Abstract
Nanoparticulate titanium dioxide (TiO2) is highly photoactive, and its function as a photocatalyst drives much of the application demand for TiO2. Because TiO2 generates reactive oxygen species (ROS) when exposed to ultraviolet radiation (UVR), nanoparticulate TiO2 has been used in antibacterial coatings and wastewater disinfection, and has been investigated as an anti-cancer agent. Oxidative stress mediated by photoactive TiO2 is the likely mechanism of its toxicity, and experiments demonstrating cytotoxicity of TiO2 have used exposure to strong artificial sources of ultraviolet radiation (UVR). In vivo tests of TiO2 toxicity with aquatic organisms have typically shown low toxicity, and results across studies have been variable. No work has demonstrated that photoactivity causes environmental toxicity of TiO2 under natural levels of UVR. Here we show that relatively low levels of ultraviolet light, consistent with those found in nature, can induce toxicity of TiO2 nanoparticles to marine phytoplankton, the most important primary producers on Earth. No effect of TiO2 on phytoplankton was found in treatments where UV light was blocked. Under low intensity UVR, ROS in seawater increased with increasing nano-TiO2 concentration. These increases may lead to increased overall oxidative stress in seawater contaminated by TiO2, and cause decreased resiliency of marine ecosystems. Phototoxicity must be considered when evaluating environmental impacts of nanomaterials, many of which are photoactive.
Introduction
Phytoplankton are the dominant primary producers in marine ecosystems [1], where they are the base of oceanic food webs and a dominant component of the global carbon cycle, as well as other biogeochemical cycles. As abundant small (0.2–200 µm) single or clustered cells with high surface-to-volume ratios suspended in water, phytoplankton have high probability of encountering suspended particles, including pollutants, especially in coastal zones where contaminants are found in highest concentrations. Phytoplankton depend on solar irradiance for photosynthetic carbon fixation, making them more vulnerable to phototoxic impacts than other groups, such as benthic organisms. Information on the impact of emerging contaminants on phytoplankton, and the potential interaction of contaminants with environmental variables such as irradiance is necessary to predict potential impacts on coastal marine food webs and the ecosystems that they support.
Nanomaterials are an important emerging class of contaminants [2], [3], [4], [5], with potentially wide-ranging ecological impacts within marine and estuarine ecosystems, the expected destination of most industrially discharged nanomaterials. [6], [7] World production of nanoparticulate TiO2 is an order of magnitude greater than the next most widely produced nanomaterial, ZnO. Estimated environmental concentrations indicate that among the most commonly used nanomaterials, TiO2 may reach highest concentrations in surface waters and pose a significant threat to aquatic ecosystems. [8], [9] Nanoparticulate TiO2 is often phototoxic to cells in vitro and consequently has been used for wastewater disinfection [10], [11] and investigated as an anti-cancer agent. [12] Oxidative stress mediated by photoactive TiO2 is the likely mechanism of its toxicity [13], [14], and experiments demonstrating cytotoxicity of TiO2 have used exposure to strong artificial sources of ultraviolet radiation (UVR). [13]
Despite the substantial body of evidence demonstrating phototoxicity of TiO2, ecotoxicological studies of this material have seldom measured or manipulated natural levels of UV light exposure in experiments. TiO2 is a photocatalyst capable of producing highly oxidizing ROS. The absorption of a photon with sufficient energy (3.2 eV for anatase) is the necessary condition for photochemical reactions to proceed at the photocatalyst surface. [14], [15] When TiO2 reaches an electronically excited state an electron (e−) is promoted from the valence band to the conduction band, generating a hole in the valence band (h+). The resulting electron-hole pair can then recombine or migrate to the surface of the particle and may react with H2O or OH− to form OH• or can directly oxidize adsorbed species. The electrons may also react with adsorbed molecular oxygen to form O2 −• ions. [15], [16], [17] In the water column, TiO2 may diffuse and adsorb to the surface of phytoplankton where the UV-activated TiO2-plankton complex could then participate in a ligand-to-metal charge transfer reaction [14], in which the phytoplankton cell wall is subject to oxidation. Other potential interactions between TiO2 and plankton may arise through diffusion of TiO2-mediated ROS from the catalyst surface onto the cell wall or into the surrounding media, where it may attack cells or organic compounds.
