Abstract
Limited work has been done to investigate the molecular mechanisms behind the process of demyelination and remyelination that occurs in response to chronic nerve compression (CNC) injury. In the present study, we investigated the expression of the transcription factors krox-20 and c-jun, positive and negative regulators of myelination, respectively. A decrease in krox-20 expression and an increase in c-jun expression in both its phosphorylated and non-phosphorylated states were observed. In addition, we investigated the role of integrins, specifically the β4 subunit of the α6β4 dimer, as a possible upstream signal transducer in the signaling cascade leading to demyelination. We detected a decrease in β4 integrin expression at 2 and 4 weeks post-injury and a concomitant relocalization to the Schmidt-Lanterman Incisures, suggesting a role for the β4 integrin in facilitating Schwann cell – extracellular matrix interaction. The observed changes in transcription factor and integrin expression are temporally correlated with the process of demyelination, and suggest further investigation to define their definitive role in regulating the myelin response to CNC injury.
Keywords: chronic nerve compression (CNC), transcription factors, krox-20, c-jun, beta 4 integrin, Schmidt-Lanterman Incisures
Introduction
Chronic nerve compression (CNC) injuries affect millions of individuals. Yet despite their prevalence, there has been little progression in the understanding of these injuries until recently. CNC injury produces sensory abnormalities within the distribution of the affected nerve including numbness, tingling, and pain. If left untreated, these symptoms may result in permanent sensory and motor loss [8]. Previous research has shown that CNC injury induces specific histological changes in the nerve including gradual demyelination [14], axonal sprouting [14], and concurrent Schwann cell proliferation and apoptosis beginning early after injury [16]. Interestingly, these histological changes occur in the absence of any overt axonal injury or macrophage recruitment commonly associated with acute nerve injuries and the process of Wallerian degeneration [12, 23]. Taken together these findings suggest that the pathology following CNC injury is primarily mediated by Schwann cell dedifferentiation and not by signals from injured axons. The molecular signals that cause the demyelination associated with CNC injury, however, have not been identified.
While the pathogenesis of chronic nerve compression has been found to be distinct from that of acute injuries [12, 14-16, 23][2-6,10,11], it is possible that demyelination in both chronic and acute insults is controlled by similar transcription factors. While it is accepted that the stimuli for modulation of transcription factors in acute nerve insults is axonal injury and the ensuing process of Wallerian degeneration, an alternate pathway must exist in eliciting a response from key transcription factors in the absence of axonal injury as seen in CNC injury. Studies have shown that c-jun upregulation in Schwann cells, regardless of phosphorylation state, is the principal negative regulator of myelination following acute injuries such as crush or transection of the nerve [25]. In tandem, levels of krox-20, a key promyelinating signal in Schwann cells, has been shown to decrease significantly after nerve injury [19, 30]. As such, we evaluated the expression of c-jun, phosphorylated c-jun, and krox-20 following CNC injury.
To identify possible upstream signal transducing elements, we investigated the response of integrins, specifically the β4 subunit of α6β4, to CNC injury. Integrins are heterodimeric membrane molecules that serve in the adhesion of cells to various extracellular matrix molecules including collagen, fibronectin, and laminin [31]. Due to their unique role as ligand-binding and mechanically-responsive signal transducers, integrins serve as attractive candidates in the signaling cascade following CNC injury. Of particular interest is the α6β4 integrin, which is the primary laminin receptor present in myelinating Schwann cells [5]. The β4 subunit of the α6β4 integrin is unique in its long cytoplasmic domain that interacts with key cell signaling pathways including the MAPK (mitogen activated protein kinase) [10]. The above characteristics of the α6β4 integrin have led many investigators to hypothesize a role for the α6β4 integrin in myelination. Selective knockout of α6β4 integrin in Schwann cells has revealed critical functions in myelin maintenance [24] and regeneration [32] following injury. As such, we evaluated the expression of the β4 subunit of the α6β4 integrin in addition to c-jun, phosphorylated c-jun, and krox-20 to begin to investigate the molecular events that coincide with demyelination following CNC injury.
