Abstract
The limited research on the functional meaning of infant EEG frequency bands has used measures of EEG power. The purpose of this study was to examine task-related changes in frontal EEG coherence measures for three infant EEG frequency bands (2–5 Hz, 6–9 Hz, 10–13 Hz) during a spatial working memory task. Eight-month-olds exhibited baseline-to-task changes in frontal EEG coherence for all infant frequency bands. Both the 2–5 Hz and the 10–13 Hz bands differentiated frontal functional connectivity during the distinct processing stages, but each band provided unique information. The 10–13 Hz band, however, was the only frequency band to distinguish frontal EEG coherence values during correct and incorrect responses. These data reveal valuable information concerning frontal functional connectivity and the functional meaning of three different infant EEG frequency bands during working memory processing.
Keywords: working memory, frontal lobe, EEG coherence, functional connectivity, infants
Working memory (WM) emerges as a function of integrated activity from distributed brain regions (e.g., Sarnthein, Petsche, Rappelsberger, Shaw, & von Stein, 1998; Thomas et al., 1999). Colombo and Cheatham (2006) have proposed that integrative frontal functioning provides the foundation for higher order cognitive processes. We postulate that frontal functional connectivity is essential for the emergence of WM during infancy. The present paper provides the essential groundwork for this hypothesis by examining three specific frequency bands and determining whether EEG coherence, a frequency-dependent measure of functional connectivity, reveals functional dissociations in infant WM processing. In the following paragraphs, we review infant EEG frequency band research and discuss the EEG coherence-WM literature.
Infant EEG frequency bands
The adult alpha 8–13 Hz rhythm is the dominant EEG rhythm during quiet wakefulness. Lindsley (1939) has proposed that the frequency of the alpha rhythm increases as a function of age. Two key research findings have suggested functional associations between the infant 6–9 Hz rhythm and the adult alpha rhythm. Spectral analysis of longitudinal data reveals that 6–9 Hz is the dominant frequency during quiet wakefulness throughout infancy (Bell & Fox, 1992; Marshall, Bar-Haim, & Fox, 2002) and early childhood (Marshall et al., 2002). Likewise, Stroganova and colleagues (1999) noted increases in 5.2–9.6 Hz amplitudes over the occipital-parietal cortex during a “lights off” period that was postulated to be equivalent to the “eyes closed” measure that typically elicits alpha rhythms over the occipital cortex of adults.
Although the 6–9 Hz band has emerged as a standard in infant EEG research (e.g., Cuevas & Bell, 2011; Orekhova, Stroganova, & Posikera, 2001), research on the functional significance of additional frequency bands is essential for a coherent understanding of infant EEG. For adult EEG, different types of cognitive processing are associated with specific patterns of task-related changes in power and coherence for particular frequency bands (e.g., Sarnthein et al., 1998; see Klimesch, 1999, for review). The functional significance of specific infant frequency bands, however, has only been examined using EEG power, which reflects the excitability of neuronal groups (e.g., Bell, 2002; Jing, Gilchrist, Badger, & Pivik, 2010). It is unknown whether measures of functional connectivity display similar frequency-dependent relations during infancy.
Using EEG power measures, Bell (2002) analyzed the functional significance of the 3–5 Hz, 6–9 Hz, and 10–12 Hz bands during an infant spatial WM task. This frequency range captures the majority of infant EEG activity (e.g., Marshall et al., 2002) and includes bands above and below the infant “standard” 6–9 Hz band. At 8 months, all three frequency bands discriminated baseline from task activation (Bell, 2002). In addition, the 3–5 Hz and 6–9 Hz bands differentiated the various processing stages of the infant WM task. Only the 6–9 Hz band, however, discriminated WM activation associated with correct and incorrect responses. Thus, the 6–9 Hz band provides more functional information for EEG power measures during an infant WM task than other frequency bands. EEG power does not provide information about functional cortico-cortical connections during WM processing.
EEG coherence and WM
Measures of functional connectivity, such as coherence, permit quantification of coupled neural activity. EEG coherence is the squared cross-correlation between two scalp electrode sites within a designated frequency band (Nunez, 1981; Thatcher, Walker, & Giudice, 1987). If the activity at two electrodes is synchronized then coherence values approach 1, and if there is no synchronization then coherence values approach 0. Accordingly, relatively lower and higher levels of coherence reflect differentiation and integration of function between two brain areas, respectively. Unlike EEG power, coherence is not affected by arousal, opening or closing of eyes, or changes in state (Thatcher, 1994).
WM research with adults and children has emphasized the role of the prefrontal cortex within a distributed fronto-parietal neural network (Linden et al., 2003; Thomas et al., 1999). For adults, tasked-related changes in fronto-parietal EEG coherence during WM processing have been found in the theta and alpha bands (e.g., Sarnthein et al., 1998; Sauseng, Klimesch, Schabus, & Doppelmayr, 2005). Likewise, 4.5-year-olds exhibit task-related increases in 6–9 Hz medial frontal-posterior EEG coherence during WM processing (Bell & Wolfe, 2007). Changes in coherence during WM processing, however, have also been found in adults for anterior short-distance coherence (upper alpha band; Sauseng et al., 2005).
Infant WM research, on the other hand, has only examined alterations in 6–9 Hz frontal coherence (i.e., medial frontal electrode pairings). Eight-month-olds exhibit WM-related decreases in frontal EEG coherence (Bell & Wolfe, 2007). More recently, Bell (in press) has found different patterns of frontal coherence for high and low WM performers at 8 months; high performers showed no task-related changes in frontal pole-medial frontal coherence and task-related increases in medial frontal-parietal coherence while low performers showed task-related decreases for both coherence pairs. Together, these findings provide evidence that 6–9 Hz frontal coherence measures are related to infant spatial WM.
More detailed WM analyses of EEG coherence, as completed by Bell (2002) using power, have not been examined during infancy. Will coherence measures exhibit similar frequency-dependent dissociations during an infant WM task as found for EEG power (Bell, 2002)? In the adult WM literature, there is evidence of dissociations in the functional meaning of EEG frequency bands for measures of power and coherence (Babiloni et al., 2004a, 2004b; Sauseng et al., 2005; i.e., functional dissociations within a particular frequency band are found with one EEG measure, but not the other). Clearly, additional infant multi-frequency band EEG research is essential to understanding the role of functional connectivity during WM processing.
