Abstract
Background
Early versus delayed autologous stem cell transplantation (SCT) results in comparable overall survival in multiple myeloma (MM) with alkylator-based therapies. It is not clear if this paradigm holds true in the context of new therapies such as immunomodulatory agents (IMiDs).
Methods
We studied 290 patients with untreated MM who received IMiD-based initial therapy; 123 with thalidomide-dexamethasone (TD) and 167 with lenalidomide-dexamethasone (LD) induction prior to SCT. Patients undergoing a stem cell harvest attempt were considered transplant eligible and included. SCT within 12 months of diagnosis and within 2 months of harvest were considered as early-SCT (n=173, 60%). SCT more than 12 months after diagnosis was considered delayed-SCT (n=112, 40%).
Results
In the delayed-SCT group 42 patients have so far undergone SCT; the median estimated time to SCT was 5.3 and 44.5 months among the early and delayed-SCT groups respectively. The 4-year OS from diagnosis was 73% in both early-SCT and delayed-SCT groups (P=0.3), and was comparable within those receiving TD (68% vs. 64%) or LD (82% vs. 86%) as initial therapy. The time to progression after SCT was similar between the early and delayed-SCT groups (20 vs. 16 months, P=NS).
Conclusion
We conclude that in transplant eligible patients receiving IMiDs as initial therapy followed by early stem cell mobilization, delayed-SCT results in similar overall survival compared with early-SCT. Importantly, we also demonstrate excellent 4-year survival of over 80% among transplant eligible patients receiving initial therapy with lenalidomide and dexamethasone regardless of the timing of the transplantation.
Keywords: Multiple Myeloma, stem cell transplantation, lenalidomide, thalidomide, delayed transplantation, survival
INTRODUCTION
The treatment approaches for multiple myeloma have undergone a dramatic change in the past decade, primarily as a result of the introduction of effective new drugs such as thalidomide, lenalidomide and bortezomib.1 Prior to the introduction of these drugs, the standard approach for patients with newly diagnosed myeloma eligible for high dose therapy has been initial therapy with dexamethasone alone or combination regimens like vincristine, adriamycin, and dexamethasone (VAD) for 4–6 months, followed by high dose melphalan and peripheral blood stem cell transplantation (SCT).2 This approach was supported by phase 3 trials demonstrating a survival improvement for SCT compared to conventional chemotherapy, primarily in patients younger than 65.3, 4 Subsequent experience suggested that patients older than 65, who are eligible for SCT also benefit from this treatment approach.5 One randomized trial also showed comparable efficacy for SCT applied early in the course of the disease or used as salvage therapy following failure of initial line of therapy.6 However, early SCT was associated with better quality of life parameters such as time without symptoms and therapy related toxicity and an early SCT was generally favored.6
The introduction of novel agents had a profound impact on the initial therapy of myeloma.7–9 Combinations of novel agents with dexamethasone, or with other conventional agents, result in response rates comparable to those seen in the context of SCT.8, 10, 11 This degree of efficacy along with the tolerability of these regimens given long term has leveled the advantages seen with early SCT and has led to increased use of SCT in a deferred fashion.12 In fact, in many of the recent trials that did not include early SCT as part of the treatment protocol more than half of the patients have opted to continue with induction therapy in favor of a delayed SCT.8 While this approach was shown to be comparable to an early SCT in the previous trials using alkylator or steroid based induction therapies, there is no comparable data in the context of induction regimens containing novel agents. There are several potential concerns extrapolating the previous data in the context of current regimens. In particular, it is not clear if patients relapsing after newer therapies will be able to obtain a meaningful response from high dose melphalan and if patients will be losing the opportunity to benefit from an effective treatment modality such as SCT. While comparisons of patients going to early SCT to those who decided to stay on initial therapy in recent clinical trials have suggested excellent outcome for both groups,8 it is not clear if the two approaches would provide equivalent benefits to patients with newly diagnosed myeloma. We examined this question in a group of patients with newly diagnosed myeloma seen at out institution that underwent induction with an IMiD-Dex combination (thalidomide-dexamethasone or lenalidomide dexamethasone).
PATIENTS AND METHODS
Four hundred and ten consecutive patients seen at Mayo Clinic between 2001 and 2008 with newly diagnosed multiple myeloma (MM), who received initial therapy with thalidomide and dexamethasone (Thal-Dex) or lenalidomide and dexamethasone (Len-Dex) formed the initial patient group for this study. Two hundred ninety patients (71%), in whom a stem cell harvest was attempted, were considered as transplant eligible and included in the current study. Patients who were started on growth factors for mobilization were included regardless of the outcome of the stem cell mobilization attempt. Patients who underwent SCT within 12 months of diagnosis and within 2 months of stem cell harvest (n=178; 61%) were considered as having an early SCT (Early group). The remaining patients (n=112; 39%), irrespective of whether an SCT was eventually performed, were considered as having a delayed SCT approach (Delayed-SCT group). Data pertaining to the patients were captured prospectively into a database, which is continuously updated and complete follow-up was available for all the patients. A small proportion of patients did not get their SCT at our institution, and response data pertaining to SCT were not available for several of these patients. All patients had provided written informed consent for use of their medical records. Approval from the Mayo Foundation Institutional Review Board was obtained in accordance with federal regulations and the Declaration of Helsinki.
The choice of the regimen depended on the time period of diagnosis and was dictated by availability of clinical trials and commercial availability of the drugs. Our standard induction regimen outside of clinical trials was Thalidomide and dexamethasone until lenalidomide became available commercially, when it was switched to lenalidomide and dexamethasone. Thalidomide was given daily at 100–400 mg along with dexamethasone 40 mg days 1–4, 9–12, and 17–20 of a 28-day cycle. Lenalidomide was given at standard dose of 25 mg daily days 1–21 along with dexamethasone either as described with thalidomide or at 40 mg weekly according to prevailing practice. The weekly dexamethasone has been the standard practice since the initial results of the E4A03 clinical trial that demonstrated better survival with the weekly dexamethasone.8 Patients routinely received thromboprophylaxis with aspirin, and in patients perceived to be at higher risk for thromboembolic events, full anticoagulation with coumadin or low molecular weight heparin was used. Bisphosphonates were routinely administered for prophylaxis against skeletal events in accordance with standard clinical practice.
