Abstract
Background
The goal of diabetes treatment is maintaining near normoglycemia based on self-monitoring of blood glucose (SMBG). In this study, an evaluation of the analytical performance of the coulometry-based Optium Omega™ glucose meter designed for SMBG has been carried out.
Methods
The assessment of precision and between-lot variability was based on glucose measurements in ethylene-diaminetetraacetic acid venous blood samples. Glucose concentrations measured in 289 fresh capillary blood samples using the Omega glucose meter and the Biosen C_line analyzer were compared.
Results
Within-run imprecision coefficient of variation for the lower and higher glucose concentrations amounted to 5.09 and 2.1%, respectively. The relative lot-dependent differences found for the lower and higher glucose concentrations were equal to 6.8 and 2.6%, respectively. The glucose meter error calculated for various concentration ranges amounted from 2.22 to 4.48%. The glucose meter error met the accuracy criteria recommended by the International Organization for Standardization and the American Diabetes Association. The Passing-Bablok agreement test and error grid analysis with 96% of results in zone A indicated good concordance of results, including glucose concentrations below 100 mg/dl.
Conclusions
The evaluated Optium Omega glucose meter fits the analytical requirements for its use in blood glucose monitoring in diabetes patients.
Keywords: diabetes mellitus, glucose meter, glucose meter error, self-monitoring of blood glucose
Introduction
Treatment goals in diabetes include maintaining near normoglycemia in patients in order to prevent the development of chronic diabetic complications. Intensive diabetes treatment requires a tight monitoring of blood glucose concentrations performed by the patients themselves [self-monitoring of blood glucose (SMBG)]. Self-monitoring of blood glucose is the basis for assessment of diabetes metabolic control and should effectively detect hypoglycemia and hyperglycemia episodes requiring prompt interventions. For these reasons, SMBG is considered an integral part of contemporary diabetes treatment.1,2
Being operated by the patients, the glucose meters used for SMBG have to combine simplicity of use and required analytical performance. The standards of analytical quality are still under debate, and according to various guidelines, the allowable limit of glucose meter error amounts from 5 to about 20%.2–5 Manufacturers are still improving the methodology of glucose assays using meters and test strips and the quality of measurement techniques to meet these performance goals. The coulometry-based electrochemical technique was applied for measurement of the reaction course on reagent strips with glucose dehydrogenase. This measurement technique has been used in the Optium Omega™ glucose meter utilizing test strips based on TrueMeasure™ technology (Abbott Diabetes Care, Inc., Alameda, CA).
The aim of this study was to evaluate the Optium Omega glucose meter’s analytical performance in clinical settings. The evaluation included within-run imprecision, between-lot variability of strips, and assessment of accuracy based on comparison with the laboratory method.
Methods
The evaluation was carried out in the Department of Diagnostics and in the Department of Metabolic Diseases, Jagiellonian University Medical College, Krakow, Poland. Glucose concentrations were measured in fresh capillary blood samples collected for routine glycemia monitoring in patients with diabetes. The patients were recruited by their physicians and nobody participating in the study had any hematological disease and/or abnormal complete blood count results. It is well known that hematocrit values can significantly affect results of whole blood glucose concentration measurements performed using various glucose meters. However, the hematocrit effect on the Optium Omega measurements was not evaluated in this study. Glucose measurements were preformed in capillary blood samples obtained by finger prick. The volume of these samples was too small for additional hematocrit measurements. Obviously, this is an important limitation of our study. All patients were informed about the additional use of their blood and the purpose of the study, and the Jagiellonian University Bioethics Commissions approved the study. During every sampling, the first blood drop obtained by finger prick was used for glucose assay on the Optium Omega meter. Three devices were used in the study. The next drop of blood from the same finger prick was transferred to the microtube containing a hemolyzing reagent, which, after mixing, was placed in the refrigerator to store before the assay using the comparative laboratory method. All these procedures were performed by trained nurses.
The Optium Omega glucose meter utilizes the glucose dehydrogenase and nicotinamide adenine dinucleotide reaction and a coulometry-based measurement technique allowing for amperometric measuring of all the electrons released during glucose oxidation. We did not study the maltose interference because these test strips do not contain pyrroloquinoline quinone as a coenzyme and are resistant to this interference.
