 13 classes of antihyperglycemic agents available.23,24 The value of SMBG for insulin-treated T2DM patients is well accepted. However, only a minority of T2DM patients are treated with insulin.An estimated 74% of these patients are treated with medications other than insulin or with no medications at all (see Figure 1).25
Figure 1.
Percentage of adults with diagnosed diabetes receiving treatment with insulin or oral medication, United States, 2007–2009.25
Targets
The most recent targets for glycemic control for most patients with T2DM from the: (1) American Association of Clinical Endocrinologists/American College of Endocrinology (AACE/ACE); (2) American Diabetes Association (ADA); (3) U.S. Department of Veterans Affairs (DoD/VA); (4) Scottish Intercollegiate Guidelines Network (SIGN); and (5) National Institute for Health and Clinical Excellence (NICE) are shown in Table 3. All these organizations endorse individualization of their glycemic targets. Therefore, an A1C that is closer to normal might be appropriate for newly diagnosed patients with diabetes who have a long life expectancy and no significant cardiovascular disease. In contrast, less stringent targets would be prudent in those with a history of severe hypoglycemia, hypoglycemia unawareness, limited life expectancy, advanced microvascular or macrovascular complications, extensive co-morbid conditions, or long-standing diabetes where goals have not been achieved despite optimal treatment.3,22,26–29
Table 3.
Glycemic Control Recommendations for Type 2 Diabetes
| Target treatment goals | AACE/ACE 201122 | ADA 20113 | DoD/VA 201026 | IDF27 | SIGN 201028 | NICE 200829 |
|---|---|---|---|---|---|---|
| A1C | ≤6.5% | <7.0% | <7.0–9%a | ≤6.5% | 6.5–7.0%b | ≤6.5–7.5%c |
| Fasting glucose | Fasting plasma glucose <110 mg/dl | Preprandial capillary plasma glucose <70–130 mg/dl | Not available | <88 mg/dl | Not available | Not available |
| PPG | 2 h PPG <140 mg/dl | Peak postprandial plasma glucose <180 mg/dl | Peak postprandial plasma glucose < 180 mg/dl | <140 mg/dl | Not available | Not available |
Dependent on life expectancy and presence/absence of complications.
The lower goal is for some newly diagnosed patients.
Goal varies depending on how many oral agents a patient is taking.
Timing
Borg and colleagues30 reported data from the A1C-derived average glucose study that inform decisions about the timing of SMBG testing. The area under the glucose curve calculated from CGM 2 h after meals correlated well with the 90 min SMBG value. Preprandial values had stronger association with A1C than postprandial glucose (PPG) values when the A1C level was elevated.
Frequency
Evidence exists that GV, especially characterized by relatively brief spikes in glycemia, may independently contribute to the risk for diabetes complications.31 Whether this association supports more frequent measures of PPG glucose remains controversial. However, as A1C levels are brought down closer to 7% and below, the contribution of PPG appears to increase.32 This observation suggests that more frequent PPG testing may be required as one approaches target glycemia. Whether decreased GV estimated by CGM is associated with independent improvements in patient satisfaction and perceived health remains to be studied.33
What do diabetes guideline writing organizations recommend for SMBG testing in those with NIT T2DM? The ADA recommends use of SMBG by stating the following: (1) “For patients using less frequent insulin injections, noninsulin therapies, or medical nutrition therapy (MNT) alone, SMBG may be useful as a guide to the success of therapy;” (2) “To achieve PPG targets, postprandial SMBG may be appropriate;” and (3) “When prescribing SMBG, ensure that patients receive initial instruction in, and routine follow-up evaluation of, SMBG technique and their ability to use data to adjust therapy.”2
Self-monitoring of blood glucose recommendations from the AACE/ACE state that patients not requiring insulin therapy may benefit from SMBG, especially to provide feedback about the effects of their lifestyle and pharmacologic therapy. The organization also states that testing frequency must be personalized, and the initial choice of an agent targeting fasting plasma glucose or PPG involves comprehensive patient assessment with emphasis given to the glycemic profile obtained by SMBG.22
The International Diabetes Federation (IDF) guideline Self-Monitoring of Blood Glucose in Non-Insulin Treated Type 2 Diabetes states that
SMBG should be used only when individuals with diabetes (and/or their caregivers) and/or their HCPs have the knowledge, skills, and willingness toincorporate SMBG monitoring and therapy adjust-ment into their diabetes care plan in order to attain agreed treatment goals; SMBG should be considered at the time of diagnosis to enhance the understanding of diabetes as part of individuals’ education and to facilitate timely treatment initiation and titration optimization; SMBG should also be considered as part of ongoing diabetes self-manage-ment education to assist people with diabetes to better understand their disease and provide a means to actively and effectively participate in its control and treatment, modifying behavioral and pharmaco-logical interventions as needed, in consultation with their HCP; SMBG protocols (intensity and frequency) should be individualized to address each individual’s specific educational/behavioral/clinical requirements (to identify/prevent/manage acute hyper- and hypo-glycaemia) and HCP requirements for data on glycemic patterns and to monitor impact of therapeutic decision making.2
This IDF guideline also states, “Although we currently have no evidence base regarding optimal SMBG regimens in non-insulin-treated T2DM, it is generally agreed that it is often not necessary to perform SMBG on a daily basis in this population,” noting,
It may be valuable for people with diabetes to perform ‘focused’ SMBG over short periods of time, initially and periodically, during the course of their disease, in order to obtain data that facilitate identification of glucose patterns that are reflective of daily glycemic control. For example, a 5-point or 7-point SMBG regimen, testing BG before and after each meal and at bedtime over the course of 1 to 3 days, may be used to create a representative glucose profile. Alternatively, a ‘staggered’ regimen can be used to obtain BG levels before and/or after alternating meals daily or every other day over a 1 to 4-week period each month.2
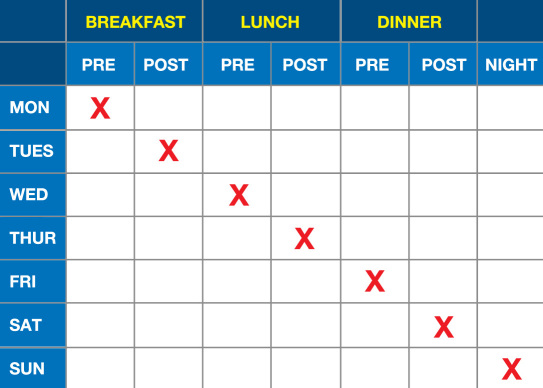
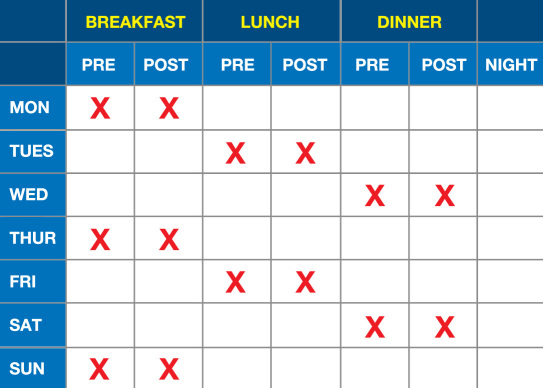
An example of a less intensive SMBG scheme is presented in Figure 2 and an example of a more intensive scheme is presented in Figure 3. These 7-day schemes can be modified from a daily testing frequency to an every-other-day testing frequency, which means that it would then require 14 days to obtain all the data points specified in these two figures.
Figure 2.
Less intensive SMBG scheme.8
Figure 3.
More intensive SMBG scheme.8
A European expert panel on standardized approaches to SMBG has recommended SMBG schemes of varying intensity across the T2DM continuum. In these schemes, the duration and frequency of SMBG performance varied depending on both the level of glycemic control and other clinical circumstances of the patient. The panel also recommended that various approaches to SMBG for T2DM patients be the subject of further clinical studies.8
Given the absence of specificity in recommendations by most professional societies, we recommend that all people with diabetes should know how to perform SMBG and should do so with a frequency and timing that is individualized, appropriate, and useful to them and to their HCPs. We also conclude that SMBG can empower patients and inform and reinforce appropriate lifestyle inter-ventions and facilitate improvements in patient/clinician selection/titration of medications. Finally, SMBG targets must be individualized, especially for NIT T2DM patients at risk for hyperglycemic or hypoglycemic events.
Incidence and Role of Self-Monitoring of Blood Glucose in Preventing Hypoglycemia with Single-Drug Regimens and Combination Regimens Consisting of Antihyperglycemic Agents Other than Secretagogues and Insulin
Key Points
Incidence of major hypoglycemia approaches zero in monotherapy and dual therapy with noninsulin secretagogues.
Incidence of minor hypoglycemia is very low in dual noninsulin secretagogue therapy (0–3%) but is high when the combination therapy includes an insulin secretagogue (4–36%).
Most clinical practice guidelines of the pharmaco-therapy of T2DM include an insulin secretagogue as the first add-on therapy to metformin.
Self-monitoring of blood glucose is neither practical nor necessary to prevent and/or recognize hypo-glycemia in patients taking one or more noninsulin secretagogues but is important in those patients whose regimens include an insulin secretagogue.
Where patients who use sulfonylurea have a high incidence of hypoglycemia (20% in the United Kingdom Prospective Diabetes Study), patients who use noninsulin secretagogues as monotherapy or combined with other noninsulin secretagogues in dual or triple therapy have a low risk of hypoglycemia. The literature was surveyed for prospective RCTs that report the incidence of major and minor hypoglycemic episodes as a percentage of patients (where percentage generally represents the proportion of patients with either type) associated with monotherapy or dual therapy with noninsulin secretagogues, and a comparison was made with combinations that included sulfonylureas. Monotherapy studies using metformin,34 thiazolidinediones,35 dipeptidyl peptidase-4 (DPP-4) inhibitors,36 glucagon-like peptide-1 (GLP-1) agonists,37,38 alpha glucosidase inhibitors (AGIs),39 a dopamine agonist (DA),40 and bile acid sequestrants (BAS)41 have no reports of major hypoglycemia and a low rate of minor hypo-glycemia, -0.2–8%, albeit most studies do not describe the criteria used for categorizing major versus minor hypoglycemia. The rate of dual therapy is similarly low. Per the opinion of the CCR-SMBG, there are 15 reasonable combinations of noninsulin secretagogues in dual therapy for T2DM (for example, a combination of GLP-1 agonist and a DPP-4 inhibitor or of either one of these with an AGI would be considered unreasonable; Table 4).