Patients typically proceeded to stem cell collection following 4–6 cycles of initial therapy with one of these regimens. Stem cells were normally collected using G-CSF priming alone with cyclophosphamide and G-CSF limited to those patients with poor response to initial therapy and in those with circulating plasma cells at the time of collection as per our clinical practice. Chemotherapy based mobilization also was used for subsequent attempts at mobilization in patients who failed the initial attempt. G-CSF was administered subcutaneously (10ug/kg) daily until the completion of peripheral blood stem cell collection with apheresis beginning on the fifth day after starting G-CSF, provided adequate peripheral blood CD34 counts were achieved. The remaining patients had stem cells collected after administration of cyclophosphamide 1.5 g/m2 per day for 2 consecutive days, followed by G-CSF at 10 ug/kg starting on day 3 and continuing through the period of granulocytopenia. A small number of patients also received plerixafor for mobilization, primarily in the past two years, for rescue attempts. All patients undergoing SCT received conditioning with melphalan alone, usually given at 200 mg/m2 divided over two days (100 mg/m2 days -2 and -1). In a few patients (n=15) melphalan was dose reduced to 140 mg/m2 because of advanced age, renal insufficiency or poor performance status. This was followed by reinfusion of all or part of the initially collected stem cells.
Responses were determined according to the International Myeloma Working Group Uniform response criteria.13 BM plasma cell labeling index (PCLI), cytogenetics, Beta 2-microglobulin and other laboratory variables were assessed pre-transplantation. PCLI (a measure of the cell proliferation) was determined using a slide-based immunoflourescence method on BM samples, as described previously and was classified as high when >1%.14
The Chi-square and Fisher exact tests were used to compare differences between nominal variables and the Mann–Whitney U-test or Kruskal–Wallis test were used for continuous variables. Kaplan–Meier analysis was used for analyzing overall and progression free survival. Differences between survival curves were tested for statistical significance using the two-tailed log-rank test or Breslow-Gehan test. Time to progression (TTP) was measured as time from transplantation to disease progression, with those dying without evidence of relapse censored at the time of death. Progression free survival (PFS) was measured as time from transplantation to disease progression or death due to any cause, with those alive and relapse free censored at last follow up. Overall survival (OS) was defined as the time from transplantation or from the date of initial diagnosis of myeloma to the date of death or last follow-up, as the case may be.
RESULTS
The median estimated follow-up for the 290 patients included in the study was 40 months (95% CI; 36.6, 42.6) from diagnosis, 42 months (95% CI; 40.1, 47.5) and 34 months (95% CI; 23.8, 39.5) respectively for the early (n=178) and delayed groups (n=112). Two hundred twenty six (78%) patients were alive at the time of analysis, with the majority of deaths related to disease progression. The baseline characteristics from diagnosis are shown in Table 1 and were similar between the two groups with the exception of higher LDH in the early transplant group. The median time to stem cell harvest was shorter among those going to an early SCT (4.9 months vs. 6.8 months; P <0.001) compared to the delayed-SCT group. Among the 112 patients in the delayed-SCT group, 41 patients (37%) have undergone SCT for relapsed or refractory disease. Sixty-nine patients in this group have received a second line therapy, including SCT. Six patients in the delayed-SCT group have died without undergoing an SCT. As expected, the median estimated time to SCT was 5.3 months (95% CI; 5.1, 5.5) among the early group compared to 44.5 months (95% CI; 28.5, 54.2) in the delayed-SCT group. Among the entire patient group, the initial therapy was Thal-Dex in 123 (43%) patients while 167 (57%) received Len-Dex. Among the 290 patients, 86 (70%) patients receiving Thal-Dex had an early SCT compared to 91 (55%) patients receiving Len-Dex; P=0.01.
Table 1.
Baseline characteristics from diagnosis
| Variable | Early SCT (n=178) |
Deferred SCT (n=112) |
P |
|---|---|---|---|
| Age at Diagnosis (Years) | 58 (29–73) | 61 (32–74) | NS |
| Gender (Male) | 57% | 66% | NS |
| Hemoglobin (gm/dL) | 10.9 (7.9–15.9) | 11.4 (8.1–14.6) | NS |
| Platelets (million/mm3) | 140 (11–418) | 154 (21–543) | NS |
| Calcium (mg/dL) | 9.1 (7–11) | 9.2 (6.8–10.7) | NS |
| Serum Creatinine (mg/dL) | 0.9 (0.5–3.7) | 1 (0.6–6.7) | NS |
| LDH (U/L) | 228 | 185 | < 0.001 |
| Marrow plasma cell % | 43 (2–90) | 40 (1–80) | NS |
| ISS: Stage 1 | 39 | 45 | NS |
| Stage 2 | 36 | 39 | |
| Stage 3 | 25 | 16 | |
| Plasma cell labeling index >= 1% | 26% | 28% | NS |
Response to primary therapy and overall survival
We first examined the outcome of patients based on the treatment approach taken, early versus delayed-SCT. A higher proportion of patients in the delayed-SCT group had deeper responses at the time of stem cell harvest; 32% of the delayed-SCT group had a VGPR or better compared to 16% of the early group (P < 0.01) (Figure 1A). The median duration of IMiD therapy was 4 months among the early group compared to 9 months among the delayed-SCT group. The 4-year overall survival estimate from diagnosis was similar for the early and the delayed-SCT group; 72.7% (95% CI; 65, 82) vs. 73.4% (95% CI; 63, 88) respectively; P = 0.3 (Figure 1B). We also compared the OS from diagnosis between the early and delayed-SCT group, based on the initial regimen. Among patients treated initially with Thal-Dex, the 4 - year OS estimate from diagnosis was 67.8% for the early group and 63.6% for the delayed-SCT group, P = 0.5 (Figure 1C). Similarly, among patients receiving Len-Dex the estimated 4 year OS was 82% for the early group compared to 86% for the delayed-SCT group, P = 0.7 (Figure 1D). We also examined the time to progression after “initial line of therapy”; induction followed by SCT or continued initial therapy with plan for delayed-SCT. The median TTP after initial line of therapy was comparable for the two groups; 25.4 months (95% CI; 23, 29) for the early group compared to 26.0 months (95% CI; 20, 36) for the delayed-SCT group (P = 0.9). Among the 69 patients in the delayed group who had a second line therapy, 42 patients have so far undergone an SCT while other patients have pursued non-SCT options until now. No OS difference has been noted between those who have received a SCT and those who have not received a SCT so far (data not shown).
Figure 1.