Our group has recently reported that although ZnO nanoparticles exhibited significant toxicity to marine phytoplankton, TiO2 showed little evidence of toxicity; these experiments were performed under standard conditions with artificial lighting. [18] Here we show that exposure to lights simulating sunlight and emitting UV led to ROS production, with toxic effects in three out of four phytoplankton species tested. To test the hypothesis that UV exposure influences toxicity of nano-TiO2 to phytoplankton, we designed experiments with two orthogonal treatments: UV exposure (2 levels: exposed, blocked), and TiO2 concentration (5 levels: 0, 1, 3, 5, 7 mg L−1). The toxicity endpoint measured was population growth rate, using four widespread species of phytoplankton representing three major groups, the diatoms (Phylum: Heterokontophyta, Class: Bacillariophyceae), green algae or chlorophytes (Phylum: Chlorophyta, Class: Chlorophyceae), and the prymnesiophytes (Phylum: Haptophyta, Class: Prymnesiophyceae).
Results
Phytoplankton growth
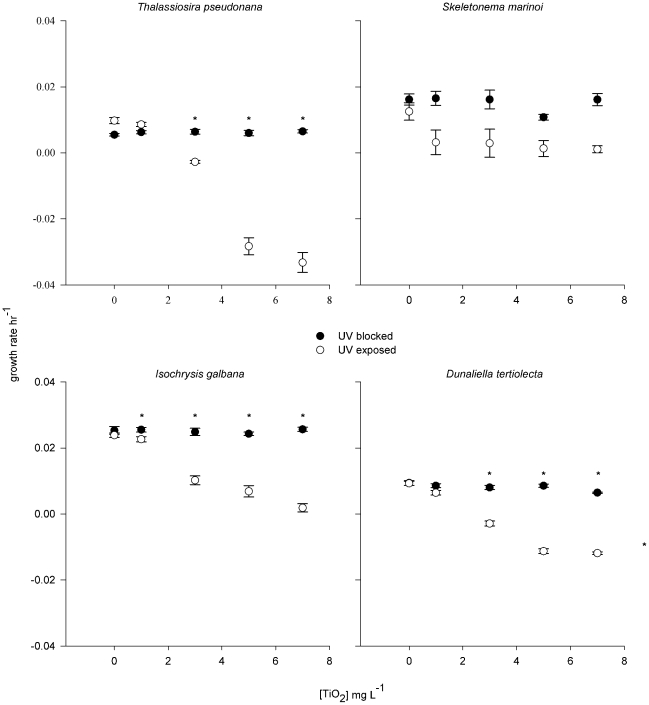
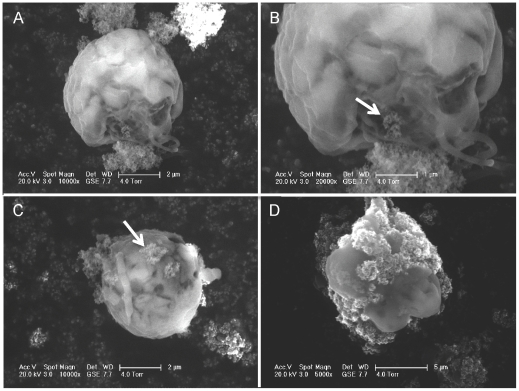
Significant suppression of population growth occurred for three out of four species in the UV-exposed treatment (Fig. 1). In one species, Isochrysis galbana, toxicity was evident at the lowest concentration tested, 1 mg L−1 (Dunnett's method, d = 2.65, p = 0.02), indicating a no-effect concentration (NOEC) <1 mg L−1. In the other two species affected, Thalassiosira pseudonana, and Dunaliella tertiolecta, significant toxicity was evident at 3 mg L−1, although a slight depression of growth rates was seen for D. tertiolecta at 1 mg L−1 (Fig. 1). No significant effect on growth rates of any species was seen in the blocked-UV treatment except in the case of I. galbana at the highest TiO2 concentration tested, 7 mg L−1. No significant effect of nano-TiO2 on growth rate was seen in any treatment for the diatom Skeletonema costatum. UVA in the exposed treatment averaged 4.5 (S.E. 0.1, n = 6) W m−2 and UVB 4.1 (S.E. 0.2, n = 6) W m−2; these levels are comparable to UV intensities near the ocean's surface (<1 m depth in coastal waters). [19] Scanning electron microscopy revealed that TiO2 nanoparticles were adhering to the surfaces of phytoplankton cells as aggregations 10's–100's nm in size (Fig. 2).
Figure 1. Effect of TiO2 nanoparticle (NP) concentration on growth rate of four species of marine phytoplankton, under UV exposure versus UV blocked treatments.
Asterisks identify means that are significantly lower than controls (Dunnett's method, P≤0.05).
Figure 2. Scanning electron micrographs showing interaction of aggregated nano-TiO2 and phytoplankton (Dunaliella tertiolecta) cells.
Arrows indicate aggregated TiO2 particles. Flagellae are visible in panels A–C.