Schwann cells in particular are highly responsive to the makeup of the extracellular matrix. For example, the various laminins [34, 35], collagens [2, 22], and gliomedin [6, 21] have been shown to influence Schwann cell processes including proliferation and myelination. As mechanical stimuli such as compression or shear stress have long been known to alter the extracellular matrix [4, 18, 20], it is possible that Schwann cells sense this altered environment using integrins. Furthermore, integrins have been implicated in the mechanotransduction of a variety of mechanical stimuli including stretch, hydrostatic pressure, shear stress, and osmotic pressure in a variety of different cell types [28]. Current research suggests that integrins transduce mechanical forces to alter gene activity in the nucleus through the use of cytoskeletal filaments [33]. As such, we hypothesize that Schwann cells respond to the mechanical stimuli provided by CNC injury via redistribution and activation of particular integrins, chiefly α6β4.
Methods
Surgical Technique
Using a gluteal-splitting approach, the right sciatic nerve of male Sprague-Dawley rats was exposed and atraumatically fitted with a biologic inert tube (Baxter Healthcare, Deerfield, IL) one centimeter in length with an internal diameter of 1.3 mm. The tube is of sufficient diameter to avoid any acute injury to the nerve. However, over time, the tube chronically compresses the nerve resulting in subsequent injury. To serve as a positive control, the same gluteal-splitting approach was used to the expose the right sciatic nerve, which was transected at the level of the sciatic notch [25]. The left sciatic nerve was mobilized and returned to its muscular bed to serve as an internal control. All experimental procedures and protocols were approved by the Institutional Animal Care and Use Committee of the University of California, Irvine.
Nerve Teasing
After harvest, nerve samples were placed in 4% PFA, followed by 10%, 20%, 30% sucrose gradient. Individual nerve fibers were then manually teased using ultra-fine forceps and a dissecting microscope.
β4/Phalloidin-TRITC Immunohistochemistry
Previous studies have identified phalloidin as a marker for Schmidt Lanterman Incisures (SLIs) due to the prevalence of f-actin at these sites [11, 29, 36]. To visualize integrin localization along the internode, samples were fixed, blocked, and then stained using monoclonal rabbit anti-β4 (1:600) overnight. After washes in PBS, samples were incubated in FITC-labeled goat anti-rabbit IgG (1:800) and Phalloidin TRITC (1:1000, Sigma).
Protein Quantification and Western Blot Analysis
Proteins were extracted at one, two, four, and 12 weeks post injury from the compressed segment of sciatic nerves with the contralateral nerve serving as a control (n=4). In addition, proteins were extracted from the distal segments of transected nerves at 1 day post transection to serve as a positive control (n=4). After the concentration of extracted samples were determined through optical density measurements using the Bradford Lowry Microassay (Bio-Rad Protein Assay Kit), appropriate amounts of sample were added to achieve 35 micrograms of protein. This methodology has been checked through the use of beta-actin and no discrepancy in the amount of protein loaded into each well has been detected. Proteins were then run on tris-HCl gels and transferred to PVDF membranes using standard procedures. Membranes were then probed with rabbit anti-c-jun (Cell Signaling Technologies, 1:1000), mouse anti-p-c-jun (Santa Cruz Biotechnologies, 1:500), rabbit anti-β4 (Santa Cruz Biotechnologies, 1:300), and rabbit anti-krox-20 (Covance 1:1000). Visualization was achieved using appropriate HRP secondary antibodies (Chemicon) and Chemiluminescence (Chemicon). Semi-quantification of Western blot was carried out using ImageJ (NIH) to obtain optical densities based on the areas under the profile curves. In total, western blot analysis was carried out for four different proteins (c-jun, p-c-jun, krox-20, and β4 integrin) at four different timepoints, not including positive controls. Proteins for the positive control were extracted 1 day post-transection. Three replicates for each protein and at each timepoint were done to ensure consistency in western blot results. Statistical significance was assessed using the standard Student’s t-test with p < 0.05 considered statistically significant.
