Abstract
Chronic methamphetamine (meth) can lead to persisting cognitive deficits in human addicts and animal models of meth addiction. Here, we examined the impact of either contingent or noncontingent meth on memory performance using an object-in-place (OIP) task, which measures the ability to detect an object relative to its location and surrounding objects. Further, we quantified monoamine transporter levels and markers of neurotoxicity within the OIP circuitry and striatum. Male Long-Evans rats received an acute meth binge (4 × 4 mg/kg i.p., 2 hour intervals) or self-administered meth (0.02 mg/infusion, i.v.; 7 days for 1 hour/day, followed by 14 days for 6 hours/day). Rats were tested for OIP recognition memory following one week of withdrawal. Subsequently, transporters for serotonin (SERT) and norepinephrine (NET) were quantified using Western blot in tissue obtained from the hippocampus, perirhinal cortex, and prefrontal cortex. In addition, striatal dopamine transporters, tyrosine hydroxylase, and glial fibrillary acidic protein were measured to assess potential neurotoxicity. Control (saline-treated) rats spent more time interacting with the objects in the changed locations. In contrast, contingent or noncontingent meth resulted in disrupted OIP performance as seen by similar amounts of time spent with all objects, regardless of location. While only acute meth binge produced signs of neurotoxicity, both meth regimens decreased SERT in the perirhinal cortex and hippocampus. Only meth self-administration resulted in a selective decrease in NET. Meth-induced changes in SERT function in the OIP circuitry may underlie memory deficits independently of overt neurotoxic effects.
Keywords: methamphetamine, recognition memory, self-administration, serotonin
1. Introduction
Methamphetamine (meth) addiction often includes a number of neurocognitive impairments that interfere with daily life and recovery. The most consistent impairments reported in meth addicts fall in the domains of episodic memory, executive functions, and information processing (Scott et al., 2007). While some of these deficits may subside with time (Salo et al., 2009; Volkow et al., 2001a), memory impairments are among the most pronounced and persistent problems in meth addiction (Ersche et al., 2006; Scott et al., 2007). Specifically, meth addicts show deficits on tests of verbal (Hoffman et al., 2006), working (Gonzalez et al., 2007), prospective (Rendell et al., 2009), and episodic (Simon et al., 2004) memory. Meth-induced memory loss may have a profound impact on treatment adherence and long term ability to prevent relapse, as the degree of memory impairment has been linked to proclivity to relapse (Simon et al., 2004). An increased understanding of the underlying neurobiology of meth-induced memory impairments is crucial for developing treatment approaches that may improve cognitive performance and overall addiction treatment outcomes.
Tests of episodic memory in rodents encompass components of what, when, and where (Dickerson and Eichenbaum, 2010; Ennaceur, 2010). Object recognition tasks can include some components of episodic memory in rodents depending on the specific task parameters (Ennaceur, 2010; Warburton and Brown, 2009). These are one-trial tasks that do not involve heuristic learning or changes in the motivational state of the subject (Ennaceur and Delacour, 1988), but instead rely on the innate tendency of rodents to explore environmental novelty (Berlyne, 1950). The ability to discern whether an item is novel or familiar is central to memory function and meth has been shown to impair this ability under multiple systemic injection regimens (Belcher et al., 2005; Belcher et al., 2008; Belcher et al., 2006; Bisagno et al., 2002; O’Dell et al., 2011) or extended access to daily self-administered meth (Reichel et al., 2011; Rogers et al., 2008).
Memory for object novelty is primarily mediated by the perirhinal (PRH) cortex and interactions with the hippocampus (Wan et al., 1999; Warburton and Brown, 2009). Although some reports indicate a role of the prefrontal cortex (PFC) in this task (Christoffersen et al., 2008; Kamei et al., 2006; Uslaner et al., 2009), excitotoxic lesions or 6-hydroxydopamine infusions in the PFC do not impact memory for novel objects in rats (Barker et al., 2007; Ennaceur and Aggleton, 1997; Mitchell and Laiacona, 1998; Nelson et al., 2011; Warburton and Brown, 2009). In contrast, intact PRH cortex is required for object recognition memory. A highly useful variant of object recognition is the object-in-place (OIP) task that relies upon a more widely diffuse PRH-PFC-hippocampal circuitry (Warburton and Brown, 2009). This task requires subjects to simultaneously remember object and place information. Further, this task incorporates the “what” and “where” components of episodic memory, as compared to the more commonly used two-item tasks that only accounts for the “what” component of an episode (Ennaceur, 2010). These memory tasks in rodent models are highly useful paradigms for the study of meth-induced changes, as episodic memory is particularly compromised in meth addicts with deficits observed in multiple aspects of episodic memory (Iudicello, 2011; Kalechstein et al., 2003; Simon et al., 2004).
Here, we tested whether meth impairs the “what” and “where” components of recognition memory in a novel object in place memory task using a single day, noncontingent meth regimen (“binge”) and an extended access meth self-administration (SA) paradigm on OIP performance. SA models of drug taking possess high face validity due to the response-contingent drug delivery (Markou et al., 1993). By employing extended daily drug access conditions, the model has emulated several hallmark characteristics of addiction including escalation of drug intake over time (Ahmed et al., 2000; Kitamura et al., 2006), compulsive drug-seeking (Vanderschuren and Everitt, 2004), and increased motivation (Paterson and Markou, 2003). Further, this translationally relevant administration procedure impacts several cognitive domains that are reminiscent of characteristics found in human meth addicts including memory (Reichel et al., 2011; Rogers et al., 2008), attention (Parsegian et al., 2011), impulsivity (Dalley et al., 2007), and sensory motor gating (Hadamitzky et al., 2011). Further, increasing the session duration can induce neurotoxic consequences (Krasnova et al., 2010). As such, this paradigm is ideally suited to study episodic memory impairments resulting from meth exposure.
The neural mechanisms involved in meth-induced memory deficits are not understood. Here, to assess the potential neural underpinnings involved in meth-induced memory deficits, we measured monoamine transporters in OIP circuitry, as cortical monoamine function has been implicated in recognition memory performance (Inagaki et al., 2010) and chronic meth abuse results in decreased striatal dopamine transporters (DAT) and serotonin transporters (SERT) in several brain regions (Sekine et al., 2006; Volkow et al., 2001b). Previous rodent studies using only a meth binge regimen showed reduced SERT in the PRH cortex and hippocampus, but these studies only looked at SERT and did not examine OIP performance or changes in the PFC (Belcher et al., 2005; O’Dell et al., 2011). Thus, in the present study, we quantified both SERT and norepinephrine transporters (NET) in the PRH cortex, PFC, and hippocampus after OIP testing in animals with a history of either meth binge or escalated meth SA. We also quantified DAT, tyrosine hydroxylase (TH), and glial fibrillary acidic protein (GFAP) in the striatum of rats that received a neurotoxic meth binge or extended access to self-administered meth.
