Abstract
Members of the Trem receptor family (Triggering receptor expressed on myeloid cells) fine-tune inflammatory responses. We previously identified one of these receptors, called Trem-like 4 (Treml4), expressed mainly in the spleen, and at high levels by CD8α+ DCs and macrophages. Like other Trem family members, Treml4 has an immunoglobulin-like extracellular domain and a short cytoplasmic tail that associates with the adaptor DAP12. To follow our initial results that Treml4-Fc fusion proteins bind necrotic cells, we now generated a knock out mouse to assess the role of Treml4 in the uptake and presentation of dying cells in vivo. Loss of Treml4 expression did not impair uptake of dying cells by CD8α+ DCs or cross-presentation of cell-associated antigen to CD8+ T cells, suggesting overlapping function between Treml4 and other receptors in vivo. To further investigate Treml4 function, we took advantage of a newly generated mAb against Treml4, and engineered its heavy chain to express 3 different antigens, i.e., ovalbumin, HIV GAGp24 and the extracellular domain of the breast cancer protein HER2. Ovalbumin directed to Treml4 was efficiently presented to CD8+ and CD4+ T cells in vivo. Anti-Treml4-GAGp24 mAbs, given along with a maturation stimulus, induced Th1 antigen-specific responses which were not observed in Treml4 knock out mice. Also, HER2 targeting using anti-Treml4 mAbs elicited combined CD4+ and CD8+ T cell immunity, and both T cells participated in resistance to a transplantable tumor. Therefore, Treml4 participates in antigen presentation in vivo, and targeting antigens with anti-Treml4 antibodies enhances immunization of otherwise naïve mice.
Keywords: Dendritic cells, Monocytes/Macrophages, Antibodies, Antigen presentation
Introduction
One feature of Dendritic cells (DCs) and macrophages is their capacity to take up dying cells, including necrotic and apoptotic cells (1, 2). The uptake of dying cells and presentation of cell-associated antigens to CD4+ T helper cells and CD8+ T cells (cross-presentation) play crucial roles in the host's responses for induction of immunity or tolerance (3). Thus, the identification of receptors that mediate binding of dead cells represents a central challenge. We have previously identified one of these receptors, called Triggering receptor expressed on myeloid cells (Trem)-like 4 (Treml4) (4). Treml4, like many other members of the Trem family, is characterized by the presence of an immunoglobulin-like extracellular domain and a short cytoplasmic tail that associates with the adaptor molecule DAP12 (5).
Members of the Trem family are known to be involved in fine-tuning of inflammatory responses (6). Accordingly, Trem1 acts synergistically with Toll-like receptors (TLR) for triggering production of cytokines (7). Also, Trem2 is involved in the clearance of apoptotic neurons and production of cytokines by macrophages (8-10), and loss-of-function mutations in human TREM2 or DAP12 cause Nasu-Hakola disease, which is characterized by dementia and bone cysts (11, 12). The function of Treml4 in vivo remains to be elucidated. We have previously found that a chimeric fusion protein, consisting of the extracellular portion of Treml4 and a human IgG1 Fc domain, binds dead cells positive for Annexin V and Propidium iodide (4). To extend this finding, but in an intact animal, we now generated a Treml4 knock out (KO) mouse. Interestingly, Treml4 loss did not result in impaired uptake of dying cells or inability to cross-present cell-associated antigens to CD8+ T cells, suggesting overlapping functions between Treml4 and other receptors for dying cells.
Our original data reveal that Treml4, both at the mRNA and protein level, is mainly expressed in the spleen (4). We have extended these results and performed careful phenotyping of splenic leukocyte populations by flow cytometry using a newly developed antibody against Treml4 (4). Taking advantage of this mAb, we further found that anti-Treml4 (α-Treml4) mAb binds to appropriate DC, macrophage, and monocytes subsets in the spleen. Also, we considered whether Treml4 has the capacity to initiate antigen uptake, processing and presentation on MHC class I and II using a novel approach that involves delivery of antigens coupled to mAbs. This approach has been shown to increase the efficiency of antigen presentation on MHC class I and II molecules ∼100-fold, and allows T cell immunization (13-15). However, many of the receptors targeted to date belong to the C-type lectin family, which are probably involved in the physiological capture of pathogens and subsequent antigen presentation. Here we show for the first time, with three different protein antigens, that similar to C-type lectin receptors, an Ig superfamily member, Treml4, can bring about antigen presentation and priming of CD4+ and CD8+ T cells in vivo.
Materials and Methods
Mice
We purchased C57BL/6J (B6), Balb/cJ and FVB/NJ mice from The Jackson Laboratory. Balb/c × C57BL/6 (C×B6) F1 mice were from Harlan. TCR-transgenic OVA-specific mice, OT-I (C57BL/6-Tg(TcraTcrb)1100Mjb/J) and OT-II (C57BL/6-Tg(TcraTcrb)425Cbn/J), and TAP-/- (B6.129S2-Taptm1Arp/J) mice were obtained from The Jackson Laboratory and crossed when necessary to CD45.1 mice in house. The targeting vector for Treml4 KO mice was designed by replacing a 1.7-kb fragment including exon 1 and 2 with an ACN cassette. The ACN cassette contains the neomycin resistance gene under the control of RNA polymerase II promoter and Cre recombinase gene under the control of angiotensin-converting enzyme promoter, flanked by loxP sites (16). Cre-mediated recombination during spermatogenesis removed the cassette leaving one loxP (Fig. 1A). The targeting construct was transfected into B6 embryonic stem (ES) cells (CY2.4). Targeted ES cells were screened by Southern blotting and subsequently injected to B6 blastocysts. The resulting male chimeric mice were bred to female B6 or C57BL/6-Tyrc-2J mice to obtain germline transmission. All mice were maintained under specific pathogen-free conditions and used at 6-8 wks of age in accordance with The Rockefeller University Animal Care and Use Committee guidelines.
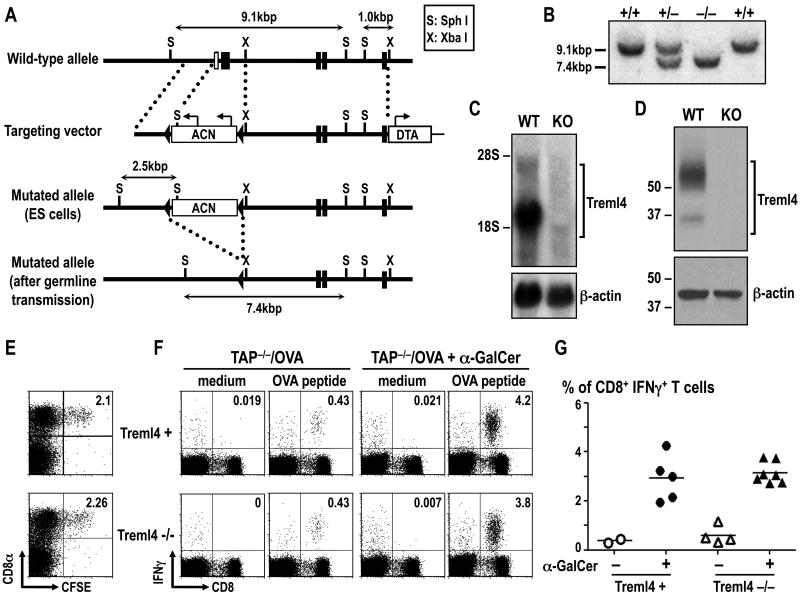
Figure 1. Generation of Treml4 KO mice.