After aspirating 6 μl of blood by the strip and waiting 5 seconds for the reaction and the measurement of glucose, the result was read from the glucose meter’s display. The meter was plasma-calibrated, which means that the results are presented as plasma glucose concentrations.
The hemolysate samples after up to 2 h storage in a temperature of 2–8 °C were used for glucose assays on a Biosen C_line glucose analyzer (EKF-diagnostics GmbH, Barleben/Magdeburg, Germany) using the glucose oxidase method with amperometric measurement technique. The Biosen glucose analyzers are used in laboratories as well as point-of-care devices. Evaluation of the analytical performance of this type of analyzer showed good agreement with other laboratory methods.6 Between-run imprecision coefficient of variation (CV) of the Biosen C_line analyzer used in this study, when assessed for normal and high glucose concentrations, was 3.2 and 1.8%, respectively. Whole blood glucose concentrations measured by the Biosen C_line were converted to the corresponding plasma values by multiplication by the constant factor 1.11.7
Within-run imprecision of the evaluated glucose meter was assessed on the basis of the results of glucose concentration measurements in 20 specimens of the same ethylenediaminetetraacetic acid (EDTA) venous blood using two glucose meters and test strips from the same lot. The glucose meter error was calculated as the bias from the results obtained using the Biosen C_line analyzer, considered the reference laboratory method. This intermethod difference between the results was expressed as the percentage of the laboratory value. In detail, the error was derived from the following formula:
This value is equal to the mean value of errors calculated for individual pairs of results in the same way. Between-lot variability was evaluated by comparison of the results obtained in the same EDTA venous blood samples using one glucose meter and test strips from different lots. We evaluated strip lot-dependent variability in a similar way to within-run imprecision, but in this case, we did not analyze results dispersion (imprecision) but rather the agreement between results obtained using strips from different lots. However, because of organizational issues, we could not perform assays using all three strip lots at both glucose concentrations and therefore we compared these lots in pairs. Differences were calculated and compared using the Bland-Altman analysis. To express the mean differences in relative values, the percentage of the average of mean concentrations obtained using test strips from compared lots was calculated. In this part of our study, we did not evaluate the acccuracy of measurements performed using strips from different lots but rather the between-lot variability assuming that, ideally, the meter and all lots of strips should give the same results in the same material. Thus, we assessed this kind of variability in this way, reporting it as absolute and relative differences between results obtained using strips from different lots. We did not use any reference glucose concentration in this analysis.
The mean values were compared using the t-test for dependent samples. Paired results were compared using unweighted linear regression analysis, correlation coefficients, Passing-Bablok agreement test, and Bland-Altman difference plot. Calculations were carried out with the Method Validator (Phillip Jordan, Royal Devon and Exeter Hospital, UK) and Excel for Windows 2003 (Microsoft Corp., Redmond, WA) programs. Statistical significance was determined by a p < .05.
Results
For mean glucose concentration values of 61.5 and 300.2 mg/dl, within-run imprecision evaluation yielded standard deviations of 3.13 and 6.31 mg/dl with coefficients of variation of 5.09 and 2.1%, respectively. Lot-to-lot variability was assessed by comparison of the results obtained in the same venous blood samples with two glucose concentrations using reagent strips from three different lots, as shown in Table 1. Relative lot-dependent differences found for lower and higher glucose concentrations amounted to 6.8 and 2.6%, respectively.
Table 1.
Between-Lot Variability of Reagent Strips
| Lot# 777410 | Lot# 784701 | Lot# 77410 | Lot# 860209 | |
|---|---|---|---|---|
| Number of tests | 20 | 20 | ||
| Minimum concentration, mg/dl | 60 | 56 | 287 | 296 |
| Maximum concentration, mg/dl | 69 | 66 | 303 | 314 |
| Mean concentration, mg/dl | 63.6a | 59.4 | 296.3a | 304.1 |
| Standard deviation, mg/dl | 2.26 | 2.39 | 4.6 | 5.35 |
| Mean difference, mg/dl | -4.2 (95% CI: -5.84 to -2.56) | 7.8 (95% CI: 4.18 to 11.4) | ||
| Relative differenceb | 6.8% | 2.6% | ||
p < .05
b The percentage of the average of mean concentrations obtained using test strips from compared lots.
