Abstract
We aimed to investigate the role of indoor office air on exposure to polyfluorinated compounds (PFCs) among office workers. Week-long, active air sampling was conducted during the winter of 2009 in 31 offices in Boston, MA. Air samples were analyzed for fluorotelomer alcohols (FTOHs), sulfonamides (FOSAs), and sulfonamidoethanols (FOSEs). Serum was collected from each participant (n=31) and analyzed for twelve PFCs including PFOA and PFOS. In air, FTOHs were present in the highest concentrations, particularly 8:2-FTOH (GM=9,920 pg/m3). FTOHs varied significantly by building with the highest levels observed in a newly constructed building. PFOA in serum was significantly correlated with air levels of 6:2-FTOH (r=0.43), 8:2-FTOH (r=0.60), and 10:2-FTOH (r=0.62). Collectively, FTOHs in air significantly predicted PFOA in serum (p < 0.001) and explained approximately 36% of the variation in serum PFOA concentrations. PFOS in serum was not associated with air levels of FOSAs/FOSEs. In conclusion, FTOH concentrations in office air significantly predict serum PFOA concentrations in office workers. Variation in PFC air concentrations by building is likely due to differences in the number, type, and age of potential sources such as carpeting, furniture and/or paint.
Introduction
Polyfluorinated compounds (PFCs) are a class of chemicals used in a range of applications for their water and stain-resistant properties. Perfluorinated carboxylic acids (PFCAs)—such as perfluorooctanoate (PFOA)—and perfluorinated sulfonates (PFSAs)—such as perfluorooctane sulfonate (PFOS)—have been used for over 50 years in the production of consumer products including carpet and upholstery stain-protectants, food-contact paper coatings, nonstick cookware, waterproofing sprays, and windshield wash.1,2 Such extensive use of PFCs can be attributed not only to their water and oil repellency, but to their unique thermal stability and resistance to degradation by chemicals, UV radiation or weather. These properties make PFCs extremely beneficial from an industrial standpoint, but accumulative and persistent contaminants from an environmental perspective.
PFCs are observed globally in air, water, wildlife, and humans.2–6 Half-lives in humans have been assessed for some compounds with estimates ranging from 2.3 years for PFOA to 7.3 years for perfluorohexane sulfonic acid (PFHxS).7,8 Investigations of health outcomes have primarily focused on exposure to PFOA and PFOS, the two most abundant PFCs in the general population. Effects from these compounds on lipid metabolism, liver health, development, reproduction, and the immune system have been reported in animal studies.5,9,10 Though there has been considerably less investigation of effects in humans, some evidence exists that PFOA and PFOS may be associated with lowered birth weight11,12 and increased cholesterol.13,14
Despite the ubiquitous presence of several PFCs in the general population, their long half-lives, and increasing evidence of potential adverse health effects, little is known about the contribution of different exposure pathways and microenvironments to human body burdens. Potential pathways of exposure include water and dietary intake, inhalation of indoor and ambient air, ingestion of indoor dust, and direct contact with PFC-containing products. Some studies have concluded that exposure to PFOA and PFOS is dominated by food intake and that exposure via consumer products is relatively small.15,16 However, such studies were limited in part by a lack of data on PFC levels in indoor air.
Further complicating exposure assessment is the hypothesis that volatile PFCA and PFSA precursors contribute to body burdens of PFOA and PFOS.17,18 Fluorotelomer alcohols (FTOHs) can be metabolized in vitro by human and animal hepatocytes and in vivo in rats and mice to form PFOA and other PFCAs.19–22 Similarly, PFOS can be formed by in vivo and in vitro metabolism of fluorinated sulfonamides (FOSAs), including sulfonamidoethanols (FOSEs).23,24 Sources of these precursor compounds to the environment are not clearly understood, though they are the main chemical residuals found in fluoropolymer products.25 (See Supplemental Material, Table 1 for chemical structures and CAS numbers of PFCs discussed in this paper.)
The primary objective of this study was to investigate the role of indoor office air on exposure to PFCs by characterizing levels of PFCs in indoor office air, including PFOA and PFOS precursors, and determining if they contribute significantly to PFC serum concentrations in office workers.
Experimental
Study Design
We recruited a convenience sample of 31 individuals living and working in the Boston, Massachusetts area of the US. Participants ranged in age from 25 to 64 years, consisted of 26 females and 5 males, and worked at least 18 hours per week in offices. Offices were located in seven buildings and categorized into three groups: Building A, Building B, and Other. Building A (6 offices) was newly built approximately one year before the study began in 2009. Building A contained new carpeting throughout hallways and offices, as well as newly purchased furniture including upholstered chairs in each of the offices. Building B (17 offices) was partially renovated approximately one year before the study began. While no new furniture or paint was purchased, new carpeting was installed throughout hallways and approximately 10% of offices. The five Other buildings (8 offices) were not known to have undergone recent renovation; six of these offices were carpeted, but most hallways were not. All 31 offices contained painted floor-to-ceiling walls, doors that were closed during evening hours, forced air ventilation and at least one desk with a computer. The average office size was 11.6 m2; two-thirds contained at least one window.
We actively sampled indoor air, particulate and gaseous phase, from each office for four-days (8 AM Monday through 8 AM Friday) during the winter of 2009 (see Supplemental Material for details of air sampling procedures). Most participants reported that office windows were closed during the entire sampling period. A trained phlebotomist collected blood at the end of each participant’s week of air sampling. A questionnaire gathered information on demographics, diet over the previous year, time spent in the office per week, and recent office renovations. We obtained informed consent prior to data collection. The study was approved by the Boston University Medical Center’s Institutional Review Board. The involvement of the Centers for Disease Control and Prevention (CDC) laboratory was limited and determined not to constitute engagement in human subjects research.
Analysis of Air Samples
Particulate and gaseous phase neutral PFCs were captured and extracted together to provide total air concentrations of the target analytes: 6:2-FTOH, 8:2-FTOH, 10:2-FTOH, MeFOSA, EtFOSA, MeFOSE, and EtFOSE. Air samples were not extracted for ionic compounds during analysis, thus measurement of the less volatile, ionic PFCs such as PFOA and PFOS was not possible. Neutral PFCs were measured by gas chromatography-positive chemical ionization mass spectrometry (GC-PCIMS). For further analytical details, see Supplemental Material.