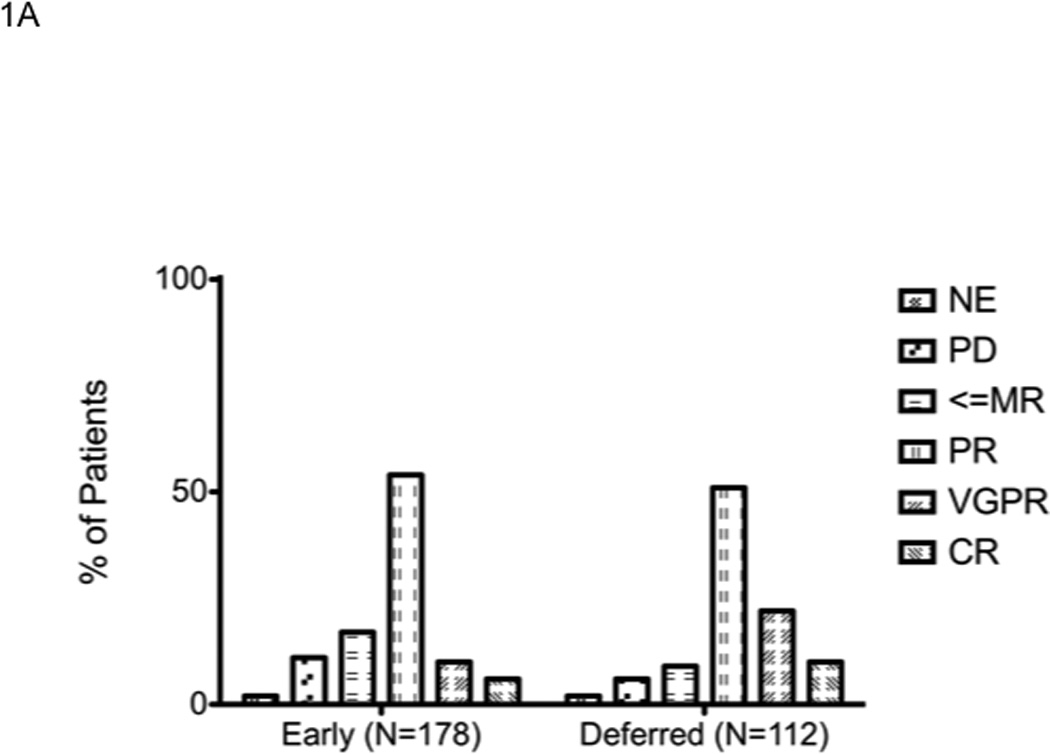
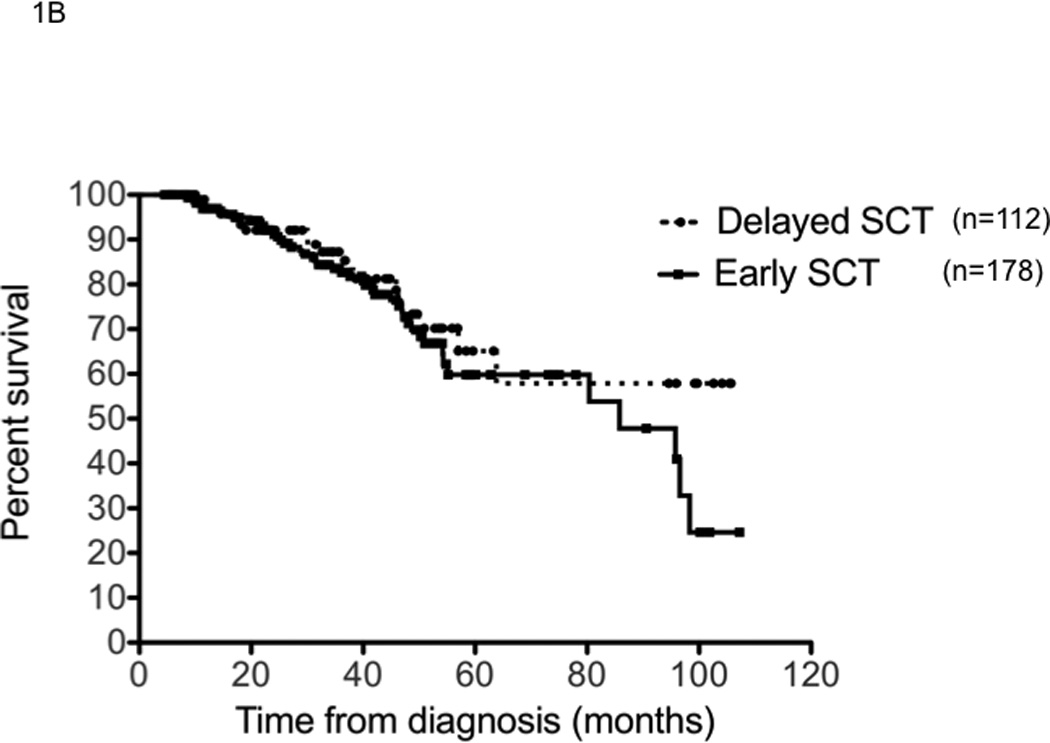
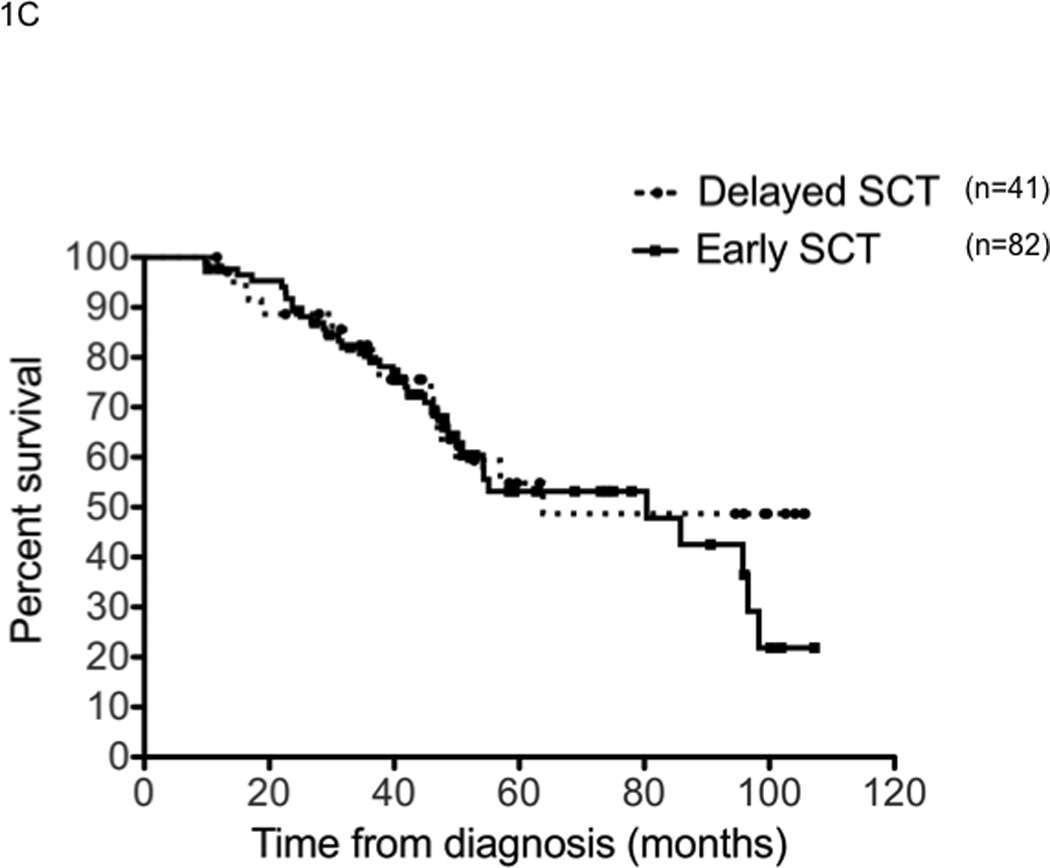
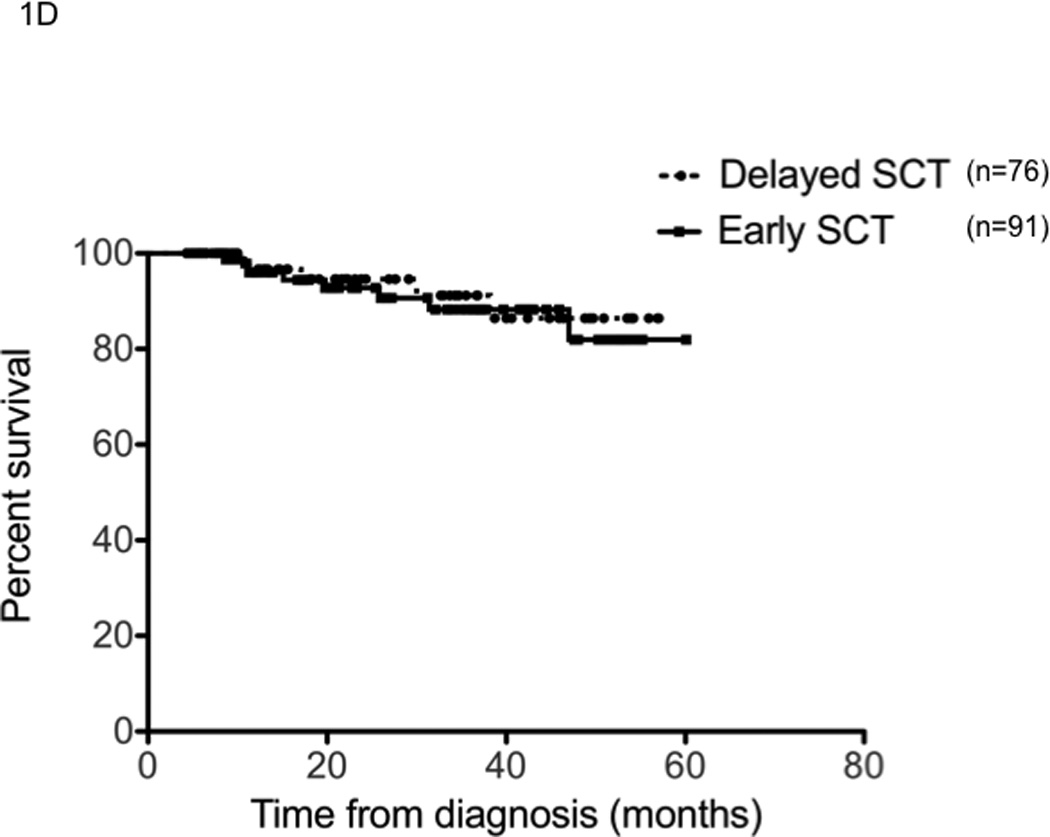
Panel A represents the response status at the time of stem cell harvest. X-axis represents the response category (NE: not evaluable; PD: progressive disease; <=MR: Minimal response or less; PR: partial response; VGPR: very good partial response; and CR: complete response) in that order (left to right) for each group of patients. Y-axis represents the proportion of patients in each response category, within each patient group. Panel B represents the overall survival from the initiation the first therapy for diagnosis of multiple myeloma for the entire patient group (n=290). Panel C represents the overall survival from the initiation the first therapy for diagnosis of multiple myeloma for patients receiving initial therapy with thalidomide and dexamethasone (n=123). Panel D represents the overall survival from the initiation the first therapy for diagnosis if multiple myeloma for patients receiving initial therapy with lenalidomide and dexamethasone (n=167).
Since the stem cell collection represented the decision-making time point for an early or delayed-SCT approach, we also examined the overall survival from that time. The 4 year overall survival estimate in the early group was 63% compared to 69% in the delayed-SCT group, P = 0.4. The survival estimates was similar between the two groups irrespective of the type of IMiD used for induction.
Efficacy of SCT
We then examined if the response to high dose therapy was comparable between those receiving an early or delayed-SCT. Forty-two patients (37%) in the delayed-SCT group who received a transplant were compared to patients in the early group (n=178). The clinical features at the time of SCT are described in Table 2. The patients going to SCT in the delayed-SCT group had higher beta 2 microglobulin and higher rate of proliferation as would be expected since more patients in this group would have relapsed disease. The overall response rate to SCT was similar between the two groups; 92% in the early group compared to 87% in the delayed-SCT group (P = NS). The complete response rate also was similar; 35% vs. 37% for the early vs. delayed-SCT group (P=NS). The engraftment kinetics were similar between the two groups with median time to neutrophil and platelet engraftment of 13 (10–34) and 15 (11–528) days respectively for the early group and 13 (10–19) and 15 (11–53) days for the delayed-SCT group.
Table 2.
