Abstract
Airway epithelial cells in the lung are the first line of defense against pathogens and environmental pollutants. Inhalation of the environmental pollutant cadmium has been linked to the development of lung cancer and chronic obstructive pulmonary disease, which are diseases characterized by chronic inflammation. To address the role of airway epithelial cells in cadmium-induced lung inflammation, we investigated how cadmium regulates secretion of interleukin 8 (IL-8) by airway epithelial cells. We show that exposure of human airway epithelial cells to subtoxic doses of cadmium in vitro promotes a characteristic inflammatory cytokine response consisting of IL-8, but not IL-1β or tumor necrosis factor-alpha. We also found that intranasal delivery of cadmium increases lung levels of the murine IL-8 homologs macrophage inflammatory protein-2 and keracinocyte-dervied chemokine and results in an influx of Gr1+ cells into the lung. We determined that inhibition of the nuclear factor-κB (NF-κB) pathway had no effect on cadmium-induced IL-8 secretion by human airway epithelial cells, suggesting that IL-8 production was mediated through an NF-κB–independent pathway. Mitogen-activated protein kinases (MAPKs) are often involved in proinflammatory signaling. Cadmium could activate the main MAPKs (i.e., p38, JNK, and Erk1/2) in human airway epithelial cells. However, only pharmacological inhibition of Erk1/2 pathway or knockdown of the expression of Erk1 and Erk2 using small interfering RNAs suppressed secretion of IL-8 induced by cadmium. Our findings identify cadmium as a potent activator of the proinflammatory cytokine IL-8 in lung epithelial cells and reveal for the first time the role of an NF-κB–independent but Erk1/2-dependent pathway in cadmium-induced lung inflammation.
Keywords: cadmium, inflammation, IL-8, lung epithelial cells, NF-κB, Erk1/2
Airway epithelial cells have long been known for their role as a barrier and the first line of defense of the lung against exogenous pathogens and pollutants (Bartlett et al., 2008; Mills et al., 1999). Compelling evidence has now established that in response to insult by exogenous products, epithelial cells secrete an array of cytokines, which in turn, help orchestrate/shape host immune responses (Cheng et al., 2007; Gershwin, 2007; Kato and Schleimer, 2007; Kauffman, 2006; Polito and Proud, 1998; Poynter et al., 2003). Several studies have shown that cytokine responses are finely regulated processes, and unique cytokine responses could be induced via specific signaling pathways triggered by a stimulus. Thus, it is crucial to understand signaling pathways triggered by individual exogenous products associated with lung diseases.
Interleukin 8 (IL-8) is a chemoattractant cytokine responsible for the recruitment of neutrophils and macrophages into the sites of inflammation. In humans, the airway epithelium is a major source of IL-8, and several lung pathologies are associated with enhanced IL-8 levels (Martin et al., 1997). For example, elevated IL-8 levels are present in the lung of chronic obstructive pulmonary disease (COPD) and cystic fibrosis patients (Chung, 2005; Larsson, 2008). The deleterious effects of localized overproduction of IL-8 in the lung result from tissue destruction by neutrophils secreting a myriad of molecules (i.e., neutrophil elastase) (Cornwell et al., 2010; Shapiro, 2002; Tetley, 2005). IL-8 can be secreted by several cell types in response to a wide variety of stimuli. Production of this cytokine by human airway epithelial cells was shown to be under the control of multiple signaling cascades, including nuclear factor-κB (NF-κB), the Mitogen-activated protein kinase (MAPK) pathways ERK, JNK, and p38 (Akhtar et al., 2003; Cheon et al., 2008; Henriquet et al., 2007; Kim et al., 2006).
Cadmium is a common air pollutant released into the environment by the burning of fossil fuels (i.e., diesel, coal) and incineration of municipal waste (Muntau and Baudo, 1992; Pan et al., 2010; Suriyawong et al., 2009; Tang et al., 2009). Cadmium also is present in fine particulate matter that has a diameter of less than 2.5 μm (PM2.5) and is inhaled due to their small size (Yoo et al., 2002). When present in draining water and/or soil, this heavy metal accumulates in tobacco leaves, and cigarette smoke can contain high amounts of cadmium (up to 2–4 μg/cigarette). Cadmium is currently ranked 7th on the priority list of hazardous substances by the Agency for Toxic Substances and Disease Registry and is widely ingested because certain foods, such as plants and seafood, contain high amounts. As a result, most previous studies focused on ingested cadmium. However, although only 5–10% of ingested cadmium is absorbed systemically, pulmonary absorption of inhaled cadmium can reach 80% (Hadley et al., 1980; Jarup et al., 1998). Cadmium inhalation has been linked to lung cancer and COPD (Kirschvink et al., 2005; Nawrot et al., 2006). The concentration of cadmium in the lung of cigarette smokers has been estimated to be in the micromolar range (≈30μM), with some region most likely exposed to higher concentrations (Chambers et al., 1998; Paakko et al., 1988, 1989). Furthermore, cadmium has recently been shown to induce lung inflammation (Kundu et al., 2009), but the underlying mechanism remains poorly understood.
Because IL-8 is a key mediator in neutrophil-mediated acute inflammation, we investigated the effect of cadmium on production of this proinflammatory cytokine in vitro in human airway epithelial cells and in mouse lungs in vivo. We also investigated the mechanism underlying cadmium-induced secretion of IL-8 focusing on airway epithelial cells.
MATERIALS AND METHODS
Materials.
Cadmium sulfate (Sigma, St Louis, MO) was dissolved in sterile water at a concentration of 100mM and was diluted in media (for in vitro studies) or PBS (for in vivo studies) to reach the indicated concentration. Lipopolysaccharide (LPS) from Pseudomonas aeruginosa was from Sigma. MAPK inhibitors were from Calbiochem (La Jolla, CA), and the NF-κB inhibitor Bay 11-7082 was from Santa Cruz Biotechnology (Santa Cruz, CA).
Tissue culture and reagents.
The human bronchial epithelial cell line Calu-3 originates from a pleural effusion in a patient with adenocarcinoma of the lung and was generated by Dr Fogh. Calu-3 cells were cultured in Dulbecco's Modified Eagle's Medium (DMEM)/F12 supplemented with 10% (vol/vol) bovine growth serum, penicillin (100 U/ml), and streptomycin (100 μg/ml). Experiments were performed using Calu-3 cells between passages 25 and 50. 16HBE14o- cells, an immortalized human bronchial epithelial cell line, were grown in DMEM containing L-glutamine, 10% fetal growth serum, and penicillin (100 U/ml) and streptomycin (100 μg/ml). Primary human small airway epithelial cells (SAEC; Clonetics, San Diego, CA) isolated from the small airway of a normal human donor were cultured in small airway growth medium (Clonetics) with SingleQuot supplements according to the manufacturer's instructions. Cells from the same donor were used in these studies and were up to three passages. All cell cultures were grown and maintained at 37°C in a 5% CO2 humidified incubator.
ELISA for analysis of cytokines released in cell supernatants.
Confluent human airway epithelial cells Calu-3 and SAEC were growth arrested for 12–24 h in serum-free media. Cells then were cultured in fresh serum-free media with cadmium or medium alone. In some experiments, cells were pretreated for 30 min with the indicated inhibitors before stimulation with cadmium at the indicated concentration. Unless specified otherwise, the experiments were carried out using MAPK inhibitors at a concentration of 20μM. Supernatants were then collected at 24 h and stored at −20°C until analysis was carried out by ELISA. CXC chemokine ligand (CXCL)-8/IL-8, macrophage inflammatory protein (MIP)-2, keracinocyte-dervied chemokine (KC), IL-6, tumor necrosis factor (TNF)-α, and IL-1β were quantified by ELISA following the manufacturer’s protocol (R&D Systems, Minneapolis, MN). The data were expressed as pg/ml. In some experiments where inhibitors were used, the data are expressed as % of control, with vehicle (dimethyl sulfoxide). Each data point represents mean from a minimum of three independent assays performed in triplicate.
Quantitative reverse transcriptase-PCR analysis.