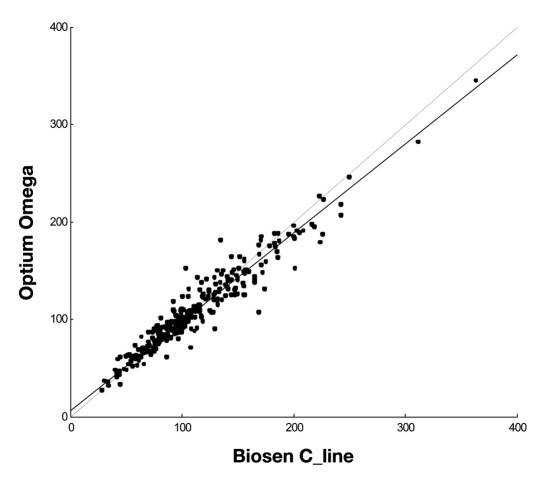
The comparison of the Optium Omega glucose meter with the reference laboratory method is shown in Table 2 and Figure 1. Across the whole range of measured concentrations from 28 to 363 mg/dl (289 samples), the glucose meter yielded slightly lower concentrations with a negative bias amounting to 2.48%. However, the Passing-Bablok agreement test did not indicate good concordance of results. An analysis of the results carried out for two groups of samples showed that in the case of samples with glucose ≤100 mg/dl, the Optium Omega yielded a positive bias of 2.22% and the Passing-Bablok indicated good agreement of the results. For samples with glucose concentrations >100 mg/dl, a negative bias equal to 4.84% and good agreement of the results were found (Table 2).
Table 2.
Comparison of Blood Glucose Concentrations Measured Using the Biosen C_line Glucose Analyzer and the Optium Omega Glucose Meter
| All samples, n = 289 | Glucose ≤100 mg/dl, n = 135 | Glucose >100 mg/dl, n = 154 | ||||
|---|---|---|---|---|---|---|
| Biosen C_line | Optium Omega | Biosen C_line | Optium Omega | Biosen C_line | Optium Omega | |
| Minimum concentration, mg/dl | 28 | 27 | 28 | 27 | 100,2 | 71 |
| Maximum concentration, mg/dl | 363 | 345 | 100 | 118 | 363 | 345 |
| Mean concentration, mg/dl | 112.9 | 110.1 | 76.6 | 78.3 | 144.7a | 137.7 |
| Standard deviation, mg/dl | 47.4 | 43.1 | 18.2 | 18.9 | 41.9 | 38,8 |
| Mean difference (error), mg/dl | -2.8 (95% CI: -5.31 to -1.97) | 1.7 (95% CI: 0.25 to 3.01) | -7.0 (95% CI: -9.47 to -4.46) | |||
| Glucose meter error | -2.48% | 2.22% | -4.84% | |||
| Correlation coefficient | 0.959 | 0.905 | 0.927 | |||
| Passing-Bablok agreement test | Slope: 0.91 (95% CI: 0.88 to 0.94) Intercept: 6.5 (95%% CI: 3.3 to 9.3) | Slope: 1.03 (95% CI: 0.96 to 1.12) Intercept: -1.6 (95% CI: -6.8 to 4.0) | Slope: 0.95 (95% CI: 0.89 to 1.01) Intercept: 0.5 (95% CI: -7.7 to 8.7) | |||
a p < .05
Figure 1.
Comparison of glucose concentrations measured using the Optium Omega glucose meter and the Biosen C_line glucose analyzer. Passing-Bablok agreement test, slope: 0.91 (95% CI: 0.88 to 0.94), intercept: 6.5 (95% CI: 3.3 to 9.3), n = 289.
