Abstract
Circulating aldosterone is increased in obesity and is associated with arterial stiffening in hypertensives and older adults. We aimed to determine whether serum aldosterone is associated with pulse wave velocity (PWV), a measure of arterial stiffness, in normotensive overweight and obese adults aged 20–45 years (n=344). We measured heart-femoral, femoral-ankle and brachial-ankle PWV. The sample was 77% female with mean BMI 32.9 kg/m2 (SD 3.9), median serum aldosterone 106.5 pg/mL (IQR 79.9, 155.5), and mean 24-hour urinary sodium excretion 185.9 mEq/day (SD 69.6). Higher serum aldosterone was not significantly correlated with any PWV measure in bivariate analysis. However, in multiple linear regression, adjusting for age, sex, race, height, heart rate, mean arterial pressure, and waist circumference, higher log aldosterone was associated with greater log heart-femoral PWV (β(se)=0.042(0.021), p=0.049). After adjusting for C-reactive protein, this association was no longer significant (β(se)=0.035(0.021), p=0.10). Circulating aldosterone may play an important role in vascular inflammation and aortic stiffening in normotensive overweight and obese adults.
Keywords: aldosterone, arterial stiffness, inflammation, obesity, RAAS
INTRODUCTION
Arterial stiffness is now recognized as an important independent predictor of incident coronary heart disease (1–3), stroke (1,3), and cardiovascular mortality (2–4). In addition to aging and hemodynamic forces, the renin-angiotensin-aldosterone system (RAAS) has been suggested to play an important role in arterial stiffening (5). Several pathophysiologic processes affected by RAAS components, such as altered elastin and collagen, increased inflammation, vascular smooth muscle cell hypertrophy, and fibrosis may underlie the vascular damage that leads to arterial stiffening (5). Aldosterone may contribute to vascular damage through its binding to mineralocorticoid receptors present in cells throughout the vasculature (6). Studies in hypertensives have reported blood pressure (BP)-independent associations between increased circulating aldosterone and reduced systemic arterial compliance (7) and increased heart-femoral pulse wave velocity (PWV) (8), a measure of aortic stiffness. Individuals with primary aldosteronism have greater arterial stiffness than either normotensives or hypertensives with normal aldosterone matched for BP and duration of hypertension, a difference that appears to be due to the increased vascular fibrosis associated with chronic aldosterone excess(9).
Recent reviews have highlighted the positive associations between aldosterone and body mass index (BMI) (10), insulin resistance (10–13), and inflammation (6,14), known risk factors for vascular changes related to early atherosclerosis in overweight and obese individuals (15). Thus it follows that, in addition to increasing BP (16), aldosterone may contribute to vascular damage in obese individuals by influencing insulin resistance and inflammation. Although there has been much research on the cardiovascular effects of aldosterone, little of this research has focused on normotensive individuals. One recent study showed a positive association between aldosterone-to-renin ratio, a marker of inappropriate aldosterone activity, and aortic PWV in healthy, normotensive young adults, but this study neither reported an association between serum aldosterone and PWV nor accounted for confounding or mediating factors (17). Furthermore, many studies of the relationship between aldosterone and vascular remodeling have failed to include a measure of dietary salt intake, an important determinant of aldosterone secretion. The aim of this study was to investigate whether serum aldosterone is positively associated with arterial stiffness, as measured by heart-femoral PWV (hfPWV), a measure of central aortic stiffness, brachial-ankle PWV (baPWV), a mixed measure of central and peripheral arterial stiffness, and femoral-ankle PWV (faPWV), a measure of peripheral arterial stiffness, in normotensive overweight and obese adults. Additionally, we aimed to determine whether these relationships could be explained by associations between higher circulating aldosterone and greater inflammation or insulin resistance.
METHODS
Study Population
This was a cross-sectional analysis of baseline data from participants enrolled in the Slow Adverse Vascular Effects (SAVE) clinical trial (NCT00366990), a single center randomized controlled trial to assess the effects of weight loss, increased physical activity, and reduced sodium intake on vascular health. Moderately overweight or obese (body mass index (BMI) 25–39.9 kg/m2) men and women (n=349) aged 20–45 years were recruited from Allegheny County, Pennsylvania during April 2007– May 2009. Participants were required to be physically inactive prior to the intervention, defined as exercising for <8 months during the past 12 months and for an average of <3 hours per week. Participants were excluded if they: 1) had diabetes (hypoglycemic medication use or fasting glucose ≥ 126 mg/dl); 2) were being treated for hypertension or had an average screening and baseline systolic blood pressure (SBP) of ≥140 or a diastolic blood pressure (DBP) ≥ 90 mmHg; 3) were on cholesterol lowering, anti-psychotic, vasoactive medications, or using vasoactive devices; 4) were pregnant or breast feeding.
For the current analysis, those with a baseline measure of serum aldosterone and at least one valid PWV measurement were included (n=344). All subjects signed informed consent, and the study design was approved by the institutional review board of the University of Pittsburgh (Pittsburgh, PA).
Design and Procedures
All participants provided self-reported demographic information and completed self- and interviewer-administered questionnaires, anthropometric measurements, fasting blood draw, 24-hour urine collection, and vascular testing.
Demographic and Physical Measures
Age, race, and smoking status were self-reported by questionnaire. For the current study, race was re-coded as black vs. non-black and smoking status as current vs. previous/never.