Real-time quantitative reverse transcriptase (RT)-PCR was employed to measure the transcript levels of the IL-8, IL-6, MIP-2, and KC genes. First-strand complementary DNAs (cDNAs) were synthesized using SuperScript reverse transcriptase (Invitrogen, Carlsbad, CA) from 2 to 4 μg of total RNA in a 20 μl reaction volume. Real-time quantitative PCR was performed with SYBR Green I PCR Master Mix in the ABI PRISM 7700 Sequence Detection System (Applied Biosystems, Carlsbad, CA). PCR amplification reactions were initially incubated at 94°C for 5 min followed by 40 cycles at 94°C for 30 s, 62°C for 1 min, and 72°C for 1 min. RT-PCR for amplifying transcripts of the IL-8, MIP-2, KC, and IL-6 genes were performed at least three times to confirm the accuracy of the results. The total synthesized cDNAs also were used to amplify the cAMP-accessory protein (CAP-1) or β-actin genes as internal standards using CAP-1 or β-actin gene-specific primers. The IL-8 and IL-6 messenger RNA (mRNA) levels were normalized to the expression of the housekeeping gene CAP-1, and MIP-2 and KC mRNA levels were normalized to the expression of the housekeeping gene β-actin. mRNA levels were expressed as relative copy number (RCN), which was calculated using with the following equation: RCN = 2−ΔCt × 100, where ΔCt = Ct(target) − Ct(housekeeping gene) (Rennolds et al., 2010).
Transfection, cell stimulation, and immunoblotting.
Calu-3 cells were transfected with Erk1 and Erk2 small interfering RNAs (siRNA) (Invitrogen) as previously described (Rennolds et al., 2008). Confluent Calu-3 and SAEC cells were placed in serum-free media overnight. Cells then were stimulated with cadmium sulfate at the concentration and time indicated. Cells were lysed in TN1 lysis buffer (50mM Tris pH 8.0, 10mM EDTA, 10mM Na4P2O7, 10mM NaF, 1% Triton-X 100, 125mM NaCl, 3mMNa3VO4, and a cocktail of protease inhibitors [Roche Applied Science, Indianapolis, IN]). The protein concentration was determined by bicinchoninic acid protein assay (Pierce, Rockford, IL). The same amount of protein present in whole-cell lysates was separated by SDS-polyacrylamide gel electrophoresis, transferred to 3 polyvinylidene fluoride membranes (BioRad, Hercules, CA), and probed with phospho Erk1/2 or phospho p38–specific antibodies (Cell Signaling Technology, Beverly, MA). Secondary antibodies (HRP-conjugated, Pierce) were diluted 1:10,000. Chemiluminescent signal was detected using West Pico (Pierce) following the manufacturer’s instructions. Membranes were reprobed with Erk1/2 or β-actin–specific antibodies to confirm that the same amount of proteins was loaded in each lane. Each Western blot is representative of at least three independent experiments.
Animals and lung tissue processing.
C57BL/6 female mice (8–10 weeks old) were used in accordance with the institutional animal welfare guidelines of the Ohio State University. Anesthetized mice received 10 nmol cadmium (in 50 μl PBS corresponding to 1-μg cadmium sulfate) or vehicle (control mice) by intranasal instillation. Twenty-four hours later, mice were sacrificed, and lung tissues were snap frozen in liquid nitrogen for protein processing and stored at −80°C until assayed. Frozen specimens (−80°C) were transferred into liquid nitrogen and shattered in a BioPulverizer (Biospec Products, Bartlesville, OK). Tissue fragments were suspended in TN1 lysis buffer to extract the proteins or Trizol to extract total RNA. In another set of experiments, mice were sacrificed 3 days after treatment, and the lungs were inflated for histology. Lung paraffin-embedded sections were subjected to hematoxylin and eosin stain or labeled with Ly-6 (GR-1), a marker for neutrophils.
Bronchoalveolar lavages.
Bronchoalveolar lavage fluids (BALF) were obtained as previously described via cannulation of the exposed trachea by infusing 0.6 ml of PBS through a 22-gauge catheter into the lungs, followed by aspiration of this fluid into a syringe (Fischer et al., 2005). A volume of 0.4 ml of fluid was consistently recovered. Cells were isolated by centrifugation, and supernatants collected were stored at −70°C until analyzed for cytokine levels. Differential cell counts were determined on cytospin-prepared slides that were stained with Wright-Giemsa stain. The percentage of neutrophils, macrophages, lymphocytes, and eosinophils in the BALF sample was calculated. The total number of neutrophils was also expressed as means ± SE for each group of animals.
Statistical analysis.
Data are expressed as mean ± SE of at least three independent experiments. Statistically significant differences were assessed using Student’s t-test, Mann-Whitney test, or ANOVA followed by Tukey’s post hoc test using JMP software. The p values < 0.05 were considered significant.
RESULTS
Cadmium Induces IL-6 and IL-8 Secretion by Primary Human Airway Epithelial Cells
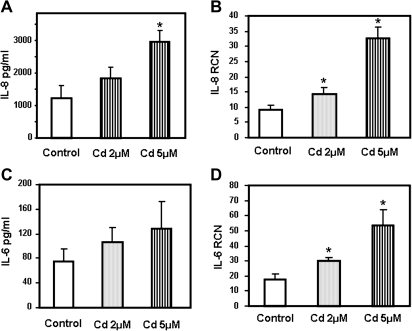
Cadmium inhalation has been associated with lung diseases where inflammation plays a key role. Therefore, we investigated whether cadmium could induce secretion of proinflammatory cytokines by human airway epithelial cells. We selected to focus on IL-8 because it is a chemotactic cytokine that plays a pivotal role in the human lung by recruiting neutrophils and macrophages to the site of inflammation. In order to investigate the effect of cadmium on cytokine secretion, primary human SAEC were incubated with 2 and 5μM cadmium. We found that cadmium-induced secretion of the proinflammatory cytokine IL-8 by SAEC in a dose-dependent manner (Fig. 1A). In fact, SAEC treated with 5μM cadmium for 4 h exhibited a threefold increase in IL-8 mRNA levels (Fig. 1B) with subsequent twofold increase in protein secretion (Fig. 1). Interestingly, cadmium differentially affected secretion of other proinflammatory cytokines. Thus, cadmium treatment induced secretion of IL-6 (Figs. 1C and 1D), but neither TNF-α nor IL-1β could be detected in the supernatants of primary human SAEC.
FIG. 1.
Cadmium induces secretion of the proinflammatory cytokines IL-8 and IL-6 by primary human SAEC. SAEC were treated for 24 h with 2 and 5μM cadmium (Cd). IL-8 (A) and IL-6 (C) present in the medium was measured by ELISA and expressed as pg/ml. SAEC were treated for 4 h with 2 and 5μM cadmium (Cd). IL-8 (B) and IL-6 (D) mRNA transcript levels were measured by real-time quantitative RT-PCR and expressed as RCN that were normalized to the housekeeping gene CAP-1. Data are expressed as mean ± SE of at least three independent experiments. *p < 0.05.
Stimulation of IL-8 Secretion by Cadmium Is a Dose- and Time-Dependent Process
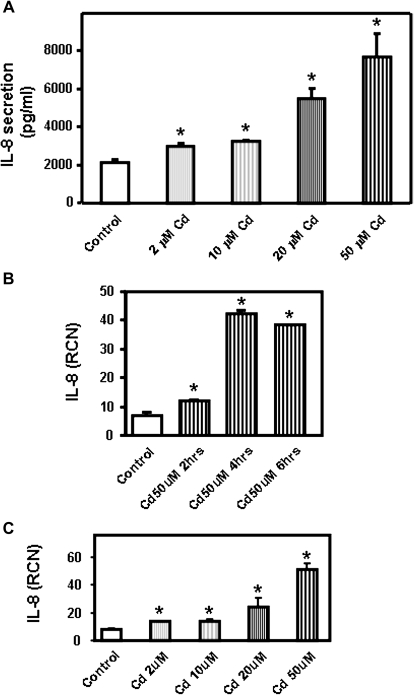
In order to elucidate the mechanism involved in secretion of IL-8, we used the human airway epithelial cell line Calu-3. Calu-3 cells differentiate into monolayers of polarized cells and express features of the airway epithelium. This cell line is a good model to study human respiratory function, structure, and inflammatory responses (Zhu et al., 2010). Because individuals could be exposed to various levels of pollutants, we investigated how varying concentrations of cadmium affect secretion of IL-8 by human airway epithelial cells. Calu-3 cells, a human subbronchial gland adenocarcinoma cell line, are resistant to xenobiotics (Hamilton et al., 2001); we therefore used concentrations of cadmium higher than for the primary human airway epithelial cells. In these studies, Calu-3 cells were incubated for 24 h with 2–50μM cadmium. It has been shown that airway epithelial cells have a basal level of IL-8 secretion. Accordingly, IL-8 was detected in Calu-3 cell supernatants in the absence of stimulation (Fig. 2A). We found that cadmium induces secretion of IL-8 in a dose-dependent manner and that IL-8 could be detected in Calu-3 cell supernatants even after incubation with the lowest cadmium dose (2μM) (Fig. 2A). We also found that the release of IL-8 was associated with an increase of IL-8 mRNA responses as soon as after 4 h incubation (Fig. 2B). At that time point, we observed a dose-dependent induction of IL-8 mRNA by cadmium (Fig. 2C).
