Abstract
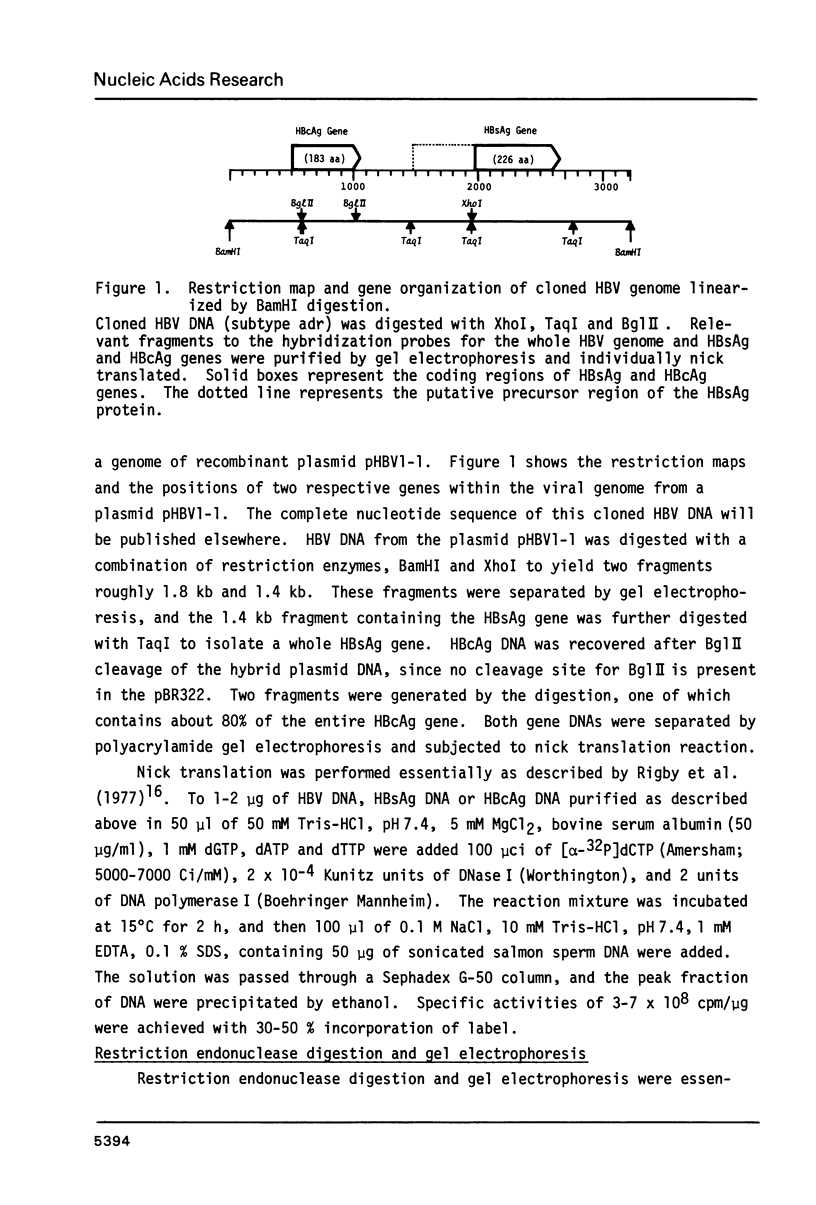
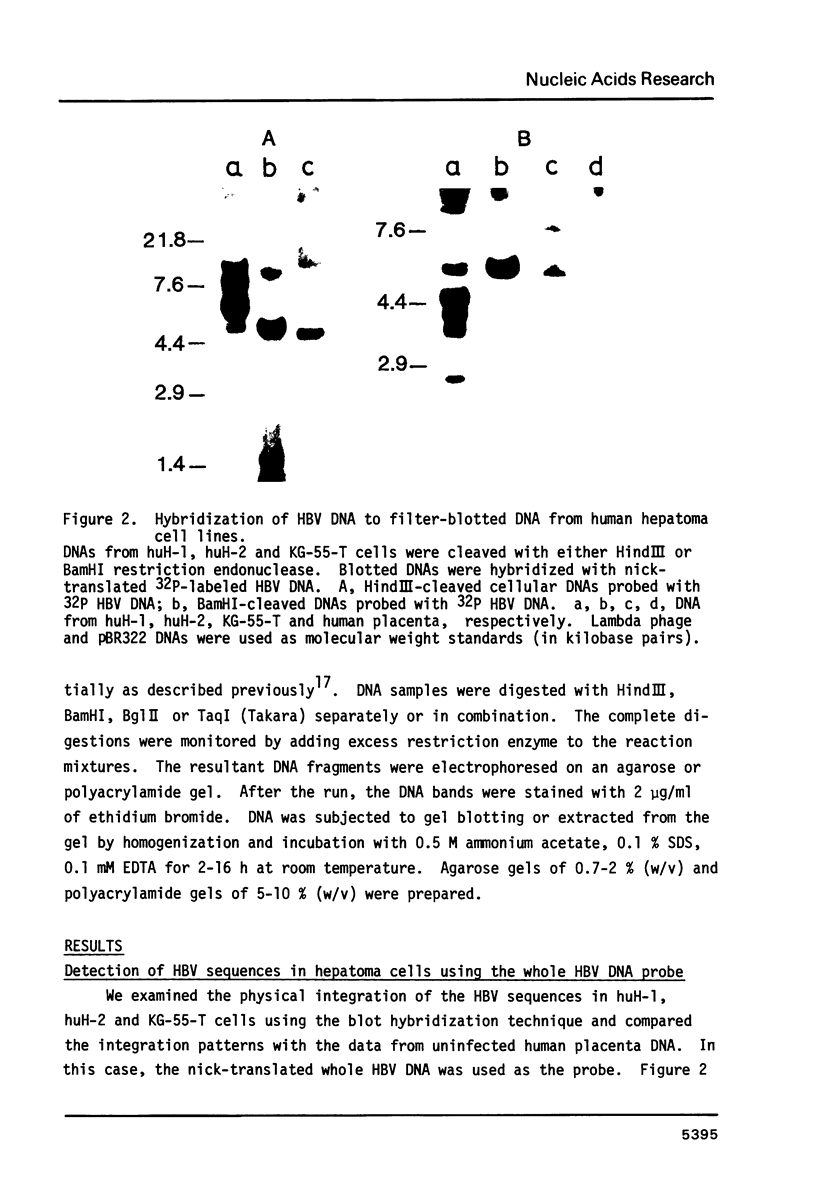
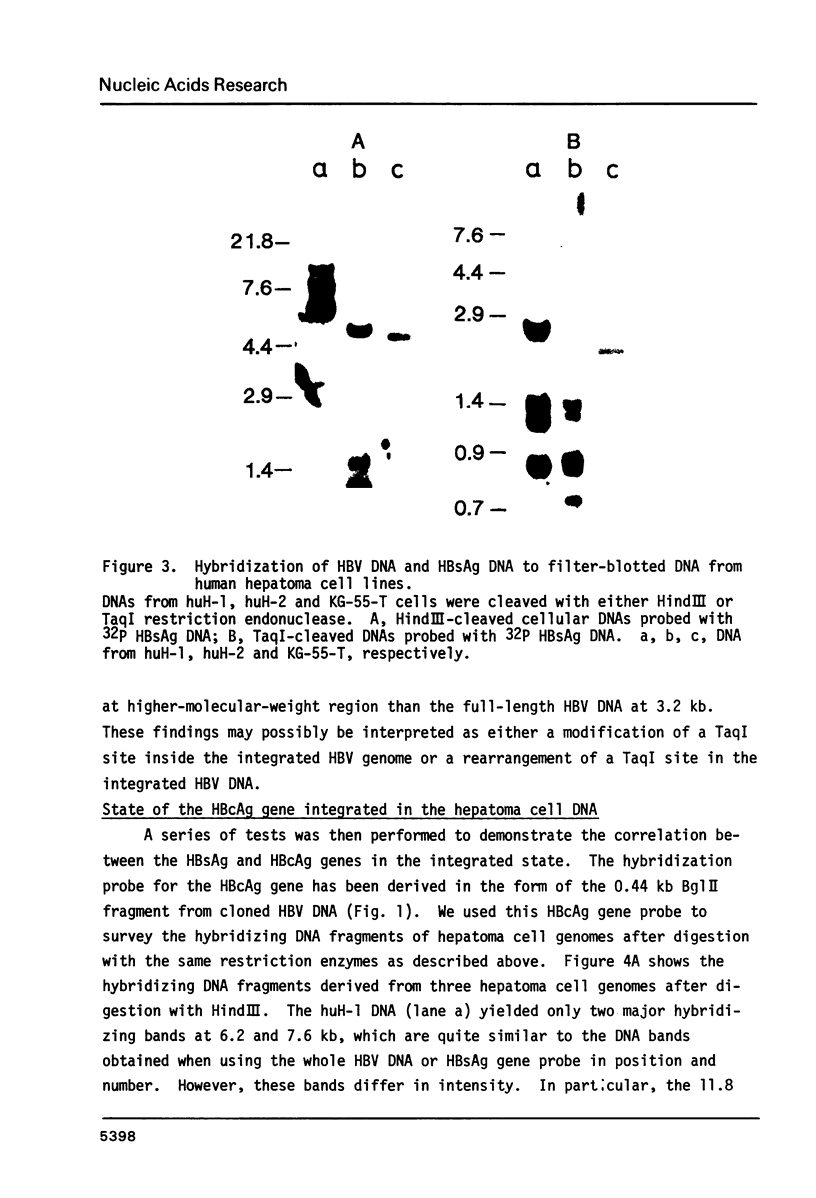
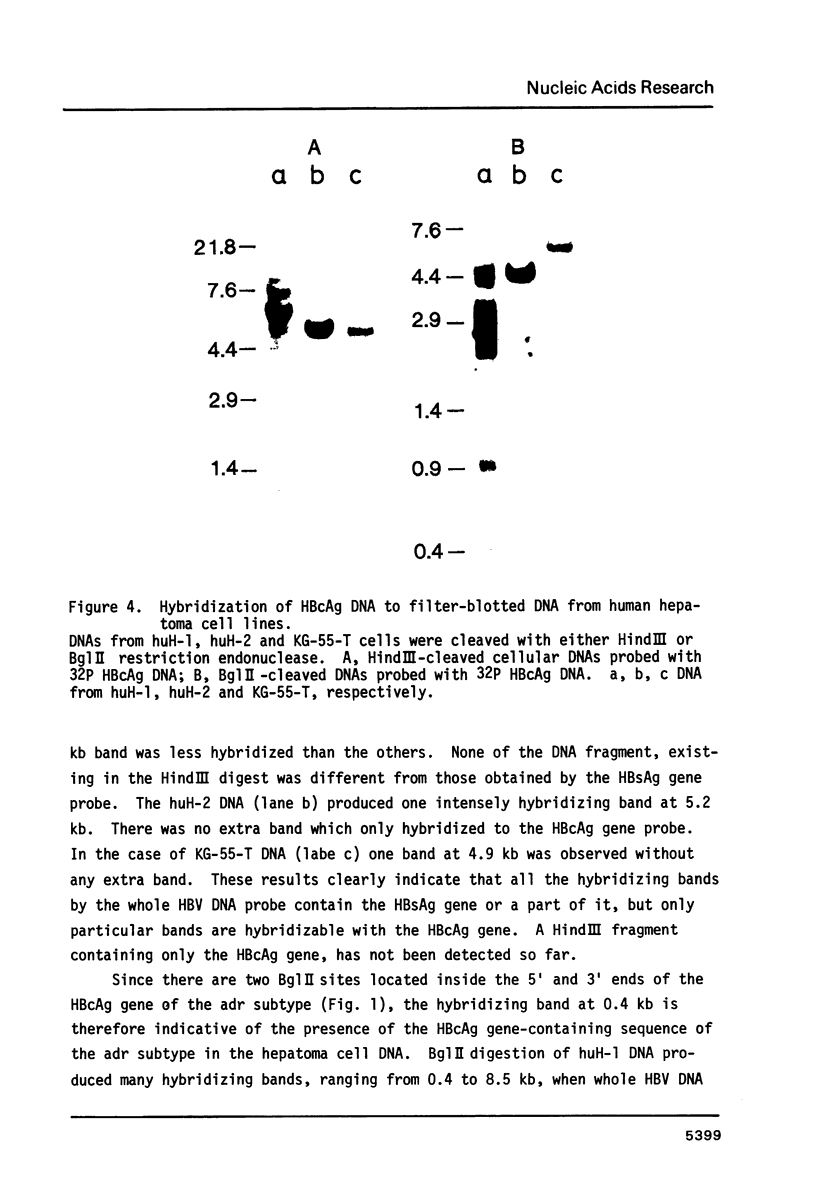
The rearrangement of integrated HBV DNA sequences in three different hepatoma cell lines, huH-1, huH-2, KG-55-T from Japanese patients, were studied by blot hybridization using whole HBV genome or a HBsAg or HBcAg DNA as a probe. The characteristic existence of multiple integration sites of HBV DNA sequences in each HindIII-restricted hepatoma cell DNA was revealed by the HBV genome probe. Detection of the isolated HBsAg gene in the HindIII fragment indicates that the integration of HBV DNA was not always related to the maintenance of the whole viral genome, and that movement of the HBsAg gene to another location occurred by rearrangement. On the other hand, the presence of the HBV DNA sequence without the intact HBcAg gene was shown in some of the HindIII fragments, when the HBcAg gene, probe was used, but a HindIII fragment, containing only the HBcAg gene, was not detected so far. The absence of the intact HBcAg gene suggests that the viral genome may lose a part of the HBcAg gene in the process of integration. This is consistent with recent findings of Ogston et al. (1982) that in Woodchuck hepatocellular carcinoma viral sequences are extensively rearranged.