Weight was measured in kilograms using a standard balance scale. Height was measured in centimeters using a calibrated stadiometer. BMI was calculated as weight in kilograms divided by height in meters squared. Waist circumference was measured in centimeters, against the participant’s skin at the narrowest part of the torso between the ribs and the iliac crest. BP was measured after participants sat quietly for 5 minutes with feet flat on the floor. A standard protocol with a mercury sphygmomanometer was used, and average BP was the average of the last 2 of 3 measurements taken 30 seconds apart.
Pulse Wave Velocity Measurement
Pulse wave velocity (PWV) measures were automatically generated using a noninvasive automated waveform analyzer (VP2000, Omron Co., Komaki, Japan). This device provides measures of carotid-femoral (cf), heart-femoral (hf), femoral-ankle (fa), and brachial-ankle (ba) PWV. Aortic stiffness was assessed by hfPWV and cfPWV and peripheral arterial stiffness by faPWV; baPWV served as a mixture of central and peripheral arterial stiffness. Following ten minutes of rest in a supine position, the participant had occlusion and monitoring cuffs placed around both arms and ankles. ECG electrodes were placed on both wrists and a microphone was placed on the left edge of the sternum. Occlusion cuffs at the brachial and tibial arteries were connected to a plethysmographic sensor that determined volume pulse form and to an oscillometric pressure sensor that measured blood pressure. The pulse volume waveforms were recorded using a semiconductor pressure sensor. Sonographers palpated the right femoral artery and the right carotid artery and placed handheld multiarray tonometers over these two pulse areas to obtain femoral and carotid pulse waveforms simultaneously. PWV (cm/sec) was calculated as the path length between arterial sites of interest divided by the time delay between the foot of the respective waveforms. For cfPWV path length, the distance between the carotid and femoral sites was measured over the surface of the body with a tape measure. The path lengths for hfPWV, faPWV and baPWV were calculated using height-based formulas (18). The faPWV and baPWV were calculated for the right and left sides and the average of both sides for each measure was used for analyses. For all PWV measures, data were collected twice for each participant, and the values were averaged. For this study, valid PWV measures were defined as between 300 m/s and 2500 m/s. Intraclass correlation coefficients (ICCs) of 0.76 (cfPWV), 0.86 (hfPWV), 0.97 (baPWV), and 0.96 (faPWV) were achieved within technicians. ICCs of 0.73 (cfPWV), 0.93 (hfPWV), 0.87(faPWV), and 0.91 (baPWV) were achieved between technicians.
24-Hour Urine Collection
24-hour urine collections were performed no more than four weeks before the PWV testing. Valid 24-hour urine collection was defined as a volume between 500 mL and 4000 mL, a collection period of ≥22 hours and ≤26 hours, and a total creatinine within the expected range (19). Because many urine collections did not meet this criteria (n=64), 24-hour urinary sodium was indexed to urinary creatinine (Na/Cr), and this ratio served as a measure of sodium intake. From April 13, 2007 to March 6, 2009, direct potentiometry was used to measure sodium, and colorimetry was used to assess creatinine levels. After this date, an indirect ion selective method was used for sodium and an alkaline picric kinetic method for creatinine.
Blood Assays
All blood assays were performed at the Heinz Laboratory at the University of Pittsburgh’s Graduate School of Public Health. A blood specimen was obtained between 7 and 11:30 am from upright participants after a fasting period of at least 9 hours.
Total cholesterol and high density lipoprotein (HDL) were determined using the enzymatic method of Allain et al. (20). HDL was determined after selective precipitation by heparin/manganese chloride and removal by centrifugation of very low density (VLDL) and low density lipoprotein (LDL)(21). LDL was calculated indirectly using the Friedewald equation. Triglycerides were assessed enzymatically using the procedure of Bucolo et al. (22).
Serum glucose was quantitatively determined by an enzymatic determination using a procedure similar to that described by Bondar and Mead (23). Insulin was measured using an RIA procedure developed by Linco Research, Inc. (St. Charles, MO, USA). Insulin resistance was estimated using the homeostasis model assessment of insulin resistance index (HOMA-IR) derived from fasting insulin and glucose values (24). HOMA-IR (mmol/L × μU/ml) = fasting glucose (mmol/L) × fasting insulin (μU/ml)/22.5.
Serum aldosterone was measured using an enzyme-linked immunoassay developed by Diagnostic Systems Laboratories, Inc. (Webster, TX, USA). Serum C-reactive protein (CRP) was measured using an enzyme-linked immunoassay developed by Alpha Diagnostic International, Inc. (San Antonio, TX, USA).
STATISTICAL METHODS
Descriptive statistics were performed to summarize study variables. Data were presented as median/inter-quartile range (IQR) or mean (SD) for continuous variables, and frequency and percentages for categorical variables. Covariates chosen for these analyses were factors of interest known to be associated with serum aldosterone and/or arterial stiffness (age, sex, race (black/non-black), mean arterial pressure (MAP), heart rate at the time of PWV measurement, BMI, waist circumference, current smoking, HDL-C, LDL-C, triglycerides, and Na/Cr). Each non-normally distributed variable was modeled as a continuous variable with natural logarithm transformation for normalization of the distribution. Associations between serum aldosterone and covariates and between each PWV measure (cfPWV, hfPWV, baPWV, and faPWV) and covariates were tested using Pearson correlation coefficients. In addition, associations between serum aldosterone and blood pressure were evaluated using multiple linear regression with adjustment for age, race, sex, and waist circumference, and associations between serum aldosterone and CRP, insulin, or HOMA-IR were evaluated using multiple linear regression with adjustment for age, race, sex, waist circumference, and MAP. Because hfPWV and cfPWV were highly correlated (r=0.82, p<0.0001), showed similar relationships with serum aldosterone, and hfPWV showed lower variability in our laboratory, only hfPWV was examined in further analyses. The first multiple linear regression model for each PWV measure included age, sex, race, height, MAP, heart rate, and waist circumference. Next, in order to include all important factors associated with PWV, we used stepwise selection in which a conservative value of p<0.15 was used for inclusion in the final model. Potential covariates considered for entry into the model were any factors correlated with serum aldosterone at p<0.3. The first order interaction between aldosterone and each covariate was checked in each PWV model. In the multivariable models, all continuous variables were centered to avoid multicollinearity. Values of p<0.05 were considered statistically significant. Statistical analyses were performed using the statistical package SAS (Statistical Analysis Software release 9.2, Cary, NC, USA).