Analysis of Serum Samples
After clotting and centrifugation, serum was recovered from whole blood and stored in polypropylene criovials at −80°C before being shipped on dry ice to CDC for analysis. PFCs (perfluorohexanoic acid [PFHxA], perfluoroheptanoic acid [PFHpA], PFOA, perfluorononanoic acid [PFNA], perfluorodecanoic acid [PFDA], perfluoroundecanoic acid [PFUA], perfluorododecanoic acid [PFDoA], PFHxS, PFOS, perfluorooctane sulfonamide [PFOSA], methyl perfluorooctane sulfonamidoacetate [N-MeFOSAA], and N-EtFOSAA) were measured using a modification of a published method based on a solid phase extraction (SPE) system linked directly on-line with HPLC isotope dilution tandem mass spectrometry.26 More details are available in Supplemental Material.
Statistical Analyses
PFC concentrations in air were blank-corrected using the mean of the three field blanks (field blanks averaged less than 1% of average sample values for all compounds except MeFOSA and EtFOSA for which field blank averages were 17% and 8% of sample values, respectively). The limit of detection (LOD) for each analyte in air was defined as three times the standard deviation of the field blanks except for EtFOSE (not detected in any of the three blanks) in which case the LOD was defined as the instrument detection limit. For both air and serum analytes, only those detected in >90% of samples were included in statistical analyses beyond summary measures. For those air and serum analytes included in further statistical analyses, values below the LOD were replaced by the LOD divided by the square root of two, a common methodology employed in exposure assessment and utilized by the CDC to report biomonitoring results from the National Health and Nutrition Examination Survey.27 Because detection frequencies were high for these compounds, requiring substitution of values below the LOD for, at most, three of thirty samples for any compound, this simple substitution method was considered appropriate.
Two air samples were excluded due to malfunctioning pump timers. One excluded sample was a duplicate, reducing the total number of offices sampled to 30 and the number of duplicate pairs to two. Percent precision was calculated as the ratio of uncertainty (square root of the average sum of squares of the difference between duplicate pairs) to the average sample value times 100. Average percent precision (PP) for the two duplicate pairs was 10.8% (range: 5.4 – 18%) for all compounds except 6:2-FTOH (PP = 42.8%) which had one highly discordant pair.
The distributions of PFC concentrations in air and blood were evaluated by visual inspection of histograms and results of the Shapiro-Wilk test and were found to be log-normally distributed. Data were natural log-transformed prior to analysis when analyzed as the dependent variable, but not when used as independent variables in regression analyses. Analyses included univariate descriptive statistics, Pearson correlations, scatterplots and testing for outliers, simple and multiple linear regression, and principal components analysis (PCA). Independent variables examined in regression analyses included building category, time spent in office (during a typical week), sex, age, and body mass index (BMI). Since outcome variables were log-transformed for regression analyses, regression coefficients (Beta values, β) presented in the text and tables can be exponentiated to represent the multiplicative change in the outcome per unit of predictor variable. PCA was performed on PFCs in air using a variance maximizing rotation (rotate = varimax) to extract components with eigenvalues greater than 1. Statistical analyses were performed using SAS 9.1. Statistical significance was tested with α= 0.05.
Results
Air concentrations
Table 1 presents the geometric means (GMs), geometric standard deviations (GSDs) and ranges of each analyte measured in office air. All analytes (6:2-FTOH, 8:2-FTOH, 10:2-FTOH, MeFOSA, EtFOSA, MeFOSE, and EtFOSE) were detected in ≥ 90% of office air samples. FTOH concentrations were considerably higher than the sulfonamides. 8:2-FTOH had the highest GM concentration (9,920 pg/m3). Among sulfonamide compounds, MeFOSE had the highest concentrations with a GM of 289 pg/m3. Strong positive correlations were observed among the three FTOHs and among the four sulfonamides. However, there was little association between the two groups except for a negative association (r = −0.36) between 8:2-FTOH and EtFOSA (Supplemental Material, Table 2).
Table 1.
Summary statistics of PFCs in office air (pg/m3) (n=30).
| Analyte | % detect | LODa | GM | (GSD) | Range |
|---|---|---|---|---|---|
| 6:2-FTOH | 93 | 19.5 | 1320 | (5.2) | <LOD – 11000 |
| 8:2-FTOH | 100 | 84.7 | 9920 | (4.7) | 283 – 70600 |
| 10:2-FTOH | 100 | 24.5 | 2850 | (3.6) | 138 – 12600 |
| EtFOSA | 97 | 1.26 | 17.0 | (2.6) | <LOD – 115 |
| MeFOSA | 100 | 0.40 | 29.1 | (2.4) | 5.93 – 162 |
| EtFOSE | 90 | 0.03 | 18.1 | (12) | <LOD – 216 |
| MeFOSE | 100 | 12.6 | 289 | (3.0) | 48.5 – 3880 |
Based on average sample volume (21.8 m3).
