Summary
Vitamin D and its analogs are potent inhibitors of colorectal cancer growth and metastasis. A number of recent studies have defined the intersections between the β-catenin-TCF pathway (a known contributor to colorectal cancer progression) and the vitamin D receptor (VDR) pathway, shedding light on the underlying mechanisms. Vitamin D also regulates the innate immune response, and as such influences susceptibility to inflammatory bowel disease, a predisposing factor in colorectal cancer. Understanding the role of vitamin D in these different contexts will enable development of next generation vitamin D analogs that will serve as both chemopreventatives and cancer therapeutics, without the accompanying side effects of hypercalcemia usually associated with high vitamin D intake. This review summarizes the mechanisms of action of vitamin D and the VDR in the context of the gastrointestinal tract and colorectal carcinogenesis.
Keywords: vitamin D, vitamin D receptor, β-catenin, colorectal cancer, Gardner Syndrome
1. Introduction
Colorectal cancer is the third leading cause of cancer-related death in the United States. It is well established that vitamin D and its analogs function as inhibitors of colorectal cancer progression but until recently the mechanisms underlying this association were unclear. Other predisposing factors for GI tract cancers include the Wnt/β-catenin/TCF oncogenic pathway, which is activated in a large proportion of colorectal cancers as well as the presence of inflammatory bowel conditions (reviewed in [1][2][49]). The past few years have led to some significant advances in our understanding of each of these different mechanisms, including the mapping of a complex series of interactions between the Wnt/β-catenin pathway and the vitamin D receptor (VDR) ([1][3][4]). Vitamin D influences both the initiation and progression of colorectal cancer via its roles in the regulation of β-catenin signaling and the innate immune response and is therefore an attractive chemopreventative agent for colorectal cancer. However, the high incidence of side effects that are unrelated to the anti-cancer actions of vitamin D is a major concern. Specifically, vitamin D has a limited therapeutic window as an anti-cancer agent due to hypercalcemia and hypercalciuria caused by elevated calcium levels (for recent review see [1]). This review is intended to update the reader on recent advances in this area and we apologize for our failure to cite many primary references, rather referring to earlier reviews.
2. Evidence for a Role for Vitamin D in the Prevention and Treatment of GI Malignancies
Epidemiological studies show an inverse relationship between the incidence of colon cancers and dietary intake or UV-activation of pre-vitamin D. This indicates that vitamin D has chemopreventative properties ([2][5][6]). Incidence of colorectal disease is associated with a high fat diet or obesity and with low vitamin D intake. VDR expression actually increases with tumor grade (until very late stage), and poor prognosis is associated with increased levels of vitamin D metabolizing enzymes ([2][7]). An anti-proliferative effect of vitamin D occurs in a wide variety of cancer cell lines and a large number of target genes have been identified that potentially contribute to the anti-proliferative VDR-driven transcriptome. These include cell cycle regulators such as p21 and p27 and regulators of apoptosis such as Bcl-2 and Bax ([2][8][9][10]).
Data from animal studies confirms the anti-cancer effect of vitamin D. In carcinogen-treated animal models vitamin D and its analogs are protective against tumor initiation and progression ([2][11]). Studies with VDR−/− mice confirm a role for the receptor in this phenomenon. VDR−/ − mice exhibit hyperplasia of colonic epithelium, but not cancer. However, VDR−/ − mice do exhibit elevated tumor incidence upon carcinogen challenge and show increased tumor burden when expressing transgenic oncogenes or mutated tumor suppressors ([2][12][13]). In contrast to many other malignancies the molecular changes underlying most colorectal cancers are quite well known, and involves the APC/β-catenin/TCF pathway with predisposition often a result of inflammatory bowel disease.
3. Mechanism of Vitamin D action in GI Cancer
Vitamin D is found in dietary sources although the majority is synthesized by UVB irradiation of the skin. It is then metabolized to 1, 25 dihydroxy vitamin D (active form of D3) in the liver and kidney. This metabolite is responsible for the well-established roles of vitamin D in calcium homeostasis via its activation of the vitamin D receptor (VDR) ([2]). The VDR is also implicated in broader roles in the regulation of genes that control cell proliferation, differentiation and apoptosis as well modulation of the immune response, and it is through these pathways that vitamin D exerts an anti-cancer effect. The VDR is central to the cancer- and most of the non-cancer-related actions of vitamin D, but there are mechanistic differences that can hopefully be exploited in order to take advantage of its beneficial chemopreventative properties.
3.1 The Classical Vitamin-D-VDR Pathway of Gene Activation
The VDR is a type II nuclear receptor that interacts with specific DNA-binding sites (vitamin D-response elements, VDREs) within the promoters of vitamin-D-responsive genes. In the absence of ligand, VDR is found bound to DNA in presence of co-repressors. Ligand binding triggers a series of conformational changes including the release of co-repressors, the formation of a VDR-RXR heterodimer, the recruitment of coactivators and the basal transcriptional machinery (reviewed in [2]). Helix 12 of the VDR AF-2 domain is critically involved in the ligand-induced conformational changes that allow interaction between VDR and RXR, and is essential for the subsequent recruitment of coactivators and gene activation ([14]).
