Abstract
We used functional magnetic resonance imaging to assess the neural correlates of autobiographical, semantic, and episodic memory retrieval in healthy young and older adults. Participants were tested with an event-related paradigm in which retrieval demand was the only factor varying between trials. A Spatiotemporal Partial Least Square (ST-PLS) analysis was conducted to identify the main patterns of activity characterizing the groups across conditions. We identified brain regions activated by all three memory conditions relative to a control condition. This pattern was expressed equally in both age groups and replicated previous findings obtained in a separate group of younger adults. We also identified regions whose activity differentiated among the different memory conditions. These patterns of differentiation were expressed less strongly in the older adults than in the young adults, a finding which was further confirmed by barycentric discriminant analysis. This analysis showed an age-related dedifferentiation in autobiographical and episodic memory tasks, but not in the semantic memory task or the control condition. These findings suggest that the activation of a common memory retrieval network is maintained with age, whereas the specific aspects of brain activity that differ with memory content are more vulnerable and less selectively engaged in older adults. Our results provide a potential neural mechanism for the well-known age differences in episodic/autobiographical memory, and preserved semantic memory, observed when older adults are compared to younger adults.
Declarative memory involves the conscious retrieval of information, and includes episodic, semantic, and autobiographical memory. Semantic memory (SM) comprises memory for factual information and general decontextualized knowledge, while episodic memory (EM) supports the rich re-experiencing of a memory’s original spatio-temporal context (Tulving, 1972, 1985). Autobiographical memory (AM) represents knowledge specific to an individual, and comprises both decontextualized personal semantics, such as a friend’s name, and highly contextualized episodic memories, such as a relative’s wedding ceremony (Brewer, 1986; Conway, 2001). In general, AM has higher personal significance and emotional valence, is more structured by general world knowledge, and operates on a longer time frame than the conventional EM tested in the laboratory (Gilboa, 2004; Wheeler et al., 1997).
Burianova and colleagues (Burianova & Grady, 2007; Burianova et al., 2010) developed a functional brain imaging paradigm to compare AM, EM, and SM directly. They identified a network of common regions supporting the retrieval of all three memory types, as well as sets of frontal and temporal brain areas specific to each memory condition. With the current study, we adopted this paradigm to explore how healthy aging affects the neural correlates of declarative memory. There is a large scientific literature documenting the effects of aging on memory, but no neuroimaging study so far has assessed these three forms of memory simultaneously within the same individuals.
Behavioural evidence indicates that SM is relatively preserved in healthy older adults (Allen et al., 2002; Craik & Jennings, 1992; Mitchell, 1989; Nilsson, 2003; Nyberg et al., 1996; Spaniol et al., 2006) as they typically perform as well as young adults on tasks of memory for general knowledge, such as naming and lexical decision-making (Allen et al., 1993; Balota & Ferraro, 1996; Mitchell, 1989), semantic priming (Laver & Burke, 1993) or multiplication verification (Allen et al., 1997). Given the relative preservation of SM in older adults, one would expect that the neural correlates of SM retrieval would also be relatively maintained. Indeed, several studies have found that brain activity during SM tasks, such as judging animacy or tests of memory for famous names, is similar in young and older adults, even though the older adults may show more frontal activity (Logan et al., 2002; Lustig et al., 2003; Nielson et al., 2006). In addition, results from an ERP study showed no age-related change in the amplitude, latency or duration of the N400 (thought to reflect lexical access) recorded during a lexical categorization task (Giaquinto et al., 2007). Similarly, Maguire and Frith (2003) found no age-related change in the neural correlates of retrieval for public events, personal facts, or general knowledge.
In contrast to their preserved SM, older adults typically perform more poorly than young adults on EM tasks, such as those in which participants must remember items from a study list (e.g. Davidson & Glisky, 2002; Mitchell, 1989). Older adults’ EM deficit may be linked to a decreased capacity to retrieve context-specific information, such as source information (Burke & Light, 1981; Mitchell et al., 2006; Spaniol et al., 2006; Spencer & Raz, 1995). Li et al. (2001) proposed that aging decreases neural responsivity, and this decrease, in turn, leads to neural dedifferentiation which is defined as a loss of distinctiveness among different cognitive states’ neural signature. Li and colleagues suggested that older adults’ memory for events and contexts are more confusable because older adults’ brain activation profiles are less distinct from one another. Neuroimaging studies of EM have reported greater activity, mainly in prefrontal regions, in older adults relative to younger adults during EM retrieval tasks (Madden et al., 1999; Morcom et al., 2007). This pattern has been interpreted as a sign of a less selective use of resources (see Rajah & D’Esposito, 2005; Grady, 2002, 2008), or as a compensatory mechanism for age reductions elsewhere in the brain (Cabeza, 2002; Grady et al., 2005; Rajah & D’Esposito, 2005). Age-related changes that suggest a differential use of strategies during EM tasks also have been reported. Studies of recognition have shown that, in older adults, successful memory is mediated by rhinal areas associated with familiarity-based recognition (Brown & Aggleton, 2001; Daselaar et al., 2006; Henson et al., 2003; but see Duarte et al., 2010) but that in young adults, successful memory is mediated by the hippocampus, a structure associated with recollection-based recognition (Cohn et al., 2009; Eldridge et al., 2000; Grady et al., 2005). Taken together, these imaging studies indicate that age effects on EM retrieval are paralleled by neural changes that may reflect less effective brain activation in older adults, as well as a variety of compensatory changes in response to this loss of effectiveness.
Like EM, AM can be affected by aging (see Piefke & Fink, 2005, for a review). In older adults, AM for personal events is typically more gist-like, lacks details (Addis et al., 2008; Levine et al., 2002; St Jacques & Levine, 2007), and is less vivid (Piolino et al., 2006). Only two studies have compared the neural correlates of AM in young and older adults. One of these (Maguire & Frith, 2003) reported that hippocampal activation was more bilateral in older than in young adults. This difference could reflect compensation, since hippocampal activation correlates with emotionality, levels of details and imagery in both young (Addis et al., 2004) and older adults (Viard et al., 2007). The other study (Donix et al., 2010) found increased occipital activity in older relative to younger adults during AM retrieval, which was interpreted as an age difference in the demands made on visual processing or imagery. Taken together, EM and AM studies suggest that an important correlate of age differences in the capacity to retrieve contextual or re-experiential details during AM and EM tasks is an alteration in medial temporal lobe activity, although altered activity in cortical regions also appears to be involved.
With this study, we wanted to assess the relative effect of aging on the neural correlates of SM, EM and AM in the same experiment, rather than in isolation, and to examine this age effect on large-scale, integrated activity in the brain. We tested young and older adults using the paradigm of Burianova and Grady (2007) to identify age-related activation changes in areas identified as parts of the common network, as well as regions that are unique to each memory type. We used Spatiotemporal Partial Least Squares (ST-PLS; Krishnan et al., In Press; McIntosh et al., 2004)—which is a multivariate statistical analysis method—in order to identify whole-brain patterns of activity related to the memory conditions and to determine how these patterns differed between younger and older adults. We chose ST-PLS, rather than a more conventional univariate analysis, because we assume that cognitive processes are the result of integrated activity across multiple brain regions that are functionally connected to one another, rather than the result of activity in any single brain region. Multivariate techniques, such as ST-PLS, are sensitive to how patterns of brain activity covary with behavioural tasks. Therefore, ST-PLS can assess commonalities, as well as differences, among groups and conditions, and this allowed us to identify the aspects of memory-related neural activity that are shared among memory conditions, as well as those that are maintained in older adults.
Based on the aging literature, we would predict larger age differences for networks involved in AM and EM than in SM. In addition, several theories of cognitive aging, such as the HAROLD model (hemispheric asymmetry reduction in the old, Cabeza 2002), the compensation-related utilization of neural circuits hypothesis (CRUNCH; Reuter-Lorenz et al., 2008), and the Scaffolding Theory of Aging and Cognition (STAC; Park & Reuter-Lorenz, 2009), would predict an over-recruitment of prefrontal regions, or alternate brain circuits, to compensate for age deficiencies and to aid performance on the tasks. If such a compensatory set of regions were to be engaged during one or more of our memory tasks, we would expect to see a network uniquely engaged by the older adults, but not younger adults. Finally, a loss of selectivity or dedifferentiation in the neural specificity of responses to the retrieval of different kinds of content might be observed. In order to quantify neural selectivity, we performed a multi-subject barycentric discriminant analysis (MUSUBADA; Abdi & Williams, 2010; Williams et al., 2010). MUSUBADA calculates how the largest patterns of whole-brain activity present in the data are expressed for each group, within each condition. These brain activity patterns, or dimensions, are analogous to the patterns picked up by ST-PLS, and thus MUSUBADA is a good complementary technique to ST-PLS. Additionally, MUSUBADA computes, for each group and condition mean, confidence ellipses which provide an intuitive display of neural specificity per group, condition, and dimension rather than an overall measure of differentiation. In our case, we would expect dedifferentiation in older adults not to affect the brain activity pattern common to all memory types, but instead to reduce the differences seen in brain patterns that distinguish among AM, EM, and SM in younger adults.
