Abstract
This study investigated the effects of chronic restraint stress and repeated cyclic estradiol pulses on hippocampal CA3 and CA1 dendritic and/or spine morphology and spatial memory in female rats. Sprague-Dawley adult female rats were ovariectomized and then injected over two days with 17β-estradiol (10µg, s.c.), which was repeated every 4–5 days. While all rats received similar estradiol injection histories, half of the rats were chronically restrained and/or given a final cyclic pulse of estradiol prior to testing on a hippocampal-dependent object placement (OP) task to assess spatial memory. OP testing was performed two days after the last restraint session, as well as when the last two estradiol pulses best captured the maximal effect on hippocampal CA1 spine density. The data revealed several novel findings: 1) chronic stress or estradiol separately facilitated spatial memory, but did not have the same effects when co-administered, 2) CA1 spine densities negatively correlated with spatial memory, and 3) repeated estradiol pulses failed to prevent stress-induced CA3 dendritic retraction. We also corroborated previous studies showing increased CA1 spine density following estradiol, chronic stress, and behavioral manipulations. The present study uniquely combined chronic stress, repeated estradiol pulses, hippocampal morphology and behavior within the same animals, allowing for correlational analyses to be performed between CA1 spine morphology and spatial memory. We demonstrate novel findings that chronic stress or estradiol pulses independently facilitate spatial memory, but not when co-administered, and that these effects may involve a balance of CA1 apical spine expression that is independent of CA3 dendritic complexity.
Keywords: stress, estradiol, spatial memory, hippocampus, object placement
Introduction
Chronic stress has a long record of influencing hippocampal morphology and function. Of particular interest are the changes in hippocampal CA3 dendritic arbors, a specialized region of neurons that receives converging input indirectly from cortical sensory regions. Chronic stress conditions that produce hippocampal CA3 dendritic retraction (Beck & Luine, 1999; Magariños & McEwen, 1995b; McKittrick et al., 2000; Sousa, Lukoyanov, Madeira, Almeida, & Paula-Barbosa, 2000; Vyas, Mitra, Shankaranarayana Rao, & Chattarji, 2002; Watanabe, Gould, Daniels, Cameron, & McEwen, 1992; Watanabe, Gould, & McEwen, 1992) also lead to impaired hippocampal function, such as poor spatial learning and memory (Conrad, Galea, Kuroda, & McEwen, 1996; Luine, Villegas, Martinez, & McEwen, 1994; Park, Campbell, & Diamond, 2001; Sousa et al., 2000; Sunanda, Shankaranarayana Rao, & Raju, 2000). When antidepressant treatment or glucocorticoid synthesis inhibitors block hippocampal CA3 dendritic retraction (Luo & Tan, 2001; Magariños & McEwen, 1995a; Watanabe, Gould, Daniels, et al., 1992; Wood, Young, Reagan, Chen, & McEwen, 2004l) or increase hippocampal volume (Czéh et al., 2001), spatial ability is restored/improved (Conrad et al., 1996; Orsetti, Colella, Dellarole, Canonico, & Ghi, 2007; Song, Che, Min-Wei, Murakami, & Matsumoto, 2006). Moreover, the developmental time course for hippocampal CA3 dendritic retraction shows that spatial learning and memory deficits are present when CA3 dendritic retraction exists (Conrad, Magariños, LeDoux, & McEwen, 1999; Hoffman et al., In Press; Sousa et al., 2000; Vyas, Pillai, & Chattarji, 2004), but not before CA3 dendritic retraction has occurred (Luine, Martinez, Villegas, Magariños, & McEwen, 1996; McLaughlin, Gomez, Baran, & Conrad, 2007). These studies strongly support a role of CA3 dendritic arbors contributing to hippocampal spatial ability.
Studies investigating the mechanisms of how chronic stress influences spatial ability typically use male subjects, including the studies discussed above, but how chronic stress influences hippocampal morphology and spatial ability appears to differ in females (for reviews, see Bowman, 2005; Conrad & Bimonte-Nelson, 2010; Luine, Beck, Bowman, Frankfurt, & Maclusky, 2007; McLaughlin, Baran, & Conrad, 2009). In gonadally-intact female rats, chronic stress produces either mild hippocampal CA3 dendritic retraction (Galea et al., 1997) or fails to alter the CA3 dendritic arbors, even when morphology is investigated at phases of the estrous cycle when ovarian hormones are highest (proestrus) or lowest (estrus) (McLaughlin et al., 2010), and yet, spatial learning and memory is unaltered or even facilitated (Beck & Luine, 2002; Bowman, Zrull, & Luine, 2001; Bowman, Micik, Gautreaux, Fernandez, & Luine, 2009; Conrad, Grote, Hobbs, & Ferayorni, 2003; Kitraki, Kremmyda, Youlatos, Alexis, & Kittas, 2004; McFadden et al., 2011). When ovarian hormones are removed by ovariectomy (OVX), chronic stress produces robust dendritic retraction in CA3 neurons, as the dendritic pruning extends beyond the traditional apical region found in males, and can be detected in the basal arbors of some of the CA3 neuronal subtypes (McLaughlin, Baran, Wright, & Conrad, 2005; McLaughlin et al., 2010). However, the same rats expressing CA3 dendritic pruning show functional or even facilitated spatial ability (McLaughlin et al., 2005). Moreover, chronic estradiol or cholesterol replacement via silastic implants in OVX females prevents chronic stress from causing hippocampal CA3 dendritic retraction and again demonstrates that spatial ability remains independent of hippocampal CA3 dendritic arborization patterns (McLaughlin et al., 2010). These studies show that unlike male subjects, females can demonstrate intact or facilitated spatial ability following chronic stress and while estrogens can be neuroprotective (for review, see, Lebesgue et al., 2009, Wise et al., 2001), a relationship among estrogens, CA3 dendritic arborization and spatial memory is still elusive.
One attractive, but not exclusive, mechanism underlying spatial ability following chronic stress in females may involve estradiol-induced changes in hippocampal CA1 spine density and morphology. Exogenous administration of estradiol or the natural peaks of estradiol or ovarian hormones during proestrus facilitate spatial abilities (Conrad & Bimonte-Nelson, 2010; Korol, 2004; Luine et al., 2007), which coincide with elevated CA1 spine density, a finding first reported by Woolley and colleagues (1990; Woolley & McEwen, 1992) and replicated by others, including our lab (Leranth, Shanabrough, & Redmond, 2002; MacLusky, Luine, Hajszan, & Leranth, 2005; McLaughlin, Bimonte-Nelson, Neisewander, & Conrad, 2008; McLaughlin et al., 2010; Silva, Mello, Freymuller, Haidar, & Baracat, 2000). More recently, we found chronic stress altered the types of CA1 spines by enhancing spines with heads or “mushroom”-shaped spines (McLaughlin et al., 2005; McLaughlin et al., 2010), which are thought to be sites for mature and strengthened synapse locations (Kasai, Matsuzaki, Noguchi, Yasumatsu, & Nakahara, 2003; Moser, 1999; Park, Zoladz, Conrad, Fleshner, & Diamond, 2008). Indeed, the density of spines with heads positively correlated with spatial memory on a Y-maze task (McLaughlin et al., 2005), and these spines with heads are sensitive to OVX (Beltrán-Campus et al., 2011). The evidence showing that estradiol and chronic stress alter CA1 dendritic spine properties and spatial performance suggests that the CA1 region may be a critical variable contributing to spatial ability in chronically stressed females.
Few studies have investigated the interaction between chronic stress and estradiol on female spatial ability. One study using gonadally-intact females reported that chronically stressed females performed better than did unstressed controls on an eight arm radial maze and that rats in proestrus displayed impaired performance regardless of chronic stress history (Bowman et al., 2001). A follow-up study investigating estradiol treatment using silastic implants, a method that releases estradiol continuously over many weeks or months, reported that estradiol facilitated radial arm maze performance in chronically stressed OVX females (Bowman et al., 2002). These studies are an important first step, but raise additional questions. Since all arms were baited, rats using non-spatial strategies must be identified and then excluded. In addition, no statistical differences were found when spatial demands were imposed using a paradigm with delays, but this manipulation occurred when putative hippocampal arborization changes could have recovered (Conrad et al., 1999; Sousa et al., 2000; Hoffman et al., In Press). In a recent study, we showed that silastic implants of estradiol facilitated spatial learning in chronically stressed OVX females in a Morris water maze when performed within two days after chronic stress ended and when CA3 dendritic retraction existed (McLaughlin et al., 2010). Together, these studies suggest that estradiol is beneficial for spatial learning and memory in chronically stressed rats. However, estradiol was given chronically and at constant levels via silastic capsules, and this continuous exposure may have activated feedback mechanisms and potentially reduced hippocampal responsiveness (Gibbs, 2000). In another case, estradiol was administered to OVX female rats 48 hr and 24 hr before spatial memory assessment and no effect of estradiol was found (McLaughlin et al., 2005). In this latter study, however, the hippocampus may have been less responsive to estradiol since increased duration between OVX and the start of estradiol injections can attenuate CA1 spine formation (McLaughlin et al., 2008). A study has yet to investigate the combination of estradiol and chronic stress on a spatial memory paradigm that: 1) maintains estradiol efficacy to influence CA1 spine density during the period of when chronic stress is given, and 2) optimizes the estradiol cyclic pulses to occur when CA1 spine density increases are maximized during spatial memory assessment. Therefore, the current study was the first to combine these variables and to investigate whether cyclic estradiol pulses would protect against stress-induced hippocampal CA3 dendritic retraction, as found with chronic and tonic levels of estradiol (McLaughlin et al., 2010), while also optimizing changes in CA1 spines.
An important consideration in the experimental design was to identify a paradigm that shows dose-dependent estradiol effects on spatial memory without compromising the effectiveness by which chronic stress and/or estradiol alter the hippocampus. Prior studies combining chronic stress and estradiol failed to show dose-dependent effects of estradiol, such as the Y-maze, or require too many training days to determine effects, such as the radial arm maze (for review of these issues, see Conrad, 2010; McLaughlin et al., 2009). Assessment of spatial ability too long after chronic stressor termination can be counter-productive because hippocampal morphology can partially recover before behavioral assessments have started (i.e., CA3 dendritic retraction reverses within ten days after chronic stress has ended, Conrad et al., 1999; Sousa et al., 2000; Vyas et al., 2004; Hoffman et al., In Press). Another consideration is that estradiol’s influence on hippocampal CA1 spine density is temporally sensitive: CA1 spine density peaks within two to three days after a two-pulse treatment and then declines thereafter (Woolley & McEwen, 1993), and increasing the time from OVX to the first treatment with estrogens decreases the effectiveness of estrogens to increase CA1 spines (Silva et al, 2003; Daniel et al., 2006; McLaughlin et al., 2008; Smith et al., 2010). Taking these important considerations in mind, we previously tested a variety of spatial tasks using a cyclic estradiol (0µg, 5µg or 10µg) administration paradigm to determine an optimal spatial task that can be used to ascertain spatial memory ability when CA1 spine density is at its greatest (McLaughlin et al., 2008). We chose to use object placement because estradiol influenced spatial memory dose-dependently (with rats given 10 µg injections of estradiol showing better spatial memory than rats given 5 µg of estradiol, which in turn outperformed rats given vehicle) and object placement can be used under the constraints imposed by our chronic stress paradigm. As a result, this experiment used object placement to study the combined effects of chronic stress and cyclic estradiol injection (10 µg) on hippocampal CA3 and CA1 dendritic or spine morphology and function in female rats.