Results
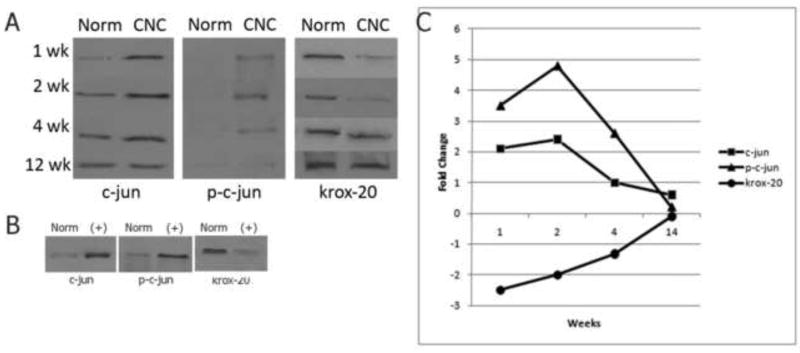
Previous studies have shown that both phosphorylated and unphosphorylated forms of the transcriptional factor c-jun play a primary role in Schwann cell demyelination following acute nerve injury [25]. As such, we evaluated the level of expression of c-jun and phosphorylated c-jun following CNC injury in order to determine if the demyelination observed in a chronic model is regulated by similar transcriptional factors. After one, two, and four weeks of CNC injury, there was a statistically significant increase (p<0.05) in the expression of both c-jun and phosphorylated c-jun (Fig.1A). The peak of c-jun and phosphorylated c-jun expression occurred at 4 weeks representing nearly a 2.5 fold and 4.9 fold increase above control levels, respectively (Fig.1C). After 12 weeks of CNC injury, the levels of c-jun and phosphorylated c-jun returned to normal (p>0.05). Transected nerves were used as a positive control for all proteins studied (Fig.1B).
Fig (1).
(A) Western blot analysis of c-jun, p-c-jun, and krox-20 expression at 1, 2, 4, and 12 weeks following CNC injury. Expression of c-jun and p-c-jun increases at 1, 2, and 4 week timepoint and returns to normal values at 12 weeks. Krox-20 expression decreases at the 1 week timepoint and similarly returns to normal values by 12 week timepoint. (B) Positive controls of c-jun, p-c-jun, and krox-20 expression. Transected nerves show appropriate increases in c-jun and p-c-jun and a concomitant decrease in krox-20 expression. All protein levels were quantified using Bradford microassay and equal amounts of protein were loaded in each well. (C) Quantification of changes in expression of c-jun, p-c-jun, and krox-20 following 1, 2, 4, and 12 weeks of CNC injury. C-jun, p-c-jun, and krox-20 levels return to normal by the 12 week timepoint.
Conversely, studies have identified krox-20 as a key positive regulator of myelination [30]. In acute injuries, a decrease in krox-20 expression is observed followed by a subsequent increase to normal levels in accordance with the process of demyelination and ensuing remyelination following acute nerve injury. As such, we evaluated the expression of krox-20 following CNC injury, and found that after one, two, and four weeks of CNC injury there was a statistically significant decrease (p<0.05) in the expression of krox-20 (Fig 1A). The lowest level of krox-20 was found one week after CNC injury and as with c-jun and phosphorylated c-jun, the expression of krox-20 returned to normal levels after 12 weeks of CNC injury (Fig. 1C).
The α6β4 integrin subunit has been shown to be important in the maintenance of myelin integrity and architecture, and in particular, the β4 subunit possesses a uniquely long cytoplasmic domain that has been shown to function in intracellular signaling [7, 24]. To determine the role of the β4 integrin in response to chronic nerve injury, we evaluated the levels of expression at 2 and 4 week timepoints following CNC injury to help determine if the β4 integrin maybe a potential signal transducer of the demyelinating response. After two and four weeks of CNC injury, there was a statistically significant (p<0.05) decrease in the expression of the β4 integrin (Fig. 2E). This decrease represented approximately 2/5 and 1/3 of control expression at two and four weeks, respectively (data not shown). This decreased level of β4 expression coincides temporally with changes observed in c-jun, phosphorylated c-jun, and krox-20 transcription factors.
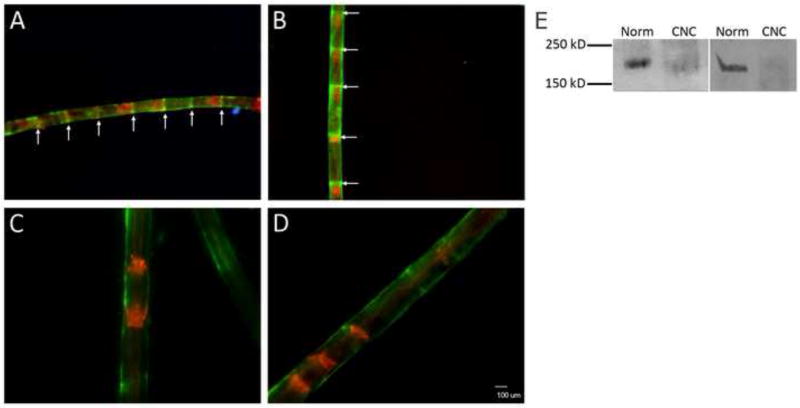
Fig (2).
