Abstract
Dendritic cells (DCs) bridge innate and adaptive immunity, but how DC-derived signals regulate T cell lineage choices remains unclear. We report that p38α MAP kinase programs DCs to drive TH17 differentiation. Deletion of p38α in DCs, but not macrophages or T cells, protects mice from TH17-mediated autoimmune neuroinflammation. p38α orchestrates expression of cytokines and co-stimulatory molecules in DCs, and further imprints T cell IL-23R signaling to promote TH17 differentiation. Moreover, p38α is required for tissue-infiltrating DCs to sustain TH17 responses. This activity of p38α is conserved between mouse and human DCs, and is dynamically regulated by pattern recognition and fungal infection. Our results identify p38α as a central pathway to integrate instructive signals in DCs for TH17 differentiation and inflammation.
Keywords: TH17 cell, dendritic cell, MAPK, autoimmune disease, fungal infection
INTRODUCTION
Dysregulation of T cell responses is the cause of autoimmune disorders. TH17 cells, a recently identified lineage of CD4+ effector T cells, play a key role in the pathogenesis of many autoimmune conditions, including multiple sclerosis (MS), an inflammatory demyelinating disease of the central nervous system (CNS), and its murine model experimental autoimmune encephalomyelitis (EAE). While the involvement of T cell-intrinsic pathways in TH17 differentiation has been described abundantly1, how TH17 development is triggered by extrinsic pathways and physiological and pathological stimuli remains poorly understood.
Dendritic cells (DCs) are the most important antigen-presenting cells (APCs) to bridge innate and adaptive immunity by triggering activation and differentiation of naïve T cells2. DCs express a repertoire of pattern recognition receptors (PRRs) that sense microbial pathogen products and endogenous ligands to initiate a signaling cascade culminating in the activation of DCs and induction of adaptive immunity. Toll-like receptors (TLRs) and downstream Myd88 signaling typically induce IFN-γ-producing TH1 cells2. A distinct group of PRRs, the C-type lectin-like receptors (CLRs), are coupled to Syk-CARD9 signaling and preferentially polarize a TH17 response3, 4. While CLR-mediated TH17 responses are important for the protective anti-fungal immunity, little evidence exists for the involvement of CLR signaling in autoimmune diseases. Consequently, how the diverse innate immune signals are sensed and transduced to shape TH17 responses and autoimmune pathogenesis remains to be established. Moreover, DCs are recruited into CNS in EAE to reactivate primed myelin-reactive T cells, thereby propagating local inflammation and disease development during EAE5, 6. The function mediated by CNS DCs is associated with their ability to mediate a TH17-polarized response7, but how this process is regulated is poorly defined.
Among the central pathways activated by innate signals are the MAP kinases (MAPKs). MAPKs, comprised of Erk, Jnk and p38, mediate an evolutionarily conserved mechanism for cellular responses to extracellular signals8. Excessive activation of MAPKs is associated with autoimmune diseases, and inhibitors of these pathways have been evaluated as new therapeutic strategies9. Despite the rapid and reversible actions, the nonspecific effects inherent in drug inhibitors make these approaches unlikely to provide definitive mechanistic insights10. Instead, genetic dissection has been highly instrumental in our understanding of the specific roles of MAPKs in the immune system. Recent work has identified functions of p38α, the most widely expressed isoform among p38 family members, in T cell intrinsic responses11, 12, including an involvement in TH17 differentiation13–17. Tissue-specific deletion in macrophages and epithelial cells also demonstrates that p38α regulates innate responses and acute inflammation18, 19. However, it remains unknown whether p38α regulates the crosstalk between innate and adaptive immunity, and whether this function is important for the pathogenesis of autoimmune disorders.
Here we report that p38α acts in DCs to drive TH17 differentiation and inflammation. p38α in DCs mediates reciprocal regulation of interleukin 6 (IL-6) and IL-27, arguably the most potent positive and negative regulators of TH17 polarization, respectively1, 20, 21, and further imprints IL-23 receptor (IL-23R) signaling in responding T cells. Not only is p38α signaling in DCs essential for the induction of TH17 differentiation, it is also required for CNS-infiltrating DCs to maintain the TH17 population at sites of inflammation. Furthermore, in contrast to previous conclusions obtained from pharmacological and dominant negative approaches13–17, we exclude a significant involvement of T cell-intrinsic p38 signaling in TH17 differentiation. Altogether, our studies identify a key mechanism of DC-mediated programming of TH17 differentiation.
RESULTS
Deletion of p38α in DCs protects mice from EAE
Although p38α is a major drug target for inflammatory disorders, the specific role for p38α in autoimmune diseases remains poorly understood. In mice that developed EAE following immunization with myelin oligodendrocyte glycoprotein peptide (MOG35–55), activity of p38 was markedly elevated in myeloid cells infiltrating the spinal cord, as compared with CNS resident cells (Fig. 1a). To determine the role of p38 in EAE, we crossed mice bearing the floxed p38α allele with Rosa26-Cre-ERT2 mice (in which a Cre-ER fusion gene was recombined into the ubiquitously expressed Rosa26 locus) to generate p38αfl/flRosa26-Cre-ERT2 mice (called “p38αCreER mice” thereafter). After tamoxifen-mediated deletion of the p38α gene, we immunized mice for the induction of EAE. As compared with wild-type controls (including Cre+ mice) that developed a severe disease, p38αCreER mice were completely resistant to EAE (Fig. 1b).
Figure 1. Deletion of p38α in DCs protects mice from EAE.
(a) p38 activity in infiltrating myeloid (CD11b+CD45hi) and CNS resident cells (CD11b+CD45lo) in the spinal cord of EAE mice (day 16). (b–e) EAE disease course in tamoxifen-treated wild-type (WT) and p38αCreER mice (b), wild-type and p38αΔT mice (c), wild-type and p38αΔMac mice (d), and wild-type and p38αΔDC mice (e). (f–h) Histopathology (f), histological scores (g) and flow cytometry (h) of spinal cord of wild-type or p38αΔDC EAE mice (day 16). Images are 10× (H&E, α-Iba1 and α-GFAP) and 20× (Luxol fast blue and α-CD3) original magnification (f). Each symbol represents an individual mouse and small horizontal lines indicate the mean (h). *P < 0.05; **P < 0.01; ***P < 0.001 (Student’s t-test). Data are representative of 2 (a,h, n=4 mice per group; d,f,g, n≥5 mice per group), 3 (b,c, n≥5 mice per group) and 4 (e, n≥5 mice per group) independent experiments. Error bars indicate SEM.
