Abstract
Introduction
Extensive evidence demonstrates that current cocaine abusers show hypoactivity in anterior cingulate and dorsolateral prefrontal cortex and respond poorly relative to drug-naïve controls on tests of executive function. Relatively little is known about the cognitive sequalae of long-term abstinence in cocaine addicts.
Methods
Here, we use a GO-NOGO task in which successful performance necessitated withholding a prepotent response to assay cognitive control in short-and long-term abstinent cocaine users (1-5 weeks and 40-102 weeks, respectively).
Results
We report significantly greater activity in prefrontal, cingulate, cerebellar and inferior frontal gyrii in abstinent cocaine users for both successful response inhibitions and errors of commission. Moreover, this relative hyperactivity was present in both abstinent groups, which, in the presence of comparable behavioral performance, suggests a functional compensation.
Conclusions
Differences between the short- and long-abstinence groups in the patterns of functional recruitment suggest different cognitive control demands at different stages in abstinence. Short-term abstinence showed increased inhibition-related dorsolateral and inferior frontal activity indicative of the need for increased inhibitory control while long-term abstinence showed increased error-related ACC activity indicative of heightened behavioral monitoring. The results suggest that the integrity of prefrontal systems that underlie cognitive control functions may be an important characteristic of successful long-term abstinence.
Keywords: Cocaine addiction, abstinence, cognitive control, response inhibition
1. Introduction
Addiction is characterized by an uncontrollable, compulsive drive to obtain and consume an abused drug, despite the profound negative health and social consequences likely to ensue (Everitt et al., 2001; Garavan and Stout, 2005; Goldstein and Volkow, 2002). Substance dependent individuals preferentially select actions that yield short-term gains, though they may lead to long-term losses (Bechara and Damasio, 2002). They are more likely to engage in risky behavior (Lane and Cherek, 2000) and show less consideration of the consequences of their actions (Petry et al., 1998). Arguably, these traits are related to executive dysfunction (Lyvers, 2000) wherein chronic cocaine users show deficits in the brain structures implicated in cognitive control of behavior, in particular, in regions thought to be the seat of higher executive brain functions (Miller and Cohen, 2001). Indeed, chronic cocaine users consistently demonstrate impairments on neuropsychological tests of executive function (Ardila et al., 1991; Di Sclafani et al., 2002; Yücel et al., 2007).
Two important aspects of executive control implicated in addiction are inhibitory control and performance monitoring (Garavan and Hester, 2007). Performance monitoring processes (e.g., error detection and conflict monitoring) have been ascribed to the anterior cingulate cortex (ACC) (Botvinick et al., 1999; Botvinick et al., 2001; Kiehl et al., 2000; MacDonald et al., 2000; Menon et al., 2001; Ruchsow et al., 2002; Ullsperger and von Cramon, 2001; van Veen and Carter, 2002).
One model of cognitive control asserts that when erroneous or conflicting behavior is detected by the ACC, it signals to lateral prefrontal cortex (PFC) regions responsible for maintaining goal-oriented behavior that greater levels of control are necessary to successfully perform a task (Botvinick et al., 2001 see Silton et al., 2010 for an alternative interpretation). Increased top-down control should reduce conflict by biasing the system away from the incorrect, conflict-causing response and towards the correct, conflict-reducing response (Botvinick et al., 2001; Fassbender et al., 2009). With regard to addiction and specifically abstinence, this monitoring process may be important in detecting risky situations or behaviors that increase the likelihood of relapse (Garavan and Stout, 2005).
Previous investigations of inhibitory control in cocaine addicts have shown reduced prefrontal activity relative to controls (Fillmore and Rush, 2002; Goldstein et al., 2001; Kaufman et al., 2003) and there is evidence that cocaine addicts appear to rely more heavily on a suboptimal cerebellar pathway to successfully inhibit a prepotent response (Hester and Garavan, 2004). These findings are consistent with theories implicating cocaine-induced damage to the mesencephalic dopamine (DA) system (Franken et al., 2005; Spanagel and Weiss, 1999). It is thought that blockade of dopamine transporters produces elevated synaptic DA levels, chronic exposure to which may account for both the reduced DA receptors and metabolism seen in users (Koob and Le Moal, 1997; Volkow et al., 1993). Inhibitory control has also been identified as a risk factor for addiction that precedes drug use (Dalley et al., 2007; Tarter et al., 2003; Verdejo-García et al., 2008). Performance and neuroimaging data on Stroop and decision-making tasks have been shown to predict likelihood of completing treatment in substance abusers (Brewer et al., 2008; Paulus et al., 2005; Streeter et al., 2008) as has cognitive functioning (Aharonovich et al., 2006; Turner et al., 2009). As these tasks are known to activate the neuronal circuits underlying cognitive control, this indicates that these circuits may play an important role in abstinence.
Previous studies of abstinent drug users have typically investigated short-term abstinence and have revealed many persistent deficits, which are more pronounced in heavy users, in the regions associated with cognitive control and reward anticipation (Bolla et al., 2004; Bolla et al., 2003). Relative to controls, abstinent cocaine abusers have been shown to have reduced metabolism in left ACC and right dorsolateral prefrontal cortex (DLPFC), and greater activation in right ACC. Indeed, activity in some of these regions predicts relapse in both abstinent cocaine and methamphetamine abusers (Kosten et al., 2006; Paulus et al., 2005; Wexler et al., 2001) with individuals showing more ACC activity at the onset of abstinence being less likely to relapse subsequently. It has previously been suggested that the general pattern of prefrontal hypoactivity in drug users may ameliorate with increasing abstinence from drug consumption (Volkow and Fowler, 2000) and indeed abstinence from cocaine use has been shown to reduce high-risk responses on a gambling task (Bartzokis et al., 2000). GO/NOGO tasks in which the GO/NOGO ratio is low thereby creating a prepotent response that is difficult to inhibit on NOGO trials provide a useful assay of cortical activity underlying inhibitory control and action monitoring. Indeed cocaine addicts have shown impaired performance in these tasks (Fillmore and Rush, 2002; Fillmore et al., 2002). We hypothesized that such a task would be useful for evaluating any functional change that may occur in the cortical circuits underlying inhibitory control and action monitoring over abstinence.
Just as not all people with a propensity to develop addiction do so, not all addicts successfully complete treatment. Indeed, treatment programs typically have very high dropout rates (Carroll et al., 1994; Simpson et al., 1999) reflecting the relapsing nature of the disease. This means that very little is known about the neurobiology of successful long-term abstinence as the high attrition and relapse rates of longitudinal studies pose significant impediments to assessing long-term abstinence effects prospectively. Another research approach is to recruit and characterize individuals known to have been abstinent for varying durations. While this approach cannot reveal whether neurobiological differences in abstinent users preceded or arose from that abstinence, it can nonetheless characterize the functioning of those who have demonstrated the ability to abstain for either short or long periods. Here, we investigate what role cognitive control may play in abstinence, both short- and long-term. We hypothesized that any changes that may occur with prolonged abstinence or any pre-existing differences that might facilitate successful abstinence would be reflected in functional brain measurements of cognitive control.
2. Materials and Methods
2.1. Participants and task design
Twenty-seven volunteers (21 male; mean age 33.2 years, range: 22-45) participated in this study, which was approved by the Institutional Review Board of the Nathan Kline Institute for Psychiatric Research (NKI). Participants gave informed written consent and were compensated for their participation.