FIG. 2.
Concentration- and time-dependent production of IL-8 by Calu-3 cells in response to cadmium. (A) Cadmium (Cd) at concentrations ranging from 2 to 50μM was added to the human bronchial epithelial cells Calu-3 for 24 h. IL-8 present in the medium was measured by ELISA and expressed as pg/ml. Calu-3 cells were treated for 2–6 h with 50μM cadmium (B) or for 4 h with 2–50μM cadmium (Ni) (C). IL-8 mRNA transcript levels were measured by real-time quantitative RT-PCR and expressed as RCN that were normalized to the housekeeping gene CAP-1. Data are expressed as mean ± SE of at least three independent experiments. *p < 0.05.
Cadmium, But Not Nickel, Induces Secretion of IL-8 and IL-6 by Human Airway Epithelial Cells Calu-3
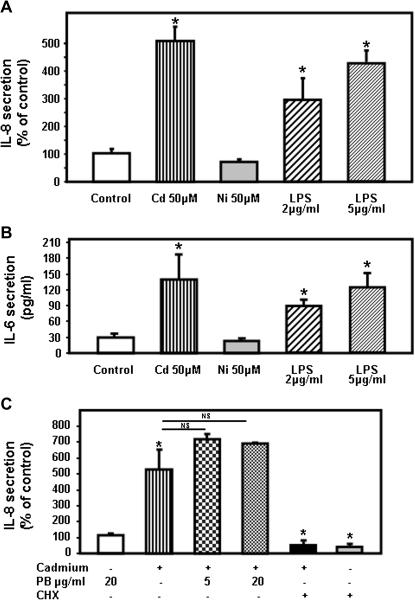
In our initial studies, the human bronchial epithelial cell line Calu-3 was incubated without or with 50μM cadmium, a dose previously reported not to affect Calu-3 cells survival (Rennolds et al., 2010). Cadmium treatment significantly increased IL-8 secretion and resulted in IL-8 responses of similar magnitude to treatment of Calu-3 cells with 5 μg/ml LPS, a dose commonly used to stimulate airway epithelial cells in vitro (MacRedmond et al., 2005) (Fig. 3A). We also confirmed that cadmium differentially affected the secretion of other proinflammatory cytokines. Thus, cadmium treatment induced secretion of IL-6 (Fig. 3B), but neither TNF-α nor IL-1β could be detected in supernatants of cells incubated with cadmium (below the detection level < 15.6 and < 3.9 pg/ml for TNF-α and IL-1β, respectively).
FIG. 3.
Cadmium induces secretion of the proinflammatory cytokines CXCL8/IL-8 by the human airway epithelial cells Calu-3. Calu-3 cells were treated for 24 h with 50μM cadmium sulfate (Cd) or nickel chloride (Ni). LPS was added at a concentration of 2 or 5 μg/ml. (A) The amount of IL-8 (pg/ml) present in the medium was measured by ELISA and expressed as % of controls. (B) IL-6 present in the medium was measured by ELISA and expressed as pg/ml. (C) Calu-3 cells were treated with cadmium that was preincubated with 5 or 20 μg/ml polymyxin B (PB) or 1 μg/ml cycloheximide (CHX). IL-8 present in the medium was measured by ELISA. Data are expressed as mean ± SE of at least three independent experiments. *p < 0.05.
It was important to establish whether the signature inflammatory responses induced by cadmium on airway epithelial cells were a general feature of inhaled heavy metals. For this purpose, we treated Calu-3 cells with nickel (Ni) because inhalation of this heavy metal has been associated with acute lung injury (Leikauf et al., 2002). In contrast to cadmium, nickel had no effect on IL-8 or IL-6 by Calu-3 cells even at a concentration of 50 or even 150μM (Figs. 3A and 3B, data not shown). It has to be noted that 150μM nickel did not affect cell viability based on lactate dehydrogenase release (16 ± 2 and 18 ± 2% for control and 150μM nickel, respectively). In order to rule out the possibility that endotoxin present in the cadmium preparation was responsible for the production of cytokines, the media containing cadmium were incubated for 1 h with 5 and 20 μg/ml polymyxin B (PB) prior to addition to the cells. As observed in Figure 3C, pretreatment with PB had no effect on cadmium-induced IL-8 secretion. We also confirmed that IL-8 released into the media required new protein synthesis by pretreating Calu-3 cells with the translation inhibitor, cycloheximide. Such treatment prevented both the basal- and cadmium-stimulated release of IL-8. These results suggest that cadmium, and not endotoxin, is responsible for the secretion of IL-8 by human airway epithelial cells.
The Cadmium-Induced Secretion of IL-8 Is Independent of NF-κB
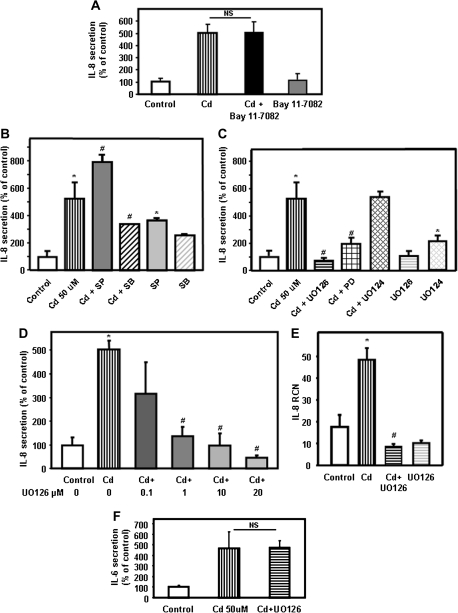
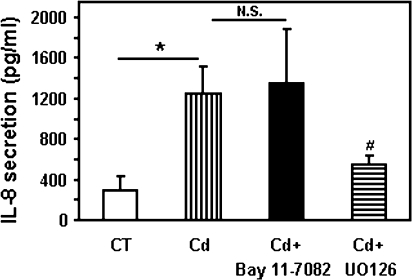
Production of IL-8 is a finely regulated process under the control of multiple signaling pathways. Because transcriptional activation of NF-κB is one of the key factors that regulate IL-8 gene expression (Hoffmann et al., 2002), we employed chemical inhibition of NF-κB to test the role of this pathway in cadmium-induced secretion of IL-8. Bay 11-7082, an irreversible inhibitor of IκB kinase, which blocks IκB-α phosphorylation and degradation, decreased the cadmium-induced secretion of IL-6 by Calu-3 cells by approximately 80% (78 ± 4%). Unexpectedly, addition of Bay 11-7082 failed to affect cadmium-induced secretion of IL-8 by Calu-3 cells (Fig. 4A). These results suggest that cadmium induces secretion of IL-8 by airway epithelial cells via a pathway independent of NF-κB.
FIG. 4.
Involvement of NF-κB and MAPK pathways on cadmium-induced secretion of IL-8. (A) Calu-3 cells were treated with 50μM cadmium for 24 h in the absence or presence of the NF-κB inhibitor Bay 11-0082 (20μM). The amount of IL-8 (pg/ml) present in the medium was measured by ELISA. To facilitate comparison between different inhibitors, IL-8 levels were expressed as % of controls with vehicle (dimethyl sulfoxide). The values for the control were 2587 ± 196 pg/ml. (B) Calu-3 cells were incubated for 24 h with 50μM cadmium in the presence of 20μM SB203580 (p38 inhibitor) or SP600125 (SP, JNK inhibitor). IL-8 present in the medium was measured by ELISA. (C) Calu-3 cells were incubated for 24 h with 50μM cadmium in the presence of 20μM UO126 (UO, MEK1/2 inhibitor), UO124 (inactive homolog of UO126), or PD98059 (PD, Erk1/2 inhibitor). IL-8 present in the medium was measured by ELISA. (D) Calu-3 cells were incubated with 50μM cadmium in the presence of 0.1–20μM UO126 (UO, MEK1/2 inhibitor). IL-8 present in the medium was measured by ELISA. (E) Calu-3 cells were incubated for 4 h with 50μM cadmium in presence or absence of 20μM UO126 (UO, MEK1/2 inhibitor) or with UO126 (UO) alone. IL-8 mRNA transcripts levels were measured by real-time quantitative RT-PCR and expressed as RCN that were normalized to the housekeeping gene CAP-1. (F) Calu-3 cells were incubated with 50μM cadmium in presence or absence of 20μM UO126 (UO, MEK1/2 inhibitor). IL-6 present in the medium was measured by ELISA and expressed as % of control. Data are expressed as mean ± SE of at least three independent experiments. *p < 0.05 compared with control cells; #p < 0.05 compared with cadmium-treated cells.