Research questions
Our baseline and spatial WM task were identical to previous infant WM EEG research to facilitate cross-experiment comparisons (e.g., Bell, 2002, in press). We chose to examine 8-month-olds because previous research has provided foundational information about EEG coherence (Bell, in press; Bell & Wolfe, 2007) and frequency-dependent changes in power as a function of WM processing (Bell, 2002) at this age. Because of the role of the frontal cortex in WM (e.g., Thomas et al., 1999), we only examined intra-hemisphere medial frontal coherence pairings, which presumably reflect functional connectivity within the fronto-parietal network (e.g., Babiloni et al., 2004b; Sarnthein et al., 1998). Based on previous infant EEG research utilizing wide band and narrow band analyses (Bell, 1998, 2002; Marshall et al., 2002), EEG data were divided into three equal-sized1 frequency bands: 2–5 Hz, 6–9 Hz, and 10–13 Hz. This study investigated the functional meaning of frontal coherence measures within each of these bands by answering the same questions as Bell’s (2002) analysis of EEG power.
Does the frequency band discriminate between baseline and task activation? For infants, there is some indication of task-related changes in 6–9 Hz frontal coherence (Bell, in press; Bell & Wolfe, 2007), but possible fluctuations in the other two bands are unknown.
Does the frequency band differentiate among various cognitive processing stages during the task? Adult theta and alpha coherence varies as a function of specific cognitive processes (Sarnthein et al., 1998; Sauseng et al., 2005). It is unknown whether this dissociation will occur for coherence measures in the infant EEG bands.
Does the frequency band distinguish correct from incorrect responses? Higher coherence is associated with correct, as compared to incorrect, responses in adult delta, theta, and alpha bands (Pavlygina, Sakharov, & Davydov, 2007; Weiss & Rappelsberger, 2000). Task-related changes in infant 6–9 Hz coherence are dependent on WM performance (Bell, in press). This frequency band may fluctuate with correct and incorrect responses.
Because of the paucity of research concerning the functional meaning of coherence measures for different infant EEG frequency bands, the answers to these questions will provide the initial framework for our understanding of WM-related changes in infant coherence values.
Method
Participants
Fifty healthy 8-month-old infants (28 male, 22 female; 46 Caucasian, 1 African-American, 1 Asian-American, 1 Hispanic, 1 Native American) were recruited from birth announcements placed in the local newspaper in a small college town in the mid-Atlantic area. Infants were born to parents with at least a high school diploma. Seventy-nine percent of the mothers had college degrees, as did 82% of the fathers. Mothers were approximately 29 years old (range 18–39) and fathers were approximately 30.5 years old (range 20–47). All infants were full term and were healthy at the time of testing. Infants were seen when they were between 8.0 and 8.75 months of age, so that only 3 weeks separated the oldest and youngest infants in the study. Parents were paid for their infants’ participation in the study.
Procedures
EEG recording
EEG was recorded during baseline and during a spatial WM task. Recordings were made from 16 left and right scalp sites: frontal pole (Fp1, Fp2), medial frontal (F3, F4), lateral frontal (F7, F8), central (C3, C4), temporal (T7, T8), lateral parietal (P7, P8), medial parietal (P3, P4), and occipital (O1, O2). All electrode sites were referenced to Cz during recording. EEG was recorded using a stretch cap (Electro-Cap, Inc.; Eaton, OH) with electrodes in the 10/20 system pattern. After the cap was placed on the head, recommended procedures regarding EEG data collection with infants and young children were followed (Pivik et al., 1993). Specifically, a small amount of abrasive was placed into each recording site and the scalp gently rubbed. Following this, conductive gel was placed in each site. Electrode impedances were measured and accepted if they were below 5 kΩ. EOG, digitized along with the EEG channels and used for subsequent artifact editing, was recorded using disposable electrodes. Electrodes were placed on the external canthus and the supra orbit of the right eye.
The electrical activity from each lead was amplified using separate SA Instrumentation Bioamps (San Diego, CA) and bandpassed from .1 to 100 Hz. Activity for each lead was displayed on the monitor of an acquisition computer. The EEG signal was digitized on-line at 512 samples per second for each channel so that the data were not affected by aliasing. The acquisition software was Snapshot-Snapstream (HEM Data Corp.; Southfield, MI) and the raw data were stored for later analyses. Prior to the recording of each subject a 10 Hz, 50 uV peak-to-peak sine wave was input through each amplifier. This calibration signal was digitized for 30 s and stored for subsequent analysis.
Baseline EEG
Baseline EEG was recorded for 1 min while the infant sat on mother’s lap. During the baseline recording, a research assistant manipulated a toy containing brightly colored balls on top of the testing table, 1.1 m in front of the infant. This procedure quieted the infant and yielded minimal eye movements and gross motor movements, thus allowing the infant to tolerate the EEG cap for the recording. Mothers were instructed to not talk to infants during the EEG recording. Immediately after baseline, the recording of EEG continued as the spatial WM task was administered.
Spatial WM task
The infant searched for a hidden toy by making an eye movement to one of two possible hiding locations. The testing apparatus was a table measuring 90 cm (L) × 60 cm (W) × 75 cm (H) and the hiding sites were bright orange and blue plastic tubs that measured 17 cm in diameter and 11 cm deep. The infant sat on the parent’s lap 1.1 m from the edge of the testing table as the experimenter manipulated a mechanical toy and hid it under one of the two (17.5 cm on either side of midline) plastic tubs. After the toy was hidden, the infant’s gaze to the hiding site was broken and brought to midline by the experimenter calling the infant’s name and asking, “Where’s the toy?” The direction of the infant’s first eye movement after being brought to midline was scored as either correct or incorrect. A video camera was placed behind and above the experimenter’s head and focused so as to maintain a close-up view of the infant’s face.
Because the infants were not allowed to manipulate the toys, the visual experience they received from the moving, mechanical toy and the smiles and praise they received (“Good job! You found it!”) from the experimenter after an eye movement to the correct tub had to provide the impetus to continue to search for the toy. For an eye movement to the incorrect tub, the infants received a sigh and sad vocalizations from the experimenter (“Oh, no. It’s not there.”).
The pattern of toy placement was determined by the infant’s performance, with initial side of hiding randomized among infants (Bell & Adams, 1999). Two consecutive successful eye movements toward the same side (e.g., toward the infant’s right) resulted in a reversal hiding, with the toy being hidden under the tub on the opposite side (e.g., toward the infant’s left; i.e., Right-Right-Left). All infants received reversal trials. Regardless of whether or not the infant was successful on the reversal trial, new “same-side” trials commenced at the reversal site and continued until two consecutive successful eye movements were executed, initiating another reversal (i.e., L-L-R). Thus, flawless performance by an infant would result in this pattern of trials: R-R-L-L-L-R-etc. In reality, most infants were not flawless in performance and some needed multiple same-side trials in order to achieve two consecutive successful eye movements prior to reversal trials (e.g., L-L-L-L-L-L-R-R-R-R-L-etc.). Assessment ceased when the infant made an eye movement toward the incorrect side in two reversal trials. The average number of trials (combining same-side and reversals) from which EEG data were collected was 18 per infant (SE=.82).