(A) Schematic diagram of the mouse Treml4 wild-type (WT) allele, targeting vector, mutated allele in ES cells and mutated allele in Treml4-deficient mice. Filled boxes and open boxes denote coding exons and non-coding exons, respectively. (B) Southern blot analysis of offspring from the heterozygote intercrosses. Genomic DNA was extracted from mouse-tails, digested with SphI, electrophoresed, and hybridized with the radio-labeled probe indicated in A. Wild-type and mutated alleles of Treml4 gene were at the 9.1-kb and 7.4-kb bands, respectively. (C) Northern blot analysis of whole splenocytes. Total RNA (10 μg) was electrophoresed, transferred to a nylon membrane, and hybridized with Treml4 cDNA or β-actin cDNA fragment as a probe. (D) Representative data of the immunoblot signals for Treml4 in B6 WT and Treml4 KO mice. Spleens were lysed and 25 μg of total protein was separated by SDS-PAGE and Treml4 expression was assayed by Western Blot using α-Treml4 mAb followed by peroxidase-conjugated α-rat mAb. To control for protein loading, the membrane was incubated with stripping buffer and immunoblotted with α-actin-HRP mAb. One experiment representative of 2 with similar results. (E) Uptake of dying cells by DCs. 20×106 CFSE-labeled dying splenocytes were injected i.v. to WT littermates (Treml4+) or Treml4 KO (Treml4-/-) mice. 2 hrs after injection, spleens were harvested and uptake of CFSE+ cells was monitored in CD8α+ and CD8α- DC subsets. Plots shown CD11chigh gated cells and are representative of two independent experiments. (F) Cross-presentation of cell-associated antigen. Control littermates (Treml4+) or Treml4 KO (Treml4-/-) mice were immunized i.v. with 20×106 dying TAP-/- splenocytes loaded with OVA (TAP-/-/OVA), in the presence or absence of the adjuvant α-GalCer. Seven days later, splenocytes from immunized mice were re-stimulated with OVA peptide (SIINFEKL) for 12 hrs with BFA, and the presence of CD8+ T cells producing IFNγ was evaluated by flow cytometry after intracellular cytokine staining. Plots shown CD3ε+ gated T cells. (G) As in F, but the % of IFNγ+ CD8+ T cells of CD3ε+ splenocytes is shown as the mean of three independent experiments. Each circle/triangle represents an individual mouse and the horizontal bar denotes the mean.
Reagents
mAbs to Treml4 (16E5 and 32D11, (4)), OLLAS peptide (17), CD4 (GK 1.5), CD8 (2.43), CD40 (IC10), and control Ig (GL117) (18) were produced from hybridoma supernatants, purified on protein G (GE Healthcare Bio-Sciences, Piscataway, NJ), and when necessary, labeled with Alexa 647 (Invitrogen, Carlsbad, CA) or EZ-Link Biotin (Pierce, Rockford, IL) per manufacturer's instructions. The following fluorescent conjugated mAbs were purchased from eBioscience (San Diego, CA) or BD Pharmingen (San Diego, CA): FITC α-B220 (RA3-6B2) and α-Ly6G (1A8), PE α-CD115 (AFS98) and α-CD11b (M1/70), PerCP-Cy5.5 α-CD8α (53-6.7) and α-F4/80 (BM8), PE-Cy7 α-Ter119 (TER-119), α-TNFα (MP6-XT22) and α-CD19 (1D3), APC-eFluor®-780 α-CD11b (M1/70), Alexa-488 α-IL-2 (JES6-5H4), eFluor®-450 and Alexa-700 α-CD3ε (500A2), Alexa-700 and PerCP-Cy5.5 α-CD4 (RM4-5), Alexa-488 and APC-eFlour®-780 α-CD11c (N418), PE-Cy7 and PE α-CD49b (DX5), PE, APC and PE-Cy7 α-IFNγ (XMG1.2). PE α-PDCA-1 (JF05-1C2.4.1) was from Miltenyi Biotec. Other reagents were Live/Dead Fixable Aqua or Violet vitality dye from Invitrogen, DAPI (Sigma-Aldrich, St. Louis, MO), and CFSE (5,6-carboxy fluorescein diacetate succinimidyl ester; Invitrogen). Overlapping (staggered by four amino acids) 15-mer peptides covering the entire HIV-GAGp17, HIV-GAGp24, the extracellular domain of the breast cancer protein HER2 (human epidermal growth factor receptor 2) or neu, or the OVA peptide (aa257-264, SIINFEKL) were synthesized by H. Zebroski in the Proteomics Resource Center of The Rockefeller University.
Tumor cell line
The neu-expressing mammary tumor cell line NT2.5 was derived from a spontaneous mammary tumor in female neu-N mice (FVB/N background)(19). The cell line was established and kindly provided by Dr. E.M. Jaffee (Johns Hopkins University School of Medicine, Baltimore, Maryland). NT2.5 tumor cells were grown in a previously defined Breast Media, which consisted of RPMI (Gibco, Invitrogen) with 20% fetal bovine serum (Gibco, Invitrogen), 1% L-glutamine, 1% non-essential amino acids, 1% Na-pyruvate, 0.5% penicillin/streptomycin, 0.02% gentamicin (Gibco, Invitrogen), and 0.2% insulin (Sigma-Aldrich), and maintained at 37°C in 5% CO2.
Cloning, engineering and production of anti-receptor mAbs
The V regions of α-Treml4 mAbs (4) were cloned from RNAs of the 32D11 hybridoma with the 5′ RACE. In brief, we used 5′ phosphorylated rat IgG1-specific oligonucleotides (GTC ATT TAC CCG GAG) or rat κ-specific oligonucleotides (GTC TAA CAC TCA TTC) to synthesize 1st strand cDNAs by Superscript III first strand Synthesis System (Invitrogen). After circularization of the cDNAs with RNA ligase (Promega, Madison, WI), PCR was performed, and then the products were cloned and sequenced. Primers for IgG1 heavy chain GCT CAA TGG CAG GAC GTT CAG ATG and CTT GTC CAC CTT GGT GCT GCT GGC, for κ light chain GTA CAG CAT GAG CAG CAC CCT CTC and GTT CAT GAG GCA CAC GAC TGA GGC. DNA for ovalbumin (OVA), HIV GAGp24 (aa133-363 derived from HIV isolate BH10), and HER2 (aa22-653) was cloned in frame into the COOH terminus of the heavy chain of α-mouse-DEC205, -Treml4, and -Control Ig, as previously described (15, 20). An OLLAS epitope sequence was inserted between the H chain and OVA (20), whereas a small linker sequence was inserted between the H chain and HIV GAGp24 or HER2 sequences (Wang et al, submitted (15, 18)). Fusion mAbs were expressed by transient transfection (calcium phosphate) in 293T cells in serum-free DMEM supplemented with Nutridoma SP (Roche, Indianapolis, IN). The mAbs were purified on protein G columns (GE Healthcare Bio-Sciences), and characterized by SDS/PAGE and Western blotting using α-mouse IgG1-HRP (Southern Biotech, Birmingham, AL), HRP-α-GAGp24 (ImmunoDiagnostics, Woburn, MA), HRP-α-OVA (Research Diagnostics Inc.), Biotin-α-HER2 (clone 42, BD Transduction Laboratories, San Jose, CA), and Biotin-α-OLLAS (produced in house) followed by HRP-conjugated streptavidin (Jackson ImmunoResearch, West Grove, PA). Receptor binding was verified using Chinese Hamster Ovary (CHO) transfected cells by FACS analysis staining with Alexa-647-labeled α-OLLAS, PE-conjugated goat α-mouse IgG (Jackson ImmunoReseach), or Alexa-488-conjugated α-HER2 mAb (clone 24D2, BioLegend, San Diego, CA).