Inhibition of the MEK1/2-Erk1/2 MAPK Pathway Suppresses IL-8 Secretion
Other pathways previously reported to control IL-8 production by human airway epithelial cells include the classical MAPK pathways Erk1/2, JNK, and p38 (Akhtar et al., 2003; Cheon et al., 2008; Henriquet et al., 2007; Kim et al., 2006). In addition, MAPKs have previously been described as regulators of the AP-1 transcription factor and linked to IL-8 gene expression (Cheon et al., 2008; Kim et al., 2006). Chemical inhibition of the JNK MAPK pathway using SP600125 did not reduce the cadmium-induced secretion of IL-8 by airway epithelial cells (Fig. 4B). On the other hand, inhibitors of the p38 MAPK pathway and MEK/Erk1/2 inhibitors altered cadmium-induced IL-8 expression by airway epithelial cells. More specifically, treatment of airway epithelial cells with SB203580, a chemical inhibitor of the p38 MAPK pathway, inhibited IL-8 secretion by approximately 50% (Fig. 4B).
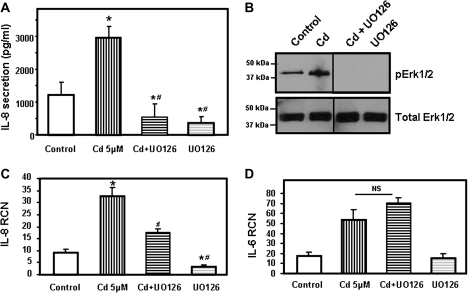
The MEK/Erk1/2 inhibitors UO126 and PD98059 suppressed the cadmium-induced IL-8 production. It is also important to indicate that the inactive homolog of UO126, UO124, had no effect on cadmium-induced secretion of IL-8 (Fig. 4C). These results further demonstrate the role of the Erk1/2 pathway in IL-8 production by airway epithelial cells exposed to cadmium.
Because our data indicate that suppression of the MEK/Erk1/2 pathway completely blocked the cadmium-induced secretion of IL-8, we addressed the influence of UO126 dose on suppression of IL-8 secretion by Calu-3 cells. For this purpose, cells were incubated with 0.1, 1, 10, and 20μM UO126 in the presence of 50μM cadmium for 24 h. The secretion of IL-8 by cadmium was decreased in a dose-dependent manner by the specific MEK inhibitor UO126 (Fig. 4D). At a dose of 0.1μM, UO126 had no effect on cadmium-induced secretion of IL-8. On the other hand, at doses > 10μM, the MEK inhibitor UO126 reduced secretion of IL-8 to a concentration below the basal level of secretion. In control experiments where Calu-3 cells were treated with UO126 alone, only UO126 concentrations above 20μM decreased the basal secretion of IL-8 (data not shown).
We further investigated the involvement of MEK/Erk1/2 pathway on cadmium-induced secretion of IL-8 by analyzing the expression of IL-8 mRNA transcripts in cells treated with the MEK/Erk1/2 inhibitor UO126. Chemical inhibition of MEK/Erk1/2 pathway significantly decreased IL-8 mRNA transcript levels (Fig. 4E).
As indicated above, cadmium also stimulated secretion of IL-6 but failed to induce IL-1β and TNF-α. Interestingly, the MEK/Erk1/2 inhibitor UO126 did not exhibit an inhibitory effect on IL-6 secretion by Calu-3 cells (Fig. 4F). Taken together, these results suggest that the MEK/Erk1/2 pathway plays a key role upstream of IL-8, but not IL-6 secretion, and upregulates IL-8 mRNA transcript levels.
We further confirmed that MEK/Erk1/2 pathway was involved in production of IL-8 in bronchial epithelial cells by using 16HBE14o- cells. As observed with Calu-3 cells, chemical inhibition of the NF-κB pathway had no effect on secretion of IL-8 upon induction with cadmium. On the other hand, inhibition of the MEK/Erk1/2 MAPK pathway using the specific inhibitor UO126 abolished the cadmium-induced secretion of IL-8 (Fig. 5). These data confirm that the MEK/Erk1/2 pathway plays a key role in regulating secretion of the proinflammatory chemokine IL-8 in human airway epithelial cells.
FIG. 5.
Inhibition of MEK/Erk1/2 pathway blocks cadmium-induced secretion of IL-8 in 16HBE14o-. The human bronchial epithelial cells 16HBE14o- were treated with 20μM cadmium for 24 h in the absence or presence of the NF-κB inhibitor, Bay 11-0082, or the MEK/Erk1/2 inhibitor, UO126. IL-8 present in the medium was measured by ELISA and expressed as pg/ml. Data are expressed as mean ± SE of at least three independent experiments. N.S., not significant; *p < 0.05 compared with control cells; #p < 0.05 compared with cadmium-treated cells.
Cadmium Induces Activation of MAPK Pathways
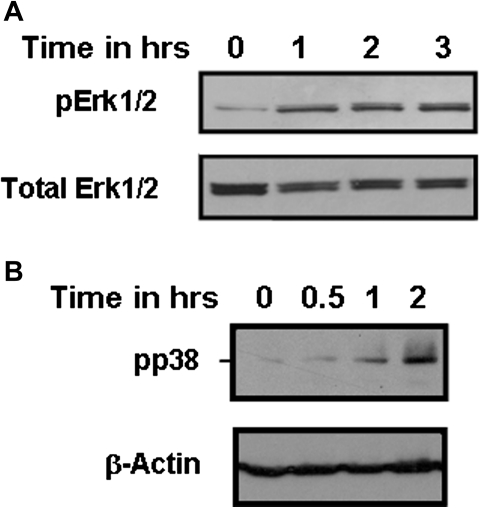
Cadmium was previously reported to activate the MAPK pathways (Kalariya et al., 2010; Misra et al., 2002; Rockwell et al., 2004). In order to confirm the role of the MEK/Erk1/2 pathway in IL-8 secretion, we first examined whether cadmium could induce phosphorylation of Erk1/2 in human airway epithelial cells Calu-3. Here, we found that cadmium could activate Erk1/2 in a time-dependent manner with a maximum phosphorylation after 3–4 h, as observed by its phosphorylation (Fig. 6A). Cadmium treatment also caused phosphorylation of p38 and c-Jun in a time- and dose-dependent manner in Calu-3 cells (Fig. 6B, data not shown).
FIG. 6.
Cadmium induces activation of the MAPKs Erk1/2 and p38 in the human bronchial epithelial cell line Calu-3. Calu-3 cells were incubated with 50μM cadmium. Phospho p38 and phospho Erk1/2 were detected by Western blot. The same amount of proteins was leaded in each lane, and the same amount of total Erk1/2 or β-actin was detected. Data are representative of at least three independent experiments.
Inhibition of the Erk1/2 Pathway Suppresses the Cadmium-Induced Secretion of IL-8 in Primary Human SAEC
In order to confirm that the MEK/Erk1/2 pathway was involved in production of IL-8 in primary human airway epithelial cells, primary SAEC were incubated with UO126. As observed with Calu-3 cells, chemical inhibition of MEK/Erk1/2 MAPK pathway abolished the basal- and cadmium-induced secretion of IL-8 (Fig. 7A). Figure 7B shows that addition of cadmium to SAEC for 2 h resulted in phosphorylation of Erk1/2 that was suppressed by the specific MEK inhibitor UO126. Inhibition of the MEK/Erk1/2 pathway significantly decreased IL-8 mRNA levels but had no effect on IL-6 mRNA transcript levels (Figs. 7C and 7D). These data confirm that the MEK/Erk1/2 pathway plays a key role in regulating secretion of the proinflammatory chemokine IL-8 in primary human airway epithelial cells.
FIG. 7.