Characteristics at transplant
| Variable | Early SCT (n=178) | Deferred SCT (n=42) |
P |
|---|---|---|---|
| Age at SCT (Years) | 58 (29–74) | 62 (36–76) | NS |
| Gender (Male) (%) | 58% | 60% | NS |
| Serum M protein (g/dL) | 0.7 (0–4.4) | 0.6 (0–7.3) | NS |
| Urine M protein (g/24 hours) | 0.06 (0–6.1) | 0.02 (0–1.2) | NS |
| Serum Creatinine (mg/dL) | 0.9 (0.5–3.9) | 1 (0.6–4.8) | NS |
| Beta 2 microglobulin > 3.5 mg/dL (%) | 13% | 30% | 0.03 |
| LDH (U/L) | 178 (116–853) | 179 (109–1216) | NS |
| Marrow plasma cell % | 7 (0–75) | 10 (1–67) | NS |
| Plasma cell labeling index >= 1% (%) | 20.00% | 41% | 0.005 |
The median time to progression following SCT was 19.7 months (95% CI; 16,22) for early group compared to 18 months (95% CI; 13,21) for the delayed-SCT group (P=0.4) (Figure 2A). This was true irrespective of whether they received thalidomide or lenalidomide. The median TTP following SCT was 25.3 months (95% CI, 22, 29) from diagnosis in the early group. The progression free survival was also comparable between the groups; 19.7 months (95% CI; 6,22) for the early group compared to 15.9 months (95% CI; 12,21) for the delayed-SCT group, P=0.09 (Figure 2B). There was a trend towards better OS post SCT for the early group, 65.7% vs. 56.3% at 4 years; P = 0.03 (Breslow Gehan) (Figure 2C).
Figure 2.
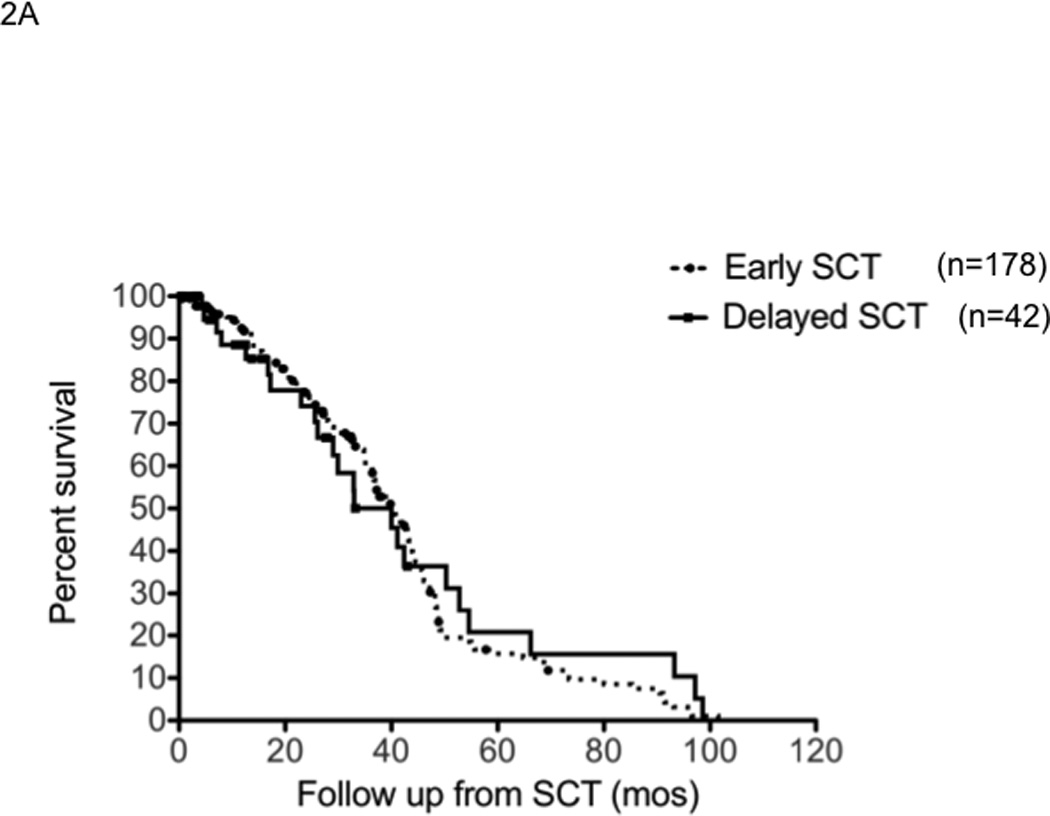
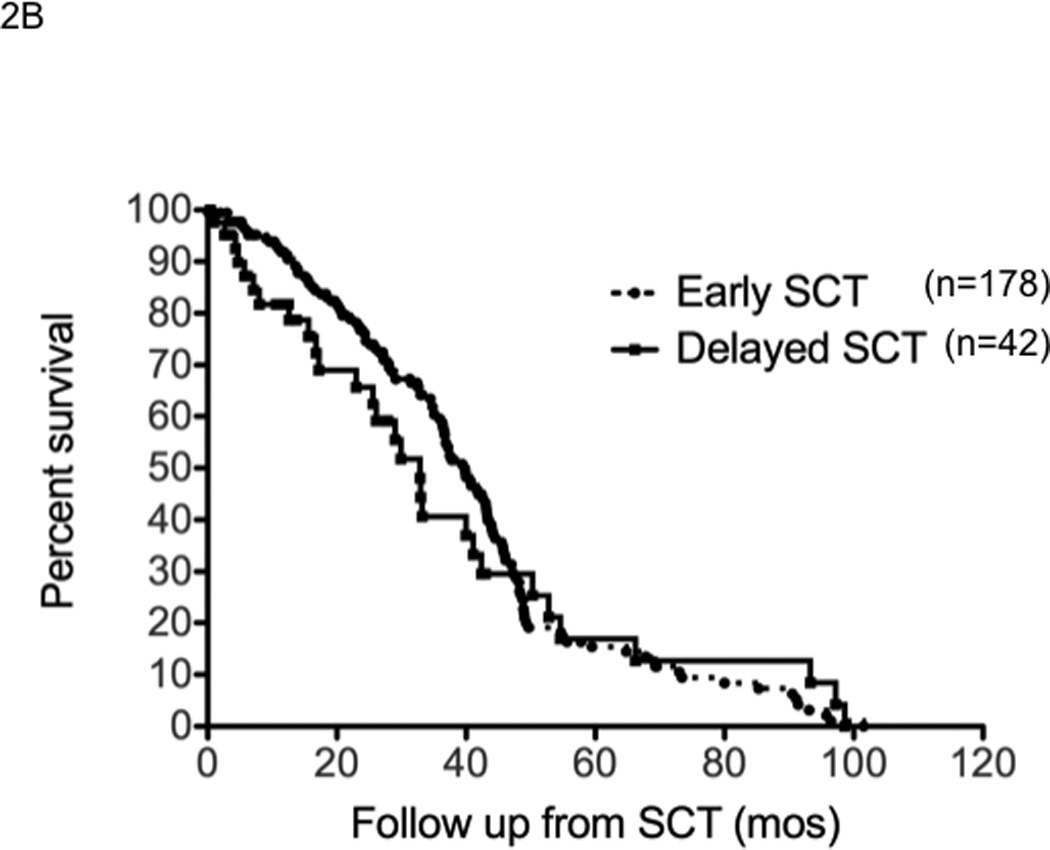
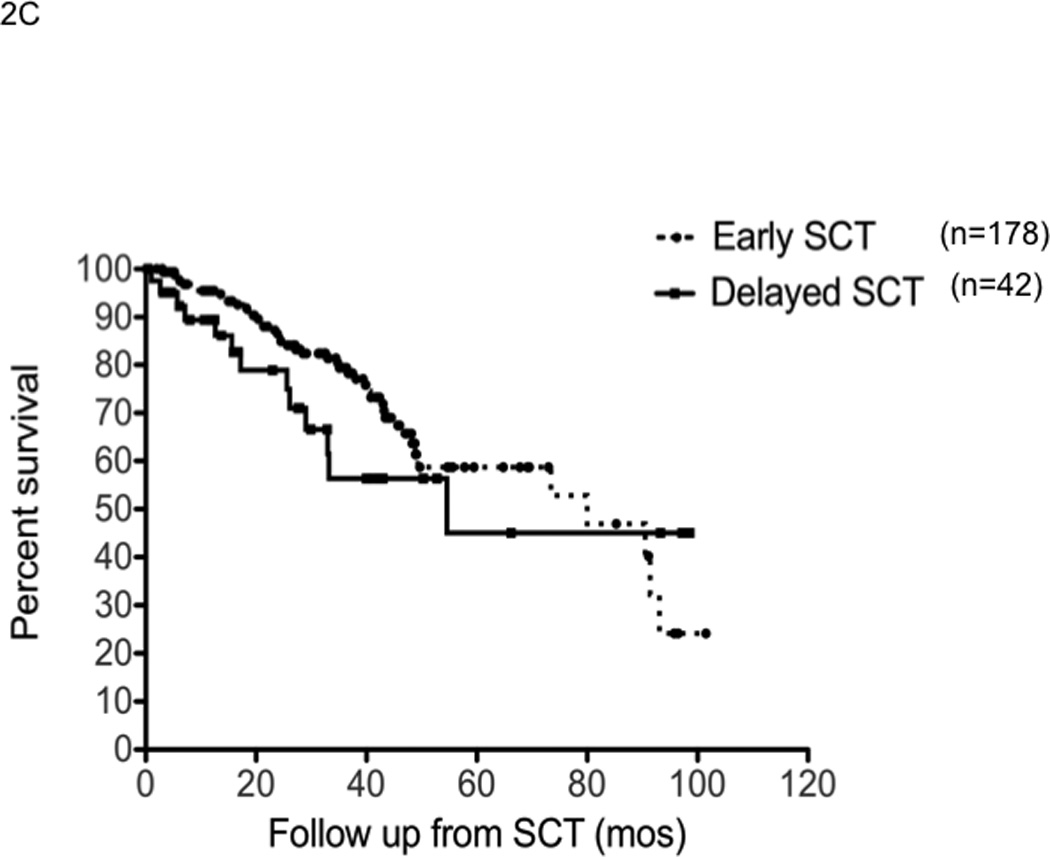
Panel A represents the time to progression following SCT in those with early (n=178) or delayed-SCT (n=42). Panel B represents the progression free survival following SCT in those with early (n=178) or delayed-SCT (n=42). Panel C represents the overall survival following SCT in those with early (n=178) or delayed-SCT (n=42).
DISCUSSION
High dose therapy and autologous stem cell transplantation continues to be an important component of the treatment approaches for multiple myeloma in transplant eligible patients.1 In fact, myeloma remains the most common indication for autologous stem cell transplantation in North America according to data reported to the CIBMTR. The wider adoption of this modality has resulted from randomized trials demonstrating a survival benefit for this approach compared to conventional non-transplant approaches. However, results of the initial trials also suggested that the improved outcome with SCT may be a reflection of the depth of response and an overall survival improvement was not consistently seen in all trials.15–17 The results of the S9321 trial suggested that the survival advantage might not persist once the complete response rates of conventional therapy improved.16 Introduction of newer, more effective therapies such as IMiDs and the proteasome inhibitor bortezomib has led to high response rates and deeper responses and has raised questions regarding the continued role of SCT in this disease. As a result of the high response rates seen at the end of the induction therapy, and low toxicity profile of current regimens, patients are increasingly opting to delay SCT and continue with initial therapy.8, 12 This raises important questions as to the efficacy of SCT when given in the context of disease refractory to the novel therapies and whether such a delay may compromise the eventual benefit a patient may obtain with this procedure.
