Abstract
The DNA polymerase-gamma (POLG) gene, which encodes the catalytic subunit of enzyme responsible for directing mitochondrial DNA replication in humans, contains a polyglutamine tract encoded by CAG repeats of varying length. The length of the CAG repeat has been associated with the risk of testicular cancer, and other genomic variants that impact mitochondrial function have been linked to breast cancer risk in African-American (AA) women. We evaluated the potential role of germline POLG-CAG repeat variants in breast cancer risk in a sample of AA women (100 cases and 100 age-matched controls) who participated in the Women's Circle of Health Study, an ongoing multi-institutional, case-control study of breast cancer. Genotyping was done by fragment analysis in a blinded manner. Results from this small study suggest the possibility of an increased risk of breast cancer in women with minor CAG repeat variants of POLG, but no statistically significant differences in CAG repeat length were observed between cases and controls (multivariate-adjusted odds ratio 1.74; 95% CI, 0.49–6.21). Our study suggests that POLG-CAG repeat length is a potential risk factor for breast cancer that needs to be explored in larger population-based studies.
Introduction
Despite the overall higher incidence of breast cancer in European-American (EA) women as compared to African-American (AA) women, AA women are more likely to be diagnosed with breast cancer before age 40 and to have tumors with aggressive pathological characteristics, including high tumor grade, lack of expression of estrogen and progesterone receptors (ER, PR) and HER2 (triple-negative breast cancers), and additional features of basal-like breast cancer (ER−/PR−/HER2−/cy5/6+/EGFR+) [1]. Triple-negative and basal-like breast cancers are recognized to be associated with a considerably poorer prognosis than other breast cancer subtypes [2]. Although the role of genetic factors, including polymorphisms in the BRCA1, BRCA2, ATM, CHEK2, p53, PTEN, NBS1, RAD50, BRIP1, and PALB2 genes have been extensively studied in breast cancer in EA populations [3], there are few studies of the role of common variants in nuclear gene(s) related to mitochondrial function in the etiology of breast cancer in AA women.
A recent study demonstrated that tumor-cell mitochondrial DNA copy (mtDNA) number correlates with tumor progression as well as patient prognosis and disease-free survival in breast cancer [4]. Cellular mtDNA copy number (or content) is controlled by the nuclear-encoded POLG gene encoding the only known mtDNA polymerase (polymerase-gamma) in humans. Our previous research suggests that mutations in the POLG gene may result in depletion of mtDNA and confer breast cancer phenotype [5]. Human POLG consists of an exonuclease domain with three exonuclease motifs, I, II and III, and a polymerase domain with three polymerase motifs, A, B and C, along with an intervening linker region [6]. The exonuclease domain of POLB, which is responsible for the proof-reading activity of the encoded enzyme, harbors a CAG repeat region in exon 2. The contraction of CAG repeats in POLG affects its expression [7], and an expanded CAG repeat sequence seems to confer toxic functions on the protein through protein-protein interactions [8]. The CAG repeat polymorphism has been studied in several diseases, including male infertility and neurodegeneration [9]–[11]. An association of the POLG-CAG repeat expansion with testicular cancer has also been reported [12]. Previous studies have also suggested that variation in the number of CAG repeats in androgen- and estrogen-receptor genes influence the risk of breast cancer [13]. However, the significance of the CAG repeat polymorphism of POLG in breast cancer has not been investigated. The purpose of this study was to evaluate the association of POLG CAG repeat length with breast cancer risk in the Women's Circle of Health Study (WCHS), an epidemiological case-control study of breast cancer in AA and EA women.
Materials and Methods
Study subjects
The data and samples from 100 AA women with breast cancer and 100 age-matched AA controls for this study were obtained from the Women's Circle of Health Study (WCHS), a case-control study specifically designed to investigate risk factors for early, aggressive breast cancer in AA women. The WCHS has been previously described [14]. Briefly, women with incident, primary breast cancer were identified through both hospital-based case ascertainment at hospitals in the New York City area and population-based case ascertainment in seven counties in northern New Jersey through the New Jersey State Cancer Registry, a National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) registry. Controls without breast cancer were identified by random-digit dialing and matched to cases by telephone prefixes and 5-year age intervals. All study participants completed an in-person, structured interview to obtain information about lifestyle, reproductive, and medical histories, demographics and other variables; AA race was determined by self-report. Participants provided either a blood or saliva sample at enrollment. Body mass index (BMI) was assessed by measuring height and weight at the interview and was calculated as weight in kilograms (kg) divided by height in meters squared (m2). All study subjects provided written informed consent for the study and use of biospecimens, and the study was approved by the Institutional Review Boards at Mount Sinai School of Medicine (MSSM) (coordinating site for NY enrollment and NYC controls), the local NYC hospital (for cases), The Cancer Institute of New Jersey (coordinating site for NJ enrollments) as well as at Roswell Park Cancer Institute IRB approval number I-120807.
