Abstract
The extent to which the progeny of one primary memory CD8 T cell differs from the progeny of one naïve CD8 T cell of the same specificity remains an unresolved question. In order to explore cell autonomous functional differences between naïve and memory CD8 T cells that are not influenced by differences in the priming environment, an experimental model has been developed in which physiological numbers of both populations of cells were co-transferred into naïve hosts before antigen-stimulation. Interestingly, naïve CD8 T cells undergo greater expansion in numbers than primary memory CD8 T cells after various infections or immunizations. The intrinsic ability of one naïve CD8 T cell to give rise to more effector CD8 T cells than one memory CD8 T cell is independent of the number and quality of primary memory CD8 T cells present in vivo. The sustained proliferation of newly activated naïve CD8 T cells contributed to their greater magnitude of expansion. In addition, longitudinal analyses of primary and secondary CD8 T cell esponses revealed that on a per cell basis naïve CD8 T cells generate higher numbers of long-lived memory cells than primary memory CD8 T cells. This enhanced ‘memory generation potential’ of responding naïve CD8 T cells occurred despite the delayed contraction of secondary CD8 T cell responses. Taken together, the data presented here revealed previously unappreciated differences between naïve and memory CD8 T cells and will help further define the functional potential for both cell types.
Introduction
Memory CD8 T cells are the surviving progeny of relatively rare naïve CD8 T cells that have been programmed to clonally expand upon encounter with cognate antigen (Ag) presented by professional antigen-presenting cells (APCs) (1–6). Only a small fraction (5–10%) of the responding cells present at the peak of the expansion phase survive to become memory (7–10). A number of studies have suggested that the protective capacity of primary memory CD8 T cells is dependent both on their absolute number and functional properties (1). Thus, controlling the quality and/or quantity of the developing memory CD8 T cell pool should represent an important goal in vaccine development.
Substantial progress has been made towards understanding the features that define primary memory CD8 T cells. In general, attributes of memory CD8 T cells such as increased representation (increase in numbers over naïve CD8 T cell repertoire), changes in distribution (enhanced surveillance at potential sites of pathogen entry), longevity (long-term survival), and function (rapid killing and cytokine producing abilities) have led to the assumption that memory CD8 T cells are qualitatively and quantitatively better than their naïve counterparts (2, 4, 11, 12). All of these attributes are indeed important factors that contribute to the increased CD8 T cell-mediated resistance to infection in immune hosts. However, the extent to which the progeny of one memory CD8 T cell differs from the progeny of one naïve CD8 T cell of the same specificity remains an unresolved and important question.
For instance, both naïve and memory CD8 T cells are capable of exponential proliferation following Ag-stimulations. Because memory CD8 T cells are present in higher numbers than naïve cells they often, but not always give rise to a higher number of secondary effectors compared to the number of primary effectors generated from the naïve CD8 T cell pool. The ability of prime-boost protocols to increase memory CD8 T cell numbers is well documented (13–15). However, experiments that examine the proliferative potential of naïve and memory CD8 T cells, while controlling for the numbers of precursor cells have yielded conflicting results (16). Despite these caveats, it has been suggested that the numerical expansion capacity of memory CD8 T cells is the same or better compared to naïve cells following Ag-stimulation (16). Importantly, data that support this conclusion are complicated by the adoptive transfer with large numbers of naïve T-cell receptor transgenic (TCR-Tg) CD8 T cells and our previous work showed that initial TCR-Tg cell precursor frequency dictates critical aspects of the CD8 T cell response to infection, including the magnitude of primary expansion (17). In addition, primary and repeatedly stimulated (ex. secondary, tertiary, quaternary) memory CD8 T cells differ substantially in their molecular signatures as well as in their functional attributes including the ability to proliferate to new Ag-stimulation (18, 19). Since loss of expansion capacity is correlated with the number of Ag-encounters, the conclusion that primary memory CD8 T cells (on a per-cell basis) are capable of equal or greater Ag-driven proliferation compared to naïve CD8 T cells warrants re-examination.
Additionally, experiments examining the kinetics of primary and secondary CD8 T cell responses have noted a prolonged contraction phase of secondary compared to primary CD8 T cell responses, suggesting differential susceptibility to apoptosis between these populations (7, 18–21). However, the assumption that due to delayed contraction the ability to generate long-lived progeny (here described as ‘memory generation potential’) is greater for one primary memory compared to one naïve CD8 T cell also remains unsupported.
In order to address intrinsic (cell autonomous) differences between naïve and memory CD8 T cells that are not the result of differences in environmental factors, we used an experimental model in which both cell types were analyzed in the same host responding to infection or immunization. Two questions were asked in this study: 1) to what extent does the expansion capacity of one naïve CD8 T cell differ from one primary memory CD8 T cell upon Ag-stimulation; and 2) does ‘memory generation potential’ in vivo differ between naïve and primary memory CD8 T cells after infection.
Our results show that naïve CD8 T cells undergo a higher magnitude of expansion than memory CD8 T cells when analyzed on a per-cell basis in various models of infection and/or immunization. Longitudinal analysis of primary and secondary CD8 T cell responses also revealed that ‘memory generation potential’ of responding naïve CD8 T cells is better than for responding primary memory CD8 T cells despite the differences in overall kinetics of both responses. Therefore, our data provide new insights into primary memory CD8 T cell function and will help further define the functional properties of primary memory CD8 T cells.
Material and Methods
Mice, bacteria, and virus infections
C57Bl/6 (B6) Thy1.2/1.2 or CD45.1/CD45.1 mice were obtained from the U.S. National Cancer institute. OT-I TCR-transgenic mice (Thy1.1/1.2 and Thy1.1/1.1) were previously described (19, 22). Pathogen-infected mice were housed in the appropriate bio safety conditions. All mice were used at 6–10 weeks of age. All animal protocols followed approved Institutional Animal Care and Use Committee (IACUC) protocols. The virulent Listeria monocytogenes strain expressing Ova257 (Vir LM-OVA) and the attenuated actA-deficient LM strain expressing Ova257 (Att LM-OVA) were grown, injected intravenously, and quantified as described (19, 23, 24). Vaccinia virus expressing the Ova257 peptide (VacV-OVA) has been described previously (25).
Dendritic cell immunizations
Splenic DC were isolated after subcutaneous injection of B6 mice with 5x106 B16 cells expressing Flt3L as previously described (26). When tumors were palpable (5 mm×5 mm), mice were injected with 2 µg LPS (Sigma-Aldrich) intravenously to mature the DC. Spleens were harvested 16 hrs later and were digested with DNase and Collagenase for 20 min at 37°C/ 5% CO2 with shaking. Spleen pieces were smashed through a nylon cell strainer (70 mm) to generate a single cell suspension, red blood cells (RBC) were lysed, and splenocytes were re-suspended in 2 parts of 10% FCS RPMI 1640 to one part B16-Flt3L conditioned medium plus recombinant GM-CSF (1000 U/mL) plus 2 µM Ova257–264 and incubated 2 hrs at 37°C/ 5% CO2. Spleen cells were washed three times and CD11c+ cells were isolated using anti-CD11c microbeads (Miltenyi Biotec). The purity and activation status of DC were determined by staining for CD11c, CD86, and MHC class II. Routinely, >90% pure CD11c+ DC were obtained. DCs were re-suspended in saline and injected intraperitoneally (i.p.).
Adoptive-transfer experiments and isolation of lymphocytes from tissues
OT-I cells were obtained from peripheral blood samples of 2–3 months old naïve OT-I mice. Contaminating memory phenotype (CD44hiCD11ahiV2+V5+) OT-I cells were always less that 5%. To generate primary memory OT-I CD8 T cells for adoptive transfer experiments, 1x103 naïve Thy1.1/1.2 or Thy1.1/1.1 OT-I cells were transferred into Thy1.2 recipients one day before VacV-OVA (3x106 PFU per mouse; i.p.) infection. For co-transfer of naive and memory OT-I cells, naïve OT-I T cells were obtained from peripheral blood of TCR-Tg OT-I mice and mixed at the indicated ratios with memory OT-I cells obtained from the spleens of VacV-OVA immunized mice at various time points after infection. The ratio of naïve to memory OT-I cells in master mix used for adoptive transfer was verified by flow cytometry before transfer. For adoptive transfer of endogenous Ova257-specific primary memory CD8 T cells splenocytes from VacV-OVA immunized mice (day 113 post infection) were used. 5x103 of Ova257-specific CD8 T cells (CD45.2) were transferred into naïve B6 (CD45.1) mice one day before Att LM-OVA infection.
For isolation of lymphocytes from secondary lymphoid organs and tertiary tissues, samples of blood were obtained by retro-orbital puncture before tissue removal. Anesthetized mice were then perfused through the left ventricle with cold PBS for 1–2 min and tissues were collected. Single cell suspension from liver, lung, spleen, lymph nodes, and bone marrow were washed with PBS before staining (27).
Abs and peptides
The following mAbs from eBioscience with the indicated specificity and with appropriate combinations in fluorochromes were used: CD8 (clone 53–6.7), Thy1.1 (OX-7 or HIS51), Thy1.2 (53–2.1), CD45.2 (104), CD127 (A7R34), CD62L (MEL-14), CD27 (LG.7F9), CD122 (5H4), anti-BrdU (PRB-1) and appropriate isotype controls.
Quantification of CD8 T cell responses and detection of BrdU uptake and activated Caspase-3/7 in Ag-specific CD8 T cells
OT-I cell responses in peripheral blood and tissues were monitored by FACS analysis for Thy1.1 positive CD8 T cells. Thy1.2 expression was used to discriminate between primary and secondary responses in the same host. Endogenous Ova257-specific CD8 T cells were detected by KbOva257 tetramers as previously described (28). To determine the rate of proliferation of Ag-specific T cells during the expansion phase, mice were i.p. injected with 2 mg BrdU on days 4,5, or 6 after Att LM-OVA infection and given 0.8 mg/ml BrdU in drinking water for an additional 24 hrs. Peripheral blood was collected and splenocytes were isolated and surface-stained for CD8 and Thy1.1, followed by fixation and permeabilization procedures as recommended in the BrdU Flow Kit (BD Biosciences). Anti-BrdU mAb was used for intracellular staining to detect BrdU uptake. To determine the proliferation rate of Ag-specific CD8 T cells during the memory maintenance phase, mice were i.p. injected with 2 mg BrdU and given 0.8 mg/mL BrdU in drinking water for an additional 8 days. BrdU uptake was determined as described above. For detection of activated caspase-3 and -7 in Ag-specific CD8 T cells, splenocytes were first surface-stained for CD8, Thy1.1, and Thy1.2. Samples were then incubated with the fluorescent inhibitor of caspases (FLICA) reagent, which binds to activated caspases, at 37° C and 5% CO2 for 60 min as recommended in the Vybrant FAM caspase-3 and -7 assay kit (Invitrogen, Carlsbad, CA).