The purpose of the current study was to investigate the effects of chronic stress and cyclic estradiol pulses on hippocampal CA3 and CA1 dendritic and spine morphology and spatial memory. Because the behavioral testing procedure can alter CA1 spine density and shape (Diamond et al., 2006; McLaughlin et al., 2008), perhaps through learning mechanisms (Diamond et al., 2006; Leuner et al., 2003; Moser et al., 1994), this study also included cohorts of rats that were not behaviorally tested. These additional groups allowed us to determine how chronic stress and estradiol pulses altered hippocampal CA3 dendritic arbors and CA1 spines without confounds from behavioral testing. We hypothesized that CA1 spine density and/or spine shape would correlate with spatial memory in chronically stressed and control rats. Moreover, we sought to determine whether cyclic administration of estradiol would prevent stress-induced CA3 dendritic retraction as reported previously when estradiol was continuously replaced via silastic implants.
Method
All procedures were approved by the Arizona State University Institutional Animal Care and Use Committee and followed guidelines listed in the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources on Life Science, National Research Council, 1996).
Subjects
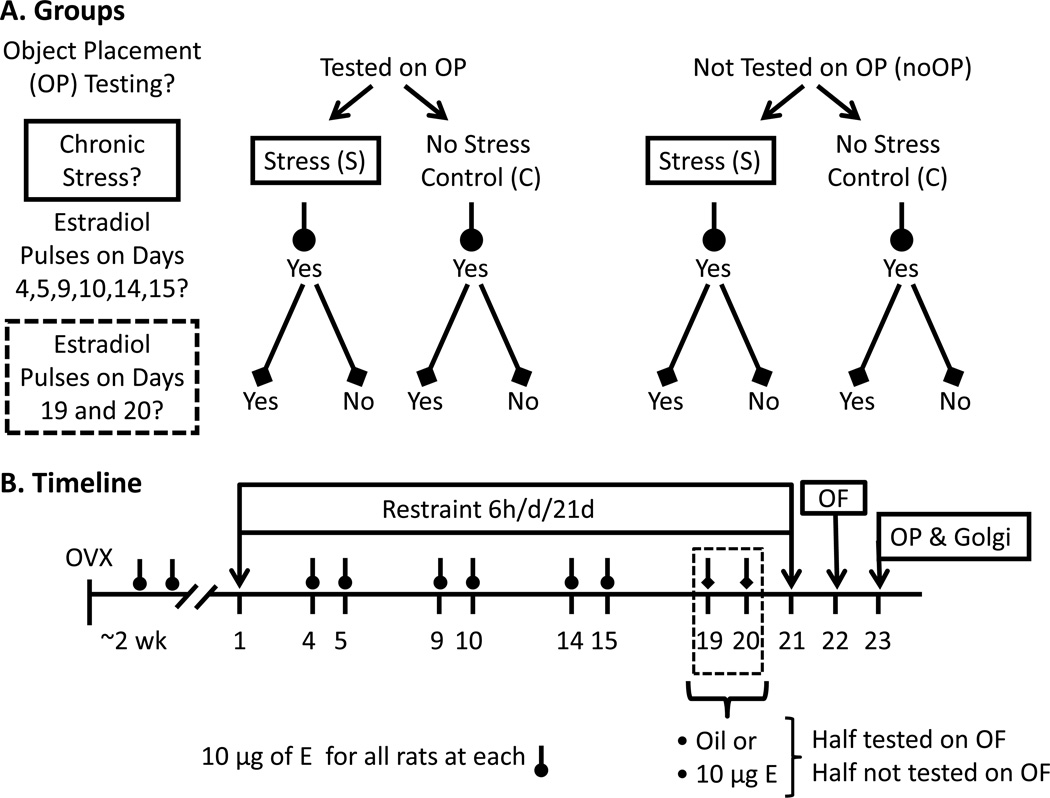
Eighty-eight female Sprague-Dawley rats, weighing 225–250 g at the time of arrival, were purchased from Charles River Labs (Hartford, CT). Rats were pair-housed in closed chambers (21–22° C) under a 12/12 light-dark cycle (lights off at 6:00AM), with access to food and water ad libitum. Rats were divided into eight experimental groups based upon stress history, whether estradiol was injected at the final two pulses, and exposure to behavioral testing. Testing was included as a variable due to research suggesting that behavioral training and/or testing can mask estrogen-induced increases in CA1 spine synapse density (Frick et al., 2004). Experimental groups included the following: control-vehicle (sesame oil) without object placement testing (CV-NoOP), control-vehicle- tested on object placement (CV-OP), control-estradiol without object placement testing (CE-NoOP), control-estradiol- tested on object placement (CE-OP), chronic stress-vehicle without object placement testing (SV-NoOP), chronic stress-vehicle- tested on object placement (SV-OP), chronic stress-estradiol without object placement testing (SE-NoOP), chronic stress-estradiol- tested on object placement (SE-OP). Figure 1A illustrates the final groupings.
Figure 1.
A. Group Illustration. In each of the testing and non-testing cohorts, a 2 × 2 design was used for chronic stress history (control or restraint stress) and final exposure to the last cyclic injections (vehicle or estradiol, 10 µg). Please note that all ovariectomized (OVX) female rats were given cyclic estradiol pulses leading up to the last two injections at 72 and 48 hours prior of assessment to maintain hippocampal responsiveness to the final injections. The final number of groups was eight. B. Experimental Timeline. After arrival, female Sprague-Dawley rats were OVX and given about two weeks to recover before starting the chronic stress procedure. Half the rats were chronically stressed by restraint for 6-hr daily for 21-days or designated as unstressed controls, denoted as days 1 to 21. Up until day 19, all rats were given cyclic estradiol (E) injections (10 µg, s.c.) to ensure a history of similar estrogen exposure prior to behavioral testing At days 19 and 20, rats were given pulse injections of either E or vehicle (sesame oil) to investigate acute effects of estradiol on OP and to determine how training may interfere with estradiol-induced changes in CA1 spines. On days 22 and 23, rats were tested on the open field (OF) and the object placement (OP) task when stress and estradiol-induced morphological changes would still be present. Within an hour of the end of OP, rats were euthanized to process the brains for Golgi.
Surgery
All rats underwent OVX surgery. Rats were administered a ketamine cocktail-based anesthesia (70 mg/kg ketamine, 6 mg/kg xylazine, 10 mg/kg acepromazine, in 0.9% sodium chloride; 1 ml/kg, i.m.) and ovaries were taken out bilaterally through a small 1 cm incision on the abdomen. Dissolvable Vicryl suture secured the muscle and wound clips held the skin. Rats were monitored under a heat lamp until they recovered by being able to move around in the recovery area after which the rats were returned to the animal colony. Approximately seven to ten days after recovery, the wound clips were removed before the restraint procedure commenced.
Chronic Restraint Stress and Handling
Restraint stress commenced two weeks following OVX surgery. Rats were placed in wire mesh restraints for 6h/d/21d. Restrainers (18 cm circumference × 24 cm long) were made in house from wire mesh (Flynn and Enslow, Inc., San Francisco, CA) with the cut ends smoothed with grip guard sealant (ACE Hardware). The open ends of the restrainers were clamped shut during the procedure with large clips on both ends. In addition, all rats were briefly handled during the last two weeks of chronic restraint to reduce any stress created between the experimenter and injections and to decrease potential anxiety for behavioral testing. Handling was kept at a minimum, with rats being held for approximately 1 minute.
Hormone Administration/Cyclic Injections
One week following OVX, all rats received cyclic injections of 10µg 17β-estradiol (Innovative Research of America, Sarosota, FL). Two estradiol injections were given 24 hours apart, every 4–5 days (Figure 1B). This pattern provided all rats with a history of cyclic estradiol treatment throughout procedure until the last set of pulse injections. Repeated estradiol injections also reduced problems associated with decreased sensitivity of the hippocampus and other brain structures that may result from extended estradiol deprivation (Daniel and Bohacek, 2010). Specifically, the absence of ovarian hormone for extended periods of time reduces the effectiveness by which estrogens could increase CA1 spine density. (Silva et al, 2003; Daniel, Hulst, & Berbling, 2006; McLaughlin et al., 2008; Smith et al., 2010). Consequently, this cyclic estradiol history ensured that rats would be primed for the final cyclic pulse of estradiol or vehicle injection.
At 72 and 48 hours prior to behavioral testing, the rats were divided into two groups, as indicated by the dotted rectangle on Figure 1A and 1B. Half of the rats received two acute pulse injections of 10 µg 17β-estradiol while the remaining half of rats received two acute pulse injections of 10 µg sesame oil (vehicle). Hereafter, rats that received the final two pulses of estradiol 72 and 48 hours prior to OP will be referred to as the repeated cyclic estradiol group, whereas the rats receiving the final two pulses of vehicle will be referred to as the vehicle group.
Open Field (OF) and Object Placement (OP) Testing
The OF was used as a measure of locomotor activity and provided an opportunity to familiarize the rats with the testing apparatus for the next day’s procedure for OP assessment that will be used to evaluate hippocampal-dependent spatial memory (Broadbent, Squire, & Clark, 2004; Ennaceur, Neave, & Aggleton, 1997). Both OF and OP protocols were similar to those previously described (Ennaceur et al., 1997; Luine et al., 2006; McLaughlin et al., 2008; Paris and Frye, 2008). To summarize, the OF consisted of a black box made of Plexiglas (61 × 61 × 38 cm) and rats received one 10 minute exploration session to help them habituate to the apparatus and assess overall activity level. In addition, the field box was separated into an inner and outer zone and distance traveled and time spent in each zone were calculated. An entry into a zone was denoted when the head of the rat completely crossed a grid. Ethovision tracking system (Noldus Information Technology Inc., Virginia) recorded exploratory activity within the box during OF testing.
Twenty-four hours after OF, the rats were tested in the OP task to determine spatial memory, which was defined by a rat spending more time with an object in a new location compared to an object in the old location. Rats were placed in a corner of the OF apparatus and the starting position was counter-balanced among groups. Two identical objects were located equidistant from the opposite corners of the box. These objects were 24cm tall and weighed approximately 0.88kg so that the rats could neither climb onto nor knock over the objects. OP testing consisted of a 3 minute session where a rat could explore the objects, given a four hour inter-trial interval, and then presented with another 3 minute session of object exploration. After every trial, each object, as well as the open field apparatus, was cleaned with Odormute to avoid odor cues within the maze. During the second trial, one of the objects was moved to a new corner, opposite of where the rat was placed at the beginning of the trial. Exploration was defined as a rat facing the object (within 2–3 cm) and actively sniffing or attentively touching the object. The total time exploring both objects was recorded. Each rat was required to explore the objects during trial 1 for a minimum of 10 s to ensure similar activity levels and motivation among the groups to be included in analyses (Beck & Luine, 1999). Three rats in the stressed-oil group (SO-OP) and one rat in the stressed-estradiol group (SET) failed to explore the objects a minimum of 10 s during the first 3 minute exposure period. Final rat numbers in the OP were: CV-OP (n = 12), CE-OP (n = 12), SV-OP (n = 9), SE-OP (n = 11).