ROS production
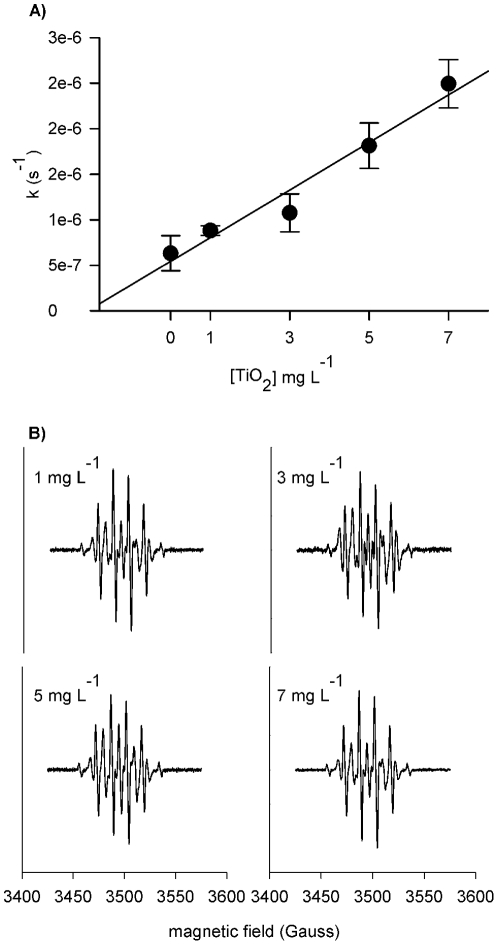
Production of OH• at low [TiO2] in seawater with simulated sunlight, measured using a coumarin probe, was up to 4.6 µM hr−1 (±0.26×103 S.E.) at the TiO2 concentrations studied (Fig. 3), around 10–20 times higher than natural OH• generation in temperate coastal waters. [20] To confirm the presence of OH•, the formation of the Dimethyl-1-pyrroline N-oxide (DMPO)-OH adduct in the presence of UV light was monitored using an in situ electroparamagnetic resonance (EPR) spin trap. The DMPO-OH adduct increased over time and with increasing [TiO2] (Fig. 3). The characteristic 1∶2∶2∶1 quartet and hyperfine constants aN = aβ H = 14.95 of the DMPO-OH spin adduct [21] were observed for all [TiO2] considered. The EPR spectra were evident after only 20 min of illumination, and coupled with the absorbance and fluorescence data, demonstrate the ability of TiO2 to produce OH• in seawater. The experimentally derived steady state [OH•] was up to 2.5×10−15 M (S.E. 0.255×1.4−16), nearly three orders of magnitude higher than that in temperate coastal waters [20].
Figure 3. Evidence of OH• production by TiO2 exposed to UVR.
(A) Photocatalytic production of OH• based on the rate of coumarin degradation. (B) Characteristic 1∶2∶2∶1 EPR spectra with aN = aβ H = 14.95 of the DMPO-OH spin adduct, produced for all TiO2 treatments, confirming the presence of OH•. The DMPO-OH adduct was not observed in the absence of TiO2.
Discussion
Our results strongly suggest that photoactivity and UVR exposure need to be considered when designing experiments to evaluate toxicity of photoactive nanomaterials. Previous work has used pre-illuminated TiO2 nanoparticles to examine potential phototoxicity to algae and daphnids; the UV light source used was too intense to directly illuminate organisms without mortality. [22] Nano-TiO2 that was pre-illuminated in dispersion using a xenon lamp for 30 min at 250 W was more toxic to daphnids than the non-illuminated material, but results were quite variable and no difference was evident for algae. Our results suggest that pre-illumination may not be an appropriate substitute for constant UV exposure in ecotoxicity experiments. Using full-spectrum lighting, as we do here, may reveal toxicity of photoactive nanomaterials where previous results were negative. Halogen lighting was shown to induce a negative effect of TiO2 on cell membranes of stream microbes; although UV levels were not measured, the authors asserted that they were environmentally relevant. [23] Although TiO2 is the best-studied nanomaterial in terms of its ecotoxicity, little work has been done on algae, and results have varied, although toxicity has generally been relatively low, with effects found at concentrations >10 mg L−1. [24] However, these experiments are typically performed under artificial fluorescent lighting that emits little UV. UV exposure has been shown to be necessary for TiO2 to act as an antibacterial agent. [25] One study has shown that toxicity of cadmium selenide/zinc selenide quantum dots to the freshwater crustacean Daphnia magna was increased with exposure to environmentally relevant levels of UV-B radiation; the cause was explained by both increased release of Cd and ROS generation. [26]
Enriched bacterial growth media has been shown to quench hydroxyl radicals, likely due to nonspecific reactions with organic and nonorganic compounds, leaving only superoxide radicals as the agent of toxicity. [25] The presence of significant quantities of OH• in our experiments shows that natural organic matter in seawater will not eliminate this form of ROS. OH• is the most biologically damaging form of ROS because it attacks all biological molecules in a diffusion-controlled fashion, with a relatively long lifetime of 10−7 s and mean diffusion distance of 4.5 nm. OH• also initiates free radical chain reactions, can oxidize membrane lipids, and denatures proteins and nucleic acids. [27], [28] In the oceans, absorption of solar radiation, particularly UVR, by dissolved organic matter in seawater leads to the photochemical production of ROS. [20] These ROS may negatively affect bacteria and phytoplankton by damaging cell membranes or inhibiting photosynthesis. [29] Marine organisms are constantly exposed to some level of oxidative stress, both from external ROS as well as ROS produced by cellular functions such as photosynthesis, and have evolved many ways to deal with this stress, including diverse antioxidant enzymes. [29]