RESULTS
The baseline characteristics of the study population are presented in Table 1. The average age was 37.9 years. Seventy-seven percent of the sample was female and sixteen percent was black. Five individuals were excluded from the analysis due to missing data on serum aldosterone (n=1) or all PWV measures (n=4). Additional data for specific PWV measures was missing due to poor quality waveforms, mainly due to the obesity of the participants (hfPWV n=9; baPWV n=6; faPWV n=12). Of all variables examined, only HOMA-IR, insulin, total cholesterol, triglycerides, and CRP were correlated with serum aldosterone (Table 2). Serum aldosterone was not correlated with SBP, DBP, or MAP, but after adjustment for age, sex, race, and waist circumference, aldosterone was significantly associated with DBP (β(se)=1.95(0.96), p=0.04) and showed a trend towards a significant association with MAP (β(se)=1.8(0.96), p=0.06). After adjustment for age, sex, race, waist circumference, and MAP, aldosterone remained significantly associated with CRP (β(se)=0.31(0.11), p=0.006), insulin (β(se)=0.17(0.05), p=0.001), and HOMA-IR (β(se)=0.16(0.05), p=0.002). Aldosterone was lower in blacks than in non-blacks (Median(IQR) 92.0 (77.1, 123.0) vs. 113 (81.5, 160), p=0.02) but was not significantly different by sex. Aldosterone did not show a significant association with any PWV measure in bivariate analysis (Table 2).
Table 1.
Characteristics of Study Participants (n=344)
| Characteristic | Mean (SD)/Median (IQR) |
|---|---|
| Age (years) | 37.9 (6.1) |
| Women (n, %) | 266 (77.3) |
| Race (n, %) | |
| Non-Black | 288 (83.7) |
| Black | 56 (16.3) |
| Current Smoker (n, %) | 32 (9.3) |
| Weight (kg) | 92.2 (14.9) |
| BMI (kg/m2) | 32.9 (3.9) |
| Waist Circumference (cm) | 100.5 (11.3) |
| SBP (mmHg) | 113.4 (10.4) |
| DBP (mmHg) | 72.9 (8.7) |
| Heart Rate (beats/min) | 64.0 (9.1) |
| Glucose (mg/dL) | 97.6 (7.9) |
| Insulin (μU/mL) | 12.5 (9.6, 17.3) |
| HOMA-IR (mmol/L × μU/mL) | 3.0 (2.2, 4.3) |
| CRP (mg/L) | 2.6 (1.3, 5.6) |
| LDL-C (mg/dL) | 123.1 (33.3) |
| HDL-C (mg/dL) | 52.8 (13.5) |
| Triglycerides (mg/dL) | 115 (78, 169) |
| Total Cholesterol (mg/dL) | 202.6 (37.5) |
| Aldosterone (pg/mL) | 106.5 (79.9, 155.5) |
| 24-hour Urinary Sodium (mEq/day) | 185.9 (69.6) |
| 24-hour Urinary Na/Cr (mEq/mg) | 0.11 (0.09, 0.15) |
| Carotid-Femoral PWV (cm/s) | 819.3 (710.5, 952) |
| Heart-Femoral PWV (cm/s) | 782.5 (711.5, 880) |
| Brachial-Ankle PWV (cm/s) | 1207.1 (132.2) |
| Femoral-Ankle PWV (cm/s) | 946.9 (103.8) |
Results are mean (SD) or median (IQR). SBP=systolic blood pressure, DBP=diastolic blood pressure, LDL-C=low density lipoprotein cholesterol, HDL-C=high density lipoprotein cholesterol, HOMA-IR= homeostasis model assessment of insulin resistance, CRP=C-reactive protein, PWV=pulse wave velocity. CRP, N=341; Triglycerides, N=343; 24-hour Urinary Sodium, N=281; 24-hour Urinary Sodium/Creatinine (Na/Cr), N=341; Carotid-Femoral PWV, N=328; Heart-Femoral PWV, N=335; Brachial-Ankle PWV, N=338; Femoral-Ankle PWV, N=322
Table 2.