2. Methods and Materials
2.1 Subjects
A total of 45 male Long-Evans rats (Charles-River) weighing 250–300 g at the time of delivery were individually housed in a temperature- and humidity-controlled vivarium on a reversed 12:12 light-dark cycle. Rats received ad libitum water throughout the study and 25 g of standard rat chow (Harlan, Indianapolis, Indiana) daily until SA stabilized, at which time animals were maintained ad libitum. Procedures were conducted in accordance with the “Guide for the Care and Use of Laboratory Rats” (Institute of Laboratory Animal Resources on Life Sciences, National Research Council) and approved by the IACUC of the Medical University of South Carolina.
2.2 Surgery
Anesthesia consisted of ketamine hydrochloride (66 mg/kg), xylazine (1.3 mg/kg), and Equithesin (0.5 ml/kg). Ketorolac (2.0 mg/kg, IP) was given preoperatively as an analgesic. One end of a silastic catheter was inserted 33 mm into the external right jugular and secured with 4.0 silk sutures. The other end ran subcutaneously and exited from a small incision just below the scapula and was attached to an infusion harness (Instech Solomon, Plymouth Meeting, Pennsylvania) that provided access to an external port for IV drug delivery. An antibiotic solution of cefazolin (10 mg/0.1 ml; Schein Pharmaceuticals, Florham Park, New Jersey) was given along with 0.1 ml 70 U/ml heparinized saline post surgery and during recovery. During SA, rats received an infusion (0.1 ml) of 10 U/ml heparinized saline (Elkins-Sinn, Cherry Hill, New Jersey) before each session. After each session, catheters were flushed with cefazolin and 0.1 ml 70 U/ml heparinized saline. Catheter patency was periodically verified with methohexital sodium (10 mg/ml dissolved in 0.9% physiological saline; Eli Lilly, Indianapolis, Indiana), a short-acting barbiturate that produces a rapid loss of muscle tone when administered intravenously.
2.3 Non-Contingent Meth Administration and Object-in-Place Recognition Memory
Figure 1A depicts the timeline for Experiment 1. Rats (N=21) received four 4 mg/kg injections (IP) of noncontingent methamphetamine hydrochloride (salt form, Sigma-Aldrich, St. Louis, Missouri) or saline at 2 hour intervals in their home cage. During abstinence, rats were handled daily and underwent OIP testing using a painted wooden box (40×40×38 cm3). Rats received two 5-min habituation sessions on days 5 and 6 following meth. The OIP test occurred 7 days after meth. During the familiarization phase (i.e., sampling phase), rats were placed in the apparatus for 5 min with 4 distinct objects positioned in adjacent corners. A memory test was conducted 90 min later by placing the rat in the apparatus for 3 min with the same objects, except that the position of two objects was changed (denoted throughout as “same” and “changed”, respectively). Object placement was counterbalanced. Time points were based on our previous study showing that meth impairs a standard 2-item recognition memory task on abstinence days 7 and 8 (Reichel et al., 2011). Behavior was recorded and stored using Noldus tracking software (EthoVision XT 6.0, Leesburg, Virginia) and an observer naive to the experimental conditions scored behavior. Object exploration was defined as sniffing or touching the object with the nose but not sitting, leaning, or standing on the objects. Objects consisted of a PVC pipe (6.4 × 3.8 cm2), a paint roller (7.5 × 2.5 cm2), a light bulb (8.9 cm), and a plastic bottle (12 cm). All objects and the apparatus were wiped down with 70% isopropyl alcohol between tests.
Figure 1.
OIP performance in rats after an acute meth binge (Experiment 1). A) Time course for Experiment 1. OIP recognition memory was tested on day 7 after treatment and tissue was collected on day 8. B) Time spent with objects in the same (open bars) and changed (hatched bars) positions for rats treated with saline or meth. Significant difference from objects in the same location is indicated (*p<0.05). C) OIP recognition index for saline (open bars) and meth (black bars) on test. Data are represented as an index between time spent exploring objects in the changed position/time with both sets of objects. Significant differences from chance exploration (†p<0.05) or control (*p<0.05) are indicated.
2.4 Self-Administered Meth and Object-in-Place Recognition Memory
Meth SA occurred in standard operant conditioning chambers (30×20×20 cm, Med Associates) housed inside sound-attenuating cubicles fitted with a fan for airflow and masking noise. Each chamber contained two retractable levers, two stimulus lights, a speaker for tone delivery, and a house light to provide general illumination. Additionally, each chamber had a balanced metal arm and spring leash attached to a swivel (Instech). Tygon® tubing extended through the leash and was connected to a 10 ml syringe mounted on an infusion pump located outside the cubicle.
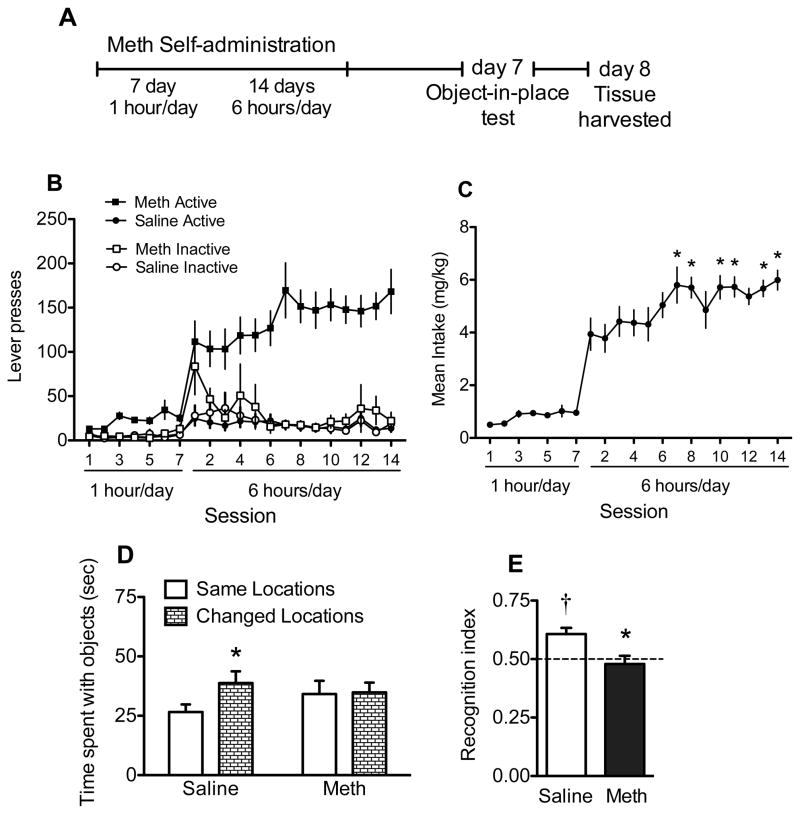
Figure 3A depicts the timeline for Experiment 2. Following at least 5 days of recovery from surgery, rats (N=24) were assigned to meth SA or yoked-saline control groups. Meth, dissolved in sterile saline, was self-administered in 1 hour/day sessions for 7 days followed by 14 days of 6 hours/day. The house light always signaled the beginning of a session and remained on throughout the session. During the sessions, a response on the active lever resulted in activation of the pump for a 2-sec meth infusion (20 μg/50 μl bolus infusion) and presentation of a stimulus complex consisting of a 5-sec tone (78 dB, 4.5 kHz) and a white stimulus light over the active lever, followed by a 20-sec time out. Yoked controls received a 50 μl bolus of saline when the matched subject received a meth infusion. Responses occurring during the time out and on the inactive lever were recorded, but had no scheduled consequences. All sessions took place during the dark cycle. During abstinence from meth, rats were handled daily but never returned to the SA chamber. OIP performance was conducted as described above for experiment 1.