3.2 Non-Classical Vitamin-D-VDR Signaling Pathways
3.2.1 The Wnt-/β-catenin/TCF Oncogenic Pathway in Colon Cancer
A substantial collection of data now demonstrates that the vitamin-D-VDR pathway intersects with the Wnt/β-catenin/TCF oncogenic pathway (reviewed in [1]). This pathway is almost universally activated very early in colon cancer. Its central player β-catenin is normally phosphorylated by, a multi-protein GSK3 β-Axin-APC complex, targeting it for degradation by the proteasome ([15][16][17]). In the presence of canonical Wnt ligands (via the Frizzled receptor-LRP-5/6 pathway), the GSK3β-Axin-APC complex is inhibited, leading to the accumulation of a pool of unphosphorylated β-catenin. This pool of β-catenin translocates to the nucleus where it interacts with TCF/LEF DNA-binding proteins to act as a transcriptional activation complex ([18][19]). Numerous secreted proteins (e.g. Frizzled-related protein, Wnt Inhibitory Factor-1, Dikkopf (DKK) family members) function to antagonize the Wnt pathway, keeping levels of activated β-catenin low. β-catenin is also recruited to the cadherin-based cell adhesion complexes at points of cell-cell contact at the cell membrane where it forms a bridge between the transmembrane cadherin and intracellular scaffolding proteins via α-catenin. This dual role of β-catenin allows changes in cell-cell contact triggered at the cell surface to be transmitted to β-catenin-dependent alterations in gene expression in the nucleus ([20], reviewed in [21]).
Activation of the Wnt-β-catenin pathway is a powerful mechanism of oncogenic transformation. For example, mutations in the APC tumor suppressor that regulates β-catenin phosphorylation are found in up to 80% of colon cancers ([22]). This dysregulation leads to elevated levels of activated nuclear β-catenin, producing altered profiles of gene expression that are favorable to oncogenesis.
3.2.2 Intersection between the Wnt-/β-catenin/TCF and Vitamin D-VDR Pathways
Studies from our laboratory and others have positioned β-catenin as a key intermediary in the preventive action of vitamin D and its analogs in colon cancer. It is now known that vitamin D represses β-catenin signaling by direct association with VDR and that β-catenin reciprocally activates VDR ([1][3][4]).
The first demonstration of a direct interaction between β-catenin and a member of the nuclear receptor superfamily concerned the retinoic acid receptor (RAR) ([23]). Subsequent work established that other nuclear receptors including VDR and AR also interact with β-catenin. Retinoic acid, androgen and vitamin D acting through the appropriate nuclear receptor all can transrepress β-catenin-TCF signaling ([1][3][4]). The extent of repression of β-catenin-TCF by the nuclear receptor is influenced by the cellular context. Repression may result from competition between VDR and TCF/LEF for binding to β-catenin or the coactivator p300 thus reducing availability of coactivators. In other contexts, repression may involve binding of liganded nuclear receptors to TCF/LEF and recruitment of co-repressors, resulting in a stronger suppression of gene expression ([1][3][24]).
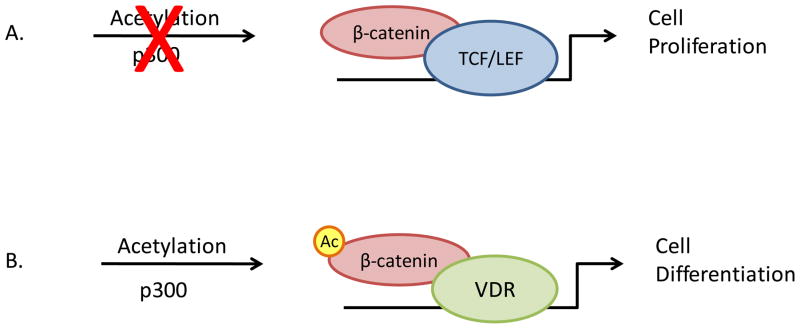
Conversely, β-catenin can also act as a co-activator for VDR-regulated promoters. This occurs via direct molecular interaction between the C-terminus of β-catenin and the AF-2 domain of the nuclear receptor ([4]). Acetylation of the β-catenin C-terminal domain discriminates between the outcomes: VDR-mediated repression of β-catenin-TCF-driven promoters versus β-catenin-dependent activation of VDR on VDRE-driven promoters ([4]). (Figure 1)
Figure 1. Schematic of p300-mediated acetylation of β-catenin differentiates between TCF- and VDR-dependent gene activation.
A. In absence of p300-mediated acetylation, β-catenin interacts with DNA binding proteins of the TCF/LEF families, activating gene expression via TCF/LEF promoter elements. This results in a pro-proliferative transcriptional profile.
B. Acetylation of β-catenin C-terminal domain promotes direct interaction between β-catenin and VDR on VDREs in vitamin D-responsive gene promoters. This leads to an anti-proliferative gene expression profile.
A specific mutation in the AF2 domain (E420Q) abrogates the ability of the VDR to activate VDRE-containing promoters using the classical co-activator-recruiting model but does not affect its interaction with β-catenin. This mutation is present in some families that exhibit hereditary vitamin D-dependent rickets (HVDDR). HVDDR individuals with the mutation do not show other symptoms normally characteristic of vitamin-D deficiency such as epidermal defects and hair loss ([25]). Work from our laboratory has determined that the E420Q mutant cannot interact with classical VDR co-activators but retains the ability to activate VDRE-dependent promoters using β-catenin as a co-activator as well as to activate heterologous promoters and can do so even in the presence VDR antagonists ([4]). These data indicate that this mutant VDR could be preferentially activated under elevated β-catenin conditions, as is the case in colon cancer.