Abstinent cocaine dependant (CD) users were patients in the Daytop Village Inc., a large therapeutic community with multiple treatment sites in the New York City area. Controls were recruited from the community via the NKI volunteer program and had no history of substance abuse disorders. Participants were recruited over the course of 5 months by a psychiatrist (JN) visiting the treatment site to make a presentation about the study. Interested participants were prescreened and signed a consent form to be enrolled in the study. The protocol described here was part of a larger study investigating the effects of abstinence on grey and white matter. All participants were screened with the Structural Clinical Interview for the DSM-IV – TR (SCID) by a psychiatrist (JN) or a SCID-certified research assistant (First et al., 2002). CD participants had no lifetime history of substance dependence (other than cocaine and nicotine) but were eligible for the study if they met criteria for abuse (lifetime or current) of other substances. Participants with any history of neurological disorders, psychiatric illness, head trauma, contra-indications for MRI, or HIV seropositivity were excluded. CD participants were excluded if they did not have continuous treatment or tested positive during the reported abstinence period. Participants early in treatment were monitored on a 24-hour basis, were subject to periodic random urine toxicology screens, and were not permitted to leave the facility without an escort. Those later in treatment were allowed leave the facility on their own recognizance but were evaluated by clinical staff (including urine toxicology) upon their return. Subjective data on drug use and abstinence history (including date of last use) were collected from participants and corroborated with records from clinical charts, lab tests and interviews with clinical staff. On the day of scanning, representatives of the Daytop Village transported participants to and from NKI where all behavioral and MRI measurements occurred.
Abstinent CD participants were divided into two groups depending on length of abstinence from their last cocaine consumption. The long-term abstinent (LA) group (n=9) had not consumed cocaine for on average 69 weeks (sd=17.49; range: 40-102). The short-term abstinent (SA) group (n=9) had refrained from consumption for on average 2.4 weeks (sd=1.34; range: 1-5.1) prior to scanning. Average length of use prior to abstinence for the LA group was 10.67 years (sd=7.63, range: 1.5-23) and for the SA group was 12.11 years (sd=5.01, range: 1-18). This difference was not significant (Welch t(13.81)= -0.47, p > 0.05). A further group of nine cocaine- naïve participants constituted the control group (see table 1).
Table 1.
Demographic characteristics for the control and abstinent cocaine groups.
| Characteristic | Control | Short | Long | Signif. | Pairwise Differences |
|---|---|---|---|---|---|
| Number of participants | 9 | 9 | 9 | ||
| Gender M/F | 7 / 2 | 7 / 2 | 7 / 2 | ||
| Age at time of scanning (years) | 30.5 / 6.7 (22.8-44.4) | 36.4 / 6.6 (25.8-45.8) | 32.8 / 8.3 (22-44.1) | ||
| Edinburgh handedness (1 = right) | 0.7 / 0.6 (-0.7-1) [1] | 0.8 / 0.4 (-0.2-1) | 0.9 / 0.1 (0.8-1) | ||
| Years of education | 16 / 3.6 (12-20) | 12.4 / 1.8 (10-16) | 10.2 / 2 (7-13) | *** | LA < C; SA < C |
| Years of use | Not Applicable | 12.1 / 5.0 (1-18) | 10.6 / 7.6 (1.5-23) | ||
| Lifetime usage (g) | Not Applicable | 3911 / 1937 (1924-8008) | 10712 / 12213 (243-33310) | ||
| Avg. weekly use just prior to treatment (g) | Not Applicable | 11.3 / 7.4 (2-25) | 29.2 / 37.3 (1-105) | ||
| Typical usage (g/week) | Not Applicable | 12.8 / 19.2 (2-63) | 19.3 / 21.2 (3-70) | ||
| Task Performance | |||||
| Percent correct responses | 50.6 / 13.1 (30.5-68.3) | 56.1 / 11.6 (36.3-76.5) | 52.4 / 9.3 (41.5-69.5) | ||
| Incorrect inhibitions (ERRORS) RTs | 272.9 / 54.2 (202.1-352.2) | 278.5 / 47.4 (216.7-359.7) | 307.3 / 76.2 (215.3-451.8) | ||
| GO Trial RTs | 315.7 / 56.1 (239.6-404.4) | 339.7 / 52.6 (271.7-411.1) | 352.1 / 50 (282.5-423.6) | ||
| Socioeconomic status | |||||
| Parental socioeconomic status | 2.1 / 1.2 (1-5) | 2.9 / 1.1 (2-5) | 2.4 / 1.2 (1-5) | ||
| Participant socioeconomic status | 2.2 / 1.1 (1-4) | 2.6 / 0.7 (1-3) | 3.7 / 0.9 (2-5) | ** | LA > SA; LA > C |
| Barratt's impulsiveness scale | |||||
| Total | 56.3 / 6.4 (46-64) | 70.4 / 13.7 (57-88) | 57.7 / 8.5 (48-70) | * | LA < SA; SA > C |
| Motor subscale | 17.6 / 3.8 (13-24) | 23.2 / 4 (17-30) | 20.1 / 4.2 (14-27) | * | SA > C |
| Attention subscale | 19.2 / 3.2 (14-23) | 22.8 / 6.6 (13-31) | 19.6 / 2.8 (14-23) | ||
| Non-planning subscale | 19.6 / 1.3 (18-22) | 24.4 / 5.7 (18-32) | 18 / 3.9 (11-22) | ** | LA < SA; SA > C |
| Buss Perry aggression scale | |||||
| Total | 45.3 / 8.4 (34-60) | 74.2 / 25.4 (41-120) | 83.3 / 16.3 (59-109) | *** | LA > C; SA > C |
| Physical subscale | 13.4 / 3.8 (9-20) | 21 / 8.1 (11-37) | 25.8 / 7.3 (15-33) | ** | LA > C |
| Verbal subscale | 10 / 2.6 (8-15) | 13.3 / 4.2 (9-20) | 15.3 / 3.6 (9-20) | * | LA > C |
| Anger subscale | 10.8 / 2.4 (8-16) | 18.4 / 8 (9-33) | 21 / 5.5 (13-28) | ** | LA > C; SA > C |
| Hostility subscale | 11.1 / 4.3 (8-21) | 21.4 / 9.2 (10-36) | 21.6 / 5.1 (13-28) | ** | LA > C; SA > C |
Entries are of the form: mean / standard deviation (min-max). The (optional) number in square brackets indicates the number of participants with no data. All observations were compared by ANOVAs. For the pairwise contrasts (Tukey's Honest Significant Difference test) C=control, LA=long and SA=short and p ≤ 0.05. Significance codes:
p ≤ 0.001
p ≤ 0.01
p ≤ 0.05 and refers to the corresponding ANOVA. Drug use information was compiled from a combination of SCID, KMSK, and clinical records.
Handedness (Oldfield, 1971) and socio-economic status (Hollingshead, 1975) of participants was assessed and participants were administered the Barratt Impulsivity test (Patton et al., 1995), the Buss Perry aggression test (Buss and Perry, 1992) and the Kreek-McHugh-Schluger-Kellogg (KMSK) scale (Kellogg et al., 2003) to assess drug use history. These assessments were included to characterize the participants and evaluate whether these traits would change with abstinence. Socioeconomic status (which includes educational status) has previously been linked with attrition rates from treatment programs (Alterman et al., 1996) and consumption of drugs of abuse (Miech and Chilcoat, 2007), as has aggression (Brook et al., 1995) and impulsivity (de Wit, 2009; Verdejo-García et al., 2008).
Participants completed a GO/NOGO task based on our earlier work (Garavan et al., 1999) and which has been shown previously to reveal functional hypoactivity in current cocaine users (Kaufman et al., 2003). The letters X and Y were serially presented, alternating at 1 Hz and participants were required to make a button press response to each letter. Responses and reaction times were recorded. Participants were instructed to withhold their response on NOGO trials, that is, trials in which the alternating pattern was broken. For example, in the stimulus train X Y X Y Y X, participants were to withhold their response to the fifth letter. The stimuli were presented for 900 ms followed by a 100 ms blank screen. Participants were instructed to respond while the letter was on the screen. Participants completed four runs each containing 315 GO and 20 NOGO stimuli totaling 1260 GO trials and 80 NOGO trials.