In the group of samples with glucose concentrations ≤76 mg/dl, for 52 of 55 (94.5%) samples, a bias lower than ±15 mg/dl was found, and in the case of 234 samples with glucose >76 mg/dl, a bias below ±20% was found for 224 (95.7%) of them. We have not conducted the entire evaluation in accordance with DIN EN ISO 15197. We only compared the obtained results with its accuracy recommendations and found that the accuracy of results obtained using the Optium Omega is equal to that recommended by the DIN EN ISO 15197.5
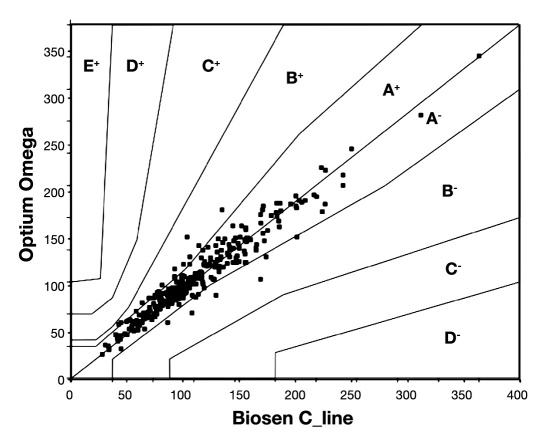
Error grid analysis yielded all measurement points reflecting paired concentrations results in zone A and B with 96% of results in zone A (Figure 2).
Figure 2.
Comparison of glucose concentrations measured using the Optium Omega glucose meter and the Biosen C_line glucose analyzer. Error grid analysis. All points are located in zones A and B.
Discussion
Glucose is one of the most difficult substances to measure, and analytical methods used for this purpose are not sufficiently harmonized.8 As glucose meters measure the concentration of glucose in fresh capillary blood, the effect of glycolysis is eliminated. However, they are influenced by several other preanalytical and analytical interfering factors limiting the accuracy of glucose monitoring. Some of these factors are common for all meters, e.g., hematocrit or blood pH, whereas others, such as maltose, are dependent on the methodology and measurement technique employed. On the other hand, the accuracy of glucose assays performed using glucose meters is of clinical importance. Studies evaluating glucose meter errors showed that the allowable error of 5% recommended by the American Diabetes Association (ADA)3 is difficult to achieve.9–11 Manufacturers are continually improving the construction of glucose meters and the properties of reagent strips. The coulometry-based measurement technique is not yet widely used in electro-chemical (sensor-type) glucose meters. To our knowledge, our study is the first trial evaluating the analytical performance of such a glucose meter in clinical settings.
For the glucose meter evaluated in our study, the Optium Omega, imprecision assessments have revealed within-run CV values of 5.09 and 2.1% for the lower and higher glucose concentrations, respectively. This level of imprecision for higher glucose concentrations meets the National Academy of Clinical Biochemistry guidelines but for lower glucose concentrations exceeds the recommended CV of ≤3.3%.4
The reagent strips’ lot-dependent variability has been reported since 1986 and considered an important factor influencing the analytical quality of measurements.12 In our study, lot-to-lot variability analysis yielded statistically significant differences between the results obtained in blood samples with two glucose concentrations using reagent strips from three different lots (Table 1). Mean values of lot-dependent differences found for lower and higher glucose concentrations amounted to 6.8 and 2.6% of average measured levels, respectively, thus below allowable accuracy error. However, this is only one of the factors contributing to the overall error of a glucose meter. The values found for the Optium Omega are lower as compared to our earlier data from the evaluation of another glucose meter13 and similar to the lot-dependent differences found by Kristensen and colleagues.14 Glucose meters are factory-calibrated and only coded according to the lot of the reagent strips used. Therefore, differences in analytical characteristics between strips from different lots cannot be compensated by any calibration procedure of the device available for the user. However, this kind of analytical variability should be detected when the analytical quality control of glucose meters is carried out properly.15