Table 4.
Fifteen Reasonable Combinations of Noninsulin Secretagogues in Dual Therapy for Type 2 Diabetes Mellitusa
| Combination Number | Drug Class 1 | Drug Class 2 |
|---|---|---|
| 1 | M | T |
| 2 | M | DPP |
| 3 | M | GLP-1 |
| 4 | M | AGI |
| 5 | M | BAS |
| 6 | M | DA |
| 7 | T | DPP |
| 8 | T | GLP-1 |
| 9 | T | DPP |
| 10 | T | AGI |
| 11 | T | BAS |
| 12 | T | DA |
| 13 | DPP | DA |
| 14 | GLP-1 | DA |
| 15 | DA | AGI |
M, metformin; T, thiazolidinedione.
Data exist on hypoglycemia rates in 9 of these 15 combinations (Table 5): (1) metformin plus a thiazolidine-dione,34,42–46 (2) metformin plus a DPP-4 inhibitor,34,36,42,44,46 (3) metformin plus a GLP-1 agonist,46–48 (4) metformin plus an AGI,49–51 (5) metformin plus a DA,40 (6) metformin plus a BAS,41,52 (7) a DPP-4 inhibitor plus a thiazolidinedione,35,53,54 (8) a GLP-1 agonist plus a thiazolidinedione,48,55 and (9) an AGI plus a thiazolidinedione.45 In these studies, the rate of major hypoglycemic episodes is zero and the percentage of patients experiencing minor hypoglycemic episodes averages 1.5% (range 0–7.1%).
Table 5.
Rates of Minor Hypoglycemia in Dual Therapy with Noninsulin Secretagoguesa
| Combination # | Drug 1 | Drug 2 | Reference | Rate for minor hypoglycemia in control group | Rate for minor hypoglycemia in treatment group | Rate ranges |
|---|---|---|---|---|---|---|
| 1 | M | T | Scott34 | M 1.2% | M + rosiglitazone 1.2% | |
| Wysham42 | M + pioglitazone 4.3% | |||||
| Pfützner43 | M + pioglitazone 1.4% | |||||
| Bolli44 | M + pioglitazone 0% | |||||
| Vlckova45 | M 0.9%b | M + pioglitazone 0.4%b | ||||
| Bergenstal46 | M 1% | M + pioglitazone 1% | ||||
| M + T = 0-4.3% | ||||||
| 2 | M | DPP-4 | Scott34 | M 1.2% | M + sitagliptin 1.2% | |
| Williams-Herman36 | Sitagliptin 3.1%b | M (or T) + sitagliptin 3.3%b | ||||
| Bergenstal46 | M + sitagliptin 3% | |||||
| Wysham42 | M + sitagliptin 0.1% | |||||
| Bolli44 | M + vildagliptin 0.3%b | |||||
| M + DPP = 0.1–3.3% | ||||||
| 3 | M | GLP-1 | Bergenstal46 | M + exenatide once-weekly 1.0% | ||
| Buse47 | M + exenatide 11% | M + liraglutide 6.2% | ||||
| Liutkus48 | M + GLP = 1–11% | |||||
| 4 | M | AGI | Rosenstock49 | M 1.6%b | M + AGI 2.6%b | |
| Jayaram50 | M + AGI 0%b | |||||
| Lin51 | M + AGI 0%b | M + AGI = 0–2.6% | ||||
| 5 | M | DA | Gaziano40 | M or sulfonylurea 0.4%b | M or sulfonylurea + bromocriptine 0.2%b | |
| M + DA = 0.2% | ||||||
| 6 | M | BAS | Zieve52 | Placebo + sulfonylurea (± M) no differenceb | Colesevelam + sulfonylurea (± M) no differenceb | |
| Bays41 | M 0% | Colesevelam + M 0.6% | ||||
| M + BAS = 0.6% | ||||||
| 7 | DPP-4 | T | Kim53 | Vildagliptin + pioglitazone 0%b | ||
| Rosenstock35 | Alogliptin + pioglitazone 3% | |||||
| Rosenstock54 | Sitagliptin 0%b | Sitagliptin + pioglitazone 1.1%b | ||||
| DPP + T = 0–3% | ||||||
| 8 | GLP-1 | T | Zinman55 | T 10.7% | GLP + T 7.1% | |
| Liutkus48 | T (or M) 1.9% | GLP + T (or M) 3.6% | ||||
| GLP + T = 3.6–7.1% | ||||||
| 9 | AGI | T | Vlckova45 | AGI + pioglitazone 0.3%b | ||
| AGI + T = 0.3% |
M, metformin; T, thiazolidinedione.
Not defined as major versus minor.
Thus, given the low incidence of hypoglycemic events, one could not sufficiently perform SMBG frequently enough to be able to predict such episodes using a predictive parameter such as the Low Blood Glucose Index.56 However, while combinations consisting exclusively of noninsulin secretagogues are becoming increasingly popular in practice, virtually all recent clinical practice guidelines for T2DM management include combinations of noninsulin secretagogues and insulin secretagogues.57–61 The percentage of patients developing hypoglycemia with such combinations of noninsulin secretagogues and insulin secretagogues is rather high. Up to 1.2% of patients have experienced major hypoglycemic episodes, and an average of 15.5% (range 4–34%) have experienced minor hypoglycemic episodes with such paired combinations as metformin plus a sulfonylurea,43,62–64 a thiazolidinedione plus a sulfonylurea,65,66 a GLP-1 agonist plus a sulfonylurea,47,67 a DPP-4 plus a sulfonylurea,68 bromocriptine plus a sulfonylurea,40 and AGI plus a sulfonylurea.69 Thus the use of SMBG seems essential when patients are treated with an insulin-secretagogue-containing combination to detect and document hypoglycemia as well as to determine when to initiate measures to treat it.
Comparison of Self-Monitoring of Blood Glucose with Continuous Glucose Monitoring
Key Points
Both SMBG and CGM provide the best outcomes if they are associated with structured educational and therapeutic programs.
In future trials comparing SMBG and CGMS, the SMBG intervention should be structured similar to that in recent trials (including STeP, ROSES, and St Carlos) and new forms of communication between patients and HCPs should be evaluated, including social media.
The role of CGM in NIT T2DM is not yet clearly established as to when or how this technology should be used in addition to—or in place of—structured SMBG.
It is difficult to design appropriate randomized controlled clinical trials to determine the effect of SMBG in isolation from other interventions. There are also few clinical trials that compare different frequencies and timings of SMBG testing regimens.70 For insulin-treated patients, SMBG is used primarily to (1) detect or confirm hypoglycemia, (2) assess the prevailing level of BG control, (3) provide guidance on making changes to ongoing therapy (i.e., altering the dose, timing, or frequency of basal insulin or making changes in therapy regimen), and (4) provide data on which immediate therapeutic decisions are made, such as adjusting the dose of rapid-acting insulin to cover a meal. Moreover, SMBG has the potential to help a patient to better understand the impact of lifestyle modifications (e.g., exercise, diet, or stress) or life events (e.g., sickness, travel, or use of high-dose corticosteroids) on glycemic control.
For NIT individuals, the role of SMBG remains controversial, because most studies have not examined the role of SMBG as an intervention on its own. In some studies, no actions were taken by intervention subjects based on the SMBG results. In only a small number of studies were patients encouraged to adjust their treatment based on SMBG values. As a corollary, some studies were based on interventions where SMBG was carried out to inform only the HCP but not the patient.
Furthermore, few studies have provided information on outcomes when stratified according to the type of treatment used. For example, SMBG could be used in a setting of various baseline medical regimens, and therefore, it could be used to determine whether one particular treatment regimen accrues added value from SMBG compared with another treatment regimen. Additionally, few studies have linked SMBG with appropriate training, feedback, or treatment modification with the potential for behavior change. Finally, there is limited guidance from trials as to whether specific patient factors are important in determining the impact of SMBG, e.g., age, gender, educational level, or socioeconomic status.
Continuous interstitial glucose monitoring provides infor-mation on direction and duration of glycemic excursions on a moment-to-moment basis. In a recent meta-analysis of real-time CGM compared with SMBG in T1DM, overall reduction in A1C was 0.30% greater with CGM.71 The meta-analysis also suggested that any improvement in the A1C level with CGM will be associated with a small reduction in hypoglycemia exposure but not severe hypoglycemic events. Continuous glucose monitoring is not a replacement for SMBG, because SMBG is still required for calibration of existing CGM systems, confirmation of suspected hypoglycemia, and dosing of insulin.
Glycemic variability has been implicated as a risk factor for development of diabetes complications.72 There is no consensus on the optimal metric for GV.73 Both postprandial SMBG values and CGM data provide information on this phenomenon. Future studies of SMBG and CGM in isolation, as well as combinations of these two diagnostic measures, will likely determine a useful definition for GV and examine its role in the development of diabetes complications.74
In a study comparing SMBG with real-time CGM in NIT T2DM subjects, after 12 weeks, A1C fell further with CGM (1.0% versus 0.5%; p = 0.006) than with SMBG, without any difference in medication or weight changes within both groups.75 Similar to data from studies in T1DM, greater duration of sensor usage was associated with greater reductions in A1C. Of note, both groups were managed by their usual HCPs. After 12 months, subjects performing SMBG one or more times per day improved their A1C levels more than those who tested less than once per day over 3 months (-0.6% versus -0.2%) and 12 months (-0.3% versus -0.0%). At the same time, patients using real-time CGM lowered their A1C levels by 1.1% over the same time period, which was significantly better than either SMBG group.76 The role of real-time CGM in NIT T2DM is not yet clearly established, and further studies are needed to assess when this technology should be used in addition to—or in place of—structured SMBG for these types of patients.