Pearson Correlations between Aldosterone and Other Factors
| Variable | Correlation Coefficient | p |
|---|---|---|
| Age | −0.05 | 0.34 |
| Female Sex | 0.10 | 0.07 |
| Black Race | −0.12 | 0.03 |
| Current Smoker | 0.03 | 0.57 |
| BMI | −0.02 | 0.77 |
| Waist Circumference | −0.04 | 0.45 |
| MAP | 0.06 | 0.26 |
| Glucose | −0.04 | 0.44 |
| Insulin* | 0.16 | 0.003 |
| HOMA-IR* | 0.15 | 0.006 |
| Total Cholesterol | 0.13 | 0.02 |
| LDL-C | 0.05 | 0.38 |
| HDL-C | 0.05 | 0.36 |
| Triglycerides* | 0.21 | <0.0001 |
| CRP* | 0.15 | 0.007 |
| 24-hour Urinary Sodium | −0.009 | 0.89 |
| 24-hour Urinary Na/Cr* | 0.06 | 0.28 |
| hfPWV* | 0.07 | 0.21 |
| baPWV* | 0.04 | 0.51 |
| faPWV* | 0.06 | 0.26 |
Aldosterone and these variables were log transformed. MAP=mean arterial pressure, LDL-C=low density lipoprotein cholesterol, HDL-C=high density lipoprotein cholesterol, HOMA-IR= homeostasis model assessment of insulin resistance, CRP=C-reactive protein, Na/Cr= sodium/creatinine ratio, hfPWV=Heart-femoral pulse wave velocity, baPWV=Brachial-ankle pulse wave velocity, faPWV=Femoral-ankle pulse wave velocity. CRP, N=341; Triglycerides, N=343; 24-hour Urinary Sodium, N=281; 24-hour Urinary Sodium/Creatinine (Na/Cr), N=341; Heart-femoral PWV, N=335; Brachial-ankle PWV, N=338; Femoral-ankle PWV, N=322
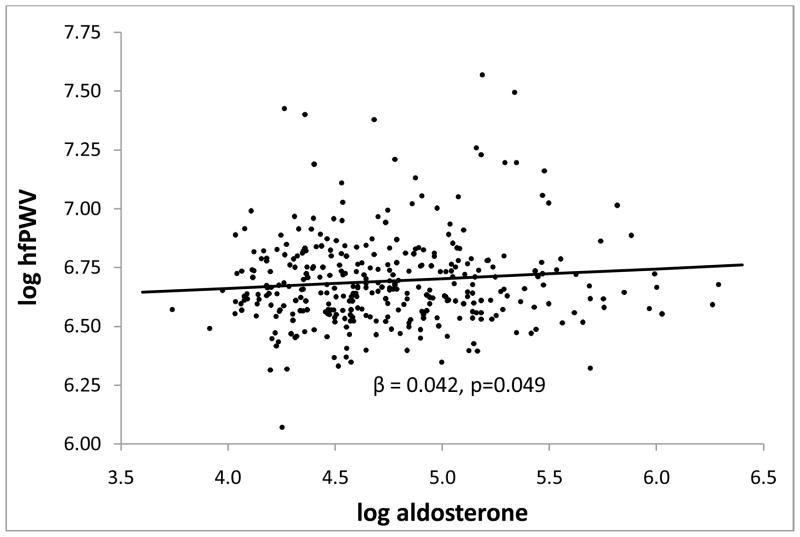
In multiple linear regression with adjustment for age, sex, race, height, MAP, heart rate, and waist circumference, aldosterone was significantly associated with aortic PWV (hfPWV) (Figure 1 and Table 3), but not with peripheral PWV (faPWV) (β(se) = 18.0(11.2), p=0.11) or mixed central/peripheral PWV (baPWV) (β(se) = 20.5(13.3), p=0.12). Though adjusting for any single covariate did not result in a significant association between aldosterone and hfPWV, adjusting simultaneously for sex and race did make evident the association between aldosterone and hfPWV. After stepwise selection of additional covariates, only CRP entered the models for hfPWV and baPWV, whereas HOMA-IR and urinary Na/Cr entered the model for faPWV (Table 3). The inclusion of CRP in the model for hfPWV removed the significance of serum aldosterone in this model. No significant interactions were found in any model and the associations between aldosterone and PWV were similar in overweight and obese subgroups. Results were similar when BMI was included instead of waist circumference in the multiple linear regression models.
Figure 1.
Association between log heart-femoral pulse wave velocity (hfPWV) in cm/s and log aldosterone in pg/mL when adjusted for age, sex, race (black/non-black), height, heart rate, waist circumference, and mean arterial pressure.
Table 3.