Development of EAE is dependent upon multiple components of the immune system. Aside from T cells, macrophages and DCs contribute to the pathogenesis by functioning as APCs as well as effector cells to cause CNS lesion. In these three cell types, p38α was expressed much higher than the other p38 isoforms (Supplementary Fig. 1a–c). To dissect the selective roles of p38α in the different immune compartments, we deleted p38α in T cells, macrophages and monocytes, and DCs by crossing p38αfl/fl mice with CD4-Cre, LysM-Cre, and CD11c-Cre mice (called p38αΔT, p38αΔMac and p38αΔDC mice, respectively). Efficient deletion of p38α was observed in these immune compartments (Supplementary Fig. 1d–g). In particular, deletion of p38α abrogated the expression and phosphorylation of p38 in DCs (Supplementary Fig. 1h), indicating that the total p38 activity in DCs is predominantly ascribed to p38α. No obvious defects in the development or homeostasis of the immune system were noticed in these mice (Supplementary Fig. 2 and data not shown). Following MOG immunization, p38αΔT and p38αΔMac mice showed comparable disease development as wild-type mice (Fig. 1c,d). In contrast, p38αΔDC mice exhibited marked reduction of disease progression (Fig. 1e). Histological analysis revealed significantly decreased inflammation and demyelination (as revealed by H&E and Luxol fast blue staining, respectively), associated with fewer numbers of CD3+ T cells, Iba1+ macrophages and microglia and GFAP+ astrocytes in the CNS of p38αΔDC mice (Fig. 1f,g). Flow cytometry analysis confirmed the reduced infiltration of CD4+ T cells and CD11b+ myeloid cells into the spinal cord of p38αΔDC mice (Fig. 1h). Thus, p38α activity in DCs is required to precipitate the autoimmune CNS disease.
p38α is required in DCs for the generation of TH17 cells in vivo
While EAE was classically considered as a TH1-dependent disease, recent studies have identified a key role for TH17 cells in disease development. CNS infiltrating T cells from p38αΔDC mice had decreased Il17a, Il17f and Il23r mRNA expression, but normal levels of Ifng, Foxp3 and Il4, at the peak of disease (Fig. 2a). Intracellular staining revealed lower frequency of IL-17+ CD4+ T cells in CNS from p38αΔDC mice, whereas the percentages of IFN-γ+ and Foxp3+ CD4+ T cells were comparable between the two groups of mice (Fig. 2b).
Figure 2. p38α deficiency in DCs impairs the generation of TH17 cells in vivo.
(a) RNA analysis of spinal cord cells of wild-type (WT) or p38αΔDC EAE mice (day 16). (b) Flow cytometry (left) and proportions (right) of IL-17+, IFN-γ+ (after PMA and ionomycin stimulation) and Foxp3+ cells in CD4+ T cells from spinal cord of wild-type or p38αΔDC EAE mice (day 16). (c) Cytokine secretion by draining LN cells from MOG-immunized wild-type or p38αΔDC mice (day 7) after ex vivo antigen stimulation for 3 days. (d) Expression of IFN-γ and IL-17 in CFSElo populations of draining LN CD4+ T cells isolated from (c), followed by CFSE labeling and then MOG stimulation for 4 days. (e) Analysis of Il17a and Ifng mRNA in draining LN cells from KLH-immunized mice after ex vivo stimulation with KLH for 48 h. (f) Proportions of IL-17+ and IFN-γ+ cells among CD4+ T cells from wild-type or p38αΔDC mouse GALTs. MLN, mesenteric lymph nodes; PP, Peyer’s patches; SI-LP, small intestine lamina propia. Each symbol represents an individual mouse and small horizontal lines indicate the mean (b,f). NS, not significant; *P < 0.05; **P < 0.01; ***P < 0.001 (Student’s t-test). Data are representative of 3 independent experiments (a–e, n≥4 mice per group; f, n=3 mice per group). Error bars indicate SEM.
To test whether the diminished TH17 generation was secondary to attenuated CNS inflammation, we analyzed T cell responses in the draining lymph nodes (LNs) at the preclinical stage of EAE (day 7 post-immunization). T cells from p38αΔDC mice expanded normally after ex vivo MOG stimulation (data not shown) but were deficient in IL-17 secretion (Fig. 2c). Also, there was a reduced frequency of IL-17+ cells among the CD4+ T cells that had proliferated in response to MOG stimulation (CFSElo cells) in immunized p38αΔDC mice in comparison to wild-type mice (Fig. 2d). These results identify a key role for p38α in DCs to promote MOG-induced TH17 responses in vivo.
To test if the requirement for DC-derived p38α signals is restricted to MOG-induced responses, we immunized wild-type and p38αΔDC mice with the protein antigen keyhole limpet hemocyanin (KLH). Draining LN cells from p38αΔDC mice had no defects in proliferation (data not shown) but showed decreased IL-17 expression in the recall response (Fig. 2e). We also examined the IL-17+ CD4+ T cells in several gut-associated lymphoid tissues (GALTs) that are known to contain a sizable population of TH17 cells in response to commensal microbiota22. CD4+ T cells from p38αΔDC mice contained a significantly decreased percentage of IL-17+ cells but normal percentage of IFN-γ+ cells (Fig. 2f). We conclude that p38α signaling in DCs is crucial to promote development of TH17 responses in vivo.
p38α in DCs impacts IL-23R expression in responding T cells
These results prompted us to examine whether p38α mediates the DC–T cell crosstalk by driving lineage differentiation of antigen-specific naïve precursors. To test this, we transferred naïve T cells (CD62LhiCD44lo; Thy1.1+) from 2D2 TCR-transgenic mice (specific for MOG35–55)23 into wild-type and p38αΔDC mice, followed by immunization with the cognate antigen emulsified in complete Freund’s adjuvant (CFA). Donor T cells isolated from p38αΔDC hosts contained fewer IL-17+ cells and expressed less Il17a mRNA in the recall response, whereas IFN-γ expression was unaltered (Fig. 3a,b). We further used T cells from a second TCR-transgenic model – the OT-II TCR-transgenic mice (specific for OVA323–339). Donor T cells development into TH17 cells was decreased in p38αΔDC hosts relative to wild-type hosts in response to OVA immunization in the presence of CFA (Fig. 3c) or incomplete Freund’s adjuvant (IFA) (Supplementary Fig. 3). Therefore, deletion of p38α in DCs impairs differentiation of antigen-specific TH17 cells in vivo.
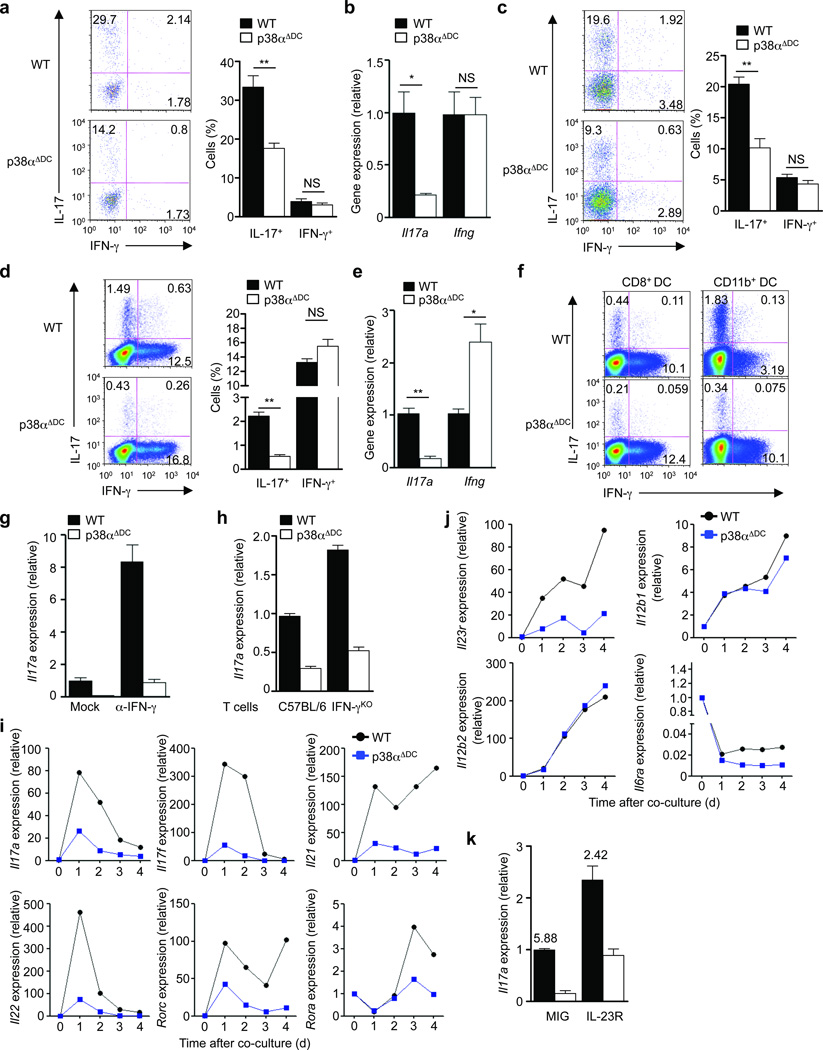
Figure 3. p38α signaling in DCs drives TH17 differentiation in vivo and in vitro and imprints IL-23R expression in responding T cells.