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beasley R. P., Hwang L. Y., Lin C. C., Chien C. S. Hepatocellular carcinoma and hepatitis B virus. A prospective study of 22 707 men in Taiwan. Lancet. 1981 Nov 21;2(8256):1129–1133. doi: 10.1016/s0140-6736(81)90585-7. [DOI] [PubMed] [Google Scholar]
- Brechot C., Pourcel C., Louise A., Rain B., Tiollais P. Presence of integrated hepatitis B virus DNA sequences in cellular DNA of human hepatocellular carcinoma. Nature. 1980 Jul 31;286(5772):533–535. doi: 10.1038/286533a0. [DOI] [PubMed] [Google Scholar]
- Chakraborty P. R., Ruiz-Opazo N., Shouval D., Shafritz D. A. Identification of integrated hepatitis B virus DNA and expression of viral RNA in an HBsAg-producing human hepatocellular carcinoma cell line. Nature. 1980 Jul 31;286(5772):531–533. doi: 10.1038/286531a0. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Edman J. C., Gray P., Valenzuela P., Rall L. B., Rutter W. J. Integration of hepatitis B virus sequences and their expression in a human hepatoma cell. Nature. 1980 Jul 31;286(5772):535–538. doi: 10.1038/286535a0. [DOI] [PubMed] [Google Scholar]
- Galibert F., Mandart E., Fitoussi F., Tiollais P., Charnay P. Nucleotide sequence of the hepatitis B virus genome (subtype ayw) cloned in E. coli. Nature. 1979 Oct 25;281(5733):646–650. doi: 10.1038/281646a0. [DOI] [PubMed] [Google Scholar]