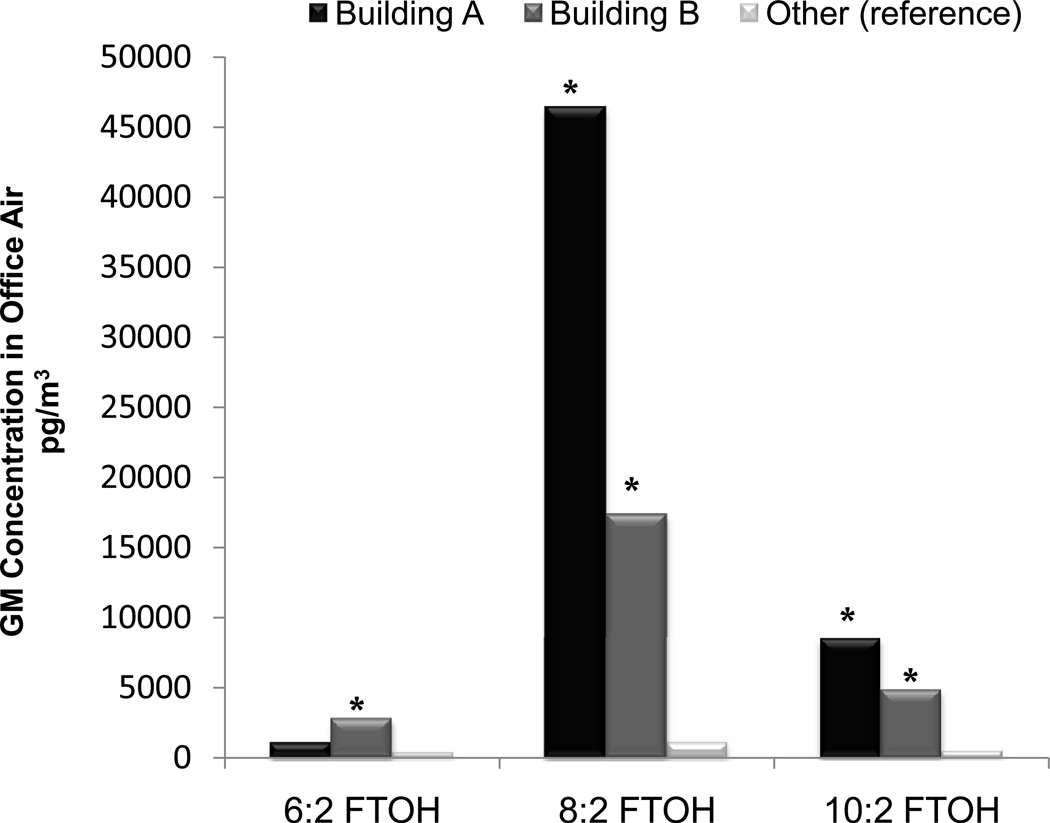
Figure 1 presents GM air concentrations of fluorotelomers by building category. Overall, building category was a significant predictor of each FTOH in air (p<0.01) in univariate regression analyses. Concentrations of 8:2-FTOH and 10:2-FTOH were highest in Building A (the new building), next highest in Building B (partially renovated), and lowest in the Other building category (un-renovated). 6:2-FTOH was significantly higher in Building B compared to the Other building category, but not significantly different between buildings A and B.
Figure 1.
Geometric mean fluorotelomer concentrations in office air by building. Asterisk (*) signifies p < 0.05 for difference from reference group in univariate regression.
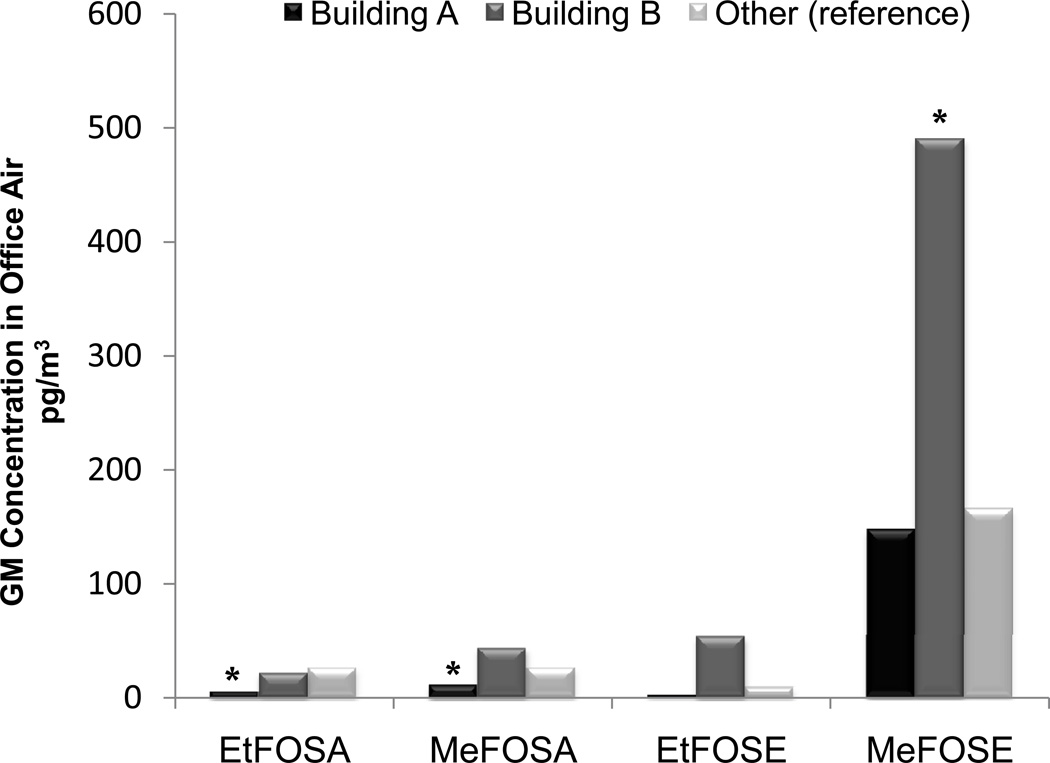
In univariate regression analyses, building category was also a significant predictor (p<0.02) of sulfonamide compounds in air (Figure 2). In contrast to FTOHs, FOSA/FOSE concentrations were lowest in Building A: significantly lower than Building B for every sulfonamide and significantly lower than the Other building category for EtFOSA and MeFOSA. Building B had the highest GM concentrations of MeFOSA, EtFOSE, and MeFOSE, though only concentrations of MeFOSE were significantly higher than the Other building category.
Figure 2.
Geometric mean perfluorosulfonamide concentrations in office air by building. Asterisk (*) signifies p < 0.05 for difference from reference group in univariate regression.