Molecular modeling techniques were used to further dissect the mechanistic differences between the different modes of VDR-dependent gene activation ([4]). In order to recruit classical co-activators upon binding of an agonist ligand (vitamin D), the VDR AF-2 domain undergoes a conformational change resulting in the full agonist conformation (closed H12 conformation). The E420Q mutation is predicted to prohibit the full closure of H12, resulting in a partial agonist conformation and preventing recruitment of classical co-activators. However, the partial agonist conformation of H12 is sufficient for activation of VDR by β-catenin, explaining the ability of E420Q VDR to activate gene promoters in presence of elevated β-catenin. Both types of activation are ligand-dependent ([4]).
VDR ligands (antagonists) that block the activation of VDRE-dependent promoters by the classical coactivator recruitment model do not prevent the interaction between β-catenin and VDR ([4]). These observations imply that vitamin D analogs can be designed that will discriminate between the two types of VDR-driven gene expression, leading to the partial agonist conformation of H12 and preferentially activating VDRE-containing promoters in the presence of high levels of activated β-catenin. As elevated β-catenin is a common occurrence in colon cancer, this strategy would lead to selective activation of anti-proliferative genes in β-catenin-expressing cancer cells. Such a strategy would offer the additional benefit of repressing β-catenin signaling through TCF sites at the same time as VDR is activated ([7]).
3.2.3 Vitamin D Analogs that Exploit the Wnt-/β-catenin/TCF Connection
The ligand-binding pocket of VDR is very large, and may be able to accommodate many different analogs ([14]). Consequently, one must be cautious when investigating ligand “independent” functions of the VDR as for the most part these are only known to be 1, 25 dihydroxy vitamin D independent and it is possible that they are still able to bind and be activated by other non-canonical ligands, perhaps in a co-activator selective manner. A large number of vitamin D analogs have been identified and screened for their ability to selectively activate the anti-cancer properties of VDR via β-catenin co-activation without promoting calcemic side-effects ([7]). Certain of these VDR analogs (partial antagonists) do not allow recruitment of classical co-activators and therefore cannot activate VDR in most cells. However they retain the ability to activate VDR in presence of β-catenin ([4]). Interestingly, these analogs do not promote the interaction of mutated VDR (E420Q) with β-catenin, suggesting a synergistic contribution to the H12 conformation from the mutation and the partial agonist ligand ([4]).
To identify additional vitamin D analogs, we performed a limited simulation study using the Common Reference Binding Mode (CRBM) strategy, which takes into account receptor/ligand “breathing” ([26]). This study identified several compounds that are predicted to activate VDR in the presence of β-catenin (unpublished observations). These compounds have the potential to preferentially activate the VDR pathway in colorectal cancer cells that express high levels of activated β-catenin. Furthermore, they would be predicted to direct β-catenin away from oncogenic binding partners (e.g. TCF/LEF) and into complexes with VDR that promote anti-proliferative gene expression profiles. A recent study reported the development of CD ring analogs of vitamin D3 that show strong inhibition of β-catenin coupled with superagonistic VDR-coactivator interactions ([27]). Such strategies might offer the additional benefit of repressing β-catenin-activated proliferative genes while simultaneously sustaining the chemopreventative actions of VDR. In support of such a model, a recent study demonstrated that a polymorphism in the VDR that is associated with protection against colon cancer (the Fok1 f/M4 polymorphism) more efficiently promoted the sequestration of β-catenin away from TCF/LEF target genes ([28] ).
3.2.4 Further Complexities
In many instances human cancer cells and tumors become resistant to the chemopreventative effects of vitamin D. This appears to be due to suppression of the VDR-mediated activation of anti-proliferative genes ([2]). Attenuated responsiveness to vitamin D may be accompanied by reduced expression or mutation of the VDR or particular RXRs. An example of this is the snail-mediated reduction of VDR expression that occurs in certain colon tumor systems ([29]). Snail RNA expression is found in 60–70% of colon tumors and is inversely correlated with VDR expression ([30][31]). As snail is a β-catenin-TCF target gene, vitamin D analogs that promote β-catenin binding to VDR instead of TCF may exhibit the additional property of preventing late stage loss of responsiveness to vitamin D. Recently the snail family member, snail 2 was also shown to cooperatively downregulate VDR expression via the β-catenin-TCF pathway ([31][32]). β-catenin-selective vitamin D analogs would be predicted to also suppress the activity of snail 2, thus alleviating another dedifferentiating influence that may account for the remaining 20% of vitamin D-resistant human colon cancers that do not show elevated snail expression.
In other cases, resistance to vitamin D therapy is accompanied by a shift in the VDR-driven transcriptome away from anti-proliferative target genes, despite the sustained presence of a functional VDR (reviewed in [2]). This suggests that other molecular mechanisms are operating to provide resistance to agents such as vitamin D. In further studies, a feedback loop was identified by which vitamin D promotes expression of the secreted protein Dikkhopf (DKK) ([33][34]). DKK family members are negative regulators of the Wnt-β-catenin pathway. Therefore this mechanism acts cooperatively to promote the anti-proliferative and pro-differentiating actions of vitamin D by keeping levels of activated β-catenin low.