Technological Capabilities and Limitations of Self-Monitoring of Blood Glucose
Key Points
Blood glucose monitor results can be adversely affected by preanalytical factors, analytical factors, and postanalytical factors.
New hardware will extend the capabilities of BG monitors.
New software will add value to SMBG results by providing instruction on what type of action to take based on preprogrammed individual factors and real-time BG data.
Preanalytical errors occur before an analytical measure-ment is performed, analytical errors occur in the act of testing, and postanalytical errors occur after the test has been performed.77,78 Seven factors account for inaccuracy in BG monitoring related to (1) patient performance, (2) strips, (3) physical environment, (4) physiology, (5) medications, (6) inherent system limitations, and (7) data processing.78–81 Preanalytical errors are more of a concern for laboratory testing of analytes than for self-monitoring, because most preanalytical errors are due to procurement, handling, and storage of samples in preparation for subsequent testing. For SMBG, most preanalytical errors are related to patient performance in obtaining a blood specimen or storing strips. Most analytical errors can be attributed to physical environmental factors, physiologic factors, and pharmacologic factors, but patient performance can also be involved. Most postanalytical errors can be attributed to data processing factors. However, a broad look at the process of testing would also include errors in interpreting and acting upon data as postanalytical errors.
Patient factors are the most common cause of BG monitor inaccuracy. Some common examples of patient factors include failure to clean the skin, failure to clean the monitor, or failure to apply an adequate blood specimen. If BG monitors are to be shared by multiple patients in a group living environment, such as a nursing home, then the monitor must be regularly disinfected and only single-use lancets should be used to avoid blood-borne virus transmission.82
Strip factors can affect BG monitor performance. Strip-to-strip variation in production can lead to measurement errors. Improper storage of strips can also lead to BG monitor error. This is a particularly severe problem in hot, humid environments if a strip vial is left open with the cap off.
Environmental factors can affect BG monitor perfor-mance.83 Strip exposure to air, high altitude (with its decreased oxygen levels), humidity, heat, or cold can all result in degraded performance of monitors. Exposure to cold can also cause vasoconstriction in a patient and lead to an increased lag time if alternate-site testing is performed.
Physiology factors can affect BG monitor performance.84,85 Extreme levels of hematocrit, as well as three other substances that are found in blood (oxygen, triglycerides, and uric acid), are known to confound accurate glucose readings in some monitors. A low hematocrit can cause falsely high glucose readings, and a high hematocrit can cause falsely low glucose readings. With BG monitors that use glucose oxidase on their strips, high ambient oxygen concentrations (such as in patients receiving oxygen therapy) can cause falsely low glucose readings, and low ambient oxygen concentrations (such as in patients with severe lung disease) can cause falsely high glucose readings. This effect of oxygen is greater when glucose levels are normal or low. Triglycerides take up volume, and in hypertriglyceridemia, the remaining volume of blood containing glucose is decreased, causing a falsely low reading with any type of monitor. Uric acid is electrochemically active and can react directly with an electrode or mediator in some BG monitors and can be falsely read as glucose if it is present in very high concentrations. The natural molecule, ascorbic acid, as well as monosaccharide molecules such as maltose, galactose, and xylose can all be read as glucose, which leads to falsely elevated readings with some glucose dehydrogenase monitors.86 Differences in assays and compensatory processes in different monitors might modify all these interferences.
Medications can affect BG monitor performance. Three examples of drugs that can affect performance of BG monitors that use glucose oxidase on their strips are acetaminophen, tolazamide, and L-dopa. Icodextrin, contained in peritoneal dialysis fluid, and maltose, contained in immunoglobulin preparations, can be falsely read as elevated glucose levels with some glucose dehydrogenase monitors.
Inherent system limitations are related to the nature of the materials used in strips and monitors. Every BG monitor has a certain degree of imprecision and bias associated with it, even when used by trained laboratory professionals, as evidenced by occasional outlier data.
Patient factors contribute to analytical error because the patient is the operator of the instrument. Unless all manufacturers’ instructions are followed, there is room for operator error. Incomplete filling of test strip wells, use of soiled monitors, and failure to use control solution are common user errors.
Postanalytical errors have been reported in situations where incorrect units of glucose concentrations are presented (i.e., substitution of mmol/liter for mg/dl or vice versa), data are not recorded or uploaded such that the data are later forgotten and not used, data are transmitted incorrectly to a computer or handheld device, or a misleading message is generated. In such a setting, the measurement may have been accurate, but the information was presented incorrectly because of software or hardware problems.
It is expected that new, more accurate BG monitors will be developed in the near future in response to an anticipated tightening of BG monitor accuracy requirements by the U.S. Food and Drug Administration.87 Next-generation BG monitors might incorporate wireless transmission to smart phones or other devices. Software is being developed for incorporation into BG monitors to provide real-time analysis of glucose data and bolus dose recommendations to patients based upon SMBG values.
Barriers to Appropriate Use of Self-Monitoring of Blood Glucose
Key Points
Knowledge barriers: HCPs must learn, and then explain to patients, what actions to take in response to SMBG data, and they must review this data with patients during visits to maintain enthusiasm for this practice.
Human factors barriers: Patients need to learn how to operate an SMBG device whose performance will not be affected by any disabilities that they might have.
Logbook barriers: Use of a logbook or a monitor data printout for patients should be encouraged to identify patterns, and data collected should be reviewed by the HCP at each visit.
Economic barriers: Patients will not use SMBG and payers will not fund SMBG unless both parties see clear benefits. Results of robust clinical trials must be disseminated to HCPs and payers to document that SMBG is a useful procedure in the management of NIT T2DM.
The four most formidable types of barriers to the appropriate use of SMBG in NIT T2DM are knowledge barriers, human factors barriers, logbook barriers, and economic barriers. Knowledge barriers require input to patients from HCPs. Human factors and logbook barriers require patients to receive training on techniques, select compatible equipment, invest time in learning proper techniques, and demonstrate motivation. Economic barriers must be overcome by presenting robust data to payers of the health care system. Well-designed trials generating outcomes and economic data are needed to remind payers of the value of this intervention.
Knowledge Barriers
Successful performance of SMBG requires that patients properly interpret the number indicated as the measure-ment result and adjust their therapy and/or lifestyle according to a plan. Patients will likely not understand why they should perform a blood sampling measurement without having any evident benefit explained to them.88 Health care professionals must learn how to adjust therapy in response to their patients’ SMBG levels, and they must then teach their patients which therapeutic measures are adequate to maintain metabolic control within an appropriate range through interpreting glycemic levels.89 They must explain the appropriate actions to take in response to SMBG levels by presenting protocols that adapt lifestyle and medications to achieve optimal glucose control. For example, if SMBG is used mainly to help the physician adjust the dosage of oral agent therapy, then infrequent measurements covering the whole day as infrequently as seven tests in the same day twice a month may be sufficient. The treating physician and the health care team must reinforce testing behavior on the part of each patient by looking at the results (in a diary or downloaded document) and discussing these at each visit. Ignoring this scrutiny will decrease motivation substantially. Finally, the consequences of each modification of therapy must be monitored.
Human Factors Barriers
Modern BG meters require increasingly less handling efforts. There is no longer a need for coding or wiping of blood. Modern meters require smaller-volume blood drops and can provide test results within seconds. Glucose meters differ in their ease of handling and readability of the numbers shown on the display. Successful performance of SMBG (defined as resulting in adequate metabolic control without acute metabolic deterioration) requires that patients perform all handling steps involved adequately. Many NIT T2DM patients are elderly and might have limited eyesight or manual capabilities as well as other disabilities.90 Patients should be encouraged to select a meter that best fits their needs, especially if they have disabilities.91 Communication with a diabetes nurse for training in using a device is very helpful.
Logbook Barriers
Use of logbooks can result in incomplete data recording, and these books may contain modified or added data.92 On the other hand, downloading data stored in the meter into a computer can be a time-consuming and cumbersome procedure, especially because there is no standardization in technology used for downloading (i.e., many different noninterchangeable hardware and software products). Improvements are required at this level for SMBG to become more widely used by HCPs. New software is being developed to present SMBG data in new and useful ways.93 Logbooks and printouts can assist patients to see glycemic patterns. Patients should be encouraged to use a logbook or software and to bring this book or a monitor data printout to each visit.
Economic Barriers
Measurement of BG without an adequate response to the data does not extract maximal value from the actions and costs of this intervention. Some payers limit access to SMBG testing supplies to save money.94 Patients who understand how BG monitoring supports them in managing their diabetes should have access to test strips at affordable costs.
Benefits of Overcoming Barriers
Self-monitoring of blood glucose can become a corner-stone in diabetes therapy in NIT T2DM if this diagnostic measure is fully implemented in programs that promote self-management through proper interpretation of and response to glycemia based on protocols that adapt lifestyle and medications to achieve optimal glucose control.95 Unfortunately, too few patients have a thorough understanding of how to react to abnormal results by making changes in lifestyle and medications. Resources, including certified diabetes educators, electronic automated management algorithms for patients, and the Internet, should be made more available to disseminate more information on how to react to glycemia.96
If these four barriers can be overcome, then SMBG could become an even more widely adopted intervention in the management of T2DM. Analysis of patient data, education of patients, simplified storage of data, and dissemination of outcomes data to HCPs and payers will all be required to help to maintain SMBG as a useful and readily available procedure for patients with NIT T2DM.
Methods and End Points for Appropriate Future Clinical Trials
Key Points
Self-monitoring of blood glucose should be linked with a structured educational and therapeutic program designed to facilitate behavior change for improving BG levels.
Hemoglobin A1c will remain a primary end point for future studies; however, secondary end points could include hypoglycemic events, hyperglycemic events, weight changes, lipid changes, time to achieve target goals, or combined end points such as a decrease in A1C plus hypoglycemic events. In addition, other biochemical and anatomic risk factors for cardiovascular disease might eventually also become targets for improvement in future clinical trials of SMBG.
Telemetry, telemedicine, and social media all have the potential to add value to SMBG and should be incorporated into selected future trials.