Cell preparation
For analysis of different leukocyte populations, spleens were cut into small pieces, incubated in a HANKS buffer (Gibco) solution containing 400 U/ml Collagenase D (Roche) and 50 μg/ml DNase I (Roche) for 25 min at 37°C. 5 mM of EDTA (Gibco) was added for the last 5 min of incubation, and the cell suspension was passed through a 70-μm cell strainer. In some experiments, cells were enriched by negative selection using α-CD19 magnetic beads and passed through LS columns (Miltenyi Biotec, Auburn, CA). For analysis of intracellular cytokine production by T cells, spleens were force-passed through a 70-μm cell strainer to obtain a homogeneous cell suspension without enzymatic digestion. Red blood cells were lysed by incubating with RBC Lysis Buffer (BioWhittaker, Walkersville, MD) for 1 min. In some experiments, splenocytes were labelled with CFSE to evaluate proliferation capacity of primed T cells (14). In brief, bulk splenocytes (2×107 cells/ml) were labeled with 2.5 μM CFSE (Invitrogen) at 37°C for 10 min. CFSE-labeled T cells were then restimulated with 0.1 μg/ml reactive or non-reactive peptide mix for 4 days, afterwards, they were further restimulated with 1 μg/ml reactive peptide mix for another 6 hrs in the presence of Brefeldin A (BFA; 10 μg/ml, Sigma-Aldrich) for evaluation of intracellular cytokines. For analysis of blood monocytes, mice were bled from the retro-orbital vein and leukocytes were enriched using lympholyte-mammal (Cedarlane Laboratories, Burlington, NC) following manufacturer's instructions.
Adoptive transfer and in vivo T cell proliferation responses
OVA-specific transgenic CD8+ or CD4+ T cells were purified from lymph nodes and spleen cell suspensions of OT-I and OT-II mice respectively by negative selection using α-F4/80, α-MHCII (TIB120), α-B220 (RA3-6B2), α-NK1.1 (PK136), and α-CD4 (GK1.5, for OT-I preparation) or α-CD8 (2.43, for OT-II preparation). T cell proliferation was evaluated by injecting individual animals with either 2-3 × 106 OT-I or 3-5 × 106 OT-II cells, labelled at 107/ml with 5 μM CFSE (Invitrogen) for 10 min at 37°C. 24 hrs later, 3 μg anti-receptor mAbs conjugated with OVA were injected s.c. footpad. CFSE dilution was evaluated 3 days later by FACS of spleen and pooled skin draining LN (popliteal, inguinal, axillary, brachial and cervical) after surface staining for CD4 (OT-II) or CD8 (OT-I) and Vα2 (OVA-specific TCR, clone B20.1). Fold clonal expansion of transgenic T cells was calculated as the ratio of the number of cells with more than one division to the number of un-divided cells in the control group, as previously described (20).
Uptake of dying cells and cross-presentation assay
To evaluate uptake of dying cells, splenocytes from B6 mice were labeled with 5 μM CFSE for 10 min at 37°C, and then induced to undergo apoptosis by osmotic shock (21). 2×107 CFSE-labeled dying cells were inoculated i.v. for 2 hrs, afterwards spleens were collected, digested with collagenase D, and analyzed by FACS for DCs containing CFSE. To measure presentation of cell associated antigens, mice were similarly injected with 2×107 OVA-pulsed and osmotically shocked TAP-/- splenocytes (TAP-/-/OVA), with or without α-GalCer (2S, 3S, 4R-1-O(α-galactopyranosyl)-2(N-hexacosanoylamino)-1,3,4-octadecanetriol) as adjuvant (22). Immune responses to OVA were measured 1 week later, by re-stimulating splenocytes for 6-12 hrs with OVA peptide (SIINFEKL) in the presence of BFA (23).
Mice inoculations
Mice were immunized i.p. once with fusion mAbs with a stimulus for DC maturation, which was 50 μg poly IC (InVivoGen, San Diego) together with 25 μg IC10 agonistic α-CD40 mAb (15). In other experiments, we used a prime/boost regimen in which α-CD40 was omitted and 50 μg poly IC (Thermo Scientific, Waltham, MA) or poly ICLC (Oncovir, Washington, DC) was the adjuvant for both prime and boost immunizations given 1 month apart. In tumor protection experiments, 10 days after the vaccine boost (day 38), mice were inoculated subcutaneously with 1 × 106 NT2.5 neu-expressing tumor cells in the shaved right flank. Tumor size was measured three times every week using a caliper. Tumor volumes were estimated according to the formula: length × (width)2 × 0.5. When animals seemed stressed, or had occurrence of tumor ulceration, or exceeded 12 mm in diameter of the tumors, the mice were sacrificed, and this was recorded as the date of death. For antibody depletion, 200 μg of α-CD4 (GK1.5) or α-CD8 (2.43) mAb or both were given to mice i.p. after boost immunization, at 9, 6, and 3 days prior to tumor challenge. Rat IgG (GL117) was given as the control mAb. Efficiency of depletion, defined as a reduction of >90% of the targeting cell subsets, was confirmed by FACS analysis of peripheral blood cells. For in vivo targeting experiments, mice were injected i.p. for 1 hr with 10 μg of α-Treml4 mAb labeled with Alexa 647.
Intracellular Cytokine Staining
Bulk splenocytes were re-stimulated with 15-mer peptide mix from HIV GAGp24 (1 μg/ml), non-reactive HIV GAGp17 (1 μg/ml), the extracellular domain of HER2, or neu peptides (1 μg/ml) in the presence of 2 μg/ml of costimulatory α-CD28 (clone 37.51, ATCC) for 6 h at 37°C, adding 10 μg/ml BFA for the last 5 hrs to allow accumulation of intracellular cytokines. Cells were washed, incubated 10 min at 4°C with 2.4G2 mAb (ATCC) to block Fcγ receptors, and stained with mAbs against surface molecules for 20 min at 4°C. Cells were then fixed, permeabilized (Cytofix/Cytoperm; BD Biosciences, USA) and stained with mAbs against cytokines. 1-3×105 live-CD3+ cells were acquired on a BD LSR II flow cytometer, and data were analyzed with FlowJo Software (Tree Star, Inc., San Carlos, CA).