In comparison to the laboratory method across the whole range of glucose concentrations measured, the Optium Omega glucose meter yielded mean error amounting to 2.48% (Table 2), thus remaining below the limit of allowable error equal to 5% recommended by the ADA.3 The accuracy of measurements using the evaluated glucose meter was equal to that required in DIN EN ISO 15197.5 The Passing-Bablok agreement test did not indicate good concordance of results (Figure 1). This test is commonly used to compare results obtained using two laboratory methods. The slope value equal to 1.0 and intercept amounting to 0.0 within the 95% confidence interval (CI) indicate significant agreement. However, the quality specifications set for glucose meters are slightly different as compared to laboratory methods. Commonly used tools evaluating the analytical performance of glucose meters include a glucose meter error and the Clarke error grid analysis. Both of these tools indicate the clinical importance of differences between the glucose meter and the laboratory method results. The error grid analysis with 96% of results located in zone A confirmed the clinical usefulness of the Optium Omega meter (Figure 2). It is noteworthy that good concordance of results was found for glucose concentrations below 100 mg/dl. The glucose meter error amounted to 2.22% and the Passing-Bablok agreement test yielded a slope of 1.0 and an intercept of 0 within the 95% confidence interval (Table 2). The Optium Omega utilizes a coulometry-based measurement technique, which is considered suitable for measuring low glucose concentrations. It was confirmed by our data, indicating reliable detecting of hypoglycemic states using the evaluated glucose meter. A good agreement of results was also found for glucose concentrations above 100 mg/dl with slightly higher glucose meter error amounting to 4.84%. In summary, the comparison of the Optium Omega glucose meter with the Biosen C_line laboratory analyzer indicated a concordance of results fitting the accuracy recommendations of ADA and International Organization for Standardization (ISO).3,5
Available glucose meters are comparable with respect to basic features such as analytical methodology or measure- ment technique. The Optium Omega, which belongs to the new generation of these devices, is plasma-calibrated and utilizes an electrochemical coulometry-based measurement technique. The overall analytical characteristics of the Optium Omega glucose meter reported here are comparable to those reported for other similar devices.13,16–21 The analytical characteristics of available glucose meters, however, still do not justify their use for diabetes diagnosis purposes.
Conclusions
The coulometry-based measurement technique applied in glucose meters seems to ensure good concordance with the laboratory method, particularly in low glucose concentrations range, with sufficient reproducibility of the obtained results. The evaluated Optium Omega glucose meter utilizing this methodology is characterized by an acceptable level of imprecision, moderate between-lot variability, and an accuracy bias that fits the require- ments recommended by ISO and ADA. Error grid analysis indicated minor clinical significance of the observed differences between the evaluated meter and the laboratory analyzer. Thus, the evaluated assay methodology applied in the Optium Omega glucose meter meets the analytical requirements for its use in blood glucose monitoring in diabetes patients.
Acknowledgments
The authors thank Mrs. Agata Porebska and Mrs. Ewa Ziarko, the nurses in the Department of Metabolic Diseases, for their helpful participation in this study, and Ms. Aleksandra Malecka for her linguistic corrections.
Glossary
Abbreviations
- (ADA)
American Diabetes Associations
- (CI)
confidence interval
- (CV)
coefficient of variation
- (EDTA)
ethylenediaminetetraacetic acid
- (ISO)
International Organization for Standardization
- (SD)
standard deviation
- (SMBG)
self-monitoring of blood glucose
Disclosures
Bogdan Solnica, Beata Kusnierz-Cabala, and Krystyna Slowinska-Solnica received consulting fees from Abbott Laboratories.
References
- 1.American Diabetes Association. Executive summary: standards of medical care in diabetes–2009. Diabetes Care. 2009;32(Suppl 1):S6–12. doi: 10.2337/dc09-S006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sacks DB, Bruns DE, Goldstein DE, Maclaren NK, McDonald JM, Parrott M. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin Chem. 2002;48(3):436–472. [PubMed] [Google Scholar]
- 3.American Diabetes Association. Consensus statement on self-monitoring of blood glucose. Diabetes Care. 1987;10(1):95–99. [PubMed] [Google Scholar]
- 4.National Academy of Clinical Biochemistry. Laboratory medicine practice guidelines. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Washington (DC): NACB; 2002. Volume 13. [Google Scholar]
- 5. International Organization for Standardization. In vitro diagnostic test systems–requirements for blood glucose monitoring systems for self-testing in managing diabetes mellitus. ISO/FDIS 15197 2003.