Serum Concentrations
As shown in Table 2, PFOA, PFNA, PFDeA, PFHxS, and PFOS were detected in greater than 90% of serum samples and, thus, were included in further analyses. The GM concentration was highest for PFOS (11 ng/mL), followed by PFOA (3.7 ng/mL), PFNA (1.6 ng/mL), PFHxS (1.5 ng/mL), and PFDeA (0.36 ng/mL). PFUA, N-MeFOSAA, N-EtFOSAA, and PFDoA were detected at 71%, 52%, 6%, and 6%, respectively, and were not further analyzed. PFHxA, PFHpA, and PFOSA had 0% detection. The five major PFCs in serum were significantly correlated with one another (0.46–0.78) except for PFDeA, which was only correlated with PFOS and PFNA (Supplemental Material, Table 3).
Table 2.
Summary statistics of PFCs in serum (ng/mL) of office workers (n=31).
| Analyte | % detect | LOD | GM | (GSD) | Range |
|---|---|---|---|---|---|
| PFHxA | 0 | 0.6 | † | † | <LOD – <LOD |
| PFHpA | 0 | 0.4 | † | † | <LOD – <LOD |
| PFOA | 100 | 0.1 | 3.7 | (1.7) | 1.1 – 8.9 |
| PFNA | 100 | 0.1 | 1.6 | (1.5) | 0.60 – 3.3 |
| PFDeA | 94 | 0.2 | 0.36 | (1.7) | <LOD – 2.5 |
| PFUA | 71 | 0.2 | 0.28 | (2.0) | <LOD – 2.8 |
| PFDoA | 6 | 0.2 | † | † | <LOD – 1.1 |
| PFHxS | 100 | 0.1 | 1.5 | (2.4) | 0.20 – 13 |
| PFOS | 100 | 0.2 | 11 | (1.8) | 2.8 – 67 |
| N-MeFOSAA | 52 | 0.2 | † | † | <LOD – 1.9 |
| N-EtFOSAA | 6 | 0.2 | † | † | <LOD – 0.80 |
| PFOSA | 0 | 0.1 | † | † | <LOD – <LOD |
Values not reported due to low percentage of detection.
Predictors of Serum PFCs
Serum PFCs followed a pattern consistent with that of the FTOHs in air with concentrations being highest in Building A workers, next highest in Building B workers, and lowest in workers of the Other building category (Table 3). Serum PFCs of workers from Buildings A and B were not significantly different from one another in univariate regression (data not shown). However, compared to the Other building category, serum concentrations of Building A workers were significantly higher for PFOA, PFOS, PFNA, and PFDeA (Table 3). Workers in Building B had significantly higher serum concentrations of PFOA and marginally significantly higher serum concentrations of PFNA than workers in Other buildings (Table 3).
Table 3.
Univariate associations between PFCs in serum of office workers and predictor variables.
| Predictor | n | lnPFOAa β (p-value) |
lnPFNAa β (p-value) |
lnPFDeAa β (p-value) |
lnPFHxSa β (p-value) |
lnPFOSa β (p-value) |
|---|---|---|---|---|---|---|
| Sex | ||||||
| Male | 5 | 0.27 (0.30) | 0.15 (0.44) | −0.01 (0.96) | 0.68 (0.12) | 0.31 (0.30) |
| Female | 26 | Reference | Reference | Reference | Reference | Reference |
| Age | ||||||
| Years | 31 | 0.009 (0.19) | 0.007 (0.19) | 0.0003 (0.97) | 0.005 (0.70) | 0.01 (0.23) |
| BMI | ||||||
| Kg/m2 | 31 | −0.002 (0.91) | 0.001 (0.96) | −0.02 (0.34) | −0.05 (0.18) | −0.008 (0.75) |
| Building | ||||||
| Building A | 6 | 0.69 (0.008) | 0.47 (0.02) | 0.65 (0.03) | 0.41 (0.41) | 0.75 (0.02) |
| Building B | 17 | 0.63 (0.003) | 0.31 (0.054) | 0.29 (0.20) | 0.22 (0.58) | 0.32 (0.20) |
| Other | 8 | Reference | Reference | Reference | Reference | Reference |
| Model r2 | 0.31 | 0.19 | 0.16 | 0.03 | 0.18 | |
| Time in office | ||||||
| Hours | 31 | 0.02 (0.02) | 0.005 (0.51) | 0.0008 (0.94) | 0.02 (0.12) | 0.02 (0.08) |
Exponentiation of β = the multiplicative increase in PFC per unit change in predictor variable (e.g., β = 0.69 is a 99% increase, or a doubling) relative to the reference group.
PFOA in serum was also positively associated with the number of hours typically spent per week in the office, which ranged from 18 to 60 hours per week. Each additional hour worked per week was associated with a 2% increase in serum PFOA (p=0.02). In this relatively homogenous population, serum concentrations of PFCs were not found to be associated with sex, age, or BMI. Dietary intake of beef, chicken, eggs, and microwave popcorn were evaluated. Those in the highest category of beef intake (4–6 servings per week) had higher serum concentrations of PFDeA, PFHxS, and PFOS than those reporting less beef intake. However, given the small number of individuals reporting high beef intake (n=3), these results are nearly anecdotal and the data are not shown. Otherwise, no associations were observed between diet and PFC concentrations in serum.
As the FTOHs and the sulfonamides measured in air were highly correlated within groups, assessing their effects in a model together may introduce collinearity. Accordingly, we performed principal components analysis on the seven compounds measured in air, yielding two factors explaining 77% of the variability in PFC air concentrations. The three FTOHs loaded onto one factor (referred to here as “PFCA-precursors”) and the four sulfonamides loaded onto the other factor (referred to here as “PFSA-precursors”). Regressing PFCs in serum on the two factors revealed a strong positive association between serum PFOA and PFCA-precursors in office air (p=0.0005) though the effect estimate (β = 0.31) is not directly interpretable due to log-transformation of FTOHs prior to PCA. Serum PFNA was also positively associated with PFCA-precursors in air, but the association was only marginally significant (p=0.10). PFDeA, PFOS, and PFHxS in serum were not significantly associated with PFCA-precursors (p=0.50, p=0.15, and p=0.35, respectively). PFSA-precursors in office air were not associated with PFC concentrations in serum.