Infants who made an eye movement toward the correct tub on reversal trials in two out of three attempts were then tested with a 2-s delay. Six infants were assessed with a delay. The delay was initiated during the time the infant’s gaze to the hiding site was broken and brought to the experimenter’s face at midline. In the delay condition, the experimenter called the infant’s name, counted out the delay period, and then asked, “Where’s the toy?” In reality, even during the “no delay” trials at the beginning of the assessment, the infants experienced a brief delay as their gaze to the tub was broken and brought to midline. This brief delay was unavoidable because of the necessity of breaking the infant’s gaze so that the direction of his/her first eye movement could be determined. Assessment terminated when the infant made an eye movement toward the incorrect tub in two out of three reversal trials at either no delay or 2-s delay.
An event marker was used in conjunction with the EEG recordings so that it was possible to mark which portions of the EEG record were associated with the specific processing stage of each trial. Thus, the “display & hide” (attention) processing stage was the time period when the experimenter manipulated the mechanical toy to capture the infant’s interest and then hid it under one of the two tubs. During “delay & search” (WM), the infant’s gaze to the hiding site was broken and brought to the experimenter’s face at midline by the experimenter calling the infant’s name and asking, “Where’s the toy?” This component of the task ended with the infant’s first eye movement. “Retrieval & reward” (emotion) consisted of the experimenter praising the infant for a correct eye movement (or sighing in the event of an incorrect one) and retrieving the toy from the tub for the infant to see. The experimenter talked to the infant during each of the three processing stages.
EEG analysis
EEG data were examined and analyzed using EEG Analysis System software developed by James Long Company (Caroga Lake, NY). First, the data were re-referenced via software to an average reference configuration (Lehmann, 1987). Average referencing, in effect, weighted all the electrode sites equally and eliminated the need for a noncephalic reference. Active (F3, F4, etc.) to reference (Cz) electrode distances vary across the scalp. The average reference configuration requires that a sufficient number of electrodes be sampled and that these electrodes be evenly distributed across the scalp. Currently, there is no agreement concerning the appropriate number of electrodes2 (Davidson, Jackson, & Larson, 2000; Hagemann, Naumann, & Thayer, 2001; Luck, 2005), although the 10/20 configuration that we used does satisfy the requirement of even scalp distribution. Average referencing is considered the optimal configuration when computing coherence between spatially distinct electrodes (Fein, Raz, Brown, & Merrin, 1988; Nunez et al., 1999). Using an average reference configuration, Grieve and colleagues (2003) found that for simulated and experimental data, measurements of coherence are less confounded by volume conduction for infants than adults.
The average reference EEG data were artifact scored for eye movements using a peak-to-peak criterion of 100 uV or greater. Artifact associated with gross motor movements over 200 uV peak-to-peak was also scored. These artifact-scored epochs were eliminated from all subsequent analyses. The data then were analyzed with a discrete Fourier transform (DFT) using a Hanning window of 1-s width and 50% overlap. Coherence between medial frontal and all other electrode sites within each hemisphere was computed using an algorithm by Saltzberg and colleagues (1986) for three frequency bands: 2–5 Hz (actually 1.5–5.5 Hz), 6–9 Hz (5.5–9.5 Hz), 10–13 Hz (9.5–13.5 Hz).
Of the original 50 infants, two cried during the spatial WM task, one was omitted due to experimenter error during the spatial WM task, and three infants did not wear the EEG cap. Thus, statistical analyses included 44 infants who contributed EEG data. Only infants with artifact-free EEG data were included in each analysis.
The artifact-free EEG data from all of the same-side and reversal trials (both correct and incorrect) were used in the analyses for research Questions 1 (Does the frequency band discriminate between baseline and task activation?) and 2 (Does the frequency band discriminate among various processing stages during the task?). Question 3 focused on task performance (Does the frequency band distinguish between correct and incorrect responses?) and, thus, differentiated between correct and incorrect eye movements.
Statistical analyses
To examine each of the research questions, the analyses consisted of repeated-measures MANOVAs with region, hemisphere, and activation (Question 1) or processing stage (Question 2) or response (Question 3) as within-subjects factors (Picton et al., 2000). Main effects and interactions with a p value of ≤ .10 are noted in the tables for information purposes; however, only p values of ≤ .05 are highlighted in the results section. Of major interest for the specific analyses associated with each question were the main effect and the interactions involving the activation (Question 1), processing stage (Question 2), and response (Question 3) within-subjects variables. For ease in examining any interactions involving these variables, follow-up MANOVAs were performed. A multivariate approach for assessing multivariate interaction effects has been suggested by Keselman (1998). A Bonferroni procedure was adopted to limit the familywise Type I error rate.
Results
Does the frequency band discriminate between baseline and task activation?
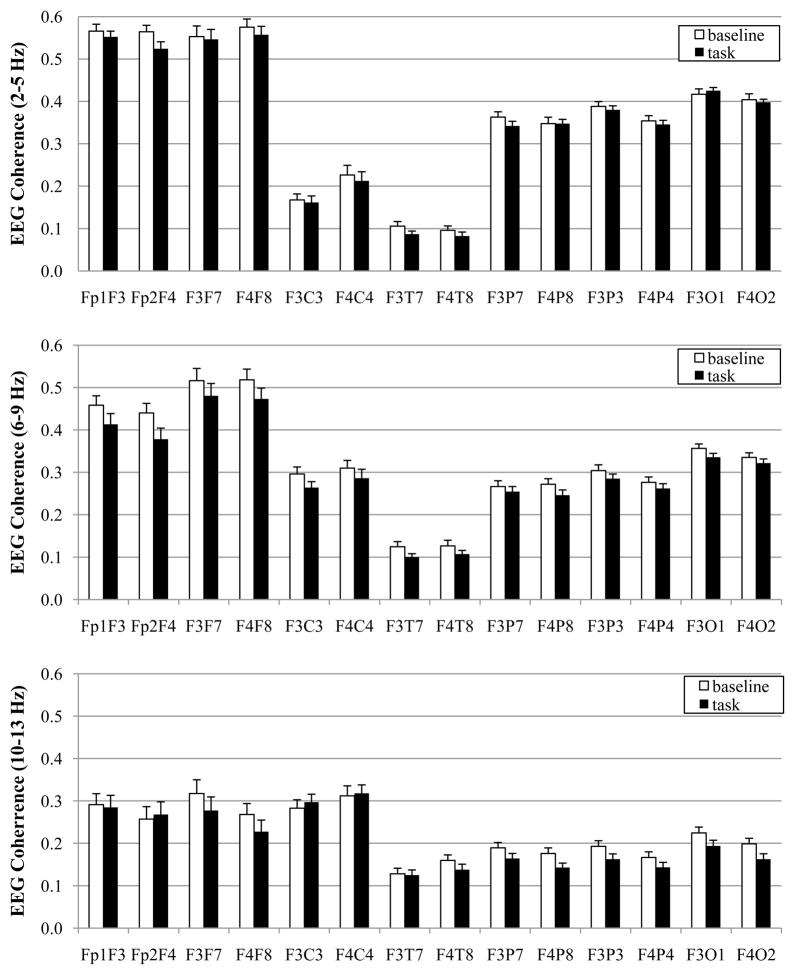
The means for EEG coherence during baseline and task are displayed in Figure 1 and the results of the MANOVA are displayed in Table 1. As can be seen from Figure 1, the three frequency bands exhibit very different coherence values in infants, with the 2–5 Hz band yielding the greatest coherence values and the 10–13 Hz the lowest coherence values. The y-axes in Figure 1 are on the same scale for each frequency band to highlight these differences.
