Abstract
In this paper, we present evidence that a multitude of mid-frontal Event-Related Potential (ERP) components partially reflect a common theta-band oscillatory process. Specifically, mid-frontal ERP components in the “N2” time range and “ERN” time range are parsimoniously characterized as reflections of theta band activities. Forty participants completed three different tasks with varying stimulus-response demands. Permutation tests were used to identify the dominant time-frequency responses of stimulus- and response-locked conditions, as well as the enhanced EEG responses to novelty, conflict, punishment and error. A dominant theta band feature was found in all conditions, and both ERP component amplitudes and theta power measures were similarly modulated by novelty, conflict, punishment and error. The findings support the hypothesis that generic and reactive mPFC processes are parsimoniously reflected by theta band activities.
Keywords: ERN, FRN, N2, Theta, Anterior Cingulate, Conflict, Reinforcement Learning
A convergence of evidence from multiple levels of the neural sciences has identified a system for action monitoring in medial prefrontal cortex (mPFC), particularly Anterior Cingulate Cortex (ACC). This system has been described as a functional node in complex processes such as adaptive control over behavior and acquisition of reinforcement contingencies, as a dynamic processing hub for attention and action selection, and as a sensitive determinant of motivational functions including emotional reactivity and willful engagement. This function of the ACC may succinctly be described by the integration of contextual cues with action selection to optimize goal-driven performance (Carter et al., 1998; Devinsky, Morrell, & Vogt, 1995; Paus, 2001; Ridderinkhof, Ullsperger, Crone, & Nieuwenhuis, 2004; Rushworth, Buckley, Behrens, Walton, & Bannerman, 2007)1. An extensive literature of human studies has detailed a series of event-related potential (ERP) components that putatively reflect mPFC/ACC operations during attention, cognitive control, feedback learning, and action selection. Specifically, we focus on ERP components relevant to formal models of reinforcement learning (Holroyd & Coles, 2002) and conflict monitoring (Yeung, Botvinick, & Cohen, 2004). These ERP features are reviewed below, and it is suggested that these components might all be reflections of general mPFC processes as indicated by frontal theta.
Stimulus-Locked Components
The only stimulus-locked components detailed here are ones that occur over mid-frontal regions in the N2 time range (negative deflections peaking ~250–350 ms post-stimulus). To characterize the variety of “N2-like” frontal voltage potentials occurring in this time range, the distinction made by Folstein and Van Petten (2008) will be used. Folstein and Van Petten (2008) described two broad classes of anterior N2s: one class involved in action selection (control N2) and another relating to attention (mismatch N2). The control-related N2 component is modulated by stimuli indicating variations in stimulus-response demand: for example, a stimulus that primes multiple competing motor responses (Yeung, Botvinick, et al., 2004). The mismatch-related N2 component is modulated by stimuli that reflect an unexpected perceptual differentiation: for example, a novel stimulus occurring in a train of standards on an oddball task. Another action monitoring component that shares features with both the control and mismatch N2 is the Feedback-Related Negativity (FRN): a fronto-central negativity occurring after feedback that indicates poor performance or a loss of value (Holroyd & Coles, 2002). Parallels between the FRN and the mismatch N2 have been frequently noted in the literature, particularly due to the similar eliciting factors and spatio-temporal patterns of these components. Holroyd, Pakzad-Vaezi, and Krigolson (2008) have recently suggested that the FRN is simply an N2 that occurs to unexpected negative feedback. As with the N2, infrequency and degree of mismatch modulate the mismatch FRN, however, it is unknown if these eliciting events reflect alterations of different underlying neural processes (Donkers, Nieuwenhuis, & van Boxtel, 2005; Donkers & van Boxtel, 2004; Holroyd, 2002). In fact, all of these fronto-central negativities appear to be sensitive to a form of expectation mismatch, although they may differ in terms of attention orientation (mismatch N2), action selection (control N2) or punishment prediction error (FRN). While these mismatch signals may reflect disparate processes in unique cognitive circumstances, these aforementioned processes have all been specifically associated with ACC function during attention orientation and/or action selection (Carter, et al., 1998; Devinsky, et al., 1995; Paus, 2001; Ridderinkhof, et al., 2004; Rushworth, et al., 2007).
Response-Locked Components
In motivated performance tasks, an error of motor commission elicits an Error-Related Negativity (ERN), a negative voltage deflection peaking around 80ms post-response (Falkenstein, Hohnsbein, Hoormann, & Blanke, 1991; Gehring, Goss, Coles, Meyer, & Donchin, 1993). A smaller negativity has been found on correct trials, sometimes termed the Correct Related Negativity (CRN). CRNs have been proposed to reflect an inherent feature of the ACC response to manual responses in a demanding task (Cavanagh, Cohen, & Allen, 2009; Vidal, Burle, Bonnet, Grapperon, & Hasbroucq, 2003; Vidal, Hasbroucq, Grapperon, & Bonnet, 2000; Yordanova, Falkenstein, Hohnsbein, & Kolev, 2004). Both the ERN and CRN are larger under conditions of increased task difficulty (Hajcak, Moser, Yeung, & Simons, 2005), but the formal differentiation between the two becomes non-distinct in ambiguous cases. The amplitude of the ERN and the CRN are reciprocally related to uncertainty (Pailing & Segalowitz, 2004), and suboptimal choices on reinforcement learning tasks yield a larger voltage potential than correct responses as the task become learned, although this suboptimal ‘error’ is much smaller than a motor error-of-commission (Frank, Woroch, & Curran, 2005; Gründler, Cavanagh, Figueroa, Frank, & Allen, 2009; Holroyd & Coles, 2002). Experimental context appears to dynamically modulate these features of response-locked ERPs along a continuum, suggesting that all of these response-related components appear to reflect a similar underlying process that is particularly sensitive to conflict or error.
Plethora of Potentials or Possible Parsimony?
To date, there is no inclusive theory or model on the collection of mid-frontal negativities commonly described as the ERN, CRN, FRN and N2. While the terminological distinctions of ‘ERN’, ‘N2’, etc. have proven useful in defining specific spatiotemporal ERP components, interpretations of the functional processes reflected by these signals may be hindered by the abundant terminology of seemingly different task-specific components. For example, a small set of cognitive functions may be shared between the reinforcement learning (Holroyd & Coles, 2002) and conflict monitoring (Botvinick, Braver, Barch, Carter, & Cohen, 2001; Yeung, Botvinick, et al., 2004) processes, although these commonalities may be obscured by methods of quantification and choice of theoretical reference frame. A parsimonious summary could propose that both the stimulus- and response-related fronto-central negativities reflect common features of the processing demands of the mPFC, especially the ACC. These features are varied across systems related to cognitive and motor control, attention, and reinforcement learning: but are especially sensitive to mismatch signals of conflict, punishment and error in the service of behavioral adaptation. Fitting with this account, a single mid-frontal EEG signal has been shown to be sensitive to these conditions, and has been suggested to be reflected within all of these aforementioned ERP components: frontal theta.
A Common Theta Substrate?
Talairach et al. (1973) described how electrical stimulation of the human ACC elicited motor actions that were integrated with environmental context, sometimes accompanied by mid-frontal theta oscillatory activities recorded in the EEG. A growing literature has identified the frontal midline theta rhythm in the generation of event related mid-frontal voltage negativites during conflict and error responses. The ACC has been shown to generate neural oscillations in the theta band (Tsujimoto, Shimazu, & Isomura, 2006; Wang, Ulbert, Schomer, Marinkovic, & Halgren, 2005; Womelsdorf et al., 2007), and these oscillations have been linked to multiple processes including memory, attention, learning and action selection (Cohen, Ridderinkhof, Haupt, Elger, & Fell, 2008; Debener et al., 2005; Marco-Pallares et al., 2008; Onton, Delorme, & Makeig, 2005; Wang, et al., 2005). The ERN has been proposed to reflect a degree of theta phase consistency and power enhancement over the medial frontal cortex (Cavanagh, et al., 2009; Luu & Tucker, 2001; Luu, Tucker, Derryberry, Reed, & Poulsen, 2003; Luu, Tucker, & Makeig, 2004; Trujillo & Allen, 2007; Yordanova, et al., 2004), as have the CRN (Burle, Roger, Allain, Vidal, & Hasbroucq, 2008; Cavanagh, et al., 2009; Yordanova, et al., 2004), the FRN (Bernat, Nelson, Holroyd, Gehring, & Patrick, 2008; Cavanagh, Frank, Klein, & Allen, 2010; Cohen, Elger, & Ranganath, 2007; Marco-Pallares, et al., 2008) and the N2 (Cavanagh et al., under review; Cohen, et al., 2008). These commonalities suggest that the distinct components defined as the ERN, CRN, FRN and N2 may reflect variants of a similar underlying neural process – namely, midfrontal theta.
Any ERP can reflect a unique combination of power enhancement and/or phase consistency of underlying processes (Fell et al., 2004; Le Van Quyen & Bragin, 2007; Makeig, Debener, Onton, & Delorme, 2004; Sauseng et al., 2007). Advanced signal processing techniques (such as wavelet convolution, short-time Fourier transform, or the Hilbert transform) can parse frequency-specific power and phase relationships within the EEG signal. While these time-frequency methods cannot alone determine if a signal is oscillatory in nature (Ritter & Becker, 2009; Sauseng, et al., 2007; Yeung, Bogacz, Holroyd, & Cohen, 2004; Yeung, Bogacz, Holroyd, Nieuwenhuis, & Cohen, 2007), they can disambiguate band-specific signals recorded at any single electrode. These methodological steps reveal dimensions of the EEG signal (frequency, power & phase) that are proposed to reflect separable physiological mechanisms for the organization and communication of neural computations (Buzsáki, 2006; Buzsaki & Draguhn, 2004; Fries, 2005; Womelsdorf, et al., 2007). In this article it will be argued that there are theoretical and practical benefits for using such a time-frequency approach to interpret the functional roles of these action monitoring ERP components. We introduce the hypothesis that mid-frontal theta phase dynamics reflect common templates for the temporal organization of neural responses to stimuli and responses, with variations on this template reflecting neural reactions to novelty, conflict, punishment and error.
The Current Study
Here we aimed to formally compare a wide variety of mid-frontal responses to stimuli and actions with multiple measures of event-related EEG. Given the broad role of the mPFC in merging cognitive and motor functions, it was hypothesized that a multitude of different action monitoring events would elicit mid-frontal EEG responses. However, like the mPFC, it was proposed that these EEG responses will be particularly sensitive to signals of novelty, conflict, punishment and error. Data-driven permutation tests were used to provide statistical evidence of dominant time-frequency responses associated with specific ERP components. Permutation tests were also used to identify the enhanced EEG responses to conditions of novelty, conflict, punishment and error. A dominant theta band feature was found in all experimental conditions, and both ERP component amplitudes and theta power measures were similarly modulated by novelty, conflict, punishment and error. The findings supported the hypothesis that generic and reactive mPFC processes are parsimoniously reflected by theta band activities.
Methods
Participants
Participants were 40 students (12 female) with a mean age of 19.18 years (SD= 1.13) who participated for course credit. All participants gave informed consent and the research ethics committee of the University of Arizona approved the study. All participants were free of past head trauma or seizures and free of current psychoactive medication use.
Procedures
First, participants filled out questionnaires and demographic information. Second, the EEG cap was applied and participants sat quietly for six minutes while resting EEG was recorded. Finally, participants completed three different sets of tasks (each described below) in a randomized counter-balanced order. The entire study lasted two hours, with each active task taking approximately 20 minutes with short self-paced breaks between tasks.