The impact of increasing background ROS levels in marine systems through introduction of nanomaterials may increase the level of oxidative stress on marine organisms and lead to added energetic costs to repair ROS-caused damage, decreasing the resiliency of marine ecosystems to other stresses, including the effects of global climate change. Oxidative stress is one of many stressors experienced by marine organisms, and some, such as thermal stresses, are rising due to climate change. [30] Since phytoplankton are hyperoxic during photosynthesis, they are already exposed to high intracellular ROS concentrations and therefore possess robust antioxidant defenses. [27], [29] Consequently, the impact of TiO2 could be even greater on non-photosynthetic organisms, and deserves further attention. ROS-induced stress has been shown to play a role in mass mortalities of fish and other organisms in red tides [31], [32], inhibition of photosynthesis in marine macrophytes [33], [34], loss of vital symbionts in sponges and corals (bleaching) [29], [35], and fertilization success and early development of marine invertebrates. [29] Oxidative stress is already higher in polluted coastal areas. [36] Increases in ROS due to nanomaterials would likely be concentrated around developed coastlines, increasing the already heavy burden of stresses on economically important nearshore ecosystems that support fisheries and recreational activities. These potential impacts should be considered in regulation of nanomaterial discharge and use.
Photoactivity is one of the major useful characteristics of nanoscale TiO2, and engineers are continually working to improve the efficiency of photocatalytic activity in this and other nanomaterials. [17] In the case of TiO2, efforts are focused particularly on enhancing photocatalytic activity in sunlight, for applications such as solar energy collection and disinfection. [37], [38] These rapid developments highlight the need to consider the mechanism of toxicity of nanomaterials, and how such mechanisms may change over time. Continual improvement in the photoactive potential of TiO2, for example, suggests that different forms, surface coatings, and dopings of this material will influence toxic effects, and that toxic effects may increase in the future. The fact that different forms of the material will be used for different applications will also influence the environmental transport and fate of the material, and should also be considered in risk analysis.
Our results highlight the need to consider UV exposure in ecotoxicity experiments on nanomaterials with photoactive potential, which includes most metal oxide nanoparticles. The well-documented thinning of the stratospheric ozone (O3) layer due to anthropogenic inputs of chlorinated fluorocarbons has caused an increase in UVR reaching the Earth's surface [39], [40], and long-term monitoring has demonstrated complex influences of local atmospheric conditions and global climate change on the amount and variability of UVR reaching the Earth's surface. [40] Interaction of changes in UVR with emerging contaminants could place additional stresses on marine ecosystems in the future, particularly in polar areas where UVR is elevated. [19]
Methods
Nanoparticles: TiO2 was acquired from Evonik Degussa Corp. (USA) and was characterized physically and chemically by the University of California Center for Environmental Implications of Nanotechnology (UC CEIN) as standard reference materials for fate and transport and toxicological studies. [41], [42] The TiO2 NPs were semi-spherical, 81% anatase, 19% rutile, and 15–30 nm in size. While the primary size of NPs was in the range from 15 to 30 nm, the NPs tend to quickly aggregate in seawater. [42] To produce 10 g L−1 stock dispersions, 10 mg of NPs were added to 1 ml of filtered (0.2 µm Millipore) natural seawater, sonicated for 30 min, vortexed briefly, and diluted to 10 mg L−1 with filtered natural seawater.
