Abstract
Ephrin-B plays an important role in neural progenitor cells to regulate self-renewal and differentiation. Cellular and embryological evidence suggest this function of ephrin-B is mediated through a PDZ-dependent reverse signaling mechanism. Here we have genetically investigated the function of PDZ-RGS3, a proposed downstream signaling mediator of ephrin-B function, and found that knockout of PDZ-RGS3 caused early cell cycle exit and precocious differentiation in neural progenitor cells of the developing cerebral cortex, reminiscent of the phenotype observed in ephrin-B1 knockout mice. This resulted in a loss of cortical neural progenitor cells during cortical neurogenesis and led to impairment in the production of late born cortical neurons. These results reveal an essential role of PDZ-RGS3 in maintaining the balance between self-renewal and differentiation of neural progenitor cells and provide genetic evidence linking PDZ-RGS3 to ephrin-B reverse signaling. As ephrin-B molecules are often differentially expressed in different types of neural progenitor/stem cells during development or in adult life, deletion of PDZ-RGS3 can achieve a uniform loss of function of the ephrin-B/RGS pathway, thereby providing a genetic tool useful for dissecting the mechanisms and functions of the ephrin-B/RGS reverse signaling pathway in neural progenitor/stem cell regulation.
Keywords: PDZ-RGS3 knockout, ephrin-B, neural progenitor cells, cerebral cortex, mammalian neurogenesis
Introduction
The ephrin/Eph family of molecules have been implicated in regulating proliferation, differentiation, survival, polarity or asymmetric division of neural progenitor/stem cells1–13. The B subfamily of ephrins is found to be associated with the neural progenitor/stem cell compartment both in embryonic and adult brains1, 7, 14–15 and is required for the maintenance of neural progenitor cells in the developing mouse cerebral cortex, acting in an autonomous manner via a reverse signaling mechanism10. Ephrin-B reverse signaling can be mediated via a phosphorylation-dependent or PDZ-dependent signaling pathway by its cytoplasmic domain16–19. One of the PDZ-containing proteins proposed to act downstream of ephrin-B is PDZ-RGS314. This protein is a member of the large family of RGS (regulator of G protein-signaling) domain containing proteins. The RGS domain is best known to act as a GTPase activating protein (GAP) to stimulate the intrinsic GTPase activity of Galpha subunit, thereby functions as a negative regulator of Galpha signaling20–21. PDZ-RGS3 contains a PDZ domain at its N-terminal end to physically interact with the cytoplasmic tail of all three known ephrin-Bs and an RGS domain at its C-terminus, and is thus expected to link ephrin-B signaling to modulation of Galpha subunit activity.
Our previous data obtained through in utero electroporation mediated dominant negative inhibition or RNA interference (RNAi)10 have implicated PDZ-RGS3 as the downstream signaling mediator of the function of ephrin-B1 in neural progenitor cell maintenance, but this role of PDZ-RGS3 has not yet been genetically tested. In addition, due to possible compensation of other remaining ephrin-B molecules, the function and mechanism of ephrin-B in neural progenitor cell self-renewal and differentiation could not be fully assessed in our previous analysis of ephrin-B1 knockout mice. In this study, we have generated a line of PDZ-RGS3 knockout mice to characterize the function of PDZ-RGS3, focusing on neural progenitor cells and neurogenesis in the developing cerebral cortex. Genetic mutation of PDZ-RGS3 is expected to avoid compensatory response in single ephrin-B knockout mice to allow a better dissection of the function of the reverse signaling of ephrin-B. We present evidence for an essential role of PDZ-RGS3 in the maintenance of neural progenitor cells. Loss of function (LOF) of PDZ-RGS3 results in a balance shift in neural progenitor cells from the state of self-renewal to differentiation. This phenotype is similar to that observed in ephrin-B1 knockout mice, implicating PDZ-RGS3 as an essential factor for the maintenance of neural progenitor cells and suggesting it is an important mediator of ephrin-B reverse signaling.
Materials and Methods
Construction of PDZ-RGS3 conditional targeting vector
Construction of targeting vector utilized a recombineering-based method for conditional knockout mutation. The cloning work was done with recombineering reagents described previously22. The BAC clone containing the PDZ-RGS3 gene was obtained from Roswell Park screening service. A 16.5 kb targeted BAC DNA was cloned by gap repair, and exons 2–5 of the PDZ-RGS3 gene in the 16.5 kb DNA were flanked by two LoxP sites. A 7kb DNA region upstream of the 5′LoxP site and a 4kb DNA region downstream of the 3′LoxP site were used as homologous recombination sequence for ES cell targeting. A phosphoglycerate kinase-1(PGK)-neo-bpA cassette flanked by FRT sites was used for positive selection with G418 as a substrate. MCI-TK was used as a negative selection cassette with ganciclovir as a substrate.
Generation of PDZ-RGS3 conditional and conventional knock out mice
Animal procedures were approved by the Institutional Animal Care and Use Committee (IACUC) and were carried out in accordance with NIH guideline, the Guide for the Care and Use of Laboratory Animals. The targeting construct was linearized with NotI and electroporated into 129/S1 ES cells. Transfectants were subjected to positive and negative selection by resistance to applied drugs23. Resistant clones carrying successful homologous recombination were screened by Southern blot hybridization (delineated by EcoR1 and Spe1 sites) using 3′ external probe 1 and 5′ internal probe 2. Three correctly targeted clones were used for injection into blastocysts and generation of chimerical mice.
The chimeras were crossed with wild-type 129/S1 to produce floxed heterozygous (floxed/+) F1 mice. Male F1 (floxed/+) mice were crossed to Hprt-Cre deleter female mice24 to obtain F2 (ΔPDZ/+) mice, in which the floxed exons (2–5) of the PDZ-RGS3 gene was deleted. Deletion of the PDZ domain (exons2–5) was verified by Southern Blot (delineated by Bcl1) using 5′ external probe 3 in the first a few litters and by RT-PCR using primers PR1 (GGGCTCTGCAAAGTGTGTTCAGAA, for PDZ-RGS3 sequence) or R1 (GTGCTTATTCACTTTGGAGGCAC, for RGS3 sequence) combined with a common 3′ primer (C primer, TGGGTGGGAGATCTTGTCCTGCA) based on the RT-PCR strategy described previously for distinguishing PDZ-RGS3 from RGS325. The loss of PDZ-RGS3 full-length protein was also confirmed with Western blot using an antibody against RGS3.