Table 4 presents the results of three multivariate predictive models of PFOA and PFOS in serum, each containing a different measure of office PFC exposure and a dichotomous measure of time spent in the office. Model 1 uses building as a surrogate of exposure to PFCs; adding time spent in office to the univariate model (shown in Table 3) increased the r2 from 0.31 to 0.39 (PFOA) and from 0.18 to 0.28 (PFOS). Model 2 examines a single analyte in air, 8:2-FTOH, combined with time in office. 8:2-FTOH predicted serum PFOA (p=0.02), but not serum PFOS (p=0.18). This model predicts a 1.1% increase in PFOA serum concentration per ng/m3 increase in 8:2-FTOH air levels and a 40% increase in PFOA serum concentration for spending 35–60 hours in the office per week vs. 18–33 hours per week. Omission of time in office from the model yielded the same β. Model 3 uses the PFCA-precursor factor (representing exposure to all three FTOHs measured in office air). This model explained 47% of the variability in PFOA serum concentrations, an increase from both the building model (Model 1) and the single FTOH model (Model 2). However, comparison with the other models is complicated by use of the log transformation before PCA. The PFCA-precursor factor did not increase the model’s ability to predict PFOS compared to Models 1 or 2; only building and time in office were significant predictors of PFOS in serum.
Table 4.
Multivariate predictive models of PFOA and PFOS in serum.
| n | lnPFOAa β (p-value) |
lnPFOSa β (p-value) |
|
|---|---|---|---|
| Model 1: Building | |||
| Building A | 6 | 0.64 (0.01) | 0.69 (0.03) |
| Building B | 17 | 0.60 (0.003) | 0.28 (0.23) |
| Other | 8 | Reference | Reference |
| Time in office | |||
| 35–60 hrs | 18 | 0.29 (0.07) | 0.38 (0.07) |
| 18–33 hrs | 13 | Reference | Reference |
| Model r2 | 0.39 | 0.28 | |
| Model 2: Air analyte | |||
| 8:2-FTOH (pg/m3) | 30 | 11×10−6 (0.02) | 7.4×10−6 (0.18) |
| Time in office | |||
| 35–60 hrs | 18 | 0.34 (0.06) | 0.47 (0.03) |
| 18–33 hrs | 12 | Reference | Reference |
| Model r2 | 0.28 | 0.21 | |
| Model 3: PCA factor | |||
| PFCA-precursorsb | 30 | 0.31 (0.0002) | 0.16 (0.14) |
| Time in office | |||
| 35–60 hrs | 18 | 0.34 (0.03) | 0.48 (0.03) |
| 18–33 hrs | 12 | Reference | Reference |
| Model r2 | 0.47 | 0.22 | |
Exponentiation of β = the multiplicative increase in PFC per unit change in predictor variable (e.g., β = 0.29 is a 34% increase) relative to the reference group.
Air concentrations were log-transformed prior to PCA making interpretation difficult for β-coefficients of the PFCA-precursor factor.
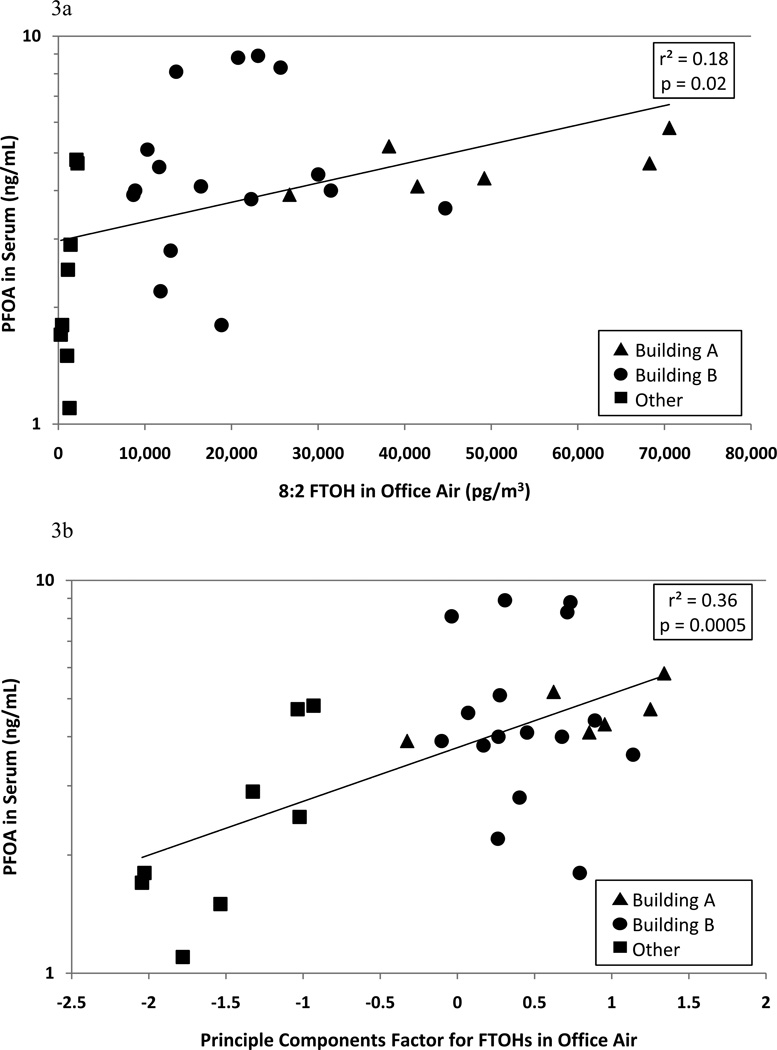
Figure 3 presents scatter plots of serum PFOA versus 8:2-FTOH in office air (r2=0.18; p=0.02) and the PFCA-precursor factor for FTOHs, collectively, in office air (r2=0.36; p=0.0005). Analysis for detection of outliers confirmed that none of the standardized residuals for the 30 sample points exceeded three. Removing the two points with the highest values of 8:2-FTOH in office air (Figure 3a) had no effect on the r2 or p-value.
Figure 3.
Scatter plots of PFOA in serum by 8:2 FTOH in office air (Figure 3a) and by the principal components factor for FTOHs in office air (Figure 3b).
