Abstract
Control of the protozoan parasite, Leishmania major, is dependent upon establishing a robust T cell response. An early event in the development of an effective T cell response is the expansion (or hypertrophy) of the lymph node draining the site of infection, although the mechanisms involved in this response are not completely understood. Here we show that lymph node hypertrophy following L. major infection in mice is associated with increased recruitment of lymphocytes to the lymph node from the blood, and that CD62L-deficient mice, which are unable to recruit cells to the lymph node, develop a chronic infection with L. major. Injection of L. major activated dendritic cells promoted lymph node hypertrophy, and this correlated with an increase in the expression of CCR7 on dendritic cells, although the upregulation of CCR7 occurred on the bystander (uninfected) dendritic cells rather than those containing parasites. We found that increased CCR7 expression was TLR9 dependent, that TLR9−/− DCs migrated less efficiently to the draining lymph node, and that TLR9−/− mice exhibited a deficit in lymph node expansion following L. major infection, as well as increased susceptibility. Taken together, these results are the first to demonstrate that activation of dendritic cells via TLR9 is essential for the induction of lymph node hypertrophy in leishmaniasis.
Introduction
Leishmania major is a protozoan parasite that is controlled by CD4+ Th1 and CD8 T cells (1). The magnitude of this immune response is regulated by several factors, including the parasite load, the induction of regulatory mechanisms, and the frequency of antigen-reactive cells. Since the frequency of a naive T cell with any particular specificity is low, the immune system depends upon the ability of naïve T cells to recirculate throughout secondary lymphoid organs, thus enhancing the potential for an appropriate T cell to come in contact with its cognate antigen. Following infection, this pathway is enhanced by the rapid expansion (or hypertrophy) of the lymph nodes draining the site of infection (dLN) (2–6). Not only does this response promote increased entry of naïve T cells, but also allows entry of effector T cells not normally able to enter LNs (7). The generation of a robust protective immune response to L. major is associated with substantial hypertrophy of the draining LN, although the factors that initiate LN expansion in leishmaniasis are not defined (8).
The importance of this response in leishmaniasis is suggested by several observations. First, mice that have been engineered to express high levels of CCL21, which leads to CCR7 downregulation and therefore inhibits T cell entry into the LNs, develop a chronic disease following infection with L. major (9). Studies in mice lacking LNs highlight the importance of LNs in resistance, as these mice developed an increased susceptibility to L. major (10). We recently correlated minimal LN expansion with the susceptibility and limited immune responses seen in mice infected with L. mexicana, further suggesting the importance of this physiologic response in establishing effective immunity (8). Finally, in addition to being protective, an exaggerated expansion of the LN can be associated with disease. For example, substantial lymphadenopathy often occurs in patients infected with L. braziliensis parasites, and while these individuals eliminate most of their parasites, they have a tendency to develop severe inflammatory lesions, sometimes with chronic metastatic mucosal disease (11, 12). These observations raise the question of how LN expansion is initiated and regulated following Leishmania infection.
Recent studies have begun to elucidate the mechanisms responsible for LN expansion following administration of adjuvants (2–5). Dendritic cells (DCs) play a critical role in initiating the response, as injection of activated DCs into the skin induces hypertrophy in the draining LN and depleting DCs abrogates adjuvant-induced LN expansion (2, 4). Once DCs initiate this response it leads to enhanced recruitment of cells from the blood to the dLN increases cellularity. Enhanced recruitment is likely mediated by increased extravasation of cells from the blood into the LN through HEVs expressing higher levels of chemokines capable of binding CCR7 on T cells, such as CCL21. In the next several days VEGF-dependent endothelial cell proliferation results in increased vascularity, specifically increasing the number of high endothelial venules through which cells gain entry to LNs. Concomitantly, there is a DC and VEGF-dependent increase in the lymphatic vessels within the LN (lymphangiogenesis) and in the tissues draining the site of inflammation (13, 14), which further increases entry of cells (such as DCs) from the tissues into the LN. Less is known about the resolution of LN hypertrophy, although recently DCs have also been shown to play a role in stabilizing the vasculature following expansion (4).
In this study we ask whether the LN hypertrophy we see following L. major infection is associated with increased cell recruitment, and what innate immune responses are involved in the response. We found that LN hypertrophy following L. major infection is associated with a substantial increase in cell recruitment throughout the first several weeks of infection, that L. major activated DCs induce this response, and that TLR9 is required for the rapid L. major induced LN hypertrophy seen after infection.
Materials and Methods
Animals
Female C57BL/6 mice were purchased from the National Cancer Institute (Fredricksburg, MD). TLR9 deficient mice were obtained from Dr. Larry Turka (University of Pennsylvania). Female B6;129S2-Selltm1Hyn/J (CD62L deficient mice) were obtained from Jackson Labs; Female B6129 mice obtained from Jackson Labs were utilized as a wild-type control for the experiments with the CD62L deficient mice. Germ-free mice are maintained in a colony at the University of Pennsylvania. Animals were housed in a specific-pathogen-free environment and tested negative for pathogens in routine screening. All experiments were conducted following the guidelines of the University of Pennsylvania institutional animal care and use committee.
Parasites and Infections
L. major V1 parasites (MHOM/IL/80/Friedlin) or L. major V1 parasites expressing red fluorescence protein (DsRed L. major) (15) were grown until stationary phase in Grace’s insect culture medium (Life Technologies, Gaithersburg, MD) supplemented with 20% heat-inactivated FBS (HyClone Laboratories, Logan, UT), 2 mM L-glutamine, and metacyclic promastigotes isolated by density gradients (16). For infection of mice, 1–2 × 106 metacyclic parasites were injected into the footpad or ear, and the course of infection monitored by measuring lesions size. Parasites were quantitated by limiting dilution.
Bone marrow-derived DCs
Bone marrow-derived dendritic cells (DC) were cultured as previously described (17). Briefly, bone marrow DC precursors were differentiated for 8–10 days in the presence of 20 ng/ml GM-CSF in RPMI 1640 containing 10% FBS, 100 U/ml penicillin/streptavidin, 0.05 mM 2-ME, and 2 mM L-glutamine. On days 8–10 of culture DCs were harvested and infected with promastigotes at a 10:1 or 5:1 ratio. After 3 hours cells were washed twice for elimination of extracellular parasites and DCs were incubated for 18 hours at 37°C. After an incubation period of 18 hours at 37°C, DCs were harvested and stained for flow cytometry as described below.
CCL21 ELISA
Draining LNs were harvested on days 3, 7 and 14 following injection of C57BL/6 mice with L. major or injection of PBS. The LNs were disrupted using a tissue lyser with 5 mm beads in PBS and protease inhibitor cocktail (Sigma). The presence of CCL21 was assessed by ELISA (R&D Systems).
Cytokine mRNA responses
Total RNA was extracted from homogenized dLN tissue with RNeasy columns, following the manufacturer’s instructions (Qiagen). The cDNA was generated using Superscript II reagents (Invitrogen Life Technologies). Real-time PCR for IFN-γ and IL-4 were performed using SYBR Green primers (Qiagen). β-actin was used as a control.
Flow cytometry
For flow cytometry, DCs were harvested, stained with fluorochrome-conjugated antibodies for surface markers (CD11c, CD11b, class II, CCR7, CD45.1 and CD45.2 (e-Bioscience)) and fixed by using 2% formaldehyde. Samples were acquired on a FACS Canto flow cytometer (BD Pharmingen), and analysis was performed using FlowJo software (Tree Star). The cells were gated based on the live cell gate and then further gated on the CD11b+CD11c+ population.
Lymphocyte homing into draining LNs
To assess cell recruitment into LNs draining the site of infection, naïve mice were sacrificed and a single cell suspension of spleen cells prepared and labeled with CFSE. 5 × 106 splenocytes were injected iv into mice that had previously been infected with L. major for various periods of time. After 18 hrs the mice were sacrificed, the LNs draining the site of infection, as well as the contralateral LNs (non-draining), were harvested and the number of cells quantitated. The presence of the donor cells was assessed in the draining LNs by analyzing the CFSE positive cells. For these experiments the CFSE label was used only to identify the transferred cells.