2.2. Scanning Parameters and Data Analysis
Functional images were acquired in contiguous 5mm transverse slices using a blipped gradient-echo echo-planar pulse sequence (TE=50ms, TR=2000ms, FOV=224mm, 64×64 matrix, 3.5×3.5 mm in-plane resolution). All scanning was conducted on a 1.5T Siemens VISION scanner (Erlangen, Germany) equipped with a 30.5-cm i.d. three-axis local gradient coil and an end-capped quadrature birdcage radio-frequency head coil. High-resolution T1-weighted MPRAGE anatomical images (TE=4.9ms, TR=11.6ms, flip angle 8°, FOV 256mm, slice thickness 1mm, matrix 256×256×180) were acquired after functional imaging to permit subsequent activation localization and spatial normalization. Stimuli were back-projected onto a screen at the participants’ feet and were viewed with the aid of prism glasses that were attached to the head coil.
Functional analyses were conducted using AFNI (Cox, 1996). After reconstruction, differences in slice acquisition timing were corrected using Fourier interpolation. The time-series was then motion corrected (least-squares alignment using three translational and three rotational parameters). Separate hemodynamic response functions for successful inhibitions (STOPS) and errors of commission (ERRORS) were then calculated using deconvolution based on each participant's behavioral data. A γ-variate function was then fitted voxelwise to these hemodynamic response functions using non-linear regression (Murphy and Garavan, 2005). Brain activation was operationally defined as the area under these event-related response functions expressed as a percentage of the area under the baseline. For this task, the baseline is implicit and reflects tonic task-related activity. The whole-brain activation maps were then warped into a standard stereotaxic (1mm3) space (Talairach and Tournoux, 1988) and spatially blurred using a 4.2 mm isotropic FWHM Gaussian filter kernel. For each of the two trial types, whole-brain one-sample t-tests against the null hypothesis of no event-related activity determined within-group activation maps separately for each group. Significant voxels passed a voxelwise statistical threshold (t=3.83, p < 0.005) and, to control for multiple comparisons, were required to be part of a larger 270 μl cluster. The volume threshold was determined by means of a Monte-Carlo simulation and resulted in 5% probability (corrected) of a cluster surviving due to chance.
In order to conduct between-group comparisons, the group activation maps were then combined to create separate OR maps for STOPs and ERRORs. An OR map included the voxels in clusters indicated as significant from any of the three constituent group maps. Mean activation levels of the clusters in the combined maps were calculated for each participant to allow a series of between-group ANOVAs to be conducted, using R (R Development Core Team, 2010), on these functionally defined ROIs. Due to significant differences (described below), total scores for the Buss Perry aggression scale and Barratt's impulsiveness scale, and scaled participant socioeconomic status were included as covariates in all fMRI regions of interest ANOVAs for STOPS, ERRORS and aggregate regions described below. Unless otherwise stated, pairwise post hoc differences between groups were assessed using Tukey's Honest Significant Difference test (HSD).
3. Results
3.1. Demographics
There were no significant between-group differences in age (F(2,24)=1.52, p > 0.05) or handedness (F(2, 23)=0.66, p > 0.05) (one subject was omitted from the latter ANOVA due to missing information; See Table 1 for complete demographic information.)
An analysis of variance revealed a significant difference on Barratt's impulsivity scale (BIS) (total) (F(2, 24)=5.42, p ≤ 0.05). Tukey's HSD revealed that the control group differed significantly from the SA group (p ≤ 0.05), the SA and LA groups also differed significantly (p ≤ 0.05), and the control and LA groups were not significantly different (p > 0.05.) ANOVAs of the Buss Perry (BP) aggression test also revealed significant differences (F(2, 24)=10.81, p ≤ 0.001). Tukey's HSD showed that the control group differed significantly from the LA (p ≤ 0.001) and SA (p ≤ 0.01) groups but that the LA and SA groups did not differ (p > 0.05). The groups did not differ on parental socioeconomic status (SES) (F(2, 24)=1.02, p>0.05) but did on participant SES (F(2, 24)=6.24, p<0.01) with the LA group being greater than both SA and control groups (p<0.05).
3.2. Behavioral Results
There were no significant between-group differences in reaction times (RTs) for incorrect inhibitions (F(2,24)=0.83, p > 0.05) or GO stimuli (F(2,24)=1.09, p > 0.05). Similarly, no between-group differences were observed in the percentage of successful inhibitions (F(2,24)=0.53, p > 0.05).
3.3. Event-related fMRI Results
3.3.1. Stops
The regions observed for STOPS (Table 2) were consistent with meta-analyses of this and similar tasks (Buchsbaum et al., 2005; Garavan et al., 2006). We observed frontal activation in inferior, middle, superior, and medial frontal gyrii and non-frontal activation in temporal, parietal, cerebellar and subcortical regions. The analysis of group differences revealed a general pattern wherein LA and SA users had greater activation than control participants. Of those regions showing significant between-group ANOVA differences, relative to controls, the users showed greater activity in predominantly prefrontal and precentral cortex with the LA group also showing increased bilateral cerebellar activity. More specifically, pairwise post hoc tests showed the SA group with greater activity than controls in the right middle frontal gyrus (RMFG), right precentral gyrus, right superior frontal gyrus (RSFG), and one right middle temporal region. The LA group showed greater activity than controls in right inferior frontal gyrus (RIFG), RMFG, right precentral gyrus, left superior temporal gyrus (LSTG), and one region in each of the right and left cerebellar tonsils.
Table 2.
Correct inhibitions (STOPS).
| Structure | Hemisphere | BA | Volume | RL | AP | IS | Significance | Pairwise differences |
|---|---|---|---|---|---|---|---|---|
| Limbic System | ||||||||
| Cingulate Gyrus | R | 24 | 323 | -1 | -8 | 31 | ||
| Posterior Cingulate | R | 23 | 367 | -4 | 27 | 23 | ||
| Frontal Lobes | ||||||||
| Inferior Frontal Gyrus | L | 47 | 707 | 34 | -24 | -4 | * | LA<SA |
| Inferior Frontal Gyrus | R | 47 | 254 | -45 | -27 | 4 | *** | LA>C; LA>SA |
| Medial Frontal Gyrus | R | 6 | 423 | -1 | -34 | 34 | ||
| Middle Frontal Gyrus | R | 9 | 983 | -42 | -9 | 35 | *** | LA>C; SA>C; LA<SA |
| Middle Frontal Gyrus | R | 10 | 292 | -38 | -52 | 5 | ||
| Middle Frontal Gyrus | R | 46 | 276 | -43 | -39 | 23 | ||
| Precentral Gyrus | L | 6 | 381 | 42 | 2 | 31 | ||
| Precentral Gyrus | R | 6 | 295 | -47 | 1 | 46 | * | LA>C; SA>C |
| Superior Frontal Gyrus | L | 10 | 421 | 31 | -51 | 22 | ** | LA<SA |
| Superior Frontal Gyrus | R | 9 | 523 | -36 | -34 | 33 | ** | SA>C |
| Superior Frontal Gyrus | R | 6 | 425 | -4 | -14 | 48 | * | |
| Temporal Lobes | ||||||||
| Middle Temporal Gyrus | R | 22 | 308 | -58 | 49 | 9 | * | SA>C; LA<SA |
| Middle Temporal Gyrus | R | 37 | 276 | -50 | 40 | -4 | ||
| Superior Temporal Gyrus | L | 22 | 275 | 54 | 8 | -5 | ** | LA<C; LA<SA |
| Superior Temporal Gyrus | R | 13 | 283 | -57 | 42 | 21 | ||
| Parietal Lobes | ||||||||
| Inferior Parietal Lobule | R | 40 | 2307 | -48 | 50 | 38 | ||
| Precuneus | R | 39 | 1315 | -31 | 59 | 39 | * | |
| Subcortical | ||||||||
| Cerebellar Tonsil | L | 513 | 35 | 39 | -41 | ** | LA>C | |
| Insula | R | 13 | 2125 | -38 | -17 | -1 | ||
| Thalamus | R | 471 | -12 | 14 | 9 | * | LA>SA | |
| Cerebellar Tonsil | R | 326 | -37 | 55 | -32 | * | LA>C; LA>SA | |
| Caudate | L | 273 | 10 | -6 | 11 |
BA is Brodmann's area, volumes are in micro-liters and center-of-mass coordinates are in the Talairach & Tournoux atlas. RL: Right-Left, AP: Anterior-Posterior, IS: Inferior-Superior. Significance codes:
p ≤ 0.001
p ≤ 0.01
p ≤ 0.05 and refers to the corresponding ANOVA.