Immunofluorescence Staining
B6 or Treml4 KO mice were inoculated with 25 μg of purified α-Treml4 mAb i.p. 3 hrs later, spleens were harvested and frozen (-80°C) in optimal cutting temperature compound (Tissue-Tek OCT; Sayura). 10-12 μm spleen sections were fixed 15 min in cold acetone, rehydrated in PBS, and blocked with 5% mouse serum in FACS buffer (PBS/2% FBS) for 1 hr at room temperature (RT). Sections were stained in a humidified chamber overnight at 4°C with anti-rat Alexa-555 (Invitrogen), washed in FACS buffer, incubated for 1 hr at RT with 5% rat serum to block free arms of primary mAb, and stained with secondary mAb. Marginal metallophilic macrophages were detected with FITC-labeled α-CD169 (AbD Serotec) followed by anti-FITC Alexa-488 (Invitrogen). Marginal zone macrophages were stained with purified α-SIGNR1 (in-house) followed by α-hamster FITC (Jackson Immunoresearch) and α-FITC Alexa-488. In all cases, B cells were detected by staining sections with Alexa-647-labeled α-B220 (eBioscience). Sections were mounted in Aqua-Poly Mount (Polysciences Inc.), examined on a Zeiss LSM 510 system (Carl Zeiss Inc.) at The Rockefeller University Bio-Imaging Resource Center, and analyzed with ImageJ (National Institutes of Health).
Statistical Analysis
Data were analyzed and charts were generated using Prism 5 GraphPad software (San Diego, CA). A Student's t test (between two groups or conditions) was applied to compare statistical significance between peptide-specific responses. A two-way ANOVA test was applied to compare differences between treated group and control groups. Results were considered statistically significant when p≤0.05.
Results
Treml4-deficient mice do not exhibit changes in the uptake and cross-presentation of dying cells
To generate Treml4 KO mice for these studies, we used gene targeting. The targeting vector used to generate Treml4 KO mice was constructed to replace exon 1 and 2 with a self-excising ACN cassette (Fig. 1A). Correctly targeted ES cells were injected into B6 blastocysts, followed by germline transmission of the mutated allele (Fig. 1B, C, D). Treml4 KO mice were born at the expected Mendelian frequency, were fertile, and appeared to be healthy over at least 6 months of observation. Treml4 is primarily expressed in the spleen (4), but Treml4 KO mice had normal numbers of splenic leukocytes including T cells, B cells, NK cells, granulocytes, monocytes, DCs and red pulp macrophages (not shown).
To investigate the in vivo function of Treml4, we evaluated whether Treml4 is involved in uptake of dying cells and cross-presentation of cell-associated antigens. B6 splenocytes were labeled with CFSE, induced to undergo apoptosis by osmotic shock (21), and then injected i.v. into WT littermates (Treml4+) or Treml4 KO (Treml4-/-) mice. Two hours later, the uptake of CFSE positive cells by CD11chigh DCs was monitored by FACS. We could detect uptake of CFSE+ dying cells in CD8α+ classical DCs in both WT littermates (Treml4+) and Treml4 KO (Treml4-/-) mice, and the frequency was not different between both strains of mice, suggesting that Treml4 is not essential for uptake of dying cells by DCs in vivo (Fig. 1E). To monitor antigen cross-presentation in vivo, WT littermates or Treml4 KO mice were injected i.v. with dying TAP-/- splenocytes loaded with OVA (TAP-/-/OVA) in the presence or absence of an adjuvant, α-GalCer. After 7 days, antigen presentation and priming of OVA-specific CD8+ T cells was monitored after in vitro re-stimulation with OVA peptide in the presence of BFA and staining of intracellular IFNγ. As shown in Fig. 1F and G, antigen specific IFNγ-producing CD8+ T cells were generated to comparable extents in Treml4 KO (Treml4-/-) mice as control littermates (Treml4+), indicating that cross-presentation was independent of Treml4 in this system. Thus, Treml4 is not essential for uptake and cross-presentation of dying cells in vivo.
Treml4 is mainly expressed on CD8α+ DCs, tissue macrophages and monocytes
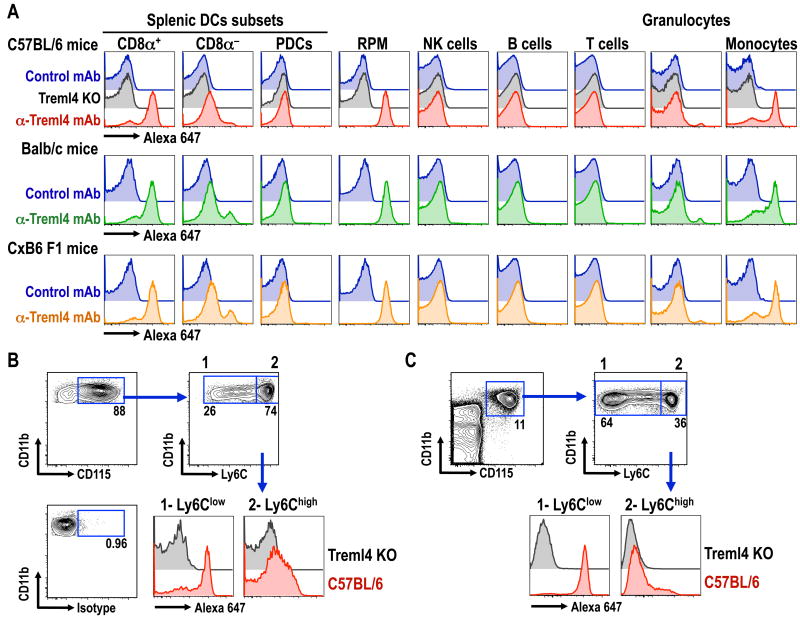
To examine the pattern of expression of Treml4 in different mouse tissues, we used a previously described multicolor flow cytometry strategy that facilitates side-by-side evaluation of distinct splenic leukocytes populations (15, 24). Consistent with our previous results (4), Treml4 was abundantly expressed on splenic CD8α+ DCs and splenic red pulp macrophages of all mouse strains analyzed (C57BL/6, Balb/c and C×B6 F1 mice), but not on B cells, T cells, NK cells or granulocytes (Fig. 2A). We found that splenic CD8α- DCs expressed low levels of Treml4, but plasmacytoid DCs (PDCs) were negative for this receptor. As previously reported, Treml4 was also found to be a good marker for Ly6Clow monocytes both in the spleen and blood (Fig. 2B & C, respectively (25)). Importantly, no staining with α-Treml4 mAb was observed on any leukocytes from Treml4 KO mice. Therefore mouse Treml4 is highly expressed on CD8α+ DCs, spleen macrophages, and Ly6Clow monocytes.
Figure 2. mAb against Treml4 labels CD8α+ DCs, red pulp macrophages and Ly6Clow monocytes.
(A) C57BL/6 (top panel), Balb/c (middle panel) or C×B6 F1 (bottom panel) splenocytes were analyzed by multicolor flow cytometry for the expression of Treml4 in different subsets of leukocytes (24). For control we used Rat IgG1 isotype control (blue histograms) or Treml4 KO mice in B6 background (gray histogram, top panel). PDCs, plasmacytoid DCs; RPM, red pulp macrophages. (B) CD115+ splenic monocytes from B6 or Treml4 KO mice were further gated on the expression of Ly6C. Ly6Clow and Ly6Chigh monocytes were analyzed for the expression of Treml4. (C) As in B, but expression of Treml4 was evaluated in Ly6Chigh and Ly6Clow blood monocytes. In all cases, one experiment of 2 with similar results is shown.