Concentrations of PFCs detected in individual serum and office air samples, by building, are available in Supplemental Material, Table 4.
Discussion
Despite the limitation of a small sample size (30 offices), this is the first systematic examination of air concentrations of PFCs in offices. Significantly different PFC concentrations were observed between buildings, with the highest FTOH air levels measured in the most recently constructed and furnished building, the next highest found in a partially renovated building, and the lowest air levels found in un-renovated buildings. We hypothesize that the differences in PFC air concentrations by building are due to off-gassing of FTOHs from new carpeting, upholstered furniture and/or paint in the renovated buildings. Dinglasan-Panlilio and Mabury22 identified residual unbound FTOHs in a commercially available carpet protector similar to industrial-scale products. 8:2-FTOH was in greatest abundance followed by 10:2-FTOH and then 6:2-FTOH, consistent with the trends we saw in office air. They concluded that the potential exists for a significant proportion of these unbound FTOHs to be released from products. The high air concentrations of FTOHs, particularly 8:2-FTOH, found in Building A in our study may thus be due to the presence of new carpeting and furniture. The low air concentrations of FOSAs/FOSEs observed in this new building may be a consequence of the absence of older carpeting and the withdrawal of sulfonamide products from the market in the early 2000s. The higher air concentrations of MeFOSE in Building B may be explained by the presence of older carpeting in 90% of offices and the fact that MeFOSE was widely used in the past as a stain repellent for carpets.28
Only a few studies have examined concentrations of neutral PFCs in indoor air. Levels of FOSAs/FOSEs measured in office air in this study were much lower than those previously measured in homes in Ottawa29 in 2002–2003 (n=59) and Norway3 in 2005 (n=4), but very similar to those measured more recently in 59 Vancouver homes.30 This difference may be attributable to the withdrawal of sulfonamides from the North American market in the early 2000s. 6:2-FTOH and 10:2-FTOH air levels were somewhat lower in the offices in our study compared to the Norwegian homes and somewhat higher than the Vancouver homes. However, the GM air concentration of 8:2-FTOH measured in our study was 3–5 times higher than those measured in these earlier studies suggesting that offices may represent a unique and important exposure environment.
Serum PFC concentrations among the 31 office workers in this study are consistent with those reported by Calafat and colleagues,4 including the decline in PFOS concentrations observed since the withdrawal from the market of its precursor compounds in the early 2000s. Geometric mean PFOS concentrations in serum of the US general population were 30.4 ng/mL in 1999–2000, 20.7 ng/mL in 2003–2004, and 17.1 ng/mL in 2005–2006.4,27 PFOS concentrations in the serum of workers in our study (collected in 2009) had a GM of 11 ng/mL. Calafat and colleagues4 observed a 100% increase in the GM concentration of PFNA from 0.5 to 1.0 ng/mL between 1999–2000 and 2003–2004. The geometric mean PFNA serum concentration found in our study, 1.6 ng/mL, could support a hypothesis of an increasing temporal trend in US body burdens of PFNA.
To our knowledge, this analysis represents the first PFC exposure assessment to measure both biologic and environmental air samples concurrently. In addition to differences in air concentrations by building, we also observed differences in serum PFCs by building, suggesting a link between the office environment and serum concentrations. We found a strong positive association between FTOH concentrations in office air and PFOA concentrations in serum. We also observed a marginally significant (p=0.10) positive association between FTOHs in office air and PFNA in serum. These results are the first empirical evidence suggesting that exposure to fluorotelomer alcohols in air contribute substantially to the body burden of PFOA and PFNA. The amount of time spent in the office was also an independent predictor of PFOA serum concentrations, providing further evidence that exposures in the office environment contribute to PFC body burden. As would be expected, serum PFOS was not associated with PFCA-precursors in office air. Concentrations of PFOS in serum were also not associated with PFSA-precursors in office air. However, serum PFOS was positively associated with both time spent in office and building, with the highest serum concentrations found in those who worked in the newly constructed Building A. For PFOS, building is likely a surrogate for some other unmeasured exposure—perhaps PFCs in dust.
It is widely recognized that PFOA and PFOS are commonly correlated in human serum, as they were in this study (r = 0.53, p = 0.002), suggesting at least some common exposure pathway. However, because concentrations in serum represent exposure from many sources and over a long period of time, it is difficult to determine the true reason for the correlation in serum of these two compounds. Interestingly, we observed a negative correlation between 8:2-FTOH and EtFOSA in office air (r = −0.36, p = 0.048), precursors of PFOA and PFOS, respectively. This was likely due, in large part, to the withdrawal of sulfonamide compounds from the market in the early 2000s and the continued use of FTOHs since that time. Supporting this theory is the fact that a building-by-building correlation analysis of 8:2-FTOH and EtFOSA revealed that the negative association is driven by data from Building A, the newly constructed office building (r = −0.74, p = 0.09). For Building B and the Other office buildings, the association between the two compounds is positive, weaker, and non-significant (Building B: r = 0.31, p = 0.24; Other: r = 0.53, p = 0.17). We observed an association between 8:2-FTOH in office air and PFOA in serum, suggesting a possible exposure pathway for PFOA body burden. The fact that a similar pathway was not observed for exposure to PFOS could mean that there are other important exposure pathways that PFOA and PFOS share (such as diet or dust) and/or that the correlation of PFOA and PFOS in serum is being strongly influenced by past exposures (when 8:2-FTOH and EtFOSA may possibly have been more correlated in office air).
While our sample size of 31 office workers is fairly small, the study was sufficiently powered to observe statistically significant differences in PFC concentrations by building as well as significant air-serum associations. Dietary factors were evaluated and not found to be significant predictors of PFCs in serum. However, dietary contributions of PFCs are unlikely to confound the observed relationship between FTOHs in office air and PFOA in serum since diet would not be expected to vary by building or be associated with PFCs measured in office air. A more important limitation is the possibility of confounding by exposure to PFOA in air (unmeasured) and to a lower extent, PFCs in office dust. Dust samples were collected from offices in this study and PFC dust results will be presented in a later manuscript. However, preliminary analyses indicate that PFOA in office dust was not correlated with FTOH concentrations in air. Office air concentrations of PFOA would likely be orders of magnitude lower than the much more volatile FTOHs.3,30 Nevertheless, while our results suggest an important contribution of FTOHs in air to PFOA in serum, we cannot rule out contributions by FTOHs in dust or PFOA in dust and air.