In this study, we were able to show that the paradigm of comparable survival with early or delayed-SCT holds true in the context of newer therapies as it did with the alkylator based regimens of the past. The results are true irrespective of the specific IMiD used, whether thalidomide or lenalidomide, further confirming that the equivalence of SCT used in an early or deferred fashion is therapy independent. One might argue that there were more patients being treated with thalidomide in the early transplant group, but the comparisons hold true irrespective of the initial drug used. The differences in proportion of patients on the two drugs in the two groups likely reflects the better tolerability of lenalidomide and the increasing comfort on the part of physicians to defer transplant with the availability of more long term data with lenalidomide. The results serve to further underscore that SCT should be considered as a therapeutic regimen, like any other drug regimen, and not necessarily as platform to base other therapies on. This concept is further supported by the comparable time to progression after “first line therapy”; whether it was induction therapy followed by an early SCT or continued primary therapy with the intent to delayed-SCT. This is not surprising given the comparable response rates and depth of response seen with early SCT versus continued primary therapy from phase 2 trials and subgroup analysis of phase 3 trials. So it is reasonable to assume that short induction therapy followed by an SCT and continued induction therapy till progression represent two comparable “regimens” in terms of the duration of disease control; and two non cross resistant approaches that can be used in either order. We only included patients receiving IMiD based induction therapy in this study, since we had very few patients being initiated on bortezomib based regimens, given the predominantly referral nature of our patient population and the preference for oral regimens. It is likely that the results will hold true for patients treated with other effective agents as well. An important finding from the current study is the 4 year overall survival of over 80% in a group of transplant eligible patients, treated with lenalidomide and dexamethasone as primary therapy with the intent of transplant either early or deferred. This is comparable to results seen with multidrug combination therapies, highlighting the need to study sequential approaches using currently available drugs versus combination approaches in prospective studies, simultaneously examining the impact on quality of life.