3.3 Calcemic Effects of Vitamin D
Vitamin D is well established as a key regulator of calcium homeostasis in the bone and intestine. These effects (part of the ‘rapid response’ to vitamin D) are known to be mediated by the VDR, but occur too rapidly (within minutes to 1 hr) to be explained by alterations in transcriptional activation profiles ([35]). A non-genomic pathway is believed to be involved in these calcemic effects of vitamin D. This mechanism involves a pool of VDR that is not resident in the cell nucleus but localized to the caveoli membranes close to the cell surface ([35]). This pool of VDR interacts with vitamin D ligand in a different conformation from the nuclear DNA-bound VDR. The interaction involves a distinct ligand-binding domain (‘rapid response’ LBD) that nevertheless overlaps with the established LBD that is involved in genomic responses, thus preventing simultaneous binding of two different ligands ([35][36]).
A longstanding challenge in the use of vitamin D chemopreventives and therapeutics for cancer has been the hypercalcemic response that accompanies high levels of vitamin D exposure. These side effects are caused by the rapid response uptake of calcium by the intestine and limit the therapeutic window for vitamin D or its analogs ([7]). In searching for new vitamin D analogs that are preferentially activated at the genomic level in the presence of elevated β-catenin, the ability of such ligands to interact with the non-genomic pool of VDR and influence the calcemic response must also be considered. By using modern molecular modeling techniques, the behavior of these ligands in both scenarios can be predicted before undertaking in vitro and in vivo biological screens. The ideal vitamin D analog will bind to the classical LBD of the VDR in a partially closed conformation, but be unable to interact with the ‘rapid response’ LBD.
3.4 Vitamin D and the Immune System
Inflammatory bowel disease (IBD) (including Crohns disease, colitis and irritable bowel syndrome) is a significant risk factor for colorectal cancer ([37]). Vitamin D and the VDR are now known to impact upon the regulation of the innate immune system that mediates such inflammatory responses in the colon and other mucosal organs.
Vitamin D acts directly on T cells and indirectly via antigen presenting cells ([10]). In autoimmune disease such as Crohns disease, T-helper (Th1) cells drive the disease by secretion of a pro-inflammatory profile of cytokines. In the presence of vitamin D, VDR directly suppresses the Th1 response by inhibiting production of IL-2 (by binding to the NF-AT site in the IL-2 promoter) and IFNγ (via a negative VDRE in the gene promoter) ([10][38][39]). In vitro studies also show that vitamin D can promote differentiation of Th2 lymphocytes. Under low vitamin D conditions these effects are reduced, leading to elevated Th1 activity and decreased regulatory T cell and Th2 activity, promoting the autoimmune Th1 response ([10]). Vitamin D also regulates the secretion of Th1-promoting cytokines IL-12 and IL-10 by dendritic cells and other antigen-presenting cells ([10]).
In animal models of Crohns disease (IL-10 −/ −), symptoms are severely exacerbated by absence of VDR and can be improved by addition of vitamin D ([10]). VDR −/ − mice also show increased sensitivity to dextran sodium sulfate (DSS) treatment, a model of mucosal injury. In addition, the response of wild type mice to (DSS) is ameliorated by vitamin D ([40]). These studies show that VDR is critical for the innate immune response in the gut. Treatment of VDR−/ − mice with AOM leads to elevated levels of GI tract tumors as well as squamous carcinomas of the transitional mucosa of the anus (Bong and Byers, unpublished observations). This susceptibility to squamous carcinoma associated with lack of VDR, may be due to the regulation of mucosal innate immunity by the vitamin D-VDR pathway.
In the innate immune response, activation of certain Toll-like receptors (TLRs), expressed on various immune and non-immune cell types, triggers direct antimicrobial activity against intracellular bacteria ([41]). In human monocytes, TLR activation leads to increased expression of VDR and the vitamin D-1-hydroxylase gene, leading to induction of the antimicrobial peptide cathelicidin ([41][42]). Cell culture studies demonstrated that adequate levels of vitamin D are required for the induction of cathelicidin ([41]). These findings are supported by clinical studies using serum from African Americans, an ethnic group known to have reduced levels of vitamin D. Monocytes cultured in this low-vitamin D serum was unable to induce cathelicidin, but addition of vitamin D in these samples restored responsiveness ([41, 42]).
Collectively, these studies show that vitamin D and the VDR play a key role in the control of the innate immune system. By these means, vitamin-D-driven pathways may be exploited by chemopreventative approaches to influence the development of inflammatory conditions that predispose to cancer as well as to prevent later progression (Figure 2). A key area of future interest will be the contribution of the classical and non-classical VDR signaling pathways to these inflammatory outcomes.
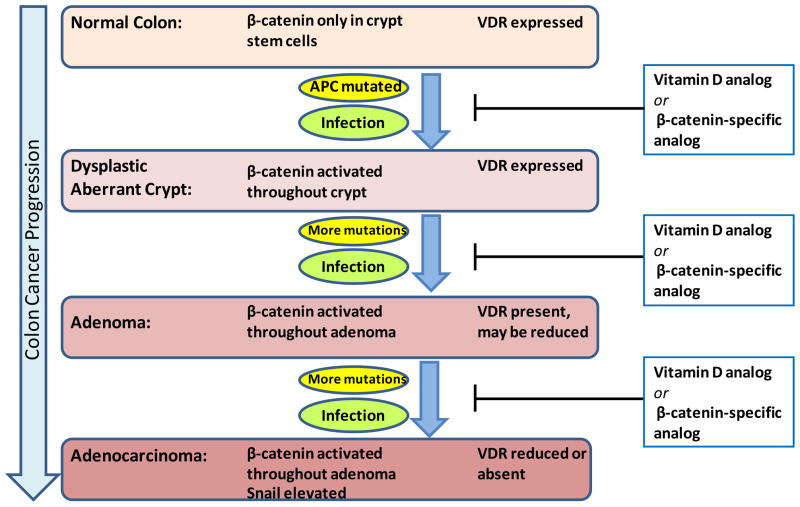
Figure 2. Illustration of the progression of colon cancer and therapeutic strategies.
