Abstract
Diabetic foot complications are increasing in prevalence worldwide. Care and attention to these complications have improved greatly. Many advanced therapies are now being investigated or taken through final stages of clinical studies worldwide. However, the data upon which assumptions regarding morbidity, healing, and mortality have been based are grossly outdated. The purpose of this brief article is to report on current data regarding neuropathic and neuroischemic wounds and to propose that the latter category of advanced-stage diabetic foot wound may now be emerging as the most commonly encountered lesion in the developed world. Unfortunately, it is still systematically excluded from most clinical study criteria. Additionally, just as in the care of cancer, we call for therapy of these advanced-stage diabetic foot ulcers to be managed in similarly interdisciplinary centers where patients may have access to potentially beneficial clinical trials.
Keywords: amputation, diabetic foot ulcers, infection, ischemia, wound healing
Introduction
Diabetic foot ulcers (DFUs) and the resulting lower-extremity amputations (LEAs) are a common, complex, costly, and disabling complication of diabetes.1,2 According to the International Working Group on the Diabetic Foot, a DFU is a full-thickness wound penetrating through the dermis (the deep vascular and collagenous inner layer of the skin) located below the ankle in a diabetes patient.3–5
The diabetic foot is biologically compromised. This results from multiple contributing factors. The major underlying causes are noted to be peripheral neuropathy and ischemia from peripheral arterial disease (PAD). In the presence of these factors, even moderate ischemia can cause ulcers and impair healing.
Etiology
Diabetic foot ulcers can be categorized as purely neuro-pathic, purely ischemic, or a combination of the two, namely, neuroischemic.6,7 The estimated current prevalence of each is 35%, 15%, and 50%, respectively.8
Foot tissues can become ischemic because of macrovascular disease (atherosclerosis) but can also be complicated by associated microvascular disease.9,10 The relationship between DFU and PAD has been explored in detail.11 Previously published DFU research often ignored PAD as a potential risk factor and/or important cause. Ischemia has gained recognition as a significant cause of DFUs, with increasing prevalence in developed countries.
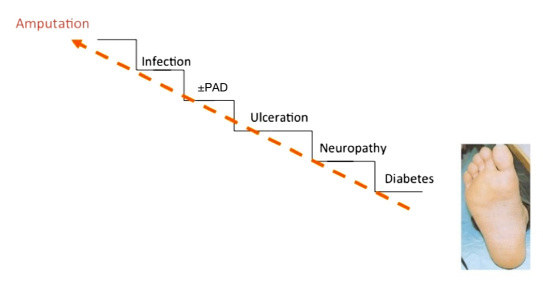
Based on an analysis conducted at the Diabetic Foot Clinic, King's College Hospital in London, there is some preliminary evidence that the prevalence of neuroischemic ulcers has been rising since the 1990s from approximately one-third of patients to over 50%, therefore becoming the most common etiology of DFUs.12 Risk for ulcers and ultimately amputations can be likened to a stairway, where each factor above contributes deleteriously to the etiologic foundation below (Figure 1).
Figure 1.
Stairway to amputation.
Additional works by Morbach and coworkers13 identified the increasing prevalence of PAD in patients with DFU. In their study, comparing centers in the developed and developing world, they identified nearly half of the population with concomitant PAD from cohorts of patients from 1998–1999. Despite these data being in existence since the late 1990s and a large, multinational prospective cohort confirming these data several years later (with data collection from 2003 to 2004),14,15 clinical studies focusing on infection and healing in people with DFUs have systematically excluded what now is likely a majority of the patients under care in centers across the developed world.
Outcomes in Neuroischemic versus Neuropathic Diabetic Foot Ulcers
The presence or absence of ischemia and PAD largely impacts the outcomes in the treatment of DFUs. Peripheral arterial disease in DFUs is associated with the most severe adverse outcomes, including lower probability of healing, longer healing times, higher probability of ulcer recurrence, greater risk of toe as well as major amputations, and potentially higher mortality.
