Abstract
Marijuana’s effects in humans are most often reported as intoxicating or therapeutic; yet, some humans report dysphoria or other negative affects. To evaluate whether differences in endocannabinoid levels might account for this variability, the present study examined whether sensitivity to cannabinoids changed when anandamide (AEA) metabolism was inhibited through administration of phenylmethyl sulfonyl fluoride (PMSF) a nonspecific irreversible amidase inhibitor. Male Long Evans rats were trained to discriminate 3 mg/kg Δ9-tetrahydrocannabinol (THC) versus vehicle in 2-lever drug discrimination procedure. ED50s for THC and CP 55,940 were lower when administered with PMSF than alone. PMSF administration also potentiated characteristic cannabimimetic effects of THC in ICR mice. Potentiation of AEA’s in vivo effects by PMSF were also observed, primarily a consequence of PMSF inhibition of the enzyme fatty acid amide hydrolase. Enhancement of the effects of THC and CP55,940 through this mechanism is unlikely, as these cannabinoids are predominantly metabolized through the P450 system. Mass spectrometry revealed that, in the presence of THC, endogenous AEA levels in the brain decreased and that this decrease was prevented by PMSF, suggesting that increased AEA levels may have acted additively with exogenously administered cannabinoids to increase cannabimimetic effects. These findings may account for the varying affect in response to marijuana in humans or cannabinoids in animals while also suggesting that metabolic inhibitors of AEA may potentiate marijuana’s intoxicating effects in humans.
Keywords: THC; anandamide; CP55,940; FAAH; drug discrimination; cannabinoid; PMSF
1 Introduction
Individuals vary substantially in their sensitivity to the subjective and abuse-related properties of marijuana, with some reporting a predominance of positive feelings associated with use and others reporting primarily dysphoria and negative affect (Zeiger et al., 2010). Not surprisingly, positive affect following marijuana use has been shown to be associated with higher levels of lifetime use and dependence (Davidson and Schenk, 1994; Le Strat et al., 2009). Causes of these differences are undoubtedly multivariate and include social and environmental, as well as biological, factors (Haberstick et al., 2010). For example, allele variants of genes that encode for CB1 receptors and for fatty acid amide hydrolase (FAAH) are associated with vulnerability to abuse of marijuana or other substances (Filbey et al., 2010; Haughey et al., 2008; Sipe et al., 2002; Zhang et al., 2004). These genetic differences may contribute to underlying variance in endocannabinoid system functioning, resulting in phenotypes that differ in their response to marijuana. This system is comprised of two identified receptors, CB1 and CB2, that are activated by at least two characterized endogenous ligands, anandamide and 2-arachidonoyl glycerol (2-AG) which primarily are degraded rapidly by their respective metabolic enzymes, FAAH and monoacylglycerol lipase (MAGL) (for review, see Mackie, 2008; Wilson and Nicoll, 2002).
THC and other psychoactive cannabinoids produce a profile of four pharmacological effects including antinociception, hypothermia, catalepsy, and suppression of locomotor activity (i.e. the "tetrad" ; Martin et al., 1991). Although potency for producing these behavioral characteristics is strongly correlated with CB1 receptor binding affinity (Compton et al., 1993a), production of the profile is not entirely selective for cannabinoid agonists, as non-cannabinoids may produce one or more of the effects (Wiley and Martin, 2003). On the other hand, cannabinoid discrimination is pharmacologically selective for psychoactive cannabinoids (Barrett et al., 1995) and represents an animal model of marijuana intoxication (Balster and Prescott, 1992). Early cannabinoid discrimination research hypothesized that AEA s rapid degradation was responsible for its inability to produce discriminative stimulus effects similar to THC in rats and monkeys (Wiley et al., 1997; Wiley et al., 1998), yet this hypothesis had limited empirical support. Recently studies have begun to unravel the discriminative stimulus effects produced by endogenous cannabinoids. Through pharmacological inhibition of FAAH, AEA has been found to produce THC-like discriminative stimulus in both rats (Solinas et al., 2007) and mice (Vann et al., 2009). Conversely, THC fully substitutes in FAAH knockout mice trained to discriminate anandamide versus vehicle (Walentiny et al., 2011). These studies have demonstrated that anandamide shares discriminative stimulus properties with THC. Given this, it also could be suggested that anandamide has the potential to produce intoxicating properties similar to marijuana; however, it remains unknown whether preventing metabolism of anandamide could alter sensitivity to the pharmacological properties of other cannabinoids (e.g., THC).
Thus the present study sought to determine whether altering endogenous anandamide levels would affect the in vivo pharmacological effects of THC in rodents. Two animal models were used to assess sensitivity to THC: (1) the tetrad battery and (2) drug discrimination. In the present study, anandamide levels were manipulated by administration of phenylmethyl sulfonamide (PMSF), a nonspecific irreversible amidase inhibitor (Desarnaud et al., 1995). Previous research has shown that PMSF increased anandamide levels in the brain following systemic administration (Wiley et al., 2000) and potentiated effects of anandamide in the tetrad battery (Compton and Martin, 1997). The results of this study suggest that brain levels of anandamide are altered in the presence of THC and that inhibiting AEA degradation leads to an apparent increase in potency of cannabinoids.
2. Methods
2.1 Subjects
Adult male Long Evans rats obtained from Harlan (Dublin, VA) were individually housed in a temperature-controlled (20–22°C) vivarium with a 12-h light/dark cycle. All rats were allowed to acclimate to the vivarium for at least a week before the experiments commenced. During the drug discrimination studies, rats were maintained within a weight range of 400–450g by restricted post-session feeding. They had water ad libitum in their home cages.
Male ICR mice (25–32 g), purchased from Harlan (Dublin, VA), were housed in groups of five. All mice were kept in a temperature-controlled (20–22°C) environment with a 14:10-h light:dark cycle and received food and water ad libitum. Separate mice were used for testing each drug dose in the in vivo procedures. All animals used in this study were cared for in accordance with the guidelines of the Institutional Animal Care and Use Committee of the Virginia Commonwealth University and the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996).
2.2 Apparatus
Rats were trained and tested in standard operant conditioning chambers (BRS/LVE Inc., Laurel, MD or Lafayette Instruments Co., Lafayette, IN) housed in sound-attenuated cubicles. Pellet dispensers delivered 45-mg BIO SERV (Frenchtown, NJ) food pellets to a food receptacle on the front wall of the chamber between two response levers. Fan motors provided ventilation and masking noise for each chamber. House lights located above the food cup were illuminated during training and testing sessions. A computer with Logic 1 interface(MED Associates, Georgia, Vermont) and MED-PC software (MED Associates) was used to control schedule contingencies and to record data.
Measurement of spontaneous activity in mice occurred in standard activity chambers interfaced with a Digiscan Animal Activity Monitor (Omnitech Electronics, Inc., Columbus, OH). A standard tail-flick apparatus and a digital thermometer (Fisher Scientific, Pittsburgh, PA) were used to measure antinociception and rectal temperature, respectively. The ring immobility device consisted of an elevated metal ring (diameter = 5.5 cm, height = 16 cm) attached to a wooden stand.
2.3 Experimental Procedures
2.3.1 Drug Discrimination
All rats were trained to press one lever following administration of 3 mg/kg THC and to press another lever after injection with vehicle each according to a fixed-ratio 10 (FR-10) schedule of food reinforcement, as described previously (Wiley et al., 2004). Completion of 10 consecutive responses on the injection-appropriate lever resulted in delivery of a food reinforcer. Each response on the incorrect lever reset the response requirement on the correct lever. The position of the drug lever was varied among the group of rats. The daily injections for each rat were administered in a double alternation sequence of THC and vehicle. Rats were injected and returned to their home cages until the start of the experimental session. Training occurred during 15-min sessions conducted 5 days a week (Monday-Friday) until the rats had met three criteria during 8 of 10 consecutive sessions: (a) first completed FR-10 on the correct lever, (b) percentage of correct-lever responding greater than 80% for the entire session, and (c) response rate greater than 0.4 responses/s.