Statistical Analysis
Statistical significance was assessed using the two-tailed t-test with a confidence interval of >95%. * indicates a p value between 0.01 and 0.05, and ** indicates a p value less than 0.01. Data are presented as mean (+/− SEM) unless otherwise stated in the figures.
Results
Naïve CD8 T cells undergo a higher magnitude of expansion than memory CD8 T cells after L. monocytogenes infection
Naïve and primary memory CD8 T cells are characterized by their ability to undergo vigorous expansion in numbers upon Ag-encounter. The prevailing assumption is that memory CD8 T cells due to their increased frequencies, tissue distribution, and function are both qualitatively and quantitatively superior to their naïve counterparts in responding to Ag. However, whether memory CD8 T cells have an enhanced proliferation capacity compared to naïve cells is still an unresolved question (reviewed in (16)). In experiments where the magnitude of the expansion of primary and secondary CD8 T cell responses were compared in different hosts, it has been shown that memory CD8 T cells have an equal or increased ability to proliferate and accumulate in vivo (21, 29, 30). Since multiple factors can influence the expansion of naïve and/or primary memory CD8 T cells (ex. precursor frequencies, type of antigen-stimulation, duration of infection and inflammation, phenotype of responding CD8 T cells (1, 2, 31–34)) we developed an experimental model to address the proliferative potential of both naïve and memory CD8 T cell populations in the same host environment (Figure 1A). The model includes: a) the adoptive transfer of physiologically relevant numbers of naïve CD8 T cells; and b) co-transfer of primary memory CD8 T cells into the same recipients to control for differences in the in vivo environment (ex. duration of infection and inflammation).
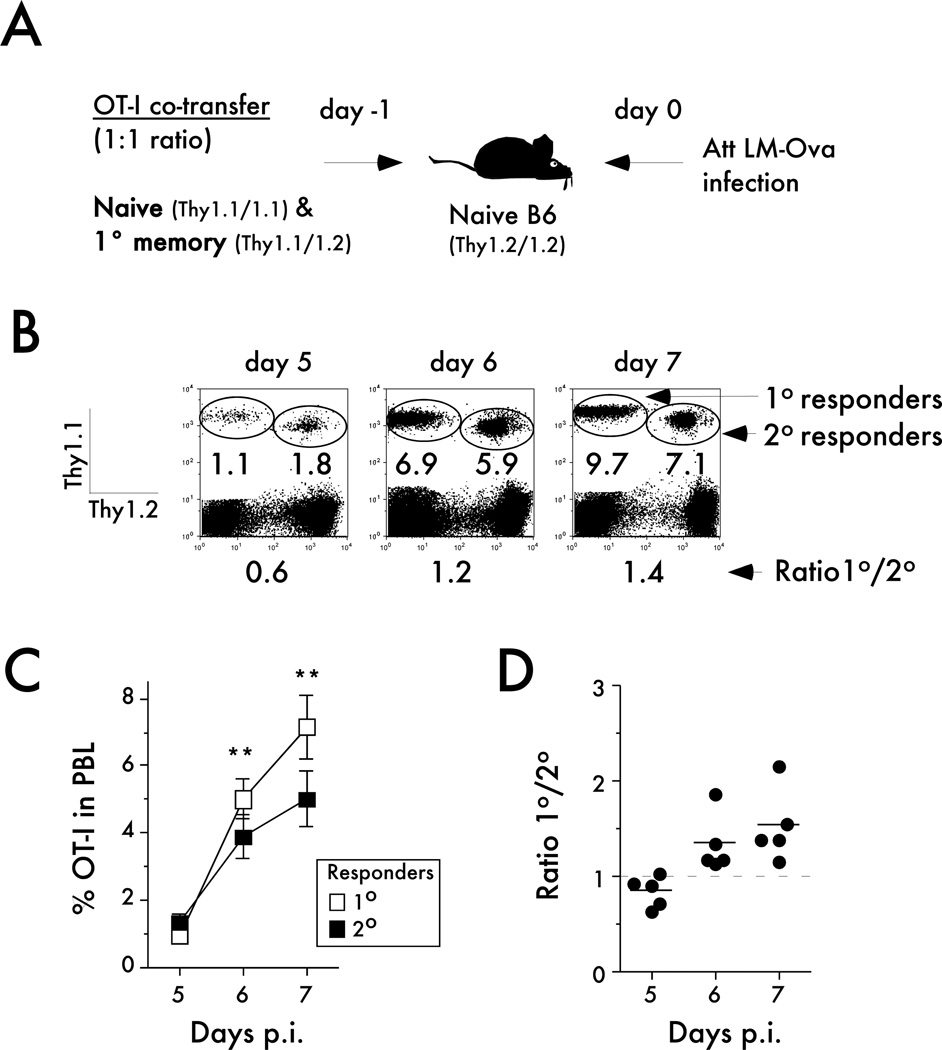
Figure 1. Naïve CD8 T cells undergo a higher magnitude of expansion than memory CD8 T cells after LM infection.
A) Experimental design. Naïve Thy1.1/1.1 OT-I (1x103) were mixed with an equal number of primary memory Thy1.1/1.2 OT-I (day 200+ after primary VacV-OVA infection) and injected into naïve B6 Thy1.2/1.2 recipients. Mice were challenged 24 hrs later with Att LM-OVA (5x106 CFU per mouse; i.v.). B) Representative plots showing the primary (Thy1.1/1.1) and secondary (Thy1.1/1.2) OT-I responses at the indicated days after Att LM-OVA infection. Numbers inside the plots indicate the percentage of primary or secondary OT-I CD8 T cells in peripheral blood, and numbers below the plots indicate the ratio of primary to secondary OT-I cells at the indicated days post infection (p.i.). C) Kinetic analysis of OT-I in peripheral blood. Data are presented as the percentage of primary or secondary OT-I cells in peripheral blood (mean +/− SEM for 5 mice per group). D) The ratio of primary to secondary OT-I cells at the indicated days after infection. Dots represent individual mice, solid lines indicate the mean, and the dashed line indicates the starting ratio of naïve and primary memory OT-I cells in the mix used for adoptive transfer before infection. The data are representative of at least three independent experiments. * indicates a p value between 0.01 and 0.05, and ** indicates a p value less than 0.01.
We adoptively co-transferred 1x103 naïve TCR-Tg OT-I CD8 T cells (Thy1.1/1.1) with the same number of primary memory OT-I cells (Thy1.1/1.2) and subsequently infected recipient mice (naïve B6 mice; Thy1.2/1.2) with a sub-lethal dose (0.1LD50) of an attenuated (act A− deficient) recombinant Listeria monocytogenes expressing the Ova257 epitope (Att LM-OVA) (Figure 1A - experimental design). Recall potential of memory CD8 T cell populations changes with time after initial stimulation (33) and to ensure that memory CD8 T cells used in the experiments were capable of vigorous secondary expansion, primary memory OT-I cells were obtained from donor mice that had been infected more than 200 days before. To determine the magnitude of expansion of each population we analyzed the percentage of primary or secondary effector OT-I cells in the peripheral blood (PBL) from days 5 to 7 post challenge (Figure 1B,C). Interestingly, the data showed that the proliferation capacity of naïve OT-I (1° responders) is greater than the capacity of memory OT-I (2° responders) after bacterial challenge (Figure 1C). Importantly, a higher ratio of primary to secondary effectors was seen in each individual mouse examined at days 6 and 7 after infection (Figure 1D). Therefore, these results suggest that the magnitude of the expansion of naïve CD8 T cells is higher than memory CD8 T cells when analyzed on a per cell basis in the same host after bacterial infection.
Naïve CD8 T cells expand in numbers more than memory CD8 T cells in response to infections or non-infectious immunization
The magnitude of naïve CD8 T cell responses varies depending on factors such as the type, route, and dose of infection as well as inflammation present during the infection (1, 12, 31). In addition, it has been recently suggested that inflammation also controls the magnitude of expansion and differentiation of secondary CD8 T cell responses but that naïve and memory CD8 T cells might differ in their susceptibility to inflammatory cues in the environment (27, 35). Therefore, we first wanted to examine if our initial findings seen after Att LM-OVA infection could be extended to additional types and doses of bacterial and viral infections as well as non-infectious priming conditions with dendritic cells (DC) (Figure 2A). Equal numbers of naïve and memory OT-I cells (d225 p.i.) were co-transferred into naïve B6 recipients followed by immunization with Att LM-OVA (5x106 or 5x104 CFU per mouse), a virulent strain of LM-OVA (5x104 CFU), Vaccinia virus expressing OVA (VacV-OVA), or dendritic cells coated with OVA peptide (DC-OVA) (Figure 2A - experimental design). The starting ratio of naïve to memory OT-I cells was determined before adoptive transfer in a master mix, which was diluted for injection of ~ 1,000 of each OT-I populations (Figure 2B). The input ratio was later used to determine how the antigen-driven proliferation changes the numbers of primary and secondary CD8 T cell responding to in vivo Ag-stimulation.