Figure 3.
SA and OIP data from rats that self-administered meth and yoked saline controls (Experiment 2). A) Time course for Experiment 2. OIP recognition memory was tested on day 7 of abstinence and tissue was collected on day 8. B) Lever responding and C) meth intake during chronic meth SA. Significant differences from first day of long access are indicated (*p<0.05). D) Time spent with objects in the same (open bars) and changed (hatched bars) positions for saline or SA meth rats. Significant difference from objects in the same location is indicated (*p<0.05). E) OIP recognition index for saline (open bars) and SA meth (black bars) on test. Data are represented as an index between time spent exploring objects in the changed position/time with both sets of objects. Significant differences from chance exploration (†p<0.05) or saline control (*p<0.05) are indicated.
2.5 Tissue Extraction and Western Blot
Rats from both experiments were rapidly decapitated and brains removed on the 8th day of abstinence from meth (Reichel et al., 2011). Brains were sectioned into 2 mm thick coronal slices obtained using a rat brain slicer (Braintree Scientific, Massachusetts), frozen in isopentane, and stored at −80°C until processed. Samples were solubilized in 1% SDS/phosphate-buffered saline containing protease inhibitors (Complete Mini, Roche Diagnostics, Indianapolis, Indiana). Equal amounts of protein (15 μg) were resolved using SDS-PAGE (4–15%) and transferred to PVDF membrane (BioRad, Hercules, California). The membrane was blocked with 5% w/v powdered milk/Tris-buffered saline/Tween-20 and probed with antibodies for DAT (1:10,000), SERT (1:1000), NET (1:1000), GFAP (1:3000), (Santa Cruz, California), or TH (1:50000, Pel Freeze, Rogers, Arizona) overnight at 4°C. After washing, the membranes were incubated with HRP-conjugated anti-rabbit (1:10000/20000, Millipore, Billerica, Massachusetts) or anti goat (1:10000/15000, Santa Cruz, California) secondary antibody at room temperature, followed by washing with Tris-buffered saline/Tween (3 × 10 min). Protein signal was detected using enhanced chemiluminescence plus (GE Healthcare, Piscataway, New Jersey). Equal loading and transfer of proteins were confirmed by probing with anti-calnexin antiserum (1/20,000, Enzo Life Sciences, Plymouth Meeting, Pennsylvania). Samples were assayed in duplicate and matched controls run on each gel. Integrated density of the bands was measured with Image J software (National Institute of Health, Bethesda, Maryland).
We quantified markers of neurotoxicity and monoamine transporters based on previous studies and the regional distribution of monoamine transporters. Therefore, in brain areas important for OIP performance (i.e., PRH-PFC-hippocampal circuitry) we quantified SERT and NET. We did not include DAT measurements within this circuitry because expression is low (Donnan et al., 1989; Richtand et al., 1995). We quantified DAT, TH, and GFAP in the dorsal striatum for an assessment of neurotoxicity. Relative to DAT, striatal NET (Sanders et al., 2005) and SERT immunoreactivity is very low (Brownstein, 1984), so we did not quantify these transporters in the striatum.
2.6 Data Analysis
Meth intake (mg/kg) was the primary dependent measure and was analyzed with a one-way repeated measures analysis of variance (ANOVA) over the 14 days of long access. Post hoc comparisons used Newman-Keuls Multiple Comparison Tests to compare the first day of long access relative to ensuing SA sessions. For the OIP tests, time spent with each object was the primary dependent measure. Time with the two objects that remained in the same locations was combined, as was the time spent with the two objects in the new locations. Object exploration was defined as sniffing or touching the object with the nose but not sitting, leaning, or standing on the object. Data were converted to a recognition index, the primary dependent measure, with the following formula: changed object exploration/changed object + same object exploration. To confirm that memory for objects in place occurred for each group, the recognition index was first compared to a hypothetical mean of 0.5. A recognition index of 0.5 indicates equal time spent exploring both sets of objects, greater than 0.5 indicates more exploration of the changed objects, and less than 0.5 indicates more exploration of the same objects. Significant differences between meth and saline groups were determined using independent t-tests. Western blot data, represented by band density values, were normalized with calnexin band immunoreactivity, expressed as a percent of saline controls, and compared with independent t-tests. All data are expressed as the mean ± SEM, and conducted with the alpha set at 0.05.
3. Results
3.1 Noncontingent Meth Binge
Initial exploration of the four objects did not differ during the familiarization session (mean ± SEM; light bulb, 22.86 ± 2.04; plastic bottle, 20.85 ± 1.86; pvc pipe, 20.34 ± 1.82; paint roller, 24.05 ± 1.69). Further, when converted to exploration ratios, rats assigned to noncontingent meth binge (0.49±0.04) or saline-treated control (0.51±0.03) had similar exploration values during familiarization. On the OIP test (Figure 1B), only control rats spent more time with objects in the changed locations relative to the same locations [t(10)=3.02, p<0.05], and meth binge rats spent the same amounts of time at each object set. Further, only control rats displayed OIP recognition memory with significantly higher recognition indices relative to the hypothetical mean of 0.5 [Figure 1C, t(10)=5.12, p<0.001]. In contrast, meth binge rats did not differ from the hypothetical mean, indicating a lack of recognition memory. When directly compared, control rats had a significantly higher recognition index relative to meth binge rats [t(19)=3.0, p<0.01].