Following successful acquisition of the discriminative stimulus, substitution tests were conducted on Tuesdays and Fridays during 15-min test sessions while training continued on Mondays, Wednesdays, and Thursdays. During test sessions, responses on either lever delivered reinforcement according to an FR-10 schedule. To be tested, rats must have completed the first FR and made at least 80% of all responses on the injection-appropriate lever on the preceding day's training session.
Dose effect curves were conducted for THC, anandamide, CP 55,940, and oleamide. All test drugs were administered 15 min after either 15 mg/kg PMSF or vehicle. THC and CP 55,940 were administered 30 min prior to testing. Anandamide and oleamide were administered 15 min prior to testing. Doses of each compound were administered in ascending order. Subsequently, an antagonist test was conducted to evaluate the ability of rimonabant (3 mg/kg) to block the THC-like discriminative stimulus effects of the 15 mg/kg PMSF and 10 mg/kg anandamide combination. Throughout the study, control tests with vehicle and 3 mg/kg THC were conducted during the week before the start of each dose-effect curve determination.
2.3.2 Mouse Tetrad
Prior to testing in the pharmacological procedures, mice were acclimated to the experimental setting (ambient temperature 22–24°C) for at least one h. Before any injections were given, baseline values were determined for rectal temperature and tail-flick latency (in s). Ten min after i.p. administration of either PMSF (30 mg/kg) or vehicle, mice were injected i.v. with anandamide, oleamide, or THC. Five minutes following anandamide, oleamide, or THC administration mice were assessed on spontaneous locomotion, tail flick, ring immobility, and rectal temperature (the “tetrad”). First, individual mice were placed into one of six locomotor activity chambers. Spontaneous activity was measured as total number of interruptions of 16 photocell beams per chamber during the 10-min test and was expressed as percent inhibition of activity of the vehicle group. Tail-flick latencies were then re-determined 35 min after drug administration. Antinociception was calculated as percent of maximum possible effect (%MPE = [(test - control latency)/ (10-control)] X 100). Control latencies typically ranged from 1.5 to 4.0 s. Rectal temperatures were reassessed 45 min after drug administration. Rectal temperature values were expressed as the difference between control temperature (before injection) and temperatures following drug administration (Δ°C). Next, at 55 min post drug administration, mice were placed on the ring immobility apparatus for 5 min. The total amount of time (in s) that the mouse remained motionless was measured. This value was divided by 300 s and multiplied by 100 to obtain a percent immobility rating. The criterion for ring immobility was the absence of all voluntary movement, including snout and whisker movement. Different mice were tested with each dose or dose combination.
2.3.3 Receptor Binding
CB1 receptor affinities of THC with and without PMSF were determined by measuring displacement of [3H]CP55,940 from its binding site in a membrane preparation from rat brain as described by Compton et al. (1993a), except that whole brain was used. Briefly, rat brains, stored at −80°C, were thawed on the day of assay, placed in 20 volumes of cold Membrane Buffer (50 mM Tris–HCl, 3 mM MgCl2, 1 mM EGTA, pH 7.4), homogenized with a Polytron and centrifuged at 48,000 × g at 4 C for 10 min. The supernatants were discarded and the pellets were re-homogenized in Membrane Buffer, centrifuged at 48,000 × g and resuspended in Assay Buffer (50 mM Tris-HCl, 3mM MgCl2, 0.2 mM EGTA, 100 mM NaCl, pH 7.4). Adenosine deaminase (final concentration=0.004 units/ml) was added to the membrane homogenates, which were then preincubated for 10 min at 30°C. Total membrane protein was measured according to (Bradford, 1976). Binding was initiated by the addition of 150 μg of membrane to test tubes containing 1 nM of [3H] CP 55,940 (79 Ci/mmol) and a sufficient quantity of buffer to bring the total incubation volume to 1 ml. Nonspecific binding was determined by the addition of 1 μM unlabeled CP 55,940. The reaction was terminated by vacuum filtration though Whatman GF/B glass fiber filter that was pre-soaked in Tris buffer containing 5g/L bovine serum albumin (Tris-BSA), followed by 3 washes with 4°C Tris-BSA. Bound radioactivity was determined by liquid scintillation spectrophotometry at 45% efficiency after extraction in ScinitSafe Econo 1 scintillation fluid.
2.3.4 Agonist-Stimulated [35S]GTPγS Binding
ICR mice were sacrificed by decapitation and cerebella were dissected out. Tissue was stored at -80°C until use. Tissue was placed in 5 mL cold membrane buffer (50 mM Tris-HCl, 3 mM MgCl2, 1 mM EGTA, pH 7.4) and homogenized. The samples were then centrifuged at 50,000 g at 5°C for 10 min. The supernatant was removed and samples were resuspended in 5mL assay buffer A (50 mM Tris-HCl, 3 mM MgCl2, 0.2 mM EGTA, 100 mM NaCl, pH 7.4). Protein concentration was determined by Bradford method (Bradford, 1976). Prior to the assay membranes (4–8 μg protein) were pre-incubated for 25 min at 30°C with adenosine deaminase (3 mU/ml) in assay buffer. Concentration-effect curves were generated by incubating the appropriate amount of membrane protein (4–8 μg) in assay buffer B (assay buffer A plus 1.25 g/L bovine serum albumin) with 0.03–60 μM of cannabinoid anandamide/THC plus or minus PMSF (900 nM) in the presence of 30 μM guanosine 5 diphosphate and 0.1 nM [35S]GTPγS in 0.5 mL total volume for 2 hours at 30°C. Basal binding was measured in the absence of an agonist and non-specific binding was measured in the presence of 20 μM unlabeled GTPγS. The reaction was terminated by vacuum filtration through Whatman GF/B glass fiber filters, followed by three washes with 4°C Tris buffer (50 mM Tris-HCl, pH 7.4). Bound radioactivity was determined by liquid scintillation spectrophotometry at 95% efficiency after 10-h extraction in ScintiSafe Econo 1 scintillation fluid.
2.3.5 Quantification of Anandamide Levels
For the first experiment, adult male ICR mice were injected i.p. with either vehicle (tween 80 7.8%, saline 92.2%) or 30 mg/kg PSMF. Twenty minutes later, mice were decapitated and whole brain was harvested and rapidly cooled by immersion in liquid nitrogen. Anandamide was then extracted using a methanol/chloroform extraction (Richardson et al., 2007). Following extraction, quantification of anandamide was conducted by LC/MS/MS using Multiple Reaction Monitoring (Richardson et al., 2007). For the second series of experiments, adult male ICR mice were injected with THC 20 mg/kg and were decapitated 0, 15, 30, 60, or 120 minutes later. Whole brain was harvested and a sagittal cut was made. Half of each brain was used to determine THC levels and the other half was used to determine endocannabinoid levels. Once extracted and cut, both halves were placed in separate plastic vials and rapidly cooled by immersion in liquid nitrogen. For THC isolation, a solvent extraction (Nyoni et al., 1996) was conducted, whereas anandamide was extracted via the method of Hardison (Hardison et al., 2006). Following extraction, quantification of THC and anandamide was conducted by LC/MS/MS (Richardson et al., 2007). Next, two groups of adult male mice were injected with 20 mg/kg THC and vehicle or 20 mg/kg THC and 30 mg/kg PSMF and were decapitated 0, 30, or 120 minutes later. Brain tissue was harvested and extractions were performed and analyzed as stated above.
2.4 Drugs and Chemicals
THC (National Institute on Drug Abuse, Bethesda, MD), oleamide (Sigma-Aldrich, St. Louis, MO), anandamide (Organix, Inc., Woburn, MA), CP 55,940 (Organix, Inc.) and PMSF (Sigma-Aldrich) were dissolved in a vehicle of 7.8% Tween-80 (Fisher Scientific, Fair Lawn, NJ), and 92.2% saline. Rimonabant (Organix, Inc.) was suspended in a vehicle of absolute ethanol, Emulphor-620 (Rhone-Poulenc, Inc., Princeton, NJ), and saline at a ratio of 1:1:18.