The β-catenin/TCF pathway is activated at an early stage in colorectal cancer by mutations in APC. Subsequently further mutations characterize the progression through to aggressive disease. In late stage disease the β-catenin target genes snail and snail 2 are elevated and suppress VDR expression. Activation of the innate immune response due to infections of the GI tract leads to inflammatory bowel disease. Low circulating levels of vitamin D increase susceptibility to pre-cancerous conditions such as inflammatory bowel disease. Analogs of vitamin D or β-catenin that direct the activation of the non-classical β-catenin-VDR pathway are predicted to act as both chemopreventatives and therapeutics at progressive stages of colorectal cancer by suppressing both the β-catenin-activated and immune response-mediated intersecting pathways.
4. Vitamin D-β-catenin Interactions beyond the GI Tract
4.1 Gardners Syndrome
Gardners Syndrome is a category within familial adenomatous polyposis (FAP), an autosomal dominant disease caused by a mutation in APC. FAP is characterized by GI polyps followed by early onset of progression to colon cancer. However, Gardners Syndrome is a more severe form of FAP, exhibiting rapid progression of GI polyps to colon cancer as well as extra-colonic manifestations including a propensity for adenomas in the cecum, osteomas, bone opacity, desmoids tumors and epidermoid cysts ([43][44]).
FAP symptoms can be mimicked in mouse models by mutations in APC (e.g. APC1638 [45]). This model is characterized by elevated levels of activated β-catenin and develops FAP-like GI polyps that progress to cancer, but does not exhibit the extra colonic symptoms of Gardners Syndrome. Recent work by our laboratory showed that expression of the APC1638 mutation on a VDR−/ − background leads to the appearance of Gardners syndrome phenotypes suggesting that Gardners syndrome represents a type of FAP in which β-catenin is activated but VDR activity is low (Bong and Byers unpublished observations). These observations suggest that Gardners syndrome patients may have mutations in VDR itself or genes responsible for vitamin D metabolism. Present studies using this novel model of Gardners Syndrome are directed toward the goal of determining whether the ligand-dependent VDR-β-catenin interaction is involved in these extra-colonic manifestations. A clear understanding of the mechanisms involved may mean that vitamin D analogs could be used as therapeutics not only for GI tract symptoms but also to alleviate the extra-colonic effects of this severe and life-threatening disorder.
4.2 Alopecia and Epidermal Tumors
A role for the vitamin D receptor in regulating hair cycle is apparent from the alopecia phenotype of HVDRR as well as from studies using VDR−/ − mice In VDR−/ − mice, cysts comprising sebocytes and keratinocytes are found in skin, and hair follicles are lacking in follicle lineage stem cells, leading to progressive hair loss. Mutants that fail to bind 1,25 dihydroxy vitamin D can rescue this phenotype indicating a “ligand” independent function of the VDR in this situation ([46]). Cross talk between the Wnt-β-catenin and VDR pathways is involved in these epidermal phenotypes.
Defective Wnt signaling influences the division of epidermal stem cells promoting epidermal keratinocyte and sebocyte lineages and inhibiting hair follicle lineages. However, the hairless phenotype of VDR−/ − mice cannot be rescued by targeted expression of β-catenin in follicle stem cells. Instead, VDR must be present in the same complex as β-catenin to activate LEF-dependent genes. Studies using the E420Q mutant of VDR demonstrated that this activation does not require classical co-activators, as VDR-E420Q can rescue the alopecia while it cannot bind co-activators ([4]). Interestingly, treatment of VDR−/ − mice with an agonist of the sonic hedgehog pathway transiently restores hair cycling, implying an intersection between the VDR-dependent and hedgehog-dependent pathways ([47]). As Wnt- and hedgehog-signaling are already linked in other systems, it is likely that a common pathway will be elucidated bringing together Wnt, hedgehog and VDR in the epidermis.
Targeted expression of constitutively activated β-catenin in the epidermis leads to epidermal tumors. The type of tumor that forms depends on the presence of VDR: in VDR+/+ mice the tumors that form are trichofolliculomas (similar to human pilomatricomas) but in the absence of VDR, activated β-catenin instead promotes formation of basal cell carcinoma ([48]). These data show that in the epidermis, the interaction between VDR and β-catenin leads to super-activation of β-catenin expression, in contrast to the attenuating effects observed in colorectal cancer cells.
5. Conclusion
The great potential of vitamin D as a chemopreventive agent for colorectal cancer has spurred investigations into the molecular mechanisms that govern its effects. Vitamin D via the VDR directly alters patterns of gene expression, and can influence the outcome between proliferation, differentiation or apoptosis. These genomic effects can be distinguished into the classical mechanism of VDR recruitment of co-activators on VDREs and the non-classical interactions with the activated β-catenin on other promoters. Detailed molecular biology analysis coupled with molecular modeling studies have shed light upon the intersection between the β-catenin-TCF and vitamin D pathways. Low circulating levels of vitamin D increase susceptibility pre-cancerous conditions such as IBD and also accelerate the progression of colonic neoplasms resulting from APC or β-catenin mutation. The contribution of the classical and non-classical/β-catenin-VDR pathways to the innate immune response and predisposition to colon cancer has yet to be clarified.