Phytoplankton: Four species of phytoplankton were used, Thalassiosira pseudonana and Skeletonema costatum (centric diatoms, Bacillariophyceae: Centrales); Dunaliella tertiolecta (Chlorophyceae: Chlamydomonadales); and Isochrysis galbana (Prymnesiophyceae: Isochrysidales). Axenic cultures were obtained from the Provasoli-Guillard National Center for Culture of Marine Phytoplankton (Bigelow Laboratory for Ocean Sciences, West Boothbay Harbor, Maine, USA), and were maintained in standard media (f/2) made with filtered (0.22 µm) natural seawater, which was autoclaved prior to inoculation. To provide inoculant for experiments, algae were incubated under cool white fluorescent lights (14∶10 light∶dark, 100–120 µmol m−2 s−1) at 20°C with aeration for 5–7 days, until log-phase growth prevailed. Cell densities were measured using a fluorometer as in vivo chlorophyll fluorescence (Trilogy, Turner Designs), which was converted to cell numbers using a standard curve based on counts done with a hemacytometer (Reichert, Buffalo NY). Standard curves were measured at the start of each experiment.
Phytoplankton exposure experiments: All experiments were conducted at 20°C, 34 ppt salinity, under the same illumination schedule described above. Fluorescent lighting fixtures fitted with UV-emitting lamps providing simulation of sunlight in the short wavelength region from 295–365 nm (UVA-340, Q-Lab Corp., Cleveland OH) were used for illumination. UV treatment had 2 levels, exposed and blocked. UV levels in the treatments were measured with a broadband radiometer (model UVX, UVP Inc. Upland CA). Blocked replicates were covered with UV-filtering acrylic (Plexiglas G UF-3, Ridout Plastics) that blocked 98% of UV levels measured under the exposed treatment. All glassware was acid-washed, rinsed with purified water (Barnstead nanopure, resistivity >18 MΩ cm), and autoclaved before use. Experiments were run in 125 ml polycarbonate flasks, media volume 50 ml, and were mixed at ∼150 rotations per minute on a rotary shaker (New Brunswick Scientific Co., NJ, USA). NP concentrations tested were 0, 1, 3, 5, 7 mg L−1, with five replicates per treatment. Flasks were inoculated with 1–2×105 cells ml−1, and cell densities were monitored every 24 hrs for 96 hours.
Data analysis: Phytoplankton population growth rates for each replicate flask were estimated as the slope of log-transformed cell count data, obtained through least-squares regression. One-way ANOVA was used to test for an overall effect of NP toxicity on growth rates. Homogeneity of variances was tested with Levene's test; all data conformed to assumptions. When ANOVA revealed significant differences among treatments, post-hoc tests were conducted with Dunnett's method, which tests for pairwise differences between each treatment and the control. Statistical analyses were performed using JMP software (Mac vers. 8.0, SAS Institute).
ROS kinetics: Hydroxylation transforms coumarin-3-carboxylic-acid (3CCA), into the fluorescent product 7-hydroxy-coumarin-3-carboxylic acid (7OH-3CCA), making this system a sensitive probe for OH• detection.[43], [44] From a stock solution of 10−2 M 3CCA (Sigma Aldrich, USA) and 1 g L−1 TiO2 aliquots were dispensed in Pacific seawater (0.2 µm filtered) to achieve a final concentration of 10−4 M 3CCA and 7, 5, 3, 1 and 0 mg L−1 TiO2 in 200 ml. The 200 ml dispersions were dispensed into polycarbonate bottles and placed on shaker tables. Bottles in triplicate were placed both directly under the UV lights and under filtered UV light (exposed and blocked treatments described above). During the first hour of the experiment, samples were taken every 15 min; subsequently samples were taken daily. After filtering (0.45 µm nylon) samples, [3CCA] was measured using UV-vis spectrometry at 280 nm (Shimadzu Biospec 1601). [7OH-3CCA] over time was used to verify the hydroxylation of 3CCA and to quantify ROS kinetics. The fluorescence data were graphed and the area under the curve was calculated to determine fluorescence intensity. Fluorescence data were then fit with a first-order rate expression and the rate constants were calculated from the characteristic plot. Production of OH• was calculated considering the stoichiometry of coumarin oxidation to 7-hydroxycoumarin by OH• using:
| (1) |
where k is the rate constant in hr−1. Mopper and Zhou [20] reported OH• rates of 95.4 nM hr−1 for temperate coastal waters and 238 nM hr−1 for upwelled coastal water. The rate of OH• production was more than 6 times greater in a seawater system with TiO2 present than in coastal waters, ostensibly with high [DOM], the most productive natural photosensitizer in seawater. [20]
The steady state concentration of OH• of coumarin, [OH]ss, was calculated using:
| (2) |
where is kex is the experimental rate constant from the 7 mg L−1 treatment and kscavenger is a scavenging coefficient. [20]
To verify that TiO2 catalyzes ROS production in seawater, electroparamagnetic resonance experiments (EPR) were conducted in situ using a well- known spin trapping technique. In situ EPR is an extremely sensitive technique that allows the direct and indirect detection and determination of ROS kinetics. EPR spin traps are ROS specific, where the first derivative of the absorbance curve provides a unique spectrum generally characteristic of a single ROS. [45]. To 1.8 ml of each TiO2 dispersion we added 0.2 mL of 100 µM 5,5-Dimethyl- 1-pyrroline N-oxide (DMPO, Sigma Aldrich, USA). 0.6 ml of the sample was then dispensed into a quartz cell and was placed directly in the EPR (Bruker EMX plus EPR Spectrometer) cavity. A xenon arc lamp (300 W m-2) was used to irradiate the sample through an optical window. Scans were taken every 5 minutes to monitor the EPR intensity.