Injected αTreml4 mAb rapidly labels CD8α+ DCs, tissue macrophage and monocytes in mice
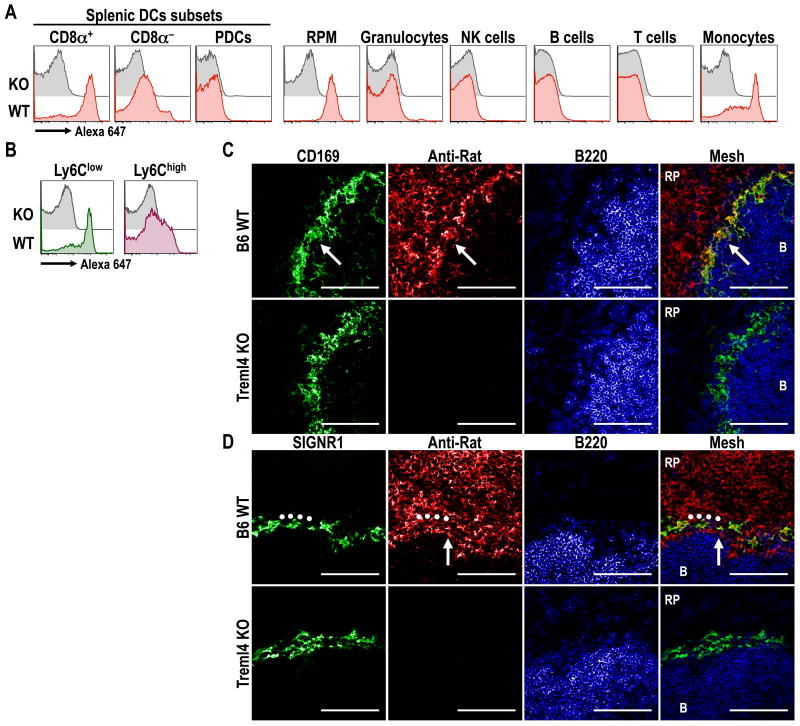
To assess if specific targeting of α-Treml4 mAb took place in vivo, B6 or Treml4 KO mice were inoculated i.p. with 10 μg of Alexa-647-labeled mAb. The uptake of labeled mAb was evaluated by multicolor flow cytometry as above, 1 h post inoculation. The injected α-Treml4 mAb labeled most CD8α+ DCs and red pulp macrophages, but only slightly labeled the CD8α- DCs (Fig. 3A). This labeling was specific since none was observed in Treml4 KO mice (Fig. 3A). In addition, Treml4 mAb was taken up by splenic monocytes, especially Ly6Clow ones (Fig 3B). Immunofluorescence staining of tissue sections showed high uptake of α-Treml4 mAb by CD169+ marginal metallophilic macrophages (Fig. 3C), but only weak capture by SIGNR1+ marginal zone macrophages (Fig. 3D), which corresponds to the described expression of Treml4 by these macrophage populations (4). All together, these results indicate that i.p. inoculation of α-Treml4 mAb faithfully targets in vivo to cells that express the receptor.
Figure 3. α-Treml4 mAb is mainly captured by CD8α+ DCs, splenic macrophages and Ly6Clow monocytes in vivo.
(A) B6 or Treml4 KO mice were inoculated i.p. with 10 μg of Alexa-647-labeled α-Treml4 mAb. Uptake of labeled mAb by different splenic dendritic cells subsets and leukocytes populations was evaluated 1 hr after inoculation by multicolor flow cytometry (15). PDCs, Plasmacytoid DCs; RPM, Red Pulp Macrophages. (B) As in A, but two populations of CD11bhigh CD115+ splenic monocytes, Ly6Clow (left panel) and Ly6Chigh (right panel), were further analyzed for the uptake of Alexa 647-labeled α-Treml4 mAb. (C-D) B6 or Treml4 KO mice were inoculated i.p. with 25 μg of purified α-Treml4 mAb. 3 hrs post inoculation, dissected spleens were cryopreserved, sectioned and stained with α-rat Alexa-555 (red) to detect the inoculated mAb. Marginal metallophilic macrophages (white arrows) were identified by staining with α-CD169 (C, green), and marginal zone macrophages (o) were identified by staining with α-SIGNR1 (D, green). To identify B cell follicles (denoted by “B”), sections were stained with B220 (blue). RP, red pulp. Scale bar: 100 μm. In all cases, one representative experiment of 2-3 with similar results is shown.
α-Treml4 mAb delivers OVA antigen for efficient presentation in vivo
To examine if Treml4 could be used to enhance antigen presentation in vivo, we genetically engineered the H chain of the mAb against Treml4 (32D11, (4)) to express OVA. As previously described (20), we added a tag sequence called OLLAS between the heavy chain and the antigen to facilitate tracking of the mAb. For comparison, we used a previously described α-DEC205-OVA mAb, and also a Control-Ig OVA fusion mAb that has no receptor affinity (GL117; (20)). The engineered heavy chains had a molecular mass of ∼110 kDa (Suppl. Fig. 1A), and reacted with α-mouse IgG1, α-OVA, and α-OLLAS by Western blotting (Suppl. Fig. 1B, (20)). By FACS, α-Treml4-OVA bound appropriately to CHO transfectants expressing Treml4, but not to untransfected CHO/NEO cells (Suppl. Fig. 1C).
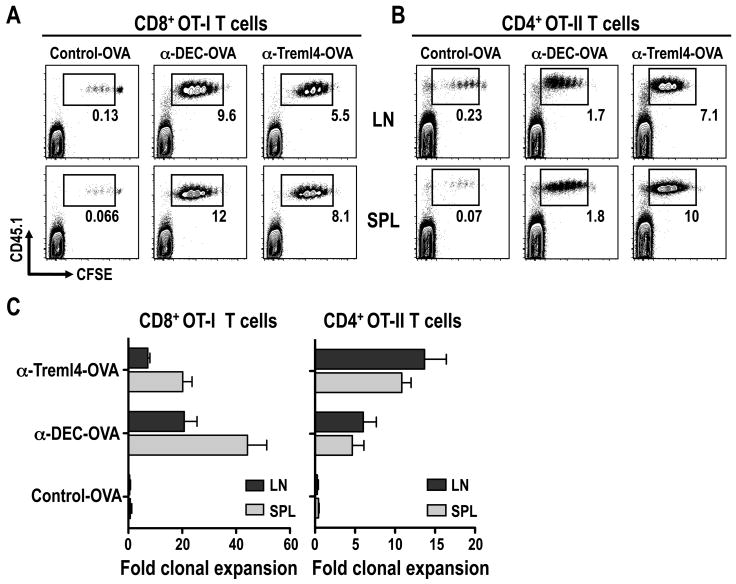
To evaluate the capacity of Treml4 to mediate presentation of OVA through the MHC I and MHC II pathway, we used CD8+ OT-I and CD4+ OT-II transgenic T cells as reporters. B6 mice were injected with 3 μg of α-Treml4-OVA s.c. footpad one day after inoculating CFSE-labeled OT-I CD8+ T cells or OT-II CD4+ T cells. After 3 days, spleen (SPL) and skin draining LN (LN) were evaluated for T cell proliferation by CFSE dilution. As shown in Fig. 4, Treml4 mediated efficient antigen presentation to CD8+ T cells (Fig. 4A) and CD4+ T cells (Fig. 4B). Virtually all T cells entered cell cycle and underwent 4-6 cell divisions after a single dose of α-Treml4-OVA mAb, while control-OVA elicited no cell division. As quantified in Fig. 4C, α-Treml4-OVA antigen delivery was less efficient than α-DEC-OVA for cross-presentation to CD8+ T cells (Fig. 4C, left panel), but more efficient for antigen presentation to CD4+ T cells (Fig. 4C, right panel). Taken together, Treml4 efficiently brings about presentation of OVA antigen on MHC I and MHC II products.