Figure 1.
EEG coherence values for baseline and spatial working memory task in 8-month-old infants at 2–5 Hz (top), 6–9 Hz (middle), and 10–13 Hz (bottom).
Table 1.
Summary of Multivariate Analyses F Values for Baseline and Task Activation Comparisons
| Activation | Pair | Hemi | Act × Pair | Act × Hemi | Pair × Hemi | A × P × H | |
|---|---|---|---|---|---|---|---|
| df | 1, 42 | 6, 37 | 1, 42 | 6, 37 | 1, 42 | 6, 37 | 6, 37 |
| 2–5 Hz | 6.18* (.13) | 233.46*** (.97) | 3.53** (.36) | ||||
| 6–9 Hz | 14.75*** (.26) | 108.13*** (.95) | 2.30+ (.27) | 3.04* (.33) | |||
| 10–13 Hz | 10.99** (.21) | 29.97*** (.83) | 2.31+ (.27) |
Note.
p ≤ .001;
p ≤ .01;
p ≤ .05;
p ≤ .10.
Effect sizes (ηp2) are in parentheses.
There was a main effect for activation for the 2–5 Hz, 6–9 Hz, and 10–13 Hz bands. Coherence values were higher during baseline than during task. There were no interactions involving activation for any frequency band.
Does the frequency band differentiate among various processing stages during the task?
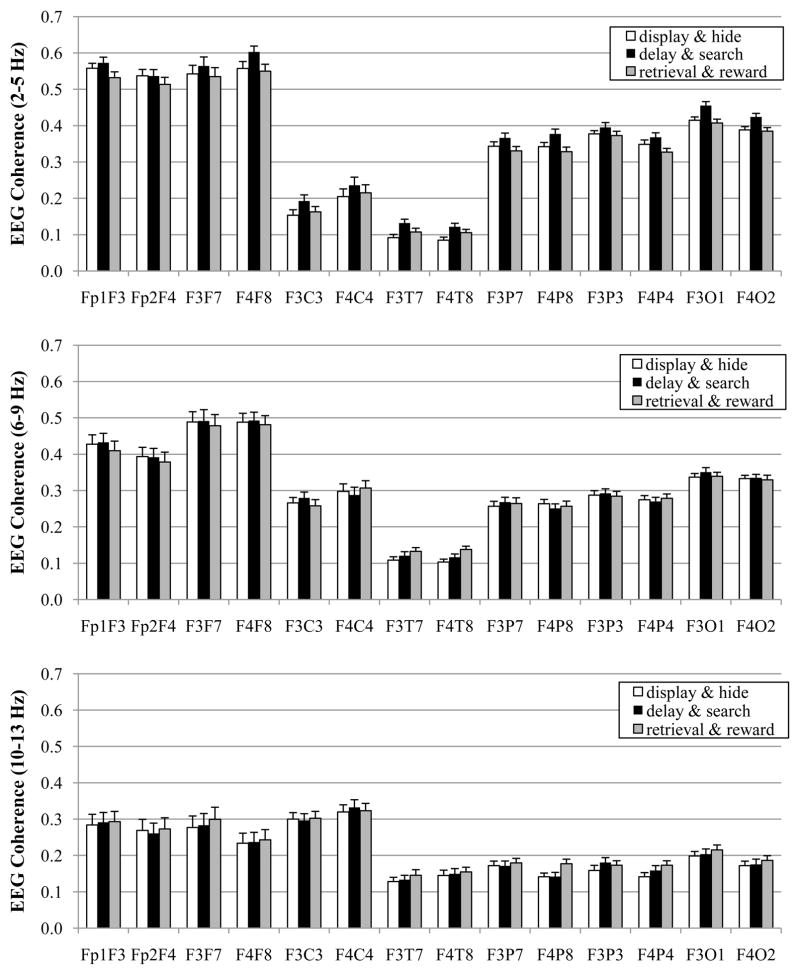
For these analyses, the processing stage factor had three levels that corresponded to the three sections of the spatial WM task: display & hide (the attention component of the task), delay & search (the WM portion), and retrieval & reward (the emotion portion). The means for EEG coherence during the three processing stages are displayed in Figure 2. Results of the MANOVA are displayed in Table 2.
Figure 2.
EEG coherence values for different processing stages during spatial working memory task at 8 months. Data are graphed with respect to frequency band: 2–5 Hz (top), 6–9 Hz (middle), and 10–13 Hz (bottom).
Table 2.
Summary of Multivariate Analyses F Values for Processing Stage Comparisons
| Stage | Pair | Hemi | Stage × Pair | Stage × Hemi | Pair × Hemi | S × P × H | |
|---|---|---|---|---|---|---|---|
| df | 2, 40 | 6, 36 | 1, 41 | 12, 30 | 2, 40 | 6, 36 | 12, 30 |
| 2–5 Hz | 18.85*** (.48) | 343.54*** (.98) | 2.46* (.50) | 8.40*** (.58) | 2.23* (.47) | ||
| 6–9 Hz | 116.92*** (.95) | 2.03+ (.45) | 2.87+ (.13) | 2.31+ (.28) | |||
| 10–13 Hz | 8.05*** (.29) | 35.31*** (.86) |
Note. ***p ≤ .001;
p ≤ .01;
p ≤ .05;
p ≤ .10.
Effect sizes (ηp2) are in parentheses.