For calculating OP exploration, an index was used (Ainge et al., 2006; Broadbent, Gaskin, Squire, & Clark, 2010; Dere, Huston, & De Souza Silva, 2005; Hammond, Tull, & Stackman, 2004; Mumby, Gaskin, Glenn, Schramek, & Lehmann, 2002), which controls for rats that have differences in total exploration. The OP index was calculated as follows:
Body Weight
Body weights were taken as a control measure to ensure that restraint was perceived as stressful because stress decreases and/or prevents weight gain (Hoffman, Armstrong, Hanna & Conrad, 2010; Kleen, Sitomer, Killeen & Conrad, 2006; Magariños and McEwen, 1995b; Wright and Conrad, 2005).
Golgi and Histology
When the rats completed the OP, they were euthanized and unperfused brain tissue was quickly removed and placed immediately into solutions, as per instructions using FD Rapid Golgistain ™ Kits (FD NeuroTechnologies, Baltimore, MD), as described in our previous studies (Hoffman et al., In Press; Kleen et al., 2006; McLaughlin et al., 2005). When finished with the FD Rapid Golgistain solutions, brains were blocked to target the hippocampus, frozen in 2-methylbutane and cut (100µm thickness, coronal sections, Microtome HM 500 OM Cryostat, 25/30 °C). Slides were left to dry for 1d in a dark location before staining. Staining involved rinsing the slides in distilled water, followed by developing solution (10min). Slide were then rinsed in distilled water, dehydrated in ascending series of ethanol, cleared in neoclear (Harleco®, Gibbstown, NJ) and coverslipped with Permount Mounting Media (Fisher Scientific International Inc.). Slides were left in the dark to dry for another 1–2wk.
Pyramidal neurons from CA3 and CA1 regions were selected for analyses. In order to be included in the study, neurons had to be fully and consistently stained throughout the entire dendritic tree and be relatively isolated from nearby neurons. A camera lucida drawing tube attached to an Olympus BX51 microscope was used to trace all neurons (320×). Dendritic length was quantified using a Scion Image Microcomputer Imaging Device Program (Scion Corporation, Frederick, MD, USA) attached to an Olympus BX 50 microscope. Hippocampal CA3 neurons were labeled as either Short-shaft (SS) or Long-shaft (LS) according to their location in the stratum pyramidale and primary apical shaft length (Fitch, Juraska, & Washington, 1989). SS and LS values were calculated for each rat to represent an average value for dendritic complexity per neuronal type (6–9 cells averaged/rat). Additionally, separate measures were taken for apical and basal CA3 neurons. Due to these criteria, the numbers of rats/group were as follows: CV-NoOP (n = 4), CV-OP (n = 8), CE-NoOP (n= 4), CE-OP (n = 6), SV-NoOP (n = 7), SV-OP (n = 8), SE-NoOP (n = 6) SE-OP (n = 6).
Hippocampal CA1 neuronal types were grouped together to generate one measure of branch points and branch length per rat. Apical and basal dendritic properties were analyzed separately. Successful staining of the CA1 region yielded 4–6 neurons, which were averaged to obtain one value per rat. Analyses on CA1 arborization included the following: CV-NoOP (n = 8), CV-OP (n = 7), CE-NoOP (n= 6), CE-OP (n = 8), SV-NoOP (n = 8), SV-OP (n = 8), SE-NoOP (n = 7) SE-OP (n = 7).
CA1 dendritic spines were categorized as either having “heads” or being “headless” (Diamond et al., 2006; McLaughlin et al., 2005; Papa, Bundman, Greenberger, & Segal, 1995). Apical and basal spines were counted separately and represented per 10µm dendritic segments. Final analyses of spine shape and density included the following subjects: CV-NoOP (n = 8), CV-OP (n = 7), CE-NoOP (n= 6), CE-OP (n = 8), SV-NoOP (n = 8), SV-OP (n = 8), SE-NoOP (n = 7) SE-OP (n = 6).
Statistical Analyses
The statistical software package, STATISTICA (Statsoft Inc., Tulsa, OK) or SPSS (v. 19) was used for the analyses using a Macintosh (OX 10.6.7). ANOVAs analyzed parametric data and were followed by Newman-Keuls posthoc tests, when p ≤ 0.05. Wilcoxon tests were performed on nonparametric data.
Results
Object Placement and Open Field
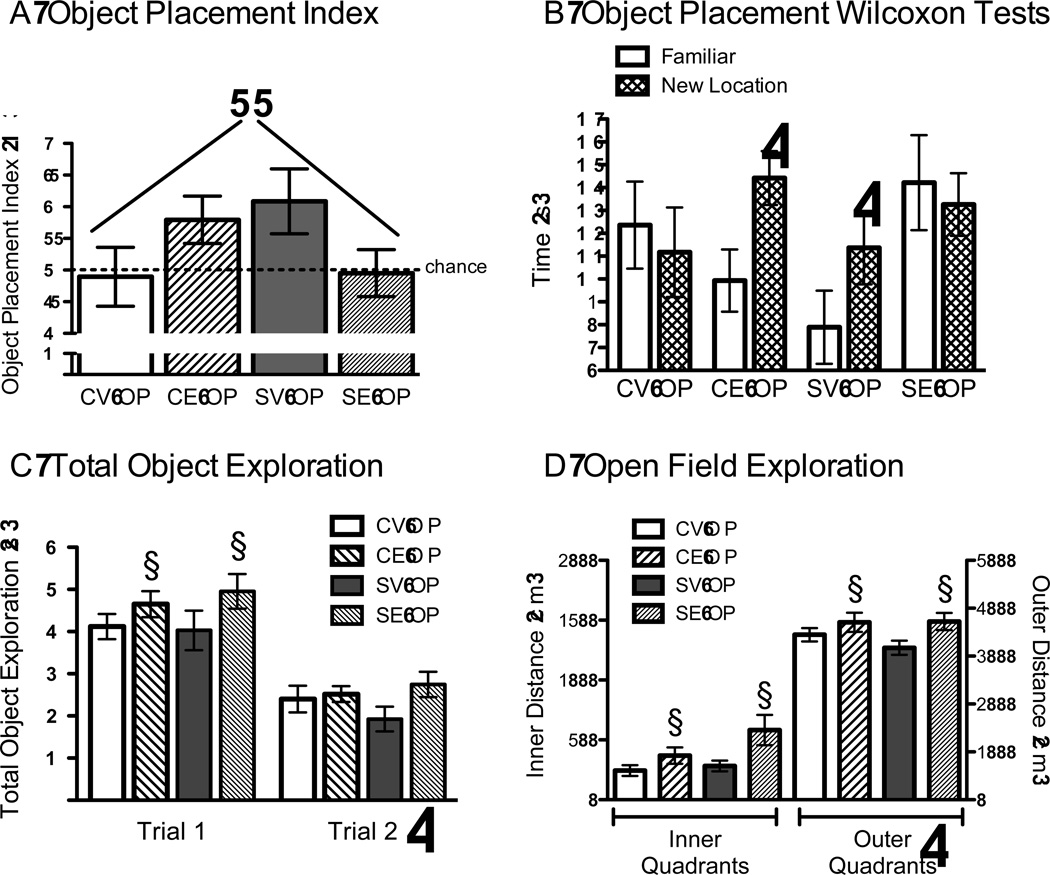
Chronic stress and 17β-estradiol each increased the OP index, but not when combined together. A 2 × 2 ANOVA for stress and estradiol treatment revealed a significant interaction between chronic stress and estradiol, F(1, 39) = 6.224, p = .01, with no significant main effects for stress or estradiol. Control rats treated with estradiol (CE-OP) and chronically stressed rats given oil vehicle (SV-OP) exhibited high and statistically similar exploration of the moved object, as indicated by the high OP index, which contrasted to the low OP index of the chronically stressed rats given estradiol (SE-OP) and the controls treated with vehicle (CV-OP, Figure 2A). While the omnibus ANOVA interaction between stress and estradiol was significant, posthoc tests failed to reveal statistically significant group differences. Therefore, time spent exploring the moved object was compared to the time spent exploring the object in the familiar location. These data were then analyzed for each treatment condition separately using Wilcoxon matched paired tests. These tests revealed that control rats treated with estradiol (CE-OP) and stressed rats treated with oil vehicle (SV-OB) spent more time exploring the object in the new location than compared to the object in the familiar location (p < .05, Figure 2B). There were no statistically significant effects for the other groups (CV-OP, SE-OP).
Figure 2.
Object Placement (OP) and Open Field (OF). A) A 2 × 2 ANOVA for stress and estradiol treatment revealed a significant interaction between chronic stress and estradiol, F(1, 39) = 6.224, p = .01, with estradiol facilitating OP in control rats and impairing OP in chronically stressed rats. ++ p = .01 for significant interaction for stress and estradiol. B) Control rats given estradiol (CE) and chronically stressed rats given vehicle (SE) showed preference for the moved object in the new location compared to the object in the familiar location, indicated by Wilcoxon tests. Controls given vehicle (CV) and chronically stressed rats given estradiol (SE) investigated both objects similarly. * p < .05 for the familiar object compared to the object in the new location. C) Total time spent exploring the objects showed that rats spent more time exploring the objects in trial 1 than they did in trial 2, * p < .001 for trial 2 vs. trial 1. Moreover, estradiol treated rats (CE, SE) spent more time exploring the objects than did the vehicle treated rats (CV, SV), but this was not observed in the second trial. § p ≤ .05 for significant main effect of estradiol. D) Estradiol (CE, SE) increased distance covered in the inner and outer quadrants than compared to vehicle (CV, SV). § p ≤ .05 for significant main effect of estradiol. * p < .05 for outer vs inner quadrant crossings. Data represent means ± S.E.M.
Another analysis was performed to determine whether the OP index could have been influenced by the total time spent exploring both objects. An omnibus ANOVA for stress and estradiol treatment across the two trials on the total time spent exploring the two objects (sum of exploration for each object) was performed and revealed a significant repeated effect of trial, F(1, 40)=109.7, p < .0001, with no other significant effects. Rats explored the objects more in trial 1 than they did in trial 2 (average object exploration in trial 1 = 44.5 ± 3.5 vs. trial 2 = 24.0 ± 2.6). Subsequent analyses investigated the two trials separately. In trial 2, a 2 × 2 ANOVA for stress and estradiol treatment on exploration of both objects revealed no statistically significant effect (p > .1), which ranged from 19.2 ± 3.0 for SV-OP to 27.5 ± 10.2 for SE-OP (Figure 2C). In addition, a 2 × 2 ANOVA was performed on the total time spent exploring the sum of both objects during trial 1 when the objects were first introduced. A marginally significant effect of estradiol was found, F(1, 40) = 3.935, p = .054, with no other significant effects. Control and stressed rats treated with estradiol (CE-OP, SE-OP) explored both objects longer than did control and stressed rats treated with vehicle (CV-OP, SV-OP, Figure 2C).
Distance covered on the OF was also analyzed to confirm estradiol’s effects on locomotor activity. A 2 × 2 × 2 ANOVA for stress and estradiol treatment across the inner and outer arenas of the OF was performed, revealing a significant main effect of estradiol treatment, F(1,44)=49.238, p < .01, and arena, F(1,44)=965.1, p < .0001, with no other significant effects. While estradiol-treated rats (CE-OP, SE-OP) covered more distance in the center or outer arena, rats of all conditions covered the most territory in the perimeter than in the center of the OF (Figure 2D).