- Huh N., Utakoji T. Production of HBs-antigen by two new human hepatoma cell lines and its enhancement by dexamethasone. Gan. 1981 Feb;72(1):178–179. [PubMed] [Google Scholar]
- Kobayashi M., Seki T., Yaginuma K., Koike K. Nucleotide sequences of small ribosomal RNA and adjacent transfer RNA genes in rat mitochondrial DNA. Gene. 1981 Dec;16(1-3):297–307. doi: 10.1016/0378-1119(81)90085-8. [DOI] [PubMed] [Google Scholar]
- Losowsky M. S. The clinical course of viral hepatitis. Clin Gastroenterol. 1980 Jan;9(1):3–21. [PubMed] [Google Scholar]
- Marion P. L., Salazar F. H., Alexander J. J., Robinson W. S. State of hepatitis B viral DNA in a human hepatoma cell line. J Virol. 1980 Feb;33(2):795–806. doi: 10.1128/jvi.33.2.795-806.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishioka K. Hepatitis B subtypes in Asia and ancestors of Alaskan natives. Lancet. 1982 Mar 27;1(8274):738–738. doi: 10.1016/s0140-6736(82)92643-5. [DOI] [PubMed] [Google Scholar]
- Nishioka Y., Leder A., Leder P. Unusual alpha-globin-like gene that has cleanly lost both globin intervening sequences. Proc Natl Acad Sci U S A. 1980 May;77(5):2806–2809. doi: 10.1073/pnas.77.5.