Figure 4. α-Treml4-OVA induces in vivo proliferation of CD8+ and CD4+ transgenic T cells.
B6 mice were injected i.v. with 2-5 × 106 CFSE-labeled OT-I (A) or OT-II (B) T cells, and 24 hrs later with 3 μg Control-, α-DEC- and α-Treml4 -OVA fusion mAbs s.c. footpad. Three days after mAb inoculation, skin draining LN (LN) and spleen (SPL) were harvested and expansion of CD8+ or CD4+ Vα2+ T cells was evaluated by FACS for CFSE dilution. Shown is the percentage of transferred CD45.1+ T cells undergoing more than one division. Plots are gated in CD4+ CD3ε+ T cells, and are representative of three experiments. (C) As in A-B, but bar graph quantified T cells undergoing more than one division (fold clonal expansion; see materials and methods) as the mean +/- SD of 4-6 animals in 3-4 different experiments.
Treml4 HIV GAGp24 fusion mAb primes Th1 CD4+ T cells
To examine the capacity of α-Treml4 mAb to immunize scarce microbial-specific T cells in the polyclonal repertoire, we introduced HIV GAGp24 (p24) by engineering the heavy chain. As controls we again used α-DEC-p24, and a Control-p24 mAb without receptor affinity as previously described (15). As shown in Suppl. Fig. 1, α-Treml4-p24 had a molecular mass of ∼75 kDa (Suppl. Fig. 1D), reacted with α-mouse IgG1 and α-p24 by Western Blotting (Suppl. Fig. 1E), and bound to CHO cells expressing the Treml4 receptor, but not untransfected CHO/NEO cells (Suppl. Fig. 1F). Thus α-Treml4 mAb, like several other mAbs, can be successfully engineered to express the HIV GAGp24 antigen.
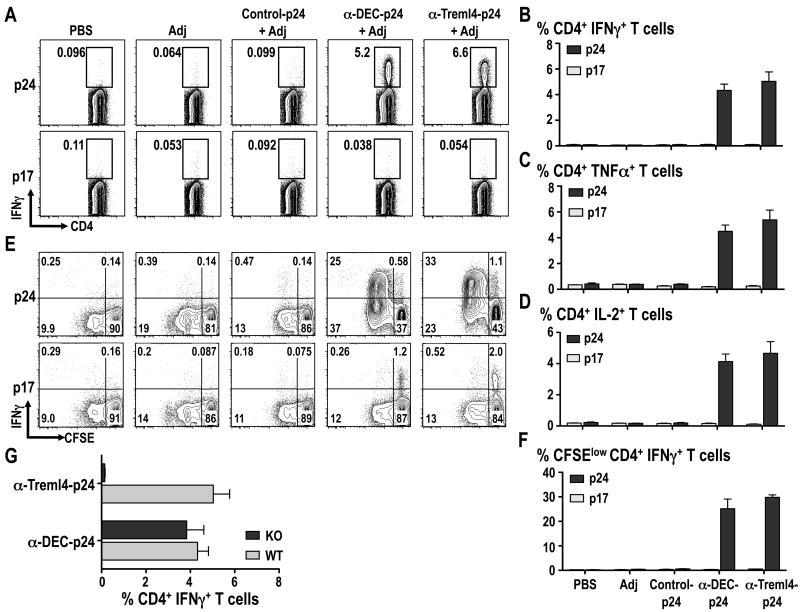
To evaluate if Treml4 could mediate priming of CD4+ T cells in vivo, we immunized B6 mice with 5 μg of α-Treml4-, α-DEC-, or Control Ig mAbs coupled with HIV GAGp24 along with 25 μg α-CD40 and 50 μg poly IC as a maturation stimulus or adjuvant (Adj). Two weeks after immunization, antigen-specific responses were evaluated by production of IFNγ, TNFα and IL-2 in response to HIV GAGp24 peptides by multicolor flow cytometry. As shown in Fig. 5A-D, α-Treml4-p24 induced significantly stronger gag-specific T cell responses compared with Control Ig-p24 with frequencies >5-6% of IFNγ-, TNFα- and IL-2- producing CD3+ CD4+ T cells after a single i.p. injection. Importantly, this antigen-specific response induced by delivery of p24 using α-Treml4 mAb was entirely Treml4 receptor dependent as shown by the lack of CD4+ T cell priming when Treml4 KO mice were used (Fig. 5G).
Figure 5. Targeting antigen through Treml4 induces HIV GAGp24 Th1 responses in vivo.
B6 mice were immunized with 5 μg of α-DEC205 or α-Treml4 mAbs conjugated with HIV GAGp24 (p24) in the presence of 50 μg poly IC and 25 μg α-CD40 (Adj). A mAb conjugated with HIV GAGp24 without receptor affinity was used as a control (Control-p24). 14 days after inoculation, splenocytes were re-stimulated in vitro with HIV GAGp17 (p17, negative control) or HIV GAGp24 peptide mix (p24) in the presence of BFA for 6 hrs. (A) Intracellular staining was performed to detect percentage of IFNγ+ in CD3+ CD4+ gated T cells. (B) Percentage of IFNγ+ CD4+T cells is shown as mean +/-SD of 4-6 experiments with 2-3 mice per group. As in B, but TNFα (C) and IL-2 (D) cytokine production were evaluated by intracellular cytokine staining in the CD3+ CD4+ gated T cells in response to HIV GAGp17 or HIV GAGp24 peptide mix. Mean +/- SD from 2 experiments with 8 mice total is shown. (E) Bulk splenocytes were CFSE-labeled and stimulated with HIV GAGp24 peptide mix (p24) or control HIV GAGp17 peptide mix (p17) for 4 days, whereupon the cells were restimulated for 6 h with HIV GAGp24 peptides in the presence of BFA to detect IFNγ in proliferated CFSElow T cells. Panels show the percentage of IFNγ+ or IFNγ- CD3+ CD4+ proliferating T cells (top and bottom, respectively) from one of two similar experiments with 4 mice per group. (F) As in E, but the % of CFSElow IFNγ+ CD4+ T cells is shown as mean +/- SD of 8 animals in two different experiments. (G) B6 or Treml4 KO mice were immunized with 5 μg of α-DEC205 or α-Treml4 mAbs conjugated with HIV GAGp24 (p24) in the presence of 50 μg poly IC and 25 μg α-CD40 (Adj) and analyzed 14 days later after re-stimulation in vitro with HIV GAGp17 (p17, negative control) or HIV GAGp24 peptide mix (p24) in the presence of BFA for 6 hrs. % of IFNγ+ CD4+ T cells is shown as mean +/- SD of 2 experiments with 4 mice total per group.