Patient-centered end points determined by validated questionnaires offer unique opportunities for strengthening the value-added case for SMBG. Some such instruments include diabetes distress index, treatment satisfaction, and fear of hypo-glycemia. These types of patient-centered end points, coupled with measures of adherence both pharmacologic and lifestyle, would strengthen the case of SMBG even when A1C changes appear relatively modest.
Treatment Algorithms
Future trials of SMBG in NIT individuals should be planned in order to assess how well this intervention can perform under optimal usage. Methodological flaws must be avoided, such as a sample size that is too small, a low baseline A1C, or failure to provide an educational program. Most importantly, the approach to SMBG should be structured for the patient, which means that SMBG should be linked to a short, simple, and clear educational program designed to facilitate behavior change to improve glucose readings. In NIT T2DM patients, this program should be focused mainly on (1) monitoring PPG levels and providing specific training in reducing meal carbohydrate load if these values are off the target and (2) monitoring preprandial BG levels and providing specific personalized training for increasing physical activity in order to improve noon or dinner BG readings if they are off target. Self-monitoring of blood glucose interventions should aim to improve patient empowerment. An additional component of a SMBG intervention could be encouragement and motivational support by the HCP to increase the frequency of structured testing and/or greater compliance with the use of increased doses of BG-lowering medications specified by treatment algorithms.
Primary End Points
A1C
To assess mean glycemia, A1C will remain the key primary end point in clinical trials of SMBG in NIT T2DM. Self-monitoring of blood glucose data can provide additional end points such as hypoglycemic events or combined end points (A1C plus hypoglycemic events) in subjects treated with insulin secretagogues. Other measures of GV might eventually become adopted for therapeutic interventions, and these can then also be applied to trials of SMBG, although the link between BG fluctuations and oxidative stress is controversial.97,98 Additional studies in a variety of populations with different characteristics would be helpful to determine which patients are most likely to benefit.
While A1C will remain the primary end point for diabetes therapy trials for the foreseeable future, the ability to show striking reductions (>1%) will become limited if the mean A1C of the diabetes population falls. The clinical usefulness of A1C as an end point of a clinical trial is dependent on a tight relationship with prevailing glycemia. However, there is emerging evidence that factors other than glycemia may impact A1C levels. Hemoglobin A1c may be affected by a variety of factors, which make interpretation of A1C levels in certain clinical situations unreliable.99
Major physiological factors that affect A1C levels or measurements of A1C include increased red cell turnover (e.g., hemolytic anemias and malaria), genetic or chemical alterations in hemoglobin (e.g., hemoglobinopathies), alterations in erythropoiesis (e.g., iron and vitamin B12 deficiency increase A1C and administration of erythro-poietin, iron, and vitamin B12 decrease A1C), and alterations in glycation (e.g., alcoholism and chronic renal failure).100 States with an increased mean age of erythrocytes will be associated with increased A1C levels, and states with a decreased mean age of erythrocytes will be associated with decreased A1C levels.101 Determining whether any of these conditions is present can be difficult, and currently, there are no agreed upon procedures and protocols on how to deal with these possibilities. The situation is compounded because the magnitude and direction of the effect of these factors may vary depending upon the type of abnormality (e.g., hemoglobinopathies) and the assay used to measure A1C.
On a population level, racial and ethnic differences in A1C have been described that do not appear to be explained by the differences in glycemia or factors described earlier.102–104 Hemoglobin A1c levels are also subject to seasonal variation, with levels being higher in cooler months and lower in warmer months in both the northern and southern hemispheres.105 Finally, increasing importance should be attributed to composite end points, particularly if they include hypoglycemia incidence and weight change.106,107
Secondary End Points
Several secondary end points, including hypoglycemic events, hyperglycemic events, weight changes, lipid changes, and time to achieve target goals, are worthy targets.108 Additional biochemical and anatomical risk factors for cardiovascular disease might eventually also become targets for improvement in future clinical trials of SMBG. Finally, although combined end points are controversial,106,107 a combination of a decrease in A1C plus a decrease in the incidence of hypoglycemia might be considered as a valid end point for a BG monitoring intervention.
Telemetry
Telemetry is the automated transmission of BG readings to a telecare center and/or physician. Feedback would occur later on a secure Web site or by way of email, text message/short message service, or phone message. Such asynchronous telemetry applications have been successful in improving test values and self-care practices.109 Such responses could provide guidance on medication dosages, diet, and physical exercise.
Telemedicine
Telemedicine uses software incorporating best practices consensus guidelines and standards from professional organizations. Such a system could provide a patient with real-time automated personalized feedback into a smart phone and facilitate patient action to improve metabolic control. This approach offers promise as a tool for affecting behavioral change. Several systems combining SMBG data uploading with instant feedback are under development.110,111 A recent trial in T2DM of real-time behavioral mobile coaching with BG data, lifestyle behaviors, and patient self-management data individually analyzed and presented with evidence-based guidelines to HCPs resulted in reduced A1C levels of 1.9% in an intervention cohort compared with 0.7 % in a usual care cohort, indicating the potential of telemedicine to improve glycemic outcomes in T2DM. The composition of study subjects with respect to whether they were insulin treated or NIT was not specified, except that no subjects used insulin pumps.112
Social Media
There may be opportunities for integrating existing social media into such clinical trials for patient education and motivation. These media also allow for capture of immediate data from participants and subsequently allow for semantic and sentiment analyses to add value to comparisons between tested systems.113
Quality-of-Life Measurement Instruments
Incorporating patient-centered end points into future trials would strengthen the value added case for patient adherence and treatment satisfaction. An effective SMBG intervention might be expected to impact positively on patient-perceived quality of life (QOL; both generic and diabetes specific), overall treatment satisfaction, and fear of hypoglycemia. Specific measures recommended to assess each of these are detailed below.
Quality of life: Many measures are available to assess various QOL aspects, including the presence and severity of mental and physical symptoms, the potential impact on day-to-day functioning (e.g., the SF-36), and broad measures of perceived wellbeing (e.g., the WHO-5). While few SMBG studies have examined such outcomes, one study did measure changes in WHO-5 scores over time. In this study, a significantly greater improvement in general wellbeing was noted by subjects who were adherent to a structured SMBG intervention compared with control subjects who were instructed to only use their meter following their physicians’ recommendations (p < .04).18 The WHO-5 is recommended for future studies in this area.114 It is a brief (only five items), well-validated measure that is used widely in studies and has been used previously to evaluate the impact of an SMBG intervention.
Diabetes-specific QOL: Of the many instruments available, the diabetes distress scale (DDS) is a 17-item self-report measure that assesses the degree of diabetes-specific emotional distress that patients may experience,115 and the 34-item revised diabetes symptom checklist assesses the presence and the degree of burden associated with common diabetes-related physical symptoms.116 The DDS has been previously shown to be sensitive to change in an SMBG intervention.117
Treatment satisfaction: The most commonly used instrument is the diabetes treatment satisfaction questionnaire (DTSQ).118 It is a brief (eight-item), well-validated, and reliable measure. While no treatment satisfaction measure has been included in SMBG studies to date, the DTSQ is recommended for inclusion in future trials.
Fear of hypoglycemia: The hypoglycemic fear survey-II is a 33-item scale that has been widely used for several decades. Validity, reliability, and responsiveness to change have been well-established. Researchers have tended to focus only on the 18-item worry subscale, but evidence suggests that inclusion of the 15-item behavior subscale (how patients may avoid common actives due to fear of hypoglycemia) may also be critical.119 Fear of hypoglycemia has not yet been examined in SMBG intervention studies, but it would definitely be worthy of investigation.
At a minimum, future studies should include measure-ment of generic QOL, using the 5-item WHO-5; diabetes-specific QOL, using the 17-item DDS; and treatment satisfaction, using the 8-item DTSQ.
Conclusions
Key Points
Self-monitoring of blood glucose is an established practice for patients with NIT T2DM, and to be most effective, it should be performed in a structured format where information obtained from this measurement is used to guide treatment.
New, high-quality efficacy data from RCTs have demonstrated efficacy of SMBG in NIT T2DM in trials reported since 2008.
Both patients and HCPs require education on how to respond to the data for SMBG to be effective.
Additional well-defined studies are needed to assess the benefits and costs of SMBG with end points not limited to A1C.
Self-monitoring of blood glucose in T2DM is a widely practiced diagnostic measure. It is important to under-stand how to extract the maximum benefit from this practice. The potential benefits of SMBG in T2DM have been scrutinized, and new insights are emerging. In this report on the role of SMBG in NIT T2DM, the current role of this diagnostic measure has been analyzed from multiple perspectives. Clinical situations have been identified wherein this practice can be helpful or not helpful and wherein additional information is needed to form a judgment on the value of this practice. In theseinstances, methods have been recommended for obtaining additional relevant information about this practice.
Where both epidemiologic and RCTs have reported contradictory results on the benefits of this practice, since 2008, a new paradigm for how this metric is to be conducted has become clear. New high-quality efficacy data from RCTs have been reported that demonstrate efficacy of SMBG in NIT T2DM. Self-monitoring of blood glucose in this patient population must be performed with a structured educational and therapeutic format in response to SMBG data. Furthermore, for SMBG to be effective, both patients and HCPs must know how to respond to the data. Self-monitoring of blood glucose can be compared with CGM, but little data exist from which to reach conclusions as to when CGM should be used in addition to, or in place of, structured SMBG. It is likely that, as data management technology evolves, future trials of SMBG will incorporate telemetry, telemedicine, and social media applications to structured responses to BG data. For SMBG to be most effective, the testing process must be conducted properly in order to avoid preventable measurement errors. It is expected that future embedded data management software and new hardware will extend the capabilities of future BG monitors to provide accurate information and even advice. The reasons for interest in the potential benefits of SMBG is that this practice, when performed properly, can provide valuable information to prevent hypoglycemia, direct lifestyle and medical treatments, and provide patient empowerment. Although SMBG can be used to identify hypoglycemia, it is not a practical or necessary intervention in NIT T2DM patients who are using one or more noninsulin secretagogues; however, this intervention is important for such patients whose regimens include an insulin secretagogue.