Multiple Linear Regression Models for Pulse Wave Velocity
| Central PWV (hfPWV) (n=332) | Mixed Central/Peripheral PWV (baPWV) (n=334) | Peripheral PWV (faPWV) (n=319) | ||||
|---|---|---|---|---|---|---|
| Standardized Coefficient | p | Standardized Coefficient | p | Standardized Coefficient | p | |
| R2 = 0.22 | R2 = 0.33 | R2 = 0.27 | ||||
| Aldosterone | 0.10 | 0.049 | 0.07 | 0.12 | 0.08 | 0.13 |
| Age | 0.35 | <0.0001 | 0.34 | <0.0001 | 0.16 | 0.001 |
| Male Sex | 0.07 | 0.30 | 0.27 | <0.0001 | 0.29 | <0.0001 |
| Black Race | 0.10 | 0.04 | 0.18 | <0.0001 | 0.13 | 0.01 |
| Height | 0.12 | 0.09 | −0.20 | 0.002 | −0.42 | <0.0001 |
| Waist Circumference | 0.07 | 0.30 | 0.09 | 0.13 | −0.02 | 0.77 |
| MAP | 0.14 | 0.01 | 0.21 | <0.0001 | 0.28 | <0.0001 |
| Heart Rate | 0.13 | 0.01 | 0.24 | <0.0001 | 0.13 | 0.01 |
| R2 = 0.24 | R2 = 0.34 | R2 = 0.29 | ||||
| Aldosterone | 0.08 | 0.10 | 0.06 | 0.22 | 0.06 | 0.23 |
| Age | 0.35 | <0.0001 | 0.33 | <0.0001 | 0.20 | <0.0001 |
| Male Sex | 0.11 | 0.12 | 0.30 | <0.0001 | 0.32 | <0.0001 |
| Black Race | 0.10 | 0.05 | 0.18 | 0.0002 | 0.13 | 0.008 |
| Height | 0.14 | 0.04 | −0.18 | 0.005 | −0.41 | <0.0001 |
| Waist Circumference | 0.002 | 0.98 | 0.04 | 0.55 | −0.09 | 0.29 |
| MAP | 0.12 | 0.008 | 0.22 | <0.0001 | 0.24 | <0.0001 |
| Heart Rate | 0.12 | 0.02 | 0.23 | <0.0001 | 0.12 | 0.03 |
| CRP | 0.14 | 0.02 | 0.12 | 0.02 | ||
| HOMA-IR | 0.13 | 0.02 | ||||
| Urinary Na/Cr | 0.08 | 0.12 | ||||
Results are from ordinary least squares regression with the listed independent variables; CRP, aldosterone, urinary sodium/creatinine, HOMA-IR, and hfPWV are log transformed; R2 = model coefficient of determination; CRP=C-reactive protein, MAP=mean arterial pressure, HOMA-IR= homeostasis model assessment of insulin resistance, Urinary Na/Cr = 24-hour urinary sodium/creatinine ratio, hfPWV=heart-femoral pulse wave velocity, baPWV=brachial-ankle pulse wave velocity, faPWV=femoral-ankle pulse wave velocity
DISCUSSION
In our community-based sample of normotensive overweight and obese young adults, we observed a significant positive association between increasing serum aldosterone and hfPWV that was independent of age, sex, race, height, MAP, heart rate, and waist circumference. This association, however, was removed after adjustment for CRP, a marker of vascular inflammation. To our knowledge, this is the first study to show 1) a positive association between serum aldosterone and aortic stiffness in normotensive young adults after adjustment for blood pressure and other CVD risk factors and 2) that the increased inflammation associated with greater circulating aldosterone may explain this association.
Our finding of positive associations between serum aldosterone and hfPWV is consistent with past studies, which have reported positive relationships between serum aldosterone and aortic PWV in hypertensives (8) and between aldosterone-to-renin ratio and aortic PWV in both normotensives and hypertensives (17,25). The studies that included normotensive individuals, however, either did not adjust for other CVD risk factors that could confound the relationship between aldosterone and PWV (17) or did not study young adults (25). Though the association between serum aldosterone and aortic stiffness in our study was not evident in simple correlation analysis, this appeared to be due to the significantly greater aortic stiffness but lower mean aldosterone level in blacks as compared to non-blacks as well as the nonsignificantly greater aortic stiffness but lower mean aldosterone level in males as compared to females. A racial difference in circulating aldosterone has been previously shown, and may be explained by increased intrarenal RAAS activity rather than systemic RAAS activity in normotensive blacks (26,27). However, the association between aldosterone and aortic stiffness showed no significant interactions with sex or race, suggesting that the relationship between serum aldosterone and arterial stiffening does not differ by these factors.
Aortic stiffening involves vascular smooth muscle hypertrophy, inflammation, and collagen accumulation, mechanisms which have been reported to be induced by aldosterone (6). Individuals with primary aldosteronism show increased aortic PWV when compared to essential hypertensives with similar BP levels, a finding that appears to be due to increased vascular fibrosis resulting from accelerated collagen turnover (9). In addition, mineralocorticoid receptor antagonists reduce aortic stiffness in patients with hypertension (28) or chronic kidney disease (29), and the reduction is at least partially BP-independent. In both animal and humans studies, aldosterone has been shown to contribute to vascular inflammation, a function that appears to be related to increased NF-κB activation and dependent on the presence of a high sodium diet (6,30). CRP, a marker of vascular inflammation, has been associated with increased aortic stiffness in healthy adults (31,32) and has been shown to act synergistically with aldosterone to increase the mechanical stiffness of endothelial cells (33), thereby increasing intravascular volume and vascular resistance. Vascular inflammation leads to increased production of collagen, degradation of the extracellular matrix, and eventually fibrosis (34). Thus, it is plausible that in overweight and obese young adults, in whom both CRP (35) and aldosterone (10) are elevated, aldosterone’s influence on aortic stiffening could be mediated by inflammation.
Our finding of a significant association between increasing serum aldosterone and increasing HOMA-IR is also in agreement with reports linking increased aldosterone to increased insulin resistance (11,12,36,37), an established risk factor for vascular changes related to atherosclerosis in overweight and obese individuals (15,38). It has been shown that aldosterone can induce insulin resistance by directly effecting sodium-glucose transport, fibrosis in insulin-sensitive and insulin-producing tissues, and pancreatic β-cell structure and function (39–41). In our study, however, insulin resistance as measured by HOMA-IR did not appear to explain the relationship between serum aldosterone and aortic stiffness.