Statistics
Statistical analysis was performed using a two-tailed Student's t test. Differences were considered significant at p< 0.05.
Results
LN hypertrophy following L. major infection involves increased recruitment of cells to the dLN
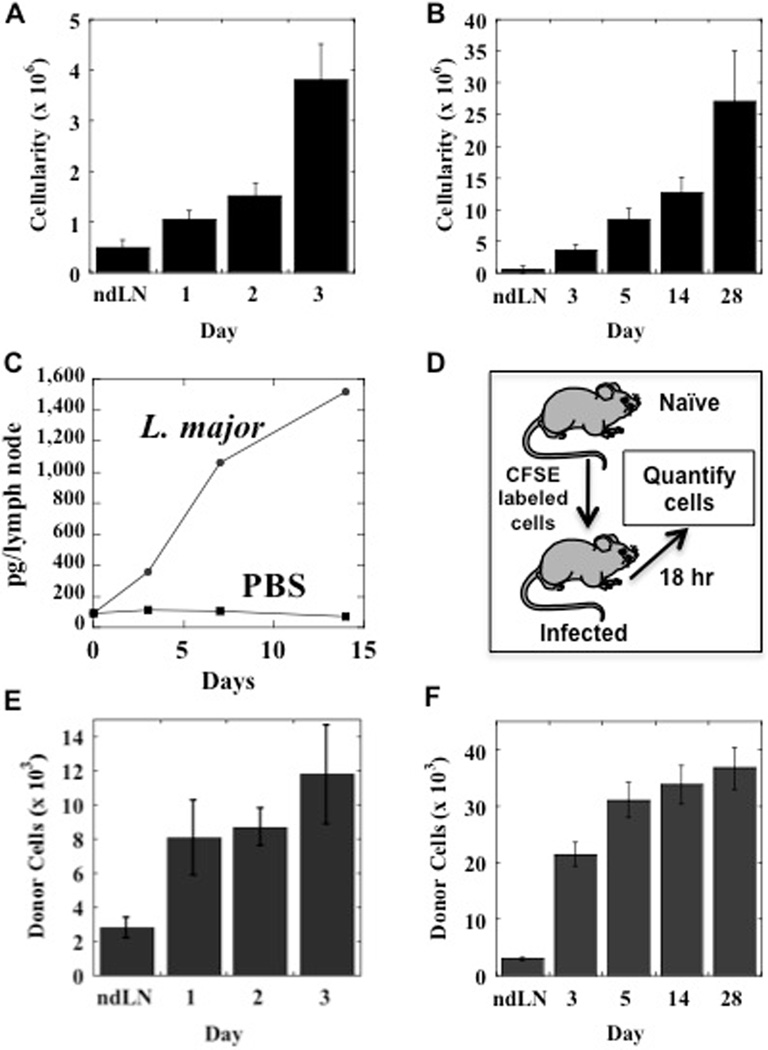
Within a few days of infection with L. major there is a substantial increase in the cellularity of the lymph node draining the site of infection, although a visible lesion is not evident for several weeks (Fig. 1A). The dLN continues to increase in cellularity throughout the course of infection, reaching 30 to 40 times normal size (Fig. 1B). While proliferation of lymphocytes in the dLNs likely contributes to the increase in LN cellularity, we were interested to know if increased recruitment of cells from the blood into the LNs was also occurring. Increased recruitment of T cells would promote a better immune response by increasing the chances that a specific T cell comes in contact with DCs presenting leishmanial antigens. T cells are able to migrate into LNs due to the expression of CD62L and the chemokine receptor, CCR7. CCR7 binds to CCL21 or CCL19, which are expressed on the endothelium. Consistent with the role of CCL21, we found that associated with L. major induced LN hypertrophy was an increase in CCL21 protein levels (Fig. 1C), which would promote increased entry of cells into the dLNs.
Figure 1.
L. major induces increased recruitment of cells to the draining LN. C57BL/6 mice were infected with L. major and the cellularity of the draining LN was assessed from day 1 to 3 (A) or day 3 to 28 (B). These responses are compared with the cellularity of non-draining LNs (ndLN). LNs were harvested from L. major or PBS injected mice at days 3, 7 and 14. The cells were dissociated, and CCL21 levels were assessed by ELISA (C). Splenocytes from naïve C57BL/6 mice were CFSE labeled and injected iv in mice that had previously been infected with L. major, and after 18 hrs the number of CFSE labeled cells quantitated in the LN draining the site of infection by flow cytometry (D). Quantitation of donor cells during the course of L. major infection from day 1 to 3 (E) or day 3 to 28 (F). The number of cells migrating into the non-draining LNs (ndLN) did not change significantly during the dcourse of the experiment, and the average is shown. The data shown are representative of 2 or more experiments.
To directly assess cell migration into LNs, we adoptively transferred CFSE labeled lymphocytes into mice that had been previously infected with L. major at various times. After 18 hrs we sacrificed the recipients, harvested the dLN and the contralateral (non-draining) LN, and quantified the recruited donor cells by flow cytometry (Fig. 1D). Within the first 24 hours of infection, there was a significant increase in the number of donor cells recruited to the dLN as compared with the non-dLN (Fig. 1E). In order to determine if the increased recruitment was transient, we examined several time points following infection, and found that increased cell recruitment was evident throughout the first 4 weeks of infection (Fig. 1F). While there was a change in the ratio of T and B cells in the dLN over time, presumably due to B cell proliferation, there was no preferential recruitment of T or B cells (data not shown). Taken together, these data indicate that L. major induces a rapid increase in dLN cellularity that is in part mediated by a sustained increase in the recruitment of lymphocytes to the dLN.
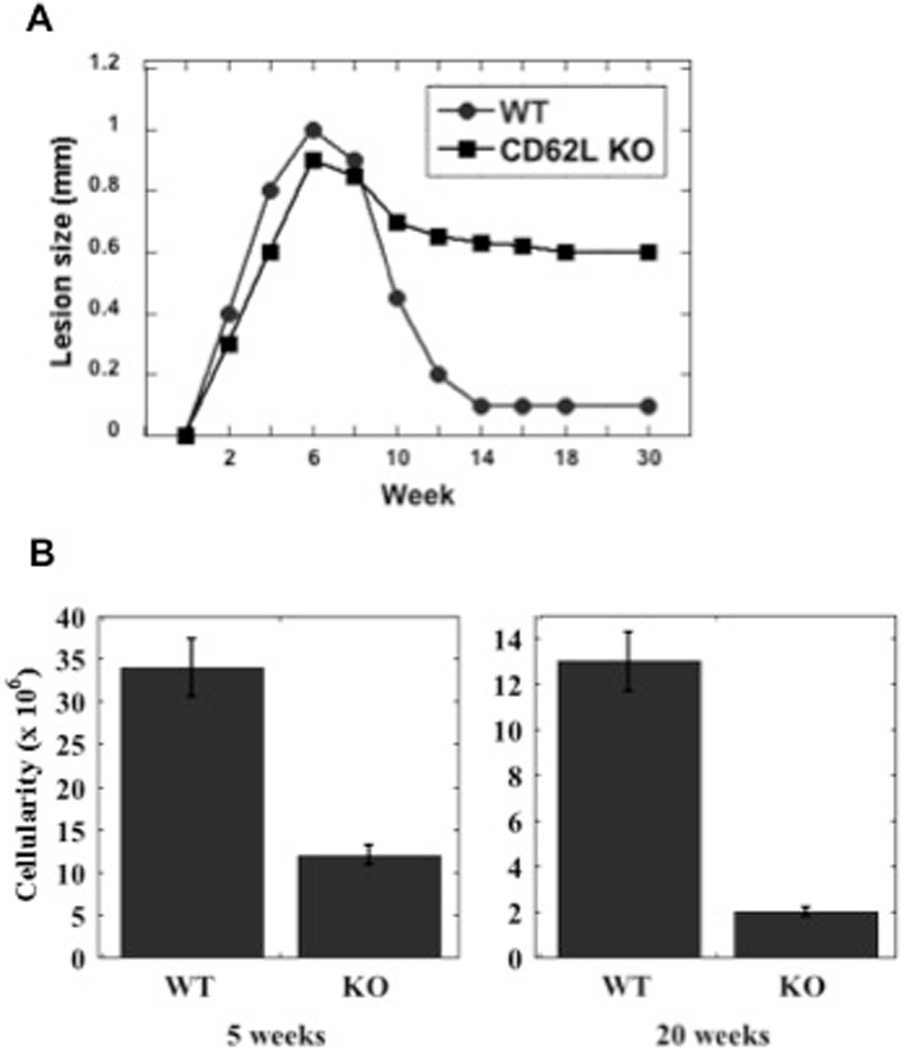
Previous studies suggested that the absence of LNs leads to increased susceptibility to L. major (9, 10). We infected CD62L deficient mice on a C57BL/6 background to confirm these observations. CD62L is expressed on the surface of cells, and is required for the efficient entry of lymphocytes into the LN via the high endothelial venules (18). Transient blockade of CD62L inhibits the development of Th2 responses in L. major infected BALB/c mice, rendering them more resistant (19). In contrast, when LN hypertrophy was completely blocked by infection of CD62L knockout mice, we found that CD62L deficient mice exhibited a relatively normal course of infection for the first few weeks of infection, but these mice were unable to resolve their infections (Fig. 2A). They maintained chronic lesions for more than 20 weeks post-infection. While parasites appeared to be cleared in the lesions of the control mice, the CD62L deficient mice maintained approximately 103 parasites in their lesions (data not shown). As expected, the dLNs were correspondingly smaller in the CD62L deficient mice both at 5 and 20 weeks following infection (Fig. 2B).