A further complicating factor in the use of vitamin D as a chemotherapeutic is the ‘rapid response’ uptake of calcium that can result in hypercalcemia and hypercaliurea. This is a non-genomic response and can be mechanistically separated from the positive influence of vitamin D on cell differentiation. Efforts are underway to identify the ideal VDR ligand that can effectively repress colorectal cancer progression while leaving calcium homeostasis unperturbed. Such a molecule may have the added advantage of suppressing the resistance to VDR vitamin D therapy that occurs during late stage colorectal disease and may therefore qualify as both a chemopreventative and a cancer therapeutic, serving the dual roles of preventing the initiation as well as progression GI tract cancers.
Acknowledgments
Financial Support: The author’s studies were funded by NIH R01CA129813, NIH R01 DK58196, NIH 1 P01 CA130821 (SWB) NIH R21CA156188 (YSB),
References
- 1.Beildeck ME, Gelmann EP, Byers SW. Cross-regulation of signaling pathways: an example of nuclear hormone receptors and the canonical Wnt pathway. Exp Cell Res. 2010;16:1763–1772. doi: 10.1016/j.yexcr.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thorne J, Campbell MJ. The vitamin D receptor in cancer. Proc Nutr Soc. 2008;67:115–127. doi: 10.1017/S0029665108006964. [DOI] [PubMed] [Google Scholar]
- 3.Shah S, Hecht A, Pestell R, Byers SW. Trans-repression of beta-catenin activity by nuclear receptors. J Biol Chem. 2003;278:48137–48145. doi: 10.1074/jbc.M307154200. [DOI] [PubMed] [Google Scholar]
- 4.Shah S, Islam MN, Dakshanamurthy S, Rizvi I, Rao M, Herrell R, et al. The molecular basis of vitamin D receptor and beta-catenin crossregulation. Mol Cell. 2006;21:799–809. doi: 10.1016/j.molcel.2006.01.037. [DOI] [PubMed] [Google Scholar]
- 5.Touvier M, Chan DS, Lau R, Aune D, Vieira R, Greenwood DC, Kampman E, Riboli E, Hercberg S, Norat T. Meta-analyses of vitamin D intake, 25-hydroxyvitamin D status, vitamin D receptor polymorphisms, and colorectal cancer risk. Cancer Epidemiol Biomarkers Prev. 2011;20:1003–1016. doi: 10.1158/1055-9965.EPI-10-1141. [DOI] [PubMed] [Google Scholar]
- 6.Campbell FC, Xu H, El-Tanani M, Crowe P, Bingham V. The yin and yang of vitamin D receptor (VDR) signaling in neoplastic progression: operational networks and tissue-specific growth control. Biochem Pharmacol. 2010;79:1–9. doi: 10.1016/j.bcp.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beildeck MR, Byers SW. Vitamin D analogues in colon cancer prevention and care. Curr Colorectal Cancer Rep. 2009;5:185–196. [Google Scholar]
- 8.Pálmer HG, Sánchez-Carbayo M, Ordóñez-Morán P, Larriba MJ, Cordón-Cardó C, Muñoz A. Genetic signatures of differentiation induced by 1alpha,25-dihydroxyvitamin D3 in human colon cancer cells. Cancer Res. 2003;63:7799–7806. [PubMed] [Google Scholar]
- 9.Wang TT, Tavera-Mendoza LE, Laperriere D, Libby E, MacLeod NB, Nagai Y, Bourdeau V, Konstorum A, Lallemant B, Zhang R, Mader S, White JH. Large-scale in silico and microarray-based identification of direct 1,25-dihydroxyvitamin D3 target genes. Mol Endocrinol. 2005;19:2685–2695. doi: 10.1210/me.2005-0106. [DOI] [PubMed] [Google Scholar]
- 10.Mullin GE, Dobs A. Vitamin d and its role in cancer and immunity: a prescription for sunlight. Nutr Clin Pract. 2007;22:305–322. doi: 10.1177/0115426507022003305. [DOI] [PubMed] [Google Scholar]
- 11.Belleli A, Shany S, Levy J, Guberman R, Lamprecht SA. A protective role of 1,25-dihydroxyvitamin D3 in chemically induced rat colon carcinogenesis. Carcinogenesis. 1992;13:2293–2298. doi: 10.1093/carcin/13.12.2293. [DOI] [PubMed] [Google Scholar]
- 12.Kallay E, Pietschmann P, Toyokuni S, Bajna E, Hahn P, Mazzucco K, Bieglmayer C, Kato S, Cross HS. Characterization of a vitamin D receptor knockout mouse as a model of colorectal hyperproliferation and DNA damage. Carcinogenesis. 2001;22:1429–1435. doi: 10.1093/carcin/22.9.1429. [DOI] [PubMed] [Google Scholar]
- 13.Zheng W, Wong KE, Zhang Z, Dougherty U, Mustafi R, Kong J, Deb DK, Zheng H, Bissonnette M, Li YC. Inactivation of the vitamin D receptor in APC(min/+) mice reveals a critical role for the vitamin D receptor in intestinal tumor growth. Int J Cancer. 2011 doi: 10.1002/ijc.25992. Epub Feb 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rochel N, Wurtz JM, Mitschler A, et al. The crystal structure of the nuclear receptor for vitamin D bound to its natural ligand. Mol Cell. 2000;5:173–179. doi: 10.1016/s1097-2765(00)80413-x. [DOI] [PubMed] [Google Scholar]