For the pair-wise contrasts (Tukey's Honest Significant Difference test) C=control, LA=long and SA=short and p ≤ 0.05.
With respect to pairwise comparisons between the user groups, the LA group displayed greater activity than the SA group in the RIFG, right thalamus, and right cerebellar tonsil. Conversely, the SA group demonstrated greater activity than the LA group in LIFG, RMFG and LSFG, right middle temporal gyrus and LSTG regions.
Based on the observation of different patterns of group effects in the left and right IFG, a 2×3 (hemisphere × group) ANOVA was conducted on the mean activation in the left and right functionally-define IFG regions. Group was not significant (F(2, 45)=1.28, p>0.05) but hemisphere (F(1, 45)=5.52, p<0.05) and the interaction term (F(2, 45)=12.51, p<0.001) were (see Figure 1). T-tests and one-way ANOVAs (and pairwise t-tests) were conducted to elucidate these observations. Within the SA group, LIFG was more active than RIFG (p<0.001); in the LA group RIFG was more active than LIFG (p<0.05). In the RIFG, a significant main effect of group was observed (F(2, 21)=10.6, p<0.001); the LA group had more activity here than the SA and control groups (p<0.05). In the LIFG, a marginal effect of group was observed (F(2, 211)=3.41, p=0.052); the SA group had more activity than the LA group (p<0.05).
Figure 1.
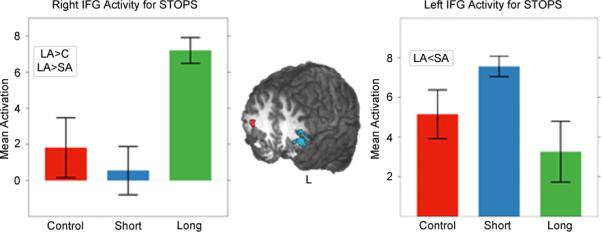
Regions in the left and right inferior frontal gyrii for STOPS analysis. Group-wise differences are shown in the charts. Error bars represent standard error of the mean.
Three regions located in BA 9 and 46 (Figure 2) were observed in right DLPFC. Given an interest in the role of DLPFC in abstinence, we aggregated their mean activity levels, weighted by cluster volume, and observed a significant main effect of group (F(2, 21)=11.80, p < 0.001). Pair-wise comparisons revealed that the LA and SA groups each displayed significantly more activity than the control group (p < 0.05).
Figure 2.
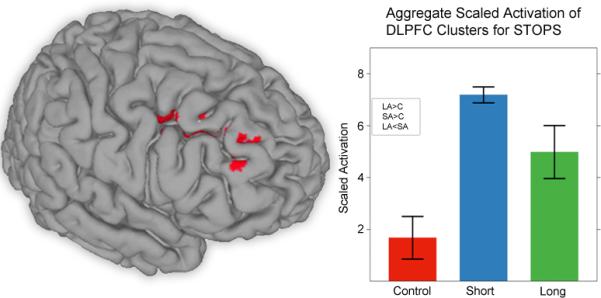
Three regions, located in RMFG, and two in RSFG, used in the aggregate STOPS DLPFC cluster, the mean activity in which was aggregated and weighted by volume. Error bars represent standard error of the mean.
3.3.2. Errors
In agreement with previous studies of error processing (Braver et al., 2001; Carter et al., 1998; Hester et al., 2004; Kiehl et al., 2000; Menon et al., 2001; Ullsperger and von Cramon, 2003), we observed error-related activation in dorsal ACC, insula, inferior and dorsolateral prefrontal cortex (PFC) (see Table 3). Significant group differences were found in prefrontal cortex, in the inferior, middle and superior frontal gyri, in the anterior cingulate, the right supramarginal gyrus and the right culmen of the cerebellum. In general, the SA and LA groups each had greater activity in these regions relative to the control group, though LA < SA in the LIFG, LA > SA in the RIFG, and LA > SA in the right culmen.
Table 3.
Errors of commission (ERRORS).
| Structure | Hemisphere | BA | Volume | RL | AP | IS | Significance | Pairwise differences |
|---|---|---|---|---|---|---|---|---|
| Limbic System | ||||||||
| Cingulate Gyrus | R | 32 | 1084 | -3 | -15 | 40 | ||
| Cingulate Gyrus | L | 32 | 1028 | 0 | -32 | 26 | ||
| Cingulate Gyrus | R | 24 | 324 | -1 | 5 | 46 | ||
| Cingulate Gyrus | L | 24 | 306 | 1 | 18 | 33 | * | SA>C |
| Frontal Lobes | ||||||||
| Inferior Frontal Gyrus◆ | L | 47 | 1185 | 35 | -23 | -6 | ** | SA>C; LA<SA |
| Inferior Frontal Gyrus | R | 9 | 376 | -46 | -1 | 22 | ** | LA>C; LA>SA |
| Middle Frontal Gyrus | R | 9 | 600 | -38 | -36 | 34 | ** | LA>C |
| Superior Frontal Gyrus | L | 9 | 312 | 37 | -43 | 30 | ** | SA>C |
| Temporal Lobes | ||||||||
| Middle Temporal Gyrus | R | 21 | 383 | -62 | 33 | -3 | ||
| Angular Gyrus | R | 39 | 390 | -43 | 63 | 36 | ||
| Parietal Lobes | ||||||||
| nSupramarginal Gyrus | L | 40 | 461 | 59 | 48 | 24 | ||
| Subcortical | ||||||||
| Insula◆ | R | 13 | 1557 | -40 | -11 | -3 | ||
| Thalamus◆ | R | 1419 | -1 | 2 | 8 | |||
| Culmen | R | 327 | -22 | 52 | -28 | *** | LA>C; LA>SA |
BA is Brodmann's area, volumes are in micro-liters and center-of-mass coordinates are in the Talairach & Tournoux atlas. RL: Right-Left, AP: Anterior-Posterior, IS: Inferior-Superior. P-values were derived from ANOVAs. Significance codes:
p ≤ 0.001
p ≤ 0.01
p ≤ 0.05 and refers to the corresponding ANOVA.
For the pair-wise contrasts (Tukey's Honest Significant Difference test) C=control, LA=long and SA=short and p ≤ 0.05.
indicates that activity was ERROR specific (p ≤ 0.05) as determined by a series of 2×3 (event-type × group) ANOVAs conducted on the activation levels within the 14 ERROR regions in both the ERRORs and STOPs conditions.
A number of error-related activations located in BA 32 and 24, shown in Figure 3, were observed in the cingulate gyrus. An aggregate of their mean activity, weighted by cluster volume, showed a marginally significant main effect of group (F(2, 21)=3.19, p =0.06). Tukey's HSD (p = 0.05) showed this difference to be driven by the LA group having greater mean activity than the controls. A planned linear contrast in the ANOVA (Control < SA < LA) showed a significant linear trend in the means of the groups (F(1, 21)=6.30, p< 0.05). Tukey's HSD also showed this effect to be driven by the LA group having greater mean activity than the controls (p < 0.05). The significant linear trend in group means was also present in this latter ANOVA (F(1, 24)=6.73, p< 0.05).
Figure 3.