Figure 2.
L. major infections in CD62L deficient mice fail to resolve their lesions. (A) B6129 (WT) or CD62L−/− mice were infected with L. major and the course of infection monitored. The results expressed are the mean (+/− SE) lesion size of 5 mice per group. (B) The cellularity of the dLN was assessed at weeks 5 and 20. The results expressed are the mean (+/− SE) number of cells in the dLN of 3 mice per group. The data shown is representative of 2 experiments.
L. major activated DCs induce LN hypertrophy
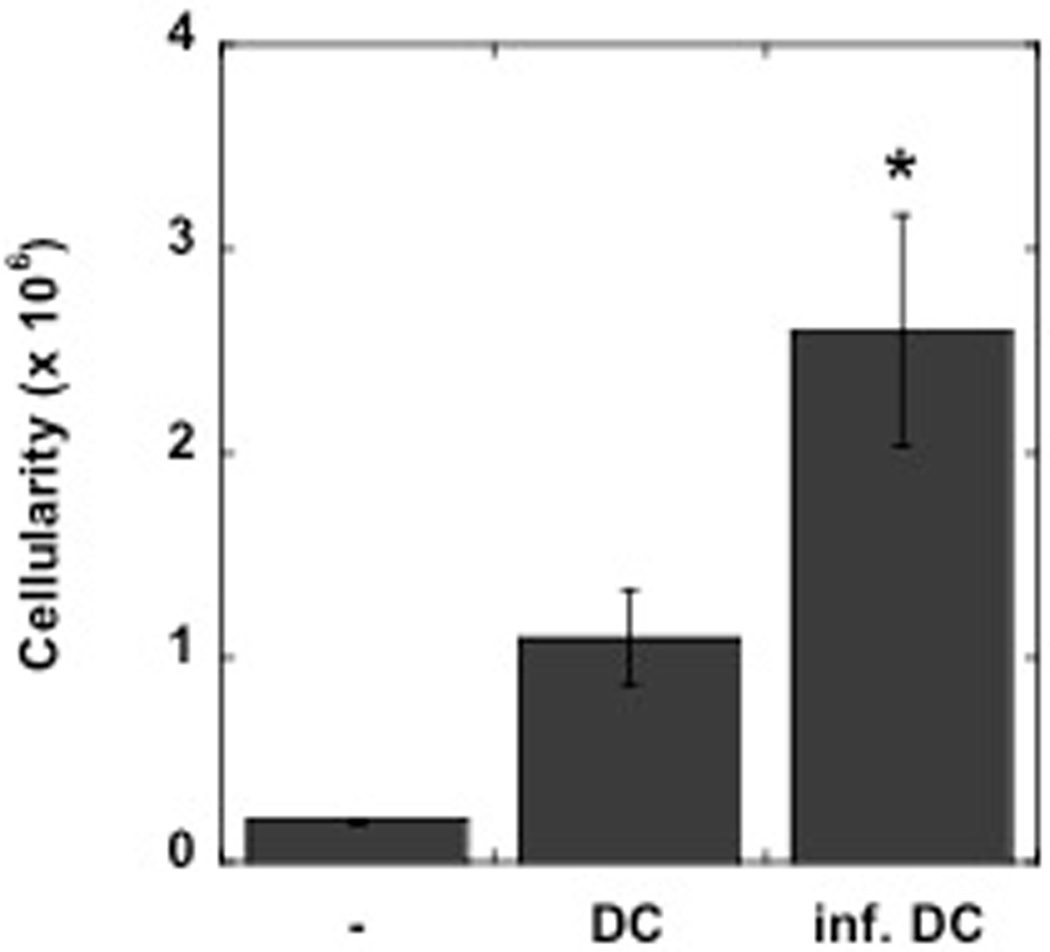
Activation of DCs in the skin induces hypertrophy in the draining LN. Thus, when LPS-matured DCs are injected into the skin they induce an increase in the cellularity of the dLN (2, 4). Therefore, we asked if DCs exposed to L. major would also induce LN hypertrophy when injected into the skin. Bone-marrow derived DCs were infected in vitro with L. major metacyclic promastigotes, leading to approximately 30–35% of the DCs containing L. major parasites. We injected the DCs exposed to the parasites into the skin, and three days later we assessed the cellularity of the dLN. We found a significant increase in the cellularity of those dLNs from mice injected with cultures of DCs that had been exposed to L. major (Fig. 3). These results indicate that L. major stimulates a population of DCs that acquire the capacity to induce LN hypertrophy.
Figure 3.
DCs activated by L. major induce LN hypertrophy. BMDCs were infected with L. major and 5 × 105 DCs or uninfected DCs were injected into the footpad of C57BL/6 mice, and the dLN harvested 3 days later. The results are the mean +/− SE of 3 mice per group, and are representative of 2 experiments.
L. major induces CCR7 expression in DCs in a TLR9 dependent manner
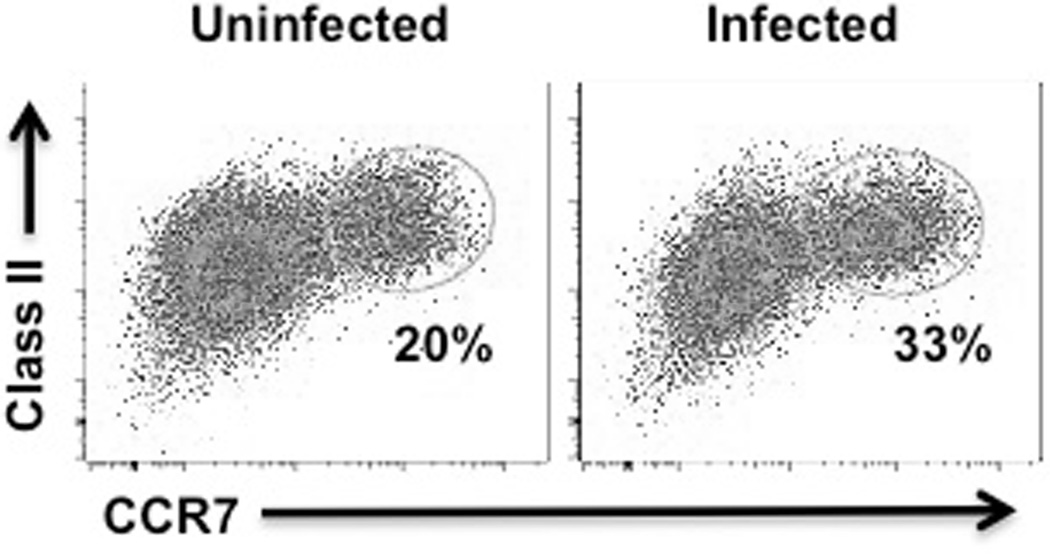
A key factor in the development of LN hypertrophy is the ability of DCs to become activated and to migrate from the tissues to the dLN, a process that is dependent upon the expression of the chemokine receptor CCR7 (2, 20). Once in the dLN, DCs promote LN expansion by directly or indirectly promoting increased blood flow to the LN. Therefore, we hypothesized that following L. major infection DCs become activated and are licensed to promote LN hypertrophy. To test this hypothesis we examined expression of MHC Class II and CCR7 on infected DCs. We found substantial upregulation of CCR7 following L. major infection (Fig. 4).