Perception and Motor Tasks
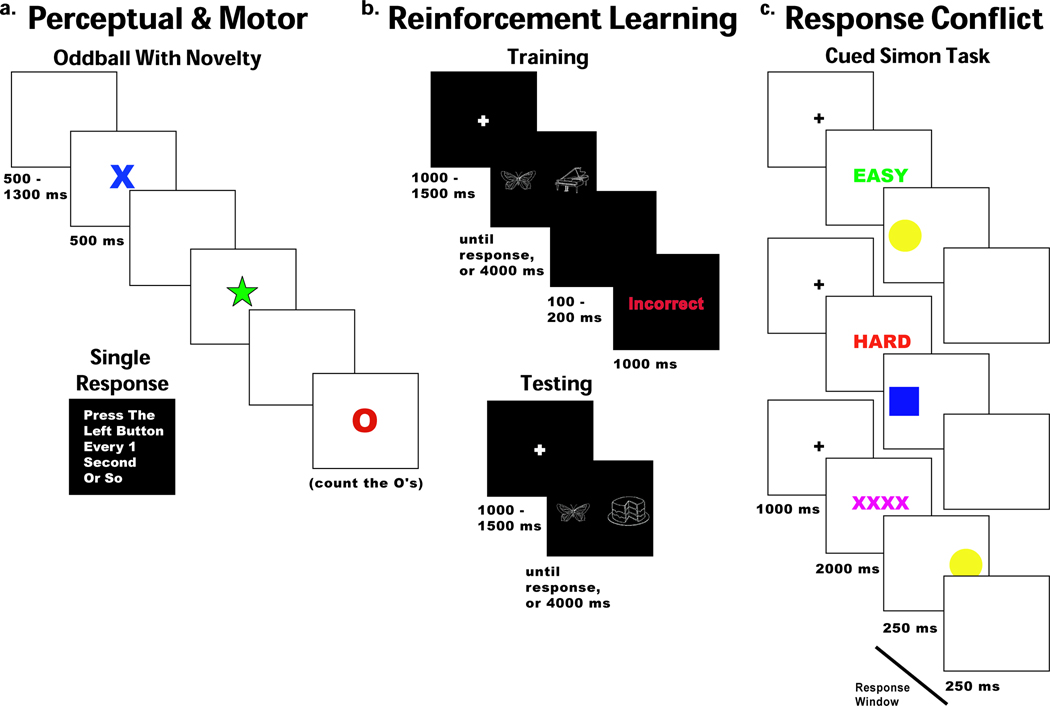
There were two mixed tasks in this set (Figure 1a). For the three oddball task blocks, participants were instructed to place their response buttons in their laps; no responses were required. Blocks consisted of 15, 20, or 25 targets (red ‘O’s) occurring at ~14% probability (60 total) that participants were instructed to count. Standards (blue ‘X’s) were presented on ~72% of trials (311 total), surprise novel stimuli (varied colored shapes) were presented on ~14% of trials (60 total). All stimuli were presented for 500ms with a jittered inter-trial interval (ITI) of 500–1300ms. The response task consisted of two blocks intermixed between oddball blocks. Each block instructed participants to pick up either the left or right button and simply press it every second or two. For EEG analysis, data were locked to the cues in the oddball task and the responses in the response tasks. These tasks provide measures of stimulus processing independent of motor responding (oddball task) and single motor responses independent of stimulus processing.
Figure 1.
Probabilistic Learning Task
The probabilistic learning task consisted of brief forced-choice training blocks consisting of sixteen trials, each followed by a subsequent testing block with sixteen trials (modified from Frank, Seeberger, & O'Reilly (2004), see Fig. 1b). There were eight of these train/test blocks. During each training block the participants were presented with two pictures (hereafter: cues), where each cue was associated with a different probabilistic chance of receiving ‘Correct’ or ‘Incorrect’ feedback. These cue pairs (and their probabilities of reward) were termed A / B (87.5% / 12.5%) and C / D (62.5% / 37.5%). All training trials began with a jittered inter-trial-interval between 1000 and 1500 ms. Each cue pair then appeared for a maximum of 4000 ms, and disappeared immediately after the choice was made. Following a button press, either ‘Correct’ or ‘Incorrect’ feedback was presented for 1000 ms (jittered between 100 and 200 ms post response). If the participant failed to make a choice within the 4000 ms, “No Response Detected” was presented. Over the course of the training block, participants typically learn to choose A over B and C over D based on adaptive responding to feedback.
During the testing blocks all possible novel combinations of cue pairs from the previous training block (e.g. AD, CB, etc.) were presented four times each (16 trials total) and no feedback was provided. All training trials began with a jittered ITI between 1000 and 1500 ms. Each cue pair then appeared for a maximum of 4000 ms, and disappeared immediately after the choice was made. These cue pairs were sorted into separate conditions based on reinforcement conflict: high conflict (consisting of both “win-win” trials (AC) and “lose-lose” trials (BD)) and low conflict (consisting of “win-lose” trials (AB or CD)). For EEG analyses, cue- and response-locked data were taken from the test phase, but feedback-locked data were taken from the training phase. Cue and response-locked trials were only included if the response times (RTs) were between 200ms and 4000ms. Response-locked high and low conflict trials consisted of correctly identified (optimal) choices, whereas the suboptimal trials condition consisted of all trials that were incorrectly identified.
Response Competition Task
A modified Simon task (Simon & Rudell, 1967) with preparatory cues was used to assess response competition processes (Figure 1c). Each trial began with an equiprobable informative cue (green ‘EASY’, red ‘HARD’ or purple ‘XXXX’), indicating that the trial would require a congruent or incongruent response, or in the case of purple X’s, that the response was equiprobably congruent or incongruent. Informative cues were presented for 2000ms, after which the imperative cue was presented to the left or right side of the screen (yellow circle for left response, blue square for right response) for 250ms, whereupon a blank screen was presented for 250 ms. Participants had this total 500ms window to respond. All trials had an ITI of 1000ms, but erroneous responses had an additional delay of 1000 ms followed by “Incorrect” feedback presented for 1000ms, and non-responses had “Faster!” feedback immediately presented for 1000 ms. This task consisted of six blocks with 48 trials each. For EEG analyses, data were time locked to informative and imperative cues, and to responses to correct congruent, correct incongruent and error trials.
Electrophysiological Recording and Processing
Scalp voltage was measured using 60 Ag/AgCl electrodes, plus two mastoid sites, referenced to a site immediately posterior to Cz using a Synamps2 system (bandpass filter 0.5–100 Hz, 500 Hz sampling rate, impedances < 10 kΩ). Data from the rest period were epoched into non-overlapping mean-centered 2000ms epochs, data from the tasks were epoched from −1500ms to +2500ms peri-event. User-identified bad epochs were marked and removed. An infomax independent components analysis was run on each task for each subject using runica from the EEGLab toolbox (Delorme & Makeig, 2004). Two experimenters reviewed the components and marked those associated with eyeblinks for removal. In all cases, a single component was removed: (82% 1st component, 13.5% 2nd component, 3.5% 3rd or 4th component, <1% other). EEG data were then re-referenced to an average reference2. Trough-to-peak measurements of standard ERPs (.5 to 15 Hz, with a −1000 to 0 ms peri-event baseline) were used to determine baseline-independent amplitudes by measuring the amplitude distance between the negative peak of the component and the preceding positive peak, with larger trough-to-peak values reflecting larger voltage potentials for the component of interest (which all consisted of negative deflections). All cue-locked ERP components of interest (i.e. N2, FRN) were measured from the peak negativity in the 200–350 ms time range and all response-locked ERP components of interest (i.e. CRN, ERN) were measured from the peak negativity in the 0–120 ms time range. Theta-band specific ERPs were also created for display by filtering the single trial EEG (4 to 8 Hz) prior to averaging. All ERPs are plotted with negative polarity upwards, by convention.
Time-frequency calculations were computed using custom-written Matlab routines (Cavanagh, et al., 2009; Cohen, et al., 2008). Time-frequency measures were computed by multiplying the fast Fourier transformed (FFT) power spectrum of single trial EEG data with the FFT power spectrum of a set of complex Morlet wavelets (defined as a Gaussian-windowed complex sine wave: e−i2πtf e−t2/(2*σ2), where t is time, f is frequency (which increased from 1 to 50Hz in 50 logarithmically spaced steps), and σ defines the width (or “cycles”) of each frequency band, set according to 4/(2πf)), and taking the inverse FFT. The end result of this process is identical to time-domain signal convolution, and it resulted in: 1) estimates of instantaneous power (the magnitude of the analytic signal), defined as Z[t] (power time series: p(t) = real[z(t)]2 + imag[z(t)]2); and, 2) phase (the phase angle) defined as φt = arctan(imag[z(t)]/real[z(t)]). The time and frequency resolutions of different center frequencies can be calculated as 2σt and 2σf (Tallon-Baudry, Bertrand, Delpuech, & Permier, 1997; Yordanova, et al., 2004). In the theta range, these resolutions ranged from 322 ms and 2Hz (centered at 4Hz) to 157ms and 4Hz (centered at 8Hz).
Each epoch was then cut in length (−500 to +1000 ms). Power was normalized by conversion to a decibel (dB) scale (10*log10[power(t)/power(baseline)]), allowing a direct comparison of effects across frequency bands. The baseline for each frequency consisted of the average power from −500 to −400 ms prior to the onset of the cues (responses for each task were baseline-corrected to task-specific pre-cue baselines, in the single response condition these were baseline corrected to the pre-cue oddball baseline). Whereas the ERPs reflect phase-locked amplitude changes, these time-frequency measures reflect total power (phase-locked and phase-varying).
Intertrial phase coherence was used to measure the consistency of phase values for a given frequency band at each point in time (Lachaux, Rodriguez, Martinerie, & Varela, 1999). Intertrial phase coherence values vary from 0 to 1, where 0 indicates random phases at that time-frequency point across trials, and 1 indicates identical phase values at that time-frequency point across trials. Intertrial phase coherence (also termed the phase locking value: PLV) at each time point is defined as:
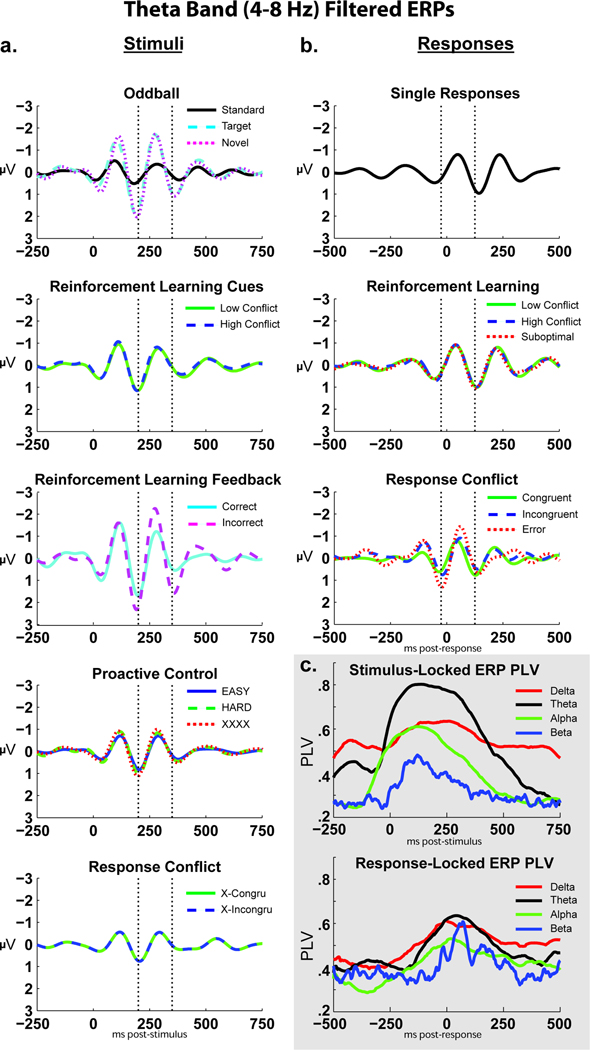
where n is the number of trials for each time and each frequency band. PLV thus reflects the extent to which oscillation phase values are consistent over trials at that point in time-frequency space (power, in contrast, represents the intensity of that signal). This investigation additionally used the Hilbert transform to compute the PLV over time between conditions for filtered ERPs to assess the degree of band-specific phase-locking in ERPs, see Figure 5c.
Figure 5.
Statistical Analysis
First, permutation tests were performed on the voltage difference over time and frequency between rest and task-specific conditions using custom-written Matlab routines. This process tested the null hypothesis that the data in the rest and task conditions are interchangeable. Results therefore indicate how task-related activity differs from intrinsic (task-unrelated) EEG processes. First, paired-sample t-tests were computed at each time-frequency point (pixel) between the grand average empirical task data and rest. This procedure matched the epoch counts between comparisons (by randomly selecting from the pool of the larger set) in order to control for unequal weightings of evidence. Since the resting data had a mean and median of 120 epochs, it was the larger set in all cases except for oddball standards. Only pixels that survived p<.05 thresholding were retained. Multiple comparison correction of the empirical tests were completed using permutation tests of weighted cluster-based thresholding, sometimes known as the “exceedance mass” (Nichols & Holmes, 2002). One thousand permutations were run for each condition. Within each permutation, t-tests were computed between data sets that had been randomly shuffled between rest and task conditions. Each permutation also used conditions with the same number of epochs. The sum of the t-values within each cluster of significant pixels (the “mass”) was used to threshold the empirical data. The top 2.5% of mass values for each of the 1000 permutations were used as the threshold, separately for positive and negative clusters, providing a two-tailed 5% alpha level of family-wise error control for multiple comparison correction. This method provides a data-driven hypothesis test that identifies where conditions differ from rest over time-frequency space. Figures 3 and 4 show these comparisons for each task and condition (versus rest).