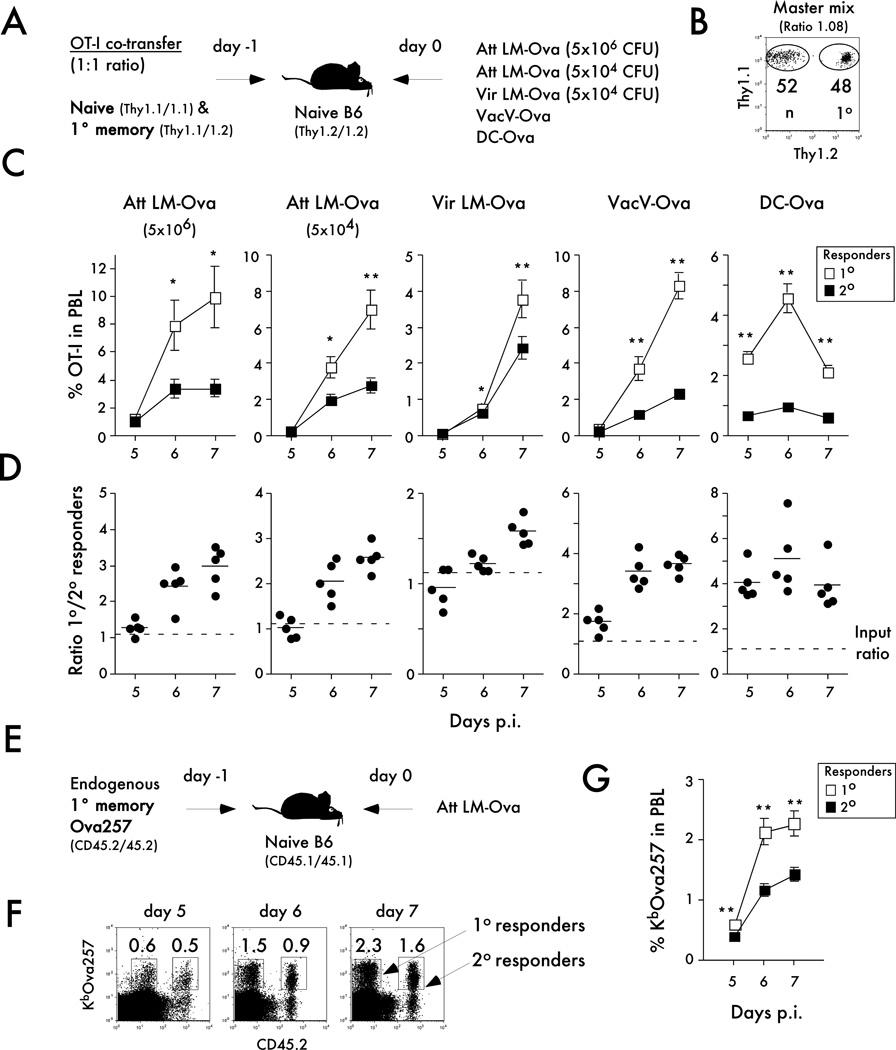
Figure 2. Naïve CD8 T cells undergo a greater magnitude of expansion than memory CD8 T cells in response to infections or non-infectious immunization.
A) Experimental design. Naïve Thy1.1/1.1 OT-I (1x103) were mixed with an equal number of primary memory Thy1.1/1.2 OT-I (day 200+ after primary VacV-OVA infection) and injected into naïve B6 Thy 1.2/1.2 recipients. Mice were challenged 24 hrs later with Att LM-OVA (5x106 CFU or 5x104 CFU per mouse; i.v.), Vir LM-OVA (5x104 CFU per mouse; i.v.), VacV-OVA (3x106 PFU per mouse; i.p.), or DC-OVA (1x106 cells per mouse; i.v.). B) Dot plot showing the mix of naïve and memory OT-I cells used for adoptive transfer. Numbers inside the plot indicate the percentage of naïve OT-I (Thy1.1/1.1) or primary memory OT-I (Thy1.1/1.2). C) Kinetic analysis of OT-I in peripheral blood at the indicated days after infection or immunization. Data are presented as the percentage of primary or secondary OT-I cells in peripheral blood (mean +/− SEM for 5 mice per group). D) The ratio of primary to secondary OT-I at the indicated days after infection or immunization. Dots represent individual mice, solid lines indicate the mean, and the dashed line indicates the starting ratio of naïve and memory OT-I cells before infection. E) Experimental design. Endogenous primary memory Ova257-specific CD8 T cells (day 113 after primary VacV-OVA infection) were transferred (5x103 cells per mouse i.v.) into naïve B6 CD45.1/CD45.1 recipients one day before Att LM-OVA (5x106 CFU; i.v.) challenge. F) Representative plots showing the primary (CD45.2 negative) and secondary (CD45.2 positive) KbOva257 endogenous CD8 T cell responses at the indicated days after Att LM-OVA infection. Numbers inside the plots indicate the percentage of primary or secondary Ova257-specific CD8 T cells in peripheral blood (PBL). G) Kinetic analysis of Ova257-specific CD8 T cells in peripheral blood at the indicated days after infection. Data are presented as the percentage of primary or secondary cells in peripheral blood (mean +/− SEM for 5 mice per group). These data are representative of two to three similar and independent experiments. * indicates a p value between 0.01 and 0.05, and ** indicates a p value less than 0.01.
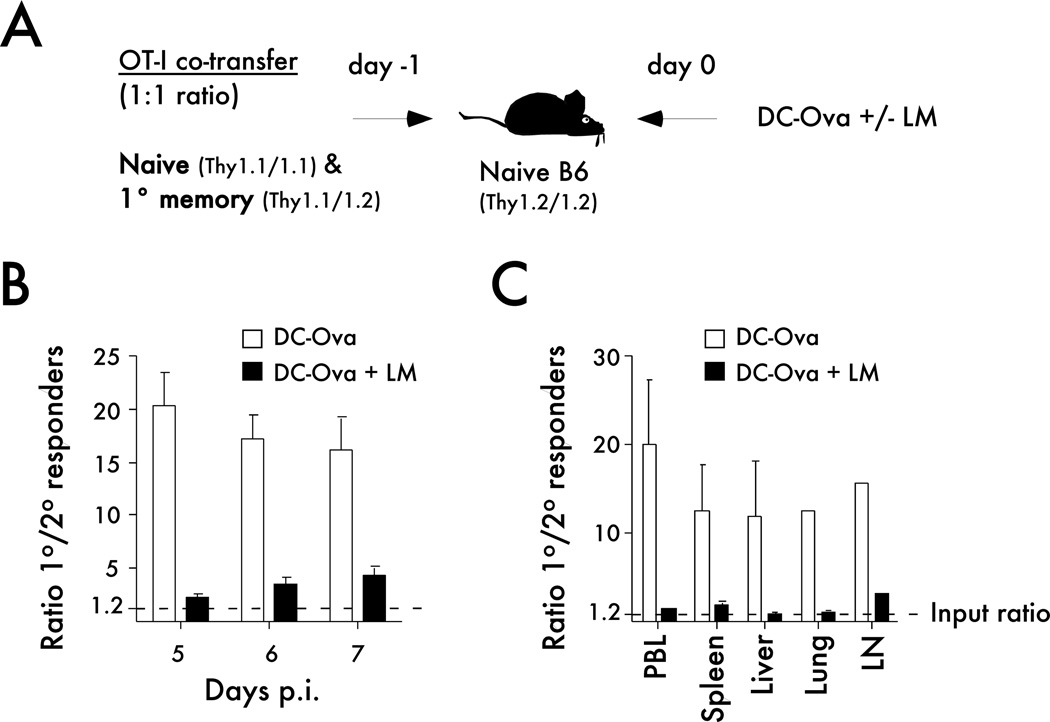
Importantly, when analyzed on a per cell basis the overall magnitude of expansion of naïve CD8 T cells was greater than primary memory CD8 T cells in all groups of mice (Figure 2C,D). However, the degree of difference in the magnitude of expansion between primary and secondary CD8 T cells depended on the specific immunization. The magnitude of expansion of the primary and secondary CD8 T cell responses were the most similar after infection with a virulent strain of LM, while differences between primary and secondary CD8 T cell responses were more pronounced after priming in an environment where systemic inflammation was substantially reduced (DC-OVA group; Figure 2C,D; (13, 31)). To determine, if the latter result was due to the systemic inflammation, mice containing equal numbers of naïve and primary memory OT-I cells were immunized with DC-OVA with or without co-infection with LM that do not express the Ova epitope (Figure 3A – experimental design). Again, naïve CD8 T cells expanded substantially more than memory CD8 T cells after immunization with DC alone. However, inducing systemic inflammation by concurrent LM infection eliminated the differences in expansion between naïve and memory CD8 T cells (Figure 3B). Similar data were obtained in secondary lymphoid organs and in peripheral tissues of mice, indicating that the observations made in the blood are consistent throughout the host (Figure 3C).
Figure 3. Systemic inflammation influences the expansion potential of primary memory CD8 T cells.
A) Experimental design. Naïve Thy1.1/1.1 OT-I cells (1x103) were mixed with an equal number of primary memory Thy1.1/1.2 OT-I cells (d350+ after primary infection) and injected into naïve B6 Thy1.2/1.2 recipients. Mice were immunized 24 hrs later with Ova257-coated dendritic cells (DC-OVA; 2x105 cells per recipient) in the presence or absence of Att LM co-infection (5x106 CFU per mouse). B) Graph showing the ratio of primary to secondary OT-I cells in PBL at the indicated days after DC-OVA immunization in the presence or absence of infection. C) The ratio of primary to secondary OT-I cells in the indicated organs at day 7 after immunization. The dotted lines in B and C indicate the ratio of naïve to primary memory OT-I cells used in the adoptive transfer mix. Representative experiment out of three similar and independent experiments is shown.
Finally, we determined if differences in expansion potential between monoclonal TCR-Tg naïve and memory CD8 T cells can be observed with polyclonal (endogenous) CD8 T cell responses. To address this question 5x103 Ova257-specific CD8 T cells (CD45.2) generated by VacV-OVA infection were transferred into naïve B6 (CD45.1) mice before Att LM-OVA infection (Figure 2E – experimental design).
Naïve B6 mice contain 100 to 200 naïve Ova257-specific CD8 T cell precursors (32, 36). Not all of the transferred cells survive in the recipient upon adoptive transfer and the percentage of the cells that survive has been referred to as ‘take’. Thus, even if ‘take’ is as low as 3–4% of transferred memory numbers (most studies suggest that ‘take’ is approximately 10% (20, 33, 37)) equal if not higher numbers of memory CD8 T cells should be present in vivo before infection in this experimental design (Figure 2E). Importantly, the endogenous naïve CD8 T cells still expanded in numbers more than transferred polyclonal primary memory CD8 T cells (Figure 2E,G).
Taken together, these data suggest that under various infections and priming conditions the magnitude of expansion of the responding naïve is greater than the magnitude of expansion of the responding primary memory CD8 T cells and indicate that when analyzed on a per cell basis naïve CD8 T cells posses a higher proliferative potential than memory CD8 T cells.