Scanning electron microscopy: Under ambient light conditions, D. tertiolecta cells were exposed to 10 mg L−1 TiO2 for one hour and then centrifuged at 5,000 RPM (Sorvall RC 5B Plus) for 20 min. The supernatant was subsequently removed and the samples were fixed in 6.8 pH phosphate buffered 3% glutaraldehyde for one hour. The cells were washed once with DI water and deposited onto EM stubs with black carbon tape (Carbon Conductive Tabs, 12 mm OD, Ted Pella). Stubs were mounted on the Peltier stage of an FEI Co. XL30 FEG ESEM (Philips Electron Optics, Eindoven, The Netherlands). Imaging was in wet mode at ∼4 Torr, 5°C, using an accelerating voltage of 10 kV. Specimens were not conductively coated prior to imaging. Identity of putative TiO2 NPs was confirmed using SEM in combination with energy-dispersive X-ray spectroscopy (FEI XL40 Sirion FEG, Sirion, USA).
Acknowledgments
The authors thank Alex Moreland and Edward Hu for help with phytoplankton toxicity experiments.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by the National Science Foundation and the United States Environmental Protection Agency under Cooperative Agreement # NSF-EF0830117, and by National Science Foundation grant EF-0742521. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Behrenfeld MJ, O'Malley RT, Siegel DA, McClain CR, Sarmiento JL, et al. Climate-driven trends in contemporary ocean productivity. Nature. 2006;444:752–755. doi: 10.1038/nature05317. [DOI] [PubMed] [Google Scholar]
- 2.Farre M, Gajda-Schrantz K, Kantiani L, Barcelo D. Ecotoxicity and analysis of nanomaterials in the aquatic environment. Analytical and Bioanalytical Chemistry. 2009;393:81–95. doi: 10.1007/s00216-008-2458-1. [DOI] [PubMed] [Google Scholar]
- 3.Klaine S, Alvarez P, Batley G, Fernandes T, Handy R, et al. Nanomaterials in the environment: Behavior, fate, bioavailability, and effects. Environmental Toxicology and Chemistry. 2008;27:1825–1851. doi: 10.1897/08-090.1. [DOI] [PubMed] [Google Scholar]
- 5.Nel A, Xia T, Madler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311:622–627. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- 6.Musee N, Thwala M, Nota N. The antibacterial effects of engineered nanomaterials: implications for wastewater treatment plants. Journal of Environmental Monitoring. 2011;13:1164–1183. doi: 10.1039/c1em10023h. [DOI] [PubMed] [Google Scholar]
- 7.Scown TM, van Aerle R, Tyler CR. Review: Do engineered nanoparticles pose a significant threat to the aquatic environment? Critical Reviews in Toxicology. 2010;40:653–670. doi: 10.3109/10408444.2010.494174. [DOI] [PubMed] [Google Scholar]
- 8.Gottschalk F, Sonderer T, Scholz R, Nowack B. Modeled environmental concentrations of engineered nanomaterials (TiO2, ZnO, Ag, CNT, fullerenes) for different regions. Environmental Science & Technology. 2009;43:9216–9222. doi: 10.1021/es9015553. [DOI] [PubMed] [Google Scholar]
- 9.Gottschalk F, Sonderer T, Scholz RW, Nowack B. Possibilities and limitations of modeling environmental exposure to engineered nanomaterials by probabilistic material flow analysis. Environmental Toxicology and Chemistry. 2010;29:1036–1048. doi: 10.1002/etc.135. [DOI] [PubMed] [Google Scholar]
- 10.Theron J, Walker J, Cloete T. Nanotechnology and water treatment: Applications and emerging opportunities. Critical Reviews in Microbiology. 2008;34:43–69. doi: 10.1080/10408410701710442. [DOI] [PubMed] [Google Scholar]
- 11.Zhang D, Li G, Yu J. Inorganic materials for photocatalytic water disinfection. Journal of Materials Chemistry. 2010;20:4529–4536. [Google Scholar]