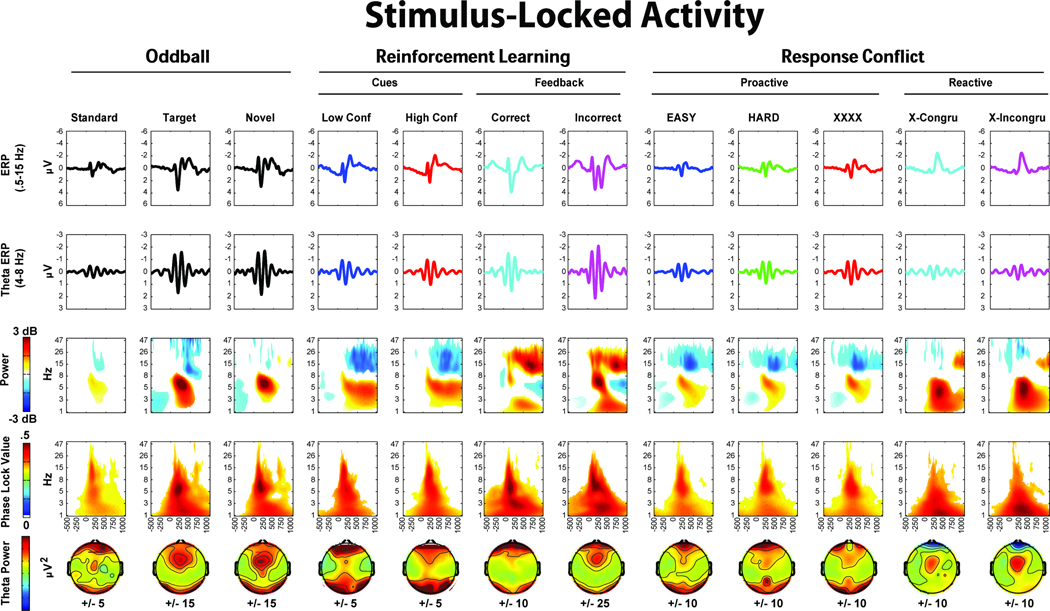
Figure 3.
Figure 4.
Next, key comparisons were made between relevant conditions to identify if novelty, conflict, punishment and error conditions demonstrate differential EEG activities than respective comparison conditions. Epoch counts were matched between conditions as described above. In all of these planned comparisons, stimulus-locked high and low conflict trials on the response conflict task were taken following non-informative (XXXX) cues to control for differential expectations that would be present on EASY or HARD trials. Differences between conditions were assessed by the permutation testing methods described above, but this time shuffling between relevant conditions (for example, shuffling between error and correct trials, see Figs 5 and 6).
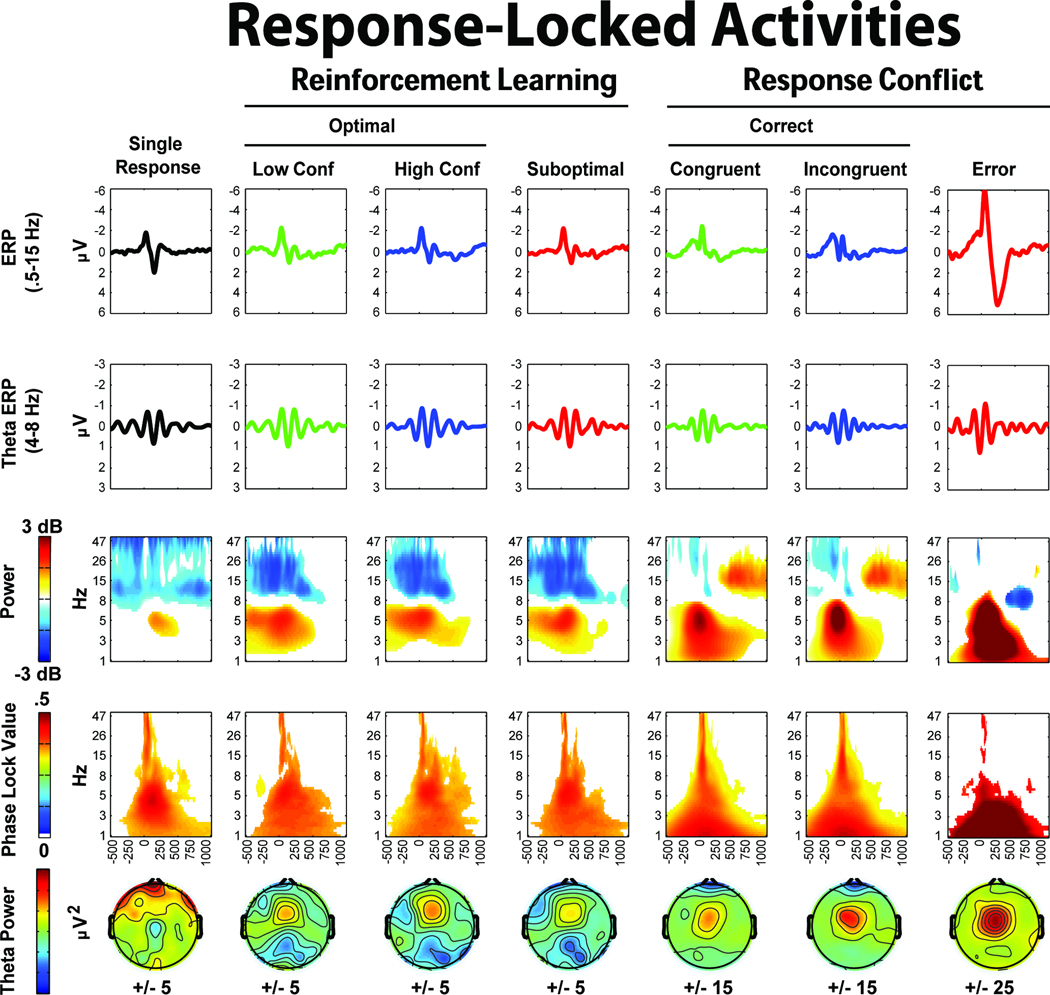
Figure 6.
Three different methods were used to compare the performance of ERP and theta power measurements. First, a factor analysis was used to examine the degree of between-measurement variation within tasks in order to determine if ERP and theta measurements loaded on similar factors. Two different factor analyses of ERP amplitudes and theta power were performed: one on all 19 variables of interest, and a second on 10 difference scores. Varimax rotation was used to derive orthogonal factors in order to highlight within-task effects. Variables were included in each factor if they had a loading greater than an absolute value of .3. Since this factor analysis includes a large number of variables with a modest number of participants, these tests should be interpreted with caution. Second, both ERP amplitudes and region-of-interest (ROI) defined theta power were used to assess differences between conditions (Figure 8). In this ROI analysis, theta power was taken from similarly-sized windows on the grand-average time plots between the time ranges that were used for ERP component selection (Figures 3 and 4; 4 to 8 Hz, stimulus-locked: 200 to 350 ms, response-locked −24 to 124 ms). Third, power analyses were computed on the theta power and ERP effect sizes for novelty, conflict, punishment and error conditions (Figure 9).
Figure 8.
Figure 9.
Results
Performance
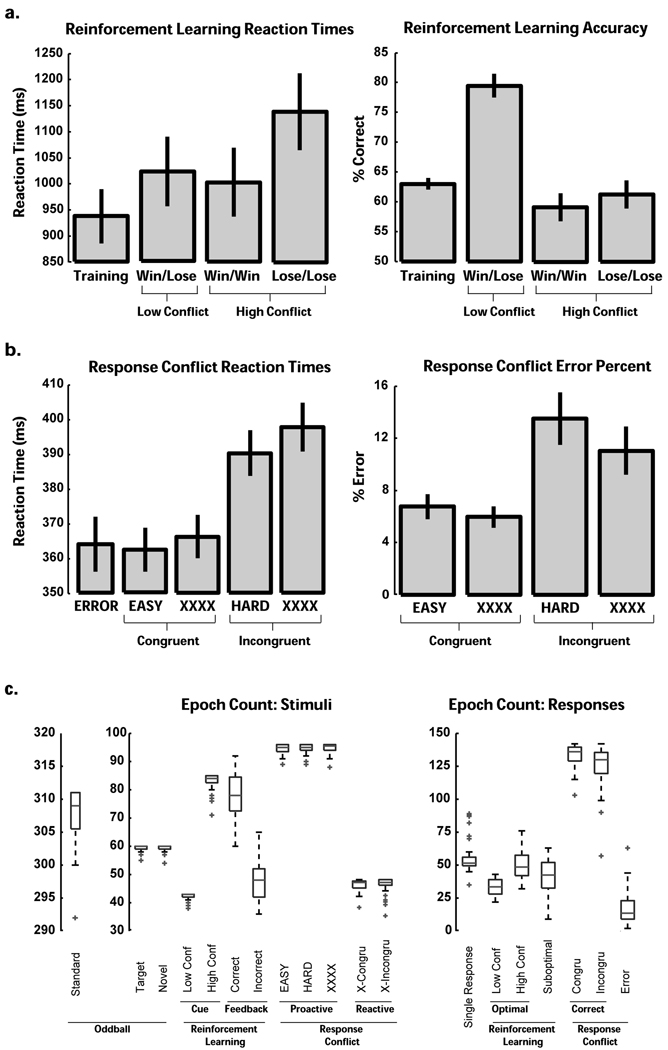
On average, participants were 100% correct in their count of oddball targets (SD = 2%). All participants were >55% accurate in the low conflict test phase condition on the reinforcement learning task. On the reinforcement learning task, high conflict trials (win-win and lose-lose) had significantly slower RTs than low conflict (win-lose) trials (t(39)=2, p<.05), and they were also characterized by poorer accuracy (t(39)=−8.94, p<.001). Whereas the accuracy difference from low conflict trials was similarly large for win-win and lose-lose valences (both t’s >−7.5, p’s<.001), RT was only slower on lose-lose trials (t(39)=3.6, p<.001), not win-win trials (t(39)<1). A highly similar pattern of effects on this same task has been found in Parkinson's patients (Cavanagh, et al., under review).
In the response conflict task, RTs were tested in a 2 (information [EASY & HARD] vs. no-information [XXXX]) * 2 (conflict: congruent vs. incongruent) GLM. There were main effects for both information (F(1,39)=6.05, p<.05) and conflict (F(1,39)=104.21, p<.001) with no interaction, such that XXXX and incongruent trials had longer RTs. Error RTs did not differ from congruent RTs (t<1). See Figure 2 for task performance means and standard errors.
Figure 2.