This study did not find an association between aldosterone and peripheral arterial stiffening, suggesting a greater influence of aldosterone on central arterial stiffening. Given the mechanisms by which aldosterone induces vascular stiffness as well as the greater density of mineralocorticoid receptors in the aorta than in the peripheral arteries (42), this finding is not surprising and is in fact in agreement with a previous study of hypertensives (8). It is important to note that our 24-hour urinary sodium data indicated that over 93% (262/281) of the study population with valid urine collections consumed more than the 2300 mg sodium/day (100 meq/day) recommended for young non-black adults in the U.S. Dietary Guidelines. Because high circulating aldosterone has been shown to induce greater vascular damage when combined with high sodium intake (43), it is possible that aldosterone’s effect on arterial stiffening was magnified by the high salt intake of the study sample, which was reflective of that of the general US population.
There are several limitations of this study. First, this was a cross-sectional study, so we could not prove the causality of the detected relationships. Second, because of the complexities of collecting 24-hour urine, only 82% of subjects had urine collections that were considered valid. However, we did not find a significant correlation between serum aldosterone and either urinary sodium excretion or urinary Na/Cr, perhaps because of the high variability of both measurements. A measure of 24-hour aldosterone excretion might have more accurately reflected chronic intravascular aldosterone exposure than a serum measurement and may have in fact been correlated with urinary sodium excretion. Another limitation is the lack of data on other measures of RAAS activity, such as plasma renin activity or serum angiotensin II, the latter of which has been shown to act synergistically with aldosterone to cause vascular inflammation (34). In addition, we had an insufficient number of blacks (n=56) and males (n=78) in this sample to stratify our results by race or sex. A notable strength of this study is that all participants were normotensive and not on hypertensive or vasoactive medications, which enabled us to evaluate the relationships between serum aldosterone and PWV independent of any treatment effects. In addition, because this study included 24-hour urine collections, we were able to evaluate the relationships between aldosterone and arterial stiffness independent of urinary Na/Cr, an approximate measure of dietary sodium intake.
In conclusion, our findings support an association between higher serum aldosterone and greater aortic stiffness in normotensive young overweight and obese adults. However, the association between aldosterone and aortic stiffness is at least partially explained by vascular inflammation. Our results suggest an important role for aldosterone in aortic stiffening, even in normotensive individuals. In light of results from randomized controlled trials of aldosterone antagonists, which have shown BP-independent reductions in arterial stiffness in patients with hypertension (28,44), future studies should investigate whether aldosterone blockade prevents CVD in at risk overweight and obese adults.
Acknowledgments
SOURCES OF SUPPORT
Dr. Ping Tepper is supported by grant U01AG12553-15. Dr. Sutton-Tyrrell is supported by grant R01 HL077525-01A2. Dr. Barinas-Mitchell is supported by grant R01 HL077525-01A2. Dr. Genevieve Woodard is supported by F31 HL09171202 from the National Heart, Lung, and Blood Institute of the National Institutes of Health. Jennifer Cooper is supported by T32 HL083825-01 from the National Heart, Lung, and Blood Institute of the National Institutes of Health.
The authors acknowledge Dr. Linda Fried, MD, MPH, for writing and editing assistance.
Footnotes
DECLARATION OF INTEREST
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Mattace-Raso FU, van der Cammen TJ, Hofman A, van Popele NM, Bos ML, Schalekamp MA, et al. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation. 2006;113:657–663. doi: 10.1161/CIRCULATIONAHA.105.555235. [DOI] [PubMed] [Google Scholar]
- 2.Boutouyrie P, Tropeano AI, Asmar R, Gautier I, Benetos A, Lacolley P, et al. Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients - A longitudinal study. Hypertension. 2002;39:10–15. doi: 10.1161/hy0102.099031. [DOI] [PubMed] [Google Scholar]
- 3.Sutton-Tyrrell K, Najjar SS, Boudreau RM, Venkitachalam L, Kupelian V, Simonsick EM, et al. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation. 2005;111:3384–3390. doi: 10.1161/CIRCULATIONAHA.104.483628. [DOI] [PubMed] [Google Scholar]
- 4.Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–1241. doi: 10.1161/01.hyp.37.5.1236. [DOI] [PubMed] [Google Scholar]