Inhibition of the Erk1/2 pathway suppresses secretion of IL-8 by cadmium in primary human SAEC. (A) Primary human SAEC were treated with 5μM cadmium for 24 h in the presence of 10μM UO126 (MEK1/2 inhibitor). IL-8 present in the medium was measured by ELISA and expressed as pg/ml. (B) SAEC were incubated with 5μM cadmium for 2 h. Phospho Erk1/2 (pErk1/2) was detected by Western blot. The same amount of proteins was leaded in each lane, and the same amount of total Erk1/2 detected. Data are representative of at least three independent experiments. (C and D) SAEC were incubated for 4 h with 5μM cadmium in the presence or absence of 10μM UO126 (MEK1/2 inhibitor) or with UO126 alone. IL-6 (C) and IL-8 (D) mRNA transcripts levels were measured by real-time quantitative RT-PCR and expressed as RCN that were normalized to the housekeeping gene CAP-1. Data are expressed as mean ± SE of at least three independent experiments. *p < 0.05 compared with control cells; #p < 0.05 compared with cadmium-treated cells.
Effect of Cadmium on Lung Inflammation In Vivo in Mice
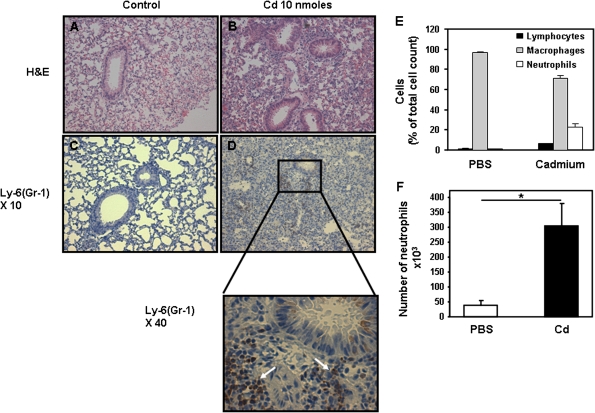
Based on data summarized above, exposure of airway epithelial cells to cadmium induces a signature inflammatory response characterized as an NF-κB–independent IL-8 response. This chemoattractant cytokine is one of the main players in inflammation in vivo due to its ability to recruit and activate neutrophils and macrophages (Pease and Sabroe, 2002). In order to establish the role of cadmium in lung inflammation in vivo, groups of mice were given either PBS or one dose of 10 nmol cadmium (or 1-μg cadmium sulfate) in PBS intranasally. Mice were sacrificed 3 days later, and lung inflammation was examined by histochemistry. As shown in Figures 8A and 8B, mice treated with cadmium exhibited marked lung inflammation with a high number of cell infiltrates. Inflammatory cells were observed in the perivascular, peribronchial, and parenchymal tissues. Staining with specific antibodies identified neutrophils and macrophages among the cells present in the lungs of cadmium-treated mice (Figs. 8C and 8D, data not shown). Airway cell hyperplasia was observed in mice that received cadmium but not in control mice that received PBS (Figs. 8A and 8B). Differential analysis of cell population in BAL revealed a major change in cell populations in the lung of mice that received cadmium (Fig. 8E). Indeed, a fivefold increase in the number of neutrophils was observed in cadmium-treated mice when compared with PBS (Fig. 8F). Conversely, eosinophils were not detected in the lung of mice treated with PBS (control) or cadmium.
FIG. 8.
In vivo delivery of cadmium leads to the influx of inflammatory cells into the lungs of mice. Groups of mice received either PBS (A and C) or 10 nmol cadmium (1-μg cadmium sulfate) (B and D) intranasally. Three days later, the mice were sacrificed and their lungs were subjected to hematoxylin and eosin (A and B) or labeled with Ly-6(GR-1) (C and D) as described in “Materials and Methods” section. Data are representative of at least three mice per group. (E) Percentage of cells present in BAL of mice 3 days after receiving PBS or cadmium (10 nmol). (F) Number of neutrophils present in BAL. Data are expressed as mean ± SE of at least three mice per group.
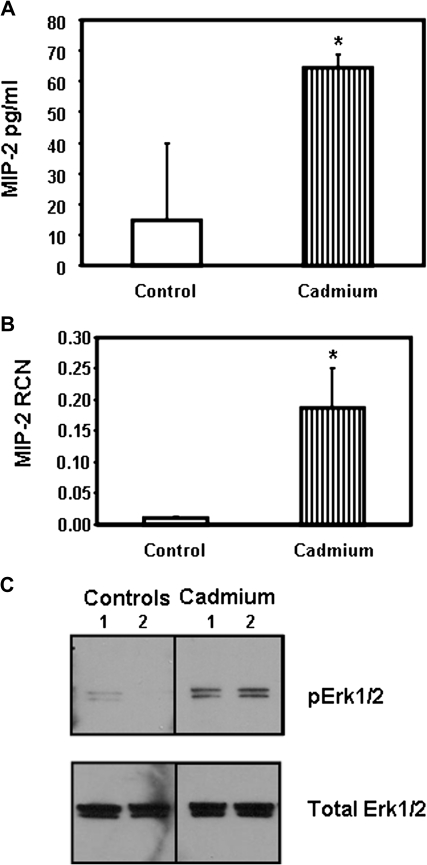
To clearly establish the role of IL-8 in lung inflammation observed in cadmium-treated mice, other groups of mice were treated with one dose of 10 nmol cadmium or PBS (control) and sacrificed 24 h later. The presence of the murine IL-8 homologs MIP-2 and KC was measured in the BAL and lung tissues. Both MIP-2 and KC were significantly increased at the mRNA and protein levels in the group of mice that received cadmium (Figs. 9A and 9B, data not shown). Finally, we investigated the ability of cadmium to activate Erk1/2 in vivo. As observed in Figure 9C, intranasal delivery of cadmium resulted in phosphorylation of Erk1/2 in the lungs of the mice. Conversely, no or very weak signal for phospho Erk1/2 could be detected in the lungs of mice that received PBS (vehicle) (Fig. 9C).
FIG. 9.
In vivo delivery of cadmium leads to activation of Erk1/2 and secretion of the IL-8 homolog MIP-2. Groups of mice received either PBS or 10 nmol cadmium intranasally. Twenty-four hours later, the mice were sacrificed. MIP-2 present in BAL (A) and MIP-2 mRNA levels present in lung samples (B) were measured by ELISA and real-time quantitative RT-PCR, respectively. Data are expressed as mean ± SE of at least three mice per group. (C) The presence of pErk1/2 in the lung samples was detected by immunoblotting that were processed as described in “Materials and Methods” section. The same amount of proteins was loaded in each lane.
Silencing of Erk1/2 Prevents Secretion of IL-8 by Human Airway Epithelial Cells
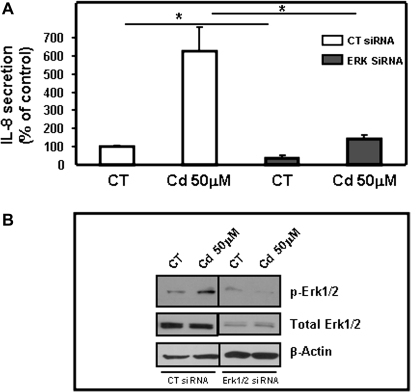
Finally, to directly confirm the role of the Erk1/2 MAPK pathway in secretion of IL-8 by human airway epithelial cells in response to cadmium, Erk1 and Erk2 were simultaneously silenced using siRNAs. Basal- and cadmium-induced secretions of IL-8 were significantly decreased upon silencing of both Erk1 and Erk2 using siRNAs (Fig. 10A). The silencing of Erk1 and Erk2 by siRNAs was confirmed by immunoblotting. Accordingly, Figure 10B shows reduced detection of phospho Erk1/2 and Erk1 and Erk2 in presence of Erk1/2 siRNAs when compared with control. Taken together, the results confirm that phosphorylation of Erk1/2 plays a key role in secretion of the proinflammatory chemokine IL-8 by airway epithelial cells in response to cadmium.
FIG. 10.
Silencing of Erk1 and 2 prevents cadmium-induced secretion of IL-8. Calu-3 cells were transiently transfected with control siRNA (CT) or a mix of siRNAs targeting Erk1 and Erk2. (A) The effect of transfection of Erk1/2 siRNAs was evaluated by measuring IL-8 secretion in response to cadmium (50μM) by ELISA. Data are expressed as % of control of at least three independent experiments. *p < 0.05. (B) The effect of siRNA transfection on pErk1/2 and total Erk1/2 expression was visualized by immunoblot in absence or presence of stimulation by cadmium (50μM). Data are representative of at least three independent experiments.