Differences in trafficking after adoptive transfer do not explain the higher magnitude of expansion of naïve CD8 T cells
The results thus far have indicated that the expansion potential of naïve CD8 T cells is greater than memory CD8 T cells when analyzed on a per-cell basis under various priming conditions. A possible explanation for this is that naïve and primary memory CD8 T cells could traffic to different areas after adoptive transfer, which might influence their priming. In order to test that idea, equal numbers of naïve and primary memory OT-I cells (3x105 each) were co-transferred and the ratio of naïve to primary memory OT-I cells in various organs 24 hrs after adoptive transfer was determined (Figure 4A - experimental design). As in the experiments before, the memory OT-I cells were obtained from donor mice more than 200 days post primary infection, and phenotypic analysis showed that the majority of them were CD127, CD62L, CD27, and CD122 positive (Figure 4B), which are characteristic of late or central memory CD8 T cells (lateM or Tcm, respectively) (3, 11). Thus, these cells should be capable of vigorous secondary expansion and trafficking to secondary lymphoid organs where priming occurs (2, 33). Figure 4C shows the ratio of naïve and primary memory OT-I cells used in the adoptive transfer mix.
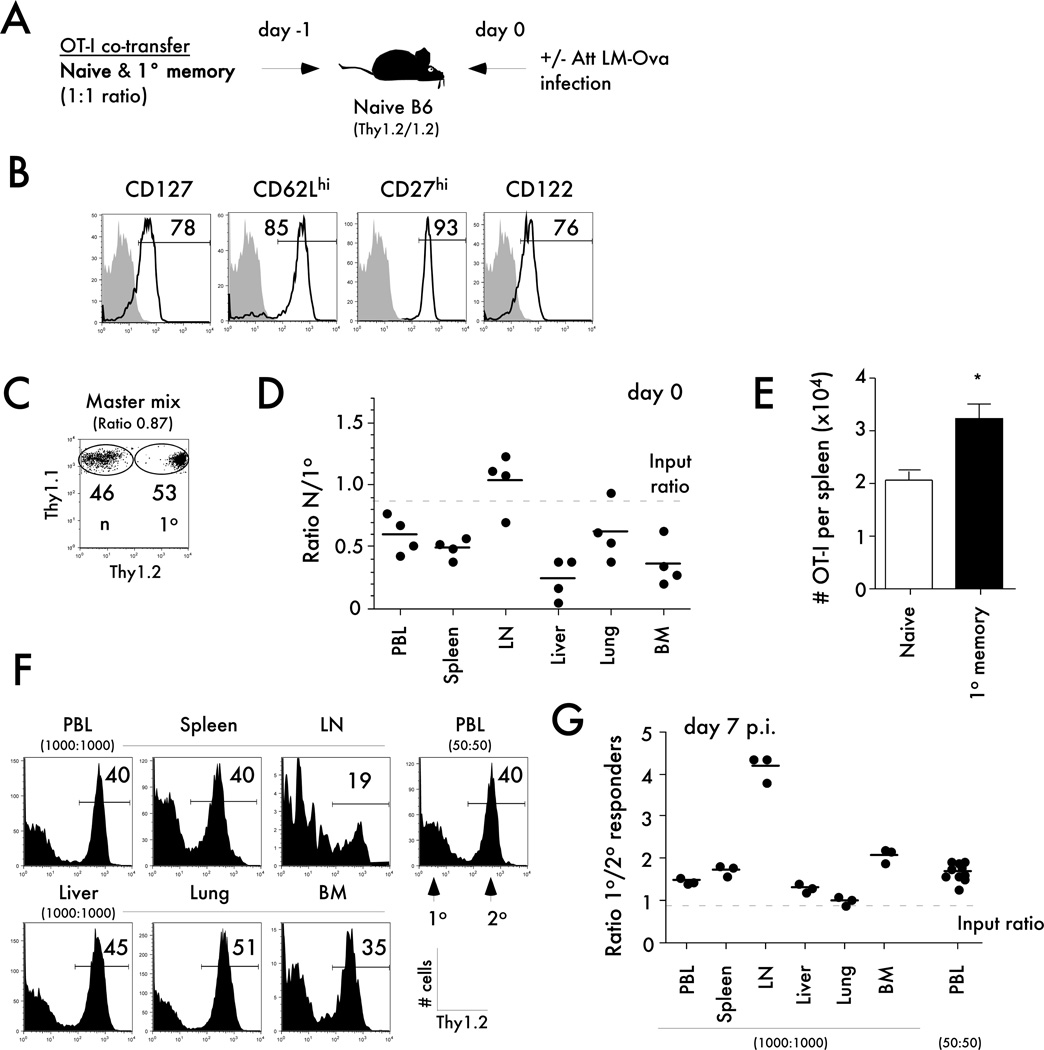
Figure 4. Differences in trafficking before infection or in tissue localization after infection do not explain the differences in expansion potential of naïve and memory CD8 T cells.
A) Experimental design. The indicated numbers of naïve Thy1.1/1.1 OT-I were mixed with an equal number of primary memory Thy1.1/1.2 OT-I (day 300+ after primary VacV-OVA infection) and injected into naïve B6 Thy1.2/1.2 recipients. Mice were challenged 24 hrs later with Att LM-OVA (5x106 CFU per mouse). B) The expression of the indicated markers was evaluated on primary memory OT-I cells. Shaded histograms represent isotype control staining and open histograms represent specific staining of gated primary memory OT-I CD8 T cells. Numbers indicate the percentage of cells positive for the indicated molecules. C) Dot plot showing the mix of naïve and memory OT-I cells used for adoptive transfer. Numbers inside the plot indicate the percentage of naïve OT-I (Thy1.1/1.1) or primary memory OT-I (Thy1.1/1.2) cells. D) The ratio of primary to secondary OT-I in the indicated organs 24 hrs after adoptive transfer of naïve OT-I and primary memory OT-I (3x105 of each cell types per recipient). Dots represent individual mice, solid lines indicate the mean, and the dashed line indicates the starting ratio of naïve and memory OT-I cells before infection. E) Total numbers of naïve or primary memory OT-I in the spleens one day after adoptive transfer. Data are presented as mean + SEM for 4 mice per group. F) Representative histograms showing the percentage of secondary among all gated OT-I cells in the indicated organs at day 7 after Att LM-OVA infection (5x106 CFU per mouse; i.v.). The total number of naïve and primary OT-I cells initially transferred is indicated. G) The ratio of primary to secondary OT-I cells in the indicated organs at day 7 p.i. Dots represent individual mice, solid lines indicate the mean, and the dashed line indicates the starting ratio of naïve and memory OT-I cells before infection. * indicates a p value between 0.01 and 0.05.
Examining the ratios of naïve to primary memory OT-I cells in various tissues after adoptive transfer revealed a higher number of primary memory cells in all organs examined except for the inguinal lymph nodes (Figure 4D). However, higher numbers of memory cells were detected in the spleen compared to naïve cells (Figure 4E). Importantly, the spleen is the major secondary lymphoid organ where priming occurs after LM infection (38). Thus, these data suggest that the differences in proliferation capacity between naïve and memory CD8 T cells are likely not explained by differences in their localization at the time of priming.
Differences in tissue localization at the peak of the response do not explain the differences in expansion potential of naïve and memory CD8 T cells
It is also possible that primary effectors are over-represented in the circulation and that overall ratios between primary and secondary effectors differ in various organs of the mice. In order to address this question, equal numbers (1x103 each) of naïve and primary memory OT-I were co-transferred into naïve mice prior to Att LM-OVA infection (Figure 4F). Consistent with our previous experiments, we found a higher number of primary effectors in PBL at day 7 post infection (Figure 4F,G). Importantly, in each organ examined primary effectors were found in higher numbers than secondary effector CD8 T cells (Figure 4F,G). Interestingly, even after the transfer of 20-fold lower numbers of both naïve and memory CD8 T cells (50 cells transfer) the expansion potential of the naïve T cell population surpasses that of primary memory CD8 T cells (Figure 4F,G). Taken together, these results suggest that primary effectors are not over-represented in circulation and that naïve CD8 T cells posses a higher proliferative potential than memory CD8 T cells when analyzed on a per-cell basis and in multiple organs of the mice.
Sustained proliferation of primary rather than increased death of secondary effectors leads to the higher magnitude of expansion of the naïve CD8 T cells
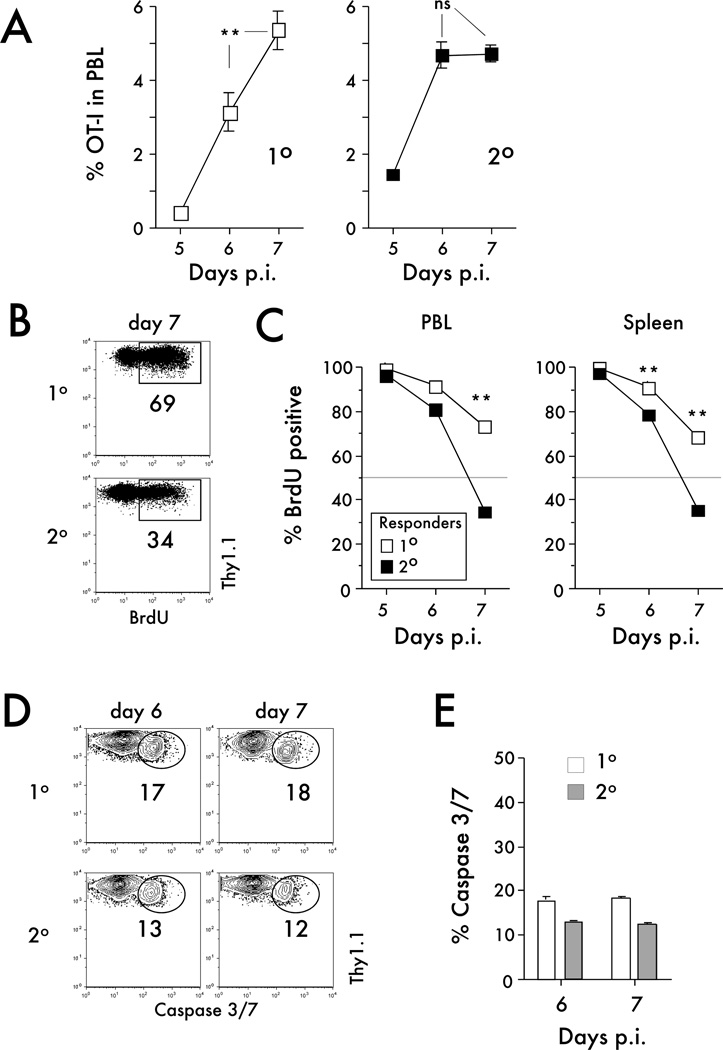
The differences in the expansion capacity of naïve and primary memory CD8 T cells after infection or immunization could be explained by differences in proliferation and/or survival of these populations after Ag stimulation. In order to test these possibilities, naïve or primary memory OT-I CD8 T cells were transferred into separate groups of naïve B6 mice before Att LM-OVA infection (Figure 5). As described before, differences in kinetics of CD8 T cell expansion were observed for primary and secondary CD8 T cell responses (Figure 5A). Importantly, even after transfer into separate mice, a statistically significant increases in numbers (from day 6 to 7 p. i.) were observed only in the PBL of mice that contained primary CD8 T cells (Figure 5A).