Current barriers to proper use of SMBG in NIT T2DM must be overcome through personalized HCP input, careful device design and selection, adoption of data management tools, and thorough analysis of existing outcomes data as well as generation of new data that will focus on proper structured use of this intervention. Knowledge barriers, human factors barriers, logbook barriers, and economic barriers all limit adoption of SMBG in this patient population. Efforts will be needed from HCPs, payers, and patients themselves for this intervention to remain widely available and to be demonstrably useful in the management of the disease. As future clinical trials are planned to assess the potential clinical benefits and economic implications of SMBG in NIT T2DM patients, new end points will need to be considered. It will be necessary to go beyond simply measuring A1C, which is an accepted surrogate end point for mean long-term glycemia but which can also be affected by various physiological, population, and seasonal factors. Examples of alternate end points that might be useful in future trials of this intervention include rates and severity of hyperglycemia and hypo-glycemia, which relate to the magnitude of GV; weight change; lipid changes; and time to therapeutic success. Patient-centered end points determined by validated questionnaires can also provide insight into QOL, treatment satisfaction, or fear of hypoglycemia associated with use of an SMBG intervention.
It cannot be overemphasized that appropriate and success-ful use of SMBG requires integration of the efforts of a diabetes health care team. This activity must combine proper operational skills by the patient, proper analytical performance by the BG monitor, development of simple data management tools by the device manufacturer, proper education accompanied by structured responses to glycemia by the HCP, and adequate reimbursement by the payer.
In conclusion, SMBG is a useful component of a manage-ment regimen for NIT T2DM if it is combined with a structured education and treatment intervention.
Acknowledgments
The authors would like to acknowledge the following people for their help with this article: Lisa Aurand, M.D., Riad Dirani, Ph.D., Serge Jabbour, M.D., FACP, FACE, Jeffrey Joseph, D.O., Praveen Raja, Ph.D., Holly Schachner, M.D., David Simmons, M.D., and Matthias Axel Schweitzer, M.D., M.B.A.
Glossary
Abbreviations
- (A1C)
hemoglobin A1c
- (AACE/ACE)
American Association of Clinical Endocrinologists/American College of Endocrinology
- (ACG)
active control group
- (ADA)
American Diabetes Association
- (AGI)
alpha glucosidase inhibitor
- (BAS)
bile acid sequestrant
- (BG)
blood glucose
- (CCR-SMBG)
Coalition for Clinical Research—Self-Monitoring of Blood Glucose
- (CGM)
continuous glucose monitoring
- (DA)
dopamine agonist
- (DDS)
diabetes distress scale
- (DPP-4)
dipeptidyl peptidase-4
- (DTSQ)
diabetes treatment satisfaction questionnaire
- (GLP-1)
glucagon-like peptide-1
- (GV)
glycemic variability
- (HCP)
health care professional
- (IDF)
International Diabetes Federation
- (NIT)
non-insulin-treated
- (PPG)
postprandial glucose
- (QOL)
quality of life
- (RCT)
randomized controlled trial
- (SMBG)
self-monitoring of blood glucose
- (STG)
structured testing group
- (T1DM)
type 1 diabetes mellitus
- (T2DM)
type 2 diabetes mellitus
References
- 1.Barnard KD, Young AJ, Waugh NR. Self monitoring of blood glucose - a survey of diabetes UK members with type 2 diabetes who use SMBG. BMC Res Notes. 2010;3:318. doi: 10.1186/1756-0500-3-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garg S, Hirsch IB. Self-monitoring of blood glucose. Int J Clin Pract Suppl. 2010;166:1–10. doi: 10.1111/j.1742-1241.2009.02271.x. [DOI] [PubMed] [Google Scholar]
- 3.American Diabetes Association. Standards of medical care in diabetes–2011. Diabetes Care. 2011;34(Suppl 1):S11–61. doi: 10.2337/dc11-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farmer A, Wade A, Goyder E, Yudkin P, French D, Craven A, Holman R, Kinmonth AL, Neil A. Impact of self monitoring of blood glucose in the management of patients with non-insulin treated diabetes: open parallel group randomised trial. BMJ. 2007;335(7611):132. doi: 10.1136/bmj.39247.447431.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Kane MJ, Bunting B, Copeland M, Coates VE, ESMON study group Efficacy of self monitoring of blood glucose in patients with newly diagnosed type 2 diabetes (ESMON study): randomised controlled trial. BMJ. 2008;336(7654):1174–1177. doi: 10.1136/bmj.39534.571644.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kleefstra N, Hortensius J, Logtenberg SJ, Slingerland RJ, Groenier KH, Houweling ST, Gans RO, van Ballegooie E, Bilo HJ. Self-monitoring of blood glucose in tablet-treated type 2 diabetic patients (ZODIAC) Neth J Med. 2010;68(1):311–316. [PubMed] [Google Scholar]
- 7.Klonoff DC, Bergenstal R, Blonde L, Boren SA, Church TS, Gaffaney J, Jovanovic L, Kendall DM, Kollman C, Kovatchev BP, Leippert C, Owens DR, Polonsky WH, Reach G, Renard E, Riddell MC, Rubin RR, Schnell O, Siminiero LM, Vigersky RA, Wilson DM, Wollitzer AO. Consensus report of the coalition for clinical research-self-monitoring of blood glucose. J Diabetes Sci Technol. 2008;2(6):1030–1053. doi: 10.1177/193229680800200612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schnell O, Alawi H, Battelino T, Ceriello A, Diem P, Felton A, Grzeszczak W, Harno K, Kempler P, Satman I, Vergès B. Addressing schemes of self-monitoring of blood glucose in type 2 diabetes: a European perspective and expert recommendation. Diabetes Technol Ther. 2011;13(9):959–965. doi: 10.1089/dia.2011.0028. [DOI] [PubMed] [Google Scholar]
- 9.Davis WA, Bruce DG, Davis TM. Does self-monitoring of blood glucose improve outcome in type 2 diabetes? The Fremantle Diabetes Study. Diabetologia. 2007;50(3):510–515. doi: 10.1007/s00125-006-0581-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tengblad A, Grodzinsky E, Lindström K, Mölstad S, Borgquist L, Ostgren CJ. Self-monitoring of blood glucose and glycaemic control in type 2 diabetes. Scand J Prim Health Care. 2007;25(3):140–146. doi: 10.1080/02813430701267413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodríguez A, Calle A, Vázquez L, Chacón F, Polavieja P, Reviriego J, CADiNI Study Group Blood glucose control and quality of health care in non-insulin-treated patients with type 2 diabetes in Spaa retrospective and cross-sectional observational study. Diabet Med. 2011;28(6):731–740. doi: 10.1111/j.1464-5491.2011.03258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karter AJ, Parker MM, Moffet HH, Spence MM, Chan J, Ettner SL, Selby JV. Longitudinal study of new and prevalent use of self-monitoring of blood glucose. Diabetes Care. 2006;29(8):1757–1763. doi: 10.2337/dc06-2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin S, Schneider B, Heinemann L, Lodwig V, Kurth HJ, Kolb H, Scherbaum WA. Self-monitoring of blood glucose in type 2diabetes and long-term outcome: an epidemiological cohort study. Diabetologia. 2006;49(2):271–278. doi: 10.1007/s00125-005-0083-5. [DOI] [PubMed] [Google Scholar]
- 14.Poolsup N, Suksomboon N, Jiamsathit W. Systematic review of the benefits of self-monitoring of blood glucose on glycemic control in type 2 diabetes patients. Diabetes Technol Ther. 2008;10(Suppl 1):S51–66. doi: 10.1089/dia.2009.0091. [DOI] [PubMed] [Google Scholar]
- 15.Towfigh A, Romanova M, Weinreb JE, Munjas B, Suttorp MJ, Zhou A, Shekelle PG. Self-monitoring of blood glucose levels in patients with type 2 diabetes mellitus not taking insula meta-analysis. Am J Manag Care. 2008;14(7):468–475. [PubMed] [Google Scholar]
- 16.St John A, Davis WA, Price CP, Davis TM. The value of self-monitoring of blood glucose: a review of recent evidence. J Diabetes Complications. 2010;24(2):129–141. doi: 10.1016/j.jdiacomp.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 17.Clar C, Barnard K, Cummins E, Royle P, Waugh N, Aberdeen Health Technology Assessment Group Self-monitoring of blood glucose in type 2 diabetes: systematic review. Health Technol Assess. 2010;14(12):1–140. doi: 10.3310/hta14120. [DOI] [PubMed] [Google Scholar]
- 18.Polonsky WH, Fisher L, Schikman CH, Hinnen DA, Parkin CG, Jelsovsky Z, Petersen B, Schweitzer M, Wagner RS. Structured self-monitoring of blood glucose significantly reduces A1C levels in poorly controlled, noninsulin-treated type 2 diabetes: results from the Structured Testing Program study. Diabetes Care. 2011;34(2):262–267. doi: 10.2337/dc10-1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Franciosi M, Lucisano G, Pellegrini F, Cantarello A, Consoli A, Cucco L, Ghidelli R, Sartore G, Sciangula L, Nicolucci A ROSES Study Group. ROSES: role of self-monitoring of blood glucose and intensive education in patients with Type 2 diabetes not receiving insulin. A pilot randomized clinical trial. Diabet Med. 2011;28(7):789–796. doi: 10.1111/j.1464-5491.2011.03268.x. [DOI] [PubMed] [Google Scholar]
- 20.Durán A, Martín P, Runkle I, Pérez N, Abad R, Fernández M, Del Valle L, Sanz MF, Calle-Pascual AL. Benefits of self-monitoring blood glucose in the management of new-onset type 2 diabetes mellitus: the St Carlos Study, a prospective randomized clinic-based interventional study with parallel groups. J Diabetes. 2010;2(3):203–211. doi: 10.1111/j.1753-0407.2010.00081.x. [DOI] [PubMed] [Google Scholar]
- 21.Kerr D. Poor numeracy: the elephant in the diabetes technology room. J Diabetes Sci Technol. 2010;4(6):1284–1287. doi: 10.1177/193229681000400601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Handelsman Y, Mechanick JI, Blonde L, Grunberger G, Bloom-garden ZT, Bray GA, Dagogo-Jack S, Davidson JA, Einhorn D, Ganda O, Garber AJ, Hirsch IB, Horton ES, Ismail-Beigi F, Jellinger PS, Jones KL, Jovanovič L, Lebovitz H, Levy P, Moghissi ES, Orzeck EA, Vinik AI, Wyne KL, AACE Task Force for Developing Diabetes Comprehensive Care Plan American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for developing a diabetes mellitus comprehensive care plan. Endocr Pract. 2011;17(Suppl 2):1–53. doi: 10.4158/ep.17.s2.1. [DOI] [PubMed] [Google Scholar]
- 23.Nicholson G, Hall GM. Diabetes mellitus: new drugs for a new epidemic. Br J Anaesth. 2011;107(1):65–73. doi: 10.1093/bja/aer120. [DOI] [PubMed] [Google Scholar]
- 24.Bennett WL, Maruthur NM, Singh S, Segal JB, Wilson LM, Chatterjee R, Marinopoulos SS, Puhan MA, Ranasinghe P, Block L, Nicholson WK, Hutfless S, Bass EB, Bolen S. Comparative effective-ness and safety of medications for type 2 diabetes: an update including new drugs and 2-drug combinations. Ann Intern Med. 2011;154(9):602–613. doi: 10.7326/0003-4819-154-9-201105030-00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Centers for Disease Control and Prevention. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf. Accessed October 23, 2011.