There was a main effect for processing stage for both the 2–5 Hz and 10–13 Hz bands, but not for the 6–9 Hz band. For the 2–5 Hz frequency band, the main effect for processing stage was superseded by Stage × Pair and Stage × Pair × Hemisphere interactions. There were no other interactions involving activation for any frequency band.
For ease in examining the Stage × Pair × Hemisphere interaction for the 2–5 Hz band, separate follow-up MANOVAs were performed on the EEG coherence values for each of the seven electrode pairs. The adjusted p value was ≤ .007 (.05/7 = .007). There was a main effect for processing stage for all electrode pairs (all F’s ≤ 7.51, all p’s ≤ .002). Pairwise comparisons for these electrode pairs were accomplished after adopting a Bonferroni procedure to control the overall level of significance (seven electrode pairs with three comparisons for each electrode pair; p = .05/21 = .002). The delay & search stage had higher EEG coherence values than the display & hide stage (all p’s ≤ .001) for medial frontal-lateral frontal, medial frontal-central, medial frontal-temporal, medial frontal-lateral parietal, and medial frontal-occipital electrode pairs. The delay & search stage had higher EEG coherence values than the retrieval & reward stage (all p’s ≤ .001) for frontal pole-medial frontal, medial frontal-lateral frontal, medial frontal-lateral parietal, medial frontal-medial parietal, and medial frontal-occipital electrode pairs.
To examine the main effect of processing stage for the 10–13 Hz band, pairwise comparisons were completed after adopting a Bonferroni procedure to control the overall level of significance (three comparisons p = .05/3 = .017). Coherence values were higher during the retrieval & reward phase than during the display & hide stage (p ≤ .001).
Does the frequency band distinguish between correct and incorrect responses?
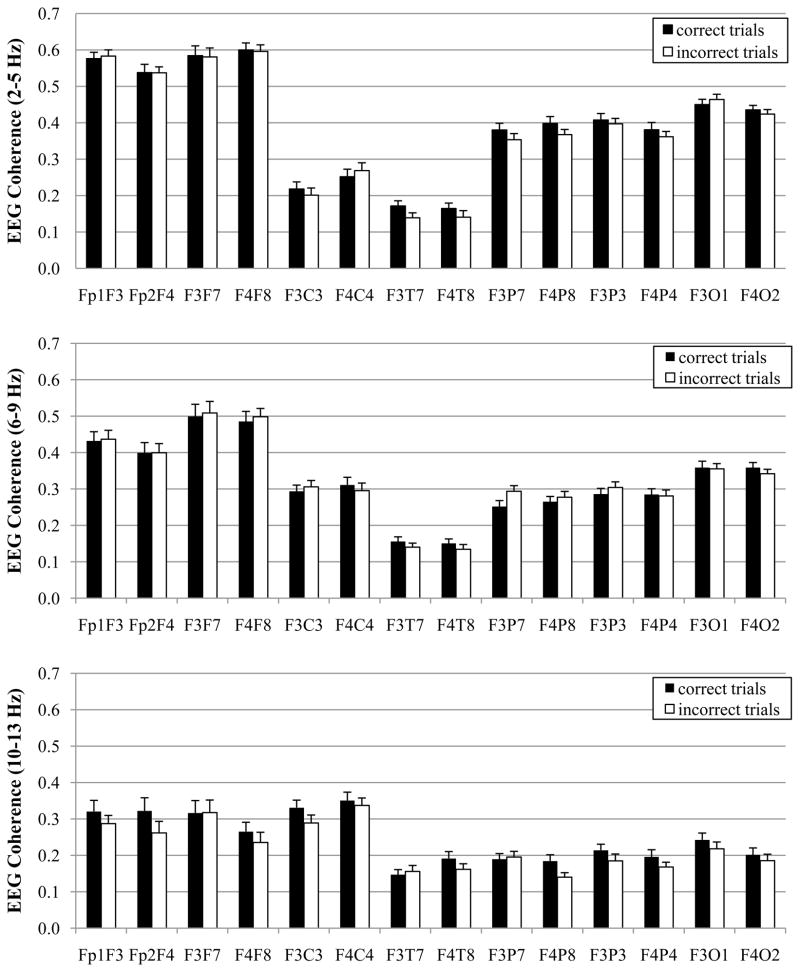
For these analyses, the response factor denoted correct and incorrect eye responses and the EEG was specific to the delay & search processing stage, when the infants’ WM cognitive skills were utilized. All 44 participants had same-side trials and 39 of the participants (89%) had artifact-free EEG data for both correct and incorrect same side trials. However, only 11 infants (25%) had artifact-free data for both correct and incorrect reversal trials. Thus, these analyses were conducted on the same-side trials. The amount of artifact-free data (i.e., the number of DFT windows) was identical for both correct and incorrect same side trials, t(38) = 1.10, p = .3. Results of the MANOVA on the EEG coherence values are displayed in Table 3 and the means are displayed in Figure 3.
Table 3.
Summary of Multivariate Analyses F Values for Correct/Incorrect Response Comparisons
| Response | Pair | Hemi | Resp × Pair | Resp × Hemi | Pair × Hemi | R × P × H | |
|---|---|---|---|---|---|---|---|
| df | 1, 38 | 6, 33 | 1, 38 | 6, 33 | 1, 38 | 6, 33 | 6, 33 |
| 2–5 Hz | 177.98*** (.97) | 5.09*** (.48) | |||||
| 6–9 Hz | 56.85*** (.91) | 2.30+ (.29) | 2.91+ (.07) | ||||
| 10–13 Hz | 9.29** (.20) | 26.26*** (.83) | 2.25+ (.29) |
Note.
p ≤ .001;
p ≤ .01;
p ≤ .05;
p ≤ .10.
Effect sizes (ηp2) are in parentheses.
Figure 3.
EEG coherence values for correct and incorrect responses during spatial working memory task with 8-month-old infants. Data are graphed with respect to frequency band: 2–5 Hz (top), 6–9 Hz (middle), and 10–13 Hz (bottom).
There was a main effect for response for the 10–13 Hz band only. The EEG coherence values were higher for correct trials than for the incorrect trials. There were no interactions involving response at any frequency band.
Summary of findings
The 2–5 Hz band discriminated baseline from task activation and differentiated among some of the processing stages for some electrode pairs. This band did not distinguish correct and incorrect responses, however. The 6–9 Hz band discriminated baseline from task activation, but did not differentiate among different processing stages during task. Neither did this band distinguish between correct and incorrect responses. The 10–13 Hz band discriminated baseline from task, distinguished among some of the processing stages, and differentiated between correct and incorrect responses.
Discussion
The data in the present study permitted the examination of dynamic changes in frontal functional connectivity associated with cognitive processing during infancy. Our findings reveal valuable information concerning frontal functional connectivity and the functional meaning of three different infant EEG frequency bands during spatial WM processing.