While we found a strong association between serum PFOA and PFCA-precursor factor, it is biologically implausible that 6:2-FTOH contributes to serum PFOA. Instead the association is probably being driven by 8:2-FTOH and 10:2-FTOH. 8:2-FTOH metabolizes to PFOA and PFNA in vivo and in vitro using rats and rat hepatocytes; 10:2-FTOH can also be metabolized to PFOA.19 Most participants from Buildings A and B had worked in those offices for little more than a year at the time of sampling. If the four days of air sampling is somewhat representative of the entire one-year exposure period, then air exposure during that period is more likely to be predictive of serum levels of PFOA with a serum half-life of 2.3 years7 than PFHxS with a serum half-life of 7.3 years.8
Some research suggests that exposure to residual FTOHs from consumer products is not likely to be a significant source of PFOA in humans. Vestergren and colleagues18 provide multiple exposure estimates based on a wide range of human behaviors. Notably, in the high-exposure scenario, precursors account for 48–55% of total daily intake for adults and teens, though much of this is attributed to migration of FTOHs from food packaging materials. However, these conclusions were based on exposure models that relied on minimal air data for PFOA-precursors (data from 4 Norwegian homes reported by Barber and colleagues3). The authors noted that particular sub-groups in the population may receive considerably higher doses from precursor compounds. We posit that residents and workers of newly renovated buildings may be one such sub-group. Importantly, Vestergren and colleagues18 stress that their conclusions are limited by lack of knowledge on the occurrence of PFOA precursors in exposure media such as indoor air and food. Our study specifically addresses this data gap and provides evidence that exposure to PFCs via indoor air in the office environment contributes to PFC body burdens.
Though previous studies have focused on PFCs in the home environment and suggest diet to be the dominant exposure pathway for PFOA,15,16 our results suggest that inhalation of indoor air may represent an important exposure pathway, particularly for office workers. Future studies of PFC exposure should aim to concurrently investigate diet and indoor exposure, but we stress the need to consider the impact of indoor air and varied microenvironments, in particular.
Supplementary Material
Acknowledgements
We thank Amal Wanigatunga, Brian Basden, and Tao Jia for technical assistance with analysis of the serum samples; Stephanie Chan, Heather Simpson and Courtney Walker for help with sample collection; and all study participants for enabling this research. This research was supported in part by grants R01ES015829 and T32ES014562 from the National Institute of Environmental Health Sciences (NIEHS). The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention, the NIEHS, or the National Institutes of Health.
References
- 1.Kissa E. Fluorinated Surfactants and Repellents. 2nd ed. New York: Marcel Dekker; 2001. [Google Scholar]
- 2.Prevedouros K, Cousins IT, Buck RC, Korzeniowski SH. Sources, fate and transport of perfluorocarboxylates. Environ Sci Technol. 2006;40:32–44. doi: 10.1021/es0512475. [DOI] [PubMed] [Google Scholar]
- 3.Barber JL, Berger U, Chaemfa C, Huber S, Jahnke A, Temme C, Jones KC. Analysis of per- and polyfluorinated alkyl substances in air samples from Northwest Europe. J Environ. Monit. 2007;9:530–541. doi: 10.1039/b701417a. [DOI] [PubMed] [Google Scholar]
- 4.Calafat AM, Wong L, Kuklenyik Z, Reidy JA, Needham LL. Polyfluoroalkyl chemicals in the U.S. population: data from the National Health and Nutrition Examination Survey (NHANES) 2003–2004 and comparisons with NHANES 1999–2000. Environ. Health Perspect. 2007;115:1596–1602. doi: 10.1289/ehp.10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lau C, Anitole K, Hodes C, Lai D, Pfahles-Hutchens A, Seed J. Perfluoroalkyl acids: a review of monitoring and toxicological findings. Toxicol. Sci. 2007;99:366–394. doi: 10.1093/toxsci/kfm128. [DOI] [PubMed] [Google Scholar]
- 6.Shoeib M, Harner T, Vlahos P. Perfluorinated chemicals in the arctic atmosphere. Environ. Sci. Technol. 2006;40:7577–7583. doi: 10.1021/es0618999. [DOI] [PubMed] [Google Scholar]
- 7.Bartell SM, Calafat AM, Lyu C, Kato K, Ryan PB, Steenland K. Rate of decline in serum PFOA concentrations after granular activated carbon filtration at two public water systems in Ohio and West Virginia. Environ. Health Perspect. 2010;118:222–228. doi: 10.1289/ehp.0901252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olsen GW, Burris JM, Ehresman DJ, Froehlich JW, Seacat AM, Butenhoff JL, Zobel LR. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ. Health Perspect. 2007;115:1298–1305. doi: 10.1289/ehp.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeWitt JC, Shnyra A, Badr MZ, Loveless SE, Hoban D, Frame SR, Cunard R, Anderson SE, Meade BJ, Peden-Adams MM, Luebke RW, Luster MI. Immunotoxicity of perfluorooctanoic acid and perfluorooctane sulfonate and the role of peroxisome proliferator-activated receptor alpha. Crit. Rev. Toxicol. 2009;39:76–94. doi: 10.1080/10408440802209804. [DOI] [PubMed] [Google Scholar]
- 10.Jensen AA, Leffers H. Emerging endocrine disrupters: perfluoroalkylated substances. Int. J. Androl. 2008;31:161–169. doi: 10.1111/j.1365-2605.2008.00870.x. [DOI] [PubMed] [Google Scholar]