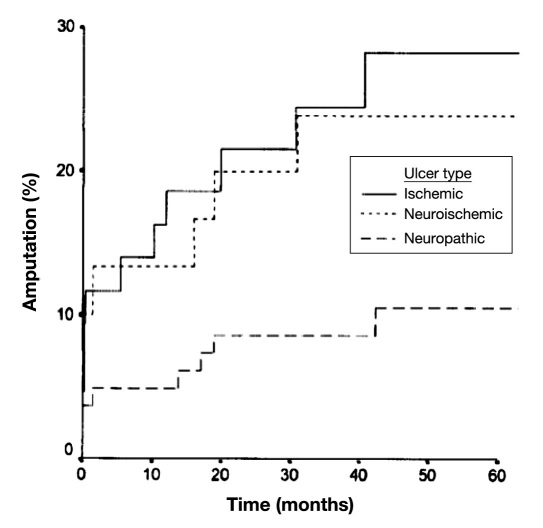
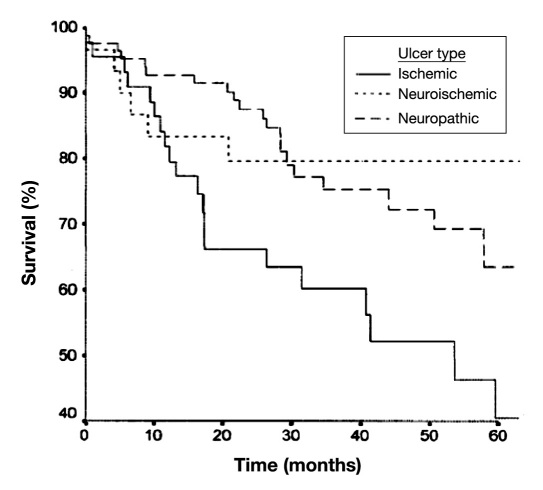
A compelling study published by Moulik and colleagues16 provides outcomes for patients with all three types of DFUs. Those with ischemic or neuroischemic disease have a much higher probability of amputation, with ischemic patients showing numerically higher mortality (Figures 2 and 3).
Figure 2.
Cumulative amputation rates for foot ulcers of various etiologies (reproduced with permission from Diabetes Care16).
Figure 3.
Cumulative survival rates for foot ulcers of various etiologies (reproduced with permission from Diabetes Care16).
The EURODIALE study14 was one of the few large prospective, international studies on outcome and deter-minants of outcome in diabetic foot disease. This study has shown that, when stratifying patients according to the presence or absence of PAD, significantly fewer wounds with PAD healed than in those without PAD (69% versus 84%, respectively). Furthermore, significant differences in clinical characteristics, outcome, and predictors of out-come in patients with and without PAD and the different pathophysiology and treatment of PAD and non-PAD ulcers led the authors to consider that DFU with and without PAD should potentially be defined as two separate disease states. Finally, the EURODIALE study confirmed that infection was significantly associated to nonhealing in individuals with PAD compared with non-PAD patients. Peripheral vascular insufficiency was previously shown to be associated with a two-fold increase of foot infection.17 Patients with infection and ischemia are nearly 90 times more likely to receive a midfoot or higher amputation compared with patients in less advanced wound stages (76.5% versus 3.5%; p < .001).6
A study commissioned by the United States Agency for Healthcare Research and Quality has shown that, among U.S. Medicare beneficiaries, the prevalence of LEA in the subpopulation of patients with diabetes and PAD was approximately three times as high as in the corresponding diabetes baseline population. This prevalence of LEA was even nearly seven times higher in nonelderly diabetes patients with PAD—many of whom likely have end-stage renal disease—compared with the prevalence in the Medicare population with diabetes.18
Outcomes of Mild/Moderate Ischemia in Diabetic Foot Ulcer Patients
The severity of PAD itself increases the risks of adverse outcomes, specifically nonhealing ulcers, amputation, and mortality. In a large cohort Swedish study, primary healing, amputation rates, and mortality were linked to the severity of the vascular insufficiency, measured by ankle or toe pressure.19
In a prospective population-based cohort study of adults with type 1 and type 2 diabetes mellitus presenting with their first foot ulcer (excluding those with severe ischemia), moderate ischemia was associated with mortality [hazard ratio = 2.74; 95% confidence interval (CI) 1.46–5.14]. Micro-vascular complications were the only explanatory factor associated with recurrent ulceration (hazard ratio = 3.34; 95% CI 1.17–9.56).20 This latter finding may have to do with skin and structural changes to the skin secondary to microvascular disease leading to a viscoelastically less robust integument less able to respond to repetitive normal and shear stress.