Methods
Participants
Fifteen young (age range = 20–33, 6 males) and fifteen older adults [age range = 63–77, 6 males; Mini-Mental State Examination (MMSE) range = 27–30, mean = 29.31] were recruited for this study. All participants were right-handed and native or fluent English speakers with either normal or corrected-to-normal vision. Exclusion criteria included poor health conditions (e.g. back problems), history of neurological or psychiatric disorders, head injury, and stroke. Informed consent was obtained in accordance with a protocol approved by Baycrest’s Research Ethics Board.
Procedure
Immediately before scanning, participants received a practice session during which they were exposed to examples of the four conditions (control, AM, EM, and SM). The study consisted of six fMRI runs of 498 seconds each. Each run consisted of 28 trials of 16 seconds (7 trials per condition); trials from each condition were randomized within the run. For each trial, a stimulus was presented (4s), followed by an inter-stimulus interval (1s), a question (10s), and an inter-trial interval (1s). The stimuli and paradigm used here are described in greater detail in Burianova and Grady (2007). For the control trials, participants were presented with a scrambled meaningless picture, which was followed by a request to press one of three response pad keys corresponding to a letter. (e.g. “Press a key that corresponds to the letter A”; “1 = A”, “2 = G”, “3 = I don’t know”).
For AM, EM, and SM trials, a photograph was presented with a cue word directing attention to the gist of the image (e.g. “poverty,” “grandparents,” “airplane”). The picture was followed by one of three types of questions (EM, AM, or SM). Conditions were randomized, and participants became aware of the condition at question onset. EM questions were about an element from the picture (e.g. “On the picture which you just saw, what was the colour of the bicycle?”). SM questions were about general knowledge related to the theme of the picture (e.g. “In which city was John F. Kennedy assassinated?”). For both these conditions, three answer choices were presented, with either button 1 or 2 corresponding to the correct answer, and button 3 corresponding to the answer “I don’t know.” During AM trials, participants were instructed to retrieve a personal event related thematically to the picture (e.g. “think of a time you were with older relatives”), and to rate the vividness of their memory for that event (e.g., 1 = “very vivid,” 2 = “somewhat vivid,” 3 = “not vivid at all”). Each photograph was presented three times over the course of the study (once for each of the three memory conditions), but never more than once per run. Accuracy was stressed over speed. The main purpose of the behavioural response was to discard incorrect trials and to insure that participants were engaged in the task. We found that, for our participants, reaction time was uncorrelated with accuracy, as measured by percent correct trials.
MRI and fMRI data acquisition
Brain images were obtained with a Siemens 3 T Trio Scanner using a Matrix 12-channel head coil. The anatomical images were acquired with a T1-weighted 3D MPRage oblique axial sequence (160 slices, 1mm thick, FOV = 256mm). Brain activity was measured using the blood oxygenation level-dependent (BOLD) response. Functional images were acquired with an EPI oblique axial sequence (TR = 2000 ms, TE = 30ms, FOV = 200 mm, Flip = 70, 28 images, 5mm thick).
Stimuli were projected onto a screen located behind the participant made visible through a mirror mounted on top of the head coil. Plastic goggles with corrective lenses were used when needed. Responses were made with the right hand using the first three buttons of a four button Fiber-Optic Response Pad System (Current Designs Inc.). Heart rate and respiration data were collected to be regressed out of the functional images.
fMRI Data Analysis
Images were reconstructed and preprocessed utilizing the Analysis of Functional Neuroimages (AFNI; Cox, 1996) and Statistical Parametric Mapping (SPM5) software’s. The images were corrected for motion associated with heart-rate and respiration, for the timing of the interleaved functional sequence (slice-timing), and for within-run head motion (co-registration). Images were normalized to standard MNI space using SPM5’s functional EPI template and smoothed with a 6-mm Gaussian filter.
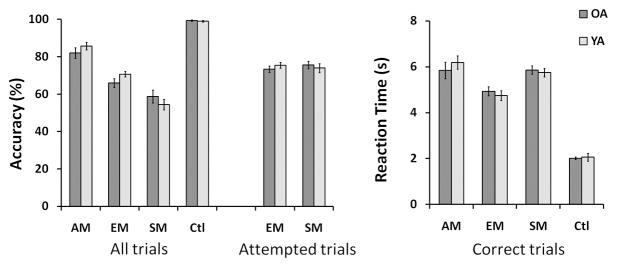
Results were analyzed with ST-PLS, which was conducted on data from both young and old adults to assess differences across conditions and age groups. For the analysis, we kept trials with correct responses for the control, SM and EM conditions, and AM trials for which participants answered “very vivid” or “somewhat vivid” (see Figure 1).
Figure 1.
For each voxel, ST-PLS calculated the percent change in signal intensity value from a trial’s first TR (the onset of the question), at each of the subsequent 7 TRs (16s). ST-PLS created a cross-block covariance matrix between changes in brain activity (for each voxel at each time point) and experimental manipulations (our task conditions: AM, SM, EM, and Ctl). A singular value decomposition was then conducted on this matrix.
The result of this analysis provided a set of latent variables (LVs), which identify how patterns of brain activity vary across the experimental conditions. Each LV accounts for a proportion of the covariance between conditions and brain activity, with the first LV accounting for the largest part. Two sets of weights called saliences are associated to each LV. The first set of saliences characterizes a contrast across the task conditions, and the second set of saliences expresses how each brain voxel’s pattern of signal change reflected the task contrast at each TR (McIntosh et al., 2004). In addition, ST-PLS calculated brain scores for each subject for each LV for each condition; brain scores are the product of each voxel’s salience by the normalized signal value for that voxel, summed across all brain voxels. These brain scores reflect how strongly a participant expresses the patterns of an LV per condition. Temporal brain scores were also calculated per participant for each task condition. These brain scores reflected how strongly the LV’s pattern of voxel salience was expressed over time, and allowed us to identify the TRs with maximal condition and group contrasts in the LV.
The significance of each LV was determined using a permutation test (McIntosh et al., 1996) with 500 permutations and the reliability of the voxel saliences was determined using bootstrap estimation of the standard errors (SE) using 100 boostrap samples (Efron & Tibshirani, 1985; McIntosh et al., 2004). Voxels with a salience/SE ratio of magnitude > 4 were considered to be reliable (Burianova & Grady, 2007; Sampson et al., 1989). Voxels with the highest salience/SE ratio within a 2cm cube centered around them were considered local maxima for each active cluster. Maxima from clusters composed of more than 20 reliably activated voxels, with a minimal distance of 10 voxels between voxel peaks, are reported in the results section in MNI coordinates. In addition, (95%) confidence intervals for the mean brain scores (mean-centered and collapsed across all TRs in the analysis window) in each condition and group were calculated with the bootstrap procedure. When two confidence intervals for two conditions did not overlap, we considered that these two conditions differed reliably.
We also analyzed the group effects using MUSUBADA, which is a variation of discriminant analysis. We used MUSUBADA to determine if a pattern recognition technique could identify (i.e. “discriminate between”) the experimental conditions and if the discriminability between these conditions would interact with age. MUSUBADA computes the average (i.e. “barycenter”) scan per condition for all the participants and performs a principal component analysis (PCA) on this set of scans. It provides discriminant factor scores for the experimental conditions that can be used to display these conditions on a PCA-like map. We also projected the barycenters of the older and younger participants for each experimental condition on a map where the distance between two conditions reflected how much the brain patterns differed between them. Finally, we used a bootstrap procedure, with both scans and participants treated as random factors, to compute 95% confidence ellipses for each barycenter in the two groups, which we then plotted on the factor score map. Ellipses from different conditions that did not overlap on a least one map are significantly (p < .05) different from each other (see Abdi et al., 2009).