In a small number of animals, the neomycin cassette was removed by crossing conditional PDZ-RGS3 mice with ROSA26FlpeR mice26. Presence of the neomycin cassette did not appear to affect the phenotype of PDZ-RGS3 conditional or deficient animals, as judged by comparison between homozygous (floxed/floxed) conditional mice or deficient mice to wild-type mice in overall appearance of embryos and brain (cortex) morphology. Homozygous conditional mice were fertile and could be recovered with the expected Mendelian ratio.
Analysis of PDZ-RGS3 knockout mice
Progenitor cell cycle exit and reentry was analyzed as described previously10. In brief, pregnant female mice were labeled (24 h) with BrdU (50 mg/kg). For BrdU birth dating, E12.5 and E15.5 pregnant female mice were labeled with BrdU (100 mg/kg). The mice were sacrificed at postnatal day 0 (P0) for analysis. Cryosections of embryonic brains (12 μm) were processed for staining on BrdU, Ki67 and other progenitor markers (Pax6 or Tbr2) or on neuronal markers (DCX, βIII-tubulin, or NeuN). Cryosections of P0 brains (16 μm) were analyzed for staining on BrdU, early born neuron maker SOX5 and late born neuron maker CUX1. Images were taken using a confocal microscope (Zeiss LSM 510 Upright 2 photon) or Olympus Inverted IX81. For neural progenitor marker expression (Pax6 and Tbr2) quantification, marker positive cells were counted in 100x optical field. For progenitor cell cycle exit, BrdU+Ki67− cells (cells exiting cell cycle) were counted against the total BrdU+ cells / 40x optical view. Quantification was done with Image-Pro 6.3 automatic measurement program. Acutely dissociated cortical cell culture was done as previously described10. Data are shown as mean +/− s.d.
In vitro self-renewal and differentiation assay
E15.5 cortices were dissociated in HBSS containing 5mM EDTA by trituration using a pipette. Dissociated cortical cells were collected in neural progenitor cell growth media (DMEM/F12, 2 mM L-Glutamine, 25 mM HEPES, 10 U/ml Heparin, Penicillin/Streptomycin, B27, 20 ng/ml bFGF, 20ng/ml EGF). Cells were plated into wells of a 24 well culture plate at 10 cells/μl27, and cultured (37°C) for 6 days. Primary spheres were first documented and then dissociated with Accutase for secondary sphere culture. For differentiation assay, neurospheres were cultured in the absence of bFGF and EGF for 3 days and were then fixed with 4% paraformaldehyde for immunostaining.
Results
Generation of PDZ-RGS3 knockout mice
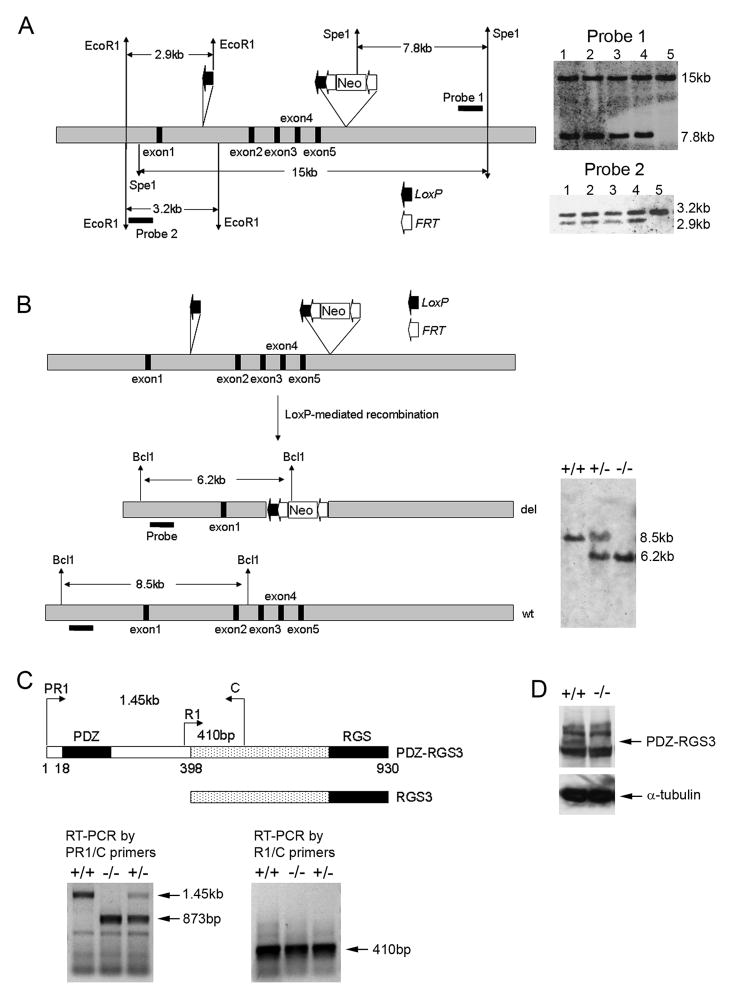
PDZ-RGS3 is a member of the RGS3 family of molecules including RGS3, PDZ-RGS3, and C2PA-RGS328. Mouse genome sequences reveal that the RGS3 genomic sequence is located on chromosome 4 and it appears that different RGS3 proteins represent differentially spliced variants of the same gene. The short RGS3 transcript is reported to be ubiquitously expressed in many tissues and is expected to have important PDZ-independent functions. To more specifically knockout the PDZ-dependent RGS3 function, we used a recombineering technique to generate a conditional targeting construct in which the exons 2–5 were flanked by LoxP sites for targeted deletion (Figure 1A). Exons 2–5 encode amino acids 18–211 which encompasses the PDZ domain in the mouse PDZ-RGS3 protein. Our previous data showed that the PDZ domain was required for binding and signaling of PDZ-RGS3 downstream of ephrin-B14. In addition, mutant PDZ-RGS3 protein lacking the PDZ domain did not seem to affect the interaction or signaling of the ephrin-B and PDZ-RGS3 complex14. Embryonic stem cells (ESC) containing the targeting sequence were obtained by homologous recombination, and conservative recombination with the genomic locus was verified by Southern blot hybridization (Figure 1A). Homologous recombinant ES cells were used to generate PDZ-RGS3 conditional mutants (PDZ-RGS3lox); PDZ-RGS3lox mice were recovered with the expected Mendelian ratio.