(a) Expression of IL-17 and IFN-γ in the MOG TCR-transgenic donor population (Thy1.1+) of draining LN cells from wild-type (WT) or p38αΔDC mice immunized with MOG + CFA. Right, the proportions of IL-17+ and IFN-γ+ populations among donor CD4+ T cells. (b) Analysis of Il17a and Ifng mRNA in draining LN cells isolated from (a) after stimulation with MOG for 48 h. (c) Expression of IL-17 and IFN-γ in the OT-II donor population (Thy1.1+) of draining LN cells from mice immunized with OVA + CFA. Right, the proportions of IL-17+ and IFN-γ+ populations among donor CD4+ T cells. (d) Expression of IL-17 and IFN-γ in OT-II T cells activated with LPS-pulsed wild-type or p38αΔDC DCs for 5 days, followed by PMA and ionomycin stimulation. Right, the proportions of IL-17+ and IFN-γ+ populations. (e) Analysis of Il17a and Ifng mRNA in T cells from (d) after brief α-CD3 stimulation. (f) Expression of IL-17 and IFN-γ in OT-II T cells activated with antigen and LPS-pulsed CD8+ DCs or CD11b+ DCs for 5 days. (g) Analysis of Il17a mRNA in T cells activated with LPS-pulsed wild-type or p38αΔDC DCs in the presence or absence of α-IFN-γ for 5 days. (h) Analysis of Il17a mRNA in T cells from C57BL/6 or IFN-αKO mice stimulated with α-CD3 and LPS-pulsed wild-type or p38αΔDC DCs for 5 days. (i,j) RNA analysis of T cells activated with wild-type or p38αΔDC DCs for various times for expression of cytokines, transcription factors (i) and cytokine receptors (j). (k) Analysis of Il17a mRNA in T cells activated with wild-type or p38αΔDC DCs and transduced with control (MIG) or IL-23R expressing retrovirus. The numbers above the bars indicate the ratios of Il17a mRNA expression between wild-type and p38αΔDC DC-stimulated T cells. NS, not significant; *P < 0.05; **P < 0.01 (Student’s t-test). Data are representative of 2 (a–c, n≥5 mice per group; f–j, n=4 mice per group), 3 (k, n=4 mice per group) and 4 (d–e, n=4 mice per group) independent experiments. Error bars indicate SEM.
We next co-cultured naïve OT-II T cells with splenic DCs in the presence of antigen and LPS, but without any exogenous cytokines, to model the physiological interaction between DCs and T cells. T cells stimulated with p38αΔDC DCs contained a significantly lower frequency of IL-17+ cells (Fig. 3d). This was associated with reduced Il17a mRNA expression (Fig. 3e) and impaired upregulation of the chemokine receptor CCR6, a selective surface marker for TH17 cells, but not of the TH1-specific receptor CXCR3 (Supplementary Fig. 4a). Similar defect was observed when a synthetic ligand for TLR2 was used instead of LPS (Supplementary Fig. 4b,c). Importantly, diminished TH17 differentiation was observed in different DC subsets lacking p38α, with the CD11b+ DC subset exhibiting a greater defect (Fig. 3f). We noticed that IFN-γ expression in OVA-specific T cells was modestly upregulated by incubation with p38αΔDC DCs in vitro (Fig. 3d–f). To test whether defective TH17 differentiation was due to IFN-γ upregulation, we added a neutralizing anti-IFN-γ antibody. This resulted in an expected overall increase of IL-17 expression, but T cells stimulated with p38αΔDC DCs remained deficient in TH17 differentiation (Fig. 3g). Moreover, lower IL-17 production was also observed in IFN-γ-deficient T cells activated with p38αΔDC DCs (Fig. 3h), thereby excluding a contribution from IFN-γ production to the p38α-dependent TH17 differentiation. Altogether, p38α has a direct role in mediating DC–T cell crosstalk for TH17 differentiation.
We next determined the mechanisms by which p38α induces TH17 differentiation following DC-T cell interaction. T cell proliferation and IL-7Rα expression (which is important for TH17 expansion24) were undisturbed when cultured with p38αΔDC DCs (data not shown). Expression of TH17-associated factors was examined by real-time PCR analysis of DC-activated T cells. T cells cultured with p38αΔDC DCs showed substantial reduction of the TH17 family cytokines including Il17a, Il17f, Il21 and Il22, and the transcription factors Rorc (encoding RORγt) and Rora during the differentiation process (Fig. 3i). Development of TH17 cells requires IL-23R, which is induced by TH17-polarizing cytokines and confers enhanced responsiveness to IL-23 to facilitate TH17 terminal differentiation and expansion25–27. T cells activated with p38αΔDC DCs were impaired to induce Il23r expression, whereas expression of other receptor chains including Il12rb1, Il12rb2 and Il6ra was unaltered (Fig. 3j). To examine the role of IL-23R in mediating DC-dependent TH17 response, we first used an IL-23R blocking antibody and found that it diminished IL-17 expression in a dose-dependent manner in T cells stimulated with wild-type DCs (Supplementary Fig. 5). To further determine whether decreased induction of IL-23R contributed to the TH17 phenotype mediated by p38αΔDC DCs, we introduced IL-23R into T cells by retroviral transduction. Overexpression of IL-23R increased Il17a expression in T cells activated with both wild-type and p38αΔDC DCs when compared with control virus-transduced cells, however the difference between wild-type and p38αΔDC DCs was only partially rescued (Fig. 3k). These results indicate that induction of IL-23R expression in responding T cells may represent one mechanism by which p38α mediates the DC–T cell crosstalk, although additional mechanisms are likely to play a role.
p38α affects IL-6, IL-27 and CD86 expression in DCs
We explored the cellular mechanisms by which p38α acts in DCs to regulate TH17 differentiation. To determine whether p38α regulates DC cytokine expression, we isolated DCs from mice at day 7 after immunization. Among the cytokines known to potentiate TH17 differentiation, Il6 was selectively decreased in splenic DCs from p38αΔDC mice after MOG immunization (Fig. 4a). This selective reduction of IL-6 was also observed in DCs after in vivo and in vitro stimulation with LPS (Fig. 4b,c). Accordingly, phosphorylation of STAT3 but not STAT4 was reduced in T cells activated with p38αΔDC DCs (Fig. 4d). Therefore, p38α activates the IL-6-STAT3 axis at the DC–T cell interface.