Results
Behavioural results
Accuracy and reaction time for each task condition are plotted per age group in Figure 1. A series of 2 (group) × 4 (task condition) ANOVAs were conducted on the percentage of correct trials, and on the mean reaction time for correct trials, respectively. The tests revealed no significant main effect of age group (F < 1), and no significant age group × condition interaction effect [accuracy: F(3, 84) = 1.914, p = .134; reaction time: F < 1] on either measure. However, both tests revealed a significant main effect of task condition [accuracy: F(3, 84) = 157.226, p < .001; reaction time: F(3, 84) = 181.481, p < .001]. Paired-sample t-tests corrected for multiple comparisons with the Holm-Bonferroni method revealed that all conditions differed significantly from each other in their mean accuracy score (p < .001, adjusted). Mean accuracy for attempted trials (excluding “I don’t know” answers) did not differ significantly between the SM and the EM conditions [t < 1]. With the exception of AM and SM (t < 1), mean reaction time for correct trials differed significantly between all the other conditions as well (p < .005, adjusted). The lack of group difference in reaction time ruled out motor response latency (i.e. button press) as a source of age differences in the BOLD response. Also, the lack of group difference in accuracy ruled out unbalanced statistical power as a confound, because both groups had equivalent numbers of trials entered in the analysis per condition.
fMRI results: ST-PLS
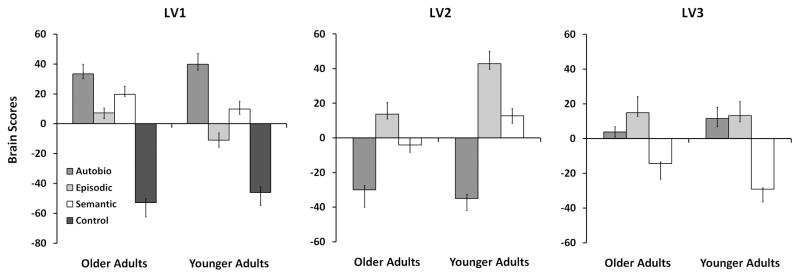
We obtained three significant LVs from our ST-PLS analysis. The first LV revealed regions whose activity differentiated the three memory conditions from the control condition. Because this LV accounted for over half of the covariance in the data, we performed an additional ST-PLS omitting the control condition for a better assessment of the differences among the memory conditions. This analysis provided two additional LVs. Figure 2 shows the mean brain scores for these three LVs, averaged per condition, for each age group. Temporal brain scores indicated that maximal differentiation of the task conditions was achieved around TRs 4 and 5, or 8–10s following the onset of the question (See Supplementary Figure). We report the brain regions with reliable contributions to the observed brain patterns at these two TRs.
Figure 2.
Common Memory Regions
The first LV of the analysis that included the control condition (LV1) accounted for the largest amount of cross-block covariance in the data (55.1%, p < .002), and identified brain regions whose level of activation differed between all memory conditions and the control condition. This pattern was expressed in both young and older adults to a similar degree. Figure 2 (left) shows the brain scores per condition for this LV in each group. In addition to showing a difference between the control and the memory conditions, the confidence intervals revealed a greater engagement of the common memory regions during AM relative to SM and EM, and greater engagement during SM than EM in both age groups. During EM, the memory regions also were significantly less engaged in young adults than in older adults.
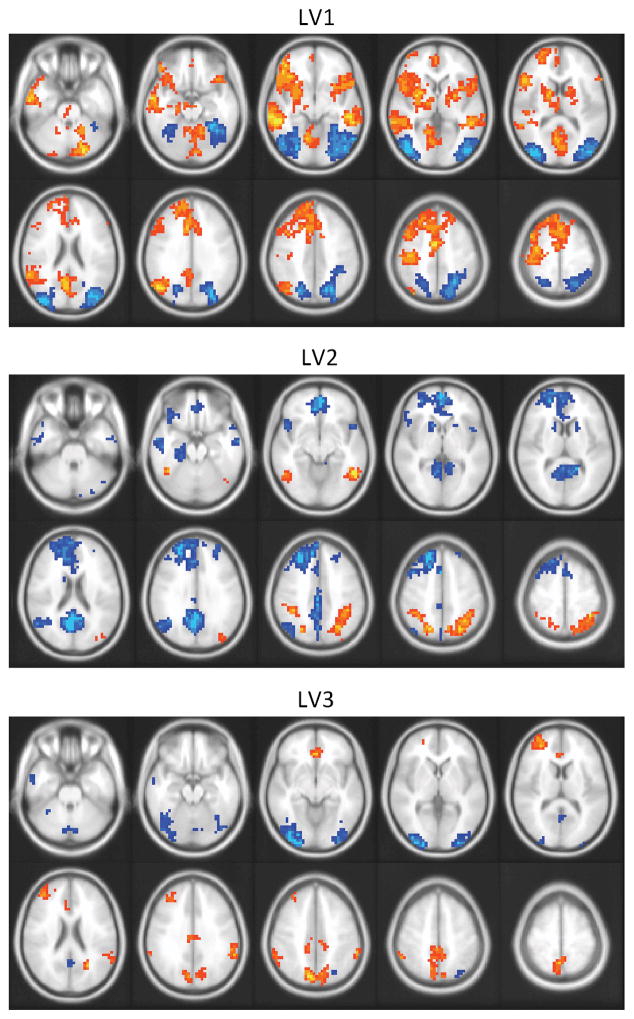
The pattern of peak voxels from this LV (See Table 1) closely resembles the common memory network proposed by Burianova and colleagues (Burianova & Grady, 2007). This network includes the middle temporal cortices, the left inferior frontal gyrus, the angular gyrus, the caudate nuclei, posterior cingulate, and the left medial temporal lobe. All of these regions were activated above baseline in the three memory conditions in both age groups (Figure 3, top). Brain regions with more activity during the retrieval period of the control task than during the memory tasks mainly consisted of bilateral occipital areas. This difference was accounted for by a deactivation from trial onset during the memory conditions, rather than by an increase during the control condition. We normalized activation to the onset of the question (immediately after the cue photograph was removed), a time point at which activity in occipital regions was elevated by the viewing of the cue photograph. Occipital activity declined when the cue was removed and the focus of the task switched to an internal retrieval process. This reduction of activity was larger during the memory trials than during the control trials, accounting for the apparently greater activity during the control condition that we observed.
Table 1.
Peak voxels of regions expressing the task contrast of LV1
| Region | Hemis | MNI Coordinates | ||||
|---|---|---|---|---|---|---|
| BA | x | y | z | BS Ratio | ||
| Memory > Control | ||||||
| Middle Temporal Gyrus | L | 21 | −60 | −8 | −24 | 13.9 |
| R | 21 | 50 | −32 | −8 | 10.8 | |
| Superior Temporal Gyrus | L | 22 | −48 | −24 | 16 | 6.7 |
| Inferior Frontal Gyrus | L | 45 | −48 | 20 | 12 | 11.7 |
| R | 47 | 52 | 16 | −4 | 8.8 | |
| SMA | L | 6 | −8 | 24 | 52 | 10.9 |
| Superior Medial Frontal Gyrus | L | 6 | −4 | 36 | 36 | 10.3 |
| Middle Frontal Gyrus | L | 8 | −44 | 16 | 48 | 10.2 |
| Precentral Gyrus | L | 4 | −44 | −20 | 56 | 8.6 |
| Retrosplenial/Posterior Cingulate | L | 23 | −8 | −56 | 16 | 11.6 |
| Angular Gyrus | L | 39 | −48 | −68 | 32 | 11.0 |
| R | 39 | 52 | −64 | 28 | 6.3 | |
| Lingual Gyrus | L | 18 | −12 | −64 | −8 | 8.1 |
| Hippocampus | L | n/a | −20 | −20 | −20 | 7.6 |
| Caudate | L | n/a | −16 | 4 | 8 | 8.7 |
| R | n/a | 16 | 8 | 8 | 7.0 | |
| Thalamus | L | n/a | −8 | −8 | 0 | 5.9 |
| Cerebellum | R | n/a | 32 | −76 | −44 | 9.9 |
| Control > Memory | ||||||
| Inferior Occipital Gyrus | L | 18 | −48 | −76 | −4 | −14.0 |
| Middle Occipital Gyrus | L | 19 | −40 | −88 | 12 | −12.8 |
| R | 19 | 44 | −80 | 0 | −12.6 | |
| Superior Parietal Lobule | R | 7 | 20 | −68 | 44 | −10.9 |
Hemis = hemisphere; BA = Brodmann’s area; x = right/left; y = anterior/posterior; z = superior/inferior; BS ratio = bootstrap ratio.