Figure 5. Sustained proliferation of primary effectors rather than increased death of secondary effectors leads to the greater magnitude of expansion of the naïve CD8 T cells.
A) Kinetic analysis of primary or secondary OT-I in peripheral blood. Naïve OT-I or primary memory OT-I cells were transferred into separate mice, and mice were challenged 24 hrs later with Att LM-OVA (5x106 CFU per recipient; i.v.). Data are presented as the percentage of primary or secondary OT-I cells in peripheral blood (mean +/− SEM for 4–10 mice per group per time point). B) Representative dot plots of BrdU staining of OT-I cells in the spleen at day 7 after Att LM-OVA infection. Numbers inside the plots indicate the percentage of OT-I cells positive for BrdU. C) Kinetic analysis of BrdU incorporation. Data are presented as the percentage of primary or secondary OT-I cells in peripheral blood or the spleen positive for BrdU (mean +/− SEM for 3–4 mice per group per time point). D) Representative dot plots of caspase-3/7 staining of primary or secondary OT-I cells in the spleens at the indicated time points after Att LM-OVA infection. Numbers indicate the percentage of OT-I cells positive for caspase-3/7. E) The percentage of primary or secondary OT-I cells positive for caspase-3/7 at the indicated days after Att LM-OVA infection (mean + SEM for 3–4 mice per group per time point). Data are representative of two independent experiments. ** indicates a p value less than 0.01; ns- not significant.
Examining the kinetics of proliferation revealed that nearly all primary and secondary effector OT-I cells incorporated BrdU from days 4–5. However, from days 6–7 in the PBL and days 5–6 and 6–7 in the spleen, a significantly higher percentage of primary effector OT-I cells incorporated BrdU than secondary effector OT-I cells (Figure 5B,C). Thus, these data suggest that primary effector CD8 T cells exhibit sustained and prolonged proliferation compared to secondary CD8 T cells.
In addition to the BrdU proliferation assay, caspase 3/7 staining (as an indication of cell death) was performed on primary and secondary OT-I cells found in the spleens at day 6 and 7 after Att LM-OVA infection. Similar percentages of primary and secondary CD8 T cells were positive for caspase 3/7 at days 6 and 7 p.i. (Figure 5D,E), suggesting that the rate of death for both primary and secondary CD8 T cell responses was similar. Therefore, these results indicate that differences in proliferation but not the rate of cell death can explain the observed differences in naïve and memory CD8 T cell expansion after infection.
Population dynamics of naïve and memory CD8 T cell responses after antigen stimulation in vivo
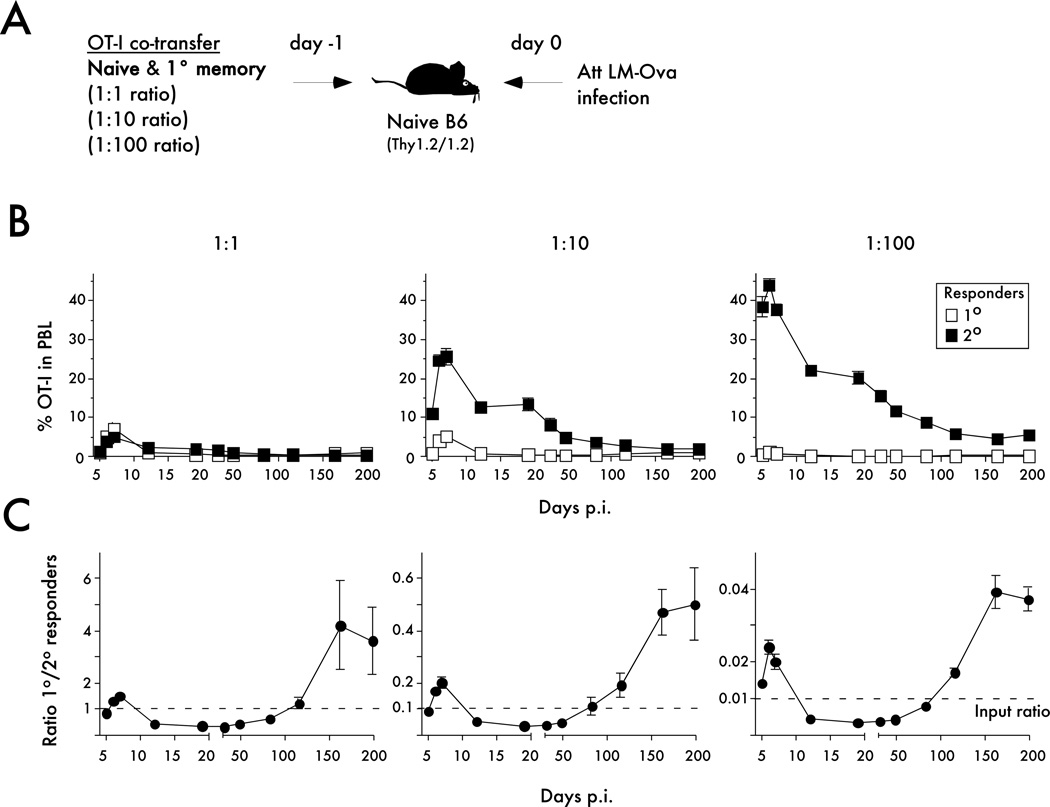
Previously, it has been shown that in contrast to the vigorous and relatively short contraction (death) phase of primary CD8 T cell responses, secondary CD8 T cells die at a substantially protracted rate (7, 18–21). Importantly, it is not known if and when contraction of secondary CD8 T cells is resolved and how the prolonged contraction influences the numbers of secondary memory CD8 T cells. In other words, is the potential for generating a stable memory CD8 T cell population different for responding naïve and primary memory CD8 T cells? To address this, low numbers of naïve (1x103 per mouse) OT-I were co-transferred with increasing numbers of primary memory CD8 T cells (naïve to memory CD8 T cell ratios: 1:1, 1:10 and 1:100) before infection with Att LM-OVA (Figure 6A – experimental design). Detailed longitudinal kinetic analysis of primary and secondary CD8 T cell responses (shown as frequencies of OT-I cells in the PBL) is presented in Figure 6B.
Figure 6. Longitudinal analysis of primary and secondary CD8 T cell responses in vivo.
A) Experimental Design. Naïve Thy1.1/1.1 OT-I (1x103) were mixed with primary memory Thy1.1/1.2 OT-I (day 200+ after primary VacV-OVA infection) at the indicated ratios and injected into naïve B6 Thy1.2/1.2 recipients. Mice were challenged 24 hrs later with Att LM-OVA (5x106 CFU per recipient; i.v.). B) Kinetic analysis of OT-I in peripheral blood at the indicated days after infection or immunization. Data are presented as the mean percentage of primary or secondary OT-I cells in PBL +/− SEM for 4 or 5 mice per group. C) Kinetic analysis of primary and secondary OT-I CD8 T cell responses presented as the ratio of primary to secondary OT-I cells in peripheral blood (mean +/− SEM for 4 or 5 mice per group). The dashed line indicates the starting ratio of naïve and memory OT-I cells transferred before infection. The data are representative of at least three similar and independent experiments.
Plotting the ratio of the primary to secondary CD8 T cell responses at various time points after infection revealed a dynamic pattern of regulation (Figure 6C). As shown above, during the expansion phase primary OT-I cells were present in higher numbers than secondary OT-I cells. During the contraction phase, secondary OT-I cells were present in higher numbers than primary OT-I cells, consistent with the prolonged contraction of secondary CD8 T cell responses. However, due to the continual decline in secondary CD8 T cell numbers, primary memory CD8 T cells were more prevalent than secondary memory OT-I when analyzed late after infection (Figure 6C). Importantly, the changes in population dynamics of primary and secondary CD8 T cell responses were not influenced by the number of memory CD8 T cells present at the time of infection (Figure 6C).