Hippocampal Morphology
CA3 Dendritic Arborization
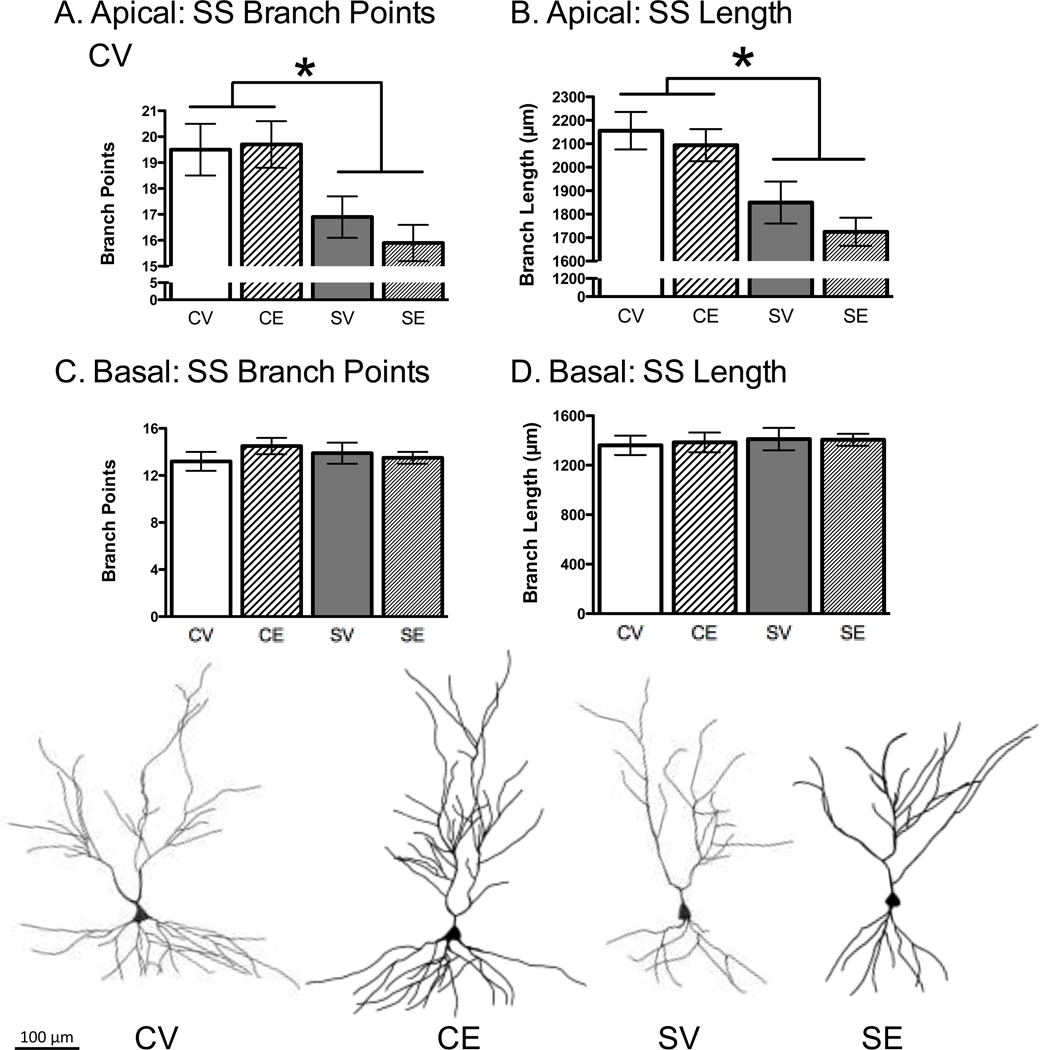
The data were collapsed across behavioral testing history (tested and non-tested on the OP) because testing history did not produce statistically significant effects on CA3 apical dendritic branch points or length (p > .10). For short-shafted CA3 neurons, chronic stress reduced the apical dendritic branch points, F(1, 45) = 13.32, p < .01 and apical branch length, F(1, 45) = 17.98, p < .01 (Figure 3A, 3B). Neither estradiol treatment nor the interaction between stress and estradiol treatment was significant, indicating that estradiol failed to prevent chronic stress from causing CA3 dendritic retraction. The basal dendritic region of the CA3 short-shafted neurons was statistically similar, as ANOVA did not show statistical effects for the basal dendritic branch points or branch length (Figure 3C, 3D). For long-shafted neurons, there were no statistically significant effects for stress or estradiol treatment on branch points and length in either the apical or basal region (Table 1).
Figure 3.
Effects of Chronic Stress and Estradiol on Hippocampal CA3 Dendritic Complexity. Chronic stress decreased CA3 apical dendritic branch points (A) and length (B) in short shafted (SS) neuron types. Estradiol had no significant effect. Chronic stress and estradiol had no effect on the SS branch points (C) and branch length (D) in the CA3 basal region. Representative CA3 SS neurons are shown for control-vehicle (CV), control-estradiol (CE), stress-vehicle (SV) and stress-estradiol (SE). * p < .05. Data represent means ± S.E.M.
Table 1.
Hippocampal CA3 Long-Shaft Dendritic Complexity
| Group | CV | CE | SV | SE |
|---|---|---|---|---|
| Apical Branch Points | 11.6 ± 1.0 | 12.7 ± 0.9 | 12.4 ± 0.9 | 12.4 ± 0.5 |
| Apical Branch Length | 1346.6 ± 129.4 | 1453.6 ± 80.1 | 1448.4 ± 96.8 | 1415.3 ± 64.0 |
| Basal Branch Points | 12.2 ± 2.3 | 12.6 ± 1.0 | 12.9 ± 0.7 | 13.6 ± 1.1 |
| Basal Branch Length | 1382.6 ± 75.4 | 1284.2 ± 77.6 | 1329.1 ± 67.2 | 1369.9 ± 91.1 |
CA1 Dendritic Arborization
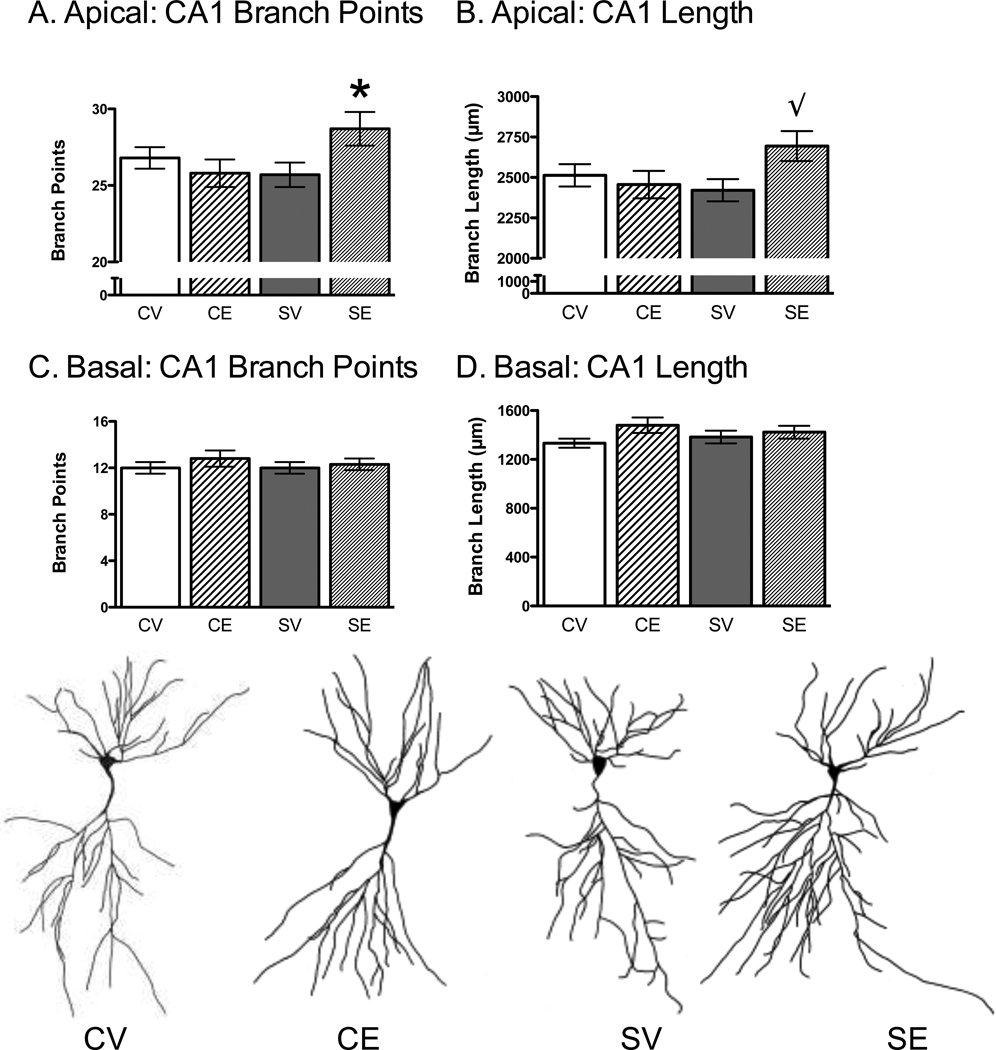
Similar to the CA3 neurons, the data from the CA1 region were collapsed across behavioral testing history (tested and non-tested on OP) because testing history did not produce statistically significant effects on CA1 dendritic branch points or length (p > .10). A 2 × 2 ANOVA for stress and estradiol treatment showed a significant interaction for CA1 apical branch points, F(1, 51) = 5.35, p < .05. Newman-Keuls post hoc analyses showed that chronic stress and estradiol (SE) increased CA1 apical branches compared to the other groups (CV, SV, CE; Figure 4A). A similar pattern was revealed for CA1 dendritic branch length, but the interaction between stress and estradiol treatment approached significance (p = .08, Figure 4B). No other significant effects were found for the CA1 apical region or for the CA1 basal region for branch points and length (p > .10, Figure 4C, 4D).
Figure 4.
Effects of Chronic Stress and Estradiol on Hippocampal CA1 Dendritic Complexity. Chronic stress and estradiol (CE) increased CA1 apical branch points (A) and length (B) without affecting the basal branch points (C) and length (D). Representative CA1 neurons are shown for control-vehicle (CV), control-estradiol (CE), stress-vehicle (SV) and stress-estradiol (SE). * p < .05 compared to the other conditions. √ p = .08 compared to the other conditions. Data represent means ± S.E.M.