Figure 3.
Regions Distinguishing Among Memory Conditions
LV2 (p < .002) accounted for 59.9% of the cross-block covariance of the matrix that did not include the control condition. This LV identified brain regions whose activity differed maximally between the AM and the EM condition in both groups, with the SM condition contributing less to the pattern of activity (Figure 2, middle). Confidence intervals revealed significantly more positive EM and SM brain scores in young adults than in older adults. In general, this pattern that differentiated AM from EM and SM was expressed to a greater degree in the young adults.
Peak voxels from regions contributing to this LV are listed in Table 2. Regions that showed greater activity during the EM than during the AM condition included the bilateral inferior frontal gyrus, middle, and superior occipital areas, as well as the right inferior temporal gyrus (Figure 3, middle). The right superior parietal lobule and the left fusiform also differentiated AM and EM because they showed greater deactivation during the AM than during the EM condition relative to the first TR in the trial.
Table 2.
Peak voxels of regions expressing the task contrast of LV2
| Region | Hemis | MNI Coordinates | ||||
|---|---|---|---|---|---|---|
| BA | x | y | z | BS Ratio | ||
| EM > AM and SM | ||||||
| Superior Parietal Lobule | R | 7 | 36 | −48 | 60 | 11.8 |
| Inferior Temporal Gyrus | R | 37 | 52 | −52 | −12 | 10.4 |
| Fusiform | L | 37 | −44 | −52 | −20 | 7.2 |
| Superior/Middle Occip Gyrus | L | 19 | −28 | −68 | 36 | 9.2 |
| Inferior Frontal Gyrus | L | 44 | −52 | 12 | 24 | 6.5 |
| R | 45 | 40 | 36 | 8 | 6.1 | |
| AM > EM and SM | ||||||
| Cuneus/precuneus | L | 31 | −8 | −64 | 20 | −20.8 |
| Angular Gyrus | L | 39 | −56 | −64 | 24 | −11.8 |
| Middle Orbital Gyrus | L | 10 | −4 | 52 | −8 | −13.8 |
| Superior Frontal Gyrus | L | 8 | −24 | 36 | 48 | −13.4 |
| Middle Frontal Gyrus | R | 9 | 28 | 44 | 32 | −8.2 |
| Inferior Frontal Gyrus | L | 47 | −40 | 32 | −20 | −6.8 |
| Middle Temporal Gyrus | L | 21 | −60 | −8 | −24 | −9.8 |
| Temporal Pole | R | 38 | 48 | 12 | −8 | −9.5 |
| L | 38 | −48 | 12 | −12 | −6.2 | |
| Hippocampus | L | n/a | −32 | −24 | −20 | −8.9 |
| R | n/a | 24 | −20 | −24 | −8.2 | |
| Caudate | L | n/a | −16 | 16 | −4 | −7.9 |
| Cerebellum | R | n/a | 44 | −64 | −44 | −10.4 |
Hemis = hemisphere; BA = Brodmann’s area; x = right/left; y = anterior/posterior; z = superior/inferior; BS ratio = bootstrap ratio.
A more extensive set of regions showed greater activity during the AM condition, relative to EM and SM. These regions (Figure 3, middle) included both ventral and dorsal portions of medial prefrontal cortex, the bilateral hippocampus (with the maximum of the left region being somewhat posterior to the hippocampal region identified by LV1), the left angular gyrus, the temporal poles, and the left caudate nucleus. This pattern of activation is consistent with the regions typically activated during AM retrieval (See Maguire, 2001; Svoboda et al., 2006 for a review).
LV3 (p < .002) accounted for 23.5% of the cross-block covariance in the matrix that did not include the control condition, and identified brain regions whose activity distinguished SM from the other two conditions in both age groups (Figure 2, right). Confidence intervals revealed significantly more negative brain scores for SM in young adults than in older adults, and more positive scores for AM, again indicating that this pattern was expressed more robustly in young adults. In addition, brain scores were significantly more positive for EM than AM in older adults, whereas there was no difference between these two conditions in young adults. Activity for EM did not differ between the groups on this LV.
Peak voxels from regions contributing to this LV are listed in Table 3. Regions showing greater activation during AM and EM than during the SM condition (Figure 3, bottom) included the left anterior cingulate cortex, the inferior parietal lobule, the left insula, and the middle frontal gyrus in both hemispheres. Activity in the left superior parietal lobule, the precuneus, and the right supramarginal gyrus also differentiated AM and EM from SM, but showed greater deactivation, relative to the first TR in the trial, during the SM condition than during the EM and AM conditions. Regions with more activity for SM than AM or EM included the left middle temporal gyrus and the cerebellum. Activity in the inferior and middle occipital gyri also was greater during SM due to stronger deactivation during the AM condition.
Table 3.
Peak voxels of regions expressing the task contrast of LV3
| Region | Hemis | MNI Coordinates | ||||
|---|---|---|---|---|---|---|
| BA | x | y | z | BS Ratio | ||
| EM and AM > SM | ||||||
| Middle Frontal Gyrus | L | 10 | −32 | 52 | 12 | 11.3 |
| L | 6 | −32 | 8 | 64 | 5.7 | |
| R | 9 | 32 | 44 | 32 | 6.8 | |
| Anterior Cingulate Cortex | L | 24 | −8 | 32 | 12 | 9.8 |
| 32 | 0 | 36 | −8 | 7.2 | ||
| Superior Parietal Lobule/Precuneus | L | 7 | −12 | −72 | 40 | 11.2 |
| Supramarginal Gyrus | R | 40 | 60 | −44 | 32 | 9.0 |
| Inferior Parietal Lobule | L | 40 | −56 | −48 | 48 | 7.9 |
| Insula | L | n/a | −44 | 16 | 0 | 7.8 |
| SM > EM and AM | ||||||
| Inferior Occipital Gyrus | L | 18 | −32 | −96 | −4 | −11.5 |
| R | 18 | 32 | −96 | −4 | −8.8 | |
| Middle Occipital Gyrus | R | 19 | 28 | −72 | 40 | −5.0 |
| Retrosplenial Cortex | R | 23 | 4 | −56 | 16 | −8.7 |
| Middle Temporal Gyrus | L | 21 | −60 | 0 | −24 | −7.4 |
| Cerebellum | R | n/a | 0 | −60 | −48 | −6.6 |
| L | n/a | −12 | −76 | −28 | −5.1 | |
Hemis = hemisphere; BA = Brodmann’s area; x = right/left; y = anterior/posterior; z = superior/inferior; BS ratio = bootstrap ratio.
In order to see whether the reduced expression of these brain activity patterns in older adults was related to task performance, we computed composite brain scores for each older participant for each of the three LVs. These composites were obtained by summing the absolute values of a subject’s brain scores for all conditions. We then correlated these brain scores with task accuracy and reaction time. None of the correlations between the composite score and task accuracy or reaction time reached significance (r =.021 to .472, p > .05, uncorrected).
fMRI results: MUSUBADA
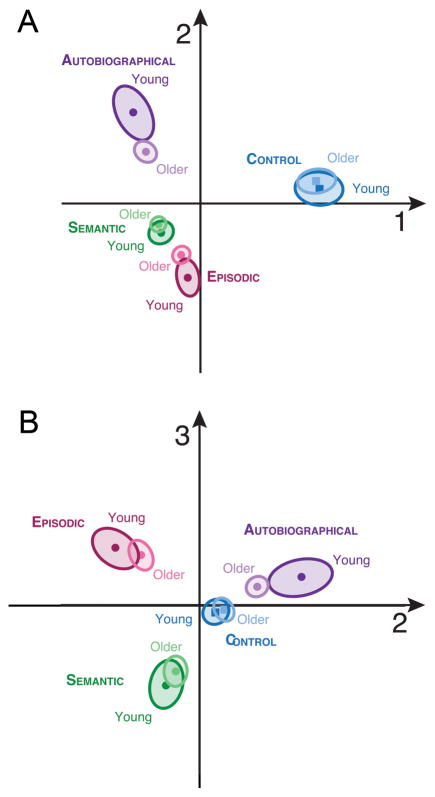
MUSUBADA identified three components which explained, respectively, 54, 27, and 19 % of the variance between the experimental conditions. Figures 4a and 4b display the maps obtained with (respectively) Dimensions 1 vs. 2, and 2 vs. 3. Dimension 1 separates the control condition from all of the memory conditions, Dimension 2 distinguishes AM from SM and EM, and Dimension 3 maximally separates EM from SM. This overall pattern mirrors the results of the ST-PLS analysis. On both discriminant maps, the largest (and significant, p < .05) differences among age groups were seen in AM, followed by EM. That is, for AM and EM, the older group projected closer to the center of the graph than did the younger group, so that the brain patterns of the older participants are less differentiated than those of the young participants in these two conditions. Although there was a tendency for the older group to fall closer to the center of the graph than the younger group for SM, the group differences did not reach significance in SM or in the control condition.