Figure 1. Generation of PDZ-RGS3 knockout mice.
(A) Illustration of targeting strategy. The exons 2–5 (black boxes), which encode the PDZ domain of PDZ-RGS3, are floxed with two LoxP sites for conditional deletion. The Neo-selection cassette is flanked with two Frt sites. ES cells that show homologous recombination of the targeting construct are confirmed by genomic Southern using two independent probes. Lanes 1–4 show four independent positive ES cell clones. Lane 5 shows wild-type ES cell control. (B) Hprt-Cre-mediated complete knockout of the PDZ sequence. Floxed PDZ-RGS3 mice were crossed with Hprt-Cre transgenic mice to obtain germline deletion of the PDZ sequence, which was confirmed by genomic Southern. (C) RT-PCR for assessing PDZ- RGS3 versus RGS3 transcript. The schematic diagram illustrates the protein domain structure of mouse PDZ-RGS3 and RGS3 proteins. Numbers indicate the amino acids of PDZ-RGS3 protein. PR1 (PDZ-RGS3 specific primer) or R1 (RGS3 specific primer) was combined with a common anchor primer (C primer) for RT-PCR. (D) Western blot on cortical cell extracts with an anti-RGS antibody shows disappearance of the fulllength band of PDZ-RGS3 in the homozygous mutant brain. Additional bands appear to be non-specific proteins.
A conventional knockout line of the PDZ-RGS3 gene was obtained by crossing the floxed PDZ-RGS3 line (PDZ-RGS3lox) with a general deleter line, Hprt-Cre transgenic mice24. The deletion of the floxed exons was confirmed by several different approaches. First, genomic Southern revealed the expected DNA pattern among littermates of knockout mice (Figure 1B). Second, using an established RT-PCR strategy for documenting different splice forms of the RGS3 transcripts25, we found that the deletion affected the PDZ containing transcript without affecting the short RGS3 transcript (Figure 1C). Finally, Western blot using an antibody raised against the C-terminus of PDZ-RGS314 showed the lack of PDZ-RGS3 full-length protein in mutant brains (Figure 1D), further confirming knockout of the PDZ-RGS3 protein.
The homozygous mutant PDZ-RGS3 knockout mice showed developmental abnormalities. During the mid-stage of cortical neurogenesis (embryonic day E15.5), we found that many homozygous mutants showed overall smaller body sizes compared to the wild-type littermates, suggesting growth retardation in the homozygous mutant embryos. The homozygous mutant embryos also showed smaller brain size compared to that of wild-type littermates. At three weeks of age, PDZ-RGS3 homozygous mutant mice were not recovered with the expected Mendelian ratio, an indication that the PDZ-RGS3 knockout resulted in early lethality (Table 1).
Table 1.
Genotype frequencies of PDZ-RGS3+/− x PDZ-RGS3+/− matingsa
| PDZ-RGS3+/− x PDZ-RGS3+/− | ||
|---|---|---|
| Genotype | Number | %b |
| +/+ | 86 | 36.4 |
| +/− | 118 | 50 |
| −/− | 32 | 13.6 |
The number and percentage of animals (including both males and females) with the indicated genotype at weaning (3 weeks) are shown.
The observed percentages of the three genotypes from PDZ-RGS3+/− x PDZ-RGS3+/− matings are statistically different from the expected 25%:50%:25% ratios (P < 0.01).
Morphological defect in the cortex of PDZ-RGS3 knockout mice
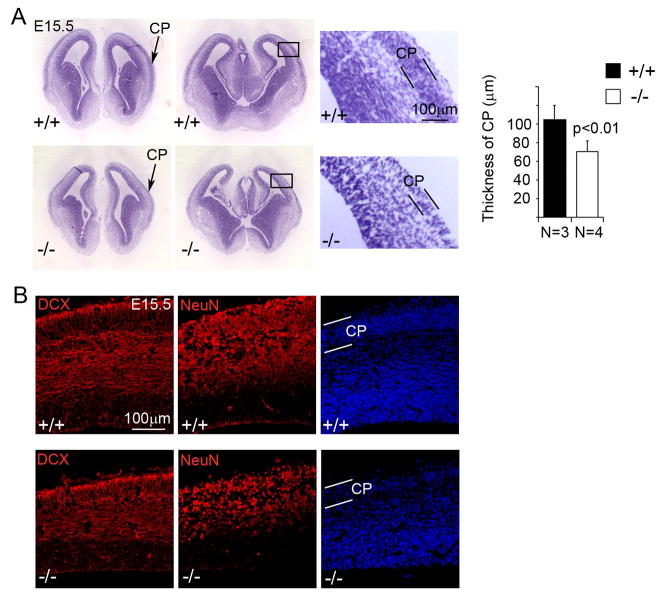
As our previous study showed that ephrin-B1 mutant mice displayed an early cell cycle exit and differentiation phenotype in cortical neural progenitor cells and that PDZ-RGS3 co-expressed with ephrin-B1 in the cortex, we asked whether knockout of PDZ-RGS3 would similarly affect cortical neural progenitor cells. We first looked at the cytoarchitecture of the mutant brains at the peak stage of cortical neurogenesis. We found that the PDZ-RGS3 homozygous mutant cortices displayed thinning along the radial dimension and showed a narrower ribbon of the forming cortical plate (CP) compared to their wild-type littermates at E15.5 (Figure 2A). Immunostaining on neuronal markers showed that, in homozygous mutant cortices, the thickness of the band of doublecortin (DCX) positive young neurons or NeuN-positive mature neurons were both noticeably reduced (Figure 2B). This morphological phenotype suggested a possible defect of neurogenesis in PDZ-RGS3 knockout mice.
Figure 2. Reduction of cortical radial thickness in the PDZ-RGS3 knockout mice.
(A) A noticeable reduction in the overall radial dimension as well as the thickness of the cortical plate (indicated by arrows in lower mag. images or by two parallel black lines in high mag. images) in the PDZ-RGS3 homozygous mutant brains at a peak stage of cortical neurogenesis (E15.5) can be seen with Nissl staining. Images show two different brain samples of PDZ-RGS3 homozygous mutant or wild-type littermates. (B) Immunostaining similarly revealed a reduction in the thickness of the band of young neurons (DCX positive) or mature neurons (NeuN positive).