Another important finding from this study is the comparable time to progression after early or delayed-SCT. This is in stark contrast to the results from the previous randomized trials that examined early versus delayed-SCT. In the MAG90 trial, a delayed-SCT was associated with a shorter TTP after SCT even though the overall survival was comparable with the two approaches.6 The type of therapy used as primary therapy in patients deciding on a delayed-SCT likely explains this. Unlike the earlier trials where patients were alkylator refractory or previously responsive to alkylating agents, patients going to a delayed-SCT in this study were all alkylator naïve at the time of SCT. In this aspect they were similar to their counterparts who elected to go for an early SCT. However, there was a trend to shorter overall survival following SCT in the delayed-SCT group. This is explained by the fact that this group of patients have “used up” one of the available treatment option since they went to transplant having relapsed following continued therapy with an IMiD and hence less likely to obtain a durable response to the treatment again.
The aspect of an early transplant approach that had traditionally tilted the decision in favor of an early transplant has been the reduced time without symptoms and side effects of therapy seen in phase 3 trials.6 However, this paradigm has changed with the newer agents, which are much better tolerated and can often be continued for prolonged duration with minimal toxicity and impact on quality of life. Hence it is not surprising that there is increased acceptance of the delayed-SCT approach. The current study, being retrospective in nature, does not allow up to compare the quality of life parameters with the two approaches. This will have to explored in the context of prospective trials comparing an early-SCT to delayed-SCT. The decision to go ahead with an early SCT or to defer transplant till relapse can be based on several factors including response to induction therapy, toxicity from treatment, physician bias, and patient perception among others. In the current study, the proportion of patients with deep responses was higher among those deciding to defer the SCT, a finding that would have undoubtedly influenced the decision. However, a recent study of patients undergoing SCT showed inferior survival for patients who obtained less than a PR to initial therapy with an IMiD unlike what had been seen in the pre-IMiD era.18 This does not necessarily contradict the current findings since over a third of patients with less than a PR opted for delayed-SCT and alternative therapies. Retrospective nature of the current study precludes an accurate determination of the reasons for early vs. delayed decision. Also, this study addresses the use of SCT primarily as the second line of therapy, as was the common practice in this patient group, and the results cannot be necessarily extrapolated to patients receiving an SCT after failing multiple lines of therapy with different newer drugs.
It is important however, to underscore that fact that nearly all patients underwent an early stem cell collection even when a delayed-SCT was planned. While slightly delayed compared to the early SCT group, the median time to collection of stem cells among the delayed-SCT group was 6.8 months representing 6–7 cycles of therapy. This is very important, as several studies have shown increased risk for collection failure in patients receiving initial therapy with lenalidomide while the impact of thalidomide and bortezomib appear to be less profound.19–22 The duration of therapy appear to be a strong predictor along with age of the risk of failure of stem cell collection.19 Had the approach been one of deferred stem cell collection at the time of stem cell transplant, the results may have been different as a higher proportion of patients would have failed to collect and hence unable to proceed to SCT.
Finally, the 4-year survival of over 80% among patients receiving lenalidomide and dexamethasone as initial therapy highlights the impact of newer therapies on patient outcome in myeloma. This is comparable to the results seen in the sub-analyses from the E4A03 clinical trial focusing on patients undergoing a stem cell transplant after lenalidomide and dexamethasone induction therapy. 23 Moreover, in this group of transplant eligible patients receiving lenalidomide and dexamethasone as initial therapy, the timing of transplant does not appear to alter the overall survival outcome.
In conclusion, this study conveys three important messages. It confirms the role of SCT as an effective treatment strategy for patients with myeloma with comparable survival outcomes when applied early in the course of disease or in a deferred manner following failure of continued initial therapy. It highlights the retained efficacy of high dose melphalan in alkylator naïve patients, demonstrating non-cross resistant mechanisms for disease control for these two approaches. Third, it demonstrates excellent long-term outcome for patients receiving initial therapy with lenalidomide and dexamethasone with overall survival of over 80% at 4 years. Thus patients have the option of delaying SCT and continuing with initial therapy if that is their preference. However, it is important to collect stem cells early on, given the reports of difficult stem cell collection in patients receiving prolonged treatment with lenalidomide. Whether such an approach will result in comparable quality of life metrics need to be studied prospectively.
Acknowledgments
This study was supported in part by Hematological Malignancies Program (Mayo Clinic Cancer Center); and CA90628 (SK) from National Cancer Institute.
SKK acknowledges research support to Mayo Clinic for conduct of clinical trials for role as principal investigator from Celgene, Millennium Pharmaceuticals, Genzyme, Novartis, Bayer and Cephalon; and advisory board participation for Merck and Celgene.
MAG has received honoraria from Easai Pharmaceuticals, Millennium Pharmaceuticals, Amgen and Celgene
AD and MQL acknowledge research support to Mayo Clinic for conduct of clinical trials for role as principal investigator from Celgene.
FG reports honoraria from Celgene.
Footnotes
Disclosure of Conflicts of Interest
SRH, FKB, DD, SVR, WH and NL have no disclosures.
Authorship Contributions
SKK and MAG designed the study, SKK, MAG and FG were involved in data collection, SKK and MAG analyzed the data; SKK, MQL, AD, FKB, SRH, DD, NL, FG, SS, SVR and MAG were involved in writing and critically reviewing the manuscript.