Spatial and temporal relocalization of the integrin β4 following CNC injury. Immunostaining of compressed (A,B) and normal (C,D) nerves 1 week (left) and 2 weeks (right) post-CNC injury. Phalloidin-TRITC (red) staining reveals F-actin at the SLIs. β4 (green) colocalization at SLIs (arrows) is seen in compressed samples but not normal. DAPI identifies Schwann cell nuclei in blue. (E) Western blot analysis of β4 expression 2 weeks (left) and 4 weeks (right) following CNC injury shows a decrease level of expression of the 205 kDa β4 compared to the normal at both timepoints.
Immunohistochemistry was performed in order to determine the spatial relocalization of the β4 integrin following CNC injury. Phalloidin-TRITC / β4 doublestaining studies reveal a spatial redistribution of the integrin at one, two, four, and eight week timepoints. Specifically, discrete localization of β4 to the Schmidt-Lanterman Incisures (SLIs) is observed at each of the timepoints evaluated from one to eight weeks post-injury (Fig 2A-D). Co-localization of β4 with the f-actin expressed at the SLIs confirms integrin turnover at these unique regions of uncompact myelin.
Discussion
As early as one week post-CNC injury, an increase in the expression of c-jun and phosphorylated c-jun as well as a concomitant decrease in the expression of krox-20 is observed. In addition, all changes in transcription factor expression returned to normal values by the twelve week timepoint. These temporal fluctuations in transcription factor expression correspond with previously reported histological changes observed in the same chronic nerve injury model and correlate with the process of demyelination. As early as two weeks after CNC injury, Schwann cells downregulate myelin proteins and undergo concurrent proliferation and apoptosis resulting in demyelination of the compressed segment [14-17]. The temporal correlation between the changes in c-jun and krox-20 levels and the previously observed histological changes suggest that the increase in c-jun expression drives the program of Schwann cell dedifferentiation. Furthermore, the antagonistic relationship between c-jun and krox-20 and their effect on myelination has been previously characterized in acute nerve injuries [25]. These findings suggest that while there are fundamental differences in the pathogenesis of acute and chronic injuries, demyelination in both injury models is under the direction of the same transcription factors.
Additionally, the change in transcription factor expression is temporally correlated with a decrease in the β4 integrin subunit. As myelinating Schwann cells are known to express the α6β4 integrin[7], the decrease in β4 is similarly consistent with the program of Schwann cell dedifferentiation. The β4 integrin has been identified as an important modulator of intracellular signaling pathways, including the MAPK pathway, which interestingly, is known to facilitate expression of the transcription factor c-jun [9, 25]. It is plausible therefore, that the expression of these transcription factors is linked via signaling cascades that are activated following CNC injury by the β4 integrin, which acts as a signal transducer following CNC injury and thus plays a role in Schwann dedifferentiation.
In support of this, spatial relocalization of the β4 integrin to the Schmidt-Lanterman Incisures suggests an alternative role for the α6β4 integrin following injury at a time when CNC induces concomitant demyelination and remyelination. Previous studies have shown an increase in the frequency of SLIs following CNC [1]. SLIs are known as unstable regions of uncompact myelin that contain a variety of cell-signaling molecules and are thought to respond most quickly to axonal perturbations. As such, the increase in SLIs has been suggested to facilitate the communication and nutritive necessities of the nerve following CNC injury. Accordingly, β4 localization at these highly reactive regions suggests a possible mechanism by which the SLIs interact with the extracellular matrix to satiate the increased communicative and metabolic demands associated with demyelination and remyelination.
In acute nerve injuries, it is generally accepted that the signal for c-jun upregulation, krox-20 downregulation, and resultant demyelination is axonal injury. CNC injury, however, is characterized by the absence of axonal injury in the early stages of injury [23]. The functional properties of integrins in activating intracellular processes in response to both injury-induced changes in the makeup of the extracellular matrix and to direct mechanical stimulation, make integrins ideal candidates as initiators of the signaling events that follow CNC injury. The α6β4 integrin is the major laminin receptor present in adult Schwann cells and has been reported as an important regulator of myelin maintenance [24]. We report here that the β4 subunit of the α6β4 integrin undergoes changes in both expression and localization at a time when there is both histologic evidence of demyelination and modulation of the appropriate transcription factors.