Noncontingent meth binge also produced signs of striatal neurotoxicity (Figure 2A) as evidenced by decreased DAT [t(17)=3.99, p<0.001] and TH [t(17)=3.86, p<0.01], and increased GFAP [t(19)=3.32, p<0.01] in meth binge rats above control values. For regions in the OIP circuitry, meth significantly decreased SERT (Figure 2B) in meth binge rats relative to controls in the PRH cortex [t(17)=2.24, p<0.05], the PFC [t(19)=1.99, p<0.05], and hippocampus [t(17)=2.10, p<0.05]. Meth binge did not result in any changes to NET in the OIP circuitry (Figure 2C).
Figure 2.
Protein levels in animals after experimenter delivered meth and saline controls. A) DAT, TH, and GFAP from striatal tissue in saline (open bars) and meth (black bars). B) SERT and C) NET protein levels in the perirhinal (PRH) cortex, prefrontal cortex (PFC), and hippocampus of saline and meth rats. Representative immunoblots depict immunoreactivity in whole tissue. Immunoreactivity was normalized to calnexin (clnx) and expressed as a percentage of saline control ± SEM. Significant differences from control are indicated (*p<0.05).
3.2 Meth Self-administration
Meth SA rats showed a distinction between active and inactive lever responding throughout the first seven days of 1 hour/day access that was even more pronounced during the 14 days of 6 hours/day (Figure 3A). Yoked-saline rats indiscriminately displayed low levels of responding on both levers. During the initial 7 days of 1 hour/day, meth intake averaged 0.82 ± 0.09 mg/kg/day. During subsequent 6 hours/day access sessions, rats escalated from an average of 3.93 ± 0.61 mg/kg on Day 1 to 5.99 ± 0.37 mg/kg by Day 14 (Figure 3C). Meth intake on the first day of long access was significantly lower than intake on days 7, 8, and 10–14 [F(13,117)=4.32, p<0.001, and post hoc, p<0.05]. Cumulative meth intake ranged from 59.68–112.53 mg/kg over the entire self-administration period.
During the familiarization of the OIP test, initial exploration of the four objects did not differ (light bulb, 30.36 ± 2.45; plastic bottle, 23.58 ± 1.68; pvc pipe, 24.12 ± 2.65; paint roller, 27.28 ± 2.14). Further, when converted to exploration ratios, similar values were seen in meth SA rats (0.49±0.02) and yoked saline control rats (0.49±0.03). On the test day (Figure 3D), yoked saline control rats spent more time with objects in the changed locations relative to the same locations [t(11)=4.19, p<0.01]. Meth SA rats spent the same amounts of time at each object set. Further, only control rats (Figure 3E) displayed OIP recognition memory with significantly higher recognition indices relative to the hypothetical mean of 0.5 [t(11)=4.05, p<0.01]. In contrast, meth SA rats did not differ from the hypothetical mean, indicating a lack of recognition memory. When directly compared, control rats had a significantly higher recognition index relative to meth SA rats [t(22)=2.93, p<0.01].
Extended meth access did not produce any markers of lasting striatal toxicity. DAT, TH, and GFAP expression were not different between groups (Figure 4A). However, meth significantly reduced SERT in the PRH cortex [t(18)=1.74, p<0.05] and hippocampus [t(20)=1.91, p<0.05], but not in the PFC (Figure 4B). Meth SA selectively reduced NET in the PFC [t(18)=1.72, p=0.05], but not in the PRH cortex or hippocampus (Figure 4C).
Figure 4.
Protein levels in animals with a history of chronic meth SA and yoked saline controls. A) DAT, TH, and GFAP from striatal tissue in saline (open bars) and SA meth (black bars). B) SERT and C) NET protein levels in the perirhinal (PRH) cortex, prefrontal (PFC), and hippocampus of saline and SA meth rats. Representative immunoblots depict immunoreactivity in whole tissue. Immunoreactivity was normalized to calnexin (clnx) and expressed as a percentage of saline control ± SEM. Significant differences from saline control are indicated (*p<0.05).
4. Discussion
A single day acute meth binge and a chronic escalating SA regimen of meth both impaired memory for objects in place and reduced SERT in the PRH-PFC-hippocampal circuitry. Intact PRH-PFC-hippocampal circuitry is necessary to assess recognition for multiple items and their association with particular locations (Barker and Warburton, 2011; Warburton and Brown, 2009). In fact, disconnection of any of these structures is sufficient to impair object in place memory, indicating the important contribution of all three regions. Even if two areas remain uncompromised, this is insufficient for optimal OIP performance (Warburton and Brown, 2009). Importantly, saline and meth rats spent similar amounts of time interacting with objects during familiarization, supporting memory impairment rather than a lack of interest in novelty reward. Similar meth SA procedures to those used in the current study produce neuroadaptive changes in each of these regions, indicating the potential debilitating impact meth has on this type of memory. For example, extended access to meth SA protocol decreased neurogenesis and reduced hippocampal granule neurons and volume (Mandyam et al., 2008), changed neuronal firing states and decreased gliogenesis in the PFC (Mandyam et al., 2007; Parsegian et al., 2011), and dysregulated glutamate (Reichel et al., 2011) and serotonin (current findings) neurotransmitter systems in the PRH cortex.
While SERT decreases have been reported previously in the PRH and hippocampus in rats treated with a neurotoxic meth regimen (O’Dell et al., 2011), we have now identified similar decreases in meth SA rats. In fact, this commonality between both meth administration regimens suggests a key role for serotonergic alterations in impaired OIP memory. Cortical serotonin activity has been well characterized for its role in affective disorders (Vaswani et al., 2003), but much less studied in cortically-based recognition memory dysfunction. Emerging evidence suggests that cortical and hippocampal SERT expression is important for memory formation (Meneses et al., 2011). With regard to recognition memory, SERT knockout rats show pronounced deficits (Olivier et al., 2009), and both chronic and acute tryptophan (i.e., serotonin precursor) depletion impaired object-recognition memory in rats (Jenkins et al., 2010; Olivier et al., 2008). Recently, Tellez and colleagues (Tellez et al., 2010) demonstrated that meth-induced SERT decreases were affected by learning experience. Specifically, rats were trained on an auto-shaping task for food pellet reward, tested in the presence of meth or saline, and SERT binding was subsequently assessed by [3H]citalopram autoradiography. Rats trained on this associative learning task had decreased SERT binding in cortical and hippocampal areas relative to untrained control rats. Meth (1 mg/kg) further reduced SERT binding in the trained rats relative to untrained rats. As such, memory formation required during associative learning may make SERT more liable to adverse meth effects.