Figure 4.
L. major infection induces increased CCR7 expression on DCs. BMDCs were infected at a 10:1 ratio with L. major for 2 hours and then cultured overnight. Cells were stained with fluorescent antibodies and results were assessed by flow cytometry. These results are representative of 3 experiments.
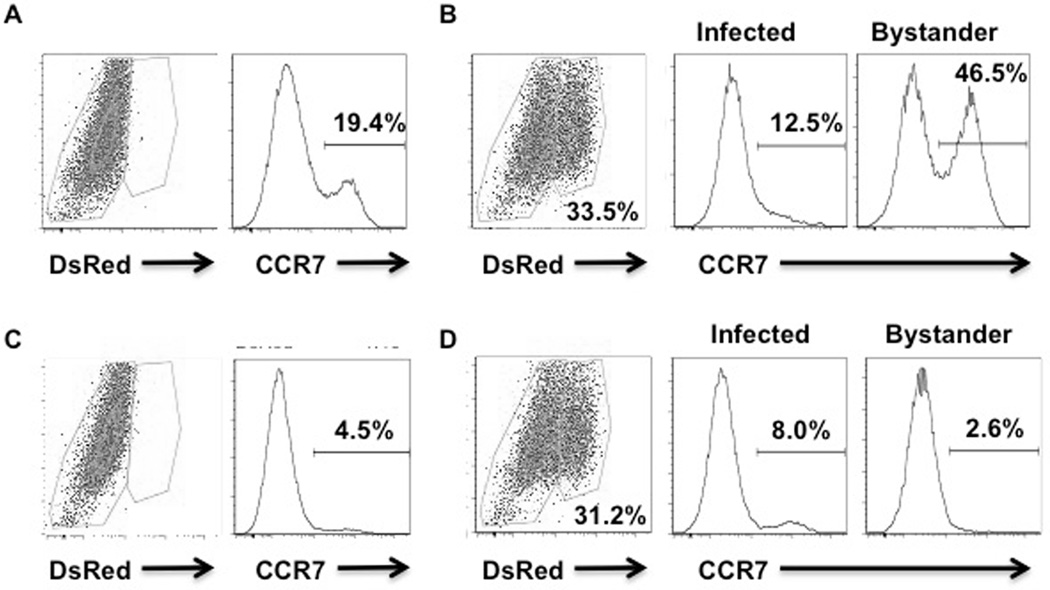
While we observed an increase in Class II and CCR7 in cultures of DCs infected with L. major, we previously reported that Leishmania induced DC activation occurs primarily in the bystander cells within the culture, i.e. the cells that are not infected (21). To determine if this was the case for CCR7 expression, we infected DCs with DsRed L. major, which allowed us to distinguish the infected and the uninfected cells within the same culture. Consistent with our previous observations, we found that upregulation of CCR7 occurred in the bystander DCs, but not the infected DCs (Fig. 5A, B).
Figure 5.
L. major infection induces increased CCR7 expression on bystander DCs in a TLR9-dependent manner. Bone marrow dendritic cell cultures from C57BL/6 (A, B) or TLR9−/− (C, D) mice were uninfected (A, C) or infected (B, D) at a 10:1 ratio with L. major for 2 hours and then incubated overnight. Cells were stained with fluorescent antibodies and results were assessed by flow cytometry. These results are representative of 3 experiments.
Previous studies found that Leishmania parasites activate DCs via TLR9 (22–24). We confirmed this by stimulating DCs with Leishmania DNA and assessing the production of cytokines. Leishmania DNA stimulated the production of IL-12 and TNF-α in WT DCs, but not in TLR9−/− DCs (data not shown). Therefore, since DC activation is required for LN hypertrophy, we hypothesized that TLR9 may similarly be critical, and investigated whether the L. major induced CCR7 upregulation on DCs was TLR9 dependent. DCs from WT or TLR9−/− mice were infected with DsRed parasites, and the expression of CCR7 assessed at 24 hr. As described above, we found that within cultures of WT DCs infected with L. major, there was a significant upregulation of CCR7 by the bystander DCs (Fig. 5A, B). In contrast, when we assessed expression of CCR7 by TLR9−/− cells, we found no significant increased expression of CCR7 (Fig. 5C, D). These results are similar to others where TLR9 promoted CCR7 upregulation in DCs (25, 26).
TLR9 is required for LN hypertrophy
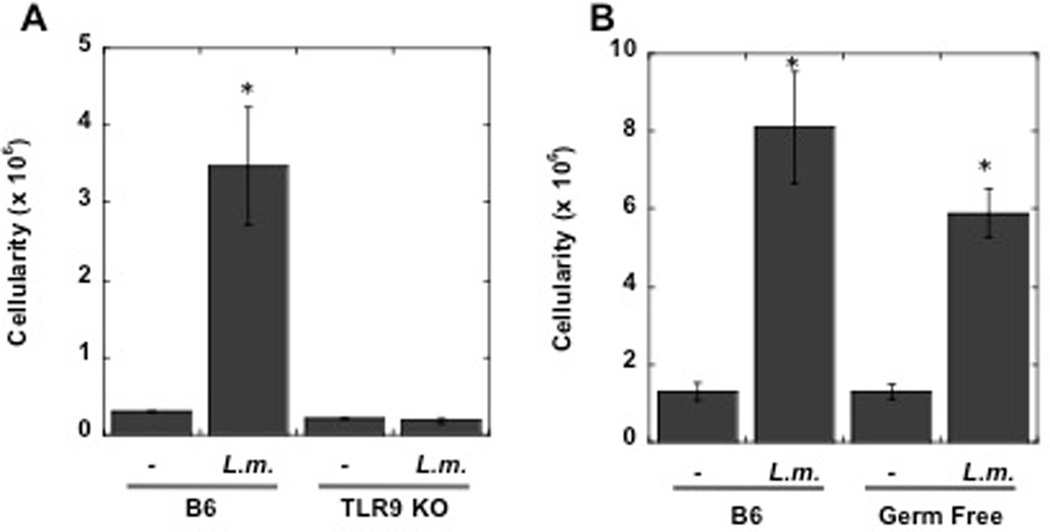
The TLR9 dependent upregulation of CCR7 in DCs suggested that LN hypertrophy may be TLR9 dependent as well. To test this hypothesis we compared the LN cellularity in WT and TLR9 −/− mice three days following L. major infection. As shown in Fig. 6A, while C57BL/6 mice infected with L. major exhibit a significant increase in cellularity by day 3, L. major infected TLR9−/− mice exhibit a minimal increase in LN cellularity.
Figure 6.
TLR9−/− mice exhibit decreased LN hypertrophy following L. major infection. C57BL/6 (B6) or TLR9−/− mice were infected with L. major or injected with killed L. major antigen and the cellularity of the dLN assessed on day 3 (A). Conventional or germ-free C57BL/6 mice were infected with L. major, and the cellularity of the dLN assessed on day 3 (B). The results expressed are the mean (+/− SE) number of cells in the dLN of 3 mice per group. The results are representative of 2 experiments.
A large and complex microbial community colonizes the skin, respiratory tract, and intestine of mammals, and the influence these microbes on the host immune system is just beginning to be evaluated (27). While L. major can activate DCs directly, it is possible that commensal-derived signals contribute to DC activation via TLR9. To determine if commensal-derived signals contribute to dLN hypertrophy, we compared LN hypertrophy induced by L. major infection in conventional C57BL/6 or germ-free mice (reared in the absence of live microbial stimuli). We found no significant difference in the cellularity of the dLN in these two groups (Fig. 6B). Thus, it appears that commensal-derived signals are not necessary for LN hypertrophy following infection with L. major.