The 10–13 Hz band appeared to be the most informative concerning frontal functional connectivity during an infant spatial WM task. This frequency band distinguished functional connectivity for correct and incorrect responses, showed variations in functional connectivity for some task processing stages, and discriminated functional connectivity during baseline and task. No other frequency band differentiated functional connectivity for correct and incorrect responses. For the 10–13 Hz band, coherence values were higher for correct responses than incorrect responses. In other words, separate cortical regions were more likely to work in conjunction during correct responses. This same pattern of EEG coherence data has been found for correct and incorrect responses in adults (Pavlygina et al., 2007; Weiss & Rappelsberger, 2000). To our knowledge, this is the first examination whether EEG coherence measures dissociate correct and incorrect responses during infancy.
Frontal functional connectivity for the 2–5 Hz and the 10–13 Hz bands varied as a function of task processing stage. For the 10–13 Hz band, EEG coherence values were higher during the retrieval & reward (emotion) phase than during the display & hide (attention) phase. Increased synchronization during the “emotion” phase as compared to the “attention” phase was unexpected. In the absence of similar comparisons within the functional connectivity literature, future research is necessary to see if this finding is consistent and place it within the appropriate framework. A different pattern of functional connectivity was found in the 2–5 Hz band; EEG coherence values were higher during the delay & search (WM) stage than the retrieval & reward (emotion) and the display & hide (attention) stages for medial frontal-lateral frontal, medial frontal-lateral parietal, and medial frontal-occipital electrode pairs. Most other electrode pairs exhibited at least one of these processing stage dissociations. There was greater functional connectivity during spatial WM processing than the other task phases. Thus, although the 2–5 Hz and the 10–13 Hz bands differentiated frontal functional connectivity during the distinct processing stages, each band provided unique information with only the 2–5 Hz band differentiating spatial WM processing from other task processing stages. The 6–9 Hz band, however, did not reveal changes in frontal functional connectivity during the different processing stages of the spatial WM task. Based on these findings, we strongly encourage future infant spatial WM research on frontal functional connectivity to take a multiple frequency band approach.
All three infant frequency bands (i.e., 2–5 Hz, 6–9 Hz, 10–13 Hz) discriminated baseline from task activation. Although this level of analysis is the most “elemental” in the present study, baseline-to-task changes are traditionally used as indicators of cognitive processing for infants, children, and adults (e.g., Bell & Wolfe, 2007; Haarmann & Cameron, 2005). Thus, it is important for spatial WM researchers to note that multiple infant frequency bands distinguish baseline and task frontal functional connectivity. Furthermore, in the present study, infants showed a “whole head effect” (i.e., there were no significant activation by region interactions); EEG coherence values were lower during the spatial WM task than during baseline for all three frequency bands. This finding replicates previous findings with the 6–9 Hz band (Bell & Wolfe, 2007).
During infant spatial WM processing, separate cortical regions were more likely to work independently, as opposed to in conjunction with each other. A different pattern of findings has been found in WM research with adults and children. Specifically, adults and children exhibit task-related increases in EEG coherence (e.g., Bell & Wolfe, 2007; Haarmann & Cameron, 2005), which are indicative of synchronized activity across distributed cortical regions. Why might different patterns of task-related changes in frontal functional connectivity be expressed during infancy as compared to later in development? From a behavioral perspective, 8-month-olds demonstrate rudimentary evidence of spatial WM; only six infants in the current sample displayed sufficient spatial WM processing to attempt the task with a 2-s delay. By 12 months of age, infants exhibit spatial WM processing with much longer delays (e.g., 10 s; Bell & Fox, 1992; Diamond, Prevor, Callender, & Druin, 1997). From a neuroanatomical perspective, during the first two years of life, there is considerable structural growth in the frontal axons and in the myelination of these projections (e.g., Deoni et al., 2011; Tsekhmistrenko, Vasil’eva, Shumeiko, & Vologirov, 2004). Thus, frontal structural connectivity likely limits frontal functional connectivity. From a psychophysiological perspective, 8-month-olds in the present study had higher coherence values (10–13 Hz) for correct, as compared to incorrect, responses. Therefore, it appears that the pattern of changes in frontal functional connectivity during correct responding (i.e., increases in EEG coherence compared to incorrect response) is associated with more mature functional connectivity.
We propose that the aforementioned differences in frontal functional connectivity between infants and adults might underlie the development of spatial WM processing. As previously mentioned, a distributed fronto-parietal neural network is activated during WM processing in adults and children (e.g., Linden et al., 2003; Thomas et al., 1999). We hypothesize that more advanced spatial WM processing relies on the integration of frontal and posterior activity (i.e., task-related increases in functional connectivity), and only a basic level of spatial WM processing is demonstrated prior to this synchronized processing. The present study, however, only assessed spatial WM processing at one point during infancy, and additional WM research with older infants (and perhaps toddlers) is necessary to assess the validity of our hypothesis.
We are intrigued by the similarities and differences when comparing the functional meaning of infant frequency bands for measures of power (Bell, 2002) and coherence (present study). As with all cross study comparisons, there are limitations; analyses are based on different groups of 8-month-olds, and slightly different frequency bands are used for the lower (2–5 Hz; 3–5 Hz) and upper (10–13 Hz; 10–12 Hz) frequency bands. The lower infant frequency band (2–5 Hz; 3–5 Hz) displayed similar functional properties for EEG power and coherence; both measures differentiated baseline from task activation as well as discriminated some of the processing stages in some regions. For the 6–9 Hz band, both EEG measures exhibited task-related changes, but only EEG power differentiated correct from incorrect responses and distinguished some of the processing stages. The opposite pattern of findings was found for the upper infant frequency band (10–13 Hz; 10–12 Hz). Although EEG power and coherence differentiated baseline from task activation, only EEG coherence differentiated correct from incorrect responses and distinguished the distinctive processing stages.
Recall that EEG power values reflect the excitability of groups of neurons and EEG coherence values indicate the amount of synchronized activity between two electrode sites. Thus, it is possible for there to be an increase in the number of active neurons (EEG power) without an increase in synchronized activity between two neuronal sites (EEG coherence) for a particular frequency band, or vice versa. Similar dissociations have been noted in the adult WM literature (Babiloni et al., 2004a, 2004b; Sauseng et al., 2005). Taken together, these findings reveal that, during infancy, EEG measures of power and coherence provide distinctive spatial WM processing information that is frequency band dependent.