- 6.Solnica B, Naskalski JW. Evaluation of analytical performance of Super G1, Super G2+ and Biosen 5030 glucose amperometric analyzers. Clin Lab. 2003;49(5-6):233–238. [PubMed] [Google Scholar]
- 7.Burnett RW, D’Orazio P, Fogh-Andersen N, Kuwa K, Külpmann WR, Larsson L, Lewnstam A, Maas AH, Mager G, Spichiger-Keller U, Scientific Division Working Group on Selective Electrodes IFCC recommendation on reporting results for blood glucose. Clinica Chimica Acta. 2001;307(1-2):205–209. doi: 10.1016/s0009-8981(01)00431-4. [DOI] [PubMed] [Google Scholar]
- 8.Gambino R. Glucose: a simple molecule that is not simple to quantify. Clin Chem. 2007;53(12):2040–2041. doi: 10.1373/clinchem.2007.094466. [DOI] [PubMed] [Google Scholar]
- 9.Grefkin G, Winter WE. Hardware and software in diabetes mellitus: performance characteristics of hand-held glucose testing devices and the application of glycemic testing to patients’ daily diabetes management. Clin Chem. 2001;47(1):11–12. [PubMed] [Google Scholar]
- 10.Skeie S, Thue G, Nerhus K, Sandberg S. Instruments for self-monitoring of blood glucose: comparisons of testing quality achieved by patients and a technician. Clin Chem. 2002;48(7):994–1003. [PubMed] [Google Scholar]
- 11.Solnica B, Naskalski JW, Sieradzki J. Analytical performance of glucometers used for routine glucose self-monitoring of diabetic patients. Clin Chim Acta. 2003;331(1-2):29–35. doi: 10.1016/s0009-8981(03)00079-2. [DOI] [PubMed] [Google Scholar]
- 12.Bradley C, Moses JL. Evaluation of blood glucose measurement techniques: locating sources of error. Diabetes Res. 1986;3(1):53–58. [PubMed] [Google Scholar]
- 13.Solnica B, Naskalski J, Gernand W. Analytical evaluation of the Optium Xido blood glucose meter. Clin Chem Lab Med. 2008;46(1):143–147. doi: 10.1515/CCLM.2008.022. [DOI] [PubMed] [Google Scholar]
- 14.Kristensen GBB, Christensen NG, Thue G, Sandberg S. Between-lot variation in external quality assessment of glucose: clinical importance and effect on participant performance evaluation. Clin Chem. 2005;51(9):1632–1636. doi: 10.1373/clinchem.2005.049080. [DOI] [PubMed] [Google Scholar]
- 15.Solnica B, Naskalski JW. Quality control of self-monitoring of blood glucose: why and how? J Diabetes Sci Technol. 2007;1(2):164–168. doi: 10.1177/193229680700100204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Savoca R, Jaworek B, Huber AR. New “plasma referenced” POCT glucose monitoring systems–are they suitable for glucose monitoring and diagnosis of diabetes? Clin Chim Acta. 2006;372(1-2):199–201. doi: 10.1016/j.cca.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 17.Solnica B, Naskalski JW. Quality control of SMBG in clinical practice. Scand J Clin Lab Invest Suppl. 2005;240:80–85. doi: 10.1080/00365510500235996. [DOI] [PubMed] [Google Scholar]
- 18.Hawkins RC. Evaluation of Roche Accu-Chek Go and Medisense Optium blood glucose meters. Clin Chim Acta. 2005;353(1-2):127–131. doi: 10.1016/j.cccn.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 19.Dai KS, Tai DY, Ho P, Chen CC, Peng WC, Chen ST, Hsu CC, Liu YP, Hsieh HC, Yang CC, Tsai MC, Mao SJ. Accuracy of the EasyTouch blood glucose self-monitoring system: a study of 516 cases. Clin Chim Acta. 2004;349(1-2):135–141. doi: 10.1016/j.cccn.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 20.Kimberly MM, Vesper HW, Caudill SP, Ethridge SF, Archibold E, Porter KH, Myers GL. Variability among five over-the-counter blood glucose monitors. Clin Chim Acta. 2006;364(1-2):292–297. doi: 10.1016/j.cca.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 21.Kristensen GB, Nerhus K, Thue G, Sandberg S. Standardized evaluation of instruments for self-monitoring of glucose by patients and a technologist. Clin Chem. 2004;50(6):1068–1071. doi: 10.1373/clinchem.2004.031575. [DOI] [PubMed] [Google Scholar]