Guanosine 5 diphosphate and bovine serum albumin, were purchased from Sigma Chemical Company (St. Louis, MO). GTPγS was purchased from Roche Diagnostics (Branchburg, NJ). [35S]GTPγS (1150–1300 Ci/mmol) was obtained from Perkin Elmer Life Sciences (Boston, MA). Scintillation fluid (ScinitSafe Econo 1) was purchased form Fisher Scientific (Pittsburgh, PA). Adenosine deaminase was purchased from Sigma Chemical Company (St. Louis, MO).
2.5 Data analysis
2.5.1 Drug Discrimination
For each test session, percentage of responses on the drug lever and response rate (responses/s) were calculated. Full substitution was defined as ≥80% THC-lever responding. ED50 values were calculated separately for each drug using least squares linear regression analysis on the linear portion of the dose-effect curve, followed by calculation of 95% confidence limits. Potency ratio values with a 95% confidence interval were calculated. Potency ratio values with a 95% confidence interval were calculated using the method of Colquhoun (1971) to compare the effects of vehicle or PMSF co-administration on THC-like discriminative stimulus effects produced by THC and CP55,940. Significance is indicated by confidence levels that do not overlap with unity (i.e., 1.0). Repeated measures ANOVAs with Dunnett s post hoc tests (α =0.05) were used to determine differences in drug lever responding during antagonism tests and response rates both compared to vehicle control. Since rats that responded less than 10 times during a test session did not press either lever a sufficient number of times to earn a reinforcer, their drug lever selection data were excluded from analysis, but their response rate data were included in mean response rate.
2.5.2 Mouse Tetrad
Antinociception was calculated as percent of maximum possible effect (%MPE = [(test - control latency)/ (10-control)] X 100). Rectal temperature values were expressed as the difference between control temperature (before injection) and temperatures following drug administration (Δ°C). Spontaneous activity was expressed as % inhibition of activity of the vehicle group. The total amount of time that the mouse remained motionless was divided by 300 sec and multiplied by 100 to obtain a percent immobility rating. ED50 values for THC and anandamide to produce % MPE, Δ°C, % inhibition, and % immobility were obtained using least squares linear regression analysis on the linear part of the dose-effect curve, followed by calculation of 95% confidence limits. Potency ratio values with a 95% confidence interval were calculated using the method of Colquhoun (1971) to compare the effects of vehicle or PMSF co-administration on cannabimimetic activity produced by THC and anandamide. Maximal effects were estimated as follows: 100% inhibition of spontaneous activity and 100% maximal possible effect in the tail flick procedure. Maximal change in rectal temperature was estimated at 6 °C (THC) or -3 °C (anandamide) and maximal percentage ring immobility was 60% (Compton et al., 1993b). ANOVAs were conducted separately for pretreatment and drug. Dunnett s test was used for post hoc comparison when appropriate. Antagonism tests also were evaluated with ANOVAs as a function of drug followed by Dunnett s test when appropriate.
2.5.3 In-vitro analysis
Data for [35S]GTPγS binding experiments are reported as mean and standard error of at least four experiments, which were each performed in triplicate. Non-specific binding was subtracted from each sample. Net stimulated [35S]GTPγS binding was defined as agonist-stimulated minus basal [35S]GTPγS binding, and percent stimulation was defined as (net-stimulated/basal [35S]GTPγS binding) × 100%. Nonlinear iterative regression analyses of agonist concentration-effect curves were performed with Prism 4.0 (GraphPad Software, Inc., San Diego, CA). For mass spectrometry data, mean and standard error were determined for THC (ng) and anandamide (pmol/g) levels in each tissue. Factorial ANOVAs followed by Tukey post hoc tests (where appropriate) were used to determine significant differences in THC and anandamide levels, respectively, across pre-treatment (vehicle or PMSF) and time. Significance was defined as p < 0.05.
3. Results
3.1 Drug Discrimination in Rats
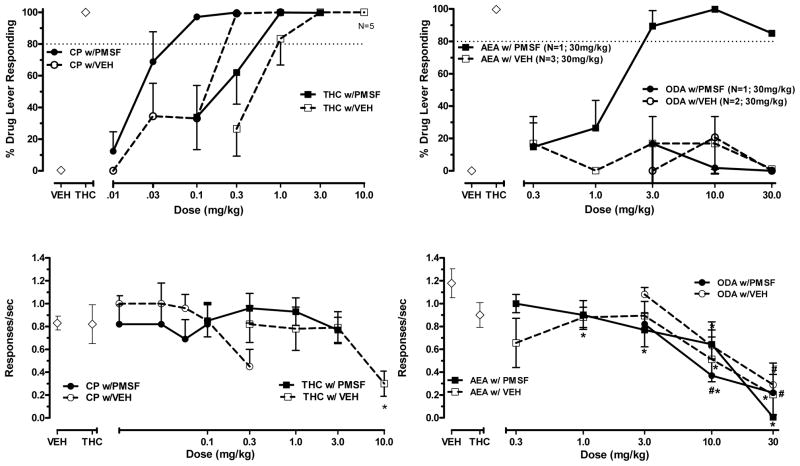
Figure 1 shows percent THC lever responding (upper panels) and response rate (lower panels) following vehicle or PMSF administration with THC, CP 55,940, anandamide, and oleamide. THC and CP 55,940 fully and dose-dependently substituted for THC when co-administered with either vehicle or with PMSF (Fig. 1, upper left panel), with higher doses of both drugs significantly decreasing responding (THC: F (4,20)=3.16, p < 0.05; CP 55,940: F (5,25)=3.23, p < 0.05) [Fig. 1, lower left panel]. In contrast, anandamide failed to substitute for THC after vehicle pre-injection (Fig. 1, upper right panel), even when tested up to doses that significantly reduced response rates (F (5,25)=6.64, p < 0.05) [as compared to vehicle; Fig. 1, lower right panel]. When anandamide was administered with PMSF, however, full and dose-dependent substitution was observed (Fig. 1, upper right panel), albeit this substitution was accompanied by significant suppression of response rates compared to vehicle control (F (5,25)=22.77, p < 0.05) [Fig. 1, lower right panel]. Substitution by the anandamide and PMSF combination was reversible by 3 mg/kg rimonabant (data not shown), suggesting CB1 receptor mediation of this effect. Oleamide failed to substitute for THC under either condition (i.e. co-administration with vehicle or PMSF) [Fig. 1, upper right panel], even up to doses that significantly suppressed response rates (oleamide with vehicle: F (3,15)=4.8, p < 0.05 and oleamide with PMSF: F (3,15)=5.6, p < 0.05) [Fig. 1, lower right panel].
Figure 1.
Effects of THC and CP 55,940 (left panels) or anandamide and oleamide (right panels) following administration of PMSF (15 mg/kg) or vehicle on percentage of THC -lever responding (upper panels) and response rates (lower panels) in rats trained to discriminate 3.0 mg/kg THC from vehicle. Points above VEH and THC represent the results of control tests with vehicle and 3 mg/kg THC conducted before each dose-effect determination. For each dose-effect curve determination, values represent the mean (±SEM) of 6 rats except where noted in legend on graph. Asterisk (*) indicates a significant difference in response rates (p<0.05) relative to the vehicle condition.
Table 1 shows ED50 values and potency ratios for THC and CP55,940 when tested with vehicle or with PMSF. Comparison of these values demonstrated that, compared to co-administration with vehicle, co-administration of PMSF significantly potentiated the potency of CP55,940 for producing THC-like discriminative stimulus effects, while PMSF potentiation of THC’s discriminative stimulus effects approached significance (lower confidence limit = 0.96).
Table 1.