DNA isolation and PCR
Genomic DNA was extracted from whole blood and evaluated for purity and concentration using a Nanodrop UV spectrophotometer and quantified on a spectrofluorometer (Gemini XS SPECTRAmax, Molecular Devices, Sunnyvale, CA, USA) using the PicoGreen dsDNA quantification kit, as per the manufacturer's instructions (Invitrogen, Carlsbad, CA, USA).. Double-stranded DNA was quantitated using a PicoGreen-based fluorometric assay. Primers (POLG_F: 6FAM-tggatgtccaatgggttgtgc and POLG_R: aagccaggtgttctgactcc) were designed to amplify a 275-bp fragment encompassing CAG repeats present in exon 2 of POLG1. The amplification was carried out in a 25 ul reaction containing approximately 50 ng of template DNA, 0.6 uM of both forward and reverse primers, and 0.5 ul AccuPrime™ Taq DNA polymerase (Invitrogen, USA) using a TECHNE TC-412 (96×0.2 ml) thermocycler (MIDSCI, USA). The PCR conditions were as follows: initial denaturation at 94°C for 4 min in 26 amplification cycles at 94°C for 10 sec, annealing at 61°C for 30 sec and 68°C for 40 sec, followed by a final extension step at 68°C for 5 min.
Genotyping assays
Fluorescently labeled fragments generated by PCR were analyzed on ABI PRISM 3130XL (Applied biosystems, USA), according to the DNA fragment analysis protocol. DNA fragment data were collected and then visualized using GeneMapper® software. The data containing the genomic location (peak size) for all samples were exported as delimited text files and blindly analyzed for the number of CAG repeats in each sample. Assay reproducibility was tested using multiple runs of both positive and negative samples with different CAG repeat variants.
Statistical Analysis
Differences between categorical variables were assessed with the chi-square test, and continuous variables were compared using Student's t-test. All statistical tests were two-sided. Age at menarche and the number of children were divided into tertiles using cut-points based on the distribution of these variables among controls. Age at first pregnancy was categorized as nulliparous, ≤19, 20–24, 25–29, or ≥30. Menopausal status at the time of cancer diagnosis was determined during the interview and categorized as premenopausal, perimenopausal, or postmenopausal. Crude and multivariate unconditional logistic regression models adjusted for age, age at menarche, age at first full-term pregnancy, menopausal status (menopausal, perimenopausal, postmenopausal), first-degree family history of breast cancer (yes/no) and BMI were used to calculate odds ratios (OR) and corresponding 95% confidence intervals (CI) as a measure of association between CAG repeats of mitochondrial DNA POLG and breast cancer risk. All statistical analyses were computed with SAS 9.2 (SAS Institute, Cary, NC, USA).
Results
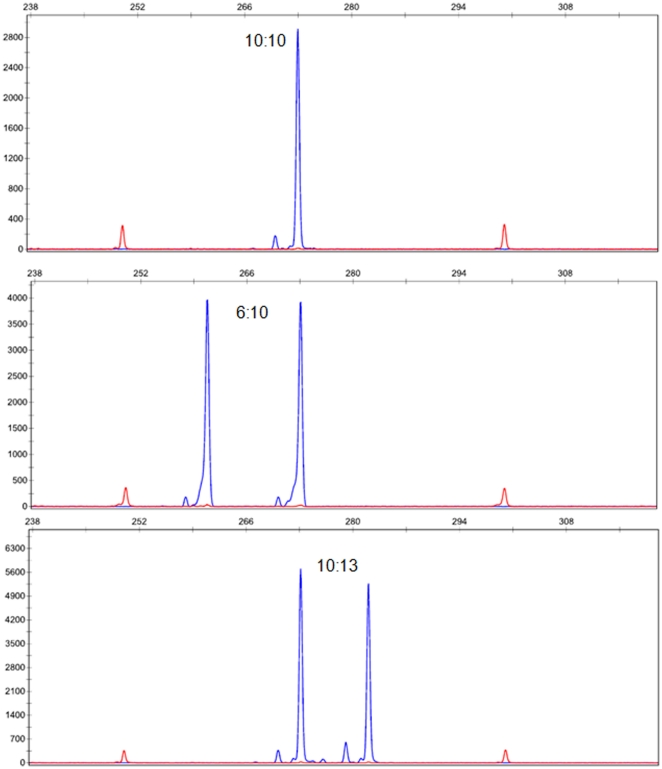
The CAG fragment analysis was done in a blinded manner and repeat length was carefully assigned based on the peak size. After the initial observation, we eliminated 12 samples because they showed poor signal due to inadequate DNA. In this subset of the WCHS, there were no statistically significant differences between cases and controls with respect to age at menarche, age at first pregnancy, number of children, menopausal status, and family history of breast cancer (Table 1). We identified 7 breast cancer cases (7%) and 6 controls (6%) in which the common 10/10 POLG-CAG repeat was absent. The common 10/10 CAG repeat occurred in 52 cases (55%) and 56 controls (60%); heterozygous 10/non-10 CAG repeats were observed in 36 cases (38%) and 31 controls (33%) (Table 2). The representative CAG repeat contraction and expansion variants are shown as electropherograms (Figure 1).