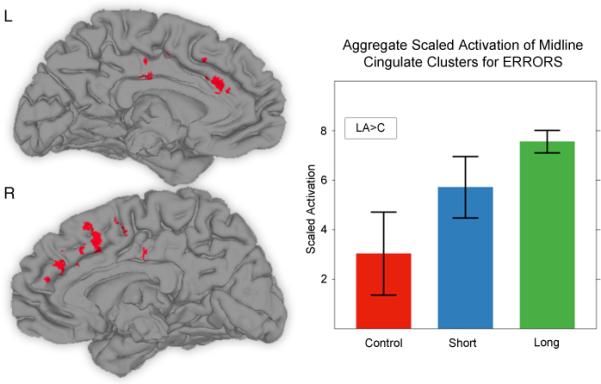
Four regions, located in left and right cingulate gyrii, used in the aggregate ERROR cingulate gyrus cluster, the mean activity in which was aggregated and scaled by volume. Error bars represent standard error of the mean.
Although activated on error trials, some error-related activation in response inhibition tasks might reflect processes that are also present on successful inhibitions (such as conflict monitoring) or may contain activation associated with the unsuccessful attempt to inhibit; in accordance with the race model of response inhibition (Logan and Cowan, 1984), the response inhibition network may activate but may not do so sufficiently soon to countermand the response (Garavan et al., 2002). Consequently, to identify error-specific activations, a series of 2×3 (event-type × group) ANOVAs were conducted on the activation levels within the 14 regions listed in Table 3. These revealed greater error-related activation in the right insula (F(1, 45)=4.77, p<0.05), right thalamus (F(1, 45)=4.30, p<0.05), and left IFG (event type: F(1, 45)=5.62, p<0.05). There were no significant interactions and there was a significant Group effect in the left IFG only (F(2, 24)=10.1, p< 0.001) which was driven by Control < SA and LA < SA (p<0.05). Similar analysis conducted on the aggregate ACC region revealed no significant effects.
4. Discussion
The present study has revealed the neuroanatomical correlates of cognitive control in abstinent cocaine users. The results demonstrate comparable performance levels and greater activation associated with inhibitory control and performance monitoring processes in abstinent cocaine users relative to controls. This stands in contrast to the poorer performance and functional hypoactivity typical of current cocaine users (Kaufman et al., 2003) and indicates an important role for the neuroanatomical integrity of cognitive control in successfully maintaining abstinence. Moreover, the two abstinent groups also differed from one another in activation levels suggesting different cognitive control demands related to abstinence duration. Cocaine abstinence may be characterized by a three-stage symptomatology (Gawin and Kleber, 1986) and proceeds from initial symptoms of withdrawal to cocaine cessation. The initial phase, generally accepted as “the crash” by cocaine addicts and psychiatrists alike, is characterized as an exhaustion (including, but not limited to, intense depression, agitation, anxiety and intense cravings) and lasts from hours to 4 days (see Weddington et al., 1990 for an alternate view of early abstinence). This is followed by a withdrawal phase ranging in duration from 1 to 10 weeks and is characterized by an initial absence of cravings in the early weeks and their return by the middle weeks. Symptoms of anhedonia, anergia and intense cravings characterize the final weeks of withdrawal. Extinction lasts indefinitely and is typified by normal mood and recurrent (spontaneous or cued) cravings. The study described here demonstrates that the processes involved in the withdrawal phase of abstinence may be different from those involved in maintaining abstinence insofar as the underlying neural circuits recruited differ between the two groups with the LA group recruiting regions more typical of non-addicts though still displaying elevated activity patterns.
Overall, there was a general trend for the user groups to display greater levels of activity than the controls. For STOPS, the SA group activated more dorsal regions of the middle and superior frontal gyrii, whereas the LA group tended to recruit more inferior regions, such as bilateral inferior frontal gyrii, typically associated with response inhibition. Inhibitory control appears to be accompanied by greater activation in frontal regions including inferior frontal gyrus, cingulate gyrus, middle frontal gyrus and precentral gyrus. The role played by these regions in inhibitory control as has been revealed by lesion studies, functional neuroimaging and transcranial magnetic stimulation studies (Aron et al., 2003; Buchsbaum et al., 2005; Chambers et al., 2006; Menon et al., 2001; Rubia et al., 2003). Previous studies in cocaine addicts have shown many of these regions to be hypoactive relative to controls (Bolla et al., 2004; Kaufman et al., 2003). In contrast, the abstinent groups in this study show more elevated activity in the aggregate DLPFC cluster; a finding that has also been observed in abstinent marijuana users (Tapert et al., 2007). This, combined with the elevated precuneus activity (an integral component of the sensorimotor network (Luppino et al., 1999)), may indicate increased frontoparietal attentional, or response selection mechanisms being brought to bear on this cognitive task.
Hyperactivity in ACC relative to posterior cingulate cortex has been linked with greater likelihood of avoiding relapse with those participants showing this hyperactivity prior to scanning having longer periods of abstinence (Kosten et al., 2006). Abstinence may place extra demands on cognitive control and, specifically, the monitoring processes subserved by the ACC suggesting that the increased activity in this region, as indicated by the linear trend in the activity of the aggregate ACC region, may be a defining characteristic of successful abstinence.
The elevation of frontal activity appears to undergo a shift from the left to right hemisphere over the course of abstinence. Activation of the LIFG has been observed in children performing a task requiring both response inhibition and interference suppression (Bunge et al., 2002). Furthermore, the left IFG has recently been shown to be important for response inhibition (Swick et al., 2008) and in a task similar to that described here, older adults have been shown to rely more on left PFC (Garavan et al., 2006). Activity observed in these regions is therefore likely to be response inhibition related. The reliance of the SA group on this region suggests that early in abstinence users may adopt an alternative cognitive strategy in that they may recruit the LIFG in a manner akin to children and older adults to achieve behavioral results similar to the other groups. Recruitment of the LIFG may be indicative of short-term abstinence whereas with prolonged abstinence a pattern topographically typical of normal, healthy controls may emerge, though with the LA group displaying elevated activity levels. Should the transition from relying on left to right IFG be a result of abstinence, it is likely this may only develop with protracted abstinence as short periods of abstinence followed by relapse may not allow this to occur (Volkow et al., 2001; Wang et al., 2004).
The involvement of the cerebellum in high-order executive functions is well documented. Focal cerebellar damage has been associated with dysexecutive impairments, personality disorders such as disinhibited and inappropriate behavior and working memory impairment (Desmond et al., 2003; Heyder et al., 2003; Parkins, 1997). We, and others, have previously hypothesized that drug abusers may develop increased cerebellar activity to compensate for reduced prefrontal activity in tasks demanding elevated levels of cognitive control (Desmond et al., 2003; Hester and Garavan, 2004). The two abstinence groups in the present study showed clusters of activity in cerebellar regions that are consistently elevated relative to controls. If, as active drug users, they came to rely on elevated cerebellar activity to compensate for reduced prefrontal activity, it appears to have been maintained into abstinence, possibly because it served a crucial role in the cognitive control system while prefrontal control system recovered from damage to the mesencephalic dopamine system inflicted by chronic cocaine use. For the STOPS, the left cerebellar regions encompass portions of Lobules VII and VIII, while the right region is located in part of Crus I of the cerebellum. These cerebellar regions have recently been shown to exhibit strong resting state functional connectivity (RSFC) correlations with contralateral DLPFC (Krienen and Buckner, 2009). Likewise the region from the ERRORs is located in Lobule VI of the cerebellum, which has been shown to exhibit strong RSFC correlations with the anterior prefrontal cortex (Krienen and Buckner, 2009).