CA1 Dendritic Spines
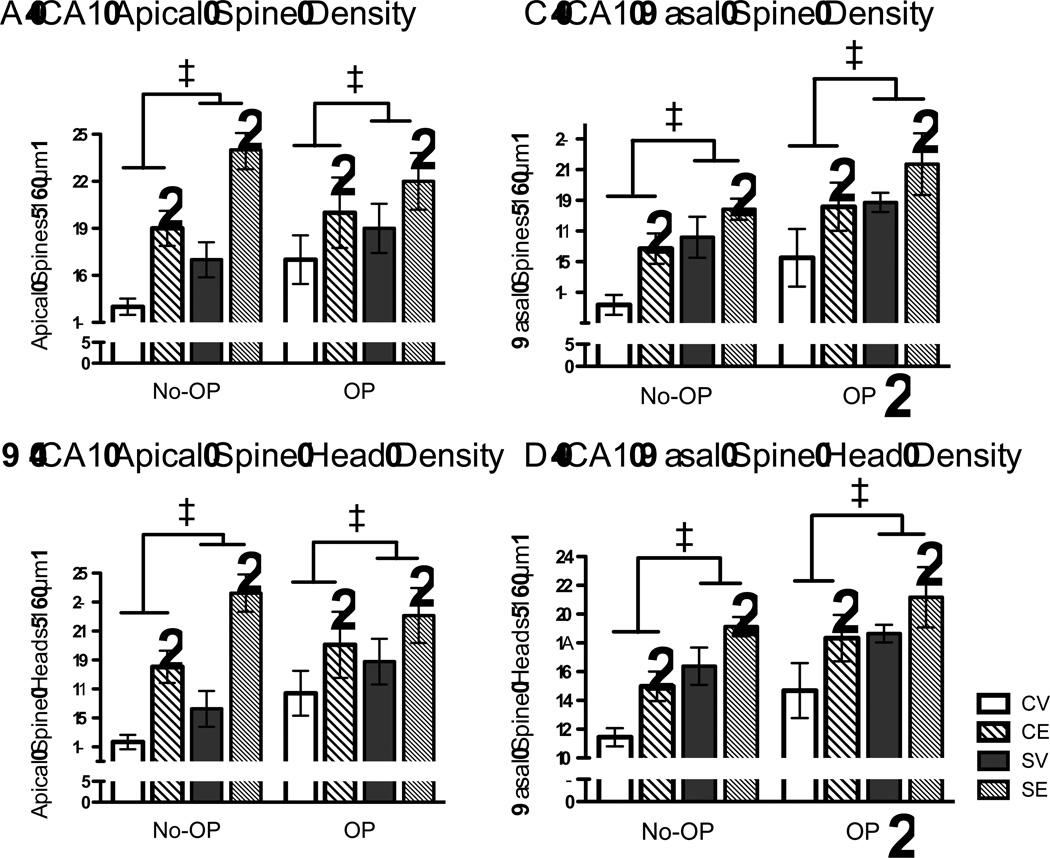
Chronic stress and estradiol influenced all of the CA1 dendritic spine properties that were measured in the apical and basal regions, with OP testing condition further modifying the CA1 basal region. A 2 × 2 × 2 ANOVA for stress, estradiol treatment and whether rats were tested on OP was performed as between subjects measures on CA1 apical spine density, apical spine head density, basal spine density and basal spine head density. Chronic stress and estradiol increased CA1 apical spine density (stress, F(1, 50) = 7.075, p = .01; estradiol, F(1, 50) = 17.329, p < .001; Figure 5A) and CA1 apical spine head density (stress, F(1, 50) = 7.125, p = .01; estradiol, F(1,50) = 20.526, p < .001, Figure 5B). No other effects or interactions were significant. For spines located in the basal region of CA1 dendrites, chronic stress, estradiol and object placement testing enhanced basal spine density (stress, F(1, 50) = 13.22, p < .001; estradiol, F(1, 50) = 114.096, p < .005; OP testing, F(1, 50) = 8.932, p < .005; Figure 5C) and basal spine head density (stress, F(1,50) = 18.259, p < .001; estradiol, F(1,50) = 11.174, p < .005; OP testing, F(1,50) = 8.593, p = .005). No other effects or interactions were significant.
Figure 5.
Effects of Chronic Stress, Estradiol and Testing Exposure on Hippocampal CA1 Dendritic Spine Number and Shape. Estradiol (CE, SE) and chronic stress (SV, SE) increased CA1 apical spine density (A), CA1 apical spine head density (B), CA1 basal spine density (C), and CA1 basal spine head density (D). Hippocampal morphology was investigated separately for rats tested on the object placement spatial paradigm (OP) or rats were left undisturbed in their home cages during this time (No-OP). OP increased the CA1 basal spine density (C) and CA1 basal spine heads (D), and these effects were a general elevation of previous numbers without causing an interaction effect. * p < .05 next to the bar graphs represent a significant main effect of estradiol, * p < .05 next to OP represents a significant main effect of OP compared to No-OP. ‡ p < .05 for significant main effect of chronic stress compared to nonstressed controls. Data represent means ± S.E.M.
Correlations between Hippocampal Dendritic and Spine Morphology with OP
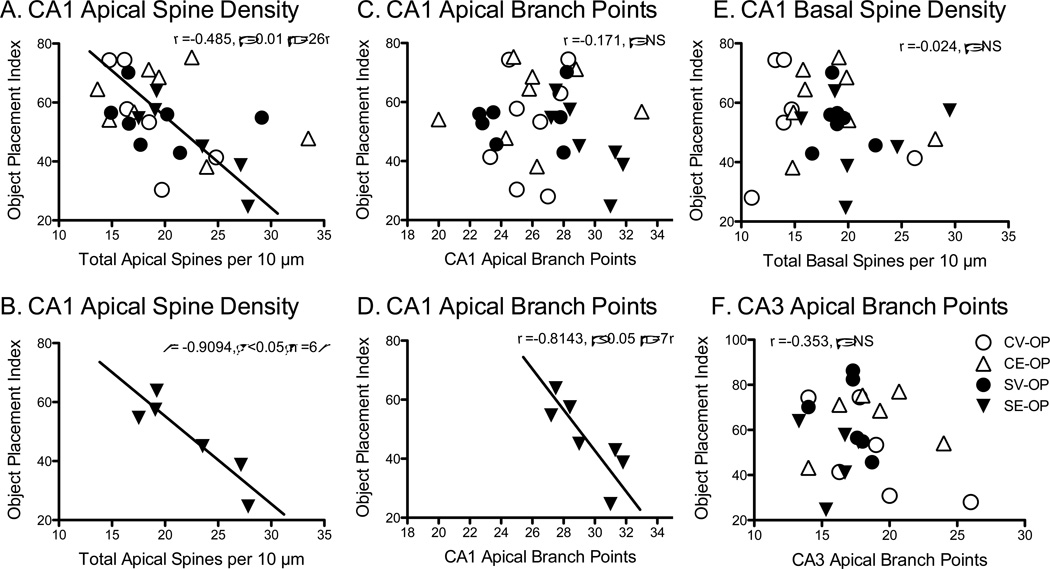
Correlations were performed on the OP index with CA1 spine density and spine shape, as well as apical dendritic complexity for the CA1 and CA3 regions. OP index inversely correlated with CA1 apical spine density (Pearson r = −.386, p < .05, n = 27, Figure 6A) and spine head density (Pearson r = −.874, p = .05, n = 27, data not shown). In these previous two analyses, one rat from the CV-OP was excluded because the data point was completely separated from the other cluster (CA1 apical spine density = 11.9 spines/10µm and OP = 28.0). Nevertheless, the SE-OP group predominately carried this effect (spine density, Pearson r = −.9094, p = .01, n = 6, Figure 6B), as no other groups exhibited a significant correlation alone. These data suggest that for the chronically stressed rats treated with estradiol, as CA1 apical spine density or heads increased, OP performance became worse. The correlation between OP and CA1 apical branch points failed to reach significance across groups. However, as found with the CA1 apical spines, correlations for each group separately showed a significant negative correlation with OP and CA1 apical dendritic branch points for the stressed rats given estradiol only (SE-OB, r = −.8143, p < .05, n = 7). These analyses reveal that stressed rats injected with estradiol exhibited greater numbers of CA1 apical dendritic spines, CA1 apical dendritic spine heads, and enhanced CA1 dendritic complexity, which correlated with decreased performance on OP. No significant effects were observed for correlations between object placement index and CA1 basal spine density, CA1 basal spine heads, CA3 apical and basal dendritic complexity with object placement. Please note that the numbers of subjects for each group slightly varied as some rats would have sufficient number of properly stained CA3 neurons, but without sufficient numbers of CA1 stained neurons, etc. Therefore, the subjects used in each correlation will vary depending upon whether the morphology was available.
Figure 6.
Correlations between Object Placement (OP) Index and Hippocampal Morphology. A) Object placement index significantly and negatively correlated with CA1 apical spine density (r = 0.485, p = .01, n = 26), which was predominately carried by the stress-estradiol (SE-OP) group in graph B (r = .909, p = .05, n=6). C) Object placement index did not correlate with CA1 apical branch points, but when the stress-estradiol (SE-OP) group was probed alone, a significant negative correlation was revealed in graph D (r = 0.814, p < .05, n=7). E) Object placement index did not correlate with CA1 basal spine density, nor for CA3 apical branch points (F).
Body weight
Chronic stress decreased body weight gain. A 2 × 2 × 2 mixed-factor ANOVA for stress and estradiol treatment, across the first and last day of restraint on body weight showed significant main effects of chronic stress F(1, 84) = 87.379, p < .001), day F(1, 84) = 656.34, p < .001, and a significant interaction between chronic stress and day F(1,84) = 448.16, p < .001). All groups had similar weights at the beginning of the restraint procedure, but the control rats (CV, CE) gained weight after 21 days, whereas the chronically stressed rats (SV, SE) did not gain weight (Table 2). While estradiol can attenuate weight gain in female rats, we did not expect differences in this paradigm because all rats were injected with estradiol until just prior the behavior testing (see Figure 1).
Table 2.
Body Weight (gms)
| Group | CV | CE | SV | SE |
|---|---|---|---|---|
| Body Weight on Day 0 | 255.4 ± 3.8 a | 259.1 ± 3.8 a | 259.2 ± 4.2 a | 260.5 ± 4.5 a |
| Body Weight on Day 21 | 315.0 ± 5.6 b | 321.0 ± 6.6 b | 266.8 ± 4.6 a | 264.4 ± 6.2 a |
Means with different letters indicate statistically significant differences compared to the other means (i.e., a ≠ b; a = a)
Discussion
This study demonstrated that chronic stress or repeated cyclic estradiol pulses can enhance hippocampal-dependent spatial memory in OVX female rats, but not when these treatments are combined. Indeed, rats that were chronically stressed and injected with the final pulses of estradiol 72 and 48 hours prior to testing (SE-OP) showed poor spatial memory on the OP task, which was similar to nonstressed controls given vehicle 72 and 48 hours prior to testing (CV-OP). As anticipated, estradiol and testing experience on the OP increased CA1 spine density and heads in the basal dendritic region. For these reasons, along with the findings from other reports (Diamond et al., 2006; Foy, Baudry, Diaz Brinton, & Thompson, 2008; Leranth et al., 2002; Leuner et al., 3003; MacLusky et al., 2005; McLaughlin et al., 2008; McLaughlin et al., 2010; Silva et al., 2000; Spencer et al., 2008; Woolley et al., 1990; Woolley & McEwen, 1992), we expected CA1 spines and/or dendrites to positively correlate with OP performance. However, CA1 apical spine density and CA1 apical branch points negatively correlated with OP performance. As CA1 apical spine density and branch points increased, performance on the OP worsened. Probing the data further revealed that this effect was predominately carried by the chronically stressed group given estradiol (SE-OP) for both CA1 apical spine density, shape and branch points. Moreover, OP performance was independent of CA3 dendritic complexity, as chronically stressed rats given vehicle or estradiol at the final two injections (SV-OP, SE-OP) exhibited CA3 dendritic retraction in the SS CA3 neurons, yet SV-OP (but not SE-OP) performed well on the OP task. Therefore, these data are the first to demonstrate that chronic stress or repeated estradiol pulses can facilitate hippocampal-dependent OP, but not when co-administered, and that these effects may involve a balance of CA1 apical spine expression that is independent of the CA3 dendritic complexity.