Table 1. Selected characteristics of Cases and Controls in WCHS with POLG-CAG repeat polymorphism.
| Characteristic | Cases (n = 95)n (%) | Controls (n = 93)n (%) | p1 |
| POLG | 0.75 | ||
| 10/10 | 52 (54.7) | 56 (60.2) | |
| 10/not 10 | 36 (37.9) | 31 (33.3) | |
| Not 10/not 10 | 7 (7.4) | 6 (6.5) | |
| Age at menarche | 0.68 | ||
| ≤12 | 50 (53.2) | 44 (47.3) | |
| 12.5–13 | 20 (21.3) | 24 (25.8) | |
| 13.5+ | 24 (25.5) | 25 (26.9) | |
| Age at first pregnancy | 0.95 | ||
| Nulliparous | 20 (21.1) | 22 (26.7) | |
| ≤19 | 23 (24.2) | 19 (20.4) | |
| 20–24 | 26 (27.4) | 25 (26.9) | |
| 25–29 | 16 (16.8) | 15 (16.1) | |
| 30+ | 10 (10.5) | 12 (12.9) | |
| Number of children | 0.86 | ||
| 0–1 | 42 (44.2) | 42 (45.2) | |
| 2–3 | 37 (39.0) | 38 (40.9) | |
| 4+ | 16 (16.8) | 13 (14.0) | |
| Menopausal Status | 0.84 | ||
| Premenopausal | 35 (42.2) | 34 (39.1) | |
| Perimenopausal | 19 (22.9) | 23 (26.4) | |
| Postmenopausal | 31 (36.5) | 31 (35.2) | |
| First degree relative with breast cancer | 0.57 | ||
| No | 80 (84.2) | 81 (87.1) | |
| Yes | 15 (15.8) | 12 (12.9) |
Chi-square test.
t-test.
Table 2. Odd ratios of POLG-CAG repeat polymorphism in WCHS.
| Crude OR(95% CI) | AdjustedOR1 (95% CI) | |
| POLG | ||
| 10/10 | 1.0 (ref) | 1.0 (ref) |
| 10/not 10 | 1.3 (0.68–2.30) | 1.5 (0.73–2.96) |
| Not 10/not 10 | 1.3 (0.40–3.98) | 1.7 (0.41–7.46) |
Adjusted for age, age at menarche, age at first full term pregnancy, menopausal status, first degree family history of breast cancer, and BMI.
Figure 1. Representative electopherogram of normal CAG (10∶10), contraction (6∶10) and expansion (10∶13) of POLG.
Compared with 10/10 repeats, the crude odds ratios (ORs) for 10/11, 10/12 repeats, and all other repeats were 1.16 (95% CI, 0.58–2.32), 0.93 (95% CI, 0.36–2.35), and 1.31 (95% CI, 0.46–3.76), respectively (Table 1). After adjustment for age, age at menarche, age at first full-term pregnancy, menopausal status, first-degree family history of breast cancer, and BMI, the adjusted ORs for 10/11 and 10/12 repeats were 1.15 (95% CI, 0.50–2.63) and 1.25 (95% CI, 0.45–3.46), respectively. In the case of CAG repeats other than 10/11 or 10/12, point estimates suggested an increased risk of breast cancer, but no statistically significant difference was observed in the frequency of CAG repeats between cases and controls (OR 1.74; 95% CI, 0.49–6.21, Table 2).