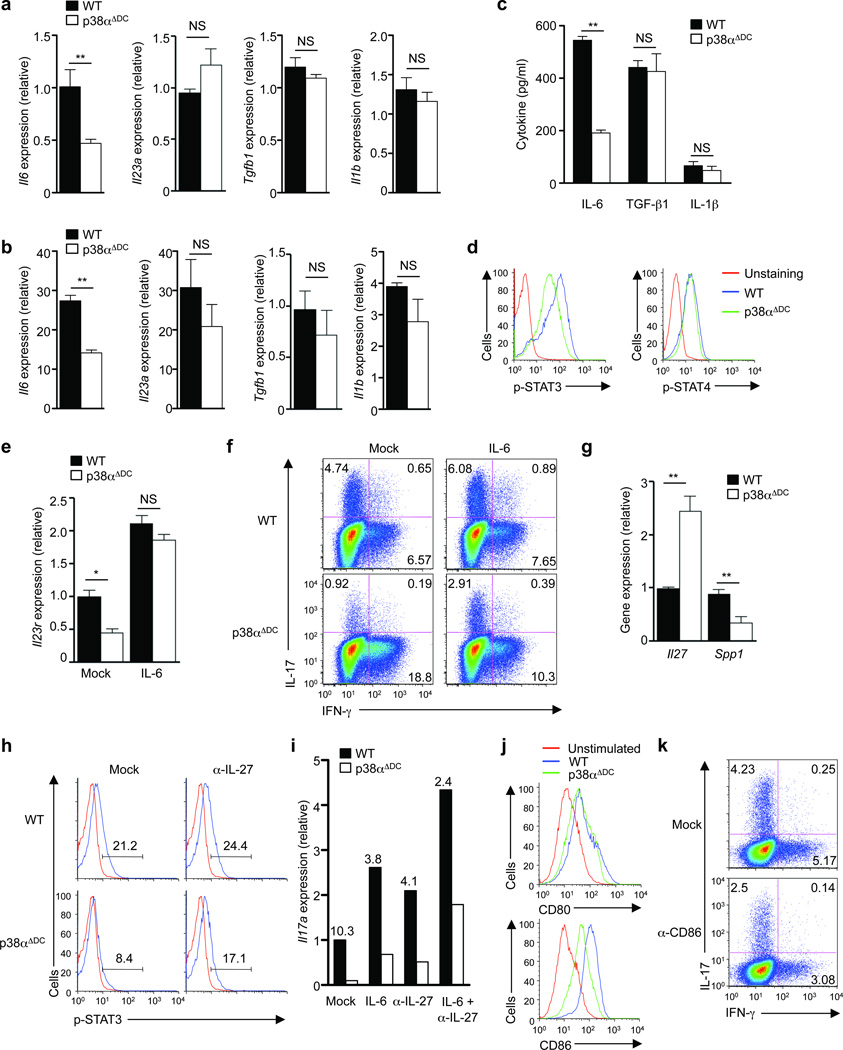
Figure 4. p38α signaling in DCs directs TH17 differentiation by regulating expression of IL-6, IL-27 and CD86.
(a) RNA analysis of wild-type (WT) or p38αΔDC splenic DCs isolated from MOG-immunized mice (day 7). (b) RNA analysis of wild-type or p38αΔDC DCs after in vivo stimulation with LPS for 5 h. The baseline level in unstimulated wild-type DCs was set as 1. (c) Cytokine production from wild-type or p38αΔDC DCs after in vitro stimulation with LPS for 24 h. (d) Levels of p-STAT3 and p-STAT4 in T cells activated with antigen and wild-type or p38αΔDC DCs for 2 days in vitro. (e) Analysis of Il23r mRNA in T cells activated with wild-type or p38αΔDC DCs in the presence or absence of IL-6. (f) Expression of IL-17 and IFN-γ in T cells stimulated with wild-type or p38αΔDC DCs in the presence or absence of IL-6 for 5 days. (g) Analysis of Il27 and Spp1 mRNA in wild-type or p38αΔDC splenic DCs from MOG-immunized mice (day 7). (h) Levels of p-STAT3 in T cells activated with wild-type or p38αΔDC DCs in the presence or absence of α-IL-27 antibodies for 2 days. Red line, isotype control. (i) Analysis of Il17a mRNA in T cells stimulated with wild-type or p38αΔDC DCs in the presence or absence of IL-6, α-IL-27 or IL-6 + α-IL-27 for 5 days. The numbers above the bars indicate the ratios of Il17a mRNA expression between wild-type and p38αΔDC DC-stimulated T cells. (j) Expression of CD80 and CD86 on wild-type or p38αΔDC splenic DCs after in vivo LPS stimulation for 5 h. (k) Expression of IL-17 and IFN-γ in T cells activated with LPS-pulsed wild-type DCs in the presence or absence of α-CD86 for 5 days. NS, not significant; *P < 0.05; **P < 0.01 (Student’s t-test). Data are representative of 2 (a,g, n≥5 mice per group; j, n=4 mice per group), 3 (f,h,i,k, n=4 mice per group) and 4 (b,c,d,e, n=4 mice per group) independent experiments. Error bars indicate SEM.
We tested the functional significance of p38α-dependent IL-6 expression. IL-6-deficient DCs, similar to p38α-deficient DCs, showed impaired ability to drive Il17a and Il23r expression in T cells (Supplementary Fig. 6a). Addition of IL-6 to p38αΔDC DCs nearly completely restored the defective Il23r expression in T cells (Fig. 4e), and increased the frequency IL-17+ cells in co-cultured T cells, although the rescue of IL-17 expression was not complete (Fig. 4f). When compared with IL-6-deficient DCs, DCs deficient in both IL-6 and p38α exhibited modestly reduced ability to induce Il17a expression (Supplementary Fig. 6b). Therefore, p38α-dependent IL-6 expression in DCs mediates TH17 generation, although additional mechanisms seem to contribute as well.
TH17 differentiation is shaped by both positive and negative polarizing cytokines. IL-27 is a potent cytokine to limit TH17 differentiation20, 21, whose expression can be repressed by osteopontin (encoded by Spp1) in DCs28, 29. Compared to wild-type cells, Il27 expression was increased and Spp1 was decreased in p38α-deficient DCs following MOG immunization (Fig. 4g). Although IL-27 can activate multiple STAT proteins, blocking IL-27 substantially restored the defective STAT3 activation in T cells induced by p38αΔDC DCs (Fig. 4h), consistent with the contrasting effects of IL-27 and IL-6 on TH17 development20, 21. Further, whereas exogenous IL-6 and blockade of IL-27 each had a modest effect to promote Il17a expression mediated by p38αΔDC DCs, a combination of both considerably restored TH17 differentiation (Fig. 4i). In addition to affecting cytokine production, p38α was required for LPS-induced expression of the DC co-stimulatory molecule CD86, but not CD80 (Fig. 4j). Blocking CD86 function in wild-type DCs diminished TH17 differentiation but did not significantly affect IFN-γ production (Fig. 4k and Supplementary Fig. 7), which was in agreement with a role for CD86 in TH17 responses30, 31. Collectively, these data illustrate that p38α exerts opposing effects on the positive and negative regulators of TH17 generation and further shapes the strength of the co-stimulatory signals, thereby orchestrating a program for DC-dependent TH17 differentiation.