In a cohort of patients with vascular insufficiency, but not candidates for revascularization, Marston and associates21 have shown a clear correlation between the severity of ischemia and the risk of limb loss. While all patients with vascular insufficiency might initially be considered candidates for revascularization, severe comorbidities, patient consent, or anatomic/technical factors might obviate revascularization in favor of nonsurgical care. This population has been largely ignored from study in the medical literature but may be one that is important to address in future works.
Taylor and coworkers22 reported on the management and outcomes of 917 neuropathic ulcers in 706 patients over 5 years. The population was divided into three groups: neuropathic, ischemic with revascularization, and ischemic without revascularization. The latter can be considered patients with mild to moderate ischemia, for whom a revascularization procedure was not justified. Out of these three groups, the ischemic patients without revasculariza-tion had the worst outcomes. Outcomes for neuropathic, ischemia (revascularized), and ischemia (not revascularized), respectively, included 5-year limb salvage of 80%, 61%, and 51% (p < .001); survival of 47%, 37%, and 24% (p = .03); amputation-free survival of 37%, 28%, and 17% (p < .001); maintenance of ambulation of 74%, 55%, and 55% (p < .001); and maintenance of independence of 82%, 72%, and 58% (p = .01).
Mortality in Ischemic Patients
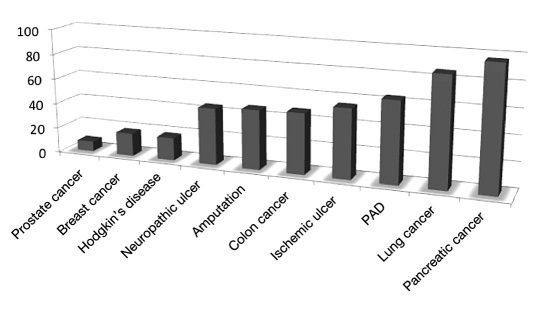
Armstrong and colleagues23 discussed the relatively high 5-year mortality rates for patients with neuropathic and ischemic DFUs and diabetes-related amputations compared with serious medical conditions, including several common types of cancer, using data gathered from multiple sources. By 5 years, 45% to 55% of patients with neuropathic and ischemic DFUs, respectively, will die. These common complications of diabetes have higher mortality rates than cancers of the prostate, breast, and colon, as well as Hodgkin's disease (Figure 4).23
Figure 4.
Relative 5-year mortality rates and comparison to major forms of cancer.
Gaps in Current Clinical Studies of Neuroischemic Diabetic Foot Ulcers
Since 2000, there have existed two main types of clinical investigations evaluating therapies for patients with DFUs. The most common have been studies evaluating various strategies to accelerate healing in DFUs. This group includes the prospective studies performed on Regranex, Dermagraft, and Apligraf and numerous other studies that did not result in approved products for the treatment of neuroischemic DFUs. These studies have employed wound healing as the primary end point and have routinely excluded patients with significant arterial disease. The other common strategy has involved angiogenic therapies designed to stimulate capillary development and address revascularization in patients with severe limb ischemia. None of these angiogenic therapies has achieved success to gain market approval. This lack of prospective wound healing studies enrolling patients with neuroischemic ulcers has resulted in a lack of treatment options for this group of patients, which comprises more than half of our collective patient population.