- 26. U.S. Department of Veterans Affairs. Management of diabetes mellitus in primary care. http://www.healthquality.va.gov/Diabetes_Mellitus.asp. Accessed October 23, 2011.
- 27. International Diabetes Federation Guideline on Self-Monitoring of Blood Glucose in Non-Insulin Treated Type 2 Diabetes. www.idf.org/website/docs/guideline_pmg_final.pdf. Accessed October 23, 2011.
- 28. Scottish Intercollegiate Guidelines Network. Management of diabetes: a national clinical guideline. http://www.sign.ac.uk/pdf/sign116.pdf. Accessed October 23, 2011.
- 29. The National Collaborating Centre for Chronic Conditions. Type 2 diabetes: national clinical guideline for management in primary and secondary care (update). http://www.nice.org.uk/nicemedia/live/11983/40803/40803.pdf. Accessed October 23, 2011.
- 30.Borg R, Kuenen JC, Carstensen B, Zheng H, Nathan DM, Heine RJ, Nerup J, Borch-Johnsen K, Witte DR, ADAG Study Group Associations between features of glucose exposure and A1C: the A1C-Derived Average Glucose (ADAG) study. Diabetes. 2010;59(7):1585–1590. doi: 10.2337/db09-1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brownlee M, Hirsch IB. Glycemic variability: a hemoglobin A1c-independent risk factor for diabetic complications. JAMA. 2006;295(14):1707–1708. doi: 10.1001/jama.295.14.1707. [DOI] [PubMed] [Google Scholar]
- 32.Monnier L, Lapinski H, Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA(1c) Diabetes Care. 2003;26(3):881–885. doi: 10.2337/diacare.26.3.881. [DOI] [PubMed] [Google Scholar]
- 33.Testa MAB L, Gill J, Turner R, Simonson D. Decreased glycemic variability during insulin therapy improves patient-centered outcomes in type 1 and type 2 diabetes. 70th Scientific Sessions of the American Diabetes Association 2010; Poster 1 LB. [Google Scholar]
- 34.Scott R, Loeys T, Davies MJ, Engel SS, Sitagliptin Study 801 Group Efficacy and safety of sitagliptin when added to ongoing metformin therapy in patients with type 2 diabetes. Diabetes Obes Metab. 2008;10(10):949–969. doi: 10.1111/j.1463-1326.2007.00839.x. [DOI] [PubMed] [Google Scholar]
- 35.Rosenstock J, Inzucchi SE, Seufert J, Fleck PR, Wilson CA, Mekki Q. Initial combination therapy with alogliptin and pioglitazone in drug-naïve patients with type 2 diabetes. Diabetes Care. 2010;33(11):2406–2408. doi: 10.2337/dc10-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williams-Herman D, Engel SS, Round E, Johnson J, Golm GT, Guo H, Musser BJ, Davies MJ, Kaufman KD, Goldstein BJ. Safety and tolerability of sitagliptin in clinical studies: a pooled analysis of data from 10,246 patients with type 2 diabetes. BMC Endocr Disord. 2010;10:7. doi: 10.1186/1472-6823-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blonde L, Russell-Jones D. The safety and efficacy of liraglutide with or without oral antidiabetic drug therapy in type 2 diabetes: an overview of the LEAD 1-5 studies. Diabetes Obes Metab. 2009;11(Suppl 3):26–34. doi: 10.1111/j.1463-1326.2009.01075.x. [DOI] [PubMed] [Google Scholar]
- 38.Linnebjerg H, Kothare PA, Skrivanek Z, de la Peña A, Atkins M, Ernest CS, Trautmann ME. Exenatide: effect of injection time on postprandial glucose in patients with type 2 diabetes. Diabet Med. 2006;23(3):240–245. doi: 10.1111/j.1464-5491.2006.01800.x. [DOI] [PubMed] [Google Scholar]
- 39.Chiasson JL, Josse RG, Hunt JA, Palmason C, Rodger NW, Ross SA, Ryan EA, Tan MH, Wolever TM. The efficacy of acarbose in the treatment of patients with non-insulin-dependent diabetes mellitus. A multicenter controlled clinical trial. Ann Intern Med. 1994;121(12):928–935. doi: 10.7326/0003-4819-121-12-199412150-00004. [DOI] [PubMed] [Google Scholar]
- 40.Gaziano JM, Cincotta AH, O’Connor CM, Ezrokhi M, Rutty D, Ma ZJ, Scranton RE. Randomized clinical trial of quick-release bromocriptine among patients with type 2 diabetes on overall safety and cardiovascular outcomes. Diabetes Care. 2010;33(7):1503–1508. doi: 10.2337/dc09-2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bays HE, Goldberg RB, Truitt KE, Jones MR. Colesevelam hydro-chloride therapy in patients with type 2 diabetes mellitus treated with metformglucose and lipid effects. Arch Intern Med. 2008;168(18):1975–1983. doi: 10.1001/archinte.168.18.1975. [DOI] [PubMed] [Google Scholar]
- 42.Wysham C, Bergenstal R, Malloy J, Yan P, Walsh B, Malone J, Taylor K. DURATION-2: efficacy and safety of switching from maximum daily sitagliptin or pioglitazone to once-weekly exenatide. Diabet Med. 2011;28(6):705–714. doi: 10.1111/j.1464-5491.2011.03301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pfützner A, Schöndorf T, Tschöpe D, Lobmann R, Merke J, Müller J, Lehmann U, Fuchs W, Forst T. PIOfix-Study: Effects of Pioglitazone/Metformin fixed combination in comparison with a combination of metformin with glimepiride on diabetic dyslipidemia. Diabetes Technol Ther. 2011;13(6):637–643. doi: 10.1089/dia.2010.0233. [DOI] [PubMed] [Google Scholar]
- 44.Bolli G, Dotta F, Rochotte E, Cohen SE. Efficacy and tolerability of vildagliptin vs. pioglitazone when added to metforma 24-week, randomized, double-blind study. Diabetes Obes Metab. 2008;10(1):82–90. doi: 10.1111/j.1463-1326.2007.00820.x. [DOI] [PubMed] [Google Scholar]
- 45.Vlckova V, Cornelius V, Kasliwal R, Wilton L, Shakir S. Hypo-glycaemia with pioglitazone: analysis of data from the prescription-event monitoring study. J Eval Clin Pract. 2010;16(6):1124–1128. doi: 10.1111/j.1365-2753.2009.01280.x. [DOI] [PubMed] [Google Scholar]
- 46.Bergenstal RM, Wysham C, Macconell L, Malloy J, Walsh B, Yan P, Wilhelm K, Malone J, Porter LE, DURATION-2 Study Group Efficacy and safety of exenatide once weekly versus sitagliptin or pioglitazone as an adjunct to metformin for treatment of type 2 diabetes (DURATION-2): a randomized trial. Lancet. 2010;376(9739):431–439. doi: 10.1016/S0140-6736(10)60590-9. [DOI] [PubMed] [Google Scholar]
- 47.Buse JB, Rosenstock J, Sesti G, Schmidt WE, Montanya E, Brett JH, Zychma M, Blonde L, LEAD-6 Study Group Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomized, parallel-group, multinational, open-label trial (LEAD-6) Lancet. 2009;374(9683):39–47. doi: 10.1016/S0140-6736(09)60659-0. [DOI] [PubMed] [Google Scholar]
- 48.Liutkus J, Rosas Guzman J, Norwood P, Pop L, Northrup J, Cao D, Trautmann M. A placebo-controlled trial of exenatide twice-daily added to thiazolidinediones alone or in combination with metformin. Diabetes Obes Metab. 2010;12(12):1058–1065. doi: 10.1111/j.1463-1326.2010.01251.x. [DOI] [PubMed] [Google Scholar]
- 49.Rosenstock J, Brown A, Fischer J, Jain A, LittleJohn T, Nadeau D, Sussman A, Taylor T, Krol A, Magner J. Efficacy and safety of acarbose in metformin-treated patients with type 2 diabetes. Diabetes Care. 1998;21(12):2050–2055. doi: 10.2337/diacare.21.12.2050. [DOI] [PubMed] [Google Scholar]
- 50.Jayaram S, Hariharan RS, Madhavan R, Periyandavar I, Samra SS. A prospective, parallel group, open-labeled, comparative, multi-centric, active controlled study to evaluate the safety, tolerability and benefits of fixed dose combination of acarbose and metformin versus metformin alone in type 2 diabetes. J Assoc Physicians India. 2010;58:679–682. [PubMed] [Google Scholar]
- 51.Lin SD, Wang JS, Hsu SR, Sheu WH, Tu ST, Lee IT, Su SL, Lin SY, Wang SY, Hsieh MC. The beneficial effect of a-glucosidase inhibitor on glucose variability compared with sulfonylurea in Taiwanese type 2 diabetic patients inadequately controlled with metformpreliminary data. J Diabetes Complications. 2011;25(5):332–338. doi: 10.1016/j.jdiacomp.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 52.Zieve FJ, Kalin ME, Schwartz SL, Jones MR, Bailey WL. Results of the glucose-lowering effect of WelChol study (GLOWS): a randomized, double-blind, placebo-controlled pilot study evaluating the effect of colesevelam hydrochloride on glycemic control in subjects with type 2 diabetes. Clin Ther. 2007;29(1):74–83. doi: 10.1016/j.clinthera.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 53.Kim SW, Baik SH, Yoon KH, Lee HW, Filozof C. Efficacy and safety of vildagliptin/pioglitazone combination therapy in Korean patients with diabetes. World J Diabetes. 2010;1(5):153–160. doi: 10.4239/wjd.v1.i5.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rosenstock J, Brazg R, Andryuk PF, Lu K, Stein P, sitagliptin study 019 group Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing pioglitazone therapy in patients with type 2 diabetes: a 24-week, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther. 2006;28(10):1556–1568. doi: 10.1016/j.clinthera.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 55.Zinman B, Gerich J, Buse JB, Lewin A, Schwartz S, Raskin P, Hale PM, Zdravkovic M, Blonde L, LEAD-4 Study Investigators Efficacy and safety of the human glucagon-like peptide-1 analog Liraglutide in combination with Metformin and Thiazolidinedione in patients With Type 2 Diabetes (LEAD-4 Met+TZD) Diabetes Care. 2009;32(7):1224–1230. doi: 10.2337/dc08-2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cox DJ, Gonder-Frederick L, Ritterband L, Clarke W, Kovatchev BP. Prediction of severe hypoglycemia. Diabetes Care. 2007;30(6):1370–1373. doi: 10.2337/dc06-1386. [DOI] [PubMed] [Google Scholar]
- 57.Rodbard HW, Jellinger PS, Davidson JA, Einhorn D, Garber AJ, Grunberger G, Handelsman Y, Horton ES, Lebovitz H, Levy P, Moghissi ES, Schwartz SS. Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control. Endocr Pract. 2009;15(6):540–559. doi: 10.4158/EP.15.6.540. [DOI] [PubMed] [Google Scholar]
- 58.Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, Zinman B, American Diabetes Association; European Association for Study of Diabetes Medical management of hyper-glycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32(1):193–203. doi: 10.2337/dc08-9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. National Institute for Health and Clinical Excellence. Type 2 diabetes - newer agents. (partial update of CG66). http://guidance.nice.org.uk/CG87. Accessed October 23, 2011.