DCs integrate TH17-instructive signals through p38α
We next investigated the nature of the upstream signals that induced p38α activation in DCs to instruct TH17 differentiation. Among PRRs, TLRs and CLRs mediate strong TH1 and TH17 responses, respectively2, 3, 32. Curdlan (an agonist for Dectin-1, a CLR that recognizes β-glucans) had a stronger in vivo adjuvant activity for the TH17 response (Fig. 5a) and induced greater upregulation of p38 activity in DCs (Fig. 5b) when compared with LPS. Deficiency of p38α in DCs diminished TH17 differentiation mediated by both LPS and curdlan, with a stronger impact on curdlan-induced differentiation (Fig. 5a). Following curdlan stimulation in vivo, antigen-specific donor T cells from p38αΔDC hosts showed reduced expression of Il17a, Il17f, Il21, Il22, Rorc and Il23r (Fig. 5c), indicating that p38α mediates CLR-induced TH17 differentiation signals in DCs.
Figure 5. p38α signaling in DCs integrates TH17-instructive signals derived from PRRs and infectious agents.
(a) Expression of IL-17 and IFN-γ in the OT-II donor population (Thy1.1+) of draining LN cells from wild-type (WT) or p38αΔDC mice immunized with OVA + LPS or curdlan. Right, the proportions of IL-17+ populations among donor CD4+ T cells. (b) Activation of p38 in splenic DCs stimulated with LPS or curdlan. Numbers below p-p38 lanes indicate band intensity relative to that of β-actin (loading control). (c) mRNA analysis of donor T cells isolated from draining LN cells of OVA + curdlan immunized mice as in (a). (d) Expression of IL-17 and IFN-γ in the donor population (Thy1.1+) of draining LN cells from mice immunized with antigen plus 2 × 107/mouse heat-killed S. cerevisiae (HKSC), C. albicans (HKCA), L. monocytogenes (HKLM) or L. pneumophila (HKLP). Right, the proportions of IL-17+ and IFN-γ+ populations among donor CD4+ T cells. (e) Activation of p38 in splenic DCs stimulated with 107/ml HKSC, HKCA, HKLM or HKLP. Numbers below p-p38 lanes indicate band intensity relative to that of p38. (f) Cytokine secretion from splenocytes isolated from live C. albicans-infected mice, followed by stimulation with HKCA for 48 h. Each symbol represents an individual mouse and small horizontal lines indicate the mean. NS, not significant; *P < 0.05 (Student’s t-test). Data are representative of 2 (a,c,d,f, n≥5 mice per group) and 3 (b,e, n=5 mice per group) independent experiments. Error bars indicate SEM.
We further compared the effects of different pathogens on p38 activation in DCs and TH17 differentiation. Prototypic bacteria such as Gram-positive Listeria monocytogenes and Gram-negative Legionella pneumophila are known to induce strong TH1-polarized responses, whereas fungi (including Candida albicans and Saccharomyces cerevisiae) appear to be the most potent pathogens characterized to elicit TH17 responses33. When the heat-killed bacteria and fungi (to avoid the variance due to differential pathogen clearance) were used as adjuvants to stimulate antigen-specific T cells in vivo, C. albicans and S. cerevisiae generated a much greater TH17 response than L. monocytogenes and L. pneumophila (Fig. 5d). This was associated with the ability of C. albicans and S. cerevisiae to strongly activate p38 in DCs (Fig. 5e). Notably, splenocytes from p38αΔDC mice produced lower amounts of IL-17 after infection with C. albicans, while IFN-γ expression was normal (Fig. 5f). Thus, fungal stimulation represents a robust activator of p38 in DCs for the induction of TH17 responses.
In agreement with reports suggesting that activation of CD40 by T cell-derived CD40L signals can promote TH17 differentiation34, 35, we detected defective TH17 differentiation induced by DCs deficient in CD40 (Supplementary Fig. 8a). Importantly, anti-CD40 strongly upregulated p38 activity in DCs (Supplementary Fig. 8b), and p38α deficiency in DCs diminished TH17 differentiation following anti-CD40 stimulation (Supplementary Fig. 8c). We conclude that p38α is a convergent point in DCs to integrate infectious and inflammatory signals to instruct TH17 differentiation.
p38α-dependent effector pathways in DC signal integration
We investigated p38α-dependent effector pathways that could mediate these diverse upstream inputs. p38α deficiency led to decreased IL-6 production from DCs stimulated with curdlan and anti-CD40 in vivo (Fig. 6a,b) and in vitro (Fig. 6c), while IL-1β and TGF-β1 levels were not altered. DC expression of Il27 and Spp1 was also affected by p38α deficiency after stimulation with LPS and curdlan, while their expression was independent of p38α in response to anti-CD40 (Supplementary Fig. 9). These results indicate that IL-6 is a shared target of p38α in DCs activated with different stimuli.
Figure 6. p38α regulates a core set of downstream effector pathways in DCs to mediate diverse upstream signals.
(a,b) Analysis of Il6 mRNA in wild-type (WT) or p38αΔDC DCs after in vivo stimulation with curdlan (a) or α-CD40 (b) for 5 h. (c) Cytokine production from wild-type or p38αΔDC DCs after in vitro stimulation with curdlan or α-CD40 for 24 h. (d) Immunoblot analysis of p38α downstream targets in LPS-stimulated wild-type or p38αΔDC DCs. p-, phosphorylated. β-actin, the loading control. (e) Immunoblot analysis of p38α and downstream targets in curdlan and α-CD40-stimulated DCs from wild-type or p38αCreER mice after treatment with tamoxifen. (f) IL-6 production from DCs stimulated with LPS, curdlan or α-CD40 in the presence of the MK2 inhibitor or vehicle for 24 h. (g,h) Intracellular staining (g) and RNA analysis (h) of IL-17 and IFN-γ expression from T cells incubated for 5 days with DCs previously pulsed with LPS in the presence of the MK2 inhibitor or vehicle. NS, not significant; *P < 0.05; **P < 0.01 (Student’s t-test). Data are representative of 2 (a,b, n≥5 mice per group), 3 (c–e, n≥4 mice per group) and 4 (f–h, n≥4 mice per group) independent experiments. Error bars indicate SEM.
To examine the signaling and transcriptional pathways involved in IL-6 expression, we assessed activation of two known p38 targets, MAPKAP kinase 2 (MK2) and MSK18, and noted that it was diminished in LPS-stimulated p38αΔDC DCs as compared with wild-type cells (Fig. 6d). Additionally, activation of two transcription factors important for IL-6 production, CREB and c-Fos36, was decreased, whereas phosphorylation of CCAAT/enhancer binding protein β (C/EBPβ) and IκBα (indicative of NF-κB activity) was not affected (Fig. 6d and Supplementary Fig. 10). MK2, MSK1, CREB and c-Fos activation was similarly diminished in p38α-deficient DCs after curdlan and anti-CD40 stimulation (Fig. 6e), indicating that a core set of molecular targets is activated by p38α in response to diverse upstream inputs. Further, treatment of wild-type DCs with an MK2 inhibitor reduced IL-6 production after stimulation with LPS, curdlan and anti-CD40 (Fig. 6f), and diminished their capacity to drive TH17 generation after stimulation with LPS (Fig. 6g,h), curdlan or anti-CD40 (data not shown). Finally, p38α and MK2 were also required for IL-6 production in DCs following stimulation with heat-killed C. albicans (Supplementary Fig. 11). These results collectively establish a p38α-MK2 signaling axis to integrate upstream signals in DCs for TH17 differentiation.