ED50s and potency ratios for THC, CP 55,940, and anandamide following vehicle and PMSF administration in rats trained to discriminate 3 mg/kg THC. * denotes significant increase in potency between conditions.
| Test | Drug & VEH ED50 in mg/kg (95% CL) | Drug & PMSF ED50 in mg/kg (95% CL) | Potency Ratio PMSF:VEH (95% CL) |
|---|---|---|---|
| THC | 0.52 (0.19–1.4) | 0.45 (0.28–0.73) | 2.7 (0.96–6.9) |
| CP 55,940 | 0.06 (0.04–0.09) | 0.05 (0.03–0.07) | 2.4* (1.7–3.6) |
| AEA | ND | 1.3 (0.8–2.2) | ND |
ED50 values are derived from the each dose effect plus the respective condition, & VEH or 15 mg/kg PMSF (column headings). Potency ratios (and 95% confidence limits [CL]) for each drug were calculated with respect to the ED50 for THC. Significance is indicated by confidence levels that do not overlap with unity (i.e., 1.0).
indicates that potencyfor producing THC-like discriminative stimulus effects is significantly different when PMSF vs. vehicle is co-administered (p<0.05). ND=not determined
3.2 Mouse tetrad effects of THC and anandamide following vehicle and PMSF administration
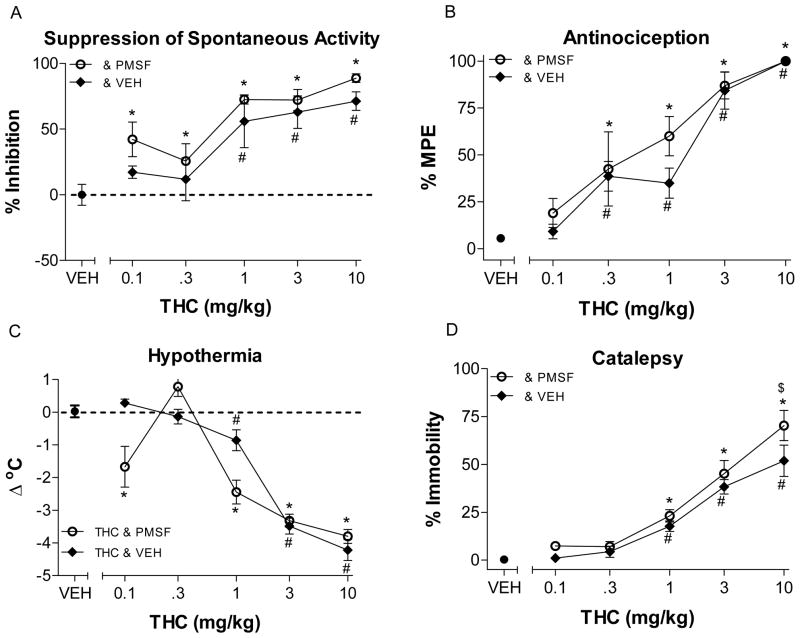
Figure 2 shows the effects of THC with vehicle and with PMSF in the tetrad test battery in mice. When administered with vehicle, THC dose-dependently decreased spontaneous activity (F (5,41)=14.94, p < 0.05) and produced antinociception (F (5,42)=31.54, p < 0.05), hypothermia (F (5,42)=42.91, p < 0.05) and catalepsy (F (5,42)=36.0, p < 0.05), whereas when THC was co-administered with PMSF, these effects were enhanced. Accordingly, this combination significantly decreased spontaneous activity (F (6,52)=17.72, p < 0.05) and produced antinociception (F (6,53)=18.93, p < 0.05), hypothermia (F (6,53)=29.71, p < 0.05) and catalepsy (F (6,53)=52.15, p < 0.05).
Figure 2.
Effects of THC following administration of PMSF 30 mg/kg (open circles, ⊖) or vehicle (filled diamonds, ◆) on spontaneous activity (panel A), antinociception (panel B), catalepsy (panel C), and rectal temperature (panel D). Mice were pretreated i.v. with THC fifteen min prior to the beginning of tests. Values represent the mean (±SEM) of 5–12 mice per group. Asterisk (*) and number symbols (#) indicate a significant difference for THC (p<0.05), relative to the vehicle condition following PMSF or vehicle co-administration, respectively.
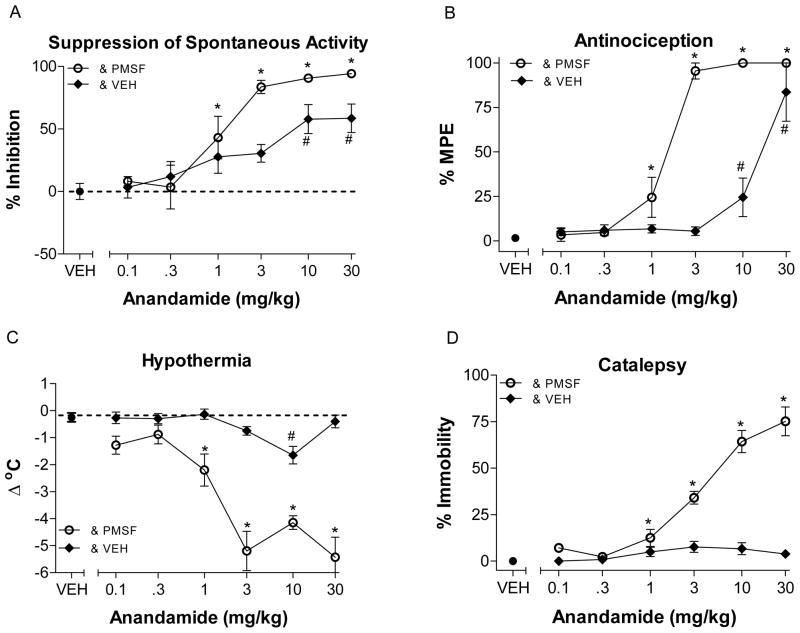
As expected, anandamide when co-administered with PMSF produced significant cannabinoid activity in all four tests, antinociception (F (7,39)=35.47, p < 0.05) and catalepsy (F (7,38)=50.35, p < 0.05) and significantly decreased spontaneous activity (F (6,33)=12.23, p < 0.05) and rectal temperature (F (7,38)=18.11, p < 0.05). However when co-administered with vehicle, anandamide significantly decreased activity (F (6,35)=9.27, p < 0.05), produced antinociception (F (5,30)=338.9, p < 0.05) at higher doses and significantly altered body temperature (F (6.35)=22.03, p < 0.05), but did not produce catalepsy (p > 0.05).
For both THC and anandamide, ED50 values were consistently lower following PMSF administration than after vehicle injection (Table 2). Further, analysis of potency ratios demonstrated that THC was significantly more potent at producing antinociception, decreasing spontaneous activity, and increasing ring immobility when co-administered with PMSF as compared to vehicle (Table 2), albeit the magnitude of these differences was small. In contrast, THC’s potency for producing hypothermia was not significantly altered byPMSF. Anandamide also produced significant potency ratios for suppression of spontaneous activity and antinociception. Potency ratios could not be determined for catalepsy and hypothermia since these effects were not exhibited following anandamide and vehicle co-administration.
Table 2.
ED50s and potency ratios for THC and anandamide (AEA) in the mouse tetrad after vehicle (VEH) and PMSF administration
| Test | THC & VEH ED50 in mg/kg (95% CL) | THC & PMSF ED50 in mg/kg (95% CL) | THC Potency Ratio (95% CL) | AEA & VEH ED50 in mg/kg (95% CL) | AEA & PMSF ED50 in mg/kg (95% CL) | AEA Potency Ratio (95% CL) |
|---|---|---|---|---|---|---|
| SA | 1.4 (0.7–2.7) | 0.4 (0.01–11.6) | 4.6* (2–13) | 9.9 (1.9–53) | 1.5 (0.8–2.5) | 7.1* (2.7–27) |
| MPE | 0.97 (0.6–1.4) | 0.5 (0.1–2.7) | 1.9* (1.03–3.89) | 13.4 (9–19) | 1.6 (0.6–2.8) | 9.2* (6.1–15.7) |
| RI | 2.1 (0.7–7) | 1.3 (0.9–1.9) | 1.6* (1.05–2.45) | (ND) | 1.49 (1–2.1) | (ND) |
| RT | 3.8 (0.5–29.9) | 2.9 (1.9–4.5) | 1.3 (0.93–1.89) | (ND) | 0.3 (0.01–19) | (ND) |
ND=not determined; SA=% inhibition of spontaneous activity; MPE=percentage of maximum possible effect in tail-flick test; RT=change in rectal temperature in (Δ°C); RI=ring immobility. ED50s for each drug are expressed in mg/kg with 95% confidence intervals in parentheses. Potency ratios (and 95% confidence limits) compare ED50 values following 15 mg/kg PMSF or VEH co-administration for each drug. Significance is indicated by confidence levels that do not overlap with unity (i.e., 1.0).
indicates a significant difference in potency between PMSF-treated and VEH-treated mice (p<0.05).