A potential limitation of our findings is related to the nature of our task, which required infants to make eye movements. The extent to which muscular eye movements and/or frontal eye field (FEF) activity influenced specific regional findings, is unknown. Indeed, FEF involvement has been noted in non-human primates during an oculomotor WM task (e.g., Funahashi, Bruce, & Goldman-Rakic, 1993). In the present study, however, distinctive regional patterns were only found for our processing stage analysis (i.e., display & hide, delay & search, retrieval & reward); whole-head effects were found for frequency bands that distinguished functional connectivity during baseline and task (all bands) as well as processing associated with correct and incorrect responses (10–13 Hz). Future research could use eye-tracking to examine whether infants exhibit different eye movements during each phase of the task and also determine the potential contribution of eye movements to the current findings.
In conclusion, all infant frequency bands examined in the present study revealed baseline-to-task changes in frontal EEG coherence during a spatial WM task. The 10–13 Hz band was the most informative concerning frontal functional connectivity during an infant spatial WM task because it also distinguished the various processing stages and differentiated correct and incorrect responses. An examination of Figures 1–3 reveals that coherence values were also the smallest for the 10–13 Hz band. A longitudinal frequency band analysis revealed increases in 10–12 Hz power from 5 months to 4 years (Marshall et al., 2002). Similar analyses have not been completed for EEG coherence; however, Cuevas and Bell (2011) has found age-related increases in most medial frontal coherence pairs between 5 and 10 months of age. Additional research is necessary to determine whether the comparatively small coherence values for the 10–13 Hz band might be a developmental phenomenon. Currently, this is the only infant spatial WM study to examine the 10–13 Hz band and measures of frontal functional connectivity. Based on these findings, further investigation of frontal EEG coherence within the 10–13 Hz band is recommended for future infant spatial WM research.
Acknowledgments
This project was funded in part by a Small Grant Award from the College of Arts and Sciences at Virginia Tech. Data analysis and manuscript preparation was supported by grant R01 HD049878 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) awarded to Martha Ann Bell. The assistance of Jonathan E. Roberts and numerous undergraduate research assistants is gratefully acknowledged.
Footnotes
Equal-sized non-overlapping frequency bands were used to ensure that the functional meaning of each band was not influenced by the size of the band.
We collected 64-channel EEG during eyes open baseline for three adults and compared coherence values for 16- and 64-channel average references. Although there were slight differences in the specific coherence values, the regional patterns were very similar.
Contributor Information
Kimberly Cuevas, Department of Psychology, Virginia Tech;.
Vinaya Raj, Department of Psychology, Virginia Tech;.
Martha Ann Bell, Department of Psychology, Virginia Tech.
References
- Babiloni C, Babiloni F, Carducci F, Cappa SF, Cincotti F, Del Percio C, Rossini PM. Human cortical responses during one-bit short-term memory. A high-resolution EEG study on delayed choice reaction time tasks. Clinical Neurophysiology. 2004a;115:161–170. doi: 10.1016/S1388-2457(03)00286-4. [DOI] [PubMed] [Google Scholar]
- Babiloni C, Babiloni F, Carducci F, Cincotti F, Vecchio F, Cola B, Rossini PM. Functional frontoparietal connectivity during short-term memory as revealed by high-resolution EEG coherence analysis. Behavioral Neuroscience. 2004b;118:687–697. doi: 10.1037/0735-7044.118.4.687. [DOI] [PubMed] [Google Scholar]
- Bell MA. The ontogeny of the EEG during infancy and childhood: Implications for cognitive development. In: Garreau B, editor. Neuroimaging in child neuropsychiatric disorders. Berlin: Springer-Verlag; 1998. pp. 97–111. [Google Scholar]
- Bell MA. Power changes in infant EEG frequency bands during a spatial working memory task. Psychophysiology. 2002;39:450–458. doi: 10.1017.S0048577201393174. doi: 10.1017.S0048577201393174. [DOI] [PubMed] [Google Scholar]
- Bell MA. A psychobiological perspective of working memory performance at 8 months of age. Child Development. doi: 10.1111/j.1467-8624.2011.01684.x. in press. [DOI] [PubMed] [Google Scholar]
- Bell MA, Adams SE. Comparable performance on looking and reaching version of the A-not-B task at 8 months of age. Infant Behavior and Development. 1999;22:221–235. [Google Scholar]
- Bell MA, Fox NA. The relations between frontal brain electrical activity and cognitive development during infancy. Child Development. 1992;63:1142–1163. [PubMed] [Google Scholar]
- Bell MA, Wolfe CD. Brain reorganization from infancy to early childhood: Evidence from EEG power and coherence during working memory tasks. Developmental Neuropsychology. 2007;31:21–38. doi: 10.1207/s15326942dn3101_2. [DOI] [PubMed] [Google Scholar]
- Colombo J, Cheatham CL. The emergence and basis of endogenous attention in infancy and early childhood. In: Kail RV, editor. Advances in child development and behavior. Vol. 34. Amsterdam: Elsevier; 2006. pp. 283–322. [DOI] [PubMed] [Google Scholar]
- Cuevas K, Bell MA. EEG and ECG from 5 to 10 months of age: Developmental changes in baseline activation and cognitive processing during a working memory task. International Journal of Psychophysiology. 2011;80:119–128. doi: 10.1016/j.ijpsycho.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ, Jackson DC, Larson CL. Human electroencephalography. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of psychophysiology. 2. Cambridge, UK: Cambridge University Press; 2000. pp. 27–52. [Google Scholar]
- Deoni SCL, Mercure E, Blasi A, Gasston D, Thomson A, Johnson M, Murphy DGM. Mapping infant brain myelination with magnetic resonance imaging. The Journal of Neuroscience. 2011;31:784–791. doi: 10.1523/JNEUROSCI.2106-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A, Prevor M, Callender G, Druin DP. Prefrontal cortex cognitive deficits in children treated early and continuously for PKU. Monographs of the Society for Research in Child Development. 1997;62(4 Serial No 252) [PubMed] [Google Scholar]