DISCUSSION
Epithelial cells play a central role in lung physiology and represent the first line of cells encountered by exogenous molecules in the lung. In addition to their role as a physical barrier, airway epithelial cells play a key role in modulating inflammatory responses that orchestrate immune responses against microbes and pollutants. Cadmium is a heavy metal present in airway pollutants. Compelling evidence indicates that cadmium inhalation leads to several lung pathologies that share the characteristic of being governed by inflammation. However, little is known about the mechanism by which heavy metals such as cadmium induce lung inflammation. Data presented herein show that human airway epithelial cells exposed to subcytotoxic concentrations of cadmium exhibit a unique profile of inflammatory responses characterized by the NF-κB–independent secretion of IL-8. We further demonstrated that this inflammatory response signature involves Erk1/2-dependent mechanisms and is associated with the recruitment of inflammatory cells in vivo.
Airway epithelial cells are known to secrete cytokines and chemokines that orchestrate host immune responses against inhaled pathogens or pollutants. Important progress made over the last couple of decades has established that bacteria and viruses induce inflammatory responses via stimulation of unique signaling pathways (Bauer et al., 2009; Gómez and Prince, 2008; Hussell and Goulding, 2010; Zeytun et al., 2010). Thus, interaction of bacterial-associated or viral pathogen–associated molecular patterns with specific host pattern recognition receptors result in inflammatory responses that reflect the pathways induced (Kawai and Akira, 2010). The airways are increasingly exposed to heavy metals, including cadmium, present in particulate matter and other environmental pollutants (Richter et al., 2009). Cadmium inhalation has been linked to lung cancer and COPD (Kirschvink et al., 2005; Nawrot et al., 2006). On the other hand, inhalation of nickel has been associated with acute lung injury (Leikauf et al., 2002). It has been shown that cadmium induces secretion of IL-8 by hepatocytes and peripheral blood mononuclear cells (Dong et al., 1998; Horiguchi et al., 1993, 2000; Souza et al., 2004). More recently, cadmium was reported to promote IL-8 secretion by human intestinal epithelial cells, human type 2 epithelial cells, and alveolar macrophages (Hyun et al., 2007; Lag et al., 2010). The amount of IL-8 secreted by the Calu-3 cells in response to cadmium was similar to the one observed with a high doses of LPS (5 μg/ml). Our results are in agreement with previous studies reporting the relative insensitivity of bronchial epithelial cells to LPS (Monick et al., 2003; Parker and Prince, 2011). We found that cadmium, but not nickel, induces IL-8 secretion by airway epithelial cells. These findings further support the ability of cadmium to promote IL-8 responses but also suggest that individual heavy metal pollutants induce unique signature inflammatory responses by airway epithelial cells. Previous studies reported that nickel could induce IL-8 production by airway epithelial cells and neutrophils (Freitas et al., 2010; Salnikow et al., 2004). However, these studies employed very high doses of nickel (> 250μM) when compared with the dose used in our study (50 and 150μM). It is well established that transcriptional activation of NF-κB is one of the key factors that regulate IL-8 gene expression (Hoffmann et al., 2002). The fact that cadmium (1) induces a cytokine not induced by another heavy metal and (2) does so via an alternative pathway strongly suggests that heavy metal pollutants could trigger unique signature inflammatory responses similarly to bacterial or viral products.
Cell lines do not always reflect the behavior of primary cells, and it is always crucial to confirm in vivo the biological significance of observations made in vitro. We have shown here that the unique inflammatory signature of cadmium, which consists of IL-8 and IL-6, but not IL-1β or TNF-α, is conserved in human airway epithelial cell lines, in primary human airway epithelial cells, but also in mouse lung in vivo. As mentioned above, cadmium previously was shown to induce IL-8 by a number of cells, including the human intestinal epithelial cell line Caco-2 (Hyun et al., 2007). In contrast to our findings with airway epithelial cells, cadmium-induced secretion of IL-8 by Caco-2 cells was reported to be mediated by an NF-κB–dependent mechanism (Hyun et al., 2007). The notion that airway epithelial cells can produce IL-8 in an NF-κB–independent fashion has been suggested by others. For example, bacterial DNA was shown to promote production of IL-8 production by a MAPK-dependent NF-κB–independent pathway (Akhtar et al., 2003). More recently, Tal et al. (2010) found a link between the amount of organic material in diesel exhaust particles and the involvement of NF-κB in IL-8 response by airway epithelial cells. Tal et al. (2010) showed that low organic– and high organic–containing diesel exhaust particles induced IL-8 release by airway epithelial cells in NF-κB–dependent and NF-κB–independent manners, respectively. Taken together, our results suggest that specific pathways control the secretion of IL-8 that depends on the stimulus as well as the cell type.
Another key result of this work is the identification of Erk1/2 as the master regulator of cadmium-induced IL-8 secretion by airway epithelial cells. Importantly, this pathway was found to control cadmium-induced IL-8 responses in both airway epithelial cell lines, primary human airway epithelial cells and in vivo in mouse lung. On the other hand, inhibition of the p38 MAPK pathway had only a slight effect on IL-8 secretion (50% inhibition) and could be because this pathway is known to stabilize the IL-8 mRNA (Holtmann et al., 1999; Winzen et al., 1999). Cadmium is released in the environment where it is mostly associated with PM2.5 (particulate matter of a diameter < 2.5 μm) or fine particulate matter that is prone to be inhaled due to its small size (Thomaidis et al., 2003). This heavy metal, which also is present in cigarette smoke (2–4 μg per cigarette), recently was shown to induce lung inflammation (Kundu et al., 2009) and has been linked to lung cancer and COPD (Kirschvink et al., 2005; Nawrot et al., 2006). It is worth indicating that the 10 nmol dose of cadmium used in our in vivo study in mice corresponds to 1 μg of cadmium sulfate and is similar to the average amount of cadmium in one cigarette. The ability of IL-8 to recruit and activate macrophages and neutrophils makes this molecule a leading contributor to chronic inflammation and neutrophil-induced tissue destruction in pathologies induced by inhalation of cadmium. Our finding that chemical inhibition of MEK1/2 (the kinase that phosphorylates Erk1/2) or silencing of Erk1 and Erk2 with siRNAs could suppress cadmium-induced IL-8 secretion by airway epithelial cells identified these molecules as potential therapeutic targets for treatment of cadmium-induced lung diseases.
In summary, we have identified an NF-κB–independent pathway for induction of CXCL8/IL-8 responses in airway epithelial cells by cadmium. Our findings suggest that targeting the MEK/Erk1/2 pathway could prevent excessive release of this proinflammatory chemokine in response to pollutants, such as cadmium. Future studies will address the efficacy of this approach in vivo.
FUNDING
National Institutes of Health (RO3 HL095442 to E.C.B., RO1 AI43197 to P.N.B., and T32 HL07946 to F.H.); American Lung Association (GRT00019086 to E.C.B.), American Heart Association (09PRE2170054 to P.M.).
Acknowledgments
We thank Dr Kathleen Hayes-Ozello for editorial assistance.