To evaluate if the immune CD4+ T cells could proliferate in response to the reactive GAG peptide pool, we labeled splenocytes with CFSE to follow the successive halving of CFSE/cell with each division. CFSE labeled splenocytes from mice primed for 2 weeks with α-Treml4-p24 in the presence of 25 μg α-CD40 + 50 μg poly IC were cultured for 4 days in the presence of p24 reactive peptide or p17 non-reactive peptide, followed by a challenge with p24 peptides. As illustrated in Fig. 5E, and quantified in Fig. 5F, the T cells responded by proliferation and IFNγ production, only to the p24 peptide mix, and not to the control p17 peptide mix. We concluded that targeting vaccine proteins with α-Treml4 mAb is effective at initiating Th1 CD4+ T cell responses.
Similar results also were obtained when we used a prime/boost regimen for immunization in the absence of α-CD40 in B6 mice (Suppl. Fig. 2).
Treml4 coupled with the breast cancer antigen HER2 elicits T Cell Immunity and Tumor Protection
To examine whether Treml4 could mount an immune response against the clinically relevant tumor antigen HER2, we genetically engineered α-Treml4 to express the extracellular domain (ECD) of the breast cancer protein HER2 (Wang et al., submitted). SDS-PAGE and Western blotting expectedly showed that the fusion heavy chain was 120 kDa and that the fusion mAb reacted to both α-mouse IgG and α-HER2 (not shown). Also, similarly to α-Treml4 mAbs conjugated with OVA and HIV GAGp24, the fusion mAb bound only to CHO cells expressing Treml4, but not CHO NEO cells (not shown).
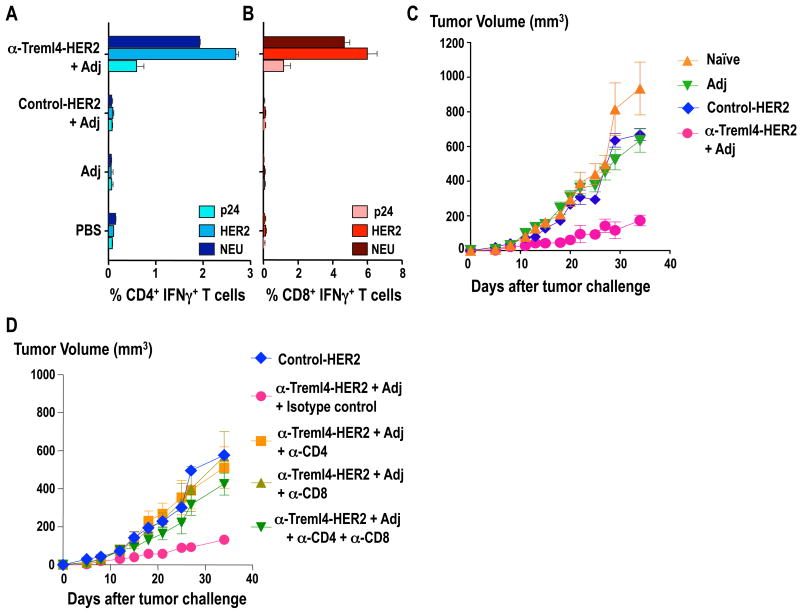
To determine the immunogenicity of the α-Treml4-HER2 mAb in vivo, FVB/N mice were immunized in a prime-boost regimen consisting of two doses administered 4 weeks apart of 5 μg of α-Treml4-HER2 mAb in the presence of 50 μg poly IC. Control groups were injected with Control-HER2 plus poly IC, poly IC alone, or PBS alone. Two weeks after boosting, total splenocytes were labeled with CFSE and re-stimulated with a non-reactive peptide pool HIV GAGp24, the reactive peptide pool HER2, and a cross-reactive peptide pool neu. 4 days later, splenocytes were re-stimulated for 6 hrs as before in the presence of BFA, and analyzed for intracellular cytokines by flow cytometry. Both CD4+ and CD8+ T cells significantly proliferated and produced IFNγ after two doses of α-Treml4-HER2, but not with Control-HER2 mAb immunization (Fig. 6A-B). Thus, α-Treml4-HER2 mAb mediates Th1 CD4+ T cell responses, as well as efficient cross-presentation and immunization of CD8+ T cells.
Figure 6. Targeting breast cancer antigen HER2 to Treml4 elicits HER2-specific T-cell immunity and tumor protection.
FVB/N mice were primed and boosted 4 weeks apart with 5 μg α-Treml4-HER2 fusion mAb in the presence of 50 μg poly IC. As control we used a mAb without receptor specificity (Control-HER2 mAb), poly IC alone, or PBS. 14 days after boost, total splenocytes were labeled with CFSE and restimulated in vitro with a non-reactive HIV GAGp24 (p24) peptide pool, a reactive HER2 peptide pool, or a cross-reactive NEU peptide pool. After 4 days, the cells were further stimulated for 6 hrs as before in the presence of BFA, and cytokines were detected by intracellular staining. Data are the % of CFSElow IFNγ+ CD4+ (A) or CD8+ (B) shown as 2 experiments with 3 mice per group. (C) 14 days after boost mice were injected s.c. into mammary fat pads with 1×106 NT2.5 transplantable breast cancer cells. Data show the tumor diameter over time and the mean of 5-10 mice per group (naïve & Adj = 5 mice; Control- & α-Treml4- HER2 = 10 mice) in two representative experiments. Significant differences were found between α-Treml4 and the control groups (P< 0.01). (D) As in C, but 9, 6, and 3 days prior to tumor challenge, group of mice were inoculated i.p. with α-CD4 depleting antibody (GK1.5), α-CD8 depleting antibody (2.43) or isotype control antibody (GL117). Mice were challenged with 1×106 NT2.5 tumor cells at day 14. Data is shown as the mean of 5 animals per group in one representative experiment.
We extended the experiments to determine whether α-Treml4-HER2 could be used to induce an anti-tumor response in vivo. We tested tumor protection in a neu-expressing mouse transplantable tumor model. In a different study, we have shown that targeting HER2 to DEC205 receptor was able to mediate efficient tumor protection by a T cell-dependent mechanism (Wang et al., submitted). To this end, we primed and boosted FVB/N mice with α-Treml4-HER2 along with poly IC as an adjuvant, as above. Ten days after the boost vaccination, mice were injected with 1×106 neu-expressing NT2.5 tumor cells. Mice were monitored for tumor growth every other day for 35 days. As shown in Fig. 6C, mice that received α-Treml4-HER2 mAb showed significantly smaller tumor volumes compared to those injected with Control-HER2, poly IC alone, or PBS (p<0.01) (Fig. 6C). This protection was mediated by both CD4+ and CD8+ T cells, since depletion of either (or both) T cells strongly abolished tumor protection (Fig. 6D). Thus, directing HER2 to Treml4 expressing cells results in significant tumor protection in vivo.