Time-Frequency Results by Task and Condition
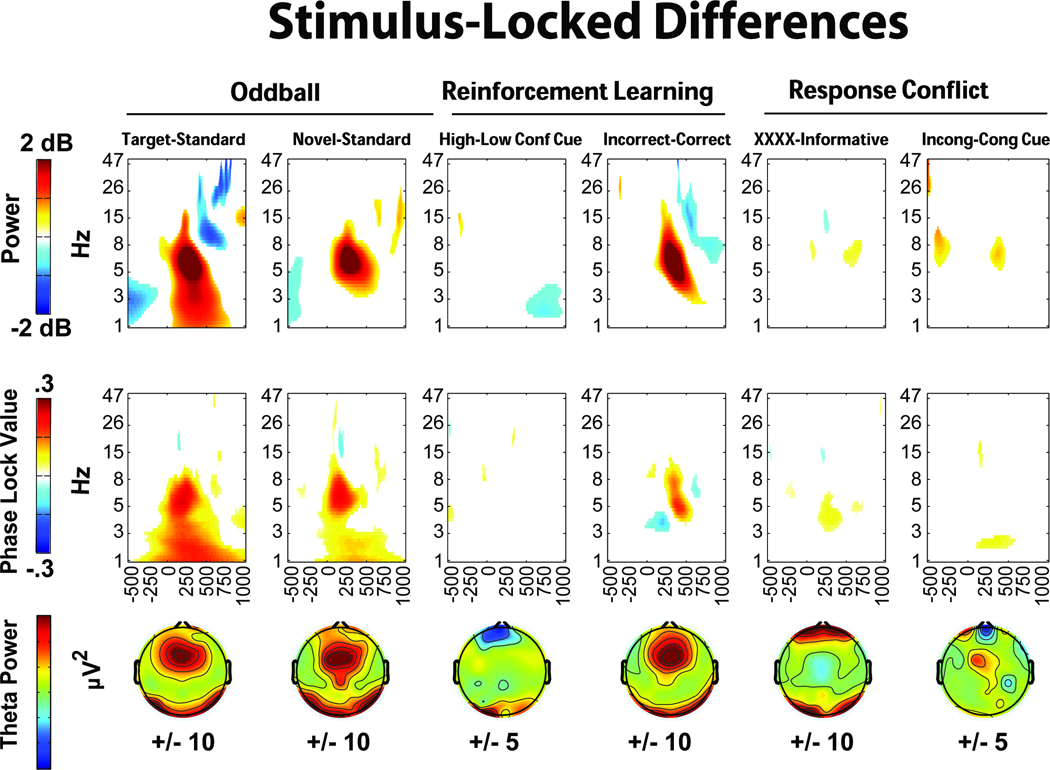
Figures 3 and 4 show stimulus- and response-locked EEG, with columns for each condition, and five rows each detailing different EEG features. All data are presented from the FCz electrode (except, of course, the topographic maps). The first row shows broad-band (.5–15 Hz) ERPs. The second row depicts theta-band (4–8Hz) filtered ERPs, showing consistent theta band phase-locking. The third and fourth rows show permutation thresholded power and intertrial phase coherence for each condition. All non-significant data have been omitted, thus the colors on these time-frequency plots reflect statistically significant changes from rest. The bottom row shows the idiosyncratically scaled topographic plots of theta power (stimulus-locked: mean over 224:276 ms, response-locked: mean over −20:80 ms), demonstrating a consistent mid-frontal focus in nearly all conditions. Supplemental Figure 1 displays the broad-band ERPs overlapped by condition. While some plots show additional frontal and occipital regions that are active on these topoplots, Supplemental Figures 5–8 demonstrate that these are features of the average reference scheme, not contamination by artifact. Across reference schemes, there is a consistent mid-frontal focus of effect.
All stimulus- and response-locked conditions show a distinct pattern of theta power increase and phase consistency, although other findings outside the theta band are also worth noting here. A peri-response power decrease in the beta band is apparent in all trials requiring responses. The time-frequency plot of correct reinforcement feedback (Figure 3) is notable in that there is only a slight theta power enhancement, even though the theta ERP demonstrates consistent phase locking and power increases. The error plot (Figure 4) shows a strong feature of delta band power and phase consistency that has been noted before (Yordanova, et al., 2004). These features are discussed in greater detail in the discussion section. In sum, there was a strong and consistent theta power enhancement and phase consistency to all instances of stimulus and response processing.
Phase-Locked Theta: Comparison between Conditions
Theta band filtered ERPs were contrasted to formally test the proposed ubiquity of phase-locked theta across different conditions during stimulus processing or response commission. Figure 5 shows the theta band filtered ERPs from the second rows of Figures 3 and 4, however here they are overlapped with each task. The time window for the N2/FRN or CRN/ERN is indicated on each plot, demonstrating that ERP features that may be described under unique circumstances (i.e. “mismatch N2”, “FRN”, “control N2”) are all reflective of a highly similar underlying pattern of phase-locked theta band dynamics. Supplemental Figures 2–4 show the same ERP plots for delta (1–4Hz), alpha (8–12Hz), and beta (12–30Hz) bands. Notably, these plots do not show a ubiquitous phase-locked feature across stimulus or response conditions, suggesting that these findings are not due to filter artifacts (c.f. Yeung, et al., 2007).
Figure 5c shows the between-condition phase consistency (PLV) for delta, theta, alpha and beta band filtered ERPs over time. In this application, the PLV quantifies the commonality of phase dynamics between conditions (as opposed to intertrial phase coherence, which acts between trials within a condition). The average theta PLV for stimuli (over 0:500 ms) was significantly higher than all other bands (t’s >3, p’s<.01, Bonferonni corrected); the average theta PLV for responses (over −100:250 ms) was significantly higher than alpha and beta bands (t’s >2.4, p’s<.05, Bonferonni corrected), but not different from the delta band (t=1: we address this notable feature of delta phase coherence in the discussion). This finding quantifies how ERPs are composed of highly similar theta phases over time to all stimulus- and response-locked processes.
Time-Frequency Indices of Novelty, Conflict, Punishment and Error
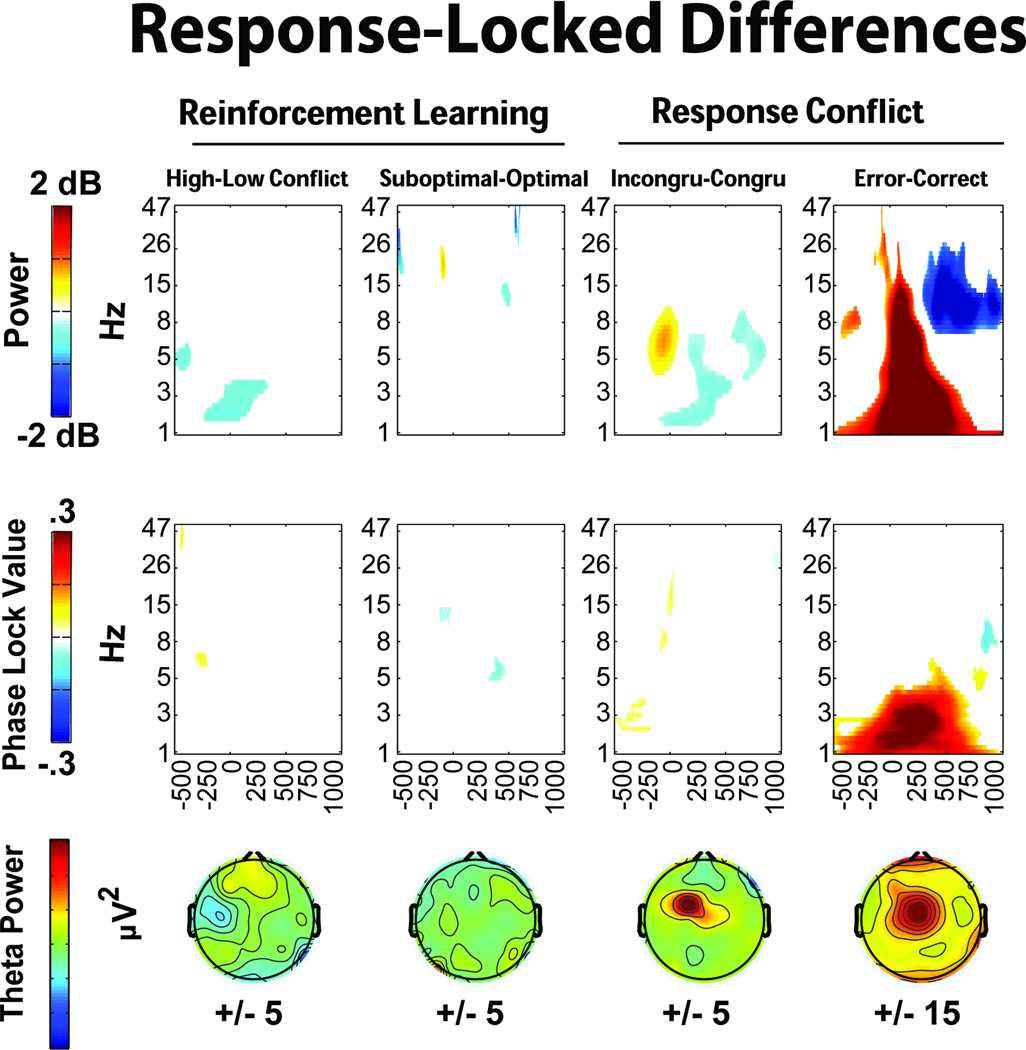
Data-driven differences between conditions are detailed in Figures 6 and 7. In the oddball task, both target and novel stimuli show increased mid-frontal theta band power and phase consistency compared to standards. In the reinforcement learning task, only punishment feedback showed an increase in power and phase consistency above its corresponding condition: there were no midfrontal theta power increases at FCz due to conflict cues. In the response conflict task, non-informative cues elicited greater theta band power and phase consistency, while imperative cues elicited greater theta power and delta intertrial phase coherence. Figure 7 depicts the response-locked differences, where high conflict responses and suboptimal choices on the reinforcement learning task failed to show notable theta power or intertrial phase coherence increases. This was a surprising outcome given previous ERP findings of reliably increased voltage negativities to suboptimal (Cavanagh, Gründler, Frank, & Allen, 2010; Frank, et al., 2005; Gründler, et al., 2009; Holroyd & Coles, 2002). In the response conflict task, both high conflict (incongruent) and error conditions demonstrated theta power increases, with errors additionally characterized by delta power and intertrial phase coherence increases.
Figure 7.
In the reinforcement learning task, conflict-related effects were also absent when each separate high conflict condition (win-win and lose-lose) were compared to the low conflict condition (win-lose). Additionally, there were no differences within or between high and low learning groups (defined by median or tertile splits based on test phase accuracy). In sum, mid-frontal theta power and phase coherence was specifically increased in conditions of novelty, punishment and error, while conflict-related conditions varied in the extent and type of theta dynamic that was enhanced (or not). The additional contribution of delta power and intertrial phase coherence on error trials was strong and unique.
Contrasting ERP and Theta Power Measurements of Novelty, Conflict, Punishment and Error
Three different methods were used to compare the performance of ERP and theta power measurements when resolving between-condition effects: 1) factor analysis, 2) planned comparisons, and 3) power analysis. First, to examine co-variance of ERP and theta power measures, factor analyses were performed with both of these measurements included. In two separate factor analyses, the raw values and the difference measures from each respective contrast condition were examined, with similar results. For simplicity, the component loadings for the difference measures are detailed in Table 1 (Supplemental Table 2 details the raw data components). Table 1 demonstrates that there was considerable between-measurement covariation within tasks, particularly for oddball novelty, Simon response conflict and error, reinforcement learning punishment, and Simon task proactive conflict effects. Although factor loadings derived from a large number of variables with a modest number of participants should be interpreted with caution, these findings provide additional evidence that theta power reflects the same variance as ERP measures to mid-frontal signals of novelty, conflict, punishment and error.
Table 1.