- 15.Munemitsu S, Albert I, Souza B, Rubinfeld B, Polakis P. Regulation of intracellular beta-catenin levels by the adenomatous polyposis coli (APC) tumor-suppressor protein. Proc Natl Acad Sci USA. 1995;92:3046–3050. doi: 10.1073/pnas.92.7.3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rubinfeld B, Albert I, Porfiri E, Fiol C, Munemitsu S, Polakis P. Binding of GSK3 beta to the APC-beta-catenin complex and regulation of complex assembly. Science. 1996;272:1023–1026. doi: 10.1126/science.272.5264.1023. [DOI] [PubMed] [Google Scholar]
- 17.Orford K, Crockett C, Jensen JP, Weissman AM, Byers SW. Serine phosphorylation-regulated ubiquitination and degradation of beta-catenin. J Biol Chem. 1997;272:24735–24738. doi: 10.1074/jbc.272.40.24735. [DOI] [PubMed] [Google Scholar]
- 18.Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W. Functional interaction of b-catenin with the transcription factor LEF-1. Nature. 1996;382:638–642. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- 19.Cong F, Schweizer L, Chamorro M, Varmus H. Requirement for a nuclear function of beta-catenin in Wnt signaling. Mol Cell Biol. 2003;23:8462–8470. doi: 10.1128/MCB.23.23.8462-8470.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barth AI, Näthke IS, Nelson WJ. Cadherins, catenins and APC protein: interplay between cytoskeletal complexes and signaling pathways. Curr Opin Cell Biol. 1997;9:683–690. doi: 10.1016/s0955-0674(97)80122-6. [DOI] [PubMed] [Google Scholar]
- 21.Gavert N, Ben-Ze’ev A. beta-Catenin signaling in biological control and cancer. J Cell Biochem. 2007;102:820–828. doi: 10.1002/jcb.21505. [DOI] [PubMed] [Google Scholar]
- 22.Kinzler KW, Nilbert MC, Vogelstein B, et al. Identification of a gene located at chromosome 5q21 that is mutated in colorectal cancers. Science. 1991;251:1366–1370. doi: 10.1126/science.1848370. [DOI] [PubMed] [Google Scholar]
- 23.Easwaran V, Pishvaian M, Salimuddin S, Byers S. Cross-regulation of beta-catenin-LEF/TCF and retinoid signaling pathways. Curr Biol. 1999;9:1415–1418. doi: 10.1016/s0960-9822(00)80088-3. [DOI] [PubMed] [Google Scholar]
- 24.Palmer HG, Gonzalez-Sancho JM, Espada J, Berciano MT, Puig I, Baulida J, et al. Vitamin D(3) promotes the differentiation of colon carcinoma cells by the induction of E-cadherin and the inhibition of beta-catenin signaling. J Cell Biol. 2001;154:369–387. doi: 10.1083/jcb.200102028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malloy PJ, Xu R, Peng L, Clark PA, Feldman D. A novel mutation in helix 12 of the vitamin D receptor impairs coactivator interaction and causes hereditary 1,25-dihydroxyvitamin D-resistant rickets without alopecia. Mol Endocrinol. 2002;16:2538–2546. doi: 10.1210/me.2002-0152. [DOI] [PubMed] [Google Scholar]
- 26.Sivanesan D, Rajnarayanan RV, Doherty J, Pattabiraman N. In-silico screening using flexible ligand binding pockets: a molecular dynamics-based approach. J Comput Aided Mol Des. 2005;19:213–228. doi: 10.1007/s10822-005-4788-9. [DOI] [PubMed] [Google Scholar]
- 27.Eelen G, Verlinden L, Bouillon R, De Clercq P, Muñoz A, Verstuyf A. CD-ring modified vitamin D3 analogs and their superagonistic action. J Steroid Biochem Mol Biol. 2010;121:417–419. doi: 10.1016/j.jsbmb.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 28.Egan JB, Thompson PA, Vitanov MV, Bartik L, Jacobs ET, Haussler MR, Gerner EW, Jurutka PW. Vitamin D receptor ligands, adenomatous polyposis coli, and the vitamin D receptor FokI polymorphism collectively modulates beta-catenin activity in colon cancer cells. Mol Carcino. 2010;49:337–352. doi: 10.1002/mc.20603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palmer HG, Larriba MJ, Garcia JM, Ordonez-Moran P, Pena C, Peiro S, et al. The transcription factor SNAIL represses vitamin D receptor expression and responsiveness in human colon cancer. Nat Med. 2004;10:917–919. doi: 10.1038/nm1095. [DOI] [PubMed] [Google Scholar]
- 30.Peña C, García JM, Silva J, García V, Rodríguez R, Alonso I, Millán I, Salas C, de Herreros AG, Muñoz A, Bonilla F. E-cadherin and vitamin D receptor regulation by SNAIL and ZEB1 in colon cancer: clinicopathological correlations. Hum Mol Genet. 2005;14:3361–3370. doi: 10.1093/hmg/ddi366. [DOI] [PubMed] [Google Scholar]