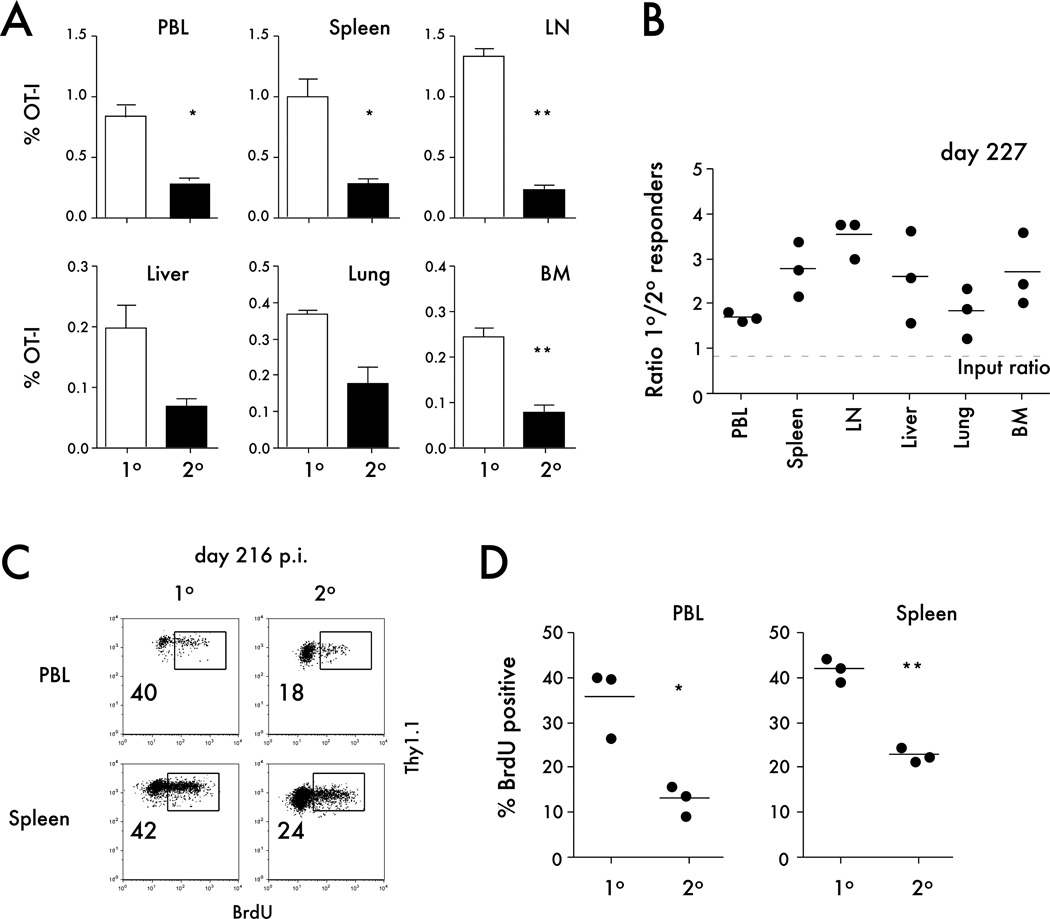
The changes in dynamics of primary and secondary CD8 T cell responses after infection could be a consequence of examining the representation of primary and secondary memory populations in PBL and may not be representative of other tissues in the host. To address this issue, the percentages of primary and secondary memory CD8 T cells were determined in secondary lymphoid organs and tertiary tissues at a memory time point (day 227 p.i.). The data clearly showed that in all organs examined a higher percentage of primary memory OT-I cells was detected compared to secondary memory OT-I cells (Figure 7A,B).
Figure 7. Tissue distribution and homeostatic proliferation of primary and secondary memory CD8 T cells analyzed in the same host.
A) The percentage of primary or secondary OT-I per indicated organ on day 227 after Att LM-OVA infection. Data are presented as mean + SEM for 3 mice per organ. B) The ratio of primary to secondary OT-I in the indicated organs at day 227 after Att LM-OVA infection. C) Representative dot plots of BrdU staining of primary or secondary OT-I cells in the spleen or peripheral blood (PBL) on day 216 after infection (the ratio of naïve to primary memory OT-I cells used for adoptive transfer was 1:10). Numbers inside the plots indicate the percentage of OT-I cells positive for BrdU. D) The percentage of BrdU positive primary or secondary OT-I cells in spleen or PBL at day 216 after Att LM-OVA infection. Dots represent individual mice, solid lines indicate the mean, and the dashed line indicates the starting ratio of naïve and memory OT-I cells transferred before infection. * indicates a p value between 0.01 and 0.05, and ** indicates a p value less than 0.01.
Memory CD8 T cell populations are maintained by a process of slow basal turnover, and it has been shown that primary memory CD8 T cells undergo higher rates of basal proliferation than secondary memory CD8 T cells (19, 39). We used BrdU incorporation to determine if differences in basal proliferation might explain the shift in primary and secondary memory CD8 T cell numbers. A significantly higher percentage of primary memory OT-I cells incorporated BrdU in the PBL and spleen when compared to secondary memory CD8 T cells (Figure 7C,D). Since basal proliferation of both primary and secondary memory CD8 T cell populations was measured in the same mice, the differences observed were not related to environmental factors, but rather represent inherent differences between these memory populations.
Taken together, these results reveal the differences in population dynamics of primary and secondary CD8 T cell responses and suggest that one naïve CD8 T cell does have a greater ‘memory generation potential’ than one primary memory CD8 T cell.
Higher proliferation potential but indistinguishable kinetics of secondary CD8 T cell responses generated from late versus early memory CD8 T cells
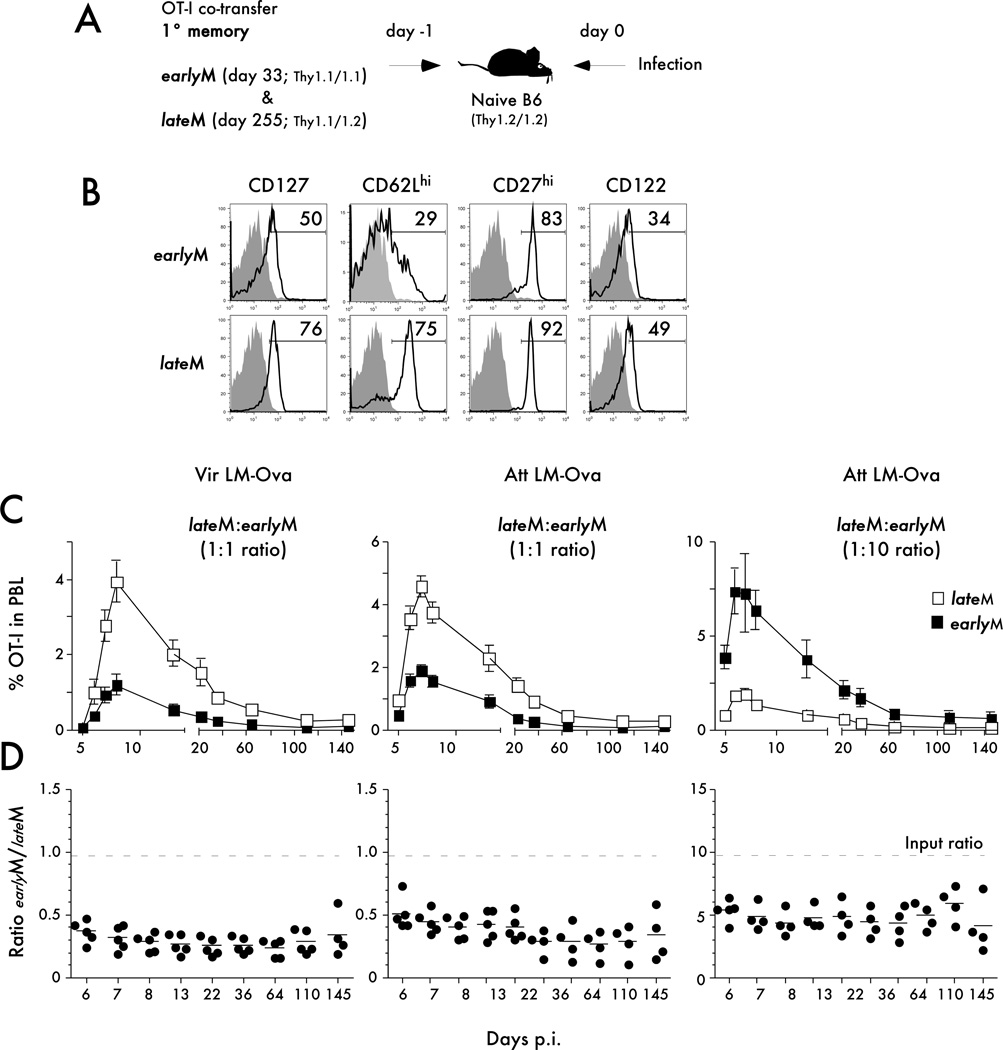
Memory CD8 T cells undergo phenotypic and functional changes throughout time after infection (1, 34, 40–42). Consequently, the quality of memory CD8 T cells used to examine the population dynamics of naïve and primary memory CD8 T cells could impact the interpretation of our results. For each of the experiments that we have discussed so far, we have used memory CD8 T cells that were at least two hundred days post primary infection. It has been shown that the majority of primary memory CD8 T cells at this time post infection have a late memory (lateM) phenotype (ex. CD62L expression) and function (ability to ‘vigorously’ expand to antigen challenge) (29, 33). To test the extent to which the observed changes in populations dynamics of primary and secondary CD8 T cell responses were influenced by the quality of primary memory CD8 T cells an additional set of adoptive co-transfer experiments was performed (Figure 8). Primary memory OT-I cell populations generated 33 days (earlyM) or 255 days after VacV-OVA infection (lateM) were co-transferred at different ratios into naïve B6 recipient mice before infection with Att LM-OVA or Vir LM-OVA infections (Figure 8A - experimental design). Phenotypic analysis of both memory OT-I populations revealed that day 33 cells express an earlyM phenotype in contrast to the lateM phenotype observed at day 255 (ex. high frequencies of cells expressing CD62L and CD27 markers) (Figure 8B).
Figure 8. Higher proliferation potential but indistinguishable kinetics of secondary CD8 T cell responses generated from late versus early primary memory CD8 T cells.
A) Experimental Design. Day 33 old primary memory (earlyM; Thy1.1/1.1 OT-I - 1x103 or 1x104) were mixed with day 250+ old primary memory (lateM Thy1.1/1.2 OT-I) at the indicated ratios and injected into naïve B6 Thy1.2/1.2 recipients. Mice were challenged 24 hrs later with Att LM-OVA or Vir LM-OVA (5x106 and 5x104 CFU per mouse, respectively). B) The expression of the indicated markers was evaluated for earlyM and lateM OT-I donors. Shaded histograms represent isotype control staining and open histograms represent staining of earlyM or lateM OT-I CD8 T cells. Numbers indicate the percentage of cells positive for the indicated molecules. C) Kinetic analysis of the earlyM or lateM OT-I response in PBL after the indicated infection. Data are presented as the mean percentage of earlyM or lateM OT-I cells in PBL +/− SEM for five mice per group. D) The ratio of earlyM or lateM OT-I in the PBL at the indicated days after infection. Dots represent individual mice, solid lines indicate the mean, and the dashed line indicates the starting ratio of earlyM and lateM OT-I cells transferred before infection. The data are representative of at least two similar and independent experiments.
The longitudinal kinetic analysis of secondary CD8 T cell responses generated either from earlyM or lateM populations revealed that the proliferation capacity of lateM CD8 T cells is higher than earlyM cells. Interestingly, despite the initial differences in the ability to expand in numbers, the ratio of earlyM and lateM remained relatively constant up to five months after infection, indicating that while lateM were able to undergo a higher magnitude of expansion than earlyM CD8 T cells (29, 33), the overall kinetics of the secondary CD8 T cell responses were nearly identical (Figure 8C,D). The same results were obtained in all groups of mice suggesting that intrinsic differences in proliferative expansion and overall kinetics of the secondary CD8 T cell responses are not influenced by the numbers of cells transferred or the virulence of the pathogen used for the infection (Figure 8C,D). Thus, the observed changes in population dynamics of primary and secondary CD8 T cell responses are not influenced by the quality of primary memory CD8 T cells used in co-transfer studies.