One of the defining neurobiological characteristics of chronic cocaine consumption is cortical hypoactivity (Volkow and Fowler, 2000). Indeed, acute administration of cocaine can prompt a return to activity levels seen in controls, making cocaine abusers’ activity almost indistinguishable from that of controls. This normalization occurred in cingulate regions previously shown to be hypoactive in cocaine abusers (Garavan et al., 2008). By contrast, the cohort of abstinent users in this study demonstrated hyperactivity relative to controls in midline cingulate performance monitoring regions (Ridderinkhof et al., 2004). It has previously been shown that fronto-parietal activity is associated with subjective awareness of errors (Hester et al., 2005) and that cocaine abusers are not as aware of their errors as drug-naïve controls (Hester et al., 2007). The elevated activity observed in this study may be functionally significant insofar as raised error-related activity tends to be present in better, more attentive individuals (Hester and Garavan, 2004). This may reflect a neuro-adaption, as increased awareness of erroneous behavior, particularly that leading to cocaine consumption, may allow more cognitive processing of error prone behavior to occur and prevent its execution, thus realizing the higher goal of maintaining abstinence. For STOPs, the SA group displayed greater activity in RDLPFC and LIFG but for errors the LA group displayed greater ACC activity that points to increased performance monitoring. Though the groups differed from one another only marginally in the aggregate ACC region, the pattern of activity in which SA is intermediate between controls and LA, revealed by the significant linear trend, is consistent with increased monitoring as abstinence proceeds. This suggests, in one possible interpretation, that in the acute phase of abstinence heightened inhibitory control is especially important and that latterly the error monitoring system plays a greater role. That is, the users may no longer need to inhibit behaviors/urges but need to actively monitor them to maintain abstinence.
Though we observed no performance differences between our three groups, this could be explained by a number of factors. The group sizes are small (nine in each), which may be too small to reveal behavioral differences between the groups. A previous application of this task to current users and controls observed significant group differences in performance (Kaufman et al., 2003). However, the groups in the present study have been abstinent for a significant period of time. The group differences revealed in the neuroimaging data above suggest that the comparable performance of the abstinent users relative to the controls may have been achieved by additional functional recruitment (Rajah and D'Esposito, 2005). Indeed, equivalent task performance may be a directly measurable consequence of this and may be facilitated by abstinence. The small sample size is a limitation of the current study (and one that is not easily corrected due to the subsequent decommissioning of the scanner) and although we have observed significant group functional differences, it will be important for future studies to replicate these effects with larger samples.
As with most studies on human clinical groups it is not possible to address the etiology of these activity pattern differences, that is, we cannot say whether the differential brain recruitment patterns reported above predate cocaine consumption and facilitate abstinence or arose as a consequence of abstinence. This ambiguity notwithstanding, the present results are one of the first to show the functional sequalae of prolonged abstinence in cocaine abusers. They demonstrate that successfully abstinent users display elevated activity in prefrontal and midline regions. Moreover, different cognitive control strategies may be required at different stages in abstinence. The withdrawal phase of abstinence may require increased inhibitory control whereas in the extinction phase this may not be as important as ongoing monitoring of behavior to prevent relapse. These results suggest that the neural systems involved in cognitive control may be apt for targeting during treatment and may increase the likelihood of prolonged successful abstinence.
Acknowledgements
Data analysis was supported by access to the IITAC high-performance computing cluster, funded by the Higher Education Authority, The National Development Plan and the Trinity Centre for High Performance Computing. The authors wish to thank Dave Deriso for help with figure preparation.
Role of the Funding Source This work was supported by NIMH grant number DA014100 awarded to Hugh Garavan. The funding agency played no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributions CGC analyzed the data and wrote the article. HG, JJF and JN designed the study. HG co-wrote the article. MS collected the MRI data. JN recruited participants. All authors have approved the manuscript.
Conflicts of Interest and Financial Disclosure The authors report no biomedical financial interests or conflicts of interest.
References
- Aharonovich E, Hasin DS, Brooks AC, Liu X, Bisaga A, Nunes EV. Cognitive deficits predict low treatment retention in cocaine dependent patients. Drug Alcohol Depend. 2006;81:313–322. doi: 10.1016/j.drugalcdep.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Alterman A, McKay J, Mulvaney F, McLellan A. Prediction of attrition from day hospital treatment in lower socioeconomic cocaine-dependent men. Drug Alcohol Depend. 1996;40:227–233. doi: 10.1016/0376-8716(95)01212-5. [DOI] [PubMed] [Google Scholar]
- Ardila A, Rosselli M, Strumwasser S. Neuropsychological deficits in chronic cocaine abusers. Int. J. Neurosci. 1991;57:73–79. doi: 10.3109/00207459109150348. [DOI] [PubMed] [Google Scholar]
- Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat. Neurosci. 2003;6:115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Beckson M, Lu PH, Nuechterlein KH, Edwards N, Mintz J. Age-related changes in frontal and temporal lobe volumes in men - a magnetic resonance imaging study. Arch. Gen. Psychiatry. 2000;58:461–465. doi: 10.1001/archpsyc.58.5.461. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H. Decision-making and addiction (part I): impaired activation of somatic states in substance dependent individuals when pondering decisions with negative future consequences. Neuropsychologia. 2002;40:1675–1689. doi: 10.1016/s0028-3932(02)00015-5. [DOI] [PubMed] [Google Scholar]
- Bolla K, Ernst M, Kiehl K, Mouratidis M, Eldreth D, Contoreggi C, Matochik J, Kurian V, Cadet J, Kimes A, Funderburk F, London E. Prefrontal cortical dysfunction in abstinent cocaine abusers. J. Neuropsychiatry Clin. Neurosci. 2004;16:456–464. doi: 10.1176/appi.neuropsych.16.4.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, London ED, Kiehl KA, Mouratidis M, Contoreggi C, Matochik JA, Kurian V, Cadet JL, Kimes AS, Funderburk FR, Ernst M. Orbitofrontal cortex dysfunction in abstinent cocaine abusers performing a decision-making task. Neuroimage. 2003;19:1085–1094. doi: 10.1016/s1053-8119(03)00113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402:179–181. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol. Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Braver TS, Barch DM, Gray JR, Molfese DL, Snyder A. Anterior cingulate cortex and response conflict: effects of frequency, inhibition and errors. Cereb. Cortex. 2001;11:825–836. doi: 10.1093/cercor/11.9.825. [DOI] [PubMed] [Google Scholar]
- Brewer JA, Worhunsky PD, Carroll KM, Rounsaville BJ, Potenza MN. Pretreatment brain activation during stroop task is associated with outcomes in cocaine-dependent patients. Biol. Psychiatry. 2008;64:998–1004. doi: 10.1016/j.biopsych.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook JS, Whiteman M, Finch S, Cohen P. Aggression, intrapsychic distress, and drug use: antecedent and intervening processes. J. Am. Acad. Child Adolesc. Psychiatry. 1995;34:1076–1084. doi: 10.1097/00004583-199508000-00018. [DOI] [PubMed] [Google Scholar]