Figure 4.
Discussion
We report the results of an experiment measuring brain activation during autobiographical (AM), episodic (EM), and semantic (SM) memory retrieval in young and older adults using a paradigm previously used for young adults (Burianova & Grady, 2007). Both the earlier study and the current one identified the same set of core regions that were engaged during all memory conditions. These regions included the inferior frontal gyrus, the middle temporal gyrus, the left superior temporal gyrus, the left thalamus, the left hippocampus, the left angular gyrus, and the caudate nucleus. We also found that the common memory regions were activated reliably in older as well as younger adults, with minimal age differences; in fact the only age difference was slightly greater engagement of the common network for EM in older adults. This result suggests that the common memory network is relatively resilient to the effects of aging, in the same way that network function and connectivity is preserved with aging in the Task-Positive Network (Grady et al., 2010), a set of fronto-parietal brain regions involved when externally driven tasks are carried out (Fox et al., 2005; Grady et al., 2010; Toro et al., 2008).
Although the common memory network was engaged strongly by both age groups, there were some subtle differences. In the first LV, which identified the common network, we actually observed greater overall differentiation among the three memory conditions and the control condition in the older adults than in the young adults, particularly in the EM and SM conditions. This result is reassuring because it clearly rules out the possibility that any dedifferentiation among the memory conditions observed in the older group is due to unspecific age-related changes in the BOLD response. However, if we consider only the three memory conditions, the first LV’s pattern was expressed more similarly among these conditions in the older than in the young adults. In other words, older adults were recruiting regions from the common memory network to a more similar extent across all memory conditions, so that their neural signature was more similar. This is consistent with the main results from LVs 2 and 3, that patterns of selective activity that distinguished AM, EM, and SM were expressed less robustly in the older adults. Interestingly, the MUSUBADA analysis showed that this loss of neural differentiation was accounted for by age-related changes in the neural signature of AM and EM, but not SM nor the control condition.
Also, MUSUBADA revealed that EM and AM were significantly less distinguishable from the SM and control conditions in the older group than in young adults. This pattern, which reflects an age reduction in the episodic neural signature of EM and AM, is consistent with the aging literature which suggests that episodic memory is disproportionally affected by aging, while semantic memory is relatively preserved (Allen et al., 2002; Spaniol et al., 2006). When narrating personal episodes, older adults produce fewer episodic elements, but equal or greater amounts of semantic elements than young adults (Addis et al., 2008; Levine et al., 2002). During memory recognition tasks, older adults rely more heavily on familiarity with the studied item than on their capacity to recollect its initial encoding, a phenomenon illustrated with self-report (e.g. Bastin & Van der Linden, 2003; Parkin & Walter, 1992; Mäntylä, 1993) and with process-dissociation procedures (Cohn et al., 2008; Java, 1996; Jennings & Jacoby, 1993; but see Davidson & Glisky, 2002). Also during recognition, older adults demonstrate an increased reliance on rhinal areas, which are associated with familiarity-based memory recognition, whereas young adults rely more on the hippocampus, which mediates recollection (Cabeza et al., 2004; Daselaar et al., 2006; Grady et al., 2005). Our results are in line with these earlier studies, and support the idea of an age-related reduction in the episodic, or richly experienced nature of both AM and EM.
Our ST-PLS results also indicated an age-related reduction in episodicity. LV2, which was expressed less robustly in the senior group, identified brain regions commonly activated during AM retrieval, such as the posterior medial cortices, left medial temporal lobe, left inferior parietal lobe, medial prefrontal cortex, and temporal poles (Maguire, 2001; Svoboda et al., 2006). The medial prefrontal and retrosplenial regions are linked to self-projection (Buckner & Carroll, 2007; St Jacques et al.; Svoboda et al., 2006; Vann et al., 2009), a phenomenon that facilitates vivid recollection. The medial temporal lobe also plays a central role in the recollection of AM and EM (Moscovitch et al., 2005), and is thought to support the retrieval and integration of AM details (Addis et al., 2004; Gilboa, 2004). The reduced distinctiveness in the engagement of these regions during AM retrieval in our older group is consistent with evidence that AM narratives lack episodic details in older adults. Also, some of the regions that are typically engaged during AM retrieval—such as the inferior parietal lobule, the medial prefrontal cortex, and the posterior cingulate—belong to the default mode network (DMN; Buckner et al., 2008; Fox et al., 2005; Raichle et al., 2001), which is activated when attention is directed towards internally driven cognitive processes. Interestingly, recent work suggests that functional connectivity within the DMN is reduced in old age (Andrews-Hanna et al., 2007; Grady et al., 2010), so the decreased robustness of LV2’s activity pattern in our older group may be linked to a general decrease in coherence, or to an increased difficulty in recruitment within the DMN.
LV3, which maximally distinguished EM from SM in both age groups, was also less robustly expressed in our older adults. Among other findings, we observed greater activity in the inferior parietal lobule during EM than during SM, a region thought to play a supportive role in recollective processes (Cabeza et al., 2008). We also observed more activity in the precuneus, a region thought to support visual imagery during EM and AM (Burgess et al., 2001; Fletcher et al., 1995; Snyder et al., 1998). It is possible that features such as temporal specificity, contextual details and visual imagery, which distinguish episodic from context-independent SM, lose some salience during normal aging and/or are retrieved less frequently.
Although our brain imaging results reflect an age-related reduction in the episodic neural signature of EM and AM, we note that we did not observe behavioural group differences on the AM or EM task (either for reaction time or accuracy). However, our behavioural measures were designed primarily to identify and exclude incorrect trials, and were not sensitive to the qualitative changes in episodic memory typically observed in aging. Also, while there was no group difference in the use of the 3-point AM vividness scale, a subjective scale cannot be considered an absolute measure of vividness. For example, St Jacques et al. (in press) also reported that they found no difference between young and older adults for the ratings of AMs retrieved in the scanner on an 8-point “reliving” scale; however, their post-scanning interviews revealed a paucity of episodic details in the older adults’ description of these AMs. In our case, the age-related changes we observed in the neural correlates of AM might indicate a difference in scale anchoring between our groups (i.e. young and older adults’ criteria used to determine which AM is rated as “somewhat vivid” or “very vivid” may differ). While our behavioural measures were not sensitive to age differences, the reduced engagement of regions known to be sensitive to detailed memory recollection, and the pattern of dedifferentiation among the memory conditions we observed in our older adults, suggest a loss of episodic distinctiveness.
This interpretation concurs with Li et al (2001), who suggested that aging is accompanied by an increase in neural noise, which leads to a loss of distinctiveness in cortical representation due to increased overlapping activation across episodic memory traces. Interestingly, several recent studies have shown age-related losses in neural distinctiveness within (Goh et al., 2010) and across stimulus categories processed by the ventral visual pathway, such as faces, places, and objects (Carp et al.; Park et al., 2004; Park et al.; Voss et al., 2008). Dennis and Cabeza (2010) also have shown that brain regions engaged distinctively during explicit and implicit learning in young adults are recruited non-discriminatively during both conditions in older adults. Our study provides additional evidence for aging-related dedifferentiation across types of processing by showing dedifferentiation across declarative memory tasks that require retrieval of different types of information.
Importantly, however, interpreting age-related dedifferentiation is not straightforward. Dedifferentiation within processing pathways may reflect a loss of distinctive representation, whereas dedifferentiation observed across modalities and/or processes may reflect either neural inefficiency or compensation. The increased brain activity observed in older relative to younger adults in the absence of age-related changes in behavior, or in the presence of a decrease in performance, is thought to reflect neural inefficiency (Grady, 2008; Morcom et al., 2007). In the case of our results, the general decrease in brain activity in comparison to our young adults, would argue against an explanation by inefficiency.