3.3 CB1 Receptor Binding and Agonist-Stimulated [35S]GTPγS Binding
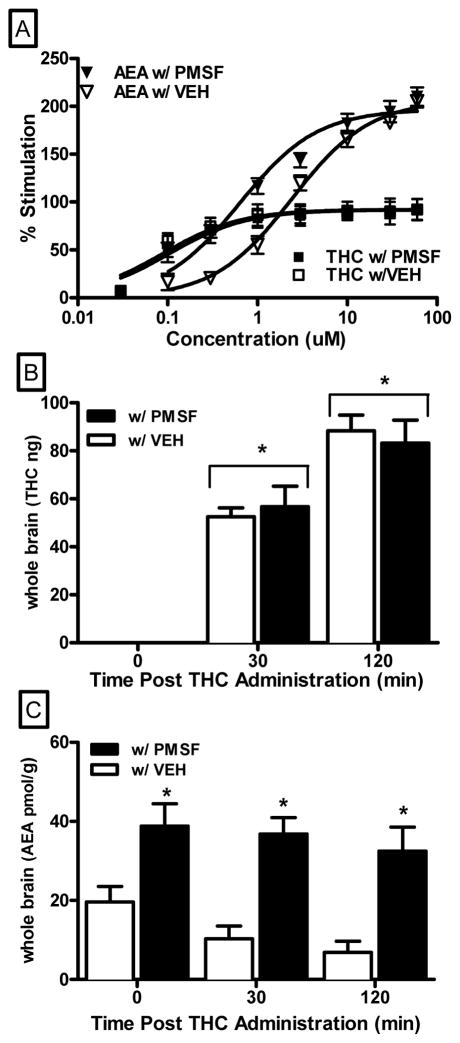
The binding affinity of THC for the CB1 receptor was not altered by PMSF (27±1 nM with vehicle and 27±2 nM with PMSF). To determine whether PMSF enhanced the ability of anandamide and THC to activate CB1 receptors, [35S]GTPγS binding assays were conducted with anandamide and THC in the presence or absence of 900 nM PMSF (Fig. 4, top panel). At this concentration, PMSF alone did not stimulate [35S]GTPγS binding (data not shown), suggesting that it did not interact with CB1 receptor directly. Curve-fitting analysis confirmed that PMSF decreased the EC50 value for anandamide from 2.42 +/− 0.29 μM to 0.63 +/− 0.15 μM, while the Emax value was unaffected (206 +/− 5.65 % versus 197 +/− 8.95 % in the absence or presence of PMSF, respectively). In contrast, 900 nM PMSF did not affect the EC50 of THC (0.10 +/− 0.04 μM in the absence of PMSF and 0.09 +/− 0.06 μM in the presence of PMSF). Hence, PMSF potentiated anandamide s effects by increasing its potency to stimulate G-protein activation of CB1 receptors whereas PMSF did not alter THC s effects on G-protein signaling, suggesting that the PMSF-induced increase in THC potency in vivo was not due to alteration of THC s direct action at the CB1 receptor.
Figure 4.
Effect of PMSF (900 nM) on anandamide and THC stimulated CB1 mediated G-protein activation (panel A). Whole brain levels of THC (ng, panel B), and anandamide (pmol/g, panel C) at 0, 30 and 120 min following 20 mg/kg i.p. injection of THC +/− 30 mg/kg PMSF . 0 time point indicates THC and anandamide levels in animals that received a PMSF injection, then a vehicle injection 20 min later, and then immediately euthanized. Asterisk (*) symbol indicates a significant difference compared to time 0 (panel B) or to vehicle treatment (panel C); i.e., main effect for time or pre-treatment, respectively.
3.4 Quantification of THC and anandamide levels
To determine whether enhancement of the in vivo pharmacological effects of anandamide and THC was related to PMSF-induced changes in brain levels of endocannabinoids or THC, mass spectrometry analysis of THC and anandamide levels was performed in mouse whole brain tissue (Fig. 4, middle and bottom panel, respectively). Administration of 20 mg/kg THC produced a significant time-dependent increase in brain levels of THC [main effect of time: F (2,54)=102.4, p < 0.05], which was not significantly altered by pre-injection with 30 mg/kg PMSF (Fig. 4, middle panel). In contrast, 30 mg/kg PMSF significantly enhanced anandamide levels following injection with 20 mg/kg THC at all time points [main effect of pre-treatment: F (1,54)=41.9, p < 0.05]. Immediately after injection of the combination of 30 mg/kg PMSF and 20 mg/kg THC, approximately twice the level of endogenous anandamide was detected in the brains of mice (33 +/− 2.9 pmol/g) compared to those of mice injected with vehicle and 20 mg/kg THC (16 +/− 1.3 pmol/g) [Fig. 4, bottom panel]. PMSF prevented time-dependent attenuation of endogenous anandamide subsequent to THC injection, as was observed with the THC and vehicle combination [Fig. 4, bottom panel]. Together, these in vitro findings strongly suggest that PMSF increased levels of endogenous anandamide by protecting it from metabolic degradation whereas it did not affect metabolism of THC.
4. Discussion
Consistent with previous reports (Gold et al., 1992; McMahon et al., 2008), THC and CP55,940 fully and dose-dependently substituted for THC. Cross-substitution of THC in rats trained to discriminate CP55,940 also has been reported (Wiley et al., 1995b), suggesting considerable overlap in the pharmacological profile of these two cannabinoids. In contrast, anandamide substituted for THC only when its metabolism was inhibited via co-administration of PMSF, coinciding with results from previous studies in which anandamide did not substitute for THC (Burkey and Nation, 1997; Jarbe et al., 2001) or substituted only when FAAH was inhibited (Solinas et al., 2007; Vann et al., 2009). Further, in most studies, as in the present one, substitution of anandamide or one of its synthetic analogs for THC occurred only at doses that concomitantly decreased overall response rates (Wiley et al., 2004; Wiley et al., 1998). CB1 receptor mediation of anandamide s THC-like discriminative stimulus effects in the present study was confirmed through rimonabant blockade of these effects. In contrast, oleamide, the sleep-inducing fatty acid amide (Cravatt et al., 1995), failed to substitute for THC at any dose, even with co-administration of PMSF. This is consistent with previous indications that oleamide has negligible affinity for CB1 receptors (Lichtman et al., 2002; Mechoulam et al., 1997).
Whereas potentiation of anandamide s substitution for THC through inhibition of its metabolism has been previously noted (Solinas et al., 2007; Vann et al., 2009), the leftward shift in the CP55,940 dose-effect curve for generalization is a novel finding. A concomitant shift in the dose-effect curve for response rate effects was not noted, suggesting selectivity for the discriminative stimulus effects. While PMSF enhanced some of THC s cannabimimetic effects in the tetrad battery, this enhancement was of lesser magnitude than that observed for THC-like discriminative stimulus effects, again suggesting a degree of selectivity. In contrast, PMSF robustly potentiated anandamide s THC-like discriminative stimulus effects and its tetrad effects, as has been reported previously (Compton and Martin, 1997; Vann et al., 2009; Wiley et al., 2000). Further, PMSF enhanced anandamide s potency at stimulating CB1 receptors (present study) and increased its binding affinity (Adams et al., 1995). While PMSF-induced inhibition of FAAH offers a ready explanation of its enhancement of all of the in vivo and in vitro pharmacological effects of anandamide reported here, THC and CP 55,940 are both degraded by cytochrome P450 enzymes rather than FAAH (Agurell et al., 1986). In addition, our data here show that THC levels in the brain increase to the same extent over time regardless of whether vehicle or PMSF is co-administered. Hence, direct interference with THC s metabolism is not a likely explanation for PMSF-induced enhancement of THC s effects.