Some questions remain as to the extent of serotonin’s involvement in the observed OIP deficits. Belcher et al. (2005) observed memory impairments in a study that compared meth, d-amphetamine, and p-chloroamphetamine and reported that only meth impaired recognition memory using a standard 2-item memory task (Belcher et al., 2005). Consistent with previous reports, a binge meth regimen decreased striatal DAT and hippocampal and perirhinal SERT. In contrast, d-amphetamine reduced striatal DAT, but not hippocampal or perirhinal SERT, while p-chloroamphetamine decreased SERT, but not DAT. The authors concluded that no single feature of meth-induced monoamine terminal damage was responsible for the memory deficits observed after a neurotoxic dosing regimen. An obvious difference between the current study and the previous one is the recognition memory task used. The OIP task engages a more extensive neurocircuitry, which may be more reliant on intact serotonin function than the traditional 2-item task. The definitive role of perirhinal and hippocampal SERT activity on object recognition memory tasks remains to be elucidated.
In contrast to SERT, self-administered meth decreased NET in the PFC, but acute experimenter delivered meth did not. While this difference is likely independent of the OIP memory deficits as both meth administration protocols impaired OIP memory, this decrease is notable given the extent of PFC-mediated tasks shown to be impaired in meth addicts (Ramos and Arnsten, 2007). Norepinephrine is highly critical for proper PFC function and is involved in several working memory and attentional tasks impaired by meth (Arnsten and Li, 2005; Sofuoglu, 2010) and normal PFC-mediated function is sensitive to even minor noradrenergic dysregulations (Arnsten, 2011). The exact neurobiological mechanisms by which meth SA decreased cortical NET remain unknown. One possibility could be a generalized degradative effect on monoamine terminals (Graham et al., 2008). However, this notion is inconsistent with the lack of overt toxicity in the meth SA animals and the lack of a similar NET downregulation in the non-contingent meth rats. Alternatively, while speculative, this long-term neuroadaptation could result from the repeated stimulation of the noradrenergic system and sensitized norepinephrine efflux in the PFC combined with the response contingency during chronic meth SA.
The relationship between meth-induced neurotoxicity, relapse, and cognitive function remains to be determined. Clinical and preclinical research has established that meth reduces striatal DAT, indicative of neurotoxic consequences (Chang et al., 2007; Hotchkiss and Gibb, 1980; Krasnova et al., 2010; Volz et al., 2007). As previously reported, we and others have shown decreased DAT (Wagner et al., 1980) and TH (Hotchkiss and Gibb, 1980) expression and elevated GFAP (Cappon et al., 2000) in the striatum of rats given an experimenter delivered binge of meth. As such, it appears that a single day administration of meth shows a consistent profile of neuronal damage that may occur in meth addicts. However, decreased DAT reported in rodent toxicity studies exceeds that reported with in vivo neuroimaging studies of meth addicts (Chang et al., 2007). In addition, even though a single day meth binge induces striatal neurotoxicity, this type of drug administration does not mimic human meth consumption, use patterns, or motivated drug seeking. Contingent models of drug delivery may not lead to the exact same neurotoxic consequences as single day binge models (Brennan et al., 2010; Schwendt et al., 2009), but they do possess higher model validity. Some studies report modest to substantial changes in DAT protein expression (Krasnova et al., 2010; Mcfadden et al., 2011; Schwendt et al., 2009), while others report no lasting changes (Shepard et al., 2006, current report). Differences between studies may be due to variations in drug dose, session length, total number of sessions, duration of withdrawal period, and behavioral experiences of the test subjects. In regards to the current results, it is important to reiterate that OIP memory impairments occured even in the absence of persistent striatal toxicity.
The memory deficits and striatal toxicity measures were dissociated in this study as both groups of rats had identical deficits in OIP memory. This dissociation is important as human meth addicts show recovery of DAT in a time dependent manner, but this recovery does not necessarily parallel that of cognitive function (Volkow et al., 2001a). Additionally, individuals exhibit cognitive impairments in multiple domains not related to striatal neurotoxicity (Kalechstein et al., 2003; Paulus et al., 2003; Simon et al., 2002; Volkow et al., 2001a) or degree of previous meth use (Cherner et al., 2010). Human studies also report increased cortical and striatal microglial activation in meth users relative to controls (Sekine et al., 2008); however, increased GFAP expression is not always evident in the brains of postmortem human meth addicts (Kitamura, 2009; Kitamura et al., 2010). In general, our findings support the notion that overt neurotoxic consequences do not need to be evident for the emergence of neurocognitive deficits in a task dependent on PRH-PFC-hippocampal circuitry. These data have potential to inform clinical assessments of memory impairments in human meth addicts by shifting focus from specific brain regions (e.g., striatal toxicity) to study of integrated circuits underlying deficits in episodic memory.
In conclusion, the current study revealed memory impairments caused by either extended access meth SA or acute experimenter-delivered meth that were independent of striatal toxicity. Further, the OIP memory task engages a more complex circuitry than previous novel object recognition tasks. Reduced SERT within the PRH-hippocampal circuitry suggests that serotonergic facilitation may be an important target for treatment medications aimed at recovery of meth-induced cognitive impairments. In contrast to SERT, decreased PFC NET is a neuroadaptation unique to meth SA and likely to be relevant to PFC-dependent attentional and motivational deficits observed in human meth addicts. Future investigations using this meth SA regimen will provide insight into potential treatment and prevention options for meth addiction.
Highlights.
A meth binge and a chronic escalating regimen of meth impaired memory for objects in place.
Meth-induced memory impairments are independent of striatal toxicity.
Serotonergic facilitation may be a target for meth-induced cognitive deficits.