In this paradigm, the amount of time exploring the displaced object more than the non-displaced object was used as a measure of spatial memory. Inherent in this task is the motivation of the rats to explore the objects in order to form a spatial representation of their location. The amount of time exploring the objects in trial 2 was statistically similar across groups, which suggested similar motivational levels during object placement testing. However, trial 1 revealed differences in total object exploration. Rats given estradiol (CE-OP, SE-OP) explored the objects more in trial 1 (Figure 2A) than did the rats given vehicle (CV-OP, SV-OP), which may have provided an advantage to the estradiol-treated rats. The increased distance covered in the OF and the higher activity level during trial 1 of OP are consistent with estradiol enhancing locomotion. Because estradiol increased locomotion in both the inner and outer zones of the OF, this effect does not appear to be strictly anxiolytic, as found by others and us (Bowman, Ferguson, & Luine, 2002; Huynh, Krigbaum, Hanna, & Conrad, 2011; Lund, Rovis, Chung, & Handa, 2005). Perhaps estradiol’s facilitatory effects on locomotion may have inadvertently given the rats an advantage in viewing the room from a variety of spatial locations. Nonetheless, estradiol was not broadly advantageous, as CE-OP showed enhanced spatial memory, while SE-OP performed at chance. Moreover, chronically stressed rats given vehicle (SV-OP) showed enhanced spatial memory even though the amount of total object exploration in trial 1 was less than that found from both estradiol treated groups. The additional requirement that rats had to explore the objects for at least 10 s in trial 1 also may have mitigated any potential motivational differences among groups. Taken together, estradiol may have facilitated spatial memory in control rats through elevating locomotion in trial 1, but this interpretation does not explain the facilitated performance of chronically stressed rats given vehicle or the poor performance of the chronically stressed rats given estradiol.
Given the past literature that estradiol and chronic stress influence hippocampal CA1 spines and morphology in a similar direction by enhancing spine density, shape, and dendritic complexity, the current study investigated whether rats exposed to both conditions of estradiol pulses and chronic stress would show even greater functional benefit on a spatial task than either condition alone. While the control rats treated with the final injections of estradiol (CE-OP) and the chronically stressed treated with the final injections of vehicle (SV-OP) showed spatial memory by preferring the displaced object over the object in the familiar location, the chronically stressed rats given the final injections of estradiol (SE-OP) showed poor spatial memory by examining both objects similarly. Moreover, CA1 spine density and CA1 apical branch points negatively correlated with OP performance with higher CA1 morphological measures producing a lower OP index. While the correlation for CA1 spine density and OP was present for all groups, the stress-estradiol group predominately carried the correlation (r = −0.9094, p < 0.05). In addition our results corroborate past reports and confirm that estrogens increase CA1 spine density and spine heads in the apical and basal regions (McLaughlin et al., 2005; McLaughlin et al., 2008; McLaughlin et al., 2010; Silva, Mello, Freymuller, Haidar, & Baracat, 2003; Woolley & McEwen, 1992, 1993; Woolley, 1998), that chronic stress increases CA1 spine density in the apical and basal spine regions (McLaughlin et al., 2005; McLaughlin et al., 2010), and that chronic stress combined with estradiol increases CA1 dendritic complexity in both OVX rats treated with estradiol or in female rats in proestrus with high estradiol (McLaughlin et al., 2010). Therefore, the consistencies of our results with many of the morphological effects in the literature reinforce the validity of our estradiol and chronic stress procedures and further demonstrate that these combined variables fail to exert mutually beneficial actions on spatial memory on OP.
Interestingly, other work has investigated potentially beneficial manipulations on hippocampal function and failed to find synergistic effects in female mice (Gresack & Frick, 2004; Gresack, Kerr, & Frick, 2007). In the studies by Gresack and Frick (2004, 2007), the actions of estrogens and environmental enrichment were investigated on cognitive ability in mice. Environmental enrichment involves exposing subjects to increased exercise opportunities, social interactions, novelty and visual cues (Rosenzweig & Bennett, 1996), which can produce robust beneficial effects on cognition (Kempermann, Kuhn, & Gage, 1997; Rampon et al., 2000; Rosenzweig, Breedlove, & Watson, 2005), including heightened hippocampal function (Wright & Conrad, 2008). However, young adult mice raised in enrichment and given estrogens failed to show enhanced object recognition (Gresack et al., 2007) or spatial working memory on a radial arm water maze (Gresack & Frick, 2004). Consequently, two separate research groups investigating the potential synergistic effect of estrogens with another manipulation (environmental enrichment or chronic stress) failed to demonstrate synergistic or additive actions. It is tempting to speculate that the rodents were already at peak performance and so any manipulation to increase or decrease the underlying neural constructs would tip this balance. For instance, excessive or abnormal synaptic plasticity has been described in many conditions including Rett syndrome and Fragile X syndrome (Johnston, 2004; Wang, Martin, & Zukin, 2010). Perhaps enhancing CA1 spine density, shape or dendritic complexity in our paradigm may have hindered optimal performance? While our investigation focused on the hippocampal CA1 and CA3 regions, it is important to recognize that other neural substrates might contribute to these behavioral alterations. Possible candidates that are sensitive to chronic stress and estrogens include the prefrontal cortex (Garrett and Wellman, 2009; Wilber et al., 2011), striatum (Sadowski et al., 2009), and amygdala (Hoffman et al., 2010), to name a few. Future studies may target these regions for putative links for how chronic stress and estradiol influence spatial memory.
An important component of our study was the careful documentation of testing effects on hippocampal CA1 spine density. Past work showed that exposure to training and testing procedures could potentially influence CA1 spine density (Beltrán-Campos et al., 2011; Bohacek & Daniel, 2007; Diamond et al., 2006; Frick et al., 2004). Consequently, we included a cohort of rats that were not tested on object placement in order to determine the status of the hippocampus morphology in rats that were tested on OP. Indeed, we found that rats tested in OP had greater CA1 apical and basal spine density and apical and basal spine head density. In contrast, other studies reported that training negated the actions of estrogens on hippocampal CA1 morphology (Bohacek & Daniel, 2007; Frick et al., 2004). In the study by Bohacek and Daniel (2007), silastic implants were used, which provided constant estradiol levels that could have reduced the responsiveness of the CA1 region. Moreover, Bohacek and Daniel’s (2007) used cholesterol, a precursor of estrogens, as a comparison control, which could have increased CA1 spines by itself or through conversion to other steroids (McLaughlin et al., 2010), potentially creating an overall null experimental outcome. For the study by Frick and colleagues (2004), estradiol benzoate was given in two injections, with the last injection 48-hrs prior to water maze testing. Either the delay from OVX to estrogen treatment (McLaughlin et al., 2010) or the aversive nature of the water maze paradigm (Conrad, 2006; Conrad, 2010; Conrad & Bimonte-Nelson, 2010) could have been sufficient to potentially make changes in CA1 spines undetectable. Therefore, the method of estrogen administration, the window of time that estrogen is absent, and the nature of the task contributed to these differing effects. Importantly, the testing paradigm had no influence on CA1 or CA3 dendritic complexity, indicating that spines are more sensitive to testing parameters than the overall dendritic arborization.
Another novel finding was a lack of estradiol’s actions to protect against stress-induced CA3 dendritic retraction. We have previously reported that silastic implants of estradiol protected against stress-induced CA3 dendritic retraction in OVX female rats (McLaughlin et al., 2010) and that OVX females expressed robust CA3 dendritic retraction (McLaughlin et al., 2005) when compared to data taken from prior reports using gonadally intact females (Galea et al., 1997; McLaughlin et al., 2010). The current study was the first to use a cyclic regimen of estradiol injections to investigate the potential protective actions against stress-induced CA3 dendritic retraction. However, our 4 to 5 week cyclic estradiol regimen failed to protect against stress-induced CA3 dendritic retraction even though CA1 dendritic spine density, shape and complexity was changed. Perhaps females need continual exposure to estrogens, baseline levels, or the window between the last estrogen exposure and the start of the next regimen should be narrowed in order to protect against stress-induced CA3 dendritic complexity (Conrad & Bimonte-Nelson, 2010). Regardless of the interpretation, the current study reveals that CA3 dendritic complexity did not correlate with spatial memory on object placement.
In summary, these data show that repeated cyclic estradiol injections and chronic stress both facilitate spatial memory on an object placement task, but that these manipulations are neither synergistic nor additive. A unique feature of this paradigm was the cyclic estradiol injection regimen to maintain responsiveness of the brain and the validation of the morphological measures within the hippocampus within the same animals. When estradiol and chronic stress improved spatial memory, these actions were independent of the condition of the hippocampal CA3 dendritic arbors. Instead, the effect on spatial memory appeared to involve the CA1 region through increasing spine density and/or heads to a point, as too much of an increase appears to be detrimental. Consequently, these findings indicate different potential mechanisms by which estradiol and chronic stress may influence spatial memory.
Acknowledgments
This research was supported by the National Institute of Mental Health MH74727 awarded to Cheryl D. Conrad, Arizona Biomedical Research Commission awarded to Cheryl D. Conrad, and Howard Hughes Medical Institute through the Undergraduate Science Education Program awarded to Mariam El-Ashmawy.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/bne.