- 60. Scottish Intercollegiate Guidelines Network. Management of diabetes. http://www.sign.ac.uk/guidelines/fulltext/116/index.html. AccessedOctober 23, 2011.
- 61. VA/DoD Clinical Practice Guideline. Management of diabetes mellitus(2010). http://www.healthquality.va.gov/diabetes/DM2010_FUL-v4e.pdf. Accessed October 23, 2011.
- 62.Gerich J, Raskin P, Jean-Louis L, Purkayastha D, Baron MA. PRESERVE-beta: two-year efficacy and safety of initial combination therapy with nateglinide or glyburide plus metformin. Diabetes Care. 2005;28(9):2093–2099. doi: 10.2337/diacare.28.9.2093. [DOI] [PubMed] [Google Scholar]
- 63.Nauck MA, Meininger G, Sheng D, Terranella L, Stein PP, Sitagliptin Study 024 Group Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, compared with the sulfonylurea, glipizide, in patients with type 2 diabetes inadequately controlled on metformin alone: a randomized, double-blind, non-inferiority trial. Diabetes Obes Metab. 2007;9(2):194–205. doi: 10.1111/j.1463-1326.2006.00704.x. [DOI] [PubMed] [Google Scholar]
- 64.Seck T, Nauck M, Sheng D, Sunga S, Davies MJ, Stein PP, Kaufman KD, Amatruda JM, Sitagliptin Study 024 Group Safety and efficacy of treatment with sitagliptin or glipizide in patients with type 2 diabetes inadequately controlled on metforma 2-year study. Int J Clin Pract. 2010;64(5):562–576. doi: 10.1111/j.1742-1241.2010.02353.x. [DOI] [PubMed] [Google Scholar]
- 65.Kipnes MS, Krosnick A, Rendell MS, Egan JW, Mathisen AL, Schneider RL. Pioglitazone hydrochloride in combination with sulfonylurea therapy improves glycemic control in patients with type 2 diabetes mellitus: a randomized, placebo-controlled study. Am J Med. 2001;111(1):10–17. doi: 10.1016/s0002-9343(01)00713-6. [DOI] [PubMed] [Google Scholar]
- 66.Rajagopalan R, Perez A, Ye Z, Khan M, Murray FT. Pioglitazone is effective therapy for elderly patients with type 2 diabetes mellitus. Drugs Aging. 2004;21(4):259–271. doi: 10.2165/00002512-200421040-00004. [DOI] [PubMed] [Google Scholar]
- 67.Marre M, Shaw J, Brändle M, Bebakar WM, Kamaruddin NA, Strand J, Zdravkovic M, Le Thi TD, Colagiuri S, LEAD-1 SU study group Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with type 2 diabetes (LEAD-1 SU) Diabet Med. 2009;26(3):268–278. doi: 10.1111/j.1464-5491.2009.02666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hermansen K, Kipnes M, Luo E, Fanurik D, Khatami H, Stein P, Sitagliptin Study 035 Group Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, in patients with type 2 diabetes mellitus inadequately controlled on glimepiride alone or on glimepiride and metformin. Diabetes Obes Metab. 2007;9(5):733–745. doi: 10.1111/j.1463-1326.2007.00744.x. [DOI] [PubMed] [Google Scholar]
- 69.Bachmann W, Petzinna D, Raptis SA, Wascher T, Westermeier T, European Acarbose Study Group Long-term improvement of metabolic control by acarbose in type 2 diabetes patients poorly controlled with maximum sulfonylurea therapy. Clin Drug Investig. 2003;23(10):679–686. doi: 10.2165/00044011-200323100-00007. [DOI] [PubMed] [Google Scholar]
- 70.Scherbaum WA, Ohmann C, Abholz HH, Dragano N, Lankisch M. Effect of the frequency of self-monitoring blood glucose in patients with type 2 diabetes treated with oral antidiabetic drugs-a multi-centre, randomized controlled trial. PLoS One. 2008;3(8):e3087. doi: 10.1371/journal.pone.0003087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pickup JC, Freeman SC, Sutton AJ. Glycaemic control in type 1 diabetes during real time continuous glucose monitoring compared with self monitoring of blood glucose: meta-analysis of randomised controlled trials using individual patient data. BMJ. 2011;343:d3805. doi: 10.1136/bmj.d3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Weber C, Schnell O. The assessment of glycemic variability and its impact on diabetes-related complications: an overview. Diabetes Technol Ther. 2009;11(10):623–633. doi: 10.1089/dia.2009.0043. [DOI] [PubMed] [Google Scholar]
- 73.Standl E, Schnell O, Ceriello A. Postprandial hyperglycemia and glycemic variability: should we care? Diabetes Care. 2011;34(Suppl 2):S120–7. doi: 10.2337/dc11-s206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Marling CR, Shubrook JH, Vernier SJ, Wiley MT, Schwartz FL. Characterizing blood glucose variability using new metrics with continuous glucose monitoring data. J Diabetes Sci Technol. 2011;5(4):871–878. doi: 10.1177/193229681100500408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ehrhardt NM, Chellappa M, Walker MS, Fonda SJ, Vigersky RA. The effect of real-time continuous glucose monitoring on glycemic control in patients with type 2 diabetes mellitus. J Diabetes Sci Technol. 2011;5(3):668–675. doi: 10.1177/193229681100500320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vigersky RA, Fonda SJ, Chellappa M, Walker MS, Ehrhardt NM. Short- and long-term effects of real-time continuous glucose monitoring in patients with type 2 diabetes mellitus. Diabetes Care. doi: 10.2337/dc11-1438. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Plebani M. Exploring the iceberg of errors in laboratory medicine. Clin Chim Acta. 2009;404(1):16–23. doi: 10.1016/j.cca.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 78.Krouwer JS, Cembrowski GS. A review of standards and statistics used to describe blood glucose monitor performance. J Diabetes Sci Technol. 2010;4(1):75–83. doi: 10.1177/193229681000400110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dungan K, Chapman J, Braithwaite SS, Buse J. Glucose measure-ment: confounding issues in setting targets for inpatient management. Diabetes Care. 2007;30(2):403–409. doi: 10.2337/dc06-1679. [DOI] [PubMed] [Google Scholar]
- 80.Ginsberg BH. Factors affecting blood glucose monitoring: sources of errors in measurement. J Diabetes Sci Technol. 2009;3(4):903–913. doi: 10.1177/193229680900300438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tonyushkina K, Nichols JH. Glucose meters: a review of technical challenges to obtaining accurate results. J Diabetes Sci Technol. 2009;3(4):971–980. doi: 10.1177/193229680900300446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Klonoff DC, Perz JF. Assisted monitoring of blood glucose: special safety needs for a new paradigm in testing glucose. J Diabetes Sci Technol. 2010;4(5):1027–1031. doi: 10.1177/193229681000400501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bamberg R, Schulman K, MacKenzie M, Moore J, Olchesky S. Effect of adverse storage conditions on performance of glucometer test strips. Clin Lab Sci. 2005;18(4):203–209. [PubMed] [Google Scholar]
- 84.Dungan K, Chapman J, Braithwaite SS, Buse J. Glucose measure-ment: confounding issues in setting targets for inpatient management. Diabetes Care. 2007;30(2):403–409. doi: 10.2337/dc06-1679. [DOI] [PubMed] [Google Scholar]
- 85.Heinemann L. Quality of glucose measurement with blood glucose meters at the point-of-care: relevance of interfering factors. Diabetes Technol Ther. 2010;12(11):847–857. doi: 10.1089/dia.2010.0076. [DOI] [PubMed] [Google Scholar]
- 86.Perera NJ, Stewart PM, Williams PF, Chua EL, Yue DK, Twigg SM. The danger of using inappropriate point-of-care glucose meters in patients on icodextrin dialysis. Diabet Med. 2011;28(10):1272–1276. doi: 10.1111/j.1464-5491.2011.03362.x. [DOI] [PubMed] [Google Scholar]
- 87.Klonoff DC. The food and drug administration is now preparing to establish tighter performance requirements for blood glucose monitors. J Diabetes Sci Technol. 2010;4(3):499–504. doi: 10.1177/193229681000400301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chudyk A, Shapiro S, Russell-Minda E, Petrella R. Self-monitoring technologies for type 2 diabetes and the prevention of cardiovascular complications: perspectives from end users. J Diabetes Sci Technol. 2011;5(2):394–401. doi: 10.1177/193229681100500229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Malemute CL, Shultz JA, Ballejos M, Butkus S, Early KB. Goal setting education and counseling practices of diabetes educators. Diabetes Educ. 2011;37(4):549–563. doi: 10.1177/0145721711410718. [DOI] [PubMed] [Google Scholar]