Discussion
CD8 T cells mediate protection to infection due to their ability to employ effector functions and undergo Ag-driven clonal expansion. CD8 T cells respond to infection by the production of cytolitic molecules and cytokines which results in destruction of infected cells and the recruitment of additional cells of the immune system to the site of infection. In regards to these processes, memory CD8 T cells are superior to naïve CD8 T cells as they are able to display their effector molecules faster (16, 30, 36). However, the precise mechanisms that allow memory CD8 T cells to kill and produce cytokines faster are not completely understood, and there is some evidence that memory CD8 T cells may exist in a ‘ready to respond’ state through steady state phosphorylation of proteins involved in signaling as well as chromatin remodeling around gene loci encoding for cytolitic molecules (43–48).
The capacity to undergo vigorous proliferation in response to infection is a critical component of protective primary and secondary CD8 T cell responses. It has been suggested that compared to naïve CD8 T cells, memory CD8 T cells may be able to encounter Ag earlier (due to differences in trafficking and tissue distribution), begin to cycle faster, and proliferate to a higher extent after Ag encounter (1–6). However, interpretation of these observations has in some instances been complicated by the experimental systems employed. In addition, recent studies have suggested that primary and repeatedly stimulated memory CD8 T cells differed substantially in their functional properties, including the ability to proliferate and re-differentiate into long-lived memory (19).
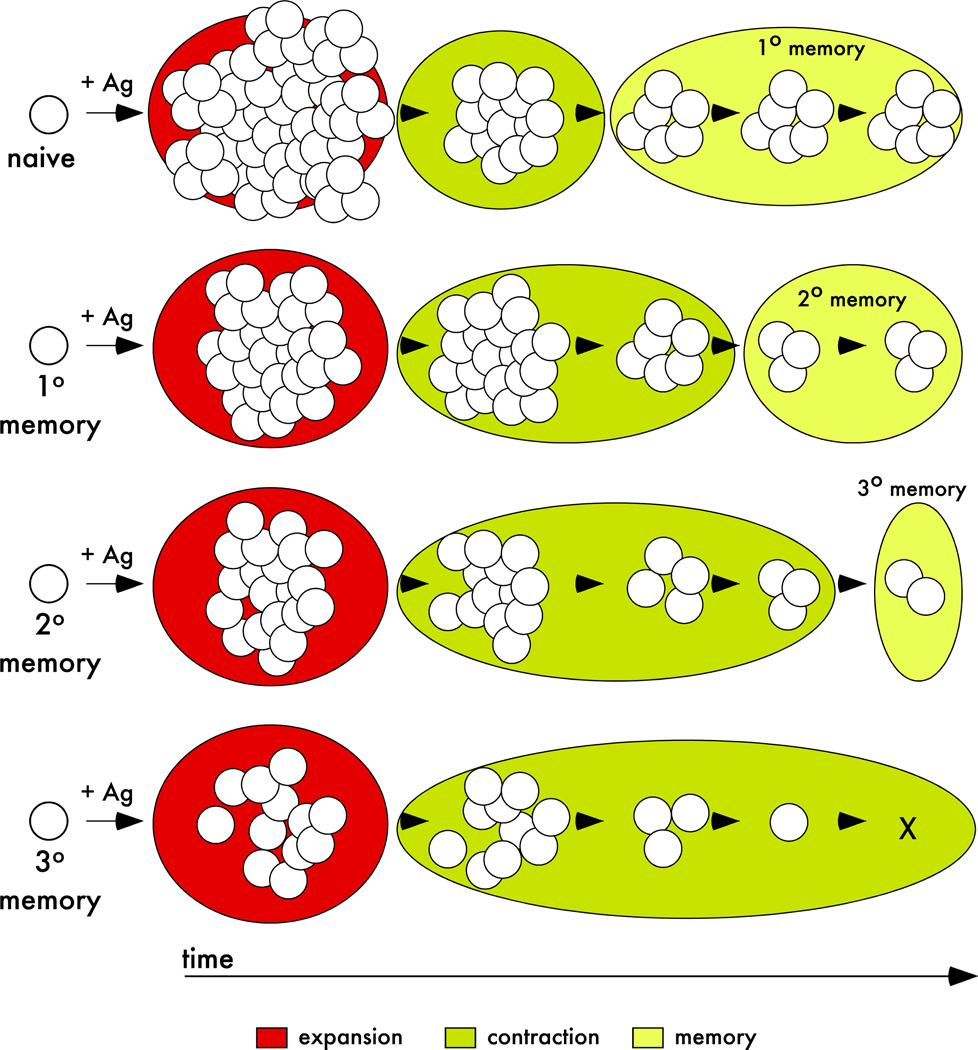
In this study, we show that naïve CD8 T cells have a greater per-cell expansion potential than primary memory CD8 T cells when analyzed in the same environment. The intrinsic ability of one naïve cell to give rise to more effector CD8 T cells than one memory CD8 T cells is independent of type and dose of infection as well as the number of primary memory cells present in vivo. In addition, the proliferation capacity of late memory (lateM; predominately CD62Lhi) is higher than capacity of early memory CD8 T cells (earlyM, predominately CD62Llow) after infection (Figure 8 and (29, 33)) confirming that differences in expansion of naïve and memory CD8 T cells are not influenced by the quality of memory CD8 T cells used in our co-transfer studies. Therefore, and in line with the notion that multiple antigen-encounters decrease the proliferation capacity of responding CD8 T cells (18, 19), even primary Ag-stimulation leads to a decreased ability of Ag-specific CD8 T cells to respond to new Ag-stimulation (Figure 9 - model).
Figure 9. Population dynamics of primary and multiple stimulated CD8 T cell responses – a model.
After antigen encounter, naïve and memory CD8 T cells (primary, secondary, and tertiary) undergo proliferative expansion. The magnitude of the expansion, duration of contraction, and ability to generate long-lived progeny (‘memory generation potential’) of naïve and/or memory CD8 T cells is dependent on the Ag-stimulation history.
However, the inflammatory milieu (signal 3 (31, 49, 50)) encountered during Ag-recognition influences the accumulation of primary and secondary CD8 T cells. The sensitivity to inflammatory cues might be greater for memory CD8 T cells since increasing inflammation during the initial priming decreases the differences in the magnitude of the expansion between primary and secondary CD8 T cell responses. Recently, we showed that inflammatory cytokines (signal 3) exert the greatest impact on proliferating CD8 T cells (26) suggesting the possibility that memory CD8 T cells, by their ability to enter cell-cycle earlier than naïve CD8 T cells, might be influenced by inflammation for a longer period of time or at the time when inflammation peaks in vivo (usually in the first few days post challenge). Although additional studies are needed to further investigate the differential susceptibility of naïve and memory CD8 T cells to inflammatory stimuli, the data presented here suggest that the choice of booster pathogen and modulation of the inflammatory milieu during Ag-restimulation might represent a viable approach to enhance the magnitude and composition of responding CD8 T cells.
By examining the population dynamics after Ag-stimulation we also showed that ‘memory generation potential’ is higher for responding naïve than primary memory CD8 T cells. Initially, contraction of the secondary CD8 T cell response was delayed leading to a greater representation of secondary than primary CD8 T cells. However, contraction over time was not reduced, but rather contraction of the secondary CD8 T cell response was protracted. As contraction of secondary CD8 T cell responses resolved, primary memory cells were actually present at greater numbers than secondary memory CD8 T cells in all organs analyzed. Again, the observed changes in population dynamics of primary and secondary CD8 T cell responses were not influenced by the quality of primary memory CD8 T cells. The decreased ability of primary memory CD8 T cells to generate long-lived progeny compared to their naïve counterparts might not be surprising since it has been shown that the ‘memory generation potential’ of multiply stimulated CD8 T cell populations decreases with every additional Ag-encounter (Figure 9 – model) (19).
In summary, the data presented here provide new insights into the functional properties of primary memory CD8 T cells that will help further delineate differences between naïve and primary memory CD8 T cells. It also establishes the functional relationship between naïve, primary and multiple stimulated memory CD8 T cells that is clearly dependent on the Ag-stimulation history, a finding that has to be taken into consideration in future vaccine development.
Acknowledgments
We thank Deepa Rai for expert technical assistance and all members of our laboratories for helpful discussions.
Footnotes
Supported by NIH grants (AI83286 – VPB; AI42767, AI46653, AI50073, AI59752 - JTH)
Disclosures
The authors have no financial conflict of interest.