References
- Ainge JA, Heron-Maxwell C, Theofilas P, Wright P, de Hoz L, Wood ER. The role of the hippocampus in object recognition in rats: Examination of the influence of task parameters and lesion size. Behavioral Brain Research. 2006;167(1):183–195. doi: 10.1016/j.bbr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Beck KD, Luine VN. Food deprivation modulates chronic stress effects on object recognition in male rats: Role of monoamines and amino acids. Brain Research. 1999;830:56–71. doi: 10.1016/s0006-8993(99)01380-3. [DOI] [PubMed] [Google Scholar]
- Beck KD, Luine VN. Sex differences in behavioral and neurochemical profiles after chronic stress: Role of housing conditions. Physiology & Behavior. 2002;75:661–673. doi: 10.1016/s0031-9384(02)00670-4. [DOI] [PubMed] [Google Scholar]
- Beltrán-Campos V, Prado-Alcalá RA, León-Jacinto U, Aguilar-Vázquez A, Quirarte GL, Ramírez-Amaya V, Díaz-Cintra S. Increase of mushroom spine density in CA1 apical dendrites produced by water maze training is prevented by ovariectomy. Brain Research. 2011;1369:119–130. doi: 10.1016/j.brainres.2010.10.105. [DOI] [PubMed] [Google Scholar]
- Bohacek J, Danielz JM. Increased daily handling of ovariectomized rats enhances performance on a radial-maze task and obscures effects of estradiol replacement. Hormones & Behavior. 2007;52(2):237–243. doi: 10.1016/j.yhbeh.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Bowman RE, Zrull MC, Luine VN. Chronic restraint stress enhances radial arm maze performance in female rats. Brain Research. 2001;904:279–289. doi: 10.1016/s0006-8993(01)02474-x. [DOI] [PubMed] [Google Scholar]
- Bowman RE, Ferguson D, Luine VN. Effects of chronic restraint stress and estradiol on open field activity, spatial memory, and monoaminergic neurotransmitters in ovariectomized rats. Neuroscience. 2002;113:401–410. doi: 10.1016/s0306-4522(02)00156-2. [DOI] [PubMed] [Google Scholar]
- Bowman RE. Stress-induced changes in spatial memory are sexually differentiated and vary across the lifespan. Journal of Neuroendocrinology. 2005;17(8):526–535. doi: 10.1111/j.1365-2826.2005.01335.x. [DOI] [PubMed] [Google Scholar]
- Bowman RE, Micik R, Gautreaux C, Fernandez L, Luine VN. Sex-dependent changes in anxiety, memory, and monoamines following one week of stress. Physioogy & Behavior. 2009;97(1):21–29. doi: 10.1016/j.physbeh.2009.01.012. [DOI] [PubMed] [Google Scholar]
- Broadbent NJ, Squire LR, Clark RE. Spatial memory, recognition memory, and the hippocampus. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(40):14515–14520. doi: 10.1073/pnas.0406344101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbent NJ, Gaskin S, Squire LR, Clark RE. Object recognition memory and the rodent hippocampus. Learning & Memory. 2010;17(1):5–11. doi: 10.1101/lm.1650110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD, Galea LAM, Kuroda Y, McEwen BS. Chronic stress impairs rat spatial memory on the Y-Maze, and this effect is blocked by tianeptine pretreatment. Behavioral Neuroscience. 1996;110(6):1321–1334. doi: 10.1037//0735-7044.110.6.1321. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Magariños AM, LeDoux JE, McEwen BS. Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behavioral Neuroscience. 1999;113(5):902–913. doi: 10.1037//0735-7044.113.5.902. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Grote KA, Hobbs RJ, Ferayorni A. Sex differences in spatial and non-spatial Y-maze performance after chronic stress. The Neurobiology of Learning and Memory. 2003;79(1):32–40. doi: 10.1016/s1074-7427(02)00018-7. [DOI] [PubMed] [Google Scholar]
- Conrad CD. What is the functional significance of chronic stress-induced CA3 dendritic retraction within the hippocampus? Behavioral and Cognitive Neuroscience Reviews. 2006;5(1):41–60. doi: 10.1177/1534582306289043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD. A critical review of chronic stress effects on spatial learning and memory. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2010;34(5):742–755. doi: 10.1016/j.pnpbp.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Bimonte-Nelson H. Impact of the hypothalamic-pituitary-adrenal/gonadal axes on trajectory of age-related cognitive decline. In: Martini L, editor. Neuroendocrinology: Pathological Situations and Diseases. 2010/06/15 ed. Vol. 182. Amsterdam, Netherlands: Elsevier; 2010. pp. 31–76. [DOI] [PubMed] [Google Scholar]
- Czéh B, Michaelis T, Watanabe T, Frahm J, de Biurrun G, van Kampen M, Bartolomucci A, Fuchs E. Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proceedings of the National Academy of Sciences. 2001;98:12796–12801. doi: 10.1073/pnas.211427898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel JM, Hulst JL, Berbling JL. Estradiol replacement enhances working memory in middle-aged rats when initiated immediately after ovariectomy but not after a long-term period of ovarian hormone deprivation. Endocrinology. 2006;147(10):607–614. doi: 10.1210/en.2005-0998. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Bohacek J. The critical period hypothesis of estrogen effects on cognition: Insights from basic research. Biochemica et Biophysica Acta. 2010;1800:1068–1076. doi: 10.1016/j.bbagen.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Dere E, Huston JP, De Souza Silva MA. Integrated memory for objects, places, and temporal order: evidence for episodic-like memory in mice. Neurobiology of Learning and Memory. 2005;84(3):214–221. doi: 10.1016/j.nlm.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Diamond DM, Campbell AM, Park CR, Woodson JC, Conrad CD, Bachstetter AD, Mervis R. Influence of predator stress on the consolidation versus retrieval of long-term spatial memory and hippocampal spinogenesis. Hippocampus. 2006;16:571–576. doi: 10.1002/hipo.20188. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Neave N, Aggleton JP. Spontaneous object recognition and object location memory in rats: the effects of lesions in the cingulate cortices, the medial prefrontal cortex, the cingulum bundle and the fornix. Experimental Brain Research. 1997;113(3):509–519. doi: 10.1007/pl00005603. [DOI] [PubMed] [Google Scholar]
- Fitch JM, Juraska JM, Washington LW. The dendritic morphology of pyramidal neurons in the rat hippocampal CA3 area. I. Cell types. Brain Research. 1989;479:105–114. doi: 10.1016/0006-8993(89)91340-1. [DOI] [PubMed] [Google Scholar]
- Foy MR, Baudry M, Diaz Brinton R, Thompson RF. Estrogen and hippocampal plasticity in rodent models. Journal of Alzheimers Disease. 2008;15(4):589–603. doi: 10.3233/jad-2008-15406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM, Fernandez SM, Bennett JC, Prange-Kiel J, MacLusky NJ, Leranth C. Behavioral training interferes with the ability of gonadal hormones to increase CA1 spine synapse density in ovariectomized female rats. European Journal of Neuroscience. 2004;19(11):3026–3032. doi: 10.1111/j.1460-9568.2004.03427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea LAM, McEwen BS, Tanapat P, Deak T, Spencer RL, Dhabhar FS. Sex differences in dendritic atrophy of CA3 pyramidal neurons in response to chronic restraint stress. Neuroscience. 1997;81(3):689–697. doi: 10.1016/s0306-4522(97)00233-9. [DOI] [PubMed] [Google Scholar]
- Garrett JE, Wellman CL. Chronic stress effects on dendritic morphology in medial prefrontal cortex: sex differences and estrogen dependence. Neuroscience. 2009;162(1):195–207. doi: 10.1016/j.neuroscience.2009.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB. Long-term treatment with estrogen and progesterone enhances acquisition of a spatial memory task by ovariectomized aged rats. Neurobiology of Aging. 2000;21(1):107–116. doi: 10.1016/s0197-4580(00)00103-2. [DOI] [PubMed] [Google Scholar]
- Gresack JE, Frick KM. Environmental enrichment reduces the mnemonic and neural benefits of estrogen. Neuroscience. 2004;128(3):459–471. doi: 10.1016/j.neuroscience.2004.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresack JE, Kerr KM, Frick KM. Short-term environmental enrichment decreases the mnemonic response to estrogen in young, but not aged, female mice. Brain Research. 2007;1160:91–101. doi: 10.1016/j.brainres.2007.05.033. [DOI] [PubMed] [Google Scholar]
- Hammond RS, Tull LE, Stackman RW. On the delay-dependent involvement of the hippocampus in object recognition memory. Neurobiology of Learning and Memory. 2004;82:26–34. doi: 10.1016/j.nlm.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Hoffman AN, Armstrong CR, Hanna JJ, Conrad CD. Chronic stress, cyclic 17beta-estradiol, and daily handling influences on fear conditioning in the female rat. The Neurobiology of Learning and Memory. 2010;94:422–433. doi: 10.1016/j.nlm.2010.08.010. [DOI] [PubMed] [Google Scholar]
- Hoffman AN, Krigbaum A, Ortiz JB, Mika A, Hutchinson KM, Bimonte-Nelson HA, Conrad CD. Recovery after chronic stress within spatial reference and working memory domains: Correspondence to hippocampal morphology. European Journal of Neuroscience. doi: 10.1111/j.1460-9568.2011.07820.x. (In Press) [DOI] [PubMed] [Google Scholar]
- Huynh TN, Krigbaum AM, Hanna JJ, Conrad CD. Sex differences and phase of light cycle modify chronic stress effects on anxiety and depressive-like behavior. Behavioural Brain Research. 2011;222(1):212–222. doi: 10.1016/j.bbr.2011.03.038. [DOI] [PubMed] [Google Scholar]
- Johnston MV. Clinical disorders of brain plasticity. Brain Devevelopment. 2004;26(2):73–80. doi: 10.1016/S0387-7604(03)00102-5. [DOI] [PubMed] [Google Scholar]
- Kasai H, Matsuzaki M, Noguchi J, Yasumatsu N, Nakahara H. Structure-stability-function relationships of dendritic spines. Trends in the Neurosciences. 2003;26(7):360–368. doi: 10.1016/S0166-2236(03)00162-0. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386(6624):493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- Kitraki E, Kremmyda O, Youlatos D, Alexis MN, Kittas C. Gender-dependent alterations in corticosteroid receptor status and spatial performance following 21 days of restraint stress. Neuroscience. 2004;125:47–55. doi: 10.1016/j.neuroscience.2003.12.024. [DOI] [PubMed] [Google Scholar]
- Kleen JK, Sitomer MT, Killeen PR, Conrad CD. Chronic stress impairs spatial memory and motivation for reward without disrupting motor ability and motivation to explore. Behavioral Neuroscience. 2006;120(4):842–851. doi: 10.1037/0735-7044.120.4.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korol DL. Role of estrogen in balancing contributions from multiple memory systems. Neurobiology of Learning and Memory. 2004;82(3):309–323. doi: 10.1016/j.nlm.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Lebesgue D, Chevaleyre V, Zukin RS, Etgen AM. Estradiol rescues neurons from global ischemia-induced cell death: multiple cellular pathways of neuroprotection. Steroids. 2009;74:555–561. doi: 10.1016/j.steroids.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leranth C, Shanabrough M, Redmond DE., Jr Gonadal hormones are responsible for maintaining the integrity of spine synapses in the CA1 hippocampal subfield of female nonhuman primates. The Journal of Comparative Neurology. 2002;447(1):34–42. doi: 10.1002/cne.10230. [DOI] [PubMed] [Google Scholar]
- Leuner B, Falduto J, Shors TJ. Associative memory formation increases the observation of dendritic spines in the hippocampus. The Journal of Neuroscience. 2003;23:659–665. doi: 10.1523/JNEUROSCI.23-02-00659.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine V, Villegas M, Martinez C, McEwen BS. Repeated stress causes reversible impairments of spatial memory performance. Brain Research. 1994;639:167–170. doi: 10.1016/0006-8993(94)91778-7. [DOI] [PubMed] [Google Scholar]
- Luine V, Martinez C, Villegas M, Magariños AM, McEwen BS. Restraint stress reversibly enhances spatial memory performance. Physiology & Behavior. 1996;59(1):27–32. doi: 10.1016/0031-9384(95)02016-0. [DOI] [PubMed] [Google Scholar]