- 31.Larriba MJ, Bonilla F, Muñoz A. The transcription factors Snail1 and Snail2 repress vitamin D receptor during colon cancer progression. J Steroid Biochem Mol Biol. 2010;121:106–109. doi: 10.1016/j.jsbmb.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 32.Larriba MJ, Martín-Villar E, García JM, Pereira F, Peña C, de Herreros AG, Bonilla F, Muñoz A. Snail2 cooperates with Snail1 in the repression of vitamin D receptor in colon cancer. Carcinogenesis. 2009;30:1459–1468. doi: 10.1093/carcin/bgp140. [DOI] [PubMed] [Google Scholar]
- 33.Aguilera O, Pena C, Garcia JM, Larriba MJ, Ordonez-Moran P, Navarro D, Barbachano A, de Silanes IL, Ballestar E, Fraga MF, Esteller M, Gamallo C, Bonilla F, Gonzalez-Sancho JM, Munoz A. The Wnt antagonist DICKKOPF-1 gene is induced by 1 alpha,25-dihydroxyvitamin D-3 associated to the differentiation of human colon cancer cells. Carcinogenesis. 2007;28:1877–1884. doi: 10.1093/carcin/bgm094. [DOI] [PubMed] [Google Scholar]
- 34.Pendas-Franco N, Aguilera O, Pereira F, Gonzalez-Sancho JM, Munoz A. Vitamin D and Wnt/beta-catenin Pathway in Colon Cancer: Role and Regulation of DICKKOPF Genes. Anticancer Research. 2008;28:2613–2623. [PubMed] [Google Scholar]
- 35.Norman AW. Vitamin D receptor: new assignments for an already busy receptor. Endocrinology. 2006;147:5542–5548. doi: 10.1210/en.2006-0946. [DOI] [PubMed] [Google Scholar]
- 36.Mizwicki MT, Bula CM, Bishop JE, Norman AW. A perspective on how the Vitamin D sterol/Vitamin D receptor (VDR) conformational ensemble model can potentially be used to understand the structure-function results of A-ring modified Vitamin D sterols. J Steroid Biochem Mol Biol. 2005;97:69–82. doi: 10.1016/j.jsbmb.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 37.Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, et al. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 38.Cippitelli M, Santoni A. Vitamin D3: a transcriptional modulator of the interferon-gamma gene. Eur J Immunol. 1998;28:3017–3030. doi: 10.1002/(SICI)1521-4141(199810)28:10<3017::AID-IMMU3017>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 39.Boonstra A. 1 Alpha,25-dihydroxyvitamin D3 has a direct effecton naive CD4(_) T cells to enhance the development of Th2 cells. J Immunol. 2001;167:4974–4980. doi: 10.4049/jimmunol.167.9.4974. [DOI] [PubMed] [Google Scholar]
- 40.Froicu M, Cantorna MT. Vitamin D and the vitamin D receptor are critical for control of the innate immune response to colonic injury. BMC Immunol. 2007;8:5. doi: 10.1186/1471-2172-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu PT, Krutzik SR, Modlin RL. Therapeutic implications of the TLR and VDR partnership. Trends Mol Med. 2007;13:117–124. doi: 10.1016/j.molmed.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 42.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, Kamen DL, Wagner M, Bals R, Steinmeyer A, Zügel U, Gallo RL, Eisenberg D, Hewison M, Hollis BW, Adams JS, Bloom BR, Modlin RL. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 43.Gómez García EB, Knoers NV. Gardner’s syndrome (familial adenomatous polyposis): a cilia-related disorder. Lancet Oncol. 2009;10:727–735. doi: 10.1016/S1470-2045(09)70167-6. [DOI] [PubMed] [Google Scholar]
- 44.Galiatsatos P, Foulkes WD. Familial adenomatous polyposis. Am J Gastroenterol. 2006;101:385–398. doi: 10.1111/j.1572-0241.2006.00375.x. [DOI] [PubMed] [Google Scholar]
- 45.Lipkin M. New rodent models for studies of chemopreventive agents. J Cell Biochem. 1997;(Suppl 28–29):144–147. [PubMed] [Google Scholar]
- 46.Li YC, Pirro AE, Amling M, Delling G, Baron R, Bronson R, Demay MB. Targeted ablation of the vitamin D receptor: an animal model of vitamin D-dependent rickets type II with alopecia. Proc Natl Acad Sci USA. 1997;94:9831–9835. doi: 10.1073/pnas.94.18.9831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Teichert A, Elalieh H, Bikle D. Disruption of the hedgehog signaling pathway contributes to the hair follicle cycling deficiency in Vdr knockout mice. J Cell Physiol. 2010;225:482–489. doi: 10.1002/jcp.22227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Palmer HG, Anjos-Afonso F, Carmeliet G, Takeda H, Watt FM. The vitamin D receptor is a Wnt effector that controls hair follicle differentiation and specifies tumor type in adult epidermis. PLoS ONE. 2008;3:e1483. doi: 10.1371/journal.pone.0001483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ullman TA, Itzkowitz SH. Intestinal inflammation and cancer. Gastroenterology. 2011;140:1807–1816. doi: 10.1053/j.gastro.2011.01.057. [DOI] [PubMed] [Google Scholar]