Loss of cortical neural progenitor cells in PDZ-RGS3 knockout mice
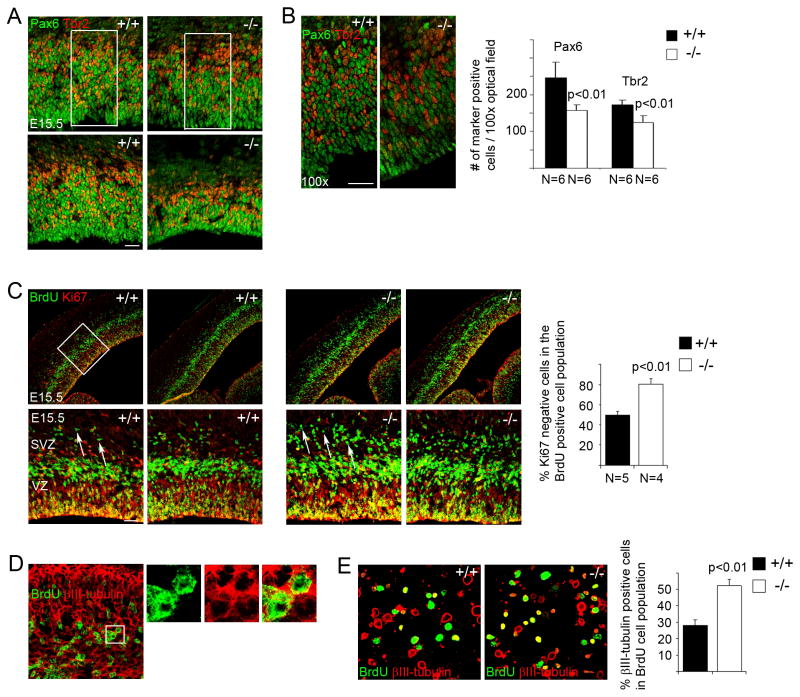
We next looked at cortical neural progenitor cells in the mutant brains. Analyses of BrdU incorporation in E15.5 brains with a short pulse of labeling (30 min) showed that knockout of PDZ-RGS3 resulted in a loss in the number of BrdU positive cells (data not shown). Immunohistochemistry on cellular markers of apical and basal progenitors29–31 showed that fewer Pax6+ or Tbr2+ cells32 were present in the cortices of the PDZ-RGS3 homozygous mutant animals (Figure 3A and 3B), indicating a loss of neural progenitor cells due to PDZ-RGS3 deletion. While we observed apparent thinning of the ventricular zone (VZ) and subventricular zone (SVZ) in some mutant cortices when compared to that of wild-type littermates (Figure 3A), this phenomenon was more subtle in many other mutant brains even when the overall thickness of the mutant cortices was thinner. On the other hand, a noticeable decrease in progenitor cell density was observed in many mutant brains – progenitors in the homozygous mutant cortex were more loosely packed than in the wild-type littermate (Figure 3A and 3B). Thus, it appears that the loss of Pax6+ and Tbr2+ neural progenitor cells due to PDZ-RGS3 deletion could result in both the reduction of the VZ/SVZ area and progenitor cell density in the cortex. Anti-cleaved caspase 3 staining in the cortex did not show obvious differences between brains of homozygotes and wild-type littermates (data not shown), suggesting that the loss of neural progenitors was not due to an elevated cell death. These results suggest that PDZ-RGS3 is important for maintaining a pool of cortical neural progenitor cells.
Figure 3. Early cell cycle exit in cortical neural progenitor cells in PDZ-RGS3 knockout mice.
(A) Images of Pax6+ and Tbr2+ neural progenitor zone of two examples of brain samples from PDZ-RGS3 homozygous mutants and wild-type littermates. Mutant brain in the bottom panel shows marked reduction in the thickness of the progenitor zone, while the one in the upper panel shows subtler difference. (B) Higher power images of the bracketed areas of (A) show progenitor cells in the homozygous mutant cortex are less packed compared to the wild-type littermate, reflecting the loss of the pool of neural progenitor cells. Quantification shows the average number of Pax6 or Tbr2 expressing cells in 100x optical fields. Scale bars, 50 μm. (C) Cell cycle exit analysis -- fraction of progenitor cells exiting cell cycle (BrdU+Ki67− cells, indicated by arrows) in the population of BrdU+ cells in brains labeled with BrdU for 24 h. Lower and higher power images of two different brain samples of PDZ-RGS3 homozygous mutant or wild-type littermates are shown. (D) BrdU and βIII-tubulin co-staining on E15.5 homozygous mutant cortex labeled with BrdU for 24 h. (E) Acutely dissociated cells obtained from 24 h BrdU-labelled cortices (E15.5) were stained for BrdU and and βIII-tubulin. The percentage of cells positive for both βIII-tubulin and BrdU in total BrdU positive cells was quantified. Error bars, s.d. Statistical analysis was done using Student’s t-test.
Early cell cycle exit in PDZ-RGS3 homozygous mutant cortices
We examined cell cycle exit and reentry in cortical neural progenitors by labeling the embryos with BrdU for 24 h33. We found that in the population of BrdU+ cells there was an increase in the number of BrdU+Ki67− cells (cells exiting the cell cycle) in the cortex of PDZ-RGS3 homozygous mutant animals (Figure 3C), suggesting that knockout of the PDZ-RGS3 gene led to an early cell cycle exit in cortical neural progenitor cells. We also observed that cells exiting the cell cycle in the PDZ-RGS3 homozygous mutant cortices were positive for βIII-tubulin (Figure 3D) or doublecortin (data not shown), markers of early migrating neurons. In acutely dissociated cell cultures derived from cortices labeled with BrdU for 24 h, more BrdU labeled cells from mutant cortices were positive for neuron markers (Figure 3E). Together, these results suggest that knockout of the PDZ-RGS3 gene induces early cell cycle exit and neuronal differentiation thereby reducing the pool of neural progenitor cells, similar to what was observed in ephrin-B1 knockout mice10.