- Buchsbaum BR, Greer S, Chang WL, Berman KF. Meta-analysis of neuroimaging studies of the Wisconsin card-sorting task and component processes. Hum. Brain Mapp. 2005;25:35–45. doi: 10.1002/hbm.20128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge SA, Dudukovic NM, Thomason ME, Vaidya CJ, Gabrieli JD. Immature frontal lobe contributions to cognitive control in children: evidence from fMRI. Neuron. 2002;33:301–311. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss AH, Perry M. The Aggression Questionnaire. J. Pers. Soc. Psychol. 1992;63:452–459. doi: 10.1037//0022-3514.63.3.452. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Rounsaville BJ, Gordon LT, Nich C, Jatlow P, Bisighini RM, Gawin FH. Psychotherapy and pharmacotherapy for ambulatory cocaine abusers. Arch. Gen. Psychiatry. 1994;51:177–187. doi: 10.1001/archpsyc.1994.03950030013002. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Chambers CD, Bellgrove MA, Stokes MG, Henderson TR, Garavan H, Robertson IH, Morris AP, Mattingley JB. Executive “brake failure” following deactivation of human frontal lobe. J. Cogn. Neurosci. 2006;18:444–455. doi: 10.1162/089892906775990606. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Lääne K, Peña Y, Murphy ER, Shah Y, Probst K, Abakumova I, Aigbirhio FI, Richards HK, Hong Y, Baron JC, Everitt BJ, Robbins TW. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H. Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addict. Biol. 2009;14:22–31. doi: 10.1111/j.1369-1600.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmond JE, Chen SH, DeRosa E, Pryor MR, Pfefferbaum A, Sullivan E. Increased frontocerebellar activation in alcoholics during verbal working memory: an fMRI study. Neuroimage. 2003;19:1510–1520. doi: 10.1016/s1053-8119(03)00102-2. [DOI] [PubMed] [Google Scholar]
- Di Sclafani V, Tolou-Shams M, Price LJ, Fein G. Neuropsychological performance of individuals dependent on crack-cocaine, or crack-cocaine and alcohol, at 6 weeks and 6 months of abstinence. Drug Alcohol Depend. 2002;66:161–171. doi: 10.1016/s0376-8716(01)00197-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Dickinson A, Robbins TW. The neuropsychological basis of addictive behaviour. Brain Res. Brain Res. Rev. 2001;36:129–138. doi: 10.1016/s0165-0173(01)00088-1. [DOI] [PubMed] [Google Scholar]
- Fassbender C, Hester R, Murphy K, Foxe JJ, Foxe DM, Garavan H. Prefrontal and midline interactions mediating behavioural control. Eur. J. Neurosci. 2009;29:181–187. doi: 10.1111/j.1460-9568.2008.06557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillmore MT, Rush CR. Impaired inhibitory control of behavior in chronic cocaine users. Drug Alcohol Depend. 2002;66:265–273. doi: 10.1016/s0376-8716(01)00206-x. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Rush CR, Hays L. Acute effects of oral cocaine on inhibitory control of behavior in humans. Drug Alcohol Depend. 2002;67:157–167. doi: 10.1016/s0376-8716(02)00062-5. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV-TR Axis I Disorders-Patient Edition (SCID-I/P, 11/2002 revision) Biometrics Research, New York State Psychiatric Institute; New York, NY: 2002. [Google Scholar]
- Franken IH, Booij J, van den Brink W. The role of dopamine in human addiction: from reward to motivated attention. Eur. J. Pharmacol. 2005;526:199–206. doi: 10.1016/j.ejphar.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Garavan H, Hester R. The role of cognitive control in cocaine dependence. Neuropsychol. Rev. 2007;17:337–345. doi: 10.1007/s11065-007-9034-x. [DOI] [PubMed] [Google Scholar]
- Garavan H, Hester R, Murphy K, Fassbender C, Kelly C. Individual differences in the functional neuroanatomy of inhibitory control. Brain Res. 2006;1105:130–142. doi: 10.1016/j.brainres.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Garavan H, Kaufman JN, Hester R. Acute effects of cocaine on the neurobiology of cognitive control. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2008;363:3267–3276. doi: 10.1098/rstb.2008.0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Murphy K, Roche RA, Stein EA. Dissociable executive functions in the dynamic control of behavior: inhibition, error detection, and correction. Neuroimage. 2002;17:1820–1829. doi: 10.1006/nimg.2002.1326. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Stein EA. Right hemispheric dominance of inhibitory control: an event-related functional MRI study. Proc. Natl. Acad. Sci. U. S. A. 1999;96:8301–8306. doi: 10.1073/pnas.96.14.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H, Stout JC. Neurocognitive insights into substance abuse. Trends Cognit. Sci. 2005;9:195–201. doi: 10.1016/j.tics.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Gawin FH, Kleber HD. Abstinence symptomatology and psychiatric diagnosis in cocaine abusers. Clinical observations. Arch. Gen. Psychiatry. 1986;43:107–113. doi: 10.1001/archpsyc.1986.01800020013003. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am. J. Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND, Wang GJ, Fowler JS, Rajaram S. Addiction changes orbitofrontal gyrus function: involvement in response inhibition. Neuroreport. 2001;12:2595–2599. doi: 10.1097/00001756-200108080-00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R, Fassbender C, Garavan H. Individual differences in error processing: a review and reanalysis of three event-related fMRI studies using the GO/NOGO task. Cereb. Cortex. 2004;14:986–994. doi: 10.1093/cercor/bhh059. [DOI] [PubMed] [Google Scholar]
- Hester R, Foxe JJ, Molholm S, Shpaner M, Garavan H. Neural mechanisms involved in error processing: a comparison of errors made with and without awareness. Neuroimage. 2005;27:602–608. doi: 10.1016/j.neuroimage.2005.04.035. [DOI] [PubMed] [Google Scholar]
- Hester R, Garavan H. Executive dysfunction in cocaine addiction: evidence for discordant frontal, cingulate, and cerebellar activity. J. Neurosci. 2004;24:11017–11022. doi: 10.1523/JNEUROSCI.3321-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R, Simões-Franklin C, Garavan H. Post-error behavior in active cocaine users: poor awareness of errors in the presence of intact performance adjustments. Neuropsychopharmacology. 2007;32:1974–1984. doi: 10.1038/sj.npp.1301326. [DOI] [PubMed] [Google Scholar]
- Heyder K, Suchan B, Daum I. Cortico-subcortical contributions to executive control. Acta Psychol. Amst. 2003;115:271–289. doi: 10.1016/j.actpsy.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Hollingshead A. Four Factor Index of Social Status. Yale University press; New Haven, Conn: 1975. [Google Scholar]
- Kaufman JN, Ross TJ, Stein EA, Garavan H. Cingulate hypoactivity in cocaine users during a GO-NOGO task as revealed by event-related functional magnetic resonance imaging. J. Neurosci. 2003;23:7839–7843. doi: 10.1523/JNEUROSCI.23-21-07839.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg SH, McHugh PF, Bell K, Schluger JH, Schluger RP, LaForge KS, Ho A, Kreek MJ. The Kreek-McHugh-Schluger-Kellogg scale: a new, rapid method for quantifying substance abuse and its possible applications. Drug Alcohol Depend. 2003;69:137–150. doi: 10.1016/s0376-8716(02)00308-3. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Liddle PF, Hopfinger JB. Error processing and the rostral anterior cingulate: an event-related fMRI study. Psychophysiology. 2000;37:216–223. [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Scanley BE, Tucker KA, Oliveto A, Prince C, Sinha R, Potenza MN, Skudlarski P, Wexler BE. Cue-induced brain activity changes and relapse in cocaine-dependent patients. Neuropsychopharmacology. 2006;31:644–650. doi: 10.1038/sj.npp.1300851. [DOI] [PubMed] [Google Scholar]
- Krienen FM, Buckner RL. Segregated fronto-cerebellar circuits revealed by intrinsic functional connectivity. Cereb. Cortex. 2009;19:2485–2497. doi: 10.1093/cercor/bhp135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane SD, Cherek DR. Analysis of risk taking in adults with a history of high risk behavior. Drug Alcohol Depend. 2000;60:179–187. doi: 10.1016/s0376-8716(99)00155-6. [DOI] [PubMed] [Google Scholar]