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogston C. W., Jonak G. J., Rogler C. E., Astrin S. M., Summers J. Cloning and structural analysis of integrated woodchuck hepatitis virus sequences from hepatocellular carcinomas of woodchucks. Cell. 1982 Jun;29(2):385–394. doi: 10.1016/0092-8674(82)90155-6. [DOI] [PubMed] [Google Scholar]
- Pasek M., Goto T., Gilbert W., Zink B., Schaller H., MacKay P., Leadbetter G., Murray K. Hepatitis B virus genes and their expression in E. coli. Nature. 1979 Dec 6;282(5739):575–579. doi: 10.1038/282575a0. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Szmuness W. Hepatocellular carcinoma and the hepatitis B virus: evidence for a causal association. Prog Med Virol. 1978;24:40–69. [PubMed] [Google Scholar]
- Tiollais P., Charnay P., Vyas G. N. Biology of hepatitis B virus. Science. 1981 Jul 24;213(4506):406–411. doi: 10.1126/science.6264599. [DOI] [PubMed] [Google Scholar]
- Valenzuela P., Gray P., Quiroga M., Zaldivar J., Goodman H. M., Rutter W. J. Nucleotide sequence of the gene coding for the major protein of hepatitis B virus surface antigen. Nature. 1979 Aug 30;280(5725):815–819. doi: 10.1038/280815a0. [DOI] [PubMed] [Google Scholar]
- Vanin E. F., Goldberg G. I., Tucker P. W., Smithies O. A mouse alpha-globin-related pseudogene lacking intervening sequences. Nature. 1980 Jul 17;286(5770):222–226. doi: 10.1038/286222a0. [DOI] [PubMed] [Google Scholar]