In vitro characterization of self-renewal and differentiation property of mutant progenitor cells
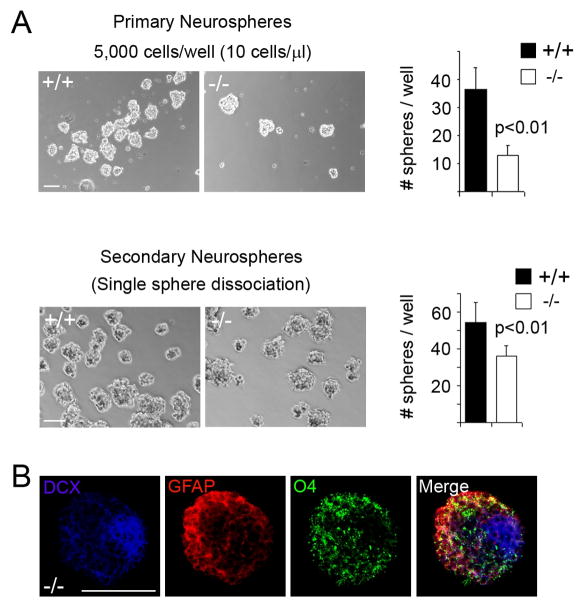
We further looked at the effect of PDZ-RGS3 LOF on self-renewal and differentiation in cortical neural progenitor cells. We collected dissociated E15.5 cortical cells from littermate brains and cultured them in vitro to assay for neurosphere formation, using culture conditions promoting clonal sphere formation27. As shown in Figure 4A, cortical cells derived from wild-type and PDZ-RGS3 homozygous mutant brains could generate neurospheres of comparable sizes, however, mutant cells contained fewer neurosphere-forming cells compared to the wild-type littermates, consistent with the observation that PDZ-RGS3 deletion resulted in a loss of self-renewing neural progenitors. To address the self-renewability further, we examined secondary sphere formation by normal and mutant cells derived from primary spheres. This was done using dissociated cells both from mixed primary spheres (data not shown) and from single sphere (Figure 4). Under both conditions, when primary spheres of the homozygous mutant cells were dissociated and replated, secondary spheres could be formed with an increased frequency, suggesting that, like wild-type neural progenitor cells, the mutant progenitor cells were able to self-renew. However, mutant cells appeared to have a lower potency to generate secondary spheres, suggesting that deletion of PDZ-RGS3 function might also affect self-renewal. When cultured in the absence of growth factors, neurospheres of the mutant progenitor cells generated neurons (DCX+), astrocytes (GFAP+) or oligodendrocytes (O4+) (Figure 4B), suggesting mutant progenitor cells maintained their capacity to differentiate into different mature neural cells under in vitro conditions.
Figure 4. In vitro self-renewal and differentiation of mutant cortical neural progenitor cells.
(A) Neurosphere assay. Dissociated cortical cells derived from E15.5 cortices were cultured in neurosphere promoting growth media. The number of primary spheres generated by mutant and wild-type cortical cells was documented. Single primary sphere was then dissociated with Accutase and replated in growth media for secondary sphere formation. Fewer cells were needed to generate secondary spheres. (B) When cultured in the absence of bFGF and EGF, mutant neurospheres were also able to differentiate into different neural cells, making neuron (DCX), astrocyte (GFAP), or oligodendrocyte (O4). Scale bars, 100 μm.
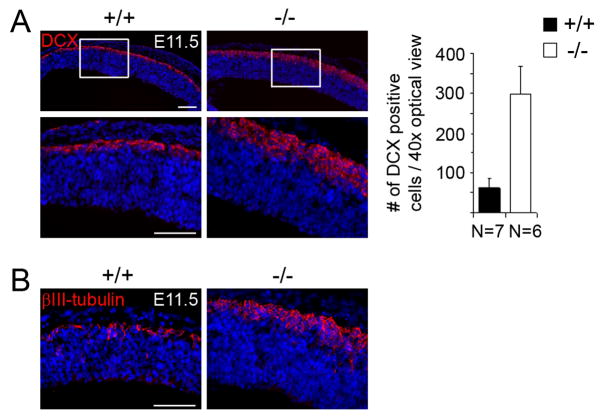
PDZ-RGS3 homozygous mutant cortex in early cortical neurogenesis
To examine whether induced differentiation caused by LOF of PDZ-RGS3 initiated earlier during corticogenesis, we examined PDZ-RGS3 homozygous mutant cortices at the starting stage of cortical neurogenesis. Immunostaining of doublecortin on E11.5 brain sections revealed a marked difference in the forming layer of young neurons amongst littermate brains. PDZ-RGS3 homozygous mutant brains showed a noticeably thicker layer of forming neurons than the wild-type littermate brains at E11.5 (Figure 5A), suggesting that PDZ-RGS3 deletion could induce precocious neuronal differentiation. A similar defect was detected with a different neuron marker, βIII-tubulin (Figure 5B). This result indicates that PDZ-RGS3 function is critical for maintaining a balance between self-renewal and differentiation in neural progenitor cells.
Figure 5. Precocious differentiation in mutant cortices at the start of neurogenesis.
Immunostaining of early neuronal marker DCX (A) or βIII-tubulin (B) showed an increased production of neurons and the resultant thickening of the cortices in homozygous PDZ-RGS3 mutant brains at the start of cortical neurogenesis. Scale bars, 100 μm.
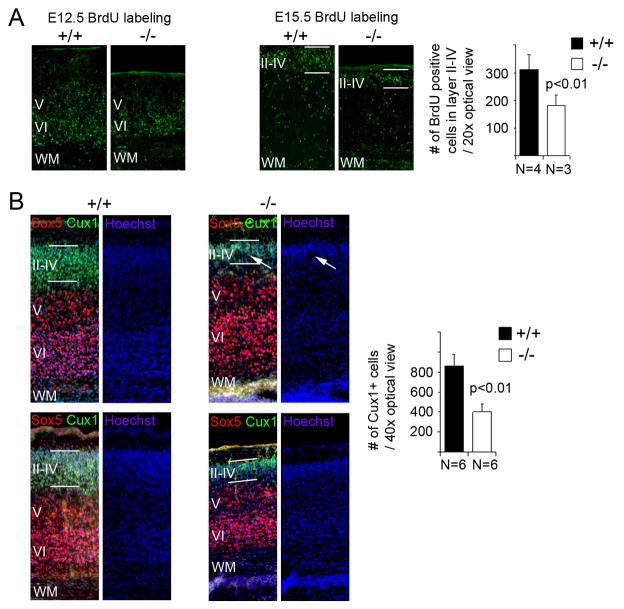
PDZ-RGS3 homozygous mutant cortex at birth
We next looked at the PDZ-RGS3 homozygous mutant cortices at birth when the laminar structure of the cortex is being laid out. To examine cortical lamination, we labeled littermates with BrdU at E12.5 or E15.5 and looked at the distribution of BrdU positive cells in the cortices at P0. As shown in Figure 6A, E12.5-labeled BrdU positive cells were mainly concentrated in the deep layers VI and V, while E15.5-labeled BrdU positive cells were mostly located in the upper layers, suggesting that cortical lamination in mutant cortices were comparable to wild-type littermates. However, in the mutant cortices, there were consistently fewer BrdU labeled neurons in the upper layers, suggesting a defect in the generation of late born neurons. To confirm that this observation was not simply due to a variation in BrdU labeling efficiency among different littermates, we further examined the deep and upper layer neurons, looking at Sox5-positive and Cux1-positive cells. While the deep layer neurons (Sox5-positive) of the PDZ-RGS3 homozygous mutant cortices showed variable degrees of difference from wild-type littermates (from similar numbers to fewer Sox5-positive cells in the mutant cortices), the upper layer neurons (Cux-1 positive) of the mutant cortices were consistently and noticeably thinner than wild-type littermates and often appeared non-uniform across the tangential plane (Figure 6B). This result indicates that the production of late born cortical neurons were impaired due to deletion of the PDZ-RGS3 gene.