Effects of p38α in CNS DCs and therapeutic targeting
During EAE, DCs are recruited into the CNS where they present antigens to myelin-specific T cells and polarize the local TH17 response5, 7. To test if CNS DCs require p38α to actively maintain the proinflammatory TH17 response, we immunized p38αCreER mice with MOG, and started tamoxifen treatment at day 7 to bypass the requirement of p38α in T cell priming in the periphery. Such treatment markedly ameliorated disease severity (Fig. 7a) and inflammation and demyelination of CNS (Fig. 7b). DCs isolated from CNS following acute deletion of p38α were impaired to induce Il17a and Il23r expression from co-cultured MOG TCR-transgenic T cells in vitro, indicating a key function for p38α in CNS DCs to mediate TH17 responses (Fig. 7c).
Figure 7. p38α activity is required for CNS-infiltrating DCs to maintain the TH17 response and is evolutionarily conserved between mouse and human DCs.
(a,b) EAE disease course (a) and histology (b) of wild-type (WT) or p38αCreER mice treated with tamoxifen at day 7 after immunization. Images are 4× (H&E) and 20× (Luxol fast blue) original magnification (b). (c) RNA analysis of MOG TCR-transgenic T cells stimulated with antigen and DCs isolated at the peak of disease from the spinal cord of wild-type or p38αCreER mice (treated with tamoxifen at day 13) for 5 days. (d) EAE disease course in wild-type or p38αΔDC mice transferred with in vitro derived TH17 cells. (e) RNA analysis of spinal cord cells from mice in (d). (f) Analysis of Il17a mRNA in T cells incubated for 5 days with mouse DCs previously pulsed with LPS in the presence of the p38 inhibitor SB203580 or vehicle. (g,h) Intracellular staining (g) and RNA analysis (h) of IL-17 and IFN-γ of human cord blood T cells incubated for 7 days with human DCs previously pulsed with LPS in the presence of SB203580 or vehicle. (i) IL-6 production from human DC stimulated with LPS in the presence of SB203580 or vehicle for 24 h. NS, not significant; *P < 0.05; **P < 0.01 (Student’s t-test). Data are representative of 2 (b–f: n≥ 4 mice per group) and 3 (a, n=5 mice per group; g–i) independent experiments. Error bars indicate SEM.
Using a TH17-polarized transfer model of EAE37, we next investigated the contribution of p38α in DCs to the effector phase of CNS inflammation. Upon adoptive transfer of TH17 effector cells into recipient mice, wild-type recipients rapidly developed EAE, whereas p38αΔDC mice showed delayed onset and reduced severity (Fig. 7d). Expression of Il17a and Il23r mRNA was lower in CNS-infiltrating cells from p38αΔDC mice than wild-type mice (Fig. 7e). These results suggest a continuous requirement for p38α activity in DCs to sustain IL-23R expression and TH17 responses at sites of inflammation.
To further evaluate the role of p38α in DCs as a therapeutic target for TH17-mediated diseases, we investigated if these regulatory pathways apply to human cells as well. DCs derived from human peripheral blood monocytes were treated with a p38 inhibitor and then cultured with naïve T cells. Similar to the mouse DCs that were used as control (Fig. 7f), blocking p38 in human DCs diminished IL-17 expression from T cells (Fig. 7g,h), as well as IL-6 production from DCs (Fig. 7i). Thus, p38 activity represents an evolutionarily conserved pathway to shape DC-dependent TH17 differentiation.
p38 in T cells is dispensable for TH17 differentiation
Several recent studies suggest an important role of p38 in T cells during TH17 generation13–17. Although deletion of p38α in T cells did not protect mice from EAE (Fig. 1c), we further tested whether p38 regulates TH17 differentiation in a T cell-intrinsic manner. Naïve T cells from p38αΔT mice differentiated normally into IL-17+ cells under TH17-polarizing conditions in vitro (Fig. 8a,b), despite the loss of p38 expression and phosphorylation in these cells (Supplementary Fig. 12a). To exclude compensation from other p38 family members, we generated mice lacking both p38α and p38β isoforms (p38αΔTp38βKO), because these two p38 isoforms are detectable in T cells (Supplementary Fig. 1a). TH17 differentiation in vitro was normal in T cells from p38αΔTp38βKO mice (Fig. 8c,d). In addition, MOG35–55 peptide immunization of wild-type, p38αΔT, p38βKO and p38αΔTp38βKO mice followed by ex vivo recall response to antigen or polyclonal stimulation showed comparable levels of IL-17 expression in T cells (Fig. 8e and Supplementary Fig. 12b). In addition, transfer of naïve T cells from p38αΔTp38βKO OT-II TCR-transgenic mice into wild-type mice followed by OVA immunization showed that T cells lacking p38α and p38β were as efficient as wild-type cells in mounting IL-17 expression (Fig. 8f). Altogether, our results exclude a T cell-intrinsic function of p38 signaling in TH17 differentiation.
Figure 8. p38α and p38β are not required for regulating T cell-intrinsic TH17 differentiation.
(a) Expression of IL-17 in wild-type (WT) or p38αΔT naïve T cells differentiated under TH17 conditions for 5 days, followed by PMA and ionomycin stimulation. (b) IL-17 secretion by T cells from (a) after α-CD3 stimulation for 24 h. (c) Expression of IL-17 in naïve T cells from wild-type or p38αΔTp38βKO mice simulated with LPS-pulsed DCs in the presence of IL-6 and TGF-β1 for 5 days, followed by PMA and ionomycin stimulation. (d) Analysis of Il17a mRNA in T cells from (c) after brief α-CD3 stimulation. (e) IL-17 expression in the CFSElo population of draining LN cells isolated from mice immunized with MOG + CFA, followed by CFSE labeling and then stimulation with MOG for 4 days. (f) Expression of IL-17 in the OT-II donor population of draining LN cells from recipients (CD45.1+) immunized with OVA + CFA. NS, not significant (Student’s t-test). Data are representative of 2–3 independent experiments (n≥3 mice per group). Error bars indicate SEM.
DISCUSSION
How instructive signals derived from the innate immune system trigger TH17 responses and inflammation, in contrast to T cell-intrinsic transcriptional mechanisms1, remains poorly understood. Here we describe that p38α integrates a diverse array of immunostimulatory signals in DCs to direct TH17 generation under autoimmune, inflammatory and infectious conditions, thereby establishing a previously unappreciated pathway of DC-dependent programming of TH17 differentiation. We show that p38α directs IL-6 and IL-27 and further shapes CD86 expression in DCs and imprints STAT3 signaling and IL-23R expression in responding T cells. Although p38α deficiency in DCs does not completely abrogate DC-dependent IL-17 production from T cells, the pathway has a critical role in disease pathogenesis and may represent an attractive therapeutic target, as both the induction and effector phases of autoimmune inflammation are highly dependent upon p38α activity in DCs.