- 12.Rozhkova E, Ulasov I, Lai B, Dimitrijevic N, Lesniak M, et al. A high-performance nanobio photocatalyst for targeted brain cancer therapy. Nano Letters. 2009;9:3337–3342. doi: 10.1021/nl901610f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnston H, Hutchison G, Christensen F, Peters S, Hankin S, et al. Identification of the mechanisms that drive the toxicity of TiO2 particulates: the contribution of physicochemical characteristics. Particle and Fibre Toxicology. 2009;6:1–27. doi: 10.1186/1743-8977-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carp O, Huisman C, Reller A. Photoinduced reactivity of titanium dioxide. Progress in Solid State Chemistry. 2004;32:33–177. [Google Scholar]
- 15.Czili H, Horvath A. Applicability of coumarin for detecting and measuring hydroxyl radicals generated by photoexcitation of TiO2 nanoparticles. Applied Catalysis B-Environmental. 2008;81:295–302. [Google Scholar]
- 16.Konstantinou IK, Albanis TA. TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: kinetic and mechanistic investigations - A review. Applied Catalysis B-Environmental. 2004;49:1–14. [Google Scholar]
- 17.Linsebigler AL, Lu GQ, Yates JT. Photocatalysis on Tio2 surfaces - principles, mechanisms, and selected results. Chemical Reviews. 1995;95:735–758. [Google Scholar]
- 18.Miller R, Lenihan H, Muller E, Tseng N, Hanna S, et al. Impacts of metal oxide nanoparticles on marine phytoplankton. Environmental Science & Technology. 2010;44:7329–7334. doi: 10.1021/es100247x. [DOI] [PubMed] [Google Scholar]
- 19.Tedetti M, Sempere R. Penetration of ultraviolet radiation in the marine environment. A review. Photochemistry and Photobiology. 2006;82:389–397. doi: 10.1562/2005-11-09-IR-733. [DOI] [PubMed] [Google Scholar]
- 20.Mopper K, Zhou XL. Hydroxyl radical photoproduction in the sea and its potential impact on marine processes. Science. 1990;250:661–664. doi: 10.1126/science.250.4981.661. [DOI] [PubMed] [Google Scholar]
- 21.Chamulitrat W, Iwahashi H, Kelman DJ, Mason RP. Evidence against the 1-2-2-1 quartet dmpo spectrum as the radical adduct of the lipid alkoxyl radical. Archives of Biochemistry and Biophysics. 1992;296:645–649. doi: 10.1016/0003-9861(92)90621-3. [DOI] [PubMed] [Google Scholar]
- 22.Hund-Rinke K, Simon M. Ecotoxic effect of photocatalytic active nanoparticles TiO2 on algae and daphnids. Environmental Science and Pollution Research. 2006;13:225–232. doi: 10.1065/espr2006.06.311. [DOI] [PubMed] [Google Scholar]
- 23.Battin TJ, Kammer FVD, Weilhartner A, Ottofuelling S, Hofmann T. Nanostructured TiO2: transport behavior and effects on aquatic microbial communities under environmental conditions. Environmental Science & Technology. 2009;43:8098–8104. doi: 10.1021/es9017046. [DOI] [PubMed] [Google Scholar]
- 24.Menard A, Drobne D, Jemec A. Ecotoxicity of nanosized TiO2: review of in vivo data. Environmental Pollution. 2011;159:677–684. doi: 10.1016/j.envpol.2010.11.027. [DOI] [PubMed] [Google Scholar]
- 25.Brunet L, Lyon DY, Hotze EM, Alvarez PJJ, Wiesner MR. Comparative photoactivity and antibacterial properties of C60 fullerenes and titanium dioxide nanoparticles. Environmental Science & Technology. 2009;43:4355–4360. doi: 10.1021/es803093t. [DOI] [PubMed] [Google Scholar]
- 26.Kim J, Park Y, Yoon TH, Yoon CS, Choi K. Phototoxicity of CdSe/ZnSe quantum dots with surface coatings of 3-mercaptopropionic acid or tri-n-octylphosphine oxide/gum arabic in Daphnia magna under environmentally relevant UV-B light. Aquatic Toxicology. 2010;97:116–124. doi: 10.1016/j.aquatox.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 27.Cadenas E. Biochemistry of oxygen toxicity. Annual Review of Biochemistry. 1989;58:79–110. doi: 10.1146/annurev.bi.58.070189.000455. [DOI] [PubMed] [Google Scholar]
- 28.Fridovich I. Oxygen toxicity: A radical explanation. Journal of Experimental Biology. 1998;201:1203–1209. doi: 10.1242/jeb.201.8.1203. [DOI] [PubMed] [Google Scholar]
- 29.Lesser MP. Oxidative stress in marine environments: Biochemistry and physiological ecology. Annual Review of Physiology. 2006;68:253–278. doi: 10.1146/annurev.physiol.68.040104.110001. [DOI] [PubMed] [Google Scholar]