Figure 6. Defect in the production of late born neurons in mutant cortices.
(A) Cortical lamination in homozygous PDZ-RGS3 mutant mice appeared normal based on BrdU birthdating observed at P0, but production of late born neurons (revealed by BrdU labeling at E15.5) in PDZ-RGS3 mutant mice appeared to be reduced comparing to the wild type littermates. (B) Fewer late born neurons (Cux1+) were present in the mutant cortices, as reflected in the reduction of the thickness of the upper cortical layers (II–IV). The late born neuron layer in the mutant cortices often appeared non-uniform across the tangential plane (indicated by white arrows). Images were two different P0 brain samples of PDZ-RGS3 homozygous mutant or wild-type littermates. WM, white matter.
Discussion
In this study we have genetically tested the function of PDZ-RGS3 in mice with a focus on neural progenitor cells of the developing cerebral cortex. We targeted exons encoding the PDZ domain of the PDZ-RGS3 gene product for homologous recombination in order to more specifically dissect the PDZ-mediated function of PDZ-RGS3 molecule, such as in the reverse signaling of ephrin-B. Our data revealed a clear neurogenesis defect in the cortices of PDZ-RGS3 knockout mice. We found that in homozygous mutant mice, there were more neurons generated at the start of cortical neurogenesis (Figure 5), but this phenomenon was reversed at a peak stage of cortical neurogenesis (Figure 2) when fewer neurons were seen. As a result, E15.5 cortices in the homozygous mutant brains showed reduced radial thickness of the entire cortex as well as the forming cortical plate (Figure 2). These apparently contradicting phenomena may have resulted from the dynamic effect of early depletion of neural progenitor cells. During the course of cortical neurogenesis, neural progenitor cells experience a transition from early prominent progenitor population expansion to later accelerated neuronal differentiation34–40. Loss of function of PDZ-RGS3 induced early cell cycle exit in cortical neural progenitor cells (Figure 3). This should be expected to contribute to the precocious differentiation and accumulation of more neurons at the early phase of neurogenesis, but this would also be expected to result in a reduction of the population of neural progenitor cells due to the lack of a proper expansion period. Consequently, when neurogenesis proceeded (e.g. at the peak neurogenesis stage E15.5), fewer neurons could be generated. Consistent with this idea, we observed that generation of late born neurons was severely affected in the postnatal brains. We also noticed that cortical neural progenitor cells derived from homozygous mutant PDZ-RGS3 brains were less potent in generating secondary spheres, compared to the wild-type cells, in a neurosphere formation assay, suggesting that LOF of PDZ-RGS3 might also affect the self-renewal of neural progenitor cells, in addition to promoting differentiation. Overall, our data revealed that PDZ-RGS3 knockout mice showed a cortical neural progenitor phenotype similar to what was observed in ephrin-B1 knockout mice --- LOF of ephrin- B1 or PDZ-RGS3 caused early cell cycle exit and neuronal differentiation of neural progenitor cells, thereby reducing the pool of self-renewing neural progenitor cells in the developing cerebral cortex. Similar cortical phenotype was also observed in RGS-insensitive G184SGαi2 knock-in mice in a separate study (Murai et al., in press). These observations support the idea that PDZ-RGS3 functions as a link to mediate ephrin-B reverse signaling, which may be achieved through coordination with Gα subunit signaling. Together, our data on ephrin-B110 and PDZ-RGS3 (this study), identify the RGS-mediated ephrin-B reverse signaling pathway as an essential axis for maintaining the balance between self-renewal and differentiation in neural progenitor cells during brain development.
We observed that the loss of cortical neural progenitor cells in the PDZ-RGS3 mutant cortices caused reduction of progenitor cell density in the VZ and SVZ in many cases, but did not always result in significant thinning of the VZ and SVZ area. This suggests that the dimension of the progenitor zone is likely independent of self-renewal or differentiation per se. We speculate that the thickness of VZ and SVZ may be encoded in the dimension of individual radial glia cell, perhaps depending on the apical-basal extent of radial processes, the radial extent of interkinetic nuclear migration, and/or the mechanisms that control radial unit formation and maintenance41. On the other hand, we observed noticeable thinning of the band of βIII-tubulin and NeuN-positive neurons at the same developmental stage, thus it appeared that the dimension of neuron layers could more directly reflect the number of cells within the region.
We observed that PDZ-RGS3 knockout mice showed more prominent cortical phenotype compared to ephrin-B1 knockout mice. For example, homozygous mutant PDZ-RGS3 mice showed a significant reduction in the radial thickness of the cortex and the cortical plate at E15.5 and displayed strong precocious differentiation at an earlier stage. These differences were likely due to compensation often associated with LOF of a gene of a multimember family. Among the three known ephrin-Bs, ephrin-B1 and ephrin-B2 are expressed in cortical neural progenitor cells. Knockout of ephrin-B1 might cause a compensatory response of ephrin-B2, whereas knockout of PDZ-RGS3 would be expected to block the reverse signaling from both ephrin-B1 and ephrin-B2, as PDZ-RGS3 binds to all known ephrin-Bs. In different neural progenitor/stem cell compartments, different combinations of ephrin-B are frequently co-expressed by neural progenitor/stem cells. Dissecting the function of ephrin-B signaling in these systems will require the generation of compound ephrin-B knockout mice. The PDZ-RGS3 knockout mice generated in this study should provide a more convenient genetic tool with respect to the study of the PDZ- and RGS-dependent ephrin-B function, as knockout of PDZ-RGS3 would be expected to uniformly block the RGS-mediated ephrin-B reverse signaling in different neural progenitor/stem cell systems.