References
- 1.Harty JT, Badovinac VP. Shaping and reshaping CD8+ T-cell memory. Nat Rev Immunol. 2008;8:107–119. doi: 10.1038/nri2251. [DOI] [PubMed] [Google Scholar]
- 2.Jameson SC, Masopust D. Diversity in T cell memory: an embarrassment of riches. Immunity. 2009;31:859–871. doi: 10.1016/j.immuni.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002;2:251–262. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- 4.Lefrancois L. Development, trafficking, function of memory T-cell subsets. Immunol Rev. 2006;211:93–103. doi: 10.1111/j.0105-2896.2006.00393.x. [DOI] [PubMed] [Google Scholar]
- 5.Sallusto F, Lanzavecchia A, Araki K, Ahmed R. From vaccines to memory and back. Immunity. 2010;33:451–463. doi: 10.1016/j.immuni.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams MA, Bevan MJ. Effector and memory CTL differentiation. Annu Rev Immunol. 2007;25:171–192. doi: 10.1146/annurev.immunol.25.022106.141548. [DOI] [PubMed] [Google Scholar]
- 7.Badovinac VP, Porter BB, Harty JT. Programmed contraction of CD8(+) T cells after infection. Nat Immunol. 2002;3:619–626. doi: 10.1038/ni804. [DOI] [PubMed] [Google Scholar]
- 8.Busch DH, Pilip IM, Vijh S, Pamer EG. Coordinate regulation of complex T cell populations responding to bacterial infection. Immunity. 1998;8:353–362. doi: 10.1016/s1074-7613(00)80540-3. [DOI] [PubMed] [Google Scholar]
- 9.Butz EA, Bevan MJ. Massive expansion of antigen-specific CD8+ T cells during an acute virus infection. Immunity. 1998;8:167–175. doi: 10.1016/s1074-7613(00)80469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murali-Krishna K, Altman JD, Suresh M, Sourdive DJ, Zajac AJ, Miller JD, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 11.Badovinac VP, Harty JT. Programming, demarcating, manipulating CD8+ T-cell memory. Immunol Rev. 2006;211:67–80. doi: 10.1111/j.0105-2896.2006.00384.x. [DOI] [PubMed] [Google Scholar]
- 12.Kaech SM, Wherry EJ. Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity. 2007;27:393–405. doi: 10.1016/j.immuni.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Badovinac VP, Messingham KA, Jabbari A, Haring JS, Harty JT. Accelerated CD8+ T-cell memory and prime-boost response after dendritic-cell vaccination. Nat Med. 2005;11:748–756. doi: 10.1038/nm1257. [DOI] [PubMed] [Google Scholar]
- 14.Pham NL, Pewe LL, Fleenor CJ, Langlois RA, Legge KL, Badovinac VP, Harty JT. Exploiting cross-priming to generate protective CD8 T-cell immunity rapidly. Proc Natl Acad Sci U S A. 2010;107:12198–12203. doi: 10.1073/pnas.1004661107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woodland DL. Jump-starting the immune system: prime-boosting comes of age. Trends Immunol. 2004;25:98–104. doi: 10.1016/j.it.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 16.DiSpirito JR, Shen H. Quick to remember, slow to forget: rapid recall responses of memory CD8+ T cells. Cell Res. 2010;20:13–23. doi: 10.1038/cr.2009.140. [DOI] [PubMed] [Google Scholar]
- 17.Badovinac VP, Haring JS, Harty JT. Initial T cell receptor transgenic cell precursor frequency dictates critical aspects of the CD8(+) T cell response to infection. Immunity. 2007;26:827–841. doi: 10.1016/j.immuni.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Masopust D, Ha SJ, Vezys V, Ahmed R. Stimulation history dictates memory CD8 T cell phenotype: implications for prime-boost vaccination. J Immunol. 2006;177:831–839. doi: 10.4049/jimmunol.177.2.831. [DOI] [PubMed] [Google Scholar]
- 19.Wirth TC, Xue HH, Rai D, Sabel JT, Bair T, Harty JT, Badovinac VP. Repetitive antigen stimulation induces stepwise transcriptome diversification but preserves a core signature of memory CD8(+) T cell differentiation. Immunity. 2010;33:128–140. doi: 10.1016/j.immuni.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Badovinac VP, Messingham KA, Hamilton SE, Harty JT. Regulation of CD8+ T cells undergoing primary and secondary responses to infection in the same host. J Immunol. 2003;170:4933–4942. doi: 10.4049/jimmunol.170.10.4933. [DOI] [PubMed] [Google Scholar]
- 21.Grayson JM, Harrington LE, Lanier JG, Wherry EJ, Ahmed R. Differential sensitivity of naive and memory CD8+ T cells to apoptosis in vivo. J Immunol. 2002;169:3760–3770. doi: 10.4049/jimmunol.169.7.3760. [DOI] [PubMed] [Google Scholar]
- 22.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 23.Pope C, Kim SK, Marzo A, Masopust D, Williams K, Jiang J, Shen H, Lefrancois L. Organ-specific regulation of the CD8 T cell response to Listeria monocytogenes infection. J Immunol. 2001;166:3402–3409. doi: 10.4049/jimmunol.166.5.3402. [DOI] [PubMed] [Google Scholar]
- 24.Wirth TC, Harty JT, Badovinac VP. Modulating numbers and phenotype of CD8+ T cells in secondary immune responses. Eur J Immunol. 2010;40:1916–1926. doi: 10.1002/eji.201040310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Restifo NP, Bacik I, Irvine KR, Yewdell JW, McCabe BJ, Anderson RW, Eisenlohr LC, Rosenberg SA, Bennink JR. Antigen processing in vivo and the elicitation of primary CTL responses. J Immunol. 1995;154:4414–4422. [PMC free article] [PubMed] [Google Scholar]
- 26.Pham NL, Badovinac VP, Harty JT. A default pathway of memory CD8 T cell differentiation after dendritic cell immunization is deflected by encounter with inflammatory cytokines during antigen-driven proliferation. J Immunol. 2009;183:2337–2348. doi: 10.4049/jimmunol.0901203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wirth TC, Martin MD, Starbeck-Miller G, Harty JT, Badovinac VP. Secondary CD8(+) T-cell responses are controlled by systemic inflammation. Eur J Immunol. 2011;41:1321–1333. doi: 10.1002/eji.201040730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Badovinac VP, Hamilton SE, Harty JT. Viral infection results in massive CD8+ T cell expansion and mortality in vaccinated perforin-deficient mice. Immunity. 2003;18:463–474. doi: 10.1016/s1074-7613(03)00079-7. [DOI] [PubMed] [Google Scholar]
- 29.Stock AT, Jones CM, Heath WR, Carbone FR. Cutting edge: central memory T cells do not show accelerated proliferation or tissue infiltration in response to localized herpes simplex virus-1 infection. J Immunol. 2006;177:1411–1415. doi: 10.4049/jimmunol.177.3.1411. [DOI] [PubMed] [Google Scholar]
- 30.Veiga-Fernandes H, Walter U, Bourgeois C, McLean A, Rocha B. Response of naive and memory CD8+ T cells to antigen stimulation in vivo. Nat Immunol. 2000;1:47–53. doi: 10.1038/76907. [DOI] [PubMed] [Google Scholar]
- 31.Haring JS, Badovinac VP, Harty JT. Inflaming the CD8+ T cell response. Immunity. 2006;25:19–29. doi: 10.1016/j.immuni.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 32.Obar JJ, Khanna KM, Lefrancois L. Endogenous naive CD8+ T cell precursor frequency regulates primary and memory responses to infection. Immunity. 2008;28:859–869. doi: 10.1016/j.immuni.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wherry EJ, Teichgraber V, Becker TC, Masopust D, Kaech SM, Antia R, von Andrian UH, Ahmed R. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 34.Williams MA, Holmes BJ, Sun JC, Bevan MJ. Developing and maintaining protective CD8+ memory T cells. Immunol Rev. 2006;211:146–153. doi: 10.1111/j.0105-2896.2006.00389.x. [DOI] [PubMed] [Google Scholar]
- 35.Obar JJ, Jellison ER, Sheridan BS, Blair DA, Pham QM, Zickovich JM, Lefrancois L. Pathogen-induced inflammatory environment controls effector and memory CD8 T cell differentiation. J Immunol. 2011 doi: 10.4049/jimmunol.1102335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haluszczak C, Akue AD, Hamilton SE, Johnson LD, Pujanauski L, Teodorovic L, Jameson SC, Kedl RM. The antigen-specific CD8+ T cell repertoire in unimmunized mice includes memory phenotype cells bearing markers of homeostatic expansion. J Exp Med. 2009;206:435–448. doi: 10.1084/jem.20081829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seedhom MO, Jellison ER, Daniels KA, Welsh RM. High Frequencies of Virus-Specific CD8+ T Cell Precursors. J Virol. 2009 doi: 10.1128/JVI.01722-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klonowski KD, Marzo AL, Williams KJ, Lee SJ, Pham QM, Lefrancois L. CD8 T cell recall responses are regulated by the tissue tropism of the memory cell and pathogen. J Immunol. 2006;177:6738–6746. doi: 10.4049/jimmunol.177.10.6738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jabbari A, Harty JT. Secondary memory CD8+ T cells are more protective but slower to acquire a central-memory phenotype. J Exp Med. 2006;203:919–932. doi: 10.1084/jem.20052237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 41.Surh CD, Boyman O, Purton JF, Sprent J. Homeostasis of memory T cells. Immunol Rev. 2006;211:154–163. doi: 10.1111/j.0105-2896.2006.00401.x. [DOI] [PubMed] [Google Scholar]
- 42.Wherry EJ, Ahmed R. Memory CD8 T-cell differentiation during viral infection. J Virol. 2004;78:5535–5545. doi: 10.1128/JVI.78.11.5535-5545.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kersh EN, Kaech SM, Onami TM, Moran M, Wherry EJ, Miceli MC, Ahmed R. TCR signal transduction in antigen-specific memory CD8 T cells. J Immunol. 2003;170:5455–5463. doi: 10.4049/jimmunol.170.11.5455. [DOI] [PubMed] [Google Scholar]
- 44.Slifka MK, Whitton JL. Functional avidity maturation of CD8(+) T cells without selection of higher affinity TCR. Nat Immunol. 2001;2:711–717. doi: 10.1038/90650. [DOI] [PubMed] [Google Scholar]
- 45.Zarozinski CC, Welsh RM. Minimal bystander activation of CD8 T cells during the virus-induced polyclonal T cell response. J Exp Med. 1997;185:1629–1639. doi: 10.1084/jem.185.9.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Araki Y, Wang Z, Zang C, Wood WH, 3rd, Schones D, Cui K, Roh TY, Lhotsky B, Wersto RP, Peng W, Becker KG, Zhao K, Weng NP. Genome-wide analysis of histone methylation reveals chromatin state-based regulation of gene transcription and function of memory CD8+ T cells. Immunity. 2009;30:912–925. doi: 10.1016/j.immuni.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kersh EN, Fitzpatrick DR, Murali-Krishna K, Shires J, Speck SH, Boss JM, Ahmed R. Rapid demethylation of the IFN-gamma gene occurs in memory but not naive CD8 T cells. J Immunol. 2006;176:4083–4093. doi: 10.4049/jimmunol.176.7.4083. [DOI] [PubMed] [Google Scholar]
- 48.Northrop JK, Thomas RM, Wells AD, Shen H. Epigenetic remodeling of the IL-2 and IFN-gamma loci in memory CD8 T cells is influenced by CD4 T cells. J Immunol. 2006;177:1062–1069. doi: 10.4049/jimmunol.177.2.1062. [DOI] [PubMed] [Google Scholar]
- 49.Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mescher MF, Curtsinger JM, Agarwal P, Casey KA, Gerner M, Hammerbeck CD, Popescu F, Xiao Z. Signals required for programming effector and memory development by CD8+ T cells. Immunol Rev. 2006;211:81–92. doi: 10.1111/j.0105-2896.2006.00382.x. [DOI] [PubMed] [Google Scholar]