- Logan GD, Cowan WB. On the ability to inhibit thought and action: a theory of an act of control. Psychol. Rev. 1984;91:295–327. doi: 10.1037/a0035230. [DOI] [PubMed] [Google Scholar]
- Luppino G, Murata A, Govoni P, Matelli M. Largely segregated parietofrontal connections linking rostral intraparietal cortex (areas AIP and VIP) and the ventral premotor cortex (areas F5 and F4). Exp. Brain Res. 1999;128:181–187. doi: 10.1007/s002210050833. [DOI] [PubMed] [Google Scholar]
- Lyvers M. “Loss of control” in alcoholism and drug addiction: a neuroscientific interpretation. Exp. Clin. Psychopharmacol. 2000;8:225–249. doi: 10.1037//1064-1297.8.2.225. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Menon V, Adleman NE, White CD, Glover GH, Reiss AL. Error-related brain activation during a Go/NoGo response inhibition task. Hum. Brain Mapp. 2001;12:131–143. doi: 10.1002/1097-0193(200103)12:3<131::AID-HBM1010>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miech R, Chilcoat H. The formation of a socioeconomic disparity: a case study of cocaine and marijuana use in the 1990s. Am. J. Prev. Med. 2007;32:S171–176. doi: 10.1016/j.amepre.2007.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Murphy K, Garavan H. Deriving the optimal number of events for an event-related fMRI study based on the spatial extent of activation. Neuroimage. 2005;27:771–777. doi: 10.1016/j.neuroimage.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Parkins EJ. Cerebellum and cerebrum in adaptive control and cognition: a review. Biol. Cybern. 1997;77:79–87. doi: 10.1007/s004220050369. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt Impulsiveness Scale. J. Clin. Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Tapert SF, Schuckit MA. Neural activation patterns of methamphetamine-dependent subjects during decision making predict relapse. Arch. Gen. Psychiatry. 2005;62:761–768. doi: 10.1001/archpsyc.62.7.761. [DOI] [PubMed] [Google Scholar]
- Petry NM, Bickel WK, Arnett M. Shortened time horizons and insensitivity to future consequences in heroin addicts. Addiction. 1998;93:729–738. doi: 10.1046/j.1360-0443.1998.9357298.x. [DOI] [PubMed] [Google Scholar]
- R Development Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2010. [Google Scholar]
- Rajah MN, D'Esposito M. Region-specific changes in prefrontal function with age: a review of PET and fMRI studies on working and episodic memory. Brain. 2005;128:1964–1983. doi: 10.1093/brain/awh608. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Brammer MJ, Taylor E. Right inferior prefrontal cortex mediates response inhibition while mesial prefrontal cortex is responsible for error detection. Neuroimage. 2003;20:351–358. doi: 10.1016/s1053-8119(03)00275-1. [DOI] [PubMed] [Google Scholar]
- Ruchsow M, Grothe J, Spitzer M, Kiefer M. Human anterior cingulate cortex is activated by negative feedback: evidence from event-related potentials in a guessing task. Neurosci. Lett. 2002;325:203–206. doi: 10.1016/s0304-3940(02)00288-4. [DOI] [PubMed] [Google Scholar]
- Silton RL, Heller W, Towers DN, Engels AS, Spielberg JM, Edgar JC, Sass SM, Stewart JL, Sutton BP, Banich MT, Miller GA. The time course of activity in dorsolateral prefrontal cortex and anterior cingulate cortex during top-down attentional control. Neuroimage. 2010;50:1292–1302. doi: 10.1016/j.neuroimage.2009.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson DD, Joe GW, Fletcher BW, Hubbard RL, Anglin MD. A national evaluation of treatment outcomes for cocaine dependence. Arch. Gen. Psychiatry. 1999;56:507–514. doi: 10.1001/archpsyc.56.6.507. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Weiss F. The dopamine hypothesis of reward: past and current status. Trends Neurosci. 1999;22:521–527. doi: 10.1016/s0166-2236(99)01447-2. [DOI] [PubMed] [Google Scholar]
- Streeter CC, Terhune D, Whitfield T, Gruber S, Sarid-Segal O, Silveri M, Tzilos G, Afshar M, Rouse E, Tian H, Renshaw PF, Ciraulo DA, Yurgelun-Todd DA. Performance on the Stroop predicts treatment compliance in cocaine-dependent individuals. Neuropsychopharmacology. 2008;33:827–836. doi: 10.1038/sj.npp.1301465. [DOI] [PubMed] [Google Scholar]
- Swick D, Ashley V, Turken AU. Left inferior frontal gyrus is critical for response inhibition. BMC Neurosci. 2008;9:102. doi: 10.1186/1471-2202-9-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain: 3-Dimensional Proportional System - an Approach to Cerebral Imaging. Thieme Medical Publishers; New York, NY: 1988. [Google Scholar]
- Tapert SF, Schweinsburg AD, Drummond SP, Paulus MP, Brown SA, Yang TT, Frank LR. Functional MRI of inhibitory processing in abstinent adolescent marijuana users. Psychopharmacology (Berl.) 2007;194:173–183. doi: 10.1007/s00213-007-0823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarter RE, Kirisci L, Mezzich A, Cornelius JR, Pajer K, Vanyukov M, Gardner W, Blackson T, Clark D. Neurobehavioral disinhibition in childhood predicts early age at onset of substance use disorder. Am. J. Psychiatry. 2003;160:1078–1085. doi: 10.1176/appi.ajp.160.6.1078. [DOI] [PubMed] [Google Scholar]
- Turner T, Larowe S, Horner M, Herron J, Malcolm R. Measures of cognitive functioning as predictors of treatment outcome for cocaine dependence. J. Subst. Abuse Treat. 2009;37:328–334. doi: 10.1016/j.jsat.2009.03.009. [DOI] [PubMed] [Google Scholar]
- Ullsperger M, von Cramon DY. Subprocesses of performance monitoring: a dissociation of error processing and response competition revealed by event-related fMRI and ERPs. Neuroimage. 2001;14:1387–1401. doi: 10.1006/nimg.2001.0935. [DOI] [PubMed] [Google Scholar]
- Ullsperger M, von Cramon DY. Error monitoring using external feedback: specific roles of the habenular complex, the reward system, and the cingulate motor area revealed by functional magnetic resonance imaging. J. Neurosci. 2003;23:4308–4314. doi: 10.1523/JNEUROSCI.23-10-04308.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Veen V, Carter CS. The timing of action-monitoring processes in the anterior cingulate cortex. J. Cogn. Neurosci. 2002;14:593–602. doi: 10.1162/08989290260045837. [DOI] [PubMed] [Google Scholar]
- Verdejo-García A, Lawrence AJ, Clark L. Impulsivity as a vulnerability marker for substance-use disorders: review of findings from high-risk research, problem gamblers and genetic association studies. Neurosci. Biobehav. Rev. 2008;32:777–810. doi: 10.1016/j.neubiorev.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, Sedler M, Gatley SJ, Miller E, Hitzemann R, Ding YS, Logan J. Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. J. Neurosci. 2001;21:9414–9418. doi: 10.1523/JNEUROSCI.21-23-09414.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb. Cortex. 2000;10:318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Hitzemann R, Logan J, Schlyer DJ, Dewey SL, Wolf AP. Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse. 1993;14:169–177. doi: 10.1002/syn.890140210. [DOI] [PubMed] [Google Scholar]
- Wang G, Volkow ND, Chang L, Miller E, Sedler M, Hitzemann R, Zhu W, Logan J, Ma Y, Fowler JS. Partial recovery of brain metabolism in methamphetamine abusers after protracted abstinence. Am. J. Psychiatry. 2004;161:242–248. doi: 10.1176/appi.ajp.161.2.242. [DOI] [PubMed] [Google Scholar]
- Weddington WW, Brown BS, Haertzen CA, Cone EJ, Dax EM, Herning RI, Michaelson BS. Changes in mood, craving, and sleep during short-term abstinence reported by male cocaine addicts. A controlled, residential study. Arch. Gen. Psychiatry. 1990;47:861–868. doi: 10.1001/archpsyc.1990.01810210069010. [DOI] [PubMed] [Google Scholar]
- Wexler BE, Gottschalk CH, Fulbright RK, Prohovnik I, Lacadie CM, Rounsaville BJ, Gore JC. Functional magnetic resonance imaging of cocaine craving. Am. J. Psychiatry. 2001;158:86–95. doi: 10.1176/appi.ajp.158.1.86. [DOI] [PubMed] [Google Scholar]
- Yücel M, Lubman DI, Solowij N, Brewer WJ. Understanding drug addiction: a neuropsychological perspective. Aust. N. Z. J. Psychiatry. 2007;41:957–968. doi: 10.1080/00048670701689444. [DOI] [PubMed] [Google Scholar]