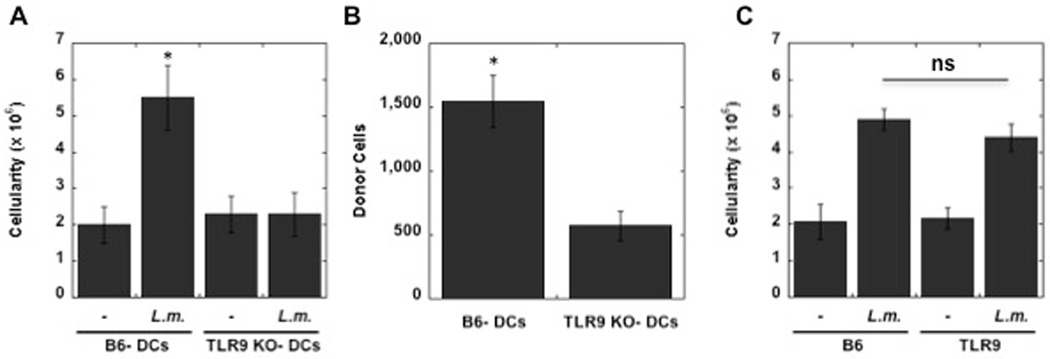
Several types of cells express TLR9 and while we and others have found that Leishmania DNA activates DCs (22–24), it was still possible that the in vivo effects we were observing were due to expression of TLR9 on cells other than DCs. To address this, we compared the ability of L. major activated WT DCs and TLR9−/− DCs to induce LN hypertrophy. DCs were exposed to L. major, and after 24 hr the cells were injected into the skin. As seen in Fig. 7A, WT DCs promoted an increase in cellularity of the dLN. In contrast, there was no increase evident when DCs from TLR9−/− mice were injected into the skin. The lack of a response by TLR9−/− DCs suggested that L. major was unable to appropriately activate TLR9−/− DCs, and thus the DCs would migrate poorly to the dLN. To test this directly, we infected WT and TLR9−/− DCs with L. major, and inoculated them into the footpad. After 3 days we harvested the dLN and quantitated the donor DCs. As seen in Fig. 7B, there were fewer infected TLR9−/− DCs, as compared with infected WT DCs, in the dLN.
Figure 7.
TLR9 expression in DCs is required and sufficient to induce LN hypertrophy. TLR9−/− DCs activated with L. major fail to induce LN hypertrophy. L. major infected DCs (5 × 105) from C57BL/6 (B6) or TLR9−/− mice, or uninfected DCs, were injected into the footpad of C57BL/6 mice, and the cellularity of the dLN assessed on day 3 (A). L. major infected DCs (1 × 106) from C57BL/6 (B6) or TLR9−/− mice, or uninfected DCs, were injected into the footpad of CD45.1 C57BL/6 mice, and the presence of the donor cells in the dLN assessed on day 3 (B). L. major infected DCs (1 × 106) from C57BL/6 (B6), or uninfected DCs, were injected into the footpad of C57BL/6 mice or TLR9−/− mice, and the cellularity of the dLN assessed on day 3 (C). The results expressed are the mean (+/− SE) number of cells in the dLN of 3 mice per group. The results are representative of 2 experiments.
While the results described above indicate that TLR9 expression in DCs is a critical component in the development of LN hypertrophy, it may be that other cells expressing TLR9 contribute to the response. To address this issue, we infected WT DCs and transferred them to either WT or TLR9−/− mice. We found that WT DCs promoted LN hypertrophy in both WT and TLR9−/− mice, indicating that TLR9 expression was only required in the DCs (Fig. 7C).
TLR9 deficient mice infected with L. major exhibit decreased LN hypertrophy for several weeks after infection
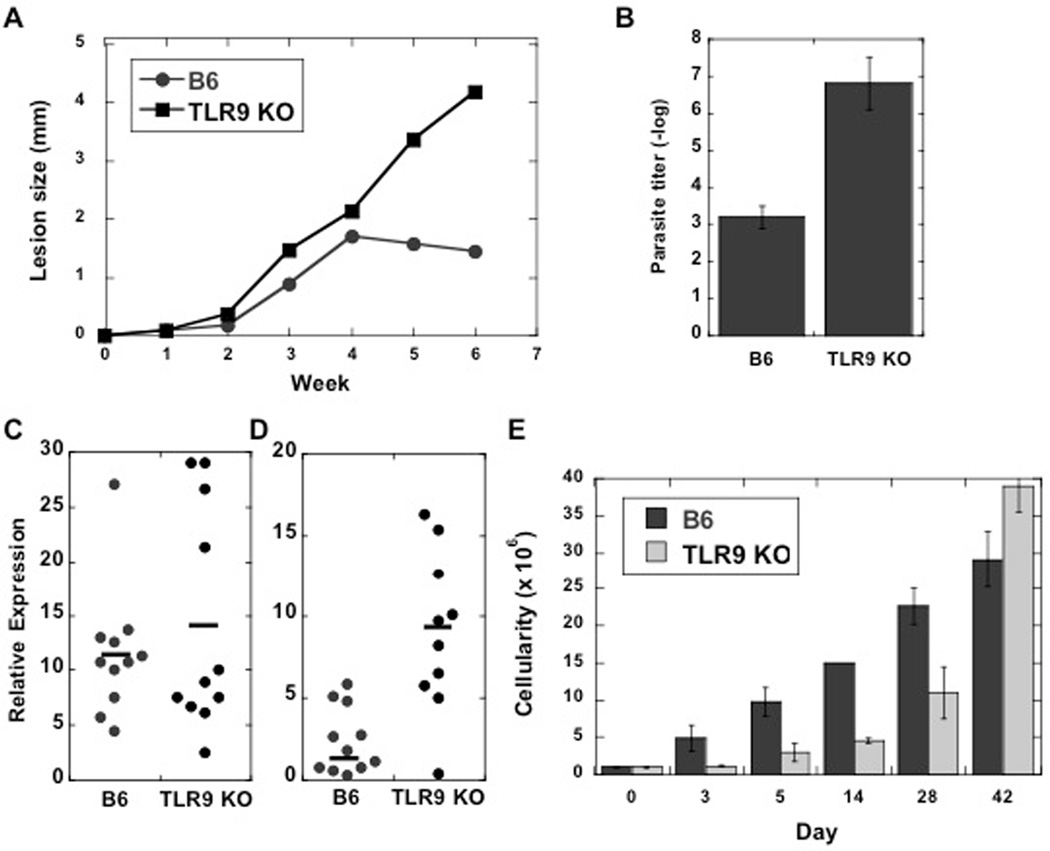
TLR9 deficient mice exhibit an increased susceptibility to L. major infection, and we were interested in determining if the deficit in LN hypertrophy extended beyond the initial few days of infection (23, 24). We infected C57BL/6 mice or TLR9−/− mice with L. major and monitored the course of infection. As reported, TLR9−/− mice develop significantly larger lesions than control mice, with substantially more parasites when assessed 6 weeks following infection (Fig. 8A, B). This increased susceptibility was associated with no significant difference in the IFN-γ mRNA levels between the controls and the TLR9−/− animals (Fig. 8C), but a significant increase in the levels of IL-4 mRNA in TLR9−/− mice (Fig. 8D). To assess whether the absence of TLR9 influenced the ability of L. major mice to induce LN hypertrophy over time, we quantitated the cellularity of the dLN during the course of infection. As seen in Fig. 8E, the dLNs from TLR9−/− were smaller than control mice throughout the first 4 weeks of infection. However, by 5 weeks, dLN cellularity in wild-type and TLR9−/− mice was similar, and as previously reported, the TLR9−/− mice were eventually able to resolve their infections (23, 24)(data not shown). Although we do not know what compensates for the absence of TLR9 at these later stages of the infection, we hypothesize that sufficient activated T cells may have slowly accumulated such that through CD40-CD40L interactions DCs become activated. This would be consistent with the findings that CD40 or CD40L is required for resistance to Leishmania parasites (28–30).