Revascularization for Patients with Diabetic Foot Ulcers and Risks of Invasive Treatment
Surgical or endovascular revascularization procedures are options frequently recommended for patients with ischemic DFUs to improve blood supply, stimulate wound healing, and prevent limb loss. While these procedures are excellent treatment choices and have resulted in high limb salvage rates, some patients are poor candidates for these invasive procedures. Given the medical condition of many patients with ischemic DFUs, they are at high risk for procedural-related complications, including renal failure, myocardial infarction, embolization, and others.
A review of over 2400 patients undergoing surgical revascularization for lower-extremity ischemia from the National Surgical Quality Improvement Program database reported a mortality rate of 2.7% and major complications in 18.7% of patients.24 While the incidence of mortality and complications are lower with endovascular methods of revascularization, these less invasive procedures are also associated with complications, including renal failure, embolization, and access vessel complications. Also, several reports have identified a lower long-term success rate for patients with diabetes undergoing endovascular interventions.25
The Way Forward: Teams and Technology
Compelling demographic data coupled with the promise for proactive intervention has led many academic centers and national organizations to develop and advocate a targeted team approach in care for this high-risk population. Likening this problem to advanced stages of cancer, patients might best be treated in similarly specialized treatment centers for the extremity. This “toe and flow” model of care, where podiatric and vascular specialists surrounded by primary and specialty diabetes care support, has gained a great deal of therapeutic traction in the United States and elsewhere. Many of the patients in these specialty centers, just as in cancer treatment centers, are enrolled in some form of clinical trial evaluating new and promising technologies.26–28 Furthermore, the concept of “remission” care, rather than frank “prevention” is a more realistic notion and may assist in communicating the exceptionally high-risk status of these patients and also the commensurate importance for frequent follow-up.29
Summary
Neuroischemic DFU is now likely the most common type of DFU seen in wound clinics in the United States and throughout the developed world. These wounds are more difficult to heal than nonischemic DFUs and are associated with a higher rate of amputation and mortality. This population of patients with neuroischemic DFUs is at high risk for complications with surgical or endovascular methods of revascularization, and currently, we have no approved lower-risk treatment options to accelerate healing in this patient group. To date, wound studies evaluating therapies for DFUs have excluded the neuroischemic wounds. Therefore, we feel that any promising treatment option for this high-risk group warrants further study in well-designed clinical trials.
Glossary
Abbreviations
- (CI)
confidence interval
- (DFU)
diabetic foot ulcer
- (LEA)
lower-extremity amputation
- (PAD)
peripheral arterial disease
References
- 1.Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA. 2005;293(2):217–228. doi: 10.1001/jama.293.2.217. [DOI] [PubMed] [Google Scholar]
- 2.Rogers LC, Lavery LA, Armstrong DG. The right to bear legs–an amendment to healthcare: how preventing amputations can save billions for the US Health-care System. J Am Podiatr Med Assoc. 2008;98(2):166–168. [PubMed] [Google Scholar]
- 3.Schaper NC. Diabetic foot ulcer classification system for research purposes: a progress report on criteria for including patients in research studies. Diabetes Metab Res Rev. 2004;20(Suppl 1):S90–S95. doi: 10.1002/dmrr.464. [DOI] [PubMed] [Google Scholar]
- 4.Jeffcoate WJ, Price P, Harding KG, International Working Group on Wound Healing and Treatments for People with Diabetic Foot Ulcers Wound healing and treatments for people with diabetic foot ulcers. Diabetes Metab Res Rev. 2004;20(Suppl 1):S78–89. doi: 10.1002/dmrr.476. [DOI] [PubMed] [Google Scholar]
- 5.International Working Group on the Diabetic Foot. International consensus on the diabetic foot. In: Apelqvist J, Bakker K, van Houtum WH, Nabuurs-Fransen MH, Schapner NC, editors. Amsterdam: Inter-national Diabetes Federation; 1999. [Google Scholar]
- 6.Armstrong DG, Lavery LA, Harkless LB. Validation of a diabetic wound classification system. The contribution of depth, infection, and ischemia to risk of amputation. Diabetes Care. 1998;21(5):855–859. doi: 10.2337/diacare.21.5.855. [DOI] [PubMed] [Google Scholar]
- 7.Edmonds ME, Foster AV. Classification and management of neuro-pathic and neuroischaemic ulcers. In: Boulton AJ, Connor H, Cavanagh PR, editors. The foot in diabetes. Chichester: John Wiley; 1994. [Google Scholar]
- 8.The Sage Group. Diabetic foot ulcers, peripheral arterial disease and critical limb ischemia. http://thesagegroup.us/pages/reports/dfu-statistics.php.