Similarly, our data show that PMSF did not change THC s CB1 receptor affinity or its activation of the receptor, suggesting that PMSF-induced potentiation of THC s in vivo pharmacological effects also is not achieved via alteration of its effects at the CB1 receptor. Given our previous observation of potentiation of the discriminative stimulus effects of THC following systemically administered anandamide (Wiley et al., 1995a), we hypothesized that PMSF might be increasing the apparent potency of THC s cannabinoid effects through an upregulation of endogenously available anandamide. Our results here appear to support this hypothesis. When vehicle was co-administered with THC, brain levels of anandamide and THC exhibited an inverse relationship over time (i.e., anandamide levels decreased as THC levels increased). When PMSF was co-administered, brain levels of anandamide remained elevated throughout the 120-min measurement period. These results suggest that maintenance of a higher level of endogenous anandamide increased the apparent potencies of THC and CP55,940 for producing THC cannabinoid effects. Given these findings, it also could be hypothesized that THC would be more potent in FAAH knockout mice. However, this was not the case in a recent study by Falenski et al., (2010) who demonstrated that THC was equally potent in both FAAH −/− and wildtype mice following acute and repeated administration. Moreover, anandamide was 10-fold less potent in producing tetrad effects as reported by Falenski et al., (2010) relative to the present study. Experimental differences may account for this discrepancy as different strains of mice (ICR versus C57BL/6) and routes of administration (i.v. versus i.p) were used in this study compared to the other study (Falenski et al., 2010). These discrepancies also might be attributable to differences in acute versus chronic FAAH inhibition. FAAH inhibition has also been shown to enhance the effects of D2 agonists in rats discriminating THC, whereby the leftward shift in the THC dose effect curve produced by quinpirole was potentiated by URB597 pretreatment (Solinas et al., 2010). The quinpirole-induced leftward shift on the THC dose effect curve was possibly attributable to release of anandamide subsequent to activation of D2 receptors, as this potentiation was reversed by rimonabant (Solinas et al., 2010).
Of note, the magnitude of PMSF-induced enhancement of the pharmacological effects of exogenous cannabinoids also was variable across measures in the present study, with enhancement most pronounced for the THC-like discriminative stimulus effects of CP55,950 and absent for response rate effects. Since previous research has shown that the discriminative stimulus and response rate effects of cannabinoids (as well as their effects in the tetrad battery) may be mediated via different mechanisms (Jarbe et al., 2001; Jarbe et al., 2003; Prescott et al., 1992), one possible explanation for this variability is variation in the amount of endogenous anandamide in different brain regions, an hypothesis that has received empirical support (Bisogno et al., 1999). Alternatively, investigators have shown that changes in local cerebral glucose utilization following THC administration across time and brain area paralleled the time course of THC-induced effects, including regional specificity in the duration of changes in motor systems versus those related to temperature regulation that corresponded to the duration of hypolocomotion and hypothermia (Freedland et al., 2002; Whitlow et al., 2002). Similar differences in G-protein activation across brain regions with differing numbers of CB1 receptors have also been reported (Breivogel et al., 1997; Selley et al., 2001; Sim et al., 1995). Together, these factors could account for differences in the magnitude of PMSF-induced facilitation across measures.
As a non-specific protease inhibitor, PMSF interacts with other proteins in addition to FAAH, including, but not limited to acetylcholine esterase (Turini et al., 1969) and trypsin (Speakman and Yarwood, 1966). As such, it is possible that activity at one of these other sites might be responsible in part for the effects observed in vivo. Although consequences of PMSF on other protein targets cannot be ruled out empirically as contributing to the findings of this study, this is likely not the case. Both models employed in the present study are largely selective for cannabinoid activity in living systems. In particular, the superb degree of pharmacological specificity afforded by THC discrimination, taken together with complimentary neurochemical data demonstrating PMSF-induced increases anandamide levels strongly suggests that the findings presented here are attributable to PMSF’s effects on the endocannabinoid system.
In summary, the results of this study showed that PMSF substantially enhanced anandamide s cannabinoid effects in the tetrad battery in mice and facilitated its substitution for THC in drug discrimination in rats. The most parsimonious explanation for these findings is that PMSF prevented degradation of anandamide, thereby prolonging its half-life. While PMSF also increased the apparent potency of CP55,940 for substitution in the drug discrimination procedure and of THC in three measures of the tetrad battery, its process for doing so was attenuation of the decrease in brain anandamide levels that accompanied THC administration in the absence of PMSF. In other words, PMSF prevented metabolism of exogenous anandamide and endogenous anandamide, with resulting increases in apparent potency of other cannabinoids. To the extent that these results can be applied to humans, a couple of implications are notable. First, these results suggest that differences in endogenous anandamide levels may represent a potential contributor to individual differences in marijuana sensitivity. In humans, sufficient differences in endocannabinoid levels conceivably could be caused by factors such as genetic variation in degradative enzyme levels (e.g., Sipe et al., 2002) or through stress-induced changes in endocannabinoid levels (Hill and McEwan, 2010). Second, the results here suggest that, while FAAH inhibitors may lack THC-like discriminative stimulus effects on their own, they may potentiate these effects in marijuana users. Given this possibility, medication development efforts in this area should proceed with due diligence.
Figure 3.
Effects of anandamide following administration of PMSF 30 mg/kg (open circles, ⊖) or vehicle (filled diamonds, ◆) on spontaneous activity (panel A), antinociception (panel B), catalepsy (panel C), and rectal temperature (panel D). Mice were pretreated i.v. with anandamide fifteen min prior to the beginning of tests. Values represent the mean (±SEM) of 5–12 mice per group. Asterisk (*) and number symbols (#) indicate a significant difference for anandamide (p<0.05), relative to the vehicle condition following PMSF or vehicle co-administration, respectively.
Highlights.
Phenylmethyl sulfonyl fluoride (PMSF), a serine protease inhibitor, elevates anandamide brain levels.
MSF administration potentiates effects of anandamide and THC in a battery of in vivo tests in mice.
When given concomitantly with PMSF, anandamide produces THC-like discriminative stimulus effects.
PMSF induced leftward shifts in generalization curves for THC and CP55,940 in THC discrimination.
Acknowledgments
Research supported by NIH/NIDA Grants DA-026449, DA-03672, and DA-09789. The authors thank Anu Mahadevan (Organix, Inc.) for providing methanandamide and anandamide, which were synthesized with support from NIH/NIDA grant DA-09789 (Project 2). The authors also thank Justin Poklis for conducting mass spectrometry analysis and Qing Tao for conducting the endocannabinoid extraction procedure. Finally, we would like to thank Dr. Laura Sim-Selley and Dr. Dana Selley for technical assistance and extremely helpful discussions.