Acknowledgments
This research was supported by NIH grants P20DA022658 (RES), F32DA029344 (CMR) and C06 RR015455. The authors thank Shannon Ghee, Stacey Sigmon, Rebecca Madell, and Amy Beth Young for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed SH, Walker JR, Koob GF. Persistent increase in the motivation to take heroin in rats with a history of drug escalation. Neuropsychopharmacology. 2000;22:413–421. doi: 10.1016/S0893-133X(99)00133-5. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT. Catecholamine influences on dorsolateral prefrontal cortical networks. Biol Psychiatry. 2011;69:e89–99. doi: 10.1016/j.biopsych.2011.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AFT, Li BM. Neurobiology of executive functions: catecholamine influences on prefrontal cortical functions. Biol Psychiatry. 2005;57:1377–1384. doi: 10.1016/j.biopsych.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Barker GRI, Bird F, Alexander V, Warburton EC. Recognition memory for objects, place, and temporal order: A disconnection analysis of the role of the medial prefrontal cortex and perirhinal cortex. J Neurosci. 2007;27:2948–2957. doi: 10.1523/JNEUROSCI.5289-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker GRI, Warburton EC. When is the hippocampus involved in recognition memory? J Neurosci. 2011;31:10721–10731. doi: 10.1523/JNEUROSCI.6413-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belcher A, O’dell S, Marshall J. Impaired object recognition memory following methamphetamine, but not p-chloroamphetamine- or d-amphetamine-induced neurotoxicity. Neuropsychopharmacology. 2005;30:2026–2034. doi: 10.1038/sj.npp.1300771. [DOI] [PubMed] [Google Scholar]
- Belcher AM, Feinstein EM, O’Dell SJ, Marshall JF. Methamphetamine influences on recognition memory: comparison of escalating and single-day dosing regimens. Neuropsychopharmacology. 2008;33:1453–1463. doi: 10.1038/sj.npp.1301510. [DOI] [PubMed] [Google Scholar]
- Belcher AM, O’Dell SJ, Marshall JF. A sensitizing regimen of methamphetamine causes impairments in a novelty preference task of object recognition. Behav Brain Res. 2006;170:167–172. doi: 10.1016/j.bbr.2006.02.025. [DOI] [PubMed] [Google Scholar]
- Berlyne DE. Novelty and curiosity as determinants of exploratory behaviour. British Journal of Psychology. 1950;41:68–80. [Google Scholar]
- Bisagno V, Ferguson D, Luine VN. Short toxic methamphetamine schedule impairs object recognition task in male rats. Brain Res. 2002;940:95–101. doi: 10.1016/s0006-8993(02)02599-4. [DOI] [PubMed] [Google Scholar]
- Brennan KA, Colussi-Mas J, Carati C, Lea RA, Fitzmaurice PS, Schenk S. Methamphetamine self-administration and the effect of contingency on monoamine and metabolite tissue levels in the rat. Brain Res. 2010;1317:137–146. doi: 10.1016/j.brainres.2009.11.069. [DOI] [PubMed] [Google Scholar]
- Brownstein MPM. Catecholamines, serotonin, acetylcholine and y-aminobutyric acid in the rat brain: biochemincal studies. In: Bjorklund AHT, editor. Handbook of Chemical Neuroanatomy: Volume 2: Classical Transmitters in the CNS. Elsevier; New York: 1984. pp. 23–54. [Google Scholar]
- Cappon GD, Pu C, Vorhees CV. Time-course of methamphetamine-induced neurotoxicity in rat caudate-putamen after single-dose treatment. Brain Res. 2000;863:106–111. doi: 10.1016/s0006-8993(00)02107-7. [DOI] [PubMed] [Google Scholar]
- Chang L, Alicata D, Ernst T, Volkow N. Structural and metabolic brain changes in the striatum associated with methamphetamine abuse. Addiction. 2007;102(Suppl 1):16–32. doi: 10.1111/j.1360-0443.2006.01782.x. [DOI] [PubMed] [Google Scholar]
- Cherner M, Suarez P, Casey C, Deiss R, Letendre S, Marcotte T, Vaida F, Atkinson JH, Grant I, Heaton RK, Group H. Methamphetamine use parameters do not predict neuropsychological impairment in currently abstinent dependent adults. Drug and Alcohol Dependence. 2010;106:154–163. doi: 10.1016/j.drugalcdep.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffersen GR, Simonyi A, Schachtman TR, Clausen B, Clement D, Bjerre VK, Mark LT, Reinholdt M, Schmith-Rasmussen K, Zink LV. MGlu5 antagonism impairs exploration and memory of spatial and non-spatial stimuli in rats. Behav Brain Res. 2008;191:235–245. doi: 10.1016/j.bbr.2008.03.032. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Lääne K, Theobald DE, Peña Y, Bruce CC, Huszar AC, Wojcieszek M, Everitt BJ, Robbins TW. Enduring deficits in sustained visual attention during withdrawal of intravenous methylenedioxymethamphetamine self-administration in rats: results from a comparative study with d-amphetamine and methamphetamine. Neuropsychopharmacology. 2007;32:1195–1206. doi: 10.1038/sj.npp.1301220. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Eichenbaum H. The episodic memory system: neurocircuitry and disorders. Neuropsychopharmacology. 2010;35:86–104. doi: 10.1038/npp.2009.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnan GA, Kaczmarczyk SJ, McKenzie JS, Kalnins RM, Chilco PJ, Mendelsohn FAO. Catecholamine uptake sites in mouse brain: distribution determined by quantitative [3H]mazindol autoradiography. Brain Research. 1989;504:64–71. doi: 10.1016/0006-8993(89)91598-9. [DOI] [PubMed] [Google Scholar]
- Ennaceur A. One-trial object recognition in rats and mice: Methodological and theoretical issues. Behav Brain Res. 2010;215:244–254. doi: 10.1016/j.bbr.2009.12.036. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Aggleton JP. The effects of neurotoxic lesions of the perirhinal cortex combined to fornix transection on object recognition memory in the rat. Behav Brain Res. 1997;88:181–193. doi: 10.1016/s0166-4328(97)02297-3. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav Brain Res. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Clark L, London M, Robbins TW, Sahakian BJ. Profile of executive and memory function associated with amphetamine and opiate dependence. Neuropsychopharmacology. 2006;31:1036–1047. doi: 10.1038/sj.npp.1300889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez R, Bechara A, Martin EM. Executive functions among individuals with methamphetamine or alcohol as drugs of choice: preliminary observations. J Clinical & Exp Neuropsychology. 2007;29:155–159. doi: 10.1080/13803390600582446. [DOI] [PubMed] [Google Scholar]
- Graham DL, Noailles PAH, Cadet JL. Differential neurochemical consequences of an escalating dose-binge regimen followed by single-day multiple-dose methamphetamine challenges. J Neurochem. 2008;105:1873–1885. doi: 10.1111/j.1471-4159.2008.05269.x. [DOI] [PubMed] [Google Scholar]