References
- Akhtar M, Watson JL, Nazli A, McKay DM. Bacterial DNA evokes epithelial IL-8 production by a MAPK-dependent, NF-kappaB-independent pathway. FASEB J. 2003;17:1319–1321. doi: 10.1096/fj.03-0950fje. [DOI] [PubMed] [Google Scholar]
- Bartlett JA, Fischer AJ, McCray PB., Jr Innate immune functions of the airway epithelium. Contrib. Microbiol. 2008;15:147–163. doi: 10.1159/000136349. [DOI] [PubMed] [Google Scholar]
- Bauer S, Muller T, Hamm S. Pattern recognition by toll-like receptors. Adv. Exp. Med. Biol. 2009;653:15–34. doi: 10.1007/978-1-4419-0901-5_2. [DOI] [PubMed] [Google Scholar]
- Chambers RC, Laurent GJ, Westergren-Thorsson G. Cadmium inhibits proteoglycan and procollagen production by cultured human lung fibroblasts. Am. J. Respir. Cell Mol. Biol. 1998;19:498–506. doi: 10.1165/ajrcmb.19.3.3242. [DOI] [PubMed] [Google Scholar]
- Cheng DS, Han W, Chen SM, Sherrill TP, Chont M, Park GY, Sheller JR, Polosukhin VV, Christman JW, Yull FE, et al. Airway epithelium controls lung inflammation and injury through the NF-kappa B pathway. J. Immunol. 2007;178:6504–6513. doi: 10.4049/jimmunol.178.10.6504. [DOI] [PubMed] [Google Scholar]
- Cheon IS, Woo SS, Kang SS, Im J, Yun CH, Chung DK, Park DK, Han SH. Peptidoglycan-mediated IL-8 expression in human alveolar type II epithelial cells requires lipid raft formation and MAPK activation. Mol. Immunol. 2008;45:1665–1673. doi: 10.1016/j.molimm.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Chung KF. Inflammatory mediators in chronic obstructive pulmonary disease. Curr. Drug Targets Inflamm. Allergy. 2005;4:619–625. doi: 10.2174/156801005774912806. [DOI] [PubMed] [Google Scholar]
- Cornwell WD, Kim V, Song C, Rogers TJ. Pathogenesis of inflammation and repair in advanced COPD. Semin. Respir. Crit. Care Med. 2010;31:257–266. doi: 10.1055/s-0030-1254066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong W, Simeonova PP, Gallucci R, Matheson J, Flood L, Wang S, Hubbs A, Luster MI. Toxic metals stimulate inflammatory cytokines in hepatocytes through oxidative stress mechanisms. Toxicol. Appl. Pharmacol. 1998;151:359–366. doi: 10.1006/taap.1998.8481. [DOI] [PubMed] [Google Scholar]
- Fischer R, McGhee JR, Vu HL, Atkinson TP, Jackson RJ, Tome D, Boyaka PN. Oral and nasal sensitization promote distinct immune responses and lung reactivity in a mouse model of peanut allergy. Am. J. Pathol. 2005;167:1621–1630. doi: 10.1016/S0002-9440(10)61246-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas M, Gomes A, Porto G, Fernandes E. Nickel induces oxidative burst, NF-kappaB activation and interleukin-8 production in human neutrophils. J. Biol. Inorg. Chem. 2010;15:1275–1283. doi: 10.1007/s00775-010-0685-3. [DOI] [PubMed] [Google Scholar]
- Gershwin LJ. Effects of allergenic extracts on airway epithelium. Curr. Allergy Asthma Rep. 2007;7:357–362. doi: 10.1007/s11882-007-0054-7. [DOI] [PubMed] [Google Scholar]
- Gómez MI, Prince A. Airway epithelial cell signaling in response to bacterial pathogens. Pediatr. Pulmonol. 2008;43:11–19. doi: 10.1002/ppul.20735. [DOI] [PubMed] [Google Scholar]
- Hadley JG, Conklin AW, Sanders CL. Rapid solubilization and translocation of 109CdO following pulmonary deposition. Toxicol. Appl. Pharmacol. 1980;54:156–160. doi: 10.1016/0041-008x(80)90016-2. [DOI] [PubMed] [Google Scholar]
- Hamilton KO, Topp E, Makagiansar I, Siahaan T, Yazdanian M, Audus KL. Multidrug resistance-associated protein-1 functional activity in Calu-3 cells. J. Pharmacol. Exp. Ther. 2001;298:1199–1205. [PubMed] [Google Scholar]
- Henriquet C, Gougat C, Combes A, Lazennec G, Mathieu M. Differential regulation of RANTES and IL-8 expression in lung adenocarcinoma cells. Lung Cancer. 2007;56:167–174. doi: 10.1016/j.lungcan.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann E, Dittrich-Breiholz O, Holtmann H, Kracht M. Multiple control of interleukin-8 gene expression. J. Leukoc. Biol. 2002;72:847–855. [PubMed] [Google Scholar]
- Holtmann H, Winzen R, Holland P, Eickemeier S, Hoffmann E, Wallach D, Malinin NL, Cooper JA, Resch K, Kracht M. Induction of interleukin-8 synthesis integrates effects on transcription and mRNA degradation from at least three different cytokine- or stress-activated signal transduction pathways. Mol. Cell. Biol. 1999;19:6742–6753. doi: 10.1128/mcb.19.10.6742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiguchi H, Harada A, Oguma E, Sato M, Homma Y, Kayama F, Fukushima M, Matsushima K. Cadmium-induced acute hepatic injury is exacerbated in human interleukin-8 transgenic mice. Toxicol. Appl. Pharmacol. 2000;163:231–239. doi: 10.1006/taap.1999.8877. [DOI] [PubMed] [Google Scholar]
- Horiguchi H, Mukaida N, Okamoto S, Teranishi H, Kasuya M, Matsushima K. Cadmium induces interleukin-8 production in human peripheral blood mononuclear cells with the concomitant generation of superoxide radicals. Lymphokine Cytokine Res. 1993;12:421–428. [PubMed] [Google Scholar]
- Hussell T, Goulding J. Structured regulation of inflammation during respiratory viral infection. Lancet Infect. Dis. 2010;10:360–366. doi: 10.1016/S1473-3099(10)70067-0. [DOI] [PubMed] [Google Scholar]
- Hyun JS, Satsu H, Shimizu M. Cadmium induces interleukin-8 production via NF-kappaB activation in the human intestinal epithelial cell, Caco-2. Cytokine. 2007;37:26–34. doi: 10.1016/j.cyto.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Jarup L, Berglund M, Elinder CG, Nordberg G, Vahter M. Health effects of cadmium exposure—A review of the literature and a risk estimate. Scand. J. Work Environ. Health. 1998;24(Suppl. 1):1–51. [PubMed] [Google Scholar]
- Kalariya NM, Nair B, Kalariya DK, Wills NK, van Kuijk FJ. Cadmium-induced induction of cell death in human lens epithelial cells: Implications to smoking associated cataractogenesis. Toxicol. Lett. 2010;198:56–62. doi: 10.1016/j.toxlet.2010.04.021. [DOI] [PubMed] [Google Scholar]
- Kato A, Schleimer RP. Beyond inflammation: Airway epithelial cells are at the interface of innate and adaptive immunity. Curr. Opin. Immunol. 2007;19:711–720. doi: 10.1016/j.coi.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffman HF. Innate immune responses to environmental allergens. Clin. Rev. Allergy Immunol. 2006;30:129–140. doi: 10.1385/criai:30:2:129. [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: Update on toll-like receptors. Nat. Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- Kim YM, Reed W, Wu W, Bromberg PA, Graves LM, Samet JM. Zn2+-induced IL-8 expression involves AP-1, JNK, and ERK activities in human airway epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006;290:L1028–L1035. doi: 10.1152/ajplung.00479.2005. [DOI] [PubMed] [Google Scholar]
- Kirschvink N, Vincke G, Fievez L, Onclinx C, Wirth D, Belleflamme M, Louis R, Cataldo D, Peck MJ, Gustin P. Repeated cadmium nebulizations induce pulmonary MMP-2 and MMP-9 production and emphysema in rats. Toxicology. 2005;211:36–48. doi: 10.1016/j.tox.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Kundu S, Sengupta S, Chatterjee S, Mitra S, Bhattacharyya A. Cadmium induces lung inflammation independent of lung cell proliferation: A molecular approach. J. Inflamm. (Lond.) 2009;6:19. doi: 10.1186/1476-9255-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lag M, Rodionov D, Ovrevik J, Bakke O, Schwarze PE, Refsnes M. Cadmium-induced inflammatory responses in cells relevant for lung toxicity: Expression and release of cytokines in fibroblasts, epithelial cells and macrophages. Toxicol. Lett. 2010;193:252–260. doi: 10.1016/j.toxlet.2010.01.015. [DOI] [PubMed] [Google Scholar]
- Larsson K. Inflammatory markers in COPD. Clin. Respir. J. 2008;2(Suppl. 1):84–87. doi: 10.1111/j.1752-699X.2008.00089.x. [DOI] [PubMed] [Google Scholar]