Abbreviations
- 2-AG
2-arachidonoyl glycerol
- AEA
anandamide
- FAAH
fatty acid amide hydrolase
- MAGL
monoacylglycerol lipase
- PMSF
phenylmethyl sulfonyl fluoride
- THC
Δ9-tetrahydrocannabinol
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams IB, Ryan W, Singer M, Thomas BF, Compton DR, Razdan RK, Martin BR. Evaluation of cannabinoid receptor binding and in vivo activities for anandamide analogs. J Pharmacol Exp Ther. 1995;273:1172–1181. [PubMed] [Google Scholar]
- Agurell S, Halldin M, Lindgren JE, Ohlsson A, Widman M, Gillespie H, Hollister L. Pharmacokinetics and metabolism of delta-1-tetrahydrocannabinol and other cannabinoids with emphasis on man. Pharmacol Rev. 1986;38:21–43. [PubMed] [Google Scholar]
- Balster RL, Prescott WR. 9-Tetrahydrocannabinol discrimination in rats as a model for cannabis intoxication. Neurosci Biobehav Rev. 1992;16:55–62. doi: 10.1016/s0149-7634(05)80051-x. [DOI] [PubMed] [Google Scholar]
- Barrett RL, Wiley JL, Balster RL, Martin BR. Pharmacological specificity of delta 9-tetrahydrocannabinol discrimination in rats. Psychopharmacology (Berl) 1995;118:419–424. doi: 10.1007/BF02245942. [DOI] [PubMed] [Google Scholar]
- Bisogno T, Berrendero F, Ambrosino G, Cebeira M, Ramos JA, Fernandez-Ruiz JJ, Di Marzo V. Brain regional distribution of endocannabinoids: implications for their biosynthesis and biological function. Biochem Biophys Res Commun. 1999;256:377–380. doi: 10.1006/bbrc.1999.0254. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Breivogel CS, Sim LJ, Childers SR. Regional differences in cannabinoid receptor/G-protein coupling in rat brain. J Pharmacol Exp Ther. 1997;282:1632–1642. [PubMed] [Google Scholar]
- Burkey RT, Nation JR. (R)-methanandamide, but not anandamide, substitutes for delta-9-THC in a drug-discrimination procedure. Exp Clin Psychopharmacol. 1997;5:195–202. doi: 10.1037//1064-1297.5.3.195. [DOI] [PubMed] [Google Scholar]
- Colquhoun D. Lectures on Biostatistics: an Introduction to Statistics with Applications in Biology and Medicine. Clarendon Press; Oxford: 1971. [Google Scholar]
- Compton DR, Martin BR. The effect of the enzyme inhibitor phenylmethylsulfonyl fluoride on the pharmacological effect of anandamide in the mouse model of cannabimimetic activity. J Pharmacol Exp Ther. 1997;283:1138–1143. [PubMed] [Google Scholar]
- Compton DR, Rice KC, De Costa BR, Razdan RK, Melvin LS, Johnson MR, Martin BR. Cannabinoid structure-activity relationships: Correlation of receptor binding and in vivo activities. J Pharmacol Exp Ther. 1993a;265:218–226. [PubMed] [Google Scholar]
- Compton DR, Rice KC, De Costa BR, Razdan RK, Melvin LS, Johnson MR, Martin BR. Cannabinoid structure-activity relationships: correlation of receptor binding and in vivo activities. J Pharmacol Exp Ther. 1993b;265:218–226. [PubMed] [Google Scholar]
- Cravatt BF, Prospero-Garcia O, Siuzdak G, Gilula NB, Henriksen SJ, Boger DL, Lerner RA. Chemical characterization of a family of brain lipids that induce sleep. Science. 1995;268:1506–1509. doi: 10.1126/science.7770779. [DOI] [PubMed] [Google Scholar]
- Davidson ES, Schenk S. Variability in subjective responses to marijuana: initial experiences of college students. Addict Behav. 1994;19:531–538. doi: 10.1016/0306-4603(94)90008-6. [DOI] [PubMed] [Google Scholar]
- Desarnaud F, Cadas H, Piomelli D. Anandamide amidohydrolase activity in rat brain microsomes. J Biol Chem. 1995;270:6030–6035. doi: 10.1074/jbc.270.11.6030. [DOI] [PubMed] [Google Scholar]
- Falenski KW, Thorpe AJ, Schlosburg JE, Cravatt BF, Abdullah RA, Smith TH, Selley DE, Lichtman AH, Sim-Selley LJ. FAAH−/− mice display differential tolerance, dependence, and cannabinoid receptor adaptation after delta-9-tetrahydrocannabinol and anandamide administration. Neuropsychopharmacology. 2010;35:1775–1787. doi: 10.1038/npp.2010.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey FM, Schacht JP, Myers US, Chavez RS, Hutchison KE. Individual and additive effects of the CNR1 and FAAH genes on brain response to marijuana cues. Neuropsychopharmacology. 2010;35:967–975. doi: 10.1038/npp.2009.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedland CS, Whitlow CT, Miller MD, Porrino LJ. Dose-dependent effects of Δ9-tetrahydrocannabinol on rates of local cerebral glucose utilization in rat. Synapse. 2002;45:131–142. doi: 10.1002/syn.10089. [DOI] [PubMed] [Google Scholar]
- Gold LH, Balster RL, Barrett RL, Britt DT, Martin BR. A comparison of the discriminative stimulus properties of delta 9-tetrahydrocannabinol and CP 55,940 in rats and rhesus monkeys. J Pharmacol Exp Ther. 1992;262:479–486. [PubMed] [Google Scholar]
- Haberstick BC, Zeiger JS, Corley RP, Hopfer CJ, Stallings MC, Rhee SH, Hewitt JK. Common and drug-specific genetic influences on subjective effects to alcohol, tobacco and marijuana use. Addiction. 2010;106:215–224. doi: 10.1111/j.1360-0443.2010.03129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardison S, Weintraub ST, Giuffrida A. Quantification of endocannabinoids in rat biological samples by GC/MS: technical and theoretical considerations. Prostaglandins Other Lipid Mediat. 2006;81:106–112. doi: 10.1016/j.prostaglandins.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Haughey HM, Marshall E, Schacht JP, Louis A, Hutchison KE. Marijuana withdrawal and craving: influence of the cannabinoid receptor 1 (CNR1) and fatty acid amide hydrolase (FAAH) genes. Addiction. 2008;103:1678–1686. doi: 10.1111/j.1360-0443.2008.02292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, McEwan BS. Involvement of the endocannabinoid system in the neurobehavioural effects of stress and glucocorticoids. Prog Neuro-Psychopharmacol Biol Psychiatr. 2010;34:791–797. doi: 10.1016/j.pnpbp.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarbe TU, Lamb RJ, Lin S, Makriyannis A. (R)-Methanandamide and delta-9-THC as discriminative stimuli in rats: tests with the cannabinoid antagonist SR-141716 and the endogenous ligand anandamide. Psychopharmacology (Berl) 2001;156:369–380. doi: 10.1007/s002130100730. [DOI] [PubMed] [Google Scholar]
- Jarbe TU, Lamb RJ, Liu Q, Makriyannis A. (R)-Methanandamide and delta-9-tetrahydrocannabinol-induced operant rate decreases in rats are not readily antagonized by SR-141716A. Eur J Pharmacol. 2003;466:121–127. doi: 10.1016/s0014-2999(03)01491-2. [DOI] [PubMed] [Google Scholar]
- Le Strat Y, Ramoz N, Horwood J, Falissard B, Hassler C, Romo L, Choquet M, Fergusson D, Gorwood P. First positive reactions to cannabis constitute a priority risk factor for cannabis dependence. Addiction. 2009;104:1710–1717. doi: 10.1111/j.1360-0443.2009.02680.x. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Hawkins EG, Griffin G, Cravatt BF. Pharmacological activity of fatty acid amides is regulated, but not mediated, by fatty acid amide hydrolase in vivo. J Pharmacol Exp Ther. 2002;302:73–79. doi: 10.1124/jpet.302.1.73. [DOI] [PubMed] [Google Scholar]
- Mackie K. Signaling via CNS cannabinoid receptors. Mol Cell Endocrinol. 2008;286:S60–65. doi: 10.1016/j.mce.2008.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin BR, Compton DR, Thomas BF, Prescott WR, Little PJ, Razdan RK, Johnson MR, Melvin LS, Mechoulam R, Ward SJ. Behavioral, biochemical, and molecular modeling evaluations of cannabinoid analogs. Pharmacol Biochem Behav. 1991;40:471–478. doi: 10.1016/0091-3057(91)90349-7. [DOI] [PubMed] [Google Scholar]