Discussion
We have utilized antigen targeting with α-mouse Treml4 mAbs to determine the capacity of this receptor for dying cells to mediate antigen presentation and immunization of naïve mice. It was feasible to engineer three proteins, OVA, HIV GAGp24, and the extracellular domain of the oncogene HER2, into the heavy chain of cloned α-Treml4 mAbs. With OVA, we studied clonal expansion of CD4+ and CD8+ TCR transgenic T cells 3 days after a dose of 3 μg fusion mAbs inoculated s.c. footpad. When we compared antigen presentation after targeting OVA using α-Treml4 or α-DEC mAbs, we found that α-Treml4-OVA was more efficient at inducing CD4+ T cell expansion while α-DEC-OVA was more efficient for CD8+ T cells. Accordingly, α-Treml4-OVA was comparable in bringing about antigen presentation to another mAb we have studied that reacts with CD8α+ DCs, i.e., α-Langerin (not shown, (20)). With HIV GAGp24 and HER2, we studied immunization of naïve mice. In order for this to take place, it was necessary to co-administer with the fusion mAb a stimulus for innate immunity; in our experiments, this was poly IC administered alone or in combination with α-CD40. As in prior work on antigen targeting to CD8α+ DCs via mAbs against DEC205, Langerin and Clec9A (15), targeting to the Ig superfamily member Treml4 greatly enhanced immunity relative to a non-reactive Ig control. The targeting was receptor specific, since immunization was ablated in Treml4 KO mice.
Treml4 is an unusual receptor for dying cells, since it is primarily expressed in spleen and not other lymphoid or non-lymphoid tissues (4). The high expression in spleen was previously attributed to high expression in red pulp macrophages and CD169+ marginal metallophilic macrophages (4). We extended the analysis of Treml4 expression to a large panel of cell types in spleen. The heightened expression on CD8α+ DC and on macrophages was confirmed. In addition, expression on splenic monocytes was noted. Blood monocytes were also positive for Treml4, particularly the Ly6Clow subset. However, peritoneal macrophages were Treml4 negative, as were liver and lymph node CD169+ sinus macrophages, as documented in our initial report (not shown and (4)). Since blood monocytes express this dying cell receptor, but spleen is the main site for Treml4 positive macrophages, we suspect the spleen sustains Treml4 on monocytes that become splenic macrophages, while other tissues do not. Alternatively, Ly6Clow monocytes may primarily be giving rise to splenic, but not other macrophages in the steady state.
We did not detect major differences in immunization outcome via Treml4 targeting relative to targeting DEC205, which is highly expressed on the CD8α+ subset of DCs. Furthermore, Treml4 was clearly able to mediate induction of an integrated and protective CD4+ and CD8+ T cell response, as noted with HER2-specific immune responses in FVB mice.
Treml4 is only one of several receptors that DCs express with the potential to react with dying cells. Others include CD36 (26), αVβ5 and αVβ3 (27, 28), and CLEC 9A (29). Of note, these receptors are specific for CD8α+ DC subset, which, in contrast to CD8α- DCs, can selectively take up and present dying cells (Fig. 1 and (30)). CLEC9A has been studied most extensively for cross-presentation of dying cell-associated antigens. The data show that CLEC9A is not required for phagocytosis, but instead contributes to cross-presentation following the uptake of dead-cell associated antigens (29). In contrast, CD36, αVβ5, and αVβ3 do not appear to contribute to the superior cross-presentation abilities of CD8α+ DCs (31). We were unable to detect alterations in either uptake or cross-presentation of cell-associated OVA in Treml4 KO mice relative to WT mice. This result suggests overlapping function between Treml4 and other dying-cell uptake receptors in vivo. Alternatively, it is possible that dying cells used in experimental studies are not the best physiological representatives of dying cells in vivo.
As of now, a human α-Treml4 mAb has not been produced. Thus, the function of this molecule and its clinical application in humans remains unclear. According to sequencing data (genebank), the human Treml4 gene contains a stop codon in the transmembrane region, which could be consistent with its release as a soluble protein (4). Treml4 also shares functional similarities with another Trem family member, Trem2, and although the expression of Treml4 has not yet been mapped in humans, Trem2 is known to be expressed on human macrophages and monocyte-derived DCs (4). It has also been shown that mouse Trem2 is involved in the clearance of apoptotic neurons by microglia (8). Likewise, we have reported that soluble Treml4 has a strong affinity to dead cells, suggesting a functional similarity between the respective molecules (4). It is therefore possible that Trem2 and Treml4 may have compensatory functions warranting further studies of both molecules in humans.
C-type lectin like receptors have been emphasized for receptor-mediated antigen targeting within mAbs. Treml4 is unique to date in that it belongs to the Ig superfamily. The use of mAb-based antigen targeting allows the faithful delivery of antigen to the cells that express the receptor, here Treml4, and it enables an assessment of the ability of that receptor to enhance antigen presentation and immunization in an intact animal. Specifically, we show that a receptor previously documented to engage with necrotic cells, can capture, present antigens and, in the presence of an innate stimulus like poly IC, induce Th1 CD4+ and CD8+ T cell immunity that promotes tumor protection.
Supplementary Material
Acknowledgments
We thank J. Adams for graphics, M. Nulty and A. Gottfried for help with the manuscript.
Grant support by NIAID AI13013 to R.M.S., and by Grant-in-Aid for Young Scientists from Ministry of Education, Culture, Sports, Science, and Technology of Japan to H.H.
Abbreviations
- DCs
Dendritic cells
- mAbs
monoclonal antibodies
- LN
Lymph nodes
- KO
knock out
- WT
wild type
References
- 1.Cambi A, Figdor C. Necrosis: C-type lectins sense cell death. Curr Biol. 2009;19:R375–378. doi: 10.1016/j.cub.2009.03.032. [DOI] [PubMed] [Google Scholar]
- 2.Poon IK, Hulett MD, Parish CR. Molecular mechanisms of late apoptotic/necrotic cell clearance. Cell Death Differ. 2010;17:381–397. doi: 10.1038/cdd.2009.195. [DOI] [PubMed] [Google Scholar]
- 3.Steinman RM, Hemmi H. Dendritic cells: translating innate to adaptive immunity. Curr Top Microbiol Immunol. 2006;311:17–58. doi: 10.1007/3-540-32636-7_2. [DOI] [PubMed] [Google Scholar]
- 4.Hemmi H, Idoyaga J, Suda K, Suda N, Kennedy K, Noda M, Aderem A, Steinman RM. A new triggering receptor expressed on myeloid cells (Trem) family member, Trem-like 4, binds to dead cells and is a DNAX activation protein 12-linked marker for subsets of mouse macrophages and dendritic cells. J Immunol. 2009;182:1278–1286. doi: 10.4049/jimmunol.182.3.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klesney-Tait J, I, Turnbull R, Colonna M. The TREM receptor family and signal integration. Nat Immunol. 2006;7:1266–1273. doi: 10.1038/ni1411. [DOI] [PubMed] [Google Scholar]
- 6.Ford JW, McVicar DW. TREM and TREM-like receptors in inflammation and disease. Curr Opin Immunol. 2009;21:38–46. doi: 10.1016/j.coi.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouchon A, Facchetti F, Weigand MA, Colonna M. TREM-1 amplifies inflammation and is a crucial mediator of septic shock. Nature. 2001;410:1103–1107. doi: 10.1038/35074114. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi K, Rochford CD, Neumann H. Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells-2. J Exp Med. 2005;201:647–657. doi: 10.1084/jem.20041611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamerman JA, Jarjoura JR, Humphrey MB, Nakamura MC, Seaman WE, Lanier LL. Inhibition of TLR and FcR responses in macrophages by triggering receptor expressed on myeloid