Discussion
Mitochondrial POLG is the only polymerase known to be involved in human mitochondrial DNA replication. POLG contains CAG trinucleotide repeats in the coding region, and CAG repeat sequences are described to be highly unstable, leading to expansion or contraction of the repeat sequences [8]. In a recent study of the POLG gene, we reported the CAG repeat expansion occurs in 20% of breast cancer patients [5]. This pilot study suggests the possibility of an association between altered POLG-CAG repeat-length and an increased risk of breast cancer in AA women.
The presence of expanded or contracted CAG repeats has been linked with several diseases, including the human polyglutamine diseases [8]. Other epidemiologic studies have shown either a positive or negative correlation of CAG repeats with sporadic breast cancer, and one study reported an inverse correlation between POLG-CAG repeat length (r = −0.81) and the age of onset of disease in Friedreich's Ataxia (FRDA) patients [13]. In another study dealing with myotonic dystrophy, no significant difference in the frequency of CAG repeats was seen between cases and controls [7]. Studies of CAG-repeat polymorphisms in antiretroviral therapy-associated peripheral neuropathy similarly showed no significant associations [15]. However, CAG repeat-length polymorphisms have been associated with male infertility; the common 10-repeat variant of the CAG repeat was found to be significantly more prevalent among men with oligospermia [9] and unexplained subfertility [16].
There are two additional reports associating POLG-CAG repeat variants with cancer risk. Interestingly, a study of CAG repeat polymorphisms in the POLG gene in testicular cancer showed 36 (74%) wild-type homozygotes and 13 (26%) lacked one or both wild-type alleles, with the 10/11 variant in 10 patients and the 10/12, 10/6 and 11/11 variants in one patient each, suggesting that variants of the DNA POLG1 gene were more frequent in testicular cancer patients than in healthy men [12]. Another study suggested that the CAG polymorphism in POLG may be a contributing factor in the pathogenesis of testicular seminoma [17]. It should be emphasized, however, that existing studies in of POLG-CAG repeat variants in cancer compared the frequency of these variants in tumor tissue to the frequency in either surrounding normal tissue or blood samples. In contrast, our study evaluated germline polymorphisms in POLG in both breast cancer cases and in controls. Results of studies may differ depending on the DNA source due to additional clonal mutations that can occur in tumor cells.
This study is the first to investigate the role of CAG repeat polymorphisms in the nuclear POLG gene, which encodes mitochondrial DNA polymerase-gamma, in breast cancer risk. While our findings suggest a possible correlation between CAG repeat length and risk of breast cancer in AA women, the size of this genetic study sample was small, as evidenced by reduced power to demonstrate significant associations of known breast cancer risk factors such as age at menarche and family history of breast cancer with cancer risk in this study. Larger, preferably population-based, studies are needed in order to draw any firm conclusions about the role of POLG-CAG variants in breast cancer risk in AA or other populations.
Acknowledgments
We thank members of the Singh laboratory for critical reading of the manuscript and Ms. Paula Jones and Carrol Thorn for editing the manuscript.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study was supported by grants from the National Institutes of Health (R01 121904 and 116430 and R21 153097) to KS. The WCHS is supported by grants from the U.S. Army Medical Research and Material Command (USARMMC) (DAMD-17-01-1-0334), the National Cancer Institute (R01 CA100598), and the Breast Cancer Research Foundation. The New Jersey State Cancer Registry (NJSCR) is a participant in the Centers for Disease Control and Prevention's National Program of Cancer Registries (NPCR) and is a National Cancer Institute SEER Expansion Registry. The authors acknowledge the Centers for Disease Control and Prevention for its support of the NJSCR under cooperative agreement DP07-703 awarded to the New Jersey Department of Health & Senior Services. The collection of State of New Jersey cancer incidence data is also supported by the National Cancer Institute's SEER Program under contract N01-PC-95001-20. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Amend K, Hicks D, Ambrosone CB. Breast cancer in African-American women: differences in tumor biology from European-American women. Cancer Res. 2006;66:8327–8330. doi: 10.1158/0008-5472.CAN-06-1927. [DOI] [PubMed] [Google Scholar]