- Hadamitzky M, Markou A, Kuczenski R. Extended access to methamphetamine self-administration affects sensorimotor gating in rats. Behav Brain Res. 2011;217:386–390. doi: 10.1016/j.bbr.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman W, Moore M, Templin R, Mcfarland B, Hitzemann R, Mitchell S. Neuropsychological function and delay discounting in methamphetamine-dependent individuals. Psychopharmacology. 2006;188:162–170. doi: 10.1007/s00213-006-0494-0. [DOI] [PubMed] [Google Scholar]
- Hotchkiss AJ, Gibb JW. Long-term effects of multiple doses of methamphetamine on tryptophan hydroxylase and tyrosine hydroxylase activity in rat brain. J Pharmacology Exp Ther. 1980;214:257–262. [PubMed] [Google Scholar]
- Inagaki T, Gautreaux C, Luine V. Acute estrogen treatment facilitates recognition memory consolidation and alters monoamine levels in memory-related brain areas. Horm Behav. 2010;58:415–426. doi: 10.1016/j.yhbeh.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iudicello JE, Weber E, Grant I, Weinborn M, Woods SP. Misremembering future intentions in methamphetamine-dependent individuals. Clinical Neuropsychology. 2011;25:269–286. doi: 10.1080/13854046.2010.546812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins TA, Elliott JJ, Ardis TC, Cahir M, Reynolds GP, Bell R, Cooper SJ. Tryptophan depletion impairs object-recognition memory in the rat: reversal by risperidone. Behav Brain Res. 2010;208:479–483. doi: 10.1016/j.bbr.2009.12.030. [DOI] [PubMed] [Google Scholar]
- Kalechstein AD, Newton TF, Green M. Methamphetamine dependence is associated with neurocognitive impairment in the initial phases of abstinence. Journal of Neuropsychiatry and Clinical Neurosciences. 2003;15:215–220. doi: 10.1176/jnp.15.2.215. [DOI] [PubMed] [Google Scholar]
- Kamei H, Nagai T, Nakano H, Togan Y, Takayanagi M, Takahashi K, Kobayashi K, Yoshida S, Maeda K, Takuma K, Nabeshima T, Yamada K. Repeated methamphetamine treatment impairs recognition memory through a failure of novelty-induced ERK1/2 activation in the prefrontal cortex of mice. Biol Psychiatry. 2006;59:75–84. doi: 10.1016/j.biopsych.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Kitamura O. Detection of methamphetamine neurotoxicity in forensic autopsy cases. Legal Medicine. 2009;11:S63–S65. doi: 10.1016/j.legalmed.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Kitamura O, Takeichi T, Wang EL, Tokunaga I, Ishigami A, Kubo S-i. Microglial and astrocytic changes in the striatum of methamphetamine abusers. Leg Med (Tokyo) 2010;12:57–62. doi: 10.1016/j.legalmed.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Kitamura O, Wee S, Specio S, Koob G, Pulvirenti L. Escalation of methamphetamine self-administration in rats: a dose-effect function. Psychopharmacology. 2006;186:48–53. doi: 10.1007/s00213-006-0353-z. [DOI] [PubMed] [Google Scholar]
- Krasnova IN, Justinova Z, Ladenheim B, Jayanthi S, McCoy MT, Barnes C, Warner JE, Goldberg SR, Cadet JL. Methamphetamine self-administration is associated with persistent biochemical alterations in striatal and cortical dopaminergic terminals in the rat. PLoS ONE. 2010;5:e8790. doi: 10.1371/journal.pone.0008790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandyam C, Wee S, Eisch A, Richardson H, Koob G. Methamphetamine self-administration and voluntary exercise have opposing effects on medial prefrontal cortex gliogenesis. J Neurosci. 2007;27:11442–11450. doi: 10.1523/JNEUROSCI.2505-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandyam CD, Wee S, Crawford EF, Eisch AJ, Richardson HN, Koob GF. Varied access to intravenous methamphetamine self-administration differentially alters adult hippocampal neurogenesis. Biol Psychiatry. 2008;64:958–965. doi: 10.1016/j.biopsych.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markou A, Weiss F, Gold L, Caine S, Schulteis G, Koob G. Animal models of drug craving. Psychopharmacology. 1993;112:163–182. doi: 10.1007/BF02244907. [DOI] [PubMed] [Google Scholar]
- McFadden L, Hadlock GC, Allen SC, Vieira-Brock PL, Stout KA, Ellis JD, Hoonakker AJ, Anderyak DM, Neilson SM, Wilkins DG, Hanson GR, Fleckenstein AE. Methamphetamine self-administration causes persistent striatal dopaminergic alterations and mitigates the deficits caused by a subsequent methamphetamine exposure. J Pharmacology Exp Ther. 2011:1–42. doi: 10.1124/jpet.111.188433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meneses A, Perez-Garcia G, Ponce-Lopez T, Tellez R, Castillo C. Serotonin transporter and memory. Neuropharmacology. 2011;61:355–363. doi: 10.1016/j.neuropharm.2011.01.018. [DOI] [PubMed] [Google Scholar]
- Mitchell JB, Laiacona J. The medial frontal cortex and temporal memory: tests using spontaneous exploratory behaviour in the rat. Behavioural Brain Research. 1998;97:107–113. doi: 10.1016/s0166-4328(98)00032-1. [DOI] [PubMed] [Google Scholar]
- Nelson AJD, Cooper MT, Thur KE, Marsden CA, Cassaday HJ. The effect of catecholaminergic depletion within the prelimbic and infralimbic medial prefrontal cortex on recognition memory for recency, location, and objects. Behavioral Neuroscience. 2011;125:396–403. doi: 10.1037/a0023337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dell SJ, Feinberg LM, Marshall JF. A neurotoxic regimen of methamphetamine impairs novelty recognition as measured by a social odor-based task. Behav Brain Res. 2011;216:396–401. doi: 10.1016/j.bbr.2010.08.022. [DOI] [PubMed] [Google Scholar]
- Olivier JDA, Jans LAW, Blokland A, Broers NJ, Homberg JR, Ellenbroek BA, Cools AR. Serotonin transporter deficiency in rats contributes to impaired object memory. Genes Brain Behav. 2009;8:829–834. doi: 10.1111/j.1601-183X.2009.00530.x. [DOI] [PubMed] [Google Scholar]
- Olivier JDA, Jans LAW, Korte-Bouws GAH, Korte SM, Deen PMT, Cools AR, Ellenbroek BA, Blokland A. Acute tryptophan depletion dose dependently impairs object memory in serotonin transporter knockout rats. Psychopharmacology. 2008;200:243–254. doi: 10.1007/s00213-008-1201-0. [DOI] [PubMed] [Google Scholar]
- Parsegian A, Glen WB, Lavin A, See RE. Methamphetamine Self-Administration Produces Attentional Set-Shifting Deficits and Alters Prefrontal Cortical Neurophysiology in Rats. Biol Psychiatry. 2011;63:253–259. doi: 10.1016/j.biopsych.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson NE, Markou A. Increased motivation for self-administered cocaine after escalated cocaine intake. Neuroreport. 2003;14:2229–2232. doi: 10.1097/00001756-200312020-00019. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Hozack N, Frank L, Brown GG, Schuckit MA. Decision making by methamphetamine-dependent subjects is associated with error-rate-independent decrease in prefrontal and parietal activation. Biol Psychiatry. 2003;53:65–74. doi: 10.1016/s0006-3223(02)01442-7. [DOI] [PubMed] [Google Scholar]