- 90.Uslan M, Blubaugh M. Analysis: including visually impaired participants in validation design studies of diabetes technology. J Diabetes Sci Technol. 2010;4(5):1236–1237. doi: 10.1177/193229681000400524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Williams AS. Universal design in diabetes care: an idea whose time has come. Diabetes Educ. 2009;35(1):45–57. doi: 10.1177/0145721708329700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kazlauskaite R, Soni S, Evans AT, Graham K, Fisher B. Accuracy of self-monitored blood glucose in type 2 diabetes. Diabetes Technol Ther. 2009;11(6):385–392. doi: 10.1089/dia.2008.0111. Jun. [DOI] [PubMed] [Google Scholar]
- 93.Rodbard D. Display of glucose distributions by date, time of day, and day of week: new and improved methods. J Diabetes Sci Technol. 2009;3(6):1388–1394. doi: 10.1177/193229680900300619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tunis SL. Cost effectiveness of self-monitoring of blood glucose (SMBG) for patients with type 2 diabetes and not on insulimpact of modelling assumptions on recent Canadian findings. Appl Health Econ Health Policy. 2011 doi: 10.2165/11594270-000000000-00000. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 95.Kaufman N. Internet and information technology use in treatment of diabetes. Int J Clin Pract Suppl. 2010;166:41–46. doi: 10.1111/j.1742-1241.2009.02277.x. [DOI] [PubMed] [Google Scholar]
- 96.Adams AS, Mah C, Soumerai SB, Zhang F, Barton MB, Ross-Degnan D. Barriers to self-monitoring of blood glucose among adults with diabetes in an HMO: a cross sectional study. BMC Health Serv Res. 2003;3(1):6. doi: 10.1186/1472-6963-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Siebel AL, Fernandez AZ, El-Osta A. Glycemic memory associated epigenetic changes. Biochem Pharmacol. 2010;80(12):1853–1859. doi: 10.1016/j.bcp.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 98.Siegelaar SE, Barwari T, Kulik W, Hoekstra JB, DeVries HJ. No relevant relationship between glucose variability and oxidative stress in well-regulated type 2 diabetes patients. J Diabetes Sci Technol. 2011;5(1):86–92. doi: 10.1177/193229681100500112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wilson DM, Xing D, Cheng J, Beck RW, Hirsch I, Kollman C, Laffel L, Lawrence JM, Mauras N, Ruedy KJ, Tsalikian E, Wolpert H, Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group Persistence of individual variations in glycated hemoglobanalysis of data from the Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Randomized Trial. Diabetes Care. 2011;34(6):1315–1317. doi: 10.2337/dc10-1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bonora E, Tuomilehto J. The pros and cons of diagnosing diabetes with A1C. Diabetes Care. 2011;34(Suppl 2):S184–90. doi: 10.2337/dc11-s216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gallagher EJ, Le Roith D, Bloomgarden Z. Review of hemoglobin A(1c) in the management of diabetes. J Diabetes. 2009;1(1):9–17. doi: 10.1111/j.1753-0407.2009.00009.x. [DOI] [PubMed] [Google Scholar]
- 102.Herman WH. Do race and ethnicity impact hemoglobin A1c independent of glycemia? J Diabetes Sci Technol. 2009;3(4):656–660. doi: 10.1177/193229680900300406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ziemer DC, Kolm P, Weintraub WS, Vaccarino V, Rhee MK, Twombly JG, Narayan KM, Koch DD, Phillips LS. Glucose-independent, black-white differences in hemoglobin A1c levels: a cross-sectional analysis of 2 studies. Ann Intern Med. 2010;152(12):770–777. doi: 10.7326/0003-4819-152-12-201006150-00004. [DOI] [PubMed] [Google Scholar]
- 104.Davidson MB, Schriger DL. Effect of age and race/ethnicity on HbA1c levels in people without known diabetes mellitus: implications for the diagnosis of diabetes. Diabetes Res Clin Pract. 2010;87(3):415–421. doi: 10.1016/j.diabres.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 105.Higgins T, Saw S, Sikaris K, Wiley CL, Cembrowski GC, Lyon AW, Khajuria A, Tran D. Seasonal variation in hemoglobin A1c: is it the same in both hemispheres? J Diabetes Sci Technol. 2009;3(4):668–671. doi: 10.1177/193229680900300408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Muchmore D. The end point is just the beginning. J Diabetes Sci Technol. 2011;5(5):1287–1289. doi: 10.1177/193229681100500538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fleming GA. Counterpoint–the end point: less is more. J Diabetes Sci Technol. 2011;5(5):1290–1293. doi: 10.1177/193229681100500539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rajpathak SN, Aggarwal V, Hu FB. Multifactorial intervention to reduce cardiovascular events in type 2 diabetes. Curr Diab Rep. 2010;10(1):16–23. doi: 10.1007/s11892-009-0084-8. [DOI] [PubMed] [Google Scholar]
- 109.Verhoeven F, Tanja-Dijkstra K, Nijland N, Eysenbach G, van Gemert-Pijnen L. Asynchronous and synchronous teleconsultation for diabetes care: a systematic literature review. J Diabetes Sci Technol. 2010;4(3):666–684. doi: 10.1177/193229681000400323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Charpentier G, Benhamou PY, Dardari D, Clergeot A, Franc S, Schaepelynck-Belicar P, Catargi B, Melki V, Chaillous L, Farret A, Bosson JL, Penfornis A, TeleDiab Study Group The Diabeo software enabling individualized insulin dose adjustments combined with telemedicine support improves HbA1c in poorly controlled type 1 diabetic patients: a 6-month, randomized, open-label, parallel-group, multicenter trial (TeleDiab 1 Study) Diabetes Care. 2011;34(3):533–539. doi: 10.2337/dc10-1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fonda SJ, Kedziora RJ, Vigersky RA, Bursell SE. Evolution of a web-based, prototype Personal Health Application for diabetes self-management. J Biomed Inform. 2010;43(5 Suppl):S17–21. doi: 10.1016/j.jbi.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 112.Quinn CC, Shardell MD, Terrin ML, Barr EA, Ballew SH, Gruber-Baldini AL. Cluster-randomized trial of a mobile phone personalized behavioral intervention for blood glucose control. Diabetes Care. 2011;34(9):1934–1942. doi: 10.2337/dc11-0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Weitzman ER, Adida B, Kelemen S, Mandl KD. Sharing data for public health research by members of an international online diabetes social network. PLoS One. 2011;6(4):e19256. doi: 10.1371/journal.pone.0019256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bech P, Olsen LR, Kjoller M, Rasmussen NK. Measuring well-being rather than the absence of distress symptoms: a comparison of the SF-36 Mental Health subscale and the WHO-Five Well-Being Scale. Int J Methods Psychiatr Res. 2003;12(2):85–91. doi: 10.1002/mpr.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Polonsky WH, Fisher L, Earles J, Dudl RJ, Lees J, Mullan J, Jackson RA. Assessing psychosocial distress in diabetes: development of the diabetes distress scale. Diabetes Care. 2005;28(3):626–631. doi: 10.2337/diacare.28.3.626. [DOI] [PubMed] [Google Scholar]
- 116.Arbuckle RA, Humphrey L, Vardeva K, Arondekar B, Danten-Viala M, Scott JA, Snoek FJ. Psychometric evaluation of the Diabetes Symptom Checklist-Revised (DSC-R)–a measure of symptom distress. Value Health. 2009;12(8):1168–1175. doi: 10.1111/j.1524-4733.2009.00571.x. [DOI] [PubMed] [Google Scholar]
- 117.Polonsky WH, Fisher L, Schikman CH, Hinnen DA, Parkin CG, Jelsovsky Z, Axel-Schweitzer M, Petersen B, Wagner RS. A structured self-monitoring of blood glucose approach in type 2 diabetes encourages more frequent, intensive, and effective physician interventions: results from the STeP Study. Diabetes Technol Ther. 2011;13(8):797–802. doi: 10.1089/dia.2011.0073. [DOI] [PubMed] [Google Scholar]
- 118.Bradley C. The Diabetes Treatment Satisfaction Questionnaire: DTSQ. In: Bradley C, editor. Handbook of psychology and diabetes: a guide to psychological measurement in diabetes research and practice. Chur: Harwood Academic Publishers; 1994. pp. 111–132. [Google Scholar]
- 119.Gonder-Frederick LA, Schmidt KM, Vajda KA, Greear ML, Singh H, Shepard JA, Cox DJ. Psychometric properties of the hypoglycemia fear survey-II for adults with type 1 diabetes. Diabetes Care. 2011;34(4):801–806. doi: 10.2337/dc10-1343. [DOI] [PMC free article] [PubMed] [Google Scholar]