- 5.Mahmud A. Arterial stiffness and the renin-anglotensin-aldosterone system. Journal of the Renin-Angiotensin-Aldosterone System. 2004;5:102–108. doi: 10.3317/jraas.2004.025. [DOI] [PubMed] [Google Scholar]
- 6.Cachofeiro V, Miana M, Heras NDL, Martin-Fernandez B, Ballesteros S, Fernandez-Tresguerres J, et al. Aldosterone and the vascular system. J Steroid Biochem Mol Biol. 2008;109:331–335. doi: 10.1016/j.jsbmb.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 7.Blacher J, Amah G, Girerd X, Kheder A, Ben Mais H, London GM, et al. Association between increased plasma levels of aldosterone and decreased systemic arterial compliance in subjects with essential hypertension. Am J Hypertens. 1997;10:1326–1334. doi: 10.1016/s0895-7061(97)00301-4. [DOI] [PubMed] [Google Scholar]
- 8.Park S, Kim JB, Shim CY, Ko YG, Choi D, Jang Y, et al. The influence of serum aldosterone and the aldosterone-renin ratio on pulse wave velocity in hypertensive patients. J Hypertens. 2007;25:1279–1283. doi: 10.1097/HJH.0b013e3280f31b6e. [DOI] [PubMed] [Google Scholar]
- 9.Bernini G, Galetta F, Franzoni F, Bardini M, Taurino C, Bernardini M, et al. Arterial stiffness, intima-media thickness and carotid artery fibrosis in patients with primary aldosteronism. J Hypertens. 2008;26:2399–2405. doi: 10.1097/HJH.0b013e32831286fd. [DOI] [PubMed] [Google Scholar]
- 10.Goodfriend TL, Egan BM, Kelley DE. Aldosterone in obesity. Endocr Res. 1998;24:789–796. doi: 10.3109/07435809809032689. [DOI] [PubMed] [Google Scholar]
- 11.Perkins JM, Davis SN. The renin-angiotensin-aldosterone system: a pivotal role in insulin sensitivity and glycemic control. [Miscellaneous] Current Opinion in Endocrinology, Diabetes & Obesity. 2008;15:147–152. doi: 10.1097/MED.0b013e3282f7026f. [DOI] [PubMed] [Google Scholar]
- 12.Lastra-Lastra G, Sowers JR, Restrepo-Erazo K, Manrique-Acevedo C, Lastra-Gonzalez G. Role of aldosterone and angiotensin II in insulin resistance: an update. Clin Endocrinol (Oxf) 2009;71:1–6. doi: 10.1111/j.1365-2265.2008.03498.x. [DOI] [PubMed] [Google Scholar]
- 13.Kraus D, Jager J, Meier B, Fasshauer M, Klein J. Aldosterone inhibits uncoupling protein-1, induces insulin resistance, and stimulates proinflammatory adipokines in adipocytes. Horm Metab Res. 2005;37:455–459. doi: 10.1055/s-2005-870240. [DOI] [PubMed] [Google Scholar]
- 14.Duprez DA. Role of the renin-angiotensin-aldosterone system in vascular remodeling and inflammation: a clinical review. J Hypertens. 2006;24:983–991. doi: 10.1097/01.hjh.0000226182.60321.69. [DOI] [PubMed] [Google Scholar]
- 15.Singhal A. Endothelial dysfunction: role in obesity-related disorders and the early origins of CVD. Proc Nutr Soc. 2005;64:15–22. doi: 10.1079/pns2004404. [DOI] [PubMed] [Google Scholar]
- 16.Kidambi S, Kotchen JM, Krishnaswami S, Grim CE, Kotchen TA. Aldosterone Contributes to Blood Pressure Variance and to Likelihood of Hypertension in Normal-Weight and Overweight African Americans. Am J Hypertens. 2009;22:1303–1308. doi: 10.1038/ajh.2009.167. [DOI] [PubMed] [Google Scholar]
- 17.Shapiro Y, Boaz M, Matas Z, Fux A, Shargorodsky M. The association between the renin-angiotensin-aldosterone system and arterial stiffness in young healthy subjects. Clin Endocrinol (Oxf) 2008;68:510–512. doi: 10.1111/j.1365-2265.2008.03176.x. [DOI] [PubMed] [Google Scholar]
- 18.Kimoto E, Shoji T, Shinohara K, Hatsuda S, Mori K, Fukumoto S, et al. Regional arterial stiffness in patients with type 2 diabetes and chronic kidney disease. J Am Soc Nephrol. 2006;17:2245–2252. doi: 10.1681/ASN.2005101038. [DOI] [PubMed] [Google Scholar]
- 19.Ellis D, Lloyd C, Becker DJ, Forrest KYZ, Orchard TJ. The changing course of diabetic nephropathy: Low-density lipoprotein cholesterol and blood pressure correlate with regression of proteinuria. Am J Kidney Dis. 1996;27:809–818. doi: 10.1016/s0272-6386(96)90518-1. [DOI] [PubMed] [Google Scholar]
- 20.Allain CC, Poon LS, Chan CS, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem. 1974;20:470–475. [PubMed] [Google Scholar]
- 21.Warnick GR, Albers JJ. A comprehensive evaluation of the heparin - manganese precipitation procedure for estimating high density lipoprotein cholesterol. J Lipid Res. 1978;19:65–76. [PubMed] [Google Scholar]
- 22.Bucolo G, David H. Quantitative Determination of Serum Triglycerides by the Use of Enzymes. Clin Chem. 1973;19:476–482. [PubMed] [Google Scholar]
- 23.Bondar RJL, Mead DC. Evaluation of Glucose-6-Phosphate Dehydrogenase from Leuconostoc mesenteroides in the Hexokinase Method for Determining Glucose in Serum. Clin Chem. 1974;20:586–590. [PubMed] [Google Scholar]
- 24.Chen H, Sullivan G, Quon MJ. Assessing the Predictive Accuracy of QUICKI as a Surrogate Index for Insulin Sensitivity Using a Calibration Model. Diabetes. 2005;54:1914–1925. doi: 10.2337/diabetes.54.7.1914. [DOI] [PubMed] [Google Scholar]