Factor analysis output of difference scores for both EEG measurements (theta power and ERP amplitude) highlighting novelty, conflict, punishment and error. There is considerable between-measurement shared variance for oddball novelty effects (component #1), Simon task response conflict and errors (#2), reinforcement learning task punishment (#3), and Simon task proactive conflict cues (#6). Other components appear to reflect within-measurement variance (#4 & #5) and an undetermined cluster (#7)
| Varimax Loading | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Component | 1 | 2 | 3 | 4 | 5 | 6 | 7 | |||||||
| Measurement (Theta or ERP) | T | E | T | E | T | E | T | E | T | E | T | E | T | E |
| % Variance | 18% | 12% | 10% | 9% | 7% | 7% | 6% | |||||||
| Target - Standard Stim | 0.85 | 0.49 | 0.39 | 0.62 | ||||||||||
| Novel - Standard Stim | 0.81 | 0.55 | 0.00 | 0.40 | ||||||||||
| High - Low Conflict Stim | 0.36 | 0.58 | 0.61 | −0.33 | ||||||||||
| Incorrect - Correct Stim | 0.66 | 0.31 | 0.38 | 0.64 | ||||||||||
| XXXX – (EASY & HARD) Stim | 0.43 | −0.61 | 0.35 | 0.87 | ||||||||||
| Incongruent – Congruent Cue Stim | 0.61 | −0.44 | 0.79 | 0.32 | ||||||||||
| High – Low Conflict Response | 0.38 | −0.44 | 0.36 | 0.74 | ||||||||||
| Suboptimal – Optimal Response | −0.66 | −0.77 | ||||||||||||
| Incongruent – Congruent Response | −0.52 | 0.69 | 0.37 | −0.39 | −0.48 | |||||||||
| Error – Correct Response | 0.77 | 0.72 | ||||||||||||
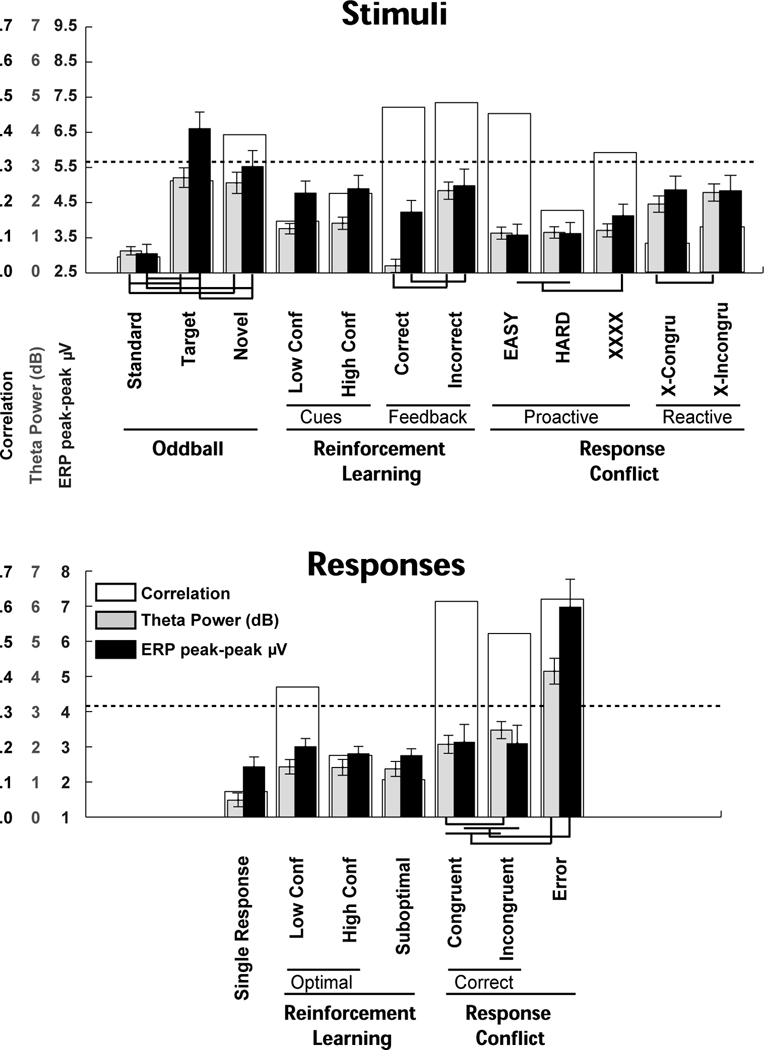
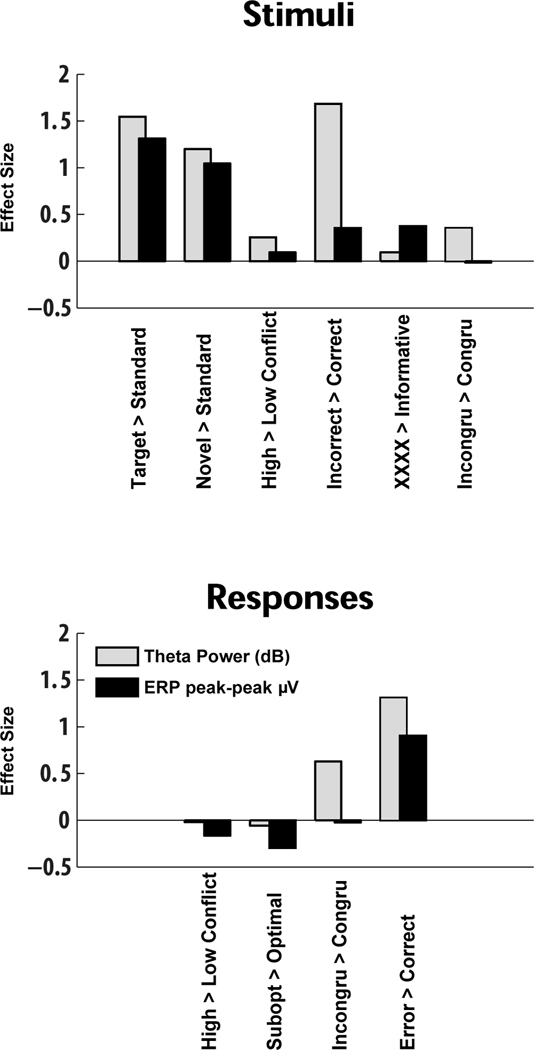
Figure 8 contrasts theta power in dB from the time-frequency plots (in grey) and peak-trough ERP amplitudes (in black) for each condition. The correlation between these measurements is shown behind the bars (in white), and lines underneath show significant a priori comparisons for novelty, conflict, punishment and error. See Supplemental Table 1 for t-test outputs. Whereas Figures 6 and 7 detail these same findings in the theta band, this plot demonstrates the functional similarity between standard ERP and theta power measurements in most, but not all conditions3. The results of the power analyses are presented in Figure 9, and demonstrate that in nearly every between-condition contrast, theta power had a larger effect size when compared to ERP amplitudes. Summarizing these analyses, it appears that theta power and ERP amplitude measurements similarly capture variance in action monitoring processes, yet time-frequency measurements provide more information and a larger effect size. Together, the findings here suggest that theta dynamics are ubiquitous to stimuli and responses, and variance within this theta feature reflects neural responses due to novelty, conflict, punishment and error.
Discussion
This study compared midfrontal EEG activities during a wide variety of executive functions related to learning and performance (action monitoring). The ERP approach has defined psychologically-relevant features of these EEG signals with varying initialisms (N2, FRN, ERN, CRN) based on timing, topography, and eliciting circumstances. While this approach has led to a robust and differentiated literature of action monitoring ERPs, this method may fail to communicate common features of these components. Fitting with the abundance of independent studies cited in the introduction, the current study suggests that mid-frontal theta is the dominant characteristic of all of these aforementioned ERP components. This commonality has important implications for interpreting the functional and computational roles of these scalp-recorded signals.
Theta: Consistency and Enhancement
Figures 3 and 4 clearly demonstrate a similar theta band feature during stimulus processing and motor responses. While other there is also significant activity in other frequency bands, there are no other band-specific phenomena that are so consistent across conditions. Topographic maps of theta power detail a variety of cortical areas that are active during stimulus and response processing, yet there is a reliable mid-frontal focus of activity. Phase-locked theta ERPs and the dominance of theta band power and intertrial phase coherence in the time-frequency representations suggest that this specific frequency band contributes strongly to mid-frontal ERP components (the stimulus-locked P2-N2 complex and the response-locked ERN/CRN). The strong theta band ERP phase consistency between conditions (Figure 5C) suggests that a similar phase-locked feature is shared between conditions. These findings provide evidence that this specific frequency and phase dynamic may reflect a common mechanism for temporal organization of neural processes during action monitoring.
Figures 6 and 7 demonstrate that mid-frontal theta power is enhanced in conditions of novelty, conflict, punishment and error. In fact, mid-frontal theta and standard ERP measures were both similarly modulated by these factors (Figure 8). While there were slight differences in statistical significance that might reflect meaningful benefits of one method over the other in certain circumstances (ERP measures differentiated target and novel oddball conditions and proactive conflict Simon task conditions; theta power differentiated stimulus and response conflict during the Simon task), both measures reliably reflected the experimental conditions of novelty, conflict, punishment and error. Even the unexpected failures to replicate previous findings (suboptimal > optimal responses in reinforcement learning)4 and to differentiate novel conditions (high vs. low conflict in stimulus- and response-locked reinforcement learning conditions) were common to both theta and ERP methods. These findings suggest that in addition to the dominant and shared theta band basis of the ERPs, both measures reflect the same psychologically meaningful constructs. This interpretation is supported by the factor analysis, which demonstrated that ERP and theta measures co-vary during novelty, conflict, punishment and error processes. Yet, the power analyses (Figure 9) suggests that theta power may be a more sensitive index of between-condition differences than ERP amplitudes. While each measurement approach has methodological advantages and disadvantages, interpretation of these signals in the context of a common theta band process offers the most parsimonious and powerful explanation of function. An appreciation of the common theta band process underlying these diverse features may offer theoretical and practical advantages.
A Theoretical Role of Theta Dynamics
Here we examine the hypothesis that mid-frontal theta phase dynamics act as common templates for the temporal organization of neural responses to stimuli and responses, with variation on this template reflecting neural reactions to novelty, conflict, punishment and error. EEG dynamics reflect physiological mechanisms for organizing and communicating neural computations. Synchronous oscillations are thought to reflect a mechanism for entrained interregional activity: rhythmic excitability may allow temporal windows of coordinated spike timing across spatially separate neural networks, presumably reflecting functional communication (Buzsáki, 2006; Buzsaki & Draguhn, 2004; Fries, 2005; Womelsdorf, et al., 2007). The ubiquity of theta-band findings across species have led to the suggestion that theta reflects a non-specific mechanism for organizing neural processes around “decision points”, such as action selection (Womelsdorf, Vinck, Leung, & Everling, 2010). The high degree of intertrial and cross-condition theta phase consistency reported here provides evidence that these theta activities to stimuli and responses reflect a common template for the temporal organization of neuronal populations during endogenous and exogenous action monitoring processes. Novelty, conflict, punishment and error responses all appear to be primarily indicated by power increases on this common organizational theme. We qualify this simplification by noting that there are slight frequency and phase differences between conditions (Figure 5) and experimental factors also elicit changes in theta phase dynamics and have varied contributions from other frequency bands (Figures 6 and 7). However, here we aim to distinguish broad effects and commonalities between conditions.
The current study demonstrated that simple button presses - where only digit on one hand is capable of executing the required action - evoke an event-related theta band power increase and phase consistency that is reflected in the ERP as a negative deflection (i.e. the CRN). This suggests that this theta response to motor selection is a reflection of a generic process of mPFC functioning: the act of making a single motor response without the possibility of response conflict (as traditionally defined) produces the theta/CRN feature. Similarly, an event-related theta/N2 dynamic is elicited by the standard stimuli in the oddball task (observing a standard visual stimulus). This pattern suggests that generic event-related endogenous and exogenous processes may be reflected by theta band dynamics in the time range of the ERN/CRN and the FRN/N2, respectively.
Experimental factors can cause dissociation in ERP component amplitudes between conditions, as do lesions (Gehring & Knight, 2000; Swick & Turken, 2002; Ullsperger & von Cramon, 2006), drug challenge (de Bruijn, Hulstijn, Verkes, Ruigt, & Sabbe, 2004; de Bruijn, Sabbe, Hulstijn, Ruigt, & Verkes, 2006; Zirnheld et al., 2004), and even clinical symptomatology (Gründler, et al., 2009). However, the basic morphology of relevant stimulus and response ERP components were retained in all of these cases, in line with the idea of common templates. It should be noted that this hypothesis of temporal organization is lacking in spatial specificity beyond the single electrode (FCz) reported here. Different conditions likely reflect an aggregation of multiple contributing sources (c.f. Cohen, et al., 2008). Indeed, distributed sources may be ideally served by a common, low frequency temporal organizational scheme.
Practical Implications for Event-Related EEG
Novel analysis methods that are well suited to time-frequency methods, including single-trial analysis, can reveal more information than the standard fixed effect analysis reported here (Cohen, 2011). As an example of a benefit of time-frequency analytic perspective, consider the EEG response to correct reinforcement feedback. The neural response to correct feedback was characterized by a very slight degree of theta power increase above rest (Fig. 3). This finding fits with the description of a Reward Positivity ERP component that has been suggested to “cancel out” the N2 component (Baker & Holroyd, 2011; Holroyd, et al., 2008), see the Figure 3 broad-band ERP ~250:500ms. While theta power was diminished during correct feedback trials, the phase-locked theta ERP and strong theta phase consistency demonstrate that the common theta phase dynamic to stimulus processing remained intact, contributing to the evidence that this theta response reflects a common temporal template. Future investigations will need to determine what part of the N2/theta process to rewards is cancelled, diminished, or overlapped by other processes: the current findings suggest that phase-related activities are retained yet power-related activities are diminished during positive reinforcement.
Another benefit of time-frequency description of event-related EEG may be a more careful summary of possibly related events. For example, the error-following Pe component (Overbeek, Nieuwenhuis, & Ridderinkhof, 2005) is often parsed into an early mid-frontal positivity (~250 ms post-error: see Fig 4, row 1, column 5) and a late posterior positivity (~400–500ms post-error) (Van Veen & Carter, 2002). Yet, the morphology of the early Pe appears to be the peak of the theta/delta deflection immediately following the ERN. While this EEG feature may reflect a psychologically unique construct that is not reflected in the ERN, it may also be a reflection of the next (180 degree) peak of the theta or delta process. A similar argument could be made about the P3a to novel stimuli: many of the voltage negativities in the N2 time range reflect parts of multi-phasic waveforms that have been associated with a temporal cascade of mismatch processing. Huster, Westerhausen, Pantev, and Konrad (2010) reviewed a number of source localization studies of the N2 and P3 in response inhibition tasks, and demonstrated that these components were associated with anterior and posterior midcingulate activation, respectively, each within a unique network of other brain activations. However, the scope of this investigation did not aim to define later, related ERP components or other components potentially associated with theta activities to mismatch or control (i.e. mismatch negativity, stop signal N2, NoGo N2). In sum, these examples demonstrate that without an appreciation of common underlying temporal-spectral features, the ERP literature may suffer from a lack of parsimonious accounting of neural responses.