Figure 8.
TLR9−/− mice are more susceptible to L. major and exhibit a deficit in LN hypertrophy for several weeks following infection. (A) C57BL/6 (B6) or TLR9−/− mice were infected with L. major and the course of infection monitored. The results expressed are the mean (+/− SE) lesion size of 5 mice per group. (B) At 6 weeks the mice were sacrificed and parasites within the lesions were quantitated by limiting dilution. These data are representative of two experiments. (C) IFN-γ and (D) IL-4 mRNA levels were assayed by qrt-PCR on cells from the dLNs of mice sacrificed at 6 weeks. Each data point represents one mouse, and the line denotes the average values. IFN-γ levels were not significantly different. IL-4 levels were significantly different (p<0.05). The cellularity of the dLNs from B6 and TLR9−/− mice were assessed during the course of infection. The data shown is representative of 3 experiments.
Discussion
Establishing an immune response is dependent upon the ability of a small number of T cells to come in contact with a small number of DCs that are presenting the relevant antigens. Adjuvants increase the chances of a successful interaction between DCs and T cells following immunization, in part by promoting a rapid increase in the cellularity of the dLN. Thus, within the first few days following inoculation of an antigen/adjuvant complex there is an increase in the recruitment of lymphocytes to the LN draining the site of immunization (4, 5, 13). This increased LN cellularity, or LN hypertrophy, requires recognition of pathogen-associated molecules that leads to DC activation. Once activated the DCs migrate to the dLN and orchestrate the response. A similar response is important for establishing immunity to pathogens, and we previously reported that LN hypertrophy occurs rapidly following infection with L. major in mice. Indeed, we show here that mice failing to exhibit normal LN hypertrophy (due to the absence of CD62L) are unable to resolve their leishmanial lesions. In this study we sought to define the factors involved in L. major induced LN hypertrophy (8). We show that, as in immunization models, early LN hypertrophy is associated with rapid recruitment of cells from the blood to the dLNs following infection, that L. major parasites induce upregulation of CCR7 in DCs, and that injection of L. major activated DCs into the skin induces LN hypertrophy. Importantly, we found that TLR9 is critical in mediating these responses. This is the first study to define the TLR ligand required for L. major- induced LN hypertrophy.
The increased recruitment of lymphocytes to the dLN of L. major infected mice was sustained from day 1 through day 28. In contrast, it was recently found that following infection with Listeria or lymphocytic choriomeningitis virus mice exhibit a transient decrease in the ability to recruit cells to the LN, in part due to decreased production of CCL21 (31). When we assessed the protein levels of CCL21 in the dLN we saw no evidence of a decrease; and correspondingly, there was no inability to recruit cells to the responding dLNs. Since the deficit in the normal migration of cells in secondary lymphoid organs following Listeria or LCMV was found to depend upon the production of IFN-γ, we hypothesize that L. major fails to induce a transient decrease in cell recruitment to the dLN because the parasite induces only a relatively modest IFN-γ response during the first several weeks of L. major infection (32).
Several studies have established that DCs play a key role in promoting LN hypertrophy following experimental immunization (2–4). Thus, DC activation, and the subsequent CCR7-dependent migration to the dLN of the DCs, is essential to promote LN hypertrophy. Because recent studies show that Leishmania parasites–and DNA from the parasites–are capable of activating DCs in a TLR9 dependent manner (22–24), we tested whether TLR9 was required for LN hypertrophy. We found that the induction of LN hypertrophy was TLR9 dependent, and that the requirement for TLR9 was to promote DC activation, since L. major WT but not TLR9−/− DCs, induced LN hypertrophy. We also found that the expression of TLR9 on DCs was sufficient to induce LN hypertrophy, as infected WT DCs transferred to TLR9−/− mice lead to LN expansion. Finally, we found that commensals associated with the skin were unlikely to contribute to the response since germ-free mice exhibited similar LN hypertrophy following L. major infection as conventional mice.
The absence of TLR9 appeared to have an effect on LN hypertrophy for many weeks after the infection suggesting that the role of TLR9 is not limited to the early interactions between the parasite and the host. Eventually, however, the TLR9−/− mice are able to resolve their lesions, possibly suggesting that other factors compensate as the infection progresses. This might involve other TLRs, since TLR2, 3, 4 and 7 have all been implicated in resistance in various models of leishmaniasis (33–36), however why these would not compensate for the absence of TLR9 earlier is not clear. An alternative possibility is that as the infection goes on sufficient activated T cells expressing CD40L accumulate, leading to DC activation via CD40-CD40L interactions. This would be consistent with the requirement for CD40 or CD40L for resolution of Leishmania infections in mice (28–30).
CCR7 expression plays an important role in facilitating the migration of cells to the dLN (2, 20, 37). The ability of naive T cells to enter LNs from the blood requires CCR7, and CCR7 on DCs promotes their migration from the tissues to the draining LN. Interestingly, however, the requirement for CCR7 differs following infection with different pathogens. Listeria infections are still controlled in the absence of CCR7, while following Toxoplasma infection CCR7-deficient mice die (38). In leishmaniasis, CCR7 appears to be required for the development of an effective immune response in leishmaniasis. For example, L. major infected CCL21 transgenic mice–which lose CCR7 signaling due to constitutive downregulation–are unable to control the infection (9). Similarly, in visceral leishmaniasis, the absence of the CCR7 ligands CCL19 and CCL21, or the loss of CCR7 on DCs, was correlated with increased pathogenesis, while immunotherapy with CCR7-expressing DCs was therapeutic (39, 40). Previous studies have indicated that L. major induces CCR7 expression on DCs (41), and our results indicate that the induction of CCR7 by L. major is TLR9 dependent, as has been shown in other systems (25, 26). Surprisingly, however, by using DsRed parasites we also found that it was the bystander DCs that upregulated CCR7 expression, rather than the infected DCs. These results are similar to our previous findings showing that bystander DCs, rather than the infected DCs, exhibit increased expression of Class II and IL-12, although both the infected and uninfected DCs produced TNF-α (21). Studies are in progress to determine how the bystander DCs become activated, and why infected DCs are unable to upregulate CCR7 expression. However, since many studies have indicated that Leishmania can impair signaling pathways in macrophages (42), these results suggest that such impairment is overcome by activating neighboring cells.
Cutaneous leishmaniasis has a wide range of clinical presentations, which depend upon the genetics of both the host and the parasite. Patients can develop small lesions that resolve rapidly, chronic single lesions that may take years to heal, and severe metastatic lesions that may result from the lack of a response (diffuse cutaneous leishmaniasis) or an exaggerated immune response (mucosal leishmaniasis). In mice, L. major induces a healing lesion in C57BL/6 animals, but a fatal infection in BALB/c mice, while L. mexicana lesions in C57BL/6 mice fail to resolve (43, 44). We previously found an association between the capacity to induce LN hypertrophy and the chronicity of L. mexicana infections in mice (8). Since L. mexicana infected mice fail to develop a robust Th1 response, we initially reasoned that administration of IL-12 would promote healing, as it does in L. major infections. However, administration of IL-12 failed to promote healing, suggesting that the inability of mice to resolve a L. mexicana infection was not simply because of the lack of IL-12 (45). This result suggested that in addition to lacking IL-12, there was something else that was defective in L. mexicana infected mice. Because L. mexicana infected mice fail to induce LN hypertrophy, we hypothesize that even when given IL-12, there are too few T cells that are responding to the infection to promote resolution of the lesions. Studies are in progress to determine why L. mexicana fails to stimulate LN hypertrophy. However, in preliminary experiments we found no increase in the susceptibility of TLR9−/− mice to L. mexicana, suggesting that L. mexicana may fail to activate this pathway in vivo, which would account for the inability of L. mexicana to promote LN hypertrophy (unpublished data).