- 9.Jeffcoate WJ, Harding KG. Diabetic foot ulcers. Lancet. 2003;361(9368):1545–1551. doi: 10.1016/S0140-6736(03)13169-8. [DOI] [PubMed] [Google Scholar]
- 10.Akbari CM, LoGerfo FW. Microvascular changes in the diabetic foot. In: Veves A, Giurini J, LoGerfo FW, editors. The diabetic foot. Totowa: Humana; 2002. [Google Scholar]
- 11.Adler AI, Boyko EJ, Ahroni JH, Smith DG. Lower-extremity amputation in diabetes. The independent effects of peripheral vascular disease, sensory neuropathy, and foot ulcers. Diabetes Care. 1999;22(7):1029–1035. doi: 10.2337/diacare.22.7.1029. [DOI] [PubMed] [Google Scholar]
- 12.Limperopoulou D, Bates M, Petrova NL, ME E. Diabetic Foot Study Group. Chalkidiki; 2005. The epidemic of neuroischaemic foot. [Google Scholar]
- 13.Morbach S, Lutale JK, Viswanathan V, Möllenberg J, Ochs HR, Rajashekar S, Ramachandran A, Abbas ZG. Regional differences in risk factors and clinical presentation of diabetic foot lesions. Diabet Med. 2004;21(1):91–95. doi: 10.1046/j.1464-5491.2003.01069.x. [DOI] [PubMed] [Google Scholar]
- 14.Prompers L, Schaper N, Apelqvist J, Edmonds M, Jude E, Mauricio D, Uccioli L, Urbancic V, Bakker K, Holstein P, Jirkovska A, Piaggesi A, Ragnarson-Tennvall G, Reike H, Spraul M, Van Acker K, Van Baal J, Van Merode F, Ferreira I, Huijberts M. Prediction of outcome in individuals with diabetic foot ulcers: focus on the differences between individuals with and without peripheral arterial disease. The EURODIALE Study. Diabetologia. 2008;51(5):747–755. doi: 10.1007/s00125-008-0940-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prompers L, Huijberts M, Apelqvist J, Jude E, Piaggesi A, Bakker K, Edmonds M, Holstein P, Jirkovska A, Mauricio D, Ragnarson Tennvall G, Reike H, Spraul M, Uccioli L, Urbancic V, Van Acker K, van Baal J, van Merode F, Schaper N. High prevalence of ischaemia, infection and serious comorbidity in patients with diabetic foot disease in Europe. Baseline results from the Eurodiale study. Diabetologia. 2007;50(1):18–25. doi: 10.1007/s00125-006-0491-1. [DOI] [PubMed] [Google Scholar]
- 16.Moulik PK, Mtonga R, Gill GV. Amputation and mortality in new-onset diabetic foot ulcers stratified by etiology. Diabetes Care. 2003;26(2):491–494. doi: 10.2337/diacare.26.2.491. [DOI] [PubMed] [Google Scholar]
- 17.Lavery LA, Armstrong DG, Wunderlich RP, Mohler MJ, Wendel CS, Lipsky BA. Risk factors for foot infections in individuals with diabetes. Diabetes Care. 2006;29(6):1288–1293. doi: 10.2337/dc05-2425. [DOI] [PubMed] [Google Scholar]
- 18.Margolis D, Malay DS, Hoffstad OJ, Leonard CE, MaCurdy T, Lopez de Nava K, Tan Y, Molina T, Siegel KL. Rockville: Quality AfHRa; 2011. Prevalence of diabetes, diabetic foot ulcer, and lower extremity amputation among Medicare beneficiaries, 2006 to 2008. [PubMed] [Google Scholar]