- 30.Hoegh-Guldberg O, Bruno JF. The impact of climate change on the world's marine ecosystems. Science (Washington D C) 2010;328:1523–1528. doi: 10.1126/science.1189930. [DOI] [PubMed] [Google Scholar]
- 31.Oda T, Ishimatsu A, Shimada M, Takeshita S, Muramatsu T. Oxygen radical mediated toxic effects of the red tide flagellate Chattonella marina on Vibrio alginolyticus. Marine Biology. 1992;112:505–509. [Google Scholar]
- 32.Yang CZ, Albright LJ, Yousif AN. Oxygen radical mediated effects of the toxic phytoplankter Heterosigma carterae on juvenile rainbow trout Oncorhynchus mykiss. Diseases of Aquatic Organisms. 1995;23:101–108. [Google Scholar]
- 33.Collen J, Davison IR. Stress tolerance and reactive oxygen metabolism in the intertidal red seaweeds Mastocarpus stellatus and Chondrus crispus. Plant Cell and Environment. 1999;22:1143–1151. [Google Scholar]
- 34.Zubia M, Robledo D, Freile-Pelegrin Y. Antioxidant activities in tropical marine macroalgae from the Yucatan Peninsula, Mexico. Journal of Applied Phycology. 2007;19:449–458. [Google Scholar]
- 35.Dunn SR, Schnitzler CE, Weis VM. Apoptosis and autophagy as mechanisms of dinoflagellate symbiont release during cnidarian bleaching: every which way you lose. Proceedings of the Royal Society Biological Sciences Series B. 2007;274:3079–3085. doi: 10.1098/rspb.2007.0711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Angel DL, Fiedler U, Eden N, Kress N, Adelung D, et al. Catalase activity in macro- and microorganisms as an indicator of biotic stress in coastal waters of the eastern Mediterranean Sea. Helgoland Marine Research. 1999;53:209–218. [Google Scholar]
- 37.He Z, Xu Q, Yang Tan TT. Understanding bactericidal performance on ambient light activated TiO(2)-InVO(4) nanostructured films. Nanoscale. 2011 doi: 10.1039/c1nr11126d. DOI: 10.1039/C1NR11126D. [DOI] [PubMed] [Google Scholar]
- 38.Gopal NO, Lo HH, Sheu SC, Ke SC. a potential site for trapping photogenerated holes on rutile TiO2 surface as revealed by EPR spectroscopy: an avenue for enhancing photocatalytic activity. Journal of the American Chemical Society. 2010;132:10982–10983. doi: 10.1021/ja909901f. [DOI] [PubMed] [Google Scholar]
- 39.Madronich S, McKenzie RL, Bjorn LO, Caldwell MM. Changes in biologically active ultraviolet radiation reaching the Earth's surface. Journal of Photochemistry and Photobiology B Biology. 1998;46:5–19. doi: 10.1016/s1011-1344(98)00182-1. [DOI] [PubMed] [Google Scholar]
- 40.McKenzie RL, Aucamp PJ, Bais AF, Bjorn LO, Ilyas M. Changes in biologically-active ultraviolet radiation reaching the Earth's surface. Photochemical & Photobiological Sciences. 2007;6:218–231. doi: 10.1039/b700017k. [DOI] [PubMed] [Google Scholar]
- 41.Godwin HA, Chopra K, Bradley KA, Cohen Y, Harthorn BH, et al. The University of California Center for the Environmental Implications of Nanotechnology. Environmental Science & Technology. 2009;43:6453–6457. doi: 10.1021/es8034544. [DOI] [PubMed] [Google Scholar]
- 42.Keller AA, Wang HT, Zhou DX, Lenihan HS, Cherr G, et al. Stability and aggregation of metal oxide nanoparticles in natural aqueous matrices. Environmental Science & Technology. 2010;44:1962–1967. doi: 10.1021/es902987d. [DOI] [PubMed] [Google Scholar]
- 43.Keller AA, Bennett SW. Comparative photoactivity of CeO(2), gamma-Fe(2)O(3), TiO(2) and ZnO in various aqueous systems. Applied Catalysis B-Environmental. 2011;102:600–607. [Google Scholar]
- 44.Horvath A, Czili H. Applicability of coumarin for detecting and measuring hydroxyl radicals generated by photoexcitation of TiO2 nanoparticles. Applied Catalysis B-Environmental. 2008;81:295–302. [Google Scholar]
- 45.Berliner LJ, Khramtsov V, Fujii H, Clanton TL. Unique in vivo applications of spin traps. Free Radical Biology and Medicine. 2001;30:489–499. doi: 10.1016/s0891-5849(00)00491-3. [DOI] [PubMed] [Google Scholar]