While failure to develop an optimal immune response can lead to chronic disease, an exaggerated immune response can also lead to severe disease. One example where there is an association between increased LN hypertrophy and the immune response is in patients infected with L. braziliensis. Infections caused by L. braziliensis are associated with a very strong immune response, involving substantial T cell proliferation and high levels of IFN-γ production (46). Moreover, in a small number of cases the parasites metastasize and the patients develop a severe non-healing form of the disease known as mucosal leishmaniasis. Interestingly, human L. braziliensis infections are often associated with a massive lymphadenopathy, which often is seen prior to the development of the primary lesion (11, 12). Understanding the mechanisms involved in LN hypertrophy is the first step in designing strategies that might lessen the exaggerated and immunopathologic response seen in these patients.
The generation of an optimal immune response depends upon ensuring that both the magnitude and type of response is appropriate to the threat. A major focus of research in experimental leishmaniasis has been on ensuring that a Th1 type response is elicited, as Th1 responses are most appropriate to eliminate Leishmania major parasites. However, in addition to getting the appropriate response, the magnitude of the response can be just as important in resolving the disease. In human leishmaniasis, chronic disease can result due to either a minimal response that fails to eliminate the parasites, or an exaggerated response where severe inflammation is uncontrolled, such as occurs in mucocutaneous leishmaniasis patients. Since the magnitude of the immune response can be influenced by LN hypertrophy, it is important to better understand how this response is regulated. We believe that defining TLR9 as a critical component for LN hypertrophy is a first step in considering how to design approaches focused on modulating the magnitude of the immune response in leishmaniasis.
Acknowledgements
The authors thank Dr. Larry Turka for the TLR9−/− mice and Dr. Yasmine Belkaid for the DsRed L. major parasites. The authors also wish to acknowledge the technical assistance of Carlos Rodriguez, Sarah Wensley and Lijun Wen.
This work was supported by the National Institutes of Health Grant AI076257 (to P.S.) and Training Grant AI055400-06 (to P.P.).
Abbreviations used in this paper
- DC
dendritic cell
- dLN
draining lymph node
References
- 1.Scott P, Artis D, Uzonna J, Zaph C. The development of effector and memory T cells in cutaneous leishmaniasis: the implications for vaccine development. Immunol Rev. 2004;201:318–338. doi: 10.1111/j.0105-2896.2004.00198.x. [DOI] [PubMed] [Google Scholar]
- 2.MartIn-Fontecha A, Sebastiani S, Hopken UE, Uguccioni M, Lipp M, Lanzavecchia A, Sallusto F. Regulation of dendritic cell migration to the draining lymph node: impact on T lymphocyte traffic and priming. J Exp Med. 2003;198:615–621. doi: 10.1084/jem.20030448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angeli V, Randolph GJ. Inflammation, lymphatic function, and dendritic cell migration. Lymphat Res Biol. 2006;4:217–228. doi: 10.1089/lrb.2006.4406. [DOI] [PubMed] [Google Scholar]
- 4.Webster B, Ekland EH, Agle LM, Chyou S, Ruggieri R, Lu TT. Regulation of lymph node vascular growth by dendritic cells. J Exp Med. 2006;203:1903–1913. doi: 10.1084/jem.20052272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soderberg KA, Payne GW, Sato A, Medzhitov R, Segal SS, Iwasaki A. Innate control of adaptive immunity via remodeling of lymph node feed arteriole. Proc Natl Acad Sci U S A. 2005;102:16315–16320. doi: 10.1073/pnas.0506190102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruddle NH, Akirav EM. Secondary lymphoid organs: responding to genetic and environmental cues in ontogeny and the immune response. J Immunol. 2009;183:2205–2212. doi: 10.4049/jimmunol.0804324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guarda G, Hons M, Soriano SF, Huang AY, Polley R, Martin-Fontecha A, Stein JV, Germain RN, Lanzavecchia A, Sallusto F. L-selectin-negative CCR7- effector and memory CD8+ T cells enter reactive lymph nodes and kill dendritic cells. Nat Immunol. 2007;8:743–752. doi: 10.1038/ni1469. [DOI] [PubMed] [Google Scholar]
- 8.Hsu AC, Scott P. Leishmania mexicana infection induces impaired lymph node expansion and Th1 cell differentiation despite normal T cell proliferation. J Immunol. 2007;179:8200–8207. doi: 10.4049/jimmunol.179.12.8200. [DOI] [PubMed] [Google Scholar]
- 9.Unsoeld H, Mueller K, Schleicher U, Bogdan C, Zwirner J, Voehringer D, Pircher H. Abrogation of CCL21 chemokine function by transgenic over-expression impairs T cell immunity to local infections. Int Immunol. 2007;19:1281–1289. doi: 10.1093/intimm/dxm098. [DOI] [PubMed] [Google Scholar]
- 10.Ehrchen JM, Roth J, Roebrock K, Varga G, Domschke W, Newberry R, Sorg C, Muller-Tidow C, Sunderkotter C, Kucharzik T, Spahn TW. The absence of cutaneous lymph nodes results in a Th2 response and increased susceptibility to Leishmania major infection in mice. Infect Immun. 2008;76:4241–4250. doi: 10.1128/IAI.01714-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barral A, Guerreiro J, Bomfim G, Correia D, Barral-Netto M, Carvalho EM. Lymphadenopathy as the first sign of human cutaneous infection by Leishmania braziliensis. Am J Trop Med Hyg. 1995;53:256–259. doi: 10.4269/ajtmh.1995.53.256. [DOI] [PubMed] [Google Scholar]
- 12.Barral A, Barral-Netto M, Almeida R, de Jesus AR, Grimaldi Junior G, Netto EM, Santos I, Bacellar O, Carvalho EM. Lymphadenopathy associated with Leishmania braziliensis cutaneous infection. Am J Trop Med Hyg. 1992;47:587–592. doi: 10.4269/ajtmh.1992.47.587. [DOI] [PubMed] [Google Scholar]
- 13.Angeli V, Ginhoux F, Llodra J, Quemeneur L, Frenette PS, Skobe M, Jessberger R, Merad M, Randolph GJ. B cell-driven lymphangiogenesis in inflamed lymph nodes enhances dendritic cell mobilization. Immunity. 2006;24:203–215. doi: 10.1016/j.immuni.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Liao S, Ruddle NH. Synchrony of high endothelial venules and lymphatic vessels revealed by immunization. J Immunol. 2006;177:3369–3379. doi: 10.4049/jimmunol.177.5.3369. [DOI] [PubMed] [Google Scholar]
- 15.Kimblin N, Peters N, Debrabant A, Secundino N, Egen J, Lawyer P, Fay MP, Kamhawi S, Sacks D. Quantification of the infectious dose of Leishmania major transmitted to the skin by single sand flies. Proc Natl Acad Sci U S A. 2008;105:10125–10130. doi: 10.1073/pnas.0802331105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spath GF, Beverley SM. A lipophosphoglycan-independent method for isolation of infective Leishmania metacyclic promastigotes by density gradient centrifugation. Exp Parasitol. 2001;99:97–103. doi: 10.1006/expr.2001.4656. [DOI] [PubMed] [Google Scholar]
- 17.Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 18.Bradley LM, Watson SR, Swain SL. Entry of naive CD4 T cells into peripheral lymph nodes requires L-selectin. J Exp Med. 1994;180:2401–2406. doi: 10.1084/jem.180.6.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laskay T, Wittmann I, Diefenbach A, Rollinghoff M, Solbach W. Control of Leishmania major infection in BALB/c mice by inhibition of early lymphocyte entry into peripheral lymph nodes. J Immunol. 1997;158:1246–1253. [PubMed] [Google Scholar]