- Ramos BP, Arnsten AFT. Adrenergic pharmacology and cognition: focus on the prefrontal cortex. Pharmacol Ther. 2007;113:523–536. doi: 10.1016/j.pharmthera.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, Schwendt M, McGinty JF, Olive MF, See RE. Loss of object recognition memory produced by extended access to methamphetamine self-administration is reversed by positive allosteric modulation of metabotropic glutamate receptor 5. Neuropsychopharmacology. 2011;36:782–792. doi: 10.1038/npp.2010.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendell PG, Mazur M, Henry JD. Prospective memory impairment in former users of methamphetamine. Psychopharmacology. 2009;203:609–616. doi: 10.1007/s00213-008-1408-0. [DOI] [PubMed] [Google Scholar]
- Richtand NM, Kelsoe JR, Segal DS, Kuczenski R. Regional quantification of dopamine transporter mRNA in rat brain using a ribonuclease protection assay. Neurosci Lett. 1995;200:73–76. doi: 10.1016/0304-3940(95)12096-m. [DOI] [PubMed] [Google Scholar]
- Rogers J, Santis S, See R. Extended methamphetamine self-administration enhances reinstatement of drug seeking and impairs novel object recognition in rats. Psychopharmacology. 2008;199:615–624. doi: 10.1007/s00213-008-1187-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salo R, Nordahl TE, Galloway GP, Moore CD, Waters C, Leamon MH. Drug abstinence and cognitive control in methamphetamine-dependent individuals. J Substance Abuse Treatment. 2009;37:292–297. doi: 10.1016/j.jsat.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders JD, Happe HK, Bylund DB, Murrin LC. Development of the norepinephrine transporter in the rat CNS. Neuroscience. 2005;130:107–117. doi: 10.1016/j.neuroscience.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Schwendt M, Rocha A, See R, Pacchioni A, Mcginty J, Kalivas P. Extended methamphetamine self-administration in rats results in a selective reduction of dopamine transporter levels in the prefrontal cortex and dorsal striatum not accompanied by marked monoaminergic depletion. J Pharmacology Exp Ther. 2009;331:555. doi: 10.1124/jpet.109.155770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JC, Woods SP, Matt GE, Meyer RA, Heaton RK, Atkinson JH, Grant I. Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychology Review. 2007;17:275–297. doi: 10.1007/s11065-007-9031-0. [DOI] [PubMed] [Google Scholar]
- Sekine Y, Ouchi Y, Sugihara G, Takei N, Yoshikawa E, Nakamura K, Iwata Y, Tsuchiya KJ, Suda S, Suzuki K, Kawai M, Takebayashi K, Yamamoto S, Matsuzaki H, Ueki T, Mori N, Gold MS, Cadet JL. Methamphetamine causes microglial activation in the brains of human abusers. J Neurosci. 2008;28:5756–5761. doi: 10.1523/JNEUROSCI.1179-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekine Y, Ouchi Y, Takei N, Yoshikawa E, Nakamura K, Futatsubashi M, Okada H, Minabe Y, Suzuki K, Iwata Y, Tsuchiya KJ, Tsukada H, Iyo M, Mori N. Brain serotonin transporter density and aggression in abstinent methamphetamine abusers. Arch Gen Psychiatry. 2006;63:90–100. doi: 10.1001/archpsyc.63.1.90. [DOI] [PubMed] [Google Scholar]
- Shepard JD, Chuang DT, Shaham Y, Morales M. Effect of methamphetamine self-administration on tyrosine hydroxylase and dopamine transporter levels in mesolimbic and nigrostriatal dopamine pathways of the rat. Psychopharmacology. 2006;185:505–513. doi: 10.1007/s00213-006-0316-4. [DOI] [PubMed] [Google Scholar]
- Simon SL, Dacey J, Glynn S, Rawson R, Ling W. The effect of relapse on cognition in abstinent methamphetamine abusers. J Substance Abuse Treatment. 2004;27:59–66. doi: 10.1016/j.jsat.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Simon SL, Domier CP, Sim T, Richardson K, Rawson RA, Ling W. Cognitive performance of current methamphetamine and cocaine abusers. J Addict Dis. 2002;21:61–74. doi: 10.1300/j069v21n01_06. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M. Cognitive enhancement as a pharmacotherapy target for stimulant addiction. Addiction. 2010;105:38–48. doi: 10.1111/j.1360-0443.2009.02791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellez R, Rocha L, Castillo C, Meneses A. Autoradiographic study of serotonin transporter during memory formation. Behav Brain Res. 2010;212:12–26. doi: 10.1016/j.bbr.2010.03.015. [DOI] [PubMed] [Google Scholar]
- Uslaner JM, Parmentier-Batteur S, Flick RB, Surles NO, Lam JSH, McNaughton CH, Jacobson MA, Hutson PH. Dose-dependent effect of CDPPB, the mGluR5 positive allosteric modulator, on recognition memory is associated with GluR1 and CREB phosphorylation in the prefrontal cortex and hippocampus. Neuropharmacology. 2009;57:531–538. doi: 10.1016/j.neuropharm.2009.07.022. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJMJ, Everitt BJ. Drug Seeking Becomes Compulsive After Prolonged Cocaine Self-Administration. Science. 2004;305:1017–1019. doi: 10.1126/science.1098975. [DOI] [PubMed] [Google Scholar]
- Vaswani M, Linda FK, Ramesh S. Role of selective serotonin reuptake inhibitors in psychiatric disorders: a comprehensive review. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:85–102. doi: 10.1016/s0278-5846(02)00338-x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang G-J, Fowler JS, Franceschi D, Sedler M, Gatley SJ, Miller E, Hitzemann R, Ding Y-S, Logan J. Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. J Neurosci. 2001a;21:9414–9418. doi: 10.1523/JNEUROSCI.21-23-09414.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Leonido-Yee M, Franceschi D, Sedler MJ, Gatley SJ, Hitzemann R, Ding YS, Logan J, Wong C, Miller EN. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. The American Journal of Psychiatry. 2001b;158:377–382. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- Volz TJ, Fleckenstein AE, Hanson GR. Methamphetamine-induced alterations in monoamine transport: implications for neurotoxicity, neuroprotection and treatment. Addiction. 2007;102(Suppl 1):44–48. doi: 10.1111/j.1360-0443.2007.01771.x. [DOI] [PubMed] [Google Scholar]
- Wagner GC, Ricaurte GA, Seiden LS, Schuster CR, Miller RJ, Westley J. Long-lasting depletions of striatal dopamine and loss of dopamine uptake sites following repeated administration of methamphetamine. Brain Research. 1980;181:151–160. doi: 10.1016/0006-8993(80)91265-2. [DOI] [PubMed] [Google Scholar]
- Wan H, Aggleton JP, Brown MW. Different contributions of the hippocampus and perirhinal cortex to recognition memory. J Neurosci. 1999;19:1142–1148. doi: 10.1523/JNEUROSCI.19-03-01142.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton EC, Brown MW. Findings from animals concerning when interactions between perirhinal cortex, hippocampus and medial prefrontal cortex are necessary for recognition memory. Neuropsychologia. 2009;48:2262–2272. doi: 10.1016/j.neuropsychologia.2009.12.022. [DOI] [PubMed] [Google Scholar]