There were two noticeable defects in the germinal zones of the brain in ephrin-B1 knockout mice --- early cell cycle exit of neural progenitor cells and disruption of radial organization in the cortex and the ganglion eminence10. These two defects did not appear to be mechanistically connected; because PDZ-RGS3 knockout mice showed cell cycle exit phenotype but did not exhibit obvious abnormality of radial organization (data not shown). Thus, in neural progenitor cells, PDZ-RGS3 appears to specifically act in the self-renewal promoting pathway downstream of ephrin-B, whereas the function of ephrin-B in maintaining a radial organization would be expected to be mediated by other effector(s). Connexin4342, the Par complex, and ZHX213 are ephrin-B-interacting proteins that are also expressed in neural progenitor cells43–45. Whether interaction between ephrin-B1 and these molecules contributes to the function of ephrin-B1 in maintaining a radial organization is an interesting question for future studies.
Acknowledgments
Funded by:
NIH grant NS052388 to Q.L.
We thank Donna Isbell, Armando Amaya and Kelley Carpenter for assistance with animal breeding and care; Mariko Lee and Brian Armstrong for helping with image acquisition; Silvia da Costa for critical reading of the manuscript. We thank the Developmental Studies Hybridoma Bank.
Footnotes
Disclosure of potential conflicts of interest
The authors indicate no potential conflicts of interest.
Author contributions:
R.X.Q.: conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing; J.W., W.T.: collection and assembly of data; Q.L.: conception and design, financial support, data analysis and interpretation, manuscript writing.
References
- 1.Conover JC, Doetsch F, Garcia-Verdugo JM, et al. Disruption of Eph/ephrin signaling affects migration and proliferation in the adult subventricular zone. Nature neuroscience. 2000;3:1091–1097. doi: 10.1038/80606. [DOI] [PubMed] [Google Scholar]
- 2.Aoki M, Yamashita T, Tohyama M. EphA receptors direct the differentiation of mammalian neural precursor cells through a mitogen-activated protein kinase-dependent pathway. The Journal of biological chemistry. 2004;279:32643–32650. doi: 10.1074/jbc.M313247200. [DOI] [PubMed] [Google Scholar]
- 3.Depaepe V, Suarez-Gonzalez N, Dufour A, et al. Ephrin signalling controls brain size by regulating apoptosis of neural progenitors. Nature. 2005;435:1244–1250. doi: 10.1038/nature03651. [DOI] [PubMed] [Google Scholar]
- 4.Holmberg J, Armulik A, Senti KA, et al. Ephrin-A2 reverse signaling negatively regulates neural progenitor proliferation and neurogenesis. Genes & development. 2005;19:462–471. doi: 10.1101/gad.326905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katakowski M, Zhang Z, Decarvalho AC, et al. EphB2 induces proliferation and promotes a neuronal fate in adult subventricular neural precursor cells. Neurosci Lett. 2005;385:204–209. doi: 10.1016/j.neulet.2005.05.060. [DOI] [PubMed] [Google Scholar]
- 6.Lee HS, Bong YS, Moore KB, et al. Dishevelled mediates ephrinB1 signalling in the eye field through the planar cell polarity pathway. Nature cell biology. 2006;8:55–63. doi: 10.1038/ncb1344. [DOI] [PubMed] [Google Scholar]
- 7.Ricard J, Salinas J, Garcia L, et al. EphrinB3 regulates cell proliferation and survival in adult neurogenesis. Molecular and cellular neurosciences. 2006;31:713–722. doi: 10.1016/j.mcn.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Chumley MJ, Catchpole T, Silvany RE, et al. EphB receptors regulate stem/progenitor cell proliferation, migration, and polarity during hippocampal neurogenesis. J Neurosci. 2007;27:13481–13490. doi: 10.1523/JNEUROSCI.4158-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Picco V, Hudson C, Yasuo H. Ephrin-Eph signalling drives the asymmetric division of notochord/neural precursors in Ciona embryos. Development (Cambridge, England) 2007;134:1491–1497. doi: 10.1242/dev.003939. [DOI] [PubMed] [Google Scholar]
- 10.Qiu R, Wang X, Davy A, et al. Regulation of neural progenitor cell state by ephrin-B. The Journal of cell biology. 2008;181:973–983. doi: 10.1083/jcb.200708091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi W, Levine M. Ephrin signaling establishes asymmetric cell fates in an endomesoderm lineage of the Ciona embryo. Development (Cambridge, England) 2008;135:931–940. doi: 10.1242/dev.011940. [DOI] [PubMed] [Google Scholar]
- 12.North HA, Zhao X, Kolk SM, et al. Promotion of proliferation in the developing cerebral cortex by EphA4 forward signaling. Development (Cambridge, England) 2009;136:2467–2476. doi: 10.1242/dev.034405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu C, Qiu R, Wang J, et al. ZHX2 Interacts with Ephrin-B and regulates neural progenitor maintenance in the developing cerebral cortex. J Neurosci. 2009;29:7404–7412. doi: 10.1523/JNEUROSCI.5841-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu Q, Sun EE, Klein RS, et al. Ephrin-B reverse signaling is mediated by a novel PDZ-RGS protein and selectively inhibits G protein-coupled chemoattraction. Cell. 2001;105:69–79. doi: 10.1016/s0092-8674(01)00297-5. [DOI] [PubMed] [Google Scholar]
- 15.Stuckmann I, Weigmann A, Shevchenko A, et al. Ephrin B1 is expressed on neuroepithelial cells in correlation with neocortical neurogenesis. J Neurosci. 2001;21:2726–2737. doi: 10.1523/JNEUROSCI.21-08-02726.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arvanitis D, Davy A. Eph/ephrin signaling: networks. Genes & development. 2008;22:416–429. doi: 10.1101/gad.1630408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pasquale EB. Eph-ephrin bidirectional signaling in physiology and disease. Cell. 2008;133:38–52. doi: 10.1016/j.cell.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 18.Klein R. Bidirectional modulation of synaptic functions by Eph/ephrin signaling. Nature neuroscience. 2009;12:15–20. doi: 10.1038/nn.2231. [DOI] [PubMed] [Google Scholar]