- Luine V, Attalla S, Mohan G, Costa A, Frankfurt M. Dietary phytoestrogens enhance spatial memory and spine density in the hippocampus and prefrontal cortex of ovariectomized rats. Brain Research. 2006;1126(1):183–187. doi: 10.1016/j.brainres.2006.07.016. [DOI] [PubMed] [Google Scholar]
- Luine VN, Beck KD, Bowman RE, Frankfurt M, Maclusky NJ. Chronic stress and neural function: accounting for sex and age. Journal of Neuroendocrinology. 2007;19(10):743–751. doi: 10.1111/j.1365-2826.2007.01594.x. [DOI] [PubMed] [Google Scholar]
- Lund TD, Rovis T, Chung WC, Handa RJ. Novel actions of estrogen receptor-beta on anxiety-related behaviors. Endocrinology. 2005;146(2):797–807. doi: 10.1210/en.2004-1158. [DOI] [PubMed] [Google Scholar]
- Luo L, Tan RX. Fluoxetine inhibits dendrite atrophy of hippocampal neurons by decreasing nitric oxide synthase expression in rat depression model. Acta Pharmacol Sin. 2001;22(10):865–870. [PubMed] [Google Scholar]
- MacLusky NJ, Luine VN, Hajszan T, Leranth C. The 17α and 17β isomers of estradiol both induce rapid spine synapse formation in the CA1 hippocampal subfield of ovariectomized female rats. Endocrinology. 2005;146(1):287–293. doi: 10.1210/en.2004-0730. [DOI] [PubMed] [Google Scholar]
- Magariños AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: Involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience. 1995a;69(1):89–98. doi: 10.1016/0306-4522(95)00259-l. [DOI] [PubMed] [Google Scholar]
- Magariños AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: Comparison of stressors. Neuroscience. 1995b;69(1):83–88. doi: 10.1016/0306-4522(95)00256-i. [DOI] [PubMed] [Google Scholar]
- McFadden LM, Paris JJ, Mitzelfelt MS, McDonough S, Frye CA, Matuszewich L. Sex-dependent effects of chronic unpredictable stress in the water maze. Physiology & Behavior. 2011;102(3–4):266–275. doi: 10.1016/j.physbeh.2010.10.022. [DOI] [PubMed] [Google Scholar]
- McKittrick CR, Magariños AM, Blanchard DC, Blanchard RJ, McEwen BS, Sakai RR. Chronic social stress reduces dendritic arbors in CA3 hippocampus and decreases binding to serotonin transporter sites. Synapse. 2000;36:85–94. doi: 10.1002/(SICI)1098-2396(200005)36:2<85::AID-SYN1>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- McLaughlin KJ, Baran SE, Wright RL, Conrad CD. Chronic stress enhances spatial memory in ovariectomized female rats despite CA3 dendritic retraction: Possible involvement of CA1 neurons. Neuroscience. 2005;135(4):1045–1054. doi: 10.1016/j.neuroscience.2005.06.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KJ, Gomez JL, Baran SE, Conrad CD. The effects of chronic stress on hippocampal morphology and function: An evaluation of chronic restraint paradigms. Brain Research. 2007;1161:56–64. doi: 10.1016/j.brainres.2007.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KJ, Bimonte-Nelson HA, Neisewander JL, Conrad CD. Assessment of estradiol influence on spatial tasks and hippocampal CA1 spines: Evidence that the duration of hormone deprivation after ovariectomy compromises 17β-estradiol effectiveness in altering CA1 spines. Hormones & Behavior. 2008;54(3):386–395. doi: 10.1016/j.yhbeh.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KJ, Baran SE, Conrad CD. Chronic Stress- and Sex-Specific Neuromorphological and Functional Changes in Limbic Structures. Molecular Neurobiology. 2009;40(2):166–182. doi: 10.1007/s12035-009-8079-7. [DOI] [PubMed] [Google Scholar]
- McLaughlin KJ, Wilson JO, Harman J, Wright RL, Wieczorek L, Gomez J, Korol DL, Conrad CD. Chronic 17β-estradiol or cholesterol prevents stress-induced hippocampal CA3 dendritic retraction in ovariectomized female rats: Possible correspondence between CA1 spine properties and spatial acquisition. Hippocampus. 2010;20:768–786. doi: 10.1002/hipo.20678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser MB, Trommald M, Andersen P. An increase in dendritic spine density on hippocampal CA1 pyramidal cells following spatial learning in adult rats suggests the formation of new synapses. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(26):12673–12675. doi: 10.1073/pnas.91.26.12673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser MB. Making more synapses: a way to store information? Cellular and Molecular Life Science. 1999;55(4):593–600. doi: 10.1007/s000180050317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumby DG, Gaskin S, Glenn MJ, Schramek TE, Lehmann H. Hippocampal damage and exploratory preferences in rats: memory for objects, places, and contexts. Learning & Memory. 2002;9(2):49–57. doi: 10.1101/lm.41302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsetti M, Colella L, Dellarole A, Canonico PL, Ghi P. Modification of spatial recognition memory and object discrimination after chronic administration of haloperidol, amitriptyline, sodium valproate or olanzapine in normal and anhedonic rats. International Journal of Neuropsychopharmacology. 2007;10(3):345–357. doi: 10.1017/S1461145706006705. [DOI] [PubMed] [Google Scholar]
- Papa M, Bundman MC, Greenberger V, Segal M. Morphological analysis of dendritic spine development in primary cultures of hippocampal neurons. Journal of Neuroscience. 1995;15:1–11. doi: 10.1523/JNEUROSCI.15-01-00001.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris JJ, Frye CA. Estrous cycle, pregnancy, and parity enhance performance of rats in object recognition or object placement tasks. Reproduction. 2008;136(1):105–115. doi: 10.1530/REP-07-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CR, Campbell AM, Diamond DM. Chronic psychosocial stress impairs learning and memory and increases sensitivity to yohimbine in rats. Biological Psychiatry. 2001;50:994–1004. doi: 10.1016/s0006-3223(01)01255-0. [DOI] [PubMed] [Google Scholar]
- Park CR, Zoladz PR, Conrad CD, Fleshner M, Diamond DM. Acute predator stress impairs the consolidation and retrieval of hippocampus-dependent memory in male and female rats. Learning & Memory. 2008;15(4):271–280. doi: 10.1101/lm.721108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampon C, Tang YP, Goodhouse J, Shimizu E, Kyin M, Tsien JZ. Enrichment induces structural changes and recovery from nonspatial memory deficits in CA1 NMDAR1-knockout mice. Nature Neuroscience. 2000;3:238–244. doi: 10.1038/72945. [DOI] [PubMed] [Google Scholar]
- Rosenzweig MR, Bennett EL. Psychobiology of plasticity: Effects of training and experience on brain and behavior. Behavioural Brain Research. 1996;78(1):57–65. doi: 10.1016/0166-4328(95)00216-2. [DOI] [PubMed] [Google Scholar]
- Rosenzweig MR, Breedlove SM, Watson NV. Biological Psychology: An introduction to behavioral, Cognitive, and Clinical Neuroscience. 4th ed. Sunderland, MA: Sinauer Associates, Inc.; 2005. [Google Scholar]
- Sadowski RN, Jackson GR, Wieczorek L, Gold PE. Effects of stress, corticosterone, and epinephrine administration on learning in place and response tasks. Behavioural Brain Research. 2009;205:19–25. doi: 10.1016/j.bbr.2009.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva I, Mello LE, Freymuller E, Haidar MA, Baracat EC. Estrogen, progestogen and tamoxifen increase synaptic density of the hippocampus of ovariectomized rats. Neuroscience Letters. 2000;291(3):183–186. doi: 10.1016/s0304-3940(00)01410-5. [DOI] [PubMed] [Google Scholar]
- Silva I, Mello LE, Freymuller E, Haidar MA, Baracat EC. Onset of estrogen replacement has a critical effect on synaptic density of CA1 hippocampus in ovariectomized adult rats. Menopause. 2003;10(5):406–411. doi: 10.1097/01.GME.0000064816.74043.E9. [DOI] [PubMed] [Google Scholar]
- Smith CC, Vedder LC, Nelson AR, Bredemann TM, McMahon LL. Duration of estrogen deprivation, not chronological age, prevents estrogen's ability to enhance hippocampal synaptic physiology. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:19543–19548. doi: 10.1073/pnas.1009307107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, Che W, Min-Wei W, Murakami Y, Matsumoto K. Impairment of the spatial learning and memory induced by learned helplessness and chronic mild stress. Pharmacology Biochemistry and Behavior. 2006;83(2):186–193. doi: 10.1016/j.pbb.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Sousa N, Lukoyanov NV, Madeira MD, Almeida OFX, Paula-Barbosa MM. Reorganization of the morphology of hippocampal neurites and synapses after stress-induced damage correlates with behavioral improvement. Neuroscience. 2000;97:253–266. doi: 10.1016/s0306-4522(00)00050-6. [DOI] [PubMed] [Google Scholar]
- Spencer JL, Waters EM, Romeo RD, Wood GE, Milner TA, McEwen BS. Uncovering the mechanisms of estrogen effects on hippocampal function. Frontiers in Neuroendocrinology. 2008;29(2):219–237. doi: 10.1016/j.yfrne.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunanda, Shankaranarayana Rao BS, Raju TR. Chronic restraint stress impairs acquisition and retention of spatial memory task in rats. Current Science. 2000;79:1581–1584. [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. The Journal of Neuroscience. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A, Pillai AG, Chattarji S. Recovery after chronic stress fails to reverse amygdaloid neuronal hypertrophy and enhanced anxiety-like behavior. Neuroscience. 2004;128(4):667–673. doi: 10.1016/j.neuroscience.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Wang DO, Martin KC, Zukin RS. Spatially restricting gene expression by local translation at synapses. Trends in the Neurosciences. 2010;33(4):173–182. doi: 10.1016/j.tins.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Gould E, Daniels DC, Cameron H, McEwen BS. Tianeptine attenuates stress-induced morphological changes in the hippocampus. European Journal of Pharmacology. 1992;222:157–162. doi: 10.1016/0014-2999(92)90830-w. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Gould E, McEwen BS. Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Research. 1992;588:341–345. doi: 10.1016/0006-8993(92)91597-8. [DOI] [PubMed] [Google Scholar]
- Wilber AA, Walker AG, Southwood CJ, Farrell MR, Lin GL, Rebec GV, Wellman CL. Chronic stress alters neural activity in medial prefrontal cortex during retrieval of extinction. Neuroscience. 2011;174:115–131. doi: 10.1016/j.neuroscience.2010.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise PM, Dubal DB, Wilson ME, Rau SW, Liu Y. Estrogens: trophic and protective factors in the adult brain. Frontiers in Neuroendocrinology. 2001;22:33–66. doi: 10.1006/frne.2000.0207. [DOI] [PubMed] [Google Scholar]
- Wood GE, Young LT, Reagan LP, Chen B, McEwen BS. Stress-induced structural remodeling in hippocampus: Prevention by lithium treatment. Proceedings of the National Academy of Sciences USA. 2004;101(11):3973–3978. doi: 10.1073/pnas.0400208101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, Gould E, Frankfurt M, McEwen BS. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. The Journal of Neuroscience. 1990;10:4035–4039. doi: 10.1523/JNEUROSCI.10-12-04035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. The Journal of Neuroscience. 1992;12(7):2549–2554. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. The Journal of Comparative Neurology. 1993;336(2):293–306. doi: 10.1002/cne.903360210. [DOI] [PubMed] [Google Scholar]
- Woolley CS. Estrogen-mediated structural and functional synaptic plasticity in the female rat hippocampus. Hormones and Behavior. 1998;34:140–148. doi: 10.1006/hbeh.1998.1466. [DOI] [PubMed] [Google Scholar]
- Wright RL, Conrad CD. Chronic stress leaves novelty-seeking intact while impairing spatial recognition memory in the Y-maze. Stress. 2005;8(2):151–154. doi: 10.1080/10253890500156663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright RL, Conrad CD. Enriched environment prevents chronic stress-induced spatial learning and memory deficits. Behavioural Brain Research. 2008;187(1):41–47. doi: 10.1016/j.bbr.2007.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]