- Leikauf GD, McDowell SA, Wesselkamper SC, Hardie WD, Leikauf JE, Korfhagen TR, Prows DR. Acute lung injury: Functional genomics and genetic susceptibility. Chest. 2002;121:70S–75S. doi: 10.1378/chest.121.3_suppl.70s. [DOI] [PubMed] [Google Scholar]
- MacRedmond R, Greene C, Taggart CC, McElvaney N, O'Neill S. Respiratory epithelial cells require toll-like receptor 4 for induction of human beta-defensin 2 by lipopolysaccharide. Respir. Res. 2005;6:116. doi: 10.1186/1465-9921-6-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LD, Rochelle LG, Fischer BM, Krunkosky TM, Adler KB. Airway epithelium as an effector of inflammation: Molecular regulation of secondary mediators. Eur. Respir. J. 1997;10:2139–2146. doi: 10.1183/09031936.97.10092139. [DOI] [PubMed] [Google Scholar]
- Mills PR, Davies RJ, Devalia JL. Airway epithelial cells, cytokines, and pollutants. Am. J. Respir. Crit. Care Med. 1999;160:S38–S43. doi: 10.1164/ajrccm.160.supplement_1.11. [DOI] [PubMed] [Google Scholar]
- Misra UK, Gawdi G, Akabani G, Pizzo SV. Cadmium-induced DNA synthesis and cell proliferation in macrophages: The role of intracellular calcium and signal transduction mechanisms. Cell. Signal. 2002;14:327–340. doi: 10.1016/s0898-6568(01)00268-6. [DOI] [PubMed] [Google Scholar]
- Monick MM, Yarovinsky TO, Powers LS, Butler NS, Carter AB, Gudmundsson G, Hunninghake GW. Respiratory syncytial virus up-regulates TLR4 and sensitizes airway epithelial cells to endotoxin. J. Biol. Chem. 2003;278:53035–53044. doi: 10.1074/jbc.M308093200. [DOI] [PubMed] [Google Scholar]
- Muntau H, Baudo R. Sources of Cadmium, Its Distribution and Turnover in the Freshwater Environment. IARC Scientific Publications No. 118, Lyon, France, pp. 1992:133–148. [PubMed] [Google Scholar]
- Nawrot T, Plusquin M, Hogervorst J, Roels HA, Celis H, Thijs L, Vangronsveld J, Van Hecke E, Staessen JA. Environmental exposure to cadmium and risk of cancer: A prospective population-based study. Lancet Oncol. 2006;7:119–126. doi: 10.1016/S1470-2045(06)70545-9. [DOI] [PubMed] [Google Scholar]
- Paakko P, Anttila S, Kokkonen P, Kalliomaki PL. Cadmium in lung tissue as marker for smoking. Lancet. 1988;1:477. doi: 10.1016/s0140-6736(88)91275-5. [DOI] [PubMed] [Google Scholar]
- Paakko P, Kokkonen P, Anttila S, Kalliomaki PL. Cadmium and chromium as markers of smoking in human lung tissue. Environ. Res. 1989;49:197–207. doi: 10.1016/s0013-9351(89)80065-9. [DOI] [PubMed] [Google Scholar]
- Pan J, Plant JA, Voulvoulis N, Oates CJ, Ihlenfeld C. Cadmium levels in Europe: Implications for human health. Environ. Geochem. Health. 2010;32:1–12. doi: 10.1007/s10653-009-9273-2. [DOI] [PubMed] [Google Scholar]
- Parker D, Prince A. Innate immunity in the respiratory epithelium. Am. J. Respir. Cell Mol. Biol. 2011;45:189–201. doi: 10.1165/rcmb.2011-0011RT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pease JE, Sabroe I. The role of interleukin-8 and its receptors in inflammatory lung disease: Implications for therapy. Am. J. Respir. Med. 2002;1:19–25. doi: 10.1007/BF03257159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polito AJ, Proud D. Epithelia cells as regulators of airway inflammation. J. Allergy Clin. Immunol. 1998;102:714–718. doi: 10.1016/s0091-6749(98)70008-9. [DOI] [PubMed] [Google Scholar]
- Poynter ME, Irvin CG, Janssen-Heininger YM. A prominent role for airway epithelial NF-kappa B activation in lipopolysaccharide-induced airway inflammation. J. Immunol. 2003;170:6257–6265. doi: 10.4049/jimmunol.170.12.6257. [DOI] [PubMed] [Google Scholar]
- Rennolds J, Butler S, Maloney K, Boyaka PN, Davis IC, Knoell DL, Parinandi NL, Cormet-Boyaka E. Cadmium regulates the expression of the CFTR chloride channel in human airway epithelial cells. Toxicol. Sci. 2010;116:349–358. doi: 10.1093/toxsci/kfq101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennolds J, Tower C, Musgrove L, Fan L, Maloney K, Clancy JP, Kirk KL, Sztul E, Cormet-Boyaka E. CFTR trafficking is mediated by the copi coat in epithelial cells. J. Biol. Chem. 2008;283:833–839. doi: 10.1074/jbc.M706504200. [DOI] [PubMed] [Google Scholar]
- Richter PA, Bishop EE, Wang J, Swahn MH. Tobacco smoke exposure and levels of urinary metals in the U.S. youth and adult population: The National Health and Nutrition Examination Survey (NHANES) 1999–2004. Int. J. Environ. Res. Public Health. 2009;6:1930–1946. doi: 10.3390/ijerph6071930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockwell P, Martinez J, Papa L, Gomes E. Redox regulates COX-2 upregulation and cell death in the neuronal response to cadmium. Cell. Signal. 2004;16:343–353. doi: 10.1016/j.cellsig.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Salnikow K, Li X, Lippmann M. Effect of nickel and iron co-exposure on human lung cells. Toxicol. Appl. Pharmacol. 2004;196:258–265. doi: 10.1016/j.taap.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Shapiro SD. Neutrophil elastase: Path clearer, pathogen killer, or just pathologic? Am. J. Respir. Cell Mol. Biol. 2002;26:266–268. doi: 10.1165/ajrcmb.26.3.f233. [DOI] [PubMed] [Google Scholar]
- Souza V, Escobar Md Mdel C, Gómez-Quiroz L, Bucio L, Hernández E, Cossio EC, Gutiérrez-Ruiz MC. Acute cadmium exposure enhances AP-1 DNA binding and induces cytokines expression and heat shock protein 70 in HepG2 cells. Toxicology. 2004;197:213–228. doi: 10.1016/j.tox.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Suriyawong A, Magee R, Peebles K, Biswas P. Energy recycling by co-combustion of coal and recovered paint solids from automobile paint operations. J. Air Waste Manag. Assoc. 2009;59:560–567. doi: 10.3155/1047-3289.59.5.560. [DOI] [PubMed] [Google Scholar]
- Tal TL, Simmons SO, Silbajoris R, Dailey L, Cho SH, Ramabhadran R, Linak W, Reed W, Bromberg PA, Samet JM. Differential transcriptional regulation of IL-8 expression by human airway epithelial cells exposed to diesel exhaust particles. Toxicol. Appl. Pharmacol. 2010;243:46–54. doi: 10.1016/j.taap.2009.11.011. [DOI] [PubMed] [Google Scholar]
- Tang J, Xiao T, Wang S, Lei J, Zhang M, Gong Y, Li H, Ning Z, He L. High cadmium concentrations in areas with endemic fluorosis: A serious hidden toxin? Chemosphere. 2009;76:300–305. doi: 10.1016/j.chemosphere.2009.03.064. [DOI] [PubMed] [Google Scholar]
- Tetley TD. Inflammatory cells and chronic obstructive pulmonary disease. Curr. Drug Targets Inflamm. Allergy. 2005;4:607–618. doi: 10.2174/156801005774912824. [DOI] [PubMed] [Google Scholar]
- Thomaidis NS, Bakeas EB, Siskos PA. Characterization of lead, cadmium, arsenic and nickel in PM(2.5) particles in the Athens atmosphere, Greece. Chemosphere. 2003;52:959–966. doi: 10.1016/S0045-6535(03)00295-9. [DOI] [PubMed] [Google Scholar]
- Winzen R, Kracht M, Ritter B, Wilhelm A, Chen CY, Shyu AB, Muller M, Gaestel M, Resch K, Holtmann H. The p38 MAP kinase pathway signals for cytokine-induced mRNA stabilization via MAP kinase-activated protein kinase 2 and an AU-rich region-targeted mechanism. EMBO J. 1999;18:4969–4980. doi: 10.1093/emboj/18.18.4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo JI, Kim KH, Jang HN, Seo YC, Seok KS, Hong JH, Jang M. The development of PM emission factor for small incinerators and boilers. Environ. Technol. 2002;23:1425–1433. doi: 10.1080/09593332508618447. [DOI] [PubMed] [Google Scholar]
- Zeytun A, Chaudhary A, Pardington P, Cary R, Gupta G. Induction of cytokines and chemokines by toll-like receptor signaling: Strategies for control of inflammation. Crit. Rev. Immunol. 2010;30:53–67. doi: 10.1615/critrevimmunol.v30.i1.40. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Chidekel A, Shaffer TH. Cultured human airway epithelial cells (calu-3): A model of human respiratory function, structure, and inflammatory responses. Crit. Care Res. Pract. 2010;2010:394578. doi: 10.1155/2010/394578. [DOI] [PMC free article] [PubMed] [Google Scholar]