If integrins modulate transcription factor expression, the question still remains as to how the α6β4 integrin itself is activated following CNC injury. One possibility is that the α6β4 integrin directly responds to the mechanical stimuli provided by compression of the nerve. Integrins have been implicated in the transduction of a variety of mechanical stimuli including stretch, hydrostatic pressure, shear stress, and osmotic pressure in various cell types [28]. Current studies propose that integrins transduce mechanical forces to alter gene activity in the nucleus through the use of cytoskeletal filaments [33]. As such, it is possible that Schwann cells are directly sensitive to mechanical stimuli via integrin activation.
Alternatively, integrins may signal Schwann cells to demyelinate secondary to alterations in the composition of the extracellular matrix. Mechanical stimuli such as compression or shear stress have long been known to alter the extracellular matrix [4, 18, 20]. For example, fibroblasts are known to alter the expression of collagens and tenascin-C in response to mechanical stimuli [27]. Furthermore, research suggests that extracellular matrix molecules are critical in the modulation of Schwann cell phenotype [3]. In support of this, the various laminins [34, 35], collagens [2, 22], and gliomedin [6, 21] have all been shown to influence Schwann cell processes including proliferation and myelination. Any changes in the makeup of the extraceullar matrix may thus influence Schwann cell phenotype following CNC injury. As bridges between intracellular activity and the extracellular matrix, integrins are likely to alert the Schwann cell to any alterations in the extracellular environment. The relocalization of integrin α6β4 to the SLIs, which facilitate interaction between the Schwann cell and the extracellular matrix, supports this idea. A rigorous evaluation of the changes that occur in the extracellular matrix following CNC injury is thus necessary. In the absence of any overt axonal pathology, demyelination is the principle pathological finding in CNC injury. Understanding the triggers for demyelination in CNC injury is thus of high importance in the development of new therapies.
Additional research is needed to explore the possible link between integrins and the transcription factors controlling myelination. Specifically, function-activating and function-blocking antibodies can be used to selectively activate or block integrin signaling in order to ascertain the influence of integrins on transcription factor expression. Such experiments will need to be carried out in both in vitro models and in murine in vivo models, which allow for the use of genetically altered knockout mice, to prove the existence of such a pathway. Recently, both a hydrostatic compression chamber model that can be used to apply hydrostatic pressure to myelinating Schwann cell and dorsal root ganglion co-cultures in vitro [26] and an in vivo CNC murine model [13] have been described. These newly described models will be critical in investigating the triggers for demyelination following CNC injury.
Acknowledgments
We are grateful to the members of the Peripheral Nerve Research Lab for all of their help. Funding for this work was provided by NIH/NINDS NS02221 & NS049203.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Berger BL, Gupta R. Demyelination secondary to chronic nerve compression injury alters Schmidt-Lanterman incisures. J Anat. 2006;209:111–118. doi: 10.1111/j.1469-7580.2006.00561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carey DJ, Todd MS. Schwann cell myelination in a chemically defined medium: demonstration of a requirement for additives that promote Schwann cell extracellular matrix formation. Brain Res. 1987;429:95–102. doi: 10.1016/0165-3806(87)90142-8. [DOI] [PubMed] [Google Scholar]
- 3.Chernousov MA, Yu WM, Chen ZL, Carey DJ, Strickland S. Regulation of Schwann cell function by the extracellular matrix. Glia. 2008;56:1498–1507. doi: 10.1002/glia.20740. [DOI] [PubMed] [Google Scholar]