- 19.Gershater MA, Löndahl M, Nyberg P, Larsson J, Thörne J, Eneroth M, Apelqvist J. Complexity of factors related to outcome of neuropathic and neuroischaemic/ischaemic diabetic foot ulcers: a cohort study. Diabetologia. 2009;52(3):398–407. doi: 10.1007/s00125-008-1226-2. [DOI] [PubMed] [Google Scholar]
- 20.Winkley K, Stahl D, Chalder T, Edmonds ME, Ismail K. Risk factors associated with adverse outcomes in a population-based prospective cohort study of people with their first diabetic foot ulcer. J Diabetes Complications. 2007;21(6):341–349. doi: 10.1016/j.jdiacomp.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 21.Marston WA, Davies SW, Armstrong B, Farber MA, Mendes RC, Fulton JJ, Keagy BA. Natural history of limbs with arterial insufficiency and chronic ulceration treated without revascularization. J Vasc Surg. 2006;44(1):108–114. doi: 10.1016/j.jvs.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 22.Taylor SM, Johnson BL, Samies NL, Rawlinson RD, Williamson LE, Davis SA, Kotrady JA, York JW, Langan EM, 3rd, Cull DL. Contemporary management of diabetic neuropathic foot ulceration: a study of 917 consecutively treated limbs. J Am Coll Surg. 2011;212(4):532–548. doi: 10.1016/j.jamcollsurg.2010.12.027. [DOI] [PubMed] [Google Scholar]
- 23.Armstrong DG, Wrobel J, Robbins JM. Guest editorial: are diabetes-related wounds and amputations worse than cancer? Int Wound J. 2007;4(4):286–287. doi: 10.1111/j.1742-481X.2007.00392.x. [DOI] [PubMed] [Google Scholar]
- 24.LaMuraglia GM, Conrad MF, Chung T, Hutter M, Watkins MT, Cambria RP. Significant perioperative morbidity accompanies contemporary infrainguinal bypass surgery: an NSQIP report. J Vasc Surg. 2009;50(2):299–304. doi: 10.1016/j.jvs.2009.01.043. Aug. [DOI] [PubMed] [Google Scholar]
- 25.Abularrage CJ, Crawford RS, Durand ML, LaMuraglia GM. Extracranial infected carotid artery aneurysm. J Vasc Surg. 2009;50(6):1484–1486. doi: 10.1016/j.jvs.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 26.Rogers LC, Andros G, Caporusso J, Harkless LB, Mills JL, Sr, Armstrong DG. Toe and flow: essential components and structure of the amputation prevention team. J Vasc Surg. 2010;52(3 Suppl):23S–27S. doi: 10.1016/j.jvs.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 27.Rogers LC, Armstrong DG. Diabetic foot ulcers: podiatry care. In: Cronenwett J, editor. Rutherford's vascular surgery. 7th ed. Amsterdam: Elsevier; 2009. [Google Scholar]
- 28.Sumpio BE, Armstrong DG, Lavery LA, Andros G, SVS/APMA writing group The role of interdisciplinary team approach in the management of the diabetic foot: a joint statement from the Society for Vascular Surgery and the American Podiatric Medical Association. J Vasc Surg. 2010;51(6):1504–1506. doi: 10.1016/j.jvs.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 29.Armstrong DG, Mills JL. Toward a change in syntax in diabetic foot care: prevention equals remission. J Am Pod Med Assoc. doi: 10.7547/1030161. Forthcoming. [DOI] [PubMed] [Google Scholar]