Delta and Beta Bands
Before moving on to further discussion of the relevance of theta band dynamics, findings in other frequency bands will be addressed. Besides the common theta feature, each time-frequency representation showed unique temporal, spatial, and frequency effects, detailing a multitude of condition-specific patterns. There was consistent beta-band power suppression before, during and after motor responses. This consistent beta power decrease to manual responses (sometimes termed desynchronization or beta blocking) stands in contrast to the notable beta power enhancement following performance feedback (Figure 3). Replicating previous findings (Cohen, et al., 2007; Marco-Pallares, et al., 2008), beta power was greater following correct than incorrect feedback (the inverse pattern is shown in Figure 7), even though this finding has not been consistently replicated (Cavanagh, Frank, et al., 2010; Christie & Tata, 2009). A functional interpretation of this feedback-specific beta response remains to be defined, although some have suggested that mediofrontal-motor cortex co-activity is increased following feedback (Cohen & Ranganath, 2007).
Delta band power increases were apparent during demanding motor responses on the response conflict task, and they were especially prevalent on errors (also see: Yordanova, et al., 2004). Delta activity could reflect error-specific processing in mPFC, the combined summation of other neural areas that contribute specifically to errors, or even variance traditionally associated with the Pe. Delta and theta bands were equally phase coherent during responses (Figure 5c), and all frequency bands increased in phase consistency immediately following a response. While these findings don’t argue against a special role for theta-band dynamics, they do indicate that there is a multitude of interactive processes during manual responses. Dissociation amongst these frequency band effects may reveal unique computational functions. For example, although responses are associated with strong theta and delta intertrial phase coherence, only theta phase dynamics are relevant to conflict-related RT adaptation (Cohen & Cavanagh, 2011), fitting with other findings that theta power predicts RT adaptation following conflict (Cavanagh, et al., under review), punishment (Cavanagh, Frank, et al., 2010), and error (Cavanagh, et al., 2009; Debener, et al., 2005).
A Generic Reflection of Cortical Processing
It is important to note that neither the mPFC nor the field of action monitoring is unique in regard to theta band reflections of active processing. Human cortical theta is generated from multiple local sites (Caplan et al., 2003; Jacobs, Hwang, Curran, & Kahana, 2006; Raghavachari et al., 2006), but the specific case of frontal midline theta has been localized to the ACC and surrounding mPFC areas using MEG (Ishii et al., 1999) and EEG (Gevins & Smith, 2000; Gevins, Smith, McEvoy, & Yu, 1997; Gevins, Smith, McEvoy, Leong, & Le, 1999; Jensen & Tesche, 2002; Onton, et al., 2005). Cortical theta has been broadly implicated in sensorimotor integration during spatial navigation (Baker & Holroyd, 2009; Caplan, et al., 2003) and episodic memory encoding and retrieval (Jacobs, et al., 2006; Kahana, Sekuler, Caplan, Kirschen, & Madsen, 1999; Klimesch, 1999; Klimesch, Doppelmayr, Schwaiger, Winkler, & Gruber, 2000; Klimesch, Sauseng, Hanslmayr, Gruber, & Freunberger, 2007; Nyhus & Curran, 2010; Raghavachari, et al., 2006; Rizzuto, Madsen, Bromfield, Schulze-Bonhage, & Kahana, 2006; Rizzuto et al., 2003; Sauseng et al., 2004), while the specific case of frontal midline theta has been implicated in decision making and working memory maintenance (Gevins, et al., 1997; Ishii, et al., 1999; Onton, et al., 2005; Sauseng, Griesmayr, Freunberger, & Klimesch, 2010). Recent studies have begun to identify how mPFC operations implicated in adaptive control may specifically contribute to these other cognitive phenomena, including conflict processing during memory retrieval (Hanslmayr, Staudigl, Aslan, & Bauml, 2010; Staudigl, Hanslmayr, & Bauml, 2010) and effortful attention during decision making (Mulert, Menzinger, Leicht, Pogarell, & Hegerl, 2005; Mulert et al., 2008). These functional and topographic distinctions further demonstrate how theta band power dynamics likely reflect a non-specific marker of active cortical operation. In this manuscript, we only address event-related mid-frontal theta responses during action monitoring tasks. It is likely that other investigations will reveal unique and dissociated functions of cortical theta to a variety of cognitive processes.
Implications for Theories of ERP Responses
Most theoretical or computational accounts of the ERN, FRN and N2 ERPs involve a comparator function. The ERN has been suggested to reflect a comparison between correct vs. actual motor responses (perhaps using an afferent motor copy: Coles, Scheffers, & Holroyd, 2001; Falkenstein, Hoormann, Christ, & Hohnsbein, 2000). In reinforcement learning, both the ERN and FRN have been suggested to reflect negative reward (punishment) prediction errors (Holroyd & Coles, 2002), as defined by reinforcement learning theory (Sutton & Barto, 1998). The eponymous mismatch N2 implies a perceptual difference between actual experience and past history or predominant category (Folstein & Van Petten, 2008), whereas the control N2 appears to be related to the occurrence of conflict, which may be more broadly defined. One common definition of conflict stands out from the comparator description: the Yeung et el., (2004) model of the ERN and conflict N2 does not invoke a comparison per se, but rather interprets the degree of co-activity between motor preparations as conflict (Hopfield, 1982). While these influential theories may appear to suggest functional differences, one can also highlight computational similarities between mismatch comparison and measures of co-activity such as free energy or entropy (Friston, 2003, 2005; O'Reilly & Munakata, 2000).
One notable exception from these theoretical accounts involves the CRN, which is not specifically accounted for in any major theories of human action monitoring, and which may not involve a comparator function or even co-active motor responses. If the CRN is interpreted as an inherent feature of theta-band phase activities during manual responses, as highlighted by the single response task response-locked theta, previous theoretical and computational accounts (mismatch or conflict) of the ERN may still be considered accurate accounts of enhanced processes that take place over an inherent background of phase-locked theta dynamics. As these theories and models continue to integrate and evolve (see: Cockburn & Frank, 2011; Holroyd & Yeung, 2011), inclusion of such biophysical details will contribute to the biological realism and accurate empirical verification of future model-based predictions.
Implications for Theories of ACC
Computational models of general ACC function have suggested this neural system acts as an evaluative node in a global computational workspace (Botvinick, et al., 2001; Dehaene, Kerszberg, & Changeux, 1998), especially when effort and vigilance are required. In both these models, errors increase vigilance and connectivity between distributed processing nodes, which then decline again as the task becomes routine – eventually leading to more errors. This account suggests that a low level, basic monitoring process underlies action monitoring, and that errors function as alarm signals when the task is not being performed as expected. Theta dynamics appear to reflect the operations of this hypothesized ACC node.
Novelty, conflict, punishment and error could all be considered signals that increase vigilance and active processing (which may be defined as surprise, effort or attention in some cases). Microelectrode recordings in dorsal ACC reflect theta dynamics in the same microdomain to different tasks and stimuli, including errors, novelty, and effortful processing (Wang, et al., 2005), fitting with other invasive recordings across species (Cohen, et al., 2008; Leung & Borst, 1987; Tsujimoto, et al., 2006; Womelsdorf, Johnston, Vinck, & Everling, 2010). A decline in EEG power has also been detailed in CRNs / theta power (Cavanagh, et al., 2009; Vidal, et al., 2003; Vidal, et al., 2000) and N2s (Eichele, Juvodden, Ullsperger, & Eichele, 2010) immediately prior to an error. Following an event, transient theta phase relations between mid-frontal and other brain areas have been proposed to reflect a communication mechanism for vigilance-instantiated cognitive control with lateral PFC (Cavanagh, et al., 2009; Cavanagh, Frank, et al., 2010; Cohen & Cavanagh, 2011; Hanslmayr et al., 2008), and sensory attention with the occipital lobe (Cohen, van Gaal, Ridderinkhof, & Lamme, 2009). Widespread intracranial phase synchrony between ACC and other sites has been detailed as well, to multiple types of eliciting stimuli (Wang, et al., 2005). Given that both ERN amplitudes and ACC activities are modulated by negative affect and anxious arousal (Etkin, Egner, & Kalisch, 2011; Olvet & Hajcak, 2008; Shackman et al., 2011), theta appears to be a sensitive index of multidimensional aspects of mPFC function.
Conclusion
A common language can be used to communicate many different things. The ubiquity of mid-frontal theta suggests that this signal reflects a generic processing mechanism for coordinating endogenous and exogenous performance-relevant information. This process is enhanced in situations typically associated with mPFC functioning: reactive responses to novelty, conflict, punishment and error. Theta-band phase dynamics may represent a biophysical mechanism for the common temporal organization of neural processes during stimulus or response processes. Variation on this theme, such as power enhancement, appears to reflect the realization of these reactive responses. These computations appear to be used to merge attentive, affective, and cognitive functions with motor selection in order to utilize environmental context during action monitoring. Theta therefore appears to reflect general operations of the mPFC during action monitoring.
Supplementary Material
Broad band (.05–15 Hz) ERPs at the FCz electrode, overlapped within each condition.
Factor analysis results of raw EEG data.
Delta band (1–4 Hz) ERPs at the FCz electrode
Alpha band (1–4 Hz) ERPs at the FCz electrode
Beta band (1–4 Hz) ERPs at the FCz electrode
Stimulus-locked topoplots as shown in Figure 3 of the main text (average reference), with additional artifact rejection (+/− 100 µV at FP1) or with an alternative reference scheme (linked mastoids). These plots demonstrate that frontal and occipital theta power seen in the topoplots are a feature of the averaged reference, and are not due to artifact contamination.
Response-locked topoplots as shown in Figure 4 with variants as above.
Stimulus-locked topoplots as shown in Figure 6 with variants as above.
Response-locked topoplots as shown in Figure 7 with variants as above.
Paired t-test data from Figure 8.
Acknowledgements
The authors thank Alhondra Felix and Katie Yeager for help running participants, Thomas Wiecki for help implementing the parallelization of the permutation tests, and the contribution of the editor Stephan Debener and three anonymous reviewers who helped the development of this manuscript.
Funding
This work was supported the National Institute of Mental Health [F31MH082560 to JFC], and by infrastructure provided by National Institute of Mental Health [R01MH066902 to JJBA].
Footnotes
While Vogt (2005) has developed a new nomenclature to parse the cingulate cortex into regions and sub-regions based on functional distinction, this investigation does not aim to interpret the spatial specificity of EEG signals or reinterpret the findings from previously published works. As such, we use the broad term ‘mPFC’ when inferring the source contribution of EEG data recorded the FCz electrode, and we use the anatomical terminology specific to each citation when detailing prior findings. Note that many subsequent descriptions of ‘ACC’ activation may actually occur in Vogt’s ‘mid cingulate’ area; not the peri and sub genual ‘anterior cingulate’ area.
All results were similar when compared to current source density-transformed EEG.
These contrasts were also performed with a mean amplitude measure of ERP power instead of the peak-trough difference, but the correlations were smaller in almost all cases.
We suspect that the failure to replicate suboptimal>optimal differences may be due to the short and simple blocks of this reinforcement learning task compared to other tasks with demonstrated successful replications (Cavanagh, Gründler, et al., 2010; Frank, et al., 2005; Gründler, et al., 2009; Holroyd & Coles, 2002).