- 20.Randolph GJ. Dendritic cell migration to lymph nodes: cytokines, chemokines, and lipid mediators. Semin Immunol. 2001;13:267–274. doi: 10.1006/smim.2001.0322. [DOI] [PubMed] [Google Scholar]
- 21.Carvalho LP, Pearce EJ, Scott P. Functional dichotomy of dendritic cells following interaction with Leishmania braziliensis: infected cells produce high levels of TNF-alpha, whereas bystander dendritic cells are activated to promote T cell responses. J Immunol. 2008;181:6473–6480. doi: 10.4049/jimmunol.181.9.6473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schleicher U, Liese J, Knippertz I, Kurzmann C, Hesse A, Heit A, Fischer JA, Weiss S, Kalinke U, Kunz S, Bogdan C. NK cell activation in visceral leishmaniasis requires TLR9, myeloid DCs, and IL-12, but is independent of plasmacytoid DCs. J Exp Med. 2007;204:893–906. doi: 10.1084/jem.20061293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liese J, Schleicher U, Bogdan C. TLR9 signaling is essential for the innate NK cell response in murine cutaneous leishmaniasis. Eur J Immunol. 2007;37:3424–3434. doi: 10.1002/eji.200737182. [DOI] [PubMed] [Google Scholar]
- 24.Abou Fakher FH, Rachinel N, Klimczak M, Louis J, Doyen N. TLR9-dependent activation of dendritic cells by DNA from Leishmania major favors Th1 cell development and the resolution of lesions. J Immunol. 2009;182:1386–1396. doi: 10.4049/jimmunol.182.3.1386. [DOI] [PubMed] [Google Scholar]
- 25.Asselin-Paturel C, Brizard G, Chemin K, Boonstra A, O'Garra A, Vicari A, Trinchieri G. Type I interferon dependence of plasmacytoid dendritic cell activation and migration. J Exp Med. 2005;201:1157–1167. doi: 10.1084/jem.20041930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pichyangkul S, Yongvanitchit K, Kum-arb U, Hemmi H, Akira S, Krieg AM, Heppner DG, Stewart VA, Hasegawa H, Looareesuwan S, Shanks GD, Miller RS. Malaria blood stage parasites activate human plasmacytoid dendritic cells and murine dendritic cells through a Toll-like receptor 9-dependent pathway. J Immunol. 2004;172:4926–4933. doi: 10.4049/jimmunol.172.8.4926. [DOI] [PubMed] [Google Scholar]
- 27.Grice EA, Kong HH, Conlan S, Deming CB, Davis J, Young AC, Bouffard GG, Blakesley RW, Murray PR, Green ED, Turner ML, Segre JA. Topographical and temporal diversity of the human skin microbiome. Science. 2009;324:1190–1192. doi: 10.1126/science.1171700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soong L, Xu JC, Grewal IS, Kima P, Sun J, Longley BJ, Jr, Ruddle NH, McMahon-Pratt D, Flavell RA. Disruption of CD40-CD40 ligand interactions results in an enhanced susceptibility to Leishmania amazonensis infection. Immunity. 1996;4:263–273. doi: 10.1016/s1074-7613(00)80434-3. [DOI] [PubMed] [Google Scholar]
- 29.Kamanaka M, Yu P, Yasui T, Yoshida K, Kawabe T, Horii T, Kishimoto T, Kikutani H. Protective role of CD40 in Leishmania major infection at two distinct phases of cell-mediated immunity. Immunity. 1996;4:275–281. doi: 10.1016/s1074-7613(00)80435-5. [DOI] [PubMed] [Google Scholar]
- 30.Campbell KA, Ovendale PJ, Kennedy MK, Fanslow WC, Reed SG, Maliszewski CR. CD40 ligand is required for protective cell-mediated immunity to Leishmania major. Immunity. 1996;4:283–289. doi: 10.1016/s1074-7613(00)80436-7. [DOI] [PubMed] [Google Scholar]
- 31.Mueller SN, Hosiawa-Meagher KA, Konieczny BT, Sullivan BM, Bachmann MF, Locksley RM, Ahmed R, Matloubian M. Regulation of homeostatic chemokine expression and cell trafficking during immune responses. Science. 2007;317:670–674. doi: 10.1126/science.1144830. [DOI] [PubMed] [Google Scholar]
- 32.Scott P, Eaton A, Gause WC, di Zhou X, Hondowicz B. Early IL-4 production does not predict susceptibility to Leishmania major. Exp Parasitol. 1996;84:178–187. doi: 10.1006/expr.1996.0103. [DOI] [PubMed] [Google Scholar]
- 33.Becker I, Salaiza N, Aguirre M, Delgado J, Carrillo-Carrasco N, Kobeh LG, Ruiz A, Cervantes R, Torres AP, Cabrera N, Gonzalez A, Maldonado C, Isibasi A. Leishmania lipophosphoglycan (LPG) activates NK cells through toll-like receptor-2. Mol Biochem Parasitol. 2003;130:65–74. doi: 10.1016/s0166-6851(03)00160-9. [DOI] [PubMed] [Google Scholar]
- 34.Kropf P, Freudenberg MA, Modolell M, Price HP, Herath S, Antoniazi S, Galanos C, Smith DF, Muller I. Toll-like receptor 4 contributes to efficient control of infection with the protozoan parasite Leishmania major. Infect Immun. 2004;72:1920–1928. doi: 10.1128/IAI.72.4.1920-1928.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paun A, Bankoti R, Joshi T, Pitha PM, Stager S. Critical role of IRF-5 in the development of T helper 1 responses to Leishmania donovani infection. PLoS Pathog. 2011;7:e1001246. doi: 10.1371/journal.ppat.1001246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Flandin JF, Chano F, Descoteaux A. RNA interference reveals a role for TLR2 and TLR3 in the recognition of Leishmania donovani promastigotes by interferon-gamma-primed macrophages. Eur J Immunol. 2006;36:411–420. doi: 10.1002/eji.200535079. [DOI] [PubMed] [Google Scholar]
- 37.Forster R, Davalos-Misslitz AC, Rot A. CCR7 and its ligands: balancing immunity and tolerance. Nat Rev Immunol. 2008;8:362–371. doi: 10.1038/nri2297. [DOI] [PubMed] [Google Scholar]
- 38.Noor S, Habashy AS, Nance JP, Clark RT, Nemati K, Carson MJ, Wilson EH. CCR7-dependent immunity during acute Toxoplasma gondii infection. Infect Immun. 2010;78:2257–2263. doi: 10.1128/IAI.01314-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ato M, Maroof A, Zubairi S, Nakano H, Kakiuchi T, Kaye PM. Loss of dendritic cell migration and impaired resistance to Leishmania donovani infection in mice deficient in CCL19 and CCL21. J Immunol. 2006;176:5486–5493. doi: 10.4049/jimmunol.176.9.5486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ato M, Stager S, Engwerda CR, Kaye PM. Defective CCR7 expression on dendritic cells contributes to the development of visceral leishmaniasis. Nat Immunol. 2002;3:1185–1191. doi: 10.1038/ni861. [DOI] [PubMed] [Google Scholar]
- 41.Steigerwald M, Moll H. Leishmania major modulates chemokine and chemokine receptor expression by dendritic cells and affects their migratory capacity. Infect Immun. 2005;73:2564–2567. doi: 10.1128/IAI.73.4.2564-2567.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Olivier M, Gregory DJ, Forget G. Subversion mechanisms by which Leishmania parasites can escape the host immune response: a signaling point of view. Clin Microbiol Rev. 2005;18:293–305. doi: 10.1128/CMR.18.2.293-305.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alexander J, Kaye PM. Immunoregulatory pathways in murine leishmaniasis: different regulatory control during Leishmania mexicana mexicana and Leishmania major infections. Clin Exp Immunol. 1985;61:674–682. [PMC free article] [PubMed] [Google Scholar]
- 44.Buxbaum LU, Denise H, Coombs GH, Alexander J, Mottram JC, Scott P. Cysteine protease B of Leishmania mexicana inhibits host Th1 responses and protective immunity. J Immunol. 2003;171:3711–3717. doi: 10.4049/jimmunol.171.7.3711. [DOI] [PubMed] [Google Scholar]
- 45.Buxbaum LU, Uzonna JE, Goldschmidt MH, Scott P. Control of New World cutaneous leishmaniasis is IL-12 independent but STAT4 dependent. Eur J Immunol. 2002;32:3206–3215. doi: 10.1002/1521-4141(200211)32:11<3206::AID-IMMU3206>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 46.Carvalho E, Correia Filho MD, Bacellar O, Almeida RP, Lessa H, Rocha H. Characterization of the immune response in subjects with self-healing cutaneous leishmaniasis. Am J Trop Med Hyg. 1995;53:273–277. doi: 10.4269/ajtmh.1995.53.273. [DOI] [PubMed] [Google Scholar]