- 19.Lai KO, Ip NY. Synapse development and plasticity: roles of ephrin/Eph receptor signaling. Current opinion in neurobiology. 2009 doi: 10.1016/j.conb.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 20.Ross EM, Wilkie TM. GTPase-activating proteins for heterotrimeric G proteins: regulators of G protein signaling (RGS) and RGS-like proteins. Annu Rev Biochem. 2000;69:795–827. doi: 10.1146/annurev.biochem.69.1.795. [DOI] [PubMed] [Google Scholar]
- 21.Siderovski DP, Willard FS. The GAPs, GEFs, and GDIs of heterotrimeric G-protein alpha subunits. Int J Biol Sci. 2005;1:51–66. doi: 10.7150/ijbs.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu P, Jenkins NA, Copeland NG. A highly efficient recombineering-based method for generating conditional knockout mutations. Genome Res. 2003;13:476–484. doi: 10.1101/gr.749203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mansour SL, Thomas KR, Capecchi MR. Disruption of the proto-oncogene int-2 in mouse embryo-derived stem cells: a general strategy for targeting mutations to non-selectable genes. Nature. 1988;336:348–352. doi: 10.1038/336348a0. [DOI] [PubMed] [Google Scholar]
- 24.Tang SH, Silva FJ, Tsark WM, et al. A Cre/loxP-deleter transgenic line in mouse strain 129S1/SvImJ. Genesis. 2002;32:199–202. doi: 10.1002/gene.10030. [DOI] [PubMed] [Google Scholar]
- 25.Kehrl JH, Srikumar D, Harrison K, et al. Additional 5′ exons in the RGS3 locus generate multiple mRNA transcripts, one of which accounts for the origin of human PDZ-RGS3. Genomics. 2002;79:860–868. doi: 10.1006/geno.2002.6773. [DOI] [PubMed] [Google Scholar]
- 26.Farley FW, Soriano P, Steffen LS, et al. Widespread recombinase expression using FLPeR (flipper) mice. Genesis. 2000;28:106–110. [PubMed] [Google Scholar]
- 27.Coles-Takabe BL, Brain I, Purpura KA, et al. Don’t look: growing clonal versus nonclonal neural stem cell colonies. Stem cells (Dayton, Ohio) 2008;26:2938–2944. doi: 10.1634/stemcells.2008-0558. [DOI] [PubMed] [Google Scholar]
- 28.Lu Q, Sun EE, Flanagan JG. Analysis of PDZ-RGS3 function in ephrin-B reverse signaling. Methods in enzymology. 2004;390:120–128. doi: 10.1016/S0076-6879(04)90008-0. [DOI] [PubMed] [Google Scholar]
- 29.Haubensak W, Attardo A, Denk W, et al. Neurons arise in the basal neuroepithelium of the early mammalian telencephalon: a major site of neurogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:3196–3201. doi: 10.1073/pnas.0308600100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Noctor SC, Martinez-Cerdeno V, Ivic L, et al. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nature neuroscience. 2004;7:136–144. doi: 10.1038/nn1172. [DOI] [PubMed] [Google Scholar]
- 31.Miyata T, Kawaguchi A, Saito K, et al. Asymmetric production of surface-dividing and non-surface-dividing cortical progenitor cells. Development (Cambridge, England) 2004;131:3133–3145. doi: 10.1242/dev.01173. [DOI] [PubMed] [Google Scholar]
- 32.Englund C, Fink A, Lau C, et al. Pax6, Tbr2, and Tbr1 are expressed sequentially by radial glia, intermediate progenitor cells, and postmitotic neurons in developing neocortex. J Neurosci. 2005;25:247–251. doi: 10.1523/JNEUROSCI.2899-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chenn A, Walsh CA. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science (New York, N Y. 2002;297:365–369. doi: 10.1126/science.1074192. [DOI] [PubMed] [Google Scholar]
- 34.McConnell SK. Constructing the cerebral cortex: neurogenesis and fate determination. Neuron. 1995;15:761–768. doi: 10.1016/0896-6273(95)90168-x. [DOI] [PubMed] [Google Scholar]
- 35.Temple S. The development of neural stem cells. Nature. 2001;414:112–117. doi: 10.1038/35102174. [DOI] [PubMed] [Google Scholar]
- 36.Gotz M, Huttner WB. The cell biology of neurogenesis. Nat Rev Mol Cell Biol. 2005;6:777–788. doi: 10.1038/nrm1739. [DOI] [PubMed] [Google Scholar]
- 37.Molyneaux BJ, Arlotta P, Menezes JR, et al. Neuronal subtype specification in the cerebral cortex. Nature reviews. 2007;8:427–437. doi: 10.1038/nrn2151. [DOI] [PubMed] [Google Scholar]
- 38.Pontious A, Kowalczyk T, Englund C, et al. Role of intermediate progenitor cells in cerebral cortex development. Developmental neuroscience. 2008;30:24–32. doi: 10.1159/000109848. [DOI] [PubMed] [Google Scholar]
- 39.Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annual review of neuroscience. 2009;32:149–184. doi: 10.1146/annurev.neuro.051508.135600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rakic P. Evolution of the neocortex: a perspective from developmental biology. Nature reviews. 2009;10:724–735. doi: 10.1038/nrn2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Torii M, Hashimoto-Torii K, Levitt P, et al. Integration of neuronal clones in the radial cortical columns by EphA and ephrin-A signalling. Nature. 2009;461:524–528. doi: 10.1038/nature08362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davy A, Bush JO, Soriano P. Inhibition of gap junction communication at ectopic Eph/ephrin boundaries underlies craniofrontonasal syndrome. PLoS Biol. 2006;4:e315. doi: 10.1371/journal.pbio.0040315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Costa MR, Wen G, Lepier A, et al. Par-complex proteins promote proliferative progenitor divisions in the developing mouse cerebral cortex. Development (Cambridge, England) 2008;135:11–22. doi: 10.1242/dev.009951. [DOI] [PubMed] [Google Scholar]
- 44.Elias LA, Wang DD, Kriegstein AR. Gap junction adhesion is necessary for radial migration in the neocortex. Nature. 2007;448:901–907. doi: 10.1038/nature06063. [DOI] [PubMed] [Google Scholar]
- 45.Bultje RS, Castaneda-Castellanos DR, Jan LY, et al. Mammalian Par3 regulates progenitor cell asymmetric division via notch signaling in the developing neocortex. Neuron. 2009;63:189–202. doi: 10.1016/j.neuron.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]