- McMahon LR, Ginsburg BC, Lamb RJ. Cannabinoid agonists differentially substitute for the discriminative stimulus effects of Delta-9-tetrahydrocannabinol in C57BL/6J mice. Psychopharmacology (Berl) 2008;198:487–495. doi: 10.1007/s00213-007-0900-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechoulam R, Fride E, Hanus L, Sheskin T, Bisogno T, Di Marzo V, Bayewitch M, Vogel Z. Anandamide may mediate sleep induction. Nature. 1997;389:25–26. doi: 10.1038/37891. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals. National Academy Press; Washington, D.C.: 1996. [Google Scholar]
- Nyoni EC, Sitaram BR, Taylor DA. Determination of delta-9-tetrahydrocannabinol levels in brain tissue using high-performance liquid chromatography with electrochemical detection. J Chromatogr B Biomed Appl. 1996;679:79–84. doi: 10.1016/0378-4347(96)00027-8. [DOI] [PubMed] [Google Scholar]
- Prescott WR, Gold LH, Martin BR. Evidence for separate neuronal mechanisms for the discriminative stimulus and catalepsy induced by delta-9-THC in the rat. Psychopharmacology (Berl) 1992;107:117–124. doi: 10.1007/BF02244975. [DOI] [PubMed] [Google Scholar]
- Richardson D, Ortori CA, Chapman V, Kendall DA, Barrett DA. Quantitative profiling of endocannabinoids and related compounds in rat brain using liquid chromatography-tandem electrospray ionization mass spectrometry. Anal Biochem. 2007;360:216–226. doi: 10.1016/j.ab.2006.10.039. [DOI] [PubMed] [Google Scholar]
- Selley DE, Rorrer WK, Breivogel CS, Zimmer AM, Zimmer A, Martin BR, Sim-Selley LJ. Agonist efficacy and receptor efficiency in heterozygous CB1 knockout mice: relationship of reduced CB1 receptor density to G-protein activation. J Neurochem. 2001;77:1048–1057. doi: 10.1046/j.1471-4159.2001.00308.x. [DOI] [PubMed] [Google Scholar]
- Sim LJ, Selley DE, Childers SR. In vitro autoradiography of receptor-activated G proteins in rat brain by agonist-stimulated guanylyl 5'-[gamma-[35S]thio]-triphosphate binding. Proc Natl Acad Sci USA. 1995;92:7242–7246. doi: 10.1073/pnas.92.16.7242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipe JC, Chiang K, Gerber AL, Beutler E, Cravatt BF. A missense mutation in human fatty acid amide hydrolase associated with problem drug use. Proc Natl Acad Sci USA. 2002;99:8394–8399. doi: 10.1073/pnas.082235799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas M, Tanda G, Justinova Z, Wertheim CE, Yasar S, Piomelli D, Vadivel SK, Makriyannis A, Goldberg SR. The endogenous cannabinoid anandamide produces delta-9-tetrahydrocannabinol-like discriminative and neurochemical effects that are enhanced by inhibition of fatty acid amide hydrolase but not by inhibition of anandamide transport. J Pharmacol Exp Ther. 2007;321:370–380. doi: 10.1124/jpet.106.114124. [DOI] [PubMed] [Google Scholar]
- Solinas M, Tanda G, Wertheim CE, Goldberg SR. Dopaminergic augmentation of delta-9-tetrahydrocannabinol (THC) discrimination: possible involvement of D 2-induced formation of anandamide. Psychopharmacology. 2010;209:191–202. doi: 10.1007/s00213-010-1789-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speakman PT, Yarwood RE. Trypsin inhibition by phenylmethylsulphonylfluoride with reduced wool as substrate. Nature. 1966;211:201–202. doi: 10.1038/211201b0. [DOI] [PubMed] [Google Scholar]
- Turini P, Kurooka S, Steer M, Corbascio AN, Singer TP. The action of phenylmethylsulfonyl fluoride on human acetylcholinesterase, chymotrypsin and trypsin. J Pharmacol Exp Ther. 1969;167:98. [PubMed] [Google Scholar]
- Vann RE, Warner JA, Bushell K, Huffman JW, Martin BR, Wiley JL. Discriminative stimulus properties of delta-9-tetrahydrocannabinol (THC) in C57Bl/6J mice. Eur J Pharmacol. 2009;615:102–107. doi: 10.1016/j.ejphar.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walentiny DM, Gamage TF, Warner JA, Nguyen TK, Grainger DB, Wiley JL, Vann RE. The endogenous cannabinoid anandamide shares discriminative stimulus effects with delta-9-tetrahydrocannabinol in fatty acid amide hydrolase knockout mice. Eur J Pharmacol. 2011;656:63–67. doi: 10.1016/j.ejphar.2011.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlow CT, Freedland CS, Porrino LJ. Metabolic mapping of the time-dependent effects of Δ9-tetrahydrocannabinol administration in the rat. Psychopharmacology. 2002;161:129–136. doi: 10.1007/s00213-002-1001-x. [DOI] [PubMed] [Google Scholar]
- Wiley J, Balster R, Martin B. Discriminative stimulus effects of anandamide in rats. Eur J Pharmacol. 1995a;276:49–54. doi: 10.1016/0014-2999(95)00010-i. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Barrett RL, Lowe J, Balster RL, Martin BR. Discriminative stimulus effects of CP 55,940 and structurally dissimilar cannabinoids in rats. Neuropharmacology. 1995b;34:669–676. doi: 10.1016/0028-3908(95)00027-4. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Dewey MA, Jefferson RG, Winckler RL, Bridgen DT, Willoughby KA, Martin BR. Influence of phenylmethylsulfonyl fluoride on anandamide brain levels and pharmacological effects. Life Sci. 2000;67:1573–1583. doi: 10.1016/s0024-3205(00)00749-9. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Golden KM, Ryan WJ, Balster RL, Razdan RK, Martin BR. Evaluation of cannabimimetic discriminative stimulus effects of anandamide and methylated fluoroanandamide in rhesus monkeys. Pharmacol Biochem Behav. 1997;58:1139–1143. doi: 10.1016/s0091-3057(97)00327-4. [DOI] [PubMed] [Google Scholar]
- Wiley JL, LaVecchia KL, Karp NE, Kulasegram S, Mahadevan A, Razdan RK, Martin BR. A comparison of the discriminative stimulus effects of delta-9-tetrahydrocannabinol and O-1812, a potent and metabolically stable anandamide analog, in rats. Exp Clin Psychopharmacol. 2004;12:173–179. doi: 10.1037/1064-1297.12.3.173. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Martin BR. Cannabinoid pharmacological properties common to other centrally acting drugs. Eur J Pharmacol. 2003;471:185–193. doi: 10.1016/s0014-2999(03)01856-9. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Ryan WJ, Razdan RK, Martin BR. Evaluation of cannabimimetic effects of structural analogs of anandamide in rats. Eur J Pharmacol. 1998;355:113–118. doi: 10.1016/s0014-2999(98)00502-0. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endocannabinoid signaling in the brain. Science. 2002;296:678–682. doi: 10.1126/science.1063545. [DOI] [PubMed] [Google Scholar]
- Zeiger JS, Haberstick BC, Corley RP, Ehringer MA, Crowley TJ, Hewitt JK, Hopfer CJ, Stallings MC, Young SE, Rhee SH. Subjective effects to marijuana associated with marijuana use in community and clinical subjects. Drug Alcohol Depend. 2010;109:161–166. doi: 10.1016/j.drugalcdep.2009.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang PW, Ishiguro H, Ohtsuki T, Hess J, Carillo F, Walther D, Onaivi ES, Arinami T, Uhl GR. Human cannabinoid receptor 1: 5' exons, candidate regulatory regions, polymorphisms, haplotypes and association with polysubstance abuse. Mol Psychiatry. 2004;9:916–931. doi: 10.1038/sj.mp.4001560. [DOI] [PubMed] [Google Scholar]