References
- Baker TE, Holroyd CB. Which way do I go? Neural activation in response to feedback and spatial processing in a virtual T-maze. Cereb Cortex. 2009;19(8):1708–1722. doi: 10.1093/cercor/bhn223. [DOI] [PubMed] [Google Scholar]
- Baker TE, Holroyd CB. Dissociated roles of the anterior cingulate cortex in reward and conflict processing as revealed by the feedback error-related negativity and N200. Biol Psychol. 2011 doi: 10.1016/j.biopsycho.2011.01.010. [DOI] [PubMed] [Google Scholar]
- Bernat EM, Nelson LD, Holroyd CB, Gehring WJ, Patrick CJ. Separating cognitive processes with principal components analysis of EEG time-frequency distributions. Proc. SPIE. 70742008 70740S. [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108(3):624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Burle B, Roger C, Allain S, Vidal F, Hasbroucq T. Error negativity does not reflect conflict: a reappraisal of conflict monitoring and anterior cingulate cortex activity. J Cogn Neurosci. 2008;20(9):1637–1655. doi: 10.1162/jocn.2008.20110. [DOI] [PubMed] [Google Scholar]
- Buzsáki G. Rhythms of the brain. Oxford; New York: Oxford University Press; 2006. [Google Scholar]
- Buzsaki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304(5679):1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- Caplan JB, Madsen JR, Schulze-Bonhage A, Aschenbrenner-Scheibe R, Newman EL, Kahana MJ. Human theta oscillations related to sensorimotor integration and spatial learning. J Neurosci. 2003;23(11):4726–4736. doi: 10.1523/JNEUROSCI.23-11-04726.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280(5364):747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Cavanagh JF, Cohen MX, Allen JJ. Prelude to and resolution of an error: EEG phase synchrony reveals cognitive control dynamics during action monitoring. J Neurosci. 2009;29(1):98–105. doi: 10.1523/JNEUROSCI.4137-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Cohen MX, Wiecki TV, Figueroa CM, Samanta JS, S J, Frank MJ. Subthalamic nucleus stimulation reverses mediofrontal influence over decision threshold. doi: 10.1038/nn.2925. (under review). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Frank MJ, Klein TJ, Allen JJB. Frontal theta links prediction errors to behavioral adaptation in reinforcement learning. NeuroImage. 2010;49(4):3198–3209. doi: 10.1016/j.neuroimage.2009.11.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Gründler TOJ, Frank MJ, Allen JJB. Altered cingulate sub-region activation accounts for task-related dissociation in ERN amplitude as a function of obsessive-compulsive symptoms. Neuropsychologia. 2010;48(7):2098–2109. doi: 10.1016/j.neuropsychologia.2010.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie GJ, Tata MS. Right frontal cortex generates reward-related theta-band oscillatory activity. Neuroimage. 2009;48(2):415–422. doi: 10.1016/j.neuroimage.2009.06.076. [DOI] [PubMed] [Google Scholar]
- Cockburn J, Frank M. Reinforcement learning, conflict monitoring, and cognitive control: An integrative model of cingulate-striatal interactions and the ERN. 2011 [Google Scholar]
- Cohen MX. It's about Time. Front Hum Neurosci. 2011;5:2. doi: 10.3389/fnhum.2011.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MX, Cavanagh JF. Single-trial regression elucidates the role of prefrontal theta oscillations in response conflict. Frontiers in Psychology. 2011;2(30):1–12. doi: 10.3389/fpsyg.2011.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MX, Elger CE, Ranganath C. Reward expectation modulates feedback-related negativity and EEG spectra. Neuroimage. 2007;35(2):968–978. doi: 10.1016/j.neuroimage.2006.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MX, Ranganath C. Reinforcement learning signals predict future decisions. J Neurosci. 2007;27(2):371–378. doi: 10.1523/JNEUROSCI.4421-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MX, Ridderinkhof KR, Haupt S, Elger CE, Fell J. Medial frontal cortex and response conflict: evidence from human intracranial EEG and medial frontal cortex lesion. Brain Res. 2008;1238:127–142. doi: 10.1016/j.brainres.2008.07.114. [DOI] [PubMed] [Google Scholar]
- Cohen MX, van Gaal S, Ridderinkhof KR, Lamme VA. Unconscious errors enhance prefrontal-occipital oscillatory synchrony. Front Hum Neurosci. 2009;3:54. doi: 10.3389/neuro.09.054.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles MG, Scheffers MK, Holroyd CB. Why is there an ERN/Ne on correct trials? Response representations, stimulus-related components, and the theory of error-processing. Biol Psychol. 2001;56(3):173–189. doi: 10.1016/s0301-0511(01)00076-x. [DOI] [PubMed] [Google Scholar]
- de Bruijn ER, Hulstijn W, Verkes RJ, Ruigt GS, Sabbe BG. Drug-induced stimulation and suppression of action monitoring in healthy volunteers. Psychopharmacology (Berl) 2004;177(1–2):151–160. doi: 10.1007/s00213-004-1915-6. [DOI] [PubMed] [Google Scholar]
- de Bruijn ER, Sabbe BG, Hulstijn W, Ruigt GS, Verkes RJ. Effects of antipsychotic and antidepressant drugs on action monitoring in healthy volunteers. Brain Res. 2006;1105(1):122–129. doi: 10.1016/j.brainres.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Debener S, Ullsperger M, Siegel M, Fiehler K, von Cramon DY, Engel AK. Trial-by-trial coupling of concurrent electroencephalogram and functional magnetic resonance imaging identifies the dynamics of performance monitoring. J Neurosci. 2005;25(50):11730–11737. doi: 10.1523/JNEUROSCI.3286-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene S, Kerszberg M, Changeux JP. A neuronal model of a global workspace in effortful cognitive tasks. Proc Natl Acad Sci U S A. 1998;95(24):14529–14534. doi: 10.1073/pnas.95.24.14529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134(1):9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118(Pt 1):279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Donkers FC, Nieuwenhuis S, van Boxtel GJ. Mediofrontal negativities in the absence of responding. Brain Res Cogn Brain Res. 2005;25(3):777–787. doi: 10.1016/j.cogbrainres.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Donkers FC, van Boxtel GJ. The N2 in go/no-go tasks reflects conflict monitoring not response inhibition. Brain Cogn. 2004;56(2):165–176. doi: 10.1016/j.bandc.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Eichele H, Juvodden HT, Ullsperger M, Eichele T. Mal-adaptation of event-related EEG responses preceding performance errors. Front Hum Neurosci. 2010;4 doi: 10.3389/fnhum.2010.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. 2011;15(2):85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoormann J, Blanke L. Effects of crossmodal divided attention on late ERP components. II. Error processing in choice reaction tasks. Electroencephalogr Clin Neurophysiol. 1991;78(6):447–455. doi: 10.1016/0013-4694(91)90062-9. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hoormann J, Christ S, Hohnsbein J. ERP components on reaction errors and their functional significance: a tutorial. Biol Psychol. 2000;51(2-3):87–107. doi: 10.1016/s0301-0511(99)00031-9. [DOI] [PubMed] [Google Scholar]
- Fell J, Dietl T, Grunwald T, Kurthen M, Klaver P, Trautner P, et al. Neural bases of cognitive ERPs: more than phase reset. J Cogn Neurosci. 2004;16(9):1595–1604. doi: 10.1162/0898929042568514. [DOI] [PubMed] [Google Scholar]
- Folstein JR, Van Petten C. Influence of cognitive control and mismatch on the N2 component of the ERP: a review. Psychophysiology. 2008;45(1):152–170. doi: 10.1111/j.1469-8986.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MJ, Seeberger LC, O'Reilly RC. By carrot or by stick: cognitive reinforcement learning in parkinsonism. Science. 2004;306(5703):1940–1943. doi: 10.1126/science.1102941. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Woroch BS, Curran T. Error-related negativity predicts reinforcement learning and conflict biases. Neuron. 2005;47(4):495–501. doi: 10.1016/j.neuron.2005.06.020. [DOI] [PubMed] [Google Scholar]
- Fries P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn Sci. 2005;9(10):474–480. doi: 10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Friston K. Learning and inference in the brain. Neural Netw. 2003;16(9):1325–1352. doi: 10.1016/j.neunet.2003.06.005. [DOI] [PubMed] [Google Scholar]
- Friston K. A theory of cortical responses. Philos Trans R Soc Lond B Biol Sci. 2005;360(1456):815–836. doi: 10.1098/rstb.2005.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring WJ, Goss B, Coles MGH, Meyer DE, Donchin E. A Neural System for Error-Detection and Compensation. Psychological Science. 1993;4(6):385–390. [Google Scholar]
- Gehring WJ, Knight RT. Prefrontal-cingulate interactions in action monitoring. Nat Neurosci. 2000;3(5):516–520. doi: 10.1038/74899. [DOI] [PubMed] [Google Scholar]
- Gevins A, Smith ME. Neurophysiological measures of working memory and individual differences in cognitive ability and cognitive style. Cereb Cortex. 2000;10(9):829–839. doi: 10.1093/cercor/10.9.829. [DOI] [PubMed] [Google Scholar]
- Gevins A, Smith ME, McEvoy L, Yu D. High-resolution EEG mapping of cortical activation related to working memory: effects of task difficulty, type of processing, and practice. Cereb Cortex. 1997;7(4):374–385. doi: 10.1093/cercor/7.4.374. [DOI] [PubMed] [Google Scholar]
- Gevins A, Smith ME, McEvoy LK, Leong H, Le J. Electroencephalographic imaging of higher brain function. Philos Trans R Soc Lond B Biol Sci. 1999;354(1387):1125–1133. doi: 10.1098/rstb.1999.0468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gründler TOJ, Cavanagh JF, Figueroa CM, Frank MJ, Allen JJB. Task-related dissociation in ERN amplitude as a function of obsessive-compulsive symptoms. Neuropsychologia. 2009;47(8–9):1978–1987. doi: 10.1016/j.neuropsychologia.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G, Moser JS, Yeung N, Simons RF. On the ERN and the significance of errors. Psychophysiology. 2005;42(2):151–160. doi: 10.1111/j.1469-8986.2005.00270.x. [DOI] [PubMed] [Google Scholar]
- Hanslmayr S, Pastotter B, Bauml KH, Gruber S, Wimber M, Klimesch W. The electrophysiological dynamics of interference during the Stroop task. J Cogn Neurosci. 2008;20(2):215–225. doi: 10.1162/jocn.2008.20020. [DOI] [PubMed] [Google Scholar]
- Hanslmayr S, Staudigl T, Aslan A, Bauml KH. Theta oscillations predict the detrimental effects of memory retrieval. Cogn Affect Behav Neurosci. 2010;10(3):329–338. doi: 10.3758/CABN.10.3.329. [DOI] [PubMed] [Google Scholar]
- Holroyd CB. A note on the Oddball N200 and the Feedback ERN. In: M. F. Ullsperger M, editor. Errors, Conflicts and the Brain. Current Opinions on Performance Monitoring. Dortmund, Germany: 2002. pp. 211–218. [Google Scholar]
- Holroyd CB, Coles MG. The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychol Rev. 2002;109(4):679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Pakzad-Vaezi KL, Krigolson OE. The feedback correct-related positivity: sensitivity of the event-related brain potential to unexpected positive feedback. Psychophysiology. 2008;45(5):688–697. doi: 10.1111/j.1469-8986.2008.00668.x. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Yeung N. An integrative theory of anterior cingulate cortex function: Option selection in hierarchical reinforcement learning. In: Mars S, Rushworth, Yeung, editors. The Neural Basis of Motivational and Cognitive Control. MIT Press; 2011. (Ed.) [Google Scholar]
- Hopfield JJ. Neural networks and physical systems with emergent collective computational abilities. Proc Natl Acad Sci U S A. 1982;79(8):2554–2558. doi: 10.1073/pnas.79.8.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huster RJ, Westerhausen R, Pantev C, Konrad C. The role of the cingulate cortex as neural generator of the N200 and P300 in a tactile response inhibition task. Hum Brain Mapp. 2010;31(8):1260–1271. doi: 10.1002/hbm.20933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii R, Shinosaki K, Ukai S, Inouye T, Ishihara T, Yoshimine T, et al. Medial prefrontal cortex generates frontal midline theta rhythm. Neuroreport. 1999;10(4):675–679. doi: 10.1097/00001756-199903170-00003. [DOI] [PubMed] [Google Scholar]
- Jacobs J, Hwang G, Curran T, Kahana MJ. EEG oscillations and recognition memory: theta correlates of memory retrieval and decision making. Neuroimage. 2006;32(2):978–987. doi: 10.1016/j.neuroimage.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Jensen O, Tesche CD. Frontal theta activity in humans increases with memory load in a working memory task. Eur J Neurosci. 2002;15(8):1395–1399. doi: 10.1046/j.1460-9568.2002.01975.x. [DOI] [PubMed] [Google Scholar]