REFERENCES
- 1.Kumar SK, Mikhael JR, Buadi FK, et al. Management of newly diagnosed symptomatic multiple myeloma: updated mayo stratification of myeloma and risk-adapted therapy (mSMART) consensus guidelines. Mayo Clin Proc. 2009 Dec;84(12):1095–1110. doi: 10.4065/mcp.2009.0603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kyle RA, Rajkumar SV. Multiple myeloma. N Engl J Med. 2004 Oct 28;351(18):1860–1873. doi: 10.1056/NEJMra041875. [DOI] [PubMed] [Google Scholar]
- 3.Attal M, Harousseau JL, Stoppa AM, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Engl J Med. 1996 Jul 11;335(2):91–97. doi: 10.1056/NEJM199607113350204. [DOI] [PubMed] [Google Scholar]
- 4.Child JA, Morgan GJ, Davies FE, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003 May 8;348(19):1875–1883. doi: 10.1056/NEJMoa022340. [DOI] [PubMed] [Google Scholar]
- 5.Kumar SK, Dingli D, Lacy MQ, et al. Autologous stem cell transplantation in patients of 70 years and older with multiple myeloma: Results from a matched pair analysis. Am J Hematol. 2008 Aug;83(8):614–617. doi: 10.1002/ajh.21191. [DOI] [PubMed] [Google Scholar]
- 6.Fermand JP, Ravaud P, Chevret S, et al. High-dose therapy and autologous peripheral blood stem cell transplantation in multiple myeloma: up-front or rescue treatment? Results of a multicenter sequential randomized clinical trial. Blood. 1998 Nov 1;92(9):3131–3136. [PubMed] [Google Scholar]
- 7.Rajkumar SV, Blood E, Vesole D, Fonseca R, Greipp PR. Eastern Cooperative Oncology G. Phase III clinical trial of thalidomide plus dexamethasone compared with dexamethasone alone in newly diagnosed multiple myeloma: a clinical trial coordinated by the Eastern Cooperative Oncology Group. J Clin Oncol. 2006 Jan 20;24(3):431–436. doi: 10.1200/JCO.2005.03.0221. [DOI] [PubMed] [Google Scholar]
- 8.Rajkumar SV, Jacobus S, Callander NS, et al. Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: an open-label randomised controlled trial. Lancet Oncol. 2010 Jan;11(1):29–37. doi: 10.1016/S1470-2045(09)70284-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harousseau J-L, Avet-Loiseau H, Attal M, et al. High Complete and Very Good Partial Response Rates with Bortezomib--Dexamethasone as Induction Prior to ASCT in Newly Diagnosed Patients with High-Risk Myeloma: Results of the IFM2005-01 Phase 3 Trial. ASH Annual Meeting Abstracts; November 20, 2009; 2009. 353-. [Google Scholar]
- 10.Harousseau JL, Mathiot C, Attal M, et al. VELCADE/Dexamethasone (Vel/D) Versus VAD as Induction Treatment Prior to Autologous Stem Cell Transplantion (ASCT) in Newly Diagnosed Multiple Myeloma (MM): Updated Results of the IFM 2005/01 Trial. Blood (ASH Annual Meeting Abstracts); November 16, 2007; 2007. 450-. [Google Scholar]
- 11.Cavo M, Tacchetti P, Patriarca F, et al. A Phase III Study of Double Autotransplantation Incorporating Bortezomib-Thalidomide-Dexamethasone (VTD) or Thalidomide-Dexamethasone (TD) for Multiple Myeloma: Superior Clinical Outcomes with VTD Compared to TD. ASH Annual Meeting Abstracts; November 20, 2009; 2009. 351-. [Google Scholar]
- 12.Lacy MQ, Gertz MA, Dispenzieri A, et al. Long-term results of response to therapy, time to progression, and survival with lenalidomide plus dexamethasone in newly diagnosed myeloma. Mayo Clin Proc. 2007 Oct;82(10):1179–1184. doi: 10.4065/82.10.1179. [DOI] [PubMed] [Google Scholar]
- 13.Durie BG, Harousseau JL, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006 Sep;20(9):1467–1473. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 14.Greipp PR, Kumar S. Plasma cell labeling index. Methods Mol Med. 2005;113:25–35. doi: 10.1385/1-59259-916-8:25. [DOI] [PubMed] [Google Scholar]
- 15.Fermand JP, Katsahian S, Divine M, et al. High-dose therapy and autologous blood stem-cell transplantation compared with conventional treatment in myeloma patients aged 55 to 65 years: long-term results of a randomized control trial from the Group Myelome-Autogreffe. J Clin Oncol. 2005 Dec 20;23(36):9227–9233. doi: 10.1200/JCO.2005.03.0551. [DOI] [PubMed] [Google Scholar]
- 16.Barlogie B, Kyle RA, Anderson KC, et al. Standard chemotherapy compared with high-dose chemoradiotherapy for multiple myeloma: final results of phase III US Intergroup Trial S9321. J Clin Oncol. 2006 Feb 20;24(6):929–936. doi: 10.1200/JCO.2005.04.5807. [DOI] [PubMed] [Google Scholar]
- 17.Blade J, Rosinol L, Sureda A, et al. High-dose therapy intensification compared with continued standard chemotherapy in multiple myeloma patients responding to the initial chemotherapy: long-term results from a prospective randomized trial from the Spanish cooperative group PETHEMA. Blood. 2005 Dec 1;106(12):3755–3759. doi: 10.1182/blood-2005-03-1301. [DOI] [PubMed] [Google Scholar]
- 18.Gertz MA, Kumar S, Lacy MQ, et al. Stem cell transplant in multiple myeloma: impact of response failure with thalidomide or lenalidomide induction. Blood. 2010 Jan 20;115(12):2348–2353. doi: 10.1182/blood-2009-07-235531. [DOI] [PubMed] [Google Scholar]
- 19.Kumar S, Dispenzieri A, Lacy MQ, et al. Impact of lenalidomide therapy on stem cell mobilization and engraftment post-peripheral blood stem cell transplantation in patients with newly diagnosed myeloma. Leukemia. 2007 Sep;21(9):2035–2042. doi: 10.1038/sj.leu.2404801. [DOI] [PubMed] [Google Scholar]
- 20.Breitkreutz I, Lokhorst HM, Raab MS, et al. Thalidomide in newly diagnosed multiple myeloma: influence of thalidomide treatment on peripheral blood stem cell collection yield. Leukemia. 2007 Jun;21(6):1294–1299. doi: 10.1038/sj.leu.2404661. [DOI] [PubMed] [Google Scholar]
- 21.Mark T, Stern J, Furst JR, et al. Stem cell mobilization with cyclophosphamide overcomes the suppressive effect of lenalidomide therapy on stem cell collection in multiple myeloma. Biol Blood Marrow Transplant. 2008 Jul;14(7):795–798. doi: 10.1016/j.bbmt.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar S, Giralt S, Stadtmauer EA, et al. Mobilization in myeloma revisited: IMWG consensus perspectives on stem cell collection following initial therapy with thalidomide-, lenalidomide-, or bortezomib-containing regimens. Blood. 2009 Aug 27;114(9):1729–1735. doi: 10.1182/blood-2009-04-205013. [DOI] [PubMed] [Google Scholar]
- 23.Vesole DH, Jacobus S, Rajkumar SV, et al. Lenalidomide Plus Low-Dose Dexamethasone (Ld): Superior One and Two Year Survival Regardless of Age Compared to Lenalidomide Plus High-Dose Dexamethasone (LD). ASH Annual Meeting Abstracts; November 19, 2010; 2010. 308-. [Google Scholar]