- 2.Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 3.Walsh T, King MC. Ten genes for inherited breast cancer. Cancer Cell. 2007;11:103–105. doi: 10.1016/j.ccr.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 4.Yu M, Zhou Y, Shi Y, Ning L, Yang Y, et al. Reduced mitochondrial DNA copy number is correlated with tumor progression and prognosis in Chinese breast cancer patients. IUBMB Life. 2007;59:450–457. doi: 10.1080/15216540701509955. [DOI] [PubMed] [Google Scholar]
- 5.Singh KK, Ayyasamy V, Owens KM, Koul MS, Vujcic M. Mutations in mitochondrial DNA polymerase- promote breast tumorigenesis. J Hum Genet. 2009;54:516–524. doi: 10.1038/jhg.2009.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nguyen KV, Ostergaard E, Ravn SH, Balslev T, Danielsen ER, et al. POLG mutations in Alpers syndrome. Neurology. 2005;65:1493–1495. doi: 10.1212/01.wnl.0000182814.55361.70. [DOI] [PubMed] [Google Scholar]
- 7.Rovio A, Tiranti V, Bednarz AL, Suomalainen A, Spelbrink JN, et al. Analysis of the trinucleotide CAG repeat from the human mitochondrial DNA polymerase gene in healthy and diseased individuals. Eur J Hum Genet. 1999;7:140–146. doi: 10.1038/sj.ejhg.5200244. [DOI] [PubMed] [Google Scholar]
- 8.Lim J, Crespo-Barreto J, Jafar-Nejad P, Bowman AB, Richman R, et al. Opposing effects of polyglutamine expansion on native protein complexes contribute to SCA1. Nature. 2008;452:713–718. doi: 10.1038/nature06731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rovio AT, Marchington DR, Donat S, Schuppe HC, Abel J, et al. Mutations at the mitochondrial DNA polymerase (POLG) locus associated with male infertility. Nat Genet. 2001;29:261–262. doi: 10.1038/ng759. [DOI] [PubMed] [Google Scholar]
- 10.Eerola J, Luoma PT, Peuralinna T, Scholz S, Paisan-Ruiz C, et al. POLG1 polyglutamine tract variants associated with Parkinson's disease. Neurosci Lett. 2010;477:1–5. doi: 10.1016/j.neulet.2010.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heidari MM, Houshmand M, Hosseinkhani S, Nafissi S, Scheiber-Mojdehkar B, et al. Association between trinucleotide CAG repeats of the DNA polymerase gene (POLG) with age of onset of Iranian Friedreich's ataxia patients. Neurol Sci. 2008;29:489–493. doi: 10.1007/s10072-008-1026-y. [DOI] [PubMed] [Google Scholar]
- 12.Nowak R, Zub R, Skoneczna I, Sikora K, Ligaj M. CAG repeat polymorphism in the DNA polymerase gene in a Polish population: an association with testicular cancer risk. Ann Oncol. 2005;16:1211–1212. doi: 10.1093/annonc/mdi205. [DOI] [PubMed] [Google Scholar]
- 13.Tsezou A, Tzetis M, Gennatas C, Giannatou E, Pampanos A, et al. Association of repeat polymorphisms in the estrogen receptors alpha, beta (ESR1, ESR2) and androgen receptor (AR) genes with the occurrence of breast cancer. Breast. 2008;17:159–166. doi: 10.1016/j.breast.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 14.Ambrosone CB, Ciupak GL, Bandera EV, Jandorf L, Bovbjerg DH, et al. Conducting Molecular Epidemiological Research in the Age of HIPAA: A Multi-Institutional Case-Control Study of Breast Cancer in African-American and European-American Women. J Oncol. 2009:871250 p. doi: 10.1155/2009/871250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen X, Goudsmit J, van der Kuyl AC. Lack of correlation between length variation in the DNA polymerase gene CAG repeat and lactic acidosis or neuropathy during antiretroviral treatment. AIDS Res Hum Retroviruses. 2002;18:531–534. doi: 10.1089/088922202753747879. [DOI] [PubMed] [Google Scholar]
- 16.Jensen M, Leffers H, Petersen JH, Nyboe AA, Jorgensen N, et al. Frequent polymorphism of the mitochondrial DNA polymerase gene (POLG) in patients with normal spermiograms and unexplained subfertility. Hum Reprod. 2004;19:65–70. doi: 10.1093/humrep/deh038. [DOI] [PubMed] [Google Scholar]
- 17.Blomberg JM, Leffers H, Petersen JH, Daugaard G, Skakkebaek NE, et al. De Association of the polymorphism of the CAG repeat in the mitochondrial DNA polymerase gamma gene (POLG) with testicular germ-cell cancer. Ann Oncol. 2008;19:1910–1914. doi: 10.1093/annonc/mdn407. [DOI] [PubMed] [Google Scholar]