- 4.Chiquet M. Regulation of extracellular matrix gene expression by mechanical stress. Matrix Biol. 1999;18:417–426. doi: 10.1016/s0945-053x(99)00039-6. [DOI] [PubMed] [Google Scholar]
- 5.Einheber S, Milner TA, Giancotti F, Salzer JL. Axonal regulation of Schwann cell integrin expression suggests a role for alpha 6 beta 4 in myelination. The Journal of cell biology. 1993;123:1223–1236. doi: 10.1083/jcb.123.5.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eshed Y, Feinberg K, Poliak S, Sabanay H, Sarig-Nadir O, Spiegel I, Bermingham JR, Jr, Peles E. Gliomedin mediates Schwann cell-axon interaction and the molecular assembly of the nodes of Ranvier. Neuron. 2005;47:215–229. doi: 10.1016/j.neuron.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 7.Feltri ML, Scherer SS, Nemni R, Kamholz J, Vogelbacker H, Scott MO, Canal N, Quaranta V, Wrabetz L. Beta 4 integrin expression in myelinating Schwann cells is polarized, developmentally regulated and axonally dependent. Development. 1994;120:1287–1301. doi: 10.1242/dev.120.5.1287. [DOI] [PubMed] [Google Scholar]
- 8.Gerritsen AA, de Vet HC, Scholten RJ, Bertelsmann FW, de Krom MC, Bouter LM. Splinting vs surgery in the treatment of carpal tunnel syndrome: a randomized controlled trial. JAMA. 2002;288:1245–1251. doi: 10.1001/jama.288.10.1245. [DOI] [PubMed] [Google Scholar]
- 9.Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- 10.Giancotti FG, Tarone G. Positional control of cell fate through joint integrin/receptor protein kinase signaling. Annu Rev Cell Dev Biol. 2003;19:173–206. doi: 10.1146/annurev.cellbio.19.031103.133334. [DOI] [PubMed] [Google Scholar]
- 11.Gould RM, Byrd AL, Barbarese E. The number of Schmidt-Lanterman incisures is more than doubled in shiverer PNS myelin sheaths. Journal of neurocytology. 1995;24:85–98. doi: 10.1007/BF01181552. [DOI] [PubMed] [Google Scholar]
- 12.Gray M, Palispis W, Popovich PG, van Rooijen N, Gupta R. Macrophage depletion alters the blood-nerve barrier without affecting Schwann cell function after neural injury. J Neurosci Res. 2007;85:766–777. doi: 10.1002/jnr.21166. [DOI] [PubMed] [Google Scholar]
- 13.Gupta, Bathen, Hazel, Palispis, Rummler, Strandberg, Mozaffar . Society for Neuroscience, 2008. Neuroscience Meeting Planner; Washington, DC: 2008. Development of a novel murine model of primary demyelinating neuropathy. [Google Scholar]
- 14.Gupta R, Rowshan K, Chao T, Mozaffar T, Steward O. Chronic nerve compression induces local demyelination and remyelination in a rat model of carpal tunnel syndrome. Exp Neurol. 2004;187:500–508. doi: 10.1016/j.expneurol.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 15.Gupta R, Rummler LS, Palispis W, Truong L, Chao T, Rowshan K, Mozaffar T, Steward O. Local down-regulation of myelin-associated glycoprotein permits axonal sprouting with chronic nerve compression injury. Exp Neurol. 2006;200:418–429. doi: 10.1016/j.expneurol.2006.02.134. [DOI] [PubMed] [Google Scholar]
- 16.Gupta R, Steward O. Chronic nerve compression induces concurrent apoptosis and proliferation of Schwann cells. J Comp Neurol. 2003;461:174–186. doi: 10.1002/cne.10692. [DOI] [PubMed] [Google Scholar]
- 17.Gupta R, Truong L, Bear D, Chafik D, Modafferi E, Hung CT. Shear stress alters the expression of myelin-associated glycoprotein (MAG) and myelin basic protein (MBP) in Schwann cells. J Orthop Res. 2005;23:1232–1239. doi: 10.1016/j.orthres.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 18.Kirwan RP, Fenerty CH, Crean J, Wordinger RJ, Clark AF, O’Brien CJ. Influence of cyclical mechanical strain on extracellular matrix gene expression in human lamina cribrosa cells in vitro. Mol Vis. 2005;11:798–810. [PubMed] [Google Scholar]
- 19.Le N, Nagarajan R, Wang JY, Araki T, Schmidt RE, Milbrandt J. Analysis of congenital hypomyelinating Egr2Lo/Lo nerves identifies Sox2 as an inhibitor of Schwann cell differentiation and myelination. Proc Natl Acad Sci U S A. 2005;102:2596–2601. doi: 10.1073/pnas.0407836102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MacKenna D, Summerour SR, Villarreal FJ. Role of mechanical factors in modulating cardiac fibroblast function and extracellular matrix synthesis. Cardiovasc Res. 2000;46:257–263. doi: 10.1016/s0008-6363(00)00030-4. [DOI] [PubMed] [Google Scholar]