- 11.Apelberg BJ, Witter FR, Herbstman JB, Calafat AM, Halden RU, Needham LL, Goldman LR. Cord serum concentrations of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in relation to weight and size at birth. Environ. Health Perspect. 2007;115:1670–1676. doi: 10.1289/ehp.10334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fei C, McLaughlin JK, Tarone RE, Olsen J. Perfluorinated chemicals and fetal growth: a study within the Danish National Birth Cohort. Environ. Health Perspect. 2007;115:1677–1682. doi: 10.1289/ehp.10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nelson JW, Hatch EE, Webster TF. Exposure to polyfluoroalkyl chemicals and cholesterol, body weight, and insulin resistance in the general U.S. population. Environ. Health Perspect. 2010;118:197–202. doi: 10.1289/ehp.0901165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steenland K, Tinker S, Frisbee S, Ducatman A, Vaccarino V. Association of perfluorooctanoic acid and perfluorooctane sulfonate with serum lipids among adults living near a chemical plant. Am. J. Epidemiol. 2009;170:1268–1278. doi: 10.1093/aje/kwp279. [DOI] [PubMed] [Google Scholar]
- 15.Trudel D, Horowitz L, Wormuth M, Scheringer M, Cousins IT, Hungerbühler K. Estimating consumer exposure to PFOS and PFOA. Risk Anal. 2008;28:251–269. doi: 10.1111/j.1539-6924.2008.01017.x. [DOI] [PubMed] [Google Scholar]
- 16.Washburn ST, Bingman TS, Braithwaite SK, Buck RC, Buxton LW, Clewell HJ, Haroun LA, Kester JE, Rickard RW, Shipp AM. Exposure assessment and risk characterization for perfluorooctanoate in selected consumer articles. Environ. Sci. Technol. 2005;39:3904–3910. doi: 10.1021/es048353b. [DOI] [PubMed] [Google Scholar]
- 17.De Silva AO, Mabury SA. Isomer distribution of perfluorocarboxylates in human blood: potential correlation to source. Environ. Sci. Technol. 2006;40:2903–2909. doi: 10.1021/es0600330. [DOI] [PubMed] [Google Scholar]
- 18.Vestergren R, Cousins IT, Trudel D, Wormuth M, Scheringer M. Estimating the contribution of precursor compounds in consumer exposure to PFOS and PFOA. Chemosphere. 2008;73:1617–1624. doi: 10.1016/j.chemosphere.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 19.Dinglasan MJA, Ye Y, Edwards EA, Mabury SA. Fluorotelomer alcohol biodegradation yields poly- and perfluorinated acids. Environ. Sci. Technol. 2004;38:2857–2864. doi: 10.1021/es0350177. [DOI] [PubMed] [Google Scholar]
- 20.Martin JW, Mabury SA, O’Brien PJ. Metabolic products and pathways of fluorotelomer alcohols in isolated rat hepatocytes. Chem. Biol. Interact. 2005;155:165–180. doi: 10.1016/j.cbi.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 21.Martin JW, Chan K, Mabury SA, O’Brien PJ. Bioactivation of fluorotelomer alcohols in isolated rat hepatocytes. Chem. Biol. Interact. 2009;177:196–203. doi: 10.1016/j.cbi.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 22.Nabb DL, Szostek B, Himmelstein MW, Mawn MP, Gargas ML, Sweeney LM, Stadler JC, Buck RC, Fasano WJ. In vitro metabolism of 8-2 fluorotelomer alcohol: interspecies comparisons and metabolic pathway refinement. Toxicol. Sci. 2007;100:333–344. doi: 10.1093/toxsci/kfm230. [DOI] [PubMed] [Google Scholar]
- 23.Martin JW, Asher BJ, Beesoon S, Benskin JP, Ross MS. PFOS or PreFOS? Are perfluorooctane sulfonate precursors (PreFOS) important determinants of human and environmental perfluorooctane sulfonate (PFOS) exposure? J Environ. Monit. 2010;12:1979. doi: 10.1039/c0em00295j. [DOI] [PubMed] [Google Scholar]
- 24.Tomy GT, Tittlemier SA, Palace VP, Budakowski WR, Braekevelt E, Brinkworth L, Friesen K. Biotransformation of N-ethyl perfluorooctanesulfonamide by rainbow trout (Onchorhynchus mykiss) liver microsomes. Environ. Sci. Technol. 2004;38:758–762. doi: 10.1021/es034550j. [DOI] [PubMed] [Google Scholar]
- 25.Dinglasan-Panlilio MJA, Mabury SA. Significant residual fluorinated alcohols present in various fluorinated materials. Environ. Sci. Technol. 2006;40:1447–1453. doi: 10.1021/es051619+. [DOI] [PubMed] [Google Scholar]
- 26.Kuklenyik Z, Needham LL, Calafat AM. Measurement of 18 perfluorinated organic acids and amides in human serum using on-line solid-phase extraction. Anal. Chem. 2005;77:6085–6091. doi: 10.1021/ac050671l. [DOI] [PubMed] [Google Scholar]
- 27.CDC. Fourth national report on human exposure to environmental chemicals. Atlanta, GA: National Center for Environmental Health, Centers for Disease Control and Prevention; 2009. http://www.cdc.gov/exposurereport/ [Google Scholar]
- 28.Olsen GW, Church TR, Miller JP, Burris JM, Hansen KJ, Lundberg JK, Armitage JB, Herron RM, Medhdizadehkashi Z, Nobiletti JB, O'Neill EM, Mandel JH, Zobel LR. Perfluorooctanesulfonate and other fluorochemicals in the serum of American Red Cross adult blood donors. Environ. Health Perspect. 2003;111:1892–1901. doi: 10.1289/ehp.6316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shoeib M, Harner T, Wilford BH, Jones KC, Zhu J. Perfluorinated sulfonamides in indoor and outdoor air and indoor dust: occurrence, partitioning, and human exposure. Environ. Sci. Technol. 2005;39:6599–6606. doi: 10.1021/es048340y. [DOI] [PubMed] [Google Scholar]
- 30.Shoeib M, Webster G, Lee SC, Harner T. Indoor air and dust concentrations of neutral and ionic perfluoroalkyl compounds (PFCs) in Vancouver, Canada. Organohalogen Compd. 2009;71:934–939. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.