- Kahana MJ, Sekuler R, Caplan JB, Kirschen M, Madsen JR. Human theta oscillations exhibit task dependence during virtual maze navigation. Nature. 1999;399(6738):781–784. doi: 10.1038/21645. [DOI] [PubMed] [Google Scholar]
- Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res Brain Res Rev. 1999;29(2–3):169–195. doi: 10.1016/s0165-0173(98)00056-3. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Schwaiger J, Winkler T, Gruber W. Theta oscillations and the ERP old/new effect: independent phenomena? Clin Neurophysiol. 2000;111(5):781–793. doi: 10.1016/s1388-2457(00)00254-6. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Sauseng P, Hanslmayr S, Gruber W, Freunberger R. Event-related phase reorganization may explain evoked neural dynamics. Neurosci Biobehav Rev. 2007;31(7):1003–1016. doi: 10.1016/j.neubiorev.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Lachaux JP, Rodriguez E, Martinerie J, Varela FJ. Measuring phase synchrony in brain signals. Hum Brain Mapp. 1999;8(4):194–208. doi: 10.1002/(SICI)1097-0193(1999)8:4<194::AID-HBM4>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Van Quyen M, Bragin A. Analysis of dynamic brain oscillations: methodological advances. Trends Neurosci. 2007;30(7):365–373. doi: 10.1016/j.tins.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Leung LW, Borst JG. Electrical activity of the cingulate cortex. I. Generating mechanisms and relations to behavior. Brain Res. 1987;407(1):68–80. doi: 10.1016/0006-8993(87)91220-0. [DOI] [PubMed] [Google Scholar]
- Luu P, Tucker DM. Regulating action: alternating activation of midline frontal and motor cortical networks. Clin Neurophysiol. 2001;112(7):1295–1306. doi: 10.1016/s1388-2457(01)00559-4. [DOI] [PubMed] [Google Scholar]
- Luu P, Tucker DM, Derryberry D, Reed M, Poulsen C. Electrophysiological responses to errors and feedback in the process of action regulation. Psychol Sci. 2003;14(1):47–53. doi: 10.1111/1467-9280.01417. [DOI] [PubMed] [Google Scholar]
- Luu P, Tucker DM, Makeig S. Frontal midline theta and the error-related negativity: neurophysiological mechanisms of action regulation. Clin Neurophysiol. 2004;115(8):1821–1835. doi: 10.1016/j.clinph.2004.03.031. [DOI] [PubMed] [Google Scholar]
- Makeig S, Debener S, Onton J, Delorme A. Mining event-related brain dynamics. Trends Cogn Sci. 2004;8(5):204–210. doi: 10.1016/j.tics.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Marco-Pallares J, Cucurell D, Cunillera T, Garcia R, Andres-Pueyo A, Munte TF, et al. Human oscillatory activity associated to reward processing in a gambling task. Neuropsychologia. 2008;46(1):241–248. doi: 10.1016/j.neuropsychologia.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Mulert C, Menzinger E, Leicht G, Pogarell O, Hegerl U. Evidence for a close relationship between conscious effort and anterior cingulate cortex activity. Int J Psychophysiol. 2005;56(1):65–80. doi: 10.1016/j.ijpsycho.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Mulert C, Seifert C, Leicht G, Kirsch V, Ertl M, Karch S, et al. Single-trial coupling of EEG and fMRI reveals the involvement of early anterior cingulate cortex activation in effortful decision making. Neuroimage. 2008;42(1):158–168. doi: 10.1016/j.neuroimage.2008.04.236. [DOI] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2002;15(1):1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyhus E, Curran T. Functional role of gamma and theta oscillations in episodic memory. Neurosci Biobehav Rev. 2010;34(7):1023–1035. doi: 10.1016/j.neubiorev.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly RC, Munakata Y. Computational explorations in cognitive neuroscience: understanding the mind by simulating the brain. Cambridge, Mass.: MIT Press; 2000. [Google Scholar]
- Olvet DM, Hajcak G. The error-related negativity (ERN) and psychopathology: Toward an endophenotype. Clin Psychol Rev. 2008 doi: 10.1016/j.cpr.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onton J, Delorme A, Makeig S. Frontal midline EEG dynamics during working memory. Neuroimage. 2005;27(2):341–356. doi: 10.1016/j.neuroimage.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Overbeek TJM, Nieuwenhuis S, Ridderinkhof KR. Dissociable components of error processing - On the functional significance of the Pe Vis-a-vis the ERN/Ne. Journal of Psychophysiology. 2005;19(4):319–329. [Google Scholar]
- Pailing PE, Segalowitz SJ. The effects of uncertainty in error monitoring on associated ERPs. Brain Cogn. 2004;56(2):215–233. doi: 10.1016/j.bandc.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Paus T. Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nat Rev Neurosci. 2001;2(6):417–424. doi: 10.1038/35077500. [DOI] [PubMed] [Google Scholar]
- Raghavachari S, Lisman JE, Tully M, Madsen JR, Bromfield EB, Kahana MJ. Theta oscillations in human cortex during a working-memory task: evidence for local generators. J Neurophysiol. 2006;95(3):1630–1638. doi: 10.1152/jn.00409.2005. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306(5695):443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Ritter P, Becker R. Detecting alpha rhythm phase reset by phase sorting: caveats to consider. Neuroimage. 2009;47(1):1–4. doi: 10.1016/j.neuroimage.2009.04.031. [DOI] [PubMed] [Google Scholar]
- Rizzuto DS, Madsen JR, Bromfield EB, Schulze-Bonhage A, Kahana MJ. Human neocortical oscillations exhibit theta phase differences between encoding and retrieval. Neuroimage. 2006;31(3):1352–1358. doi: 10.1016/j.neuroimage.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Rizzuto DS, Madsen JR, Bromfield EB, Schulze-Bonhage A, Seelig D, Aschenbrenner-Scheibe R, et al. Reset of human neocortical oscillations during a working memory task. Proc Natl Acad Sci U S A. 2003;100(13):7931–7936. doi: 10.1073/pnas.0732061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth MF, Buckley MJ, Behrens TE, Walton ME, Bannerman DM. Functional organization of the medial frontal cortex. Curr Opin Neurobiol. 2007;17(2):220–227. doi: 10.1016/j.conb.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Sauseng P, Griesmayr B, Freunberger R, Klimesch W. Control mechanisms in working memory: a possible function of EEG theta oscillations. Neurosci Biobehav Rev. 2010;34(7):1015–1022. doi: 10.1016/j.neubiorev.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W, Doppelmayr M, Hanslmayr S, Schabus M, Gruber WR. Theta coupling in the human electroencephalogram during a working memory task. Neurosci Lett. 2004;354(2):123–126. doi: 10.1016/j.neulet.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W, Gruber WR, Hanslmayr S, Freunberger R, Doppelmayr M. Are event-related potential components generated by phase resetting of brain oscillations? A critical discussion. Neuroscience. 2007;146(4):1435–1444. doi: 10.1016/j.neuroscience.2007.03.014. [DOI] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci. 2011;12(3):154–167. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon JR, Rudell AP. Auditory S-R compatibility: the effect of an irrelevant cue on information processing. J Appl Psychol. 1967;51(3):300–304. doi: 10.1037/h0020586. [DOI] [PubMed] [Google Scholar]
- Staudigl T, Hanslmayr S, Bauml KH. Theta oscillations reflect the dynamics of interference in episodic memory retrieval. J Neurosci. 2010;30(34):11356–11362. doi: 10.1523/JNEUROSCI.0637-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton RS, Barto AG. Reinforcement learning: an introduction. Cambridge, Mass.: MIT Press; 1998. [Google Scholar]
- Swick D, Turken U. Dissociation between conflict detection and error monitoring in the human anterior cingulate cortex. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(25):16354–16359. doi: 10.1073/pnas.252521499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Bancaud J, Geier S, Bordas-Ferrer M, Bonis A, Szikla G, et al. The cingulate gyrus and human behaviour. Electroencephalogr Clin Neurophysiol. 1973;34(1):45–52. doi: 10.1016/0013-4694(73)90149-1. [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O, Delpuech C, Permier J. Oscillatory gamma-band (30–70 Hz) activity induced by a visual search task in humans. J Neurosci. 1997;17(2):722–734. doi: 10.1523/JNEUROSCI.17-02-00722.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trujillo LT, Allen JJ. Theta EEG dynamics of the error-related negativity. Clin Neurophysiol. 2007;118(3):645–668. doi: 10.1016/j.clinph.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Tsujimoto T, Shimazu H, Isomura Y. Direct recording of theta oscillations in primate prefrontal and anterior cingulate cortices. J Neurophysiol. 2006;95(5):2987–3000. doi: 10.1152/jn.00730.2005. [DOI] [PubMed] [Google Scholar]
- Ullsperger M, von Cramon DY. The role of intact frontostriatal circuits in error processing. J Cogn Neurosci. 2006;18(4):651–664. doi: 10.1162/jocn.2006.18.4.651. [DOI] [PubMed] [Google Scholar]
- Van Veen V, Carter CS. The timing of action-monitoring processes in the anterior cingulate cortex. J Cogn Neurosci. 2002;14(4):593–602. doi: 10.1162/08989290260045837. [DOI] [PubMed] [Google Scholar]
- Vidal F, Burle B, Bonnet M, Grapperon J, Hasbroucq T. Error negativity on correct trials: a reexamination of available data. Biol Psychol. 2003;64(3):265–282. doi: 10.1016/s0301-0511(03)00097-8. [DOI] [PubMed] [Google Scholar]
- Vidal F, Hasbroucq T, Grapperon J, Bonnet M. Is the 'error negativity' specific to errors? Biol Psychol. 2000;51(2–3):109–128. doi: 10.1016/s0301-0511(99)00032-0. [DOI] [PubMed] [Google Scholar]
- Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci. 2005;6(7):533–544. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Ulbert I, Schomer DL, Marinkovic K, Halgren E. Responses of human anterior cingulate cortex microdomains to error detection, conflict monitoring, stimulus-response mapping, familiarity, and orienting. J Neurosci. 2005;25(3):604–613. doi: 10.1523/JNEUROSCI.4151-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womelsdorf T, Johnston K, Vinck M, Everling S. Theta-activity in anterior cingulate cortex predicts task rules and their adjustments following errors. Proc Natl Acad Sci U S A. 2010;107(11):5248–5253. doi: 10.1073/pnas.0906194107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womelsdorf T, Schoffelen JM, Oostenveld R, Singer W, Desimone R, Engel AK, et al. Modulation of neuronal interactions through neuronal synchronization. Science. 2007;316(5831):1609–1612. doi: 10.1126/science.1139597. [DOI] [PubMed] [Google Scholar]
- Womelsdorf T, Vinck M, Leung LS, Everling S. Selective theta-synchronization of choice-relevant information subserves goal-directed behavior. Front Hum Neurosci. 2010;4:210. doi: 10.3389/fnhum.2010.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung N, Bogacz R, Holroyd CB, Cohen JD. Detection of synchronized oscillations in the electroencephalogram: an evaluation of methods. Psychophysiology. 2004;41(6):822–832. doi: 10.1111/j.1469-8986.2004.00239.x. [DOI] [PubMed] [Google Scholar]
- Yeung N, Bogacz R, Holroyd CB, Nieuwenhuis S, Cohen JD. Theta phase resetting and the error-related negativity. Psychophysiology. 2007;44(1):39–49. doi: 10.1111/j.1469-8986.2006.00482.x. [DOI] [PubMed] [Google Scholar]
- Yeung N, Botvinick MM, Cohen JD. The neural basis of error detection: conflict monitoring and the error-related negativity. Psychol Rev. 2004;111(4):931–959. doi: 10.1037/0033-295x.111.4.939. [DOI] [PubMed] [Google Scholar]
- Yordanova J, Falkenstein M, Hohnsbein J, Kolev V. Parallel systems of error processing in the brain. Neuroimage. 2004;22(2):590–602. doi: 10.1016/j.neuroimage.2004.01.040. [DOI] [PubMed] [Google Scholar]
- Zirnheld PJ, Carroll CA, Kieffaber PD, O'Donnell BF, Shekhar A, Hetrick WP. Haloperidol impairs learning and error-related negativity in humans. J Cogn Neurosci. 2004;16(6):1098–1112. doi: 10.1162/0898929041502779. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Broad band (.05–15 Hz) ERPs at the FCz electrode, overlapped within each condition.
Factor analysis results of raw EEG data.
Delta band (1–4 Hz) ERPs at the FCz electrode
Alpha band (1–4 Hz) ERPs at the FCz electrode
Beta band (1–4 Hz) ERPs at the FCz electrode
Stimulus-locked topoplots as shown in Figure 3 of the main text (average reference), with additional artifact rejection (+/− 100 µV at FP1) or with an alternative reference scheme (linked mastoids). These plots demonstrate that frontal and occipital theta power seen in the topoplots are a feature of the averaged reference, and are not due to artifact contamination.
Response-locked topoplots as shown in Figure 4 with variants as above.
Stimulus-locked topoplots as shown in Figure 6 with variants as above.
Response-locked topoplots as shown in Figure 7 with variants as above.
Paired t-test data from Figure 8.