Adaptive immunity is controlled by DC-derived signals at multiple checkpoints that dictate the activation and differentiation of T cell-mediated immune responses2. The few identified innate immune pathways that potentiate the TH17 response, such as Dectin-Syk-CARD signaling3, 4, transduce a specific type of pathogen-derived signals. In addition, their involvements in autoimmune diseases are unclear. Additional DCs signaling pathways, such as MKP-1 and Wnt-β-catenin signaling, have been shown to downregulate TH17 differentiation, although they also influence multiple other T cell lineages32, 38. Here we show that p38α is potently activated in DCs by TH17-instructive innate signals via engagement of PRRs, as well as T cell-dependent CD40 signals, to induce IL-6 production. Moreover, p38α regulates IL-27 downstream of innate (TLR and CLR) but not adaptive (CD40) signals, and further shapes co-stimulatory signals. Therefore, p38α orchestrates a program for DC-dependent TH17 differentiation under inflammatory conditions, although its role under steady state requires additional testing.
DCs are sparse in the healthy CNS, but are markedly increased in number during CNS inflammation. Whereas CNS DCs have been implicated in driving TH17 responses and precipitating inflammation5–7, opposing evidence also exists39, 40. Among distinct subsets of DCs, the CD11b+ myeloid DCs are most efficient at driving TH17 differentiation in the CNS7. Consistent with this observation, we found that CD11b+ DCs are strongly dependent on p38α activity to direct TH17 differentiation as compared with CD8+ DCs. By identifying a molecular pathway in CNS DCs to drive TH17 responses and disease pathogenesis, our findings provide critical genetic evidence supporting the key proinflammatory function of CNS DCs in vivo.
p38 has been shown to act in a T cell-intrinsic manner for TH17 development, as IL-17 expression was diminished after pharmacological inhibition of p38 or expression of a dominant negative p38 transgene13–17. However, by using T cell-specific deletion of p38α in multiple in vitro and in vivo systems, and after excluding genetic redundancy among the two p38 isoforms expressed by T cells (p38α and p38β), we found no evidence for a T cell-intrinsic function of p38 in TH17 responses. The function of p38 described in T cells previously could result from nonspecific actions of the experimental approaches, or the inability to distinguish the effects in T cells from those in APCs. Our results support a selective role of p38α in DC-mediated, but not T cell-intrinsic, TH17 differentiation, although our results do not exclude the function of p38 in T cells under other conditions11, 12. Moreover, p38α activity in other cell types also contributes to EAE, as systemic ablation but not DC-specific deletion of p38α results in complete protection from EAE.
p38α is by far the most extensively investigated protein kinase target for the development of anti-inflammatory drugs in the pharmaceutical industry, but severe side effects have prevented clinical advancement of p38α inhibitors9. Our findings have potential implications for the use of the vast number of p38α inhibitors that are already available. First, our results indicate that p38α inhibitors would be effective for TH17-mediated diseases. Heterogeneity in T cell effector responses is a common feature of autoimmune diseases, and both TH17 and TH1 responses can mediate MS and EAE. Notably, IFN-β, the most frequently prescribed drug for relapsing-remitting MS, is ineffective in 30–50% patients with a prevalent TH17 response41. Second, our results suggest that selectively targeting DCs is sufficient for therapeutic efficacy, and the use of novel drug-delivery vehicles to target p38α inhibitors to specific tissues or cell types is a promising strategy to avoid undesired side effects42. Therefore, p38α-dependent regulation of DC functions and TH17 responses can be further explored for innovative autoimmune therapies.
Supplementary Material
ACKNOWLEDGEMENTS
The authors acknowledge D.R. Green for critical reading of the manuscript, C. Cloer and S. Shrestha for help with animal colony management, R. K. Subbarao Malireddi for help with fungal infection, B. Reizis for CD11c-Cre mice, T. Ludwig for Rosa26-Cre-ERT2 mice, V.K. Kuchroo for the 2D2 MOG TCR-transgenic mice, S. Arthur for p38βKO mice, D.R. Littman for the IL-23R expressing retrovirus, and St. Jude Immunology FACS core facility for cell sorting. This work was supported by NIH R01 NS064599, National Multiple Sclerosis Society RG4180-A-1, and Cancer Research Institute (to H.C.).
Footnotes
AUTHOR CONTRIBUTIONS
G.H. designed and performed cellular and molecular experiments and in vivo models, and contributed to writing the manuscript; Y.W. designed and performed biochemical and gene expression analyses and contributed to cellular experiments; P.V. contributed to histopathology analysis; T.D.K. contributed to infectious models; K.O. contributed mouse models; H.C. designed experiments, wrote the manuscript, and provided overall direction.
REFERENCES
- 1.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 2.Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science. 2010;327:291–295. doi: 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.LeibundGut-Landmann S, et al. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol. 2007;8:630–638. doi: 10.1038/ni1460. [DOI] [PubMed] [Google Scholar]
- 4.Mocsai A, Ruland J, Tybulewicz VL. The SYK tyrosine kinase: a crucial player in diverse biological functions. Nat Rev Immunol. 2010;10:387–402. doi: 10.1038/nri2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greter M, et al. Dendritic cells permit immune invasion of the CNS in an animal model of multiple sclerosis. Nat Med. 2005;11:328–334. doi: 10.1038/nm1197. [DOI] [PubMed] [Google Scholar]
- 6.McMahon EJ, Bailey SL, Castenada CV, Waldner H, Miller SD. Epitope spreading initiates in the CNS in two mouse models of multiple sclerosis. Nat Med. 2005;11:335–339. doi: 10.1038/nm1202. [DOI] [PubMed] [Google Scholar]
- 7.Bailey SL, Schreiner B, McMahon EJ, Miller SD. CNS myeloid DCs presenting endogenous myelin peptides 'preferentially' polarize CD4+ T(H)-17 cells in relapsing EAE. Nat Immunol. 2007;8:172–180. doi: 10.1038/ni1430. [DOI] [PubMed] [Google Scholar]
- 8.Huang G, Shi LZ, Chi H. Regulation of JNK and p38 MAPK in the immune system: signal integration, propagation and termination. Cytokine. 2009;48:161–169. doi: 10.1016/j.cyto.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen P. Targeting protein kinases for the development of anti-inflammatory drugs. Curr Opin Cell Biol. 2009;21:317–324. doi: 10.1016/j.ceb.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 10.Bain J, et al. The selectivity of protein kinase inhibitors; a further update. Biochem J. 2007;408:297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berenson LS, Yang J, Sleckman BP, Murphy TL, Murphy KM. Selective requirement of p38alpha MAPK in cytokine-dependent, but not antigen receptor-dependent, Th1 responses. J Immunol. 2006;176:4616–4621. doi: 10.4049/jimmunol.176.8.4616. [DOI] [PubMed] [Google Scholar]