- 21.Maertens B, Hopkins D, Franzke CW, Keene DR, Bruckner-Tuderman L, Greenspan DS, Koch M. Cleavage and oligomerization of gliomedin, a transmembrane collagen required for node of ranvier formation. J Biol Chem. 2007;282:10647–10659. doi: 10.1074/jbc.M611339200. [DOI] [PubMed] [Google Scholar]
- 22.Moya F, Bunge MB, Bunge RP. Schwann cells proliferate but fail to differentiate in defined medium. Proc Natl Acad Sci U S A. 1980;77:6902–6906. doi: 10.1073/pnas.77.11.6902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mozaffar T, Strandberg E, Abe K, Hilgenberg LG, Smith MA, Gupta R. Neuromuscular junction integrity after chronic nerve compression injury. J Orthop Res. 2008 doi: 10.1002/jor.20704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nodari A, Previtali SC, Dati G, Occhi S, Court FA, Colombelli C, Zambroni D, Dina G, Del Carro U, Campbell KP, Quattrini A, Wrabetz L, Feltri ML. Alpha6beta4 integrin and dystroglycan cooperate to stabilize the myelin sheath. J Neurosci. 2008;28:6714–6719. doi: 10.1523/JNEUROSCI.0326-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parkinson DB, Bhaskaran A, Arthur-Farraj P, Noon LA, Woodhoo A, Lloyd AC, Feltri ML, Wrabetz L, Behrens A, Mirsky R, Jessen KR. c-Jun is a negative regulator of myelination. The Journal of cell biology. 2008;181:625–637. doi: 10.1083/jcb.200803013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rummler L, Palispis W, Gupta R. Society for Neuroscience 2008. Washington DC: 2008. A novel hydrostatic compression chamber model for the study of chronic nerve compression injuries in vitro - 351.9. [Google Scholar]
- 27.Sarasa-Renedo A, Chiquet M. Mechanical signals regulating extracellular matrix gene expression in fibroblasts. Scand J Med Sci Sports. 2005;15:223–230. doi: 10.1111/j.1600-0838.2005.00461.x. [DOI] [PubMed] [Google Scholar]
- 28.Schwartz MA, Desimone DW. Cell adhesion receptors in mechanotransduction. Current opinion in cell biology. 2008 doi: 10.1016/j.ceb.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith-Slatas C, Barbarese E. Myelin basic protein gene dosage effects in the PNS. Molecular and cellular neurosciences. 2000;15:343–354. doi: 10.1006/mcne.1999.0829. [DOI] [PubMed] [Google Scholar]
- 30.Topilko P, Schneider-Maunoury S, Levi G, Baron-Van Evercooren A, Chennoufi AB, Seitanidou T, Babinet C, Charnay P. Krox-20 controls myelination in the peripheral nervous system. Nature. 1994;371:796–799. doi: 10.1038/371796a0. [DOI] [PubMed] [Google Scholar]
- 31.van der Flier A, Sonnenberg A. Function and interactions of integrins. Cell Tissue Res. 2001;305:285–298. doi: 10.1007/s004410100417. [DOI] [PubMed] [Google Scholar]
- 32.Van der Zee CE, Kreft M, Beckers G, Kuipers A, Sonnenberg A. Conditional deletion of the Itgb4 integrin gene in Schwann cells leads to delayed peripheral nerve regeneration. J Neurosci. 2008;28:11292–11303. doi: 10.1523/JNEUROSCI.3068-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang N, Tytell JD, Ingber DE. Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nat Rev Mol Cell Biol. 2009;10:75–82. doi: 10.1038/nrm2594. [DOI] [PubMed] [Google Scholar]
- 34.Yu WM, Feltri ML, Wrabetz L, Strickland S, Chen ZL. Schwann cell-specific ablation of laminin gamma1 causes apoptosis and prevents proliferation. J Neurosci. 2005;25:4463–4472. doi: 10.1523/JNEUROSCI.5032-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu WM, Yu H, Chen ZL. Laminins in peripheral nerve development and muscular dystrophy. Mol Neurobiol. 2007;35:288–297. doi: 10.1007/s12035-007-0026-x. [DOI] [PubMed] [Google Scholar]
- 36.Zimmermann H. Accumulation of synaptic vesicle proteins and cytoskeletal specializations at the peripheral node of Ranvier. Microscopy research and technique. 1996;34:462–473. doi: 10.1002/(SICI)1097-0029(19960801)34:5<462::AID-JEMT6>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]