On the other hand, neural task-related changes present in older adults, and absent in young adults, that correlate with good task performance in seniors are considered compensatory (see Grady, 2008, for a review). With these criteria, some dedifferentiation can be considered compensatory (Hommet et al., 2008; Li et al., 2001; Rajah & McIntosh, 2008). Dennis and Cabeza (2010) proposed that normal aging causes a decrease in brain function which leads to a loss of competition between different systems or processes. This loss of competition results in a pooling of competing resources in order to compensate for the loss of function, and this causes neural dedifferentiation. In carrying out our tasks, it is possible that older adults—as an attempt to pool resources across systems—engaged a more similar set of brain regions across memory conditions than the young adults.
Finally, we should note that we found little evidence in our older adults for the kind of over-recruitment of brain activity that features prominently in the HAROLD, CRUNCH, and STAC models (Cabeza, 2002; Reuter-Lorenz & Park, 2010). These theories generally emphasize situations in which older adults recruit some brain area, such as the prefrontal cortex, to a greater extent than younger adults, thereby using alternate circuitry in order to maintain adequate levels of cognitive function. Our results, as noted above, do not easily fit with the type of compensatory over-recruitment suggested by these theories and other reports in the literature. The only over-recruitment that we observed in our older group was more engagement of the common network for EM than was seen in the younger group. This could reflect over-recruitment of the common network to compensate for under-recruitment of the networks specific to each condition, along the lines of CRUNCH and STAC, as there were no age differences in performance. On the other hand, the brain activity measures did not correlate with performance in our older group, which does not support an explanation in terms of compensation on an individual participant basis. This lack of correlation could be due to a number of factors, including, as noted above, a lack of sensitivity of our behavioral measures to age-related changes in memory. It is also possible that the over-recruitment of the common network during EM in the older adults is only partially compensatory, or is not compensatory for memory at all, but for some other, non-memory process engaged during our tasks (de Chastelaine et al., in press). It also may be easier to find correlations between measures of brain activity and performance when performance is closely tied to experimentally-driven task demands, unlike performance on our tasks, which was heavily influenced by personal knowledge and experience. Clearly further work is needed to determine if there are correlations between the degree of dedifferentiated brain activity during memory retrieval in older adults and the content or detailed nature of retrieved memories.
Conclusion
In a group of healthy older adults, we found evidence for dedifferentiation in the neural signatures of episodic and autobiographical memory, but not for semantic memory. Although our results indicate that regions identified as parts of a common memory retrieval network are activated normally in older adults, we found that selective brain activity associated with the memory conditions is diminished in old age. Dedifferentiation was expressed by a loss of specificity in the episodic and autobiographical memory conditions, consistent with a literature indicating that context-specific memory is most readily disrupted by aging, whereas semantic memory is relatively preserved. The dedifferentiation we observed may reflect a pooling of resources across memory conditions that is associated with an aging-related decline in the ability to differentially represent episodes, or their content, resulting in less richly detailed memories.
Supplementary Material
Acknowledgments
The authors would like to thank Magda Wojtowicz, John Anderson, Charisa Ng, Nick Hoang, Ricky Tong, Annette Weekes-Holder, Roshan Guna, Patricia Van Roon, and all our participants for their help with the project. This study was supported by a grant from the Canadian Institute for Health Research (CIHR; Grant number: MOP14036) held by CG, and by a graduate scholarship from the National Science and Engineering Council of Canada (NSERC) awarded to MS-L.
References
- Abdi H, Dunlop JP, Williams LJ. How to compute reliability estimates and display confidence and tolerance intervals for pattern classifiers using the Bootstrap and 3-way multidimensional scaling (DISTATIS) Neuroimage. 2009;45:89–95. doi: 10.1016/j.neuroimage.2008.11.008. [DOI] [PubMed] [Google Scholar]
- Abdi H, Williams LJ. Barycentric discriminant analysis (BADIA) In: Salkind NJ, Dougherty DM, Frey B, editors. Encyclopedia of Research Design. Sage; Thousand Oaks (CA): 2010. pp. 64–75. [Google Scholar]
- Addis DR, Moscovitch M, Crawley AP, McAndrews MP. Recollective qualities modulate hippocampal activation during autobiographical memory retrieval. Hippocampus. 2004;14:752–762. doi: 10.1002/hipo.10215. [DOI] [PubMed] [Google Scholar]
- Addis DR, Wong AT, Schacter DL. Age-related changes in the episodic simulation of future events. Psychol Sci. 2008;19:33–41. doi: 10.1111/j.1467-9280.2008.02043.x. [DOI] [PubMed] [Google Scholar]
- Allen PA, Madden DJ, Weber TA, Groth KE. Influence of age and processing stage on visual word recognition. Psychol Aging. 1993;8:274–282. doi: 10.1037//0882-7974.8.2.274. [DOI] [PubMed] [Google Scholar]
- Allen PA, Sliwinski M, Bowie T, Madden DJ. Differential age effects in semantic and episodic memory. J Gerontol B Psychol Sci Soc Sci. 2002;57:173–186. doi: 10.1093/geronb/57.2.p173. [DOI] [PubMed] [Google Scholar]
- Allen PA, Smith AF, Jerge KA, Vires-Collins H. Age differences in mental multiplication: evidence for peripheral but not central decrements. J Gerontol B Psychol Sci Soc Sci. 1997;52:81–90. doi: 10.1093/geronb/52b.2.p81. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, Buckner RL. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56:924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balota DA, Ferraro FR. Lexical, Sublexical, and Implicit Memory Processes in Healthy Young and Healthy Older Adults and in Individuals With Dementia of the Alzheimer Type. Neuropsychology. 1996;10:82–95. [Google Scholar]
- Bastin C, Van der Linden M. The contribution of recollection and familiarity to recognition memory: a study of the effects of test format and aging. Neuropsychology. 2003;17:14–24. [PubMed] [Google Scholar]
- Brewer WF. What is autobiographical memory? In: DCR, editor. Autobiographical memory. Cambridge University Press; Cambridge, England: 1986. pp. 25–49. [Google Scholar]
- Brown MW, Aggleton JP. Recognition memory: what are the roles of the perirhinal cortex and hippocampus? Nat Rev Neurosci. 2001;2:51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Carroll DC. Self-projection and the brain. Trends Cogn Sci. 2007;11:49–57. doi: 10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Burgess N, Maguire EA, Spiers HJ, O’Keefe J. A temporoparietal and prefrontal network for retrieving the spatial context of lifelike events. Neuroimage. 2001;14:439–453. doi: 10.1006/nimg.2001.0806. [DOI] [PubMed] [Google Scholar]
- Burianova H, Grady CL. Common and unique neural activations in autobiographical, episodic, and semantic retrieval. J Cogn Neurosci. 2007;19:1520–1534. doi: 10.1162/jocn.2007.19.9.1520. [DOI] [PubMed] [Google Scholar]
- Burianova H, McIntosh AR, Grady CL. A common functional brain network for autobiographical, episodic, and semantic memory retrieval. Neuroimage. 2010;49:865–874. doi: 10.1016/j.neuroimage.2009.08.066. [DOI] [PubMed] [Google Scholar]