- 25.Lieb W, Larson MG, Benjamin EJ, Yin X, Tofler GH, Selhub J, et al. Multimarker Approach to Evaluate Correlates of Vascular Stiffness The Framingham Heart Study. Circulation. 2009;119:37–43. doi: 10.1161/CIRCULATIONAHA.108.816108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pratt JH, Rebhun JF, Zhou L, Ambrosius WT, Newman SA, Gomez-Sanchez CE, et al. Levels of mineralocorticoids in whites and blacks. Hypertension. 1999;34:315–319. doi: 10.1161/01.hyp.34.2.315. [DOI] [PubMed] [Google Scholar]
- 27.Price DA, Fisher ND. The renin-angiotensin system in blacks: active, passive, or what? Curr Hypertens Rep. 2003;5:225–230. doi: 10.1007/s11906-003-0025-x. [DOI] [PubMed] [Google Scholar]
- 28.Mahmud A, Feely J. Aldosterone-to-renin ratio, arterial stiffness, and the response to aldosterone antagonism in essential hypertension. Am J Hypertens. 2005;18:50–55. doi: 10.1016/j.amjhyper.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 29.Edwards NC, Steeds RP, Stewart PM, Ferro CJ, Townend JN. Effect of Spironolactone on Left Ventricular Mass and Aortic Stiffness in Early-Stage Chronic Kidney Disease A Randomized Controlled Trial. J Am Coll Cardiol. 2009;54:505–512. doi: 10.1016/j.jacc.2009.03.066. [DOI] [PubMed] [Google Scholar]
- 30.Joffe HV, Adler GK. Effect of aldosterone and mineralocorticoid receptor blockade on vascular inflammation. Heart Fail Rev. 2005;10:31–37. doi: 10.1007/s10741-005-2346-0. [DOI] [PubMed] [Google Scholar]
- 31.Yasmin, McEniery CM, Wallace S, Mackenzie IS, Cockcroft JR, Wilkinson IB. C-reactive protein is associated with arterial stiffness in apparently healthy individuals. Arterioscler Thromb Vasc Biol. 2004;24:969–974. doi: 10.1161/01.ATV.zhq0504.0173. [DOI] [PubMed] [Google Scholar]
- 32.Kullo IJ, Seward JB, Bailey KR, Bielak LF, Grossardt BR, Sheedy PF, 2nd , et al. C-reactive protein is related to arterial wave reflection and stiffness in asymptomatic subjects from the community. Am J Hypertens. 2005;18:1123–1129. doi: 10.1016/j.amjhyper.2005.03.730. [DOI] [PubMed] [Google Scholar]
- 33.Kusche-Vihrog K, Urbanova K, Blanque A, Wilhelmi M, Schillers H, Kliche K, et al. C-reactive protein makes human endothelium stiff and tight. Hypertension. 2011;57:231–237. doi: 10.1161/HYPERTENSIONAHA.110.163444. [DOI] [PubMed] [Google Scholar]
- 34.Brown NJ. Aldosterone and vascular inflammation. Hypertension. 2008;51:161–167. doi: 10.1161/HYPERTENSIONAHA.107.095489. [DOI] [PubMed] [Google Scholar]
- 35.Nguyen XM, Lane J, Smith BR, Nguyen NT. Changes in inflammatory biomarkers across weight classes in a representative US population: a link between obesity and inflammation. J Gastrointest Surg. 2009;13:1205–1212. doi: 10.1007/s11605-009-0904-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goodfriend TL, Kelley DE, Goodpaster BH, Winters SJ. Visceral obesity and insulin resistance are associated with plasma aldosterone levels in women. Obes Res. 1999;7:355–362. doi: 10.1002/j.1550-8528.1999.tb00418.x. [DOI] [PubMed] [Google Scholar]
- 37.Garg UC, Arnett DK, Folsom AR, Province MA, Williams RR, Eckfeldt JH. Lack of association between platelet glycoprotein IIb/IIIa receptor PlA polymorphism and coronary artery disease or carotid intima-media thickness. Thromb Res. 1998;89:85–89. doi: 10.1016/s0049-3848(97)00295-8. [DOI] [PubMed] [Google Scholar]
- 38.Muniyappa R, Montagnani M, Koh KK, Quon MJ. Cardiovascular actions of insulin. Endocr Rev. 2007;28:463–491. doi: 10.1210/er.2007-0006. [DOI] [PubMed] [Google Scholar]
- 39.Hayden MR, Sowers JR. Pancreatic renin-angiotensin-aldosterone system in the cardiometabolic syndrome and type 2 diabetes mellitus. J Cardiometab Syndr. 2008;3:129–131. doi: 10.1111/j.1559-4572.2008.00006.x. [DOI] [PubMed] [Google Scholar]
- 40.Mosso LM, Carvajal CA, Maiz A, Ortiz EH, Castillo CR, Artigas RA, et al. A possible association between primary aldosteronism and a lower beta-cell function. J Hypertens. 2007;25:2125–2130. doi: 10.1097/HJH.0b013e3282861fa4. [DOI] [PubMed] [Google Scholar]
- 41.Cooper SA, Whaley-Connell A, Habibi J, Wei Y, Lastra G, Manrique C, et al. Renin-angiotensin-aldosterone system and oxidative stress in cardiovascular insulin resistance. Am J Physiol Heart Circ Physiol. 2007;293:H2009–2023. doi: 10.1152/ajpheart.00522.2007. [DOI] [PubMed] [Google Scholar]
- 42.Lombes M, Oblin ME, Gasc JM, Baulieu EE, Farman N, Bonvalet JP. Immunohistochemical and Biochemical-Evidence for A Cardiovascular Mineralocorticoid Receptor. Circ Res. 1992;71:503–510. doi: 10.1161/01.res.71.3.503. [DOI] [PubMed] [Google Scholar]
- 43.Stowasser M. New perspectives on the role of aldosterone excess in cardiovascular disease. Clin Exp Pharmacol Physiol. 2001;28:783–791. doi: 10.1046/j.1440-1681.2001.03523.x. [DOI] [PubMed] [Google Scholar]
- 44.Davies J, Gavin A, Band M, Morris A, Struthers A. Spironolactone reduces brachial pulse wave velocity and PIIINP levels in hypertensive diabetic patients. Br J Clin Pharmacol. 2005;59:520–523. doi: 10.1111/j.1365-2125.2005.02363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]