- Fein G, Raz J, Brown FF, Merrin EL. Common reference coherence data confounded by power and phase effects. Electroencephalography and Clinical Neurophysiology. 1988;69:581–584. doi: 10.1016/0013-4694(88)90171-x. [DOI] [PubMed] [Google Scholar]
- Funahashi S, Bruce CJ, Goldman-Rakic PS. Dorsolateral prefrontal lesions and oculomotor delayed-response performance: Evidence for mnemonic “scotomas”. Journal of Neuroscience. 1993;13:1479–1497. doi: 10.1523/JNEUROSCI.13-04-01479.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieve PG, Emerson RG, Fifer WP, Isler JR, Stark RI. Spatial correlation of the infant and adult electroencephalogram. Clinical Neurophysiology. 2003;114:1594–1608. doi: 10.1016/S1388-2457(03)00122-6. [DOI] [PubMed] [Google Scholar]
- Haarmann HJ, Cameron KA. Active maintenance of sentence meaning in working memory: Evidence from EEG coherences. International Journal of Psychophysiology. 2005;57:115–128. doi: 10.1016/j.ijpsycho.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Hagemann D, Naumann E, Thayer JF. The quest for the EEG reference revisited: A glance from brain asymmetry research. Psychophysiology. 2001;38:847–857. [PubMed] [Google Scholar]
- Jing H, Gilchrist JM, Badger TM, Pivik RT. A longitudinal study of differences in electroencephalographic activity among breastfed, milk formula-fed, and soy formula-fed infants during the first year of life. Early Human Development. 2010;86:119–125. doi: 10.1016/j.earlhumdev.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Keselman HJ. Testing treatment effects in repeated measures designs: An update for psychophysiological researchers. Psychophysiology. 1998;35:470–478. [PubMed] [Google Scholar]
- Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: A review and analysis. Brain Research Reviews. 1999;29:169–195. doi: 10.1016/s0165-0173(98)00056-3. [DOI] [PubMed] [Google Scholar]
- Lehmann D. Principles of spatial analysis. In: Gevins AS, Remond A, editors. Handbook of electroencephalography and clinical neuropsychology, rev. ser. Vol. 1: Methods of analysis of brain electrical and magnetic signals. Amsterdam: Elsevier; 1987. pp. 309–354. [Google Scholar]
- Linden DEJ, Bittner RA, Muckli L, Waltz JA, Kriegeskorte N, Goebel R, Munk MHJ. Cortical capacity constraints for visual working memory: dissociation of fMRI load effects in a fronto-parietal network. NeuroImage. 2003;20:1518–1530. doi: 10.1016/j.neuroimage.2003.07.021. [DOI] [PubMed] [Google Scholar]
- Lindsley DB. A longitudinal study of the occipital alpha rhythm in normal children: Frequency and amplitude standards. Journal of General Psychology. 1939;55:197–213. [Google Scholar]
- Luck SJ. An Introduction to the Event-Related Potential Technique. Cambridge, MA: MIT Press; 2005. [Google Scholar]
- Marshall PJ, Bar-Haim Y, Fox NA. Development of the EEG from 5 months to 4 years of age. Clinical Neurophysiology. 2002;113:1199–1208. doi: 10.1016/s1388-2457(02)00163-3. [DOI] [PubMed] [Google Scholar]
- Nunez P. Electrical fields of the brain. New York: Oxford; 1981. [Google Scholar]
- Nunez PL, Silberstein RB, Shi Z, Carpenter MR, Srinivasan R, Tucker DM, …Wijesinghe RS. EEG coherency II: experimental comparisons of multiple measures. Clinical Neurophysiology. 1999;110:469–486. doi: 10.1016/s1388-2457(98)00043-1. [DOI] [PubMed] [Google Scholar]
- Orekhova EV, Stroganova TA, Posikera IN. Alpha activity as an index of cortical inhibition during sustained internally controlled attention in infants. Clinical Neurophysiology. 2001;112:740–749. doi: 10.1016/s1388-2457(01)00502-8. [DOI] [PubMed] [Google Scholar]
- Pavlygina RA, Sakharov DS, Davydov VI. EEG characteristics determining the quality of subsequent recognition of visual images. Human Physiology. 2007;33:27–33. doi: 10.1134/S0362119707010045. [DOI] [PubMed] [Google Scholar]
- Picton TW, Bentin S, Berg P, Donchin E, Hillyard SA, Johnson R, …Taylor MJ. Guidelines for using human event-related potentials to study cognition: Recording standards and publication criteria. Psychophysiology. 2000;37:127–152. [PubMed] [Google Scholar]
- Pivik RT, Broughton RJ, Coppola R, Davidson RJ, Fox NA, Nuwer MR. Guidelines for the recording and quantitative analysis of electroencephalographic activity in research contexts. Psychophysiology. 1993;30:547–558. doi: 10.1111/j.1469-8986.1993.tb02081.x. [DOI] [PubMed] [Google Scholar]
- Saltzberg B, Burton WD, Jr, Burch NR, Fletcher J, Michaels R. Electrophysiological measures of regional neural interactive coupling. Linear and nonlinear dependence relationships among multiple channel electroencephalographic recordings. International Journal of Bio-medical Computing. 1986;18:77–87. doi: 10.1016/0020-7101(86)90050-4. [DOI] [PubMed] [Google Scholar]
- Sarnthein J, Petsche H, Rappelsberger P, Shaw GL, von Stein A. Synchronization between prefrontal and posterior association cortex during human working memory. Proceedings of the National Academy of Science. 1998;95:7092–7096. doi: 10.1073/pnas.95.12.7092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W, Schabus M, Doppelmayr M. Fronto-parietal EEG coherence in theta and upper alpha reflect central executive functions of working memory. International Journal of Psychophysiology. 2005;57:97–103. doi: 10.1016/j.ijpsycho.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Stroganova TA, Orekhova EV, Posikera IN. EEG alpha rhythm in infants. Clinical Neurophysiology. 1999;110:997–1012. doi: 10.1016/s1388-2457(98)00009-1. [DOI] [PubMed] [Google Scholar]
- Thatcher RW. Cyclic cortical reorganization: Origins of human cognitive development. In: Dawson G, Fischer KW, editors. Human behavior and the developing brain. New York: Guilford; 1994. pp. 232–266. [Google Scholar]
- Thatcher RW, Walker RA, Giudice S. Human cerebral hemispheres develop at different rates and ages. Science. 1987;236:1110–1113. doi: 10.1126/science.3576224. [DOI] [PubMed] [Google Scholar]
- Thomas KM, King SW, Franzen PL, Welsh TF, Berkowitz AL, Noll DC, …Casey BJ. A developmental functional MRI study of spatial working memory. NeuroImage. 1999;10:327–338. doi: 10.1006/nimg.1999.0466. [DOI] [PubMed] [Google Scholar]
- Tsekhmistrenko TA, Vasil’eva VA, Shumeiko NS, Vologirov AS. Quantitative changes in the fibroarchitectonics of the human cortex from birth to the age of 12 years. Neuroscience and Behavioral Physiology. 2004;34:983–988. doi: 10.1023/b:neab.0000042657.80112.7b. [DOI] [PubMed] [Google Scholar]
- Weiss S, Rappelsberger P. Long-range EEG synchronization during word encoding correlates with successful memory performance. Cognitive Brain Research. 2000;9:299–312. doi: 10.1016/s0926-6410(00)00011-2. [DOI] [PubMed] [Google Scholar]