- Burke DM, Light LL. Memory and aging: the role of retrieval processes. Psychol Bull. 1981;90:513–514. [PubMed] [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol Aging. 2002;17:85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Ciaramelli E, Olson IR, Moscovitch M. The parietal cortex and episodic memory: an attentional account. Nat Rev Neurosci. 2008;9:613–625. doi: 10.1038/nrn2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Daselaar SM, Dolcos F, Prince SE, Budde M, Nyberg L. Task-independent and task-specific age effects on brain activity during working memory, visual attention and episodic retrieval. Cereb Cortex. 2004;14:364–375. doi: 10.1093/cercor/bhg133. [DOI] [PubMed] [Google Scholar]
- Carp J, Park J, Polk TA, Park DC. Age differences in neural distinctiveness revealed by multi-voxel pattern analysis. Neuroimage. doi: 10.1016/j.neuroimage.2010.04.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn M, Emrich SM, Moscovitch M. Age-related deficits in associative memory: the influence of impaired strategic retrieval. Psychol Aging. 2008;23:93–103. doi: 10.1037/0882-7974.23.1.93. [DOI] [PubMed] [Google Scholar]
- Cohn M, Moscovitch M, Lahat A, McAndrews MP. Recollection versus strength as the primary determinant of hippocampal engagement at retrieval. Proc Natl Acad Sci U S A. 2009;106:22451–22455. doi: 10.1073/pnas.0908651106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway MA. Sensory-perceptual episodic memory and its context: autobiographical memory. Philos Trans R Soc Lond B Biol Sci. 2001;356:1375–1384. doi: 10.1098/rstb.2001.0940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craik FIM, Jennings JM. Human memory. In: Craik FIM, Salthouse TA, editors. The handbook of aging and cognition. Erlbaum; Hillsdale, NJ: 1992. pp. 51–110. [Google Scholar]
- Daselaar SM, Fleck MS, Dobbins IG, Madden DJ, Cabeza R. Effects of healthy aging on hippocampal and rhinal memory functions: an event-related fMRI study. Cereb Cortex. 2006;16:1771–1782. doi: 10.1093/cercor/bhj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson PS, Glisky EL. Neuropsychological correlates of recollection and familiarity in normal aging. Cogn Affect Behav Neurosci. 2002;2:174–186. doi: 10.3758/cabn.2.2.174. [DOI] [PubMed] [Google Scholar]
- de Chastelaine M, Wang TH, Minton B, Muftuler LT, Rugg MD. The Effects of Age, Memory Performance, and Callosal Integrity on the Neural Correlates of Successful Associative Encoding. Cereb Cortex. doi: 10.1093/cercor/bhq294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis NA, Cabeza R. Age-related dedifferentiation of learning systems: an fMRI study of implicit and explicit learning. Neurobiol Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donix M, Poettrich K, Weiss PH, Werner A, von Kummer R, Fink GR, Holthoff VA. Age-dependent differences in the neural mechanisms supporting long-term declarative memories. Arch Clin Neuropsychol. 2010;25:383–395. doi: 10.1093/arclin/acq037. [DOI] [PubMed] [Google Scholar]
- Duarte A, Graham KS, Henson RN. Age-related changes in neural activity associated with familiarity, recollection and false recognition. Neurobiol Aging. 2010;31:1814–1830. doi: 10.1016/j.neurobiolaging.2008.09.014. [DOI] [PubMed] [Google Scholar]
- Efron B, Tibshirani R. The bootstrap method for assessing statistical accuracy. Behaviormetrika. 1985;17:1–35. [Google Scholar]
- Eldridge LL, Knowlton BJ, Furmanski CS, Bookheimer SY, Engel SA. Remembering episodes: a selective role for the hippocampus during retrieval. Nat Neurosci. 2000;3:1149–1152. doi: 10.1038/80671. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Frith CD, Baker SC, Shallice T, Frackowiak RS, Dolan RJ. The mind’s eye--precuneus activation in memory-related imagery. Neuroimage. 1995;2:195–200. doi: 10.1006/nimg.1995.1025. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaquinto S, Ranghi F, Butler S. Stability of word comprehension with age. An electrophysiological study. Mech Ageing Dev. 2007;128:628–636. doi: 10.1016/j.mad.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Gilboa A. Autobiographical and episodic memory--one and the same? Evidence from prefrontal activation in neuroimaging studies. Neuropsychologia. 2004;42:1336–1349. doi: 10.1016/j.neuropsychologia.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Goh JO, Suzuki A, Park DC. Reduced neural selectivity increases fMRI adaptation with age during face discrimination. Neuroimage. 2010;51:336–344. doi: 10.1016/j.neuroimage.2010.01.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL. Age-related differences in face processing: a meta-analysis of three functional neuroimaging experiments. Can J Exp Psychol. 2002;56:208–220. doi: 10.1037/h0087398. [DOI] [PubMed] [Google Scholar]
- Grady CL. Cognitive neuroscience of aging. Ann N Y Acad Sci. 2008;1124:127–144. doi: 10.1196/annals.1440.009. [DOI] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Craik FI. Task-related activity in prefrontal cortex and its relation to recognition memory performance in young and old adults. Neuropsychologia. 2005;43:1466–1481. doi: 10.1016/j.neuropsychologia.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Grady CL, Protzner AB, Kovacevic N, Strother SC, Afshin-Pour B, Wojtowicz M, Anderson JA, Churchill N, McIntosh AR. A Multivariate Analysis of Age-Related Differences in Default Mode and Task-Positive Networks across Multiple Cognitive Domains. Cereb Cortex. 2010;20:1432–1447. doi: 10.1093/cercor/bhp207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson RN, Cansino S, Herron JE, Robb WG, Rugg MD. A familiarity signal in human anterior medial temporal cortex? Hippocampus. 2003;13:301–304. doi: 10.1002/hipo.10117. [DOI] [PubMed] [Google Scholar]
- Hommet C, Destrieux C, Constans T, Berrut G. Aging and hemispheric cerebral lateralization. Psychol Neuropsychiatr Vieil. 2008;6:49–56. doi: 10.1684/pnv.2008.0114. [DOI] [PubMed] [Google Scholar]
- Java RI. Effects of age on state of awareness following implicit and explicit word-association tasks. Psychol Aging. 1996;11:108–111. doi: 10.1037//0882-7974.11.1.108. [DOI] [PubMed] [Google Scholar]
- Jennings JM, Jacoby LL. Automatic versus intentional uses of memory: aging, attention, and control. Psychol Aging. 1993;8:283–293. doi: 10.1037//0882-7974.8.2.283. [DOI] [PubMed] [Google Scholar]
- Krishnan A, Williams LJ, McIntosh AR, Abdi H. Partial Least Squares (PLS) methods for neuroimaging: A tutorial and review. Neuroimage. doi: 10.1016/j.neuroimage.2010.07.034. [DOI] [PubMed] [Google Scholar]
- Laver GD, Burke DM. Why do semantic priming effects increase in old age? A meta-analysis. Psychol Aging. 1993;8:34–43. doi: 10.1037//0882-7974.8.1.34. [DOI] [PubMed] [Google Scholar]
- Levine B, Svoboda E, Hay JF, Winocur G, Moscovitch M. Aging and autobiographical memory: dissociating episodic from semantic retrieval. Psychol Aging. 2002;17:677–689. [PubMed] [Google Scholar]
- Li SC, Lindenberger U, Sikstrom S. Aging cognition: from neuromodulation to representation. Trends Cogn Sci. 2001;5:479–486. doi: 10.1016/s1364-6613(00)01769-1. [DOI] [PubMed] [Google Scholar]
- Logan JM, Sanders AL, Snyder AZ, Morris JC, Buckner RL. Under-recruitment and nonselective recruitment: dissociable neural mechanisms associated with aging. Neuron. 2002;33:827–840. doi: 10.1016/s0896-6273(02)00612-8. [DOI] [PubMed] [Google Scholar]
- Lustig C, Snyder AZ, Bhakta M, O’Brien KC, McAvoy M, Raichle ME, Morris JC, Buckner RL. Functional deactivations: change with age and dementia of the Alzheimer type. Proc Natl Acad Sci U S A. 2003;100:14504–14509. doi: 10.1073/pnas.2235925100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Turkington TG, Provenzale JM, Denny LL, Hawk TC, Gottlob LR, Coleman RE. Adult age differences in the functional neuroanatomy of verbal recognition memory. Hum Brain Mapp. 1999;7:115–135. doi: 10.1002/(SICI)1097-0193(1999)7:2<115::AID-HBM5>3.0.CO;2-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA. Neuroimaging studies of autobiographical event memory. Philos Trans R Soc Lond B Biol Sci. 2001;356:1441–1451. doi: 10.1098/rstb.2001.0944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Frith CD. Aging affects the engagement of the hippocampus during autobiographical memory retrieval. Brain. 2003;126:1511–1523. doi: 10.1093/brain/awg157. [DOI] [PubMed] [Google Scholar]