- 12.Jirmanova L, Giardino Torchia ML, Sarma ND, Mittelstadt PR, Ashwell JD. Lack of the T-cell-specific alternative p38 activation pathway reduces autoimmunity and inflammation. Blood. 2011;118:3280–3289. doi: 10.1182/blood-2011-01-333039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noubade R, et al. Activation of P38 MAPK in CD4 T cells controls IL-17 production and autoimmune encephalomyelitis. Blood. 2011;118:290–300. doi: 10.1182/blood-2011-02-336552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gulen MF, et al. The Receptor SIGIRR Suppresses Th17 Cell Proliferation via Inhibition of the Interleukin-1 Receptor Pathway and mTOR Kinase Activation. Immunity. 2010;32:54–66. doi: 10.1016/j.immuni.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu L, et al. Role of SMAD and non-SMAD signals in the development of Th17 and regulatory T cells. J Immunol. 2010;184:4295–4306. doi: 10.4049/jimmunol.0903418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li L, Kim J, Boussiotis VA. IL-1beta-mediated signals preferentially drive conversion of regulatory T cells but not conventional T cells into IL-17-producing cells. J Immunol. 2010;185:4148–4153. doi: 10.4049/jimmunol.1001536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Commodaro AG, et al. p38{alpha} MAP kinase controls IL-17 synthesis in vogt-koyanagi-harada syndrome and experimental autoimmune uveitis. Invest Ophthalmol Vis Sci. 2010;51:3567–3574. doi: 10.1167/iovs.09-4393. [DOI] [PubMed] [Google Scholar]
- 18.Kim C, et al. The kinase p38 alpha serves cell type-specific inflammatory functions in skin injury and coordinates pro- and anti-inflammatory gene expression. Nat Immunol. 2008;9:1019–1027. doi: 10.1038/ni.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang YJ, et al. Macrophage deletion of p38alpha partially impairs lipopolysaccharide-induced cellular activation. J Immunol. 2008;180:5075–5082. doi: 10.4049/jimmunol.180.7.5075. [DOI] [PubMed] [Google Scholar]
- 20.Stumhofer JS, et al. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7:937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 21.Batten M, et al. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol. 2006;7:929–936. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 22.Ivanov II, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bettelli E, et al. Myelin oligodendrocyte glycoprotein-specific T cell receptor transgenic mice develop spontaneous autoimmune optic neuritis. J Exp Med. 2003;197:1073–1081. doi: 10.1084/jem.20021603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu X, et al. Crucial role of interleukin-7 in T helper type 17 survival and expansion in autoimmune disease. Nat Med. 2010;16:191–197. doi: 10.1038/nm.2077. [DOI] [PubMed] [Google Scholar]
- 25.Zhou L, et al. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGeachy MJ, et al. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol. 2009;10:314–324. doi: 10.1038/ni.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghoreschi K, et al. Generation of pathogenic T(H)17 cells in the absence of TGF-beta signalling. Nature. 2010;467:967–971. doi: 10.1038/nature09447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murugaiyan G, Mittal A, Weiner HL. Identification of an IL-27/osteopontin axis in dendritic cells and its modulation by IFN-gamma limits IL-17-mediated autoimmune inflammation. Proc Natl Acad Sci U S A. 2010;107:11495–11500. doi: 10.1073/pnas.1002099107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shinohara ML, Kim JH, Garcia VA, Cantor H. Engagement of the type I interferon receptor on dendritic cells inhibits T helper 17 cell development: role of intracellular osteopontin. Immunity. 2008;29:68–78. doi: 10.1016/j.immuni.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park H, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Odobasic D, Leech MT, Xue JR, Holdsworth SR. Distinct in vivo roles of CD80 and CD86 in the effector T-cell responses inducing antigen-induced arthritis. Immunology. 2008;124:503–513. doi: 10.1111/j.1365-2567.2007.02802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang G, Wang Y, Shi LZ, Kanneganti TD, Chi H. Signaling by the phosphatase MKP-1 in dendritic cells imprints distinct effector and regulatory T cell fates. Immunity. 2011;35:45–58. doi: 10.1016/j.immuni.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van de Veerdonk FL, et al. The macrophage mannose receptor induces IL-17 in response to Candida albicans. Cell Host Microbe. 2009;5:329–340. doi: 10.1016/j.chom.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 34.Perona-Wright G, et al. A pivotal role for CD40-mediated IL-6 production by dendritic cells during IL-17 induction in vivo. J Immunol. 2009;182:2808–2815. doi: 10.4049/jimmunol.0803553. [DOI] [PubMed] [Google Scholar]
- 35.Iezzi G, et al. CD40-CD40L cross-talk integrates strong antigenic signals and microbial stimuli to induce development of IL-17-producing CD4+ T cells. Proc Natl Acad Sci U S A. 2009;106:876–881. doi: 10.1073/pnas.0810769106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yin F, et al. Noncanonical cAMP pathway and p38 MAPK mediate beta2-adrenergic receptor-induced IL-6 production in neonatal mouse cardiac fibroblasts. J Mol Cell Cardiol. 2006;40:384–393. doi: 10.1016/j.yjmcc.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 37.Shi LZ, et al. HIF1a-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J Exp Med. 2011;208:1367–1376. doi: 10.1084/jem.20110278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Manicassamy S, et al. Activation of beta-catenin in dendritic cells regulates immunity versus tolerance in the intestine. Science. 2010;329:849–853. doi: 10.1126/science.1188510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suter T, et al. The brain as an immune privileged site: dendritic cells of the central nervous system inhibit T cell activation. Eur J Immunol. 2003;33:2998–3006. doi: 10.1002/eji.200323611. [DOI] [PubMed] [Google Scholar]
- 40.Heppner FL, et al. Experimental autoimmune encephalomyelitis repressed by microglial paralysis. Nat Med. 2005;11:146–152. doi: 10.1038/nm1177. [DOI] [PubMed] [Google Scholar]
- 41.Axtell RC, Raman C, Steinman L. Interferon-beta exacerbates Th17-mediated inflammatory disease. Trends Immunol. 2011;32:272–277. doi: 10.1016/j.it.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sy JC, et al. Sustained release of a p38 inhibitor from non-inflammatory microspheres inhibits cardiac dysfunction. Nat Mater. 2008;7:863–868. doi: 10.1038/nmat2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Caton ML, Smith-Raska MR, Reizis B. Notch-RBP-J signaling controls the homeostasis of CD8- dendritic cells in the spleen. J Exp Med. 2007;204:1653–1664. doi: 10.1084/jem.20062648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu G, et al. The receptor S1P1 overrides regulatory T cell-mediated immune suppression through Akt-mTOR. Nat Immunol. 2009;10:769–777. doi: 10.1038/ni.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu G, Yang K, Burns S, Shrestha S, Chi H. The S1P(1)-mTOR axis directs the reciprocal differentiation of T(H)1 and T(reg) cells. Nat Immunol. 2010;11:1047–1056. doi: 10.1038/ni.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nishida K, et al. p38alpha mitogen-activated protein kinase plays a critical role in cardiomyocyte survival but not in cardiac hypertrophic growth in response to pressure overload. Mol Cell Biol. 2004;24:10611–10620. doi: 10.1128/MCB.24.24.10611-10620.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beardmore VA, et al. Generation and characterization of p38beta (MAPK11) gene-targeted mice. Mol Cell Biol. 2005;25:10454–10464. doi: 10.1128/MCB.25.23.10454-10464.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lefrancois L, Lycke N. Isolation of mouse small intestinal intraepithelial lymphocytes, Peyer's patch, and lamina propria cells. Curr Protoc Immunol. 2001;Chapter 3(Unit 3):19. doi: 10.1002/0471142735.im0319s17. [DOI] [PubMed] [Google Scholar]
- 49.Yang K, Neale G, Green DR, He W, Chi H. The tumor suppressor Tsc1 enforces quiescence of naive T cells to promote immune homeostasis and function. Nat Immunol. 2011;12:888–897. doi: 10.1038/ni.2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.