- Mantyla T. Priming effects in prospective memory. Memory. 1993;1:203–218. doi: 10.1080/09658219308258233. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Bookstein FL, Haxby JV, Grady CL. Spatial pattern analysis of functional brain images using partial least squares. Neuroimage. 1996;3:143–157. doi: 10.1006/nimg.1996.0016. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Chau WK, Protzner AB. Spatiotemporal analysis of event-related fMRI data using partial least squares. Neuroimage. 2004;23:764–775. doi: 10.1016/j.neuroimage.2004.05.018. [DOI] [PubMed] [Google Scholar]
- Mitchell DB. How many memory systems? Evidence from aging. J Exp Psychol Learn Mem Cogn. 1989;15:31–49. doi: 10.1037//0278-7393.15.1.31. [DOI] [PubMed] [Google Scholar]
- Mitchell KJ, Raye CL, Johnson MK, Greene EJ. An fMRI investigation of short-term source memory in young and older adults. Neuroimage. 2006;30:627–633. doi: 10.1016/j.neuroimage.2005.09.039. [DOI] [PubMed] [Google Scholar]
- Morcom AM, Li J, Rugg MD. Age effects on the neural correlates of episodic retrieval: increased cortical recruitment with matched performance. Cereb Cortex. 2007;17:2491–2506. doi: 10.1093/cercor/bhl155. [DOI] [PubMed] [Google Scholar]
- Moscovitch M, Rosenbaum RS, Gilboa A, Addis DR, Westmacott R, Grady C, McAndrews MP, Levine B, Black S, Winocur G, Nadel L. Functional neuroanatomy of remote episodic, semantic and spatial memory: a unified account based on multiple trace theory. J Anat. 2005;207:35–66. doi: 10.1111/j.1469-7580.2005.00421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielson KA, Douville KL, Seidenberg M, Woodard JL, Miller SK, Franczak M, Antuono P, Rao SM. Age-related functional recruitment for famous name recognition: an event-related fMRI study. Neurobiol Aging. 2006;27:1494–1504. doi: 10.1016/j.neurobiolaging.2005.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson LG. Memory function in normal aging. Acta Neurol Scand Suppl. 2003;179:7–13. doi: 10.1034/j.1600-0404.107.s179.5.x. [DOI] [PubMed] [Google Scholar]
- Nyberg L, Backman L, Erngrund K, Olofsson U, Nilsson LG. Age differences in episodic memory, semantic memory, and priming: relationships to demographic, intellectual, and biological factors. J Gerontol B Psychol Sci Soc Sci. 1996;51:234–240. doi: 10.1093/geronb/51b.4.p234. [DOI] [PubMed] [Google Scholar]
- Park DC, Polk TA, Park R, Minear M, Savage A, Smith MR. Aging reduces neural specialization in ventral visual cortex. Proc Natl Acad Sci U S A. 2004;101:13091–13095. doi: 10.1073/pnas.0405148101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, Reuter-Lorenz P. The adaptive brain: aging and neurocognitive scaffolding. Annu Rev Psychol. 2009;60:173–196. doi: 10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Carp J, Hebrank A, Park DC, Polk TA. Neural specificity predicts fluid processing ability in older adults. J Neurosci. 30:9253–9259. doi: 10.1523/JNEUROSCI.0853-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin AJ, Walter BM. Recollective experience, normal aging, and frontal dysfunction. Psychol Aging. 1992;7:290–298. doi: 10.1037//0882-7974.7.2.290. [DOI] [PubMed] [Google Scholar]
- Piefke M, Fink GR. Recollections of one’s own past: the effects of aging and gender on the neural mechanisms of episodic autobiographical memory. Anat Embryol (Berl) 2005;210:497–512. doi: 10.1007/s00429-005-0038-0. [DOI] [PubMed] [Google Scholar]
- Piolino P, Desgranges B, Clarys D, Guillery-Girard B, Taconnat L, Isingrini M, Eustache F. Autobiographical memory, autonoetic consciousness, and self-perspective in aging. Psychol Aging. 2006;21:510–525. doi: 10.1037/0882-7974.21.3.510. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajah MN, D’Esposito M. Region-specific changes in prefrontal function with age: a review of PET and fMRI studies on working and episodic memory. Brain. 2005;128:1964–1983. doi: 10.1093/brain/awh608. [DOI] [PubMed] [Google Scholar]
- Rajah MN, McIntosh AR. Age-related differences in brain activity during verbal recency memory. Brain Res. 2008;1199:111–125. doi: 10.1016/j.brainres.2007.12.051. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Cappell KA. Neurocogntive aging and the compensation hypothesis. Current Directions in Psychological Science. 2008;18:177–182. [Google Scholar]
- Reuter-Lorenz PA, Park DC. Human neuroscience and the aging mind: a new look at old problems. J Gerontol B Psychol Sci Soc Sci. 2010;65:405–415. doi: 10.1093/geronb/gbq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson PD, Streissguth AP, Barr HM, Bookstein FL. Neurobehavioral effects of prenatal alcohol: Part II. Partial least squares analysis. Neurotoxicol Teratol. 1989;11:477–491. doi: 10.1016/0892-0362(89)90025-1. [DOI] [PubMed] [Google Scholar]
- Snyder LH, Grieve KL, Brotchie P, Andersen RA. Separate body-and world-referenced representations of visual space in parietal cortex. Nature. 1998;394:887–891. doi: 10.1038/29777. [DOI] [PubMed] [Google Scholar]
- Spaniol J, Madden DJ, Voss A. A diffusion model analysis of adult age differences in episodic and semantic long-term memory retrieval. J Exp Psychol Learn Mem Cogn. 2006;32:101–117. doi: 10.1037/0278-7393.32.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer WD, Raz N. Differential effects of aging on memory for content and context: a meta-analysis. Psychol Aging. 1995;10:527–539. doi: 10.1037//0882-7974.10.4.527. [DOI] [PubMed] [Google Scholar]
- St Jacques PL, Conway MA, Lowder MW, Cabeza R. Watching My Mind Unfold versus Yours: An fMRI Study Using a Novel Camera Technology to Examine Neural Differences in Self-projection of Self versus Other Perspectives. J Cogn Neurosci. doi: 10.1162/jocn.2010.21518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Jacques PL, Levine B. Ageing and autobiographical memory for emotional and neutral events. Memory. 2007;15:129–144. doi: 10.1080/09658210601119762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Jacques PL, Rubin DC, Cabeza R. Age-related effects on the neural correlates of autobiographical memory retrieval. Neurobiol Aging. doi: 10.1016/j.neurobiolaging.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda E, McKinnon MC, Levine B. The functional neuroanatomy of autobiographical memory: a meta-analysis. Neuropsychologia. 2006;44:2189–2208. doi: 10.1016/j.neuropsychologia.2006.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toro R, Fox PT, Paus T. Functional coactivation map of the human brain. Cereb Cortex. 2008;18:2553–2559. doi: 10.1093/cercor/bhn014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E. Episodic and semantic memory. In: Tulving E, Donaldson W, editors. Organization of memory. Academic Press; New York: 1972. pp. 381–403. [Google Scholar]
- Tulving E. Memory and consciousness. Canadian Psychologist. 1985;26:1–12. [Google Scholar]
- Vann SD, Aggleton JP, Maguire EA. What does the retrosplenial cortex do? Nature reviews. Neuroscience. 2009;10:792–802. doi: 10.1038/nrn2733. [DOI] [PubMed] [Google Scholar]
- Viard A, Piolino P, Desgranges B, Chetelat G, Lebreton K, Landeau B, Young A, De La Sayette V, Eustache F. Hippocampal activation for autobiographical memories over the entire lifetime in healthy aged subjects: an fMRI study. Cereb Cortex. 2007;17:2453–2467. doi: 10.1093/cercor/bhl153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss MW, Erickson KI, Chaddock L, Prakash RS, Colcombe SJ, Morris KS, Doerksen S, Hu L, McAuley E, Kramer AF. Dedifferentiation in the visual cortex: an fMRI investigation of individual differences in older adults. Brain Res. 2008;1244:121–131. doi: 10.1016/j.brainres.2008.09.051. [DOI] [PubMed] [Google Scholar]
- Wheeler MA, Stuss DT, Tulving E. Toward a theory of episodic memory: the frontal lobes and autonoetic consciousness. Psychol Bull. 1997;121:331–354. doi: 10.1037/0033-2909.121.3.331. [DOI] [PubMed] [Google Scholar]
- Williams LJ, Abdi H, French R, Orange JB. A tutorial on MultiBlock Discriminant Correspondence Analysis (MUDICA): A new method for analyzing discourse data from clinical populations. Journal of Speech Language and Hearing Research. 2010;53:1372–1393. doi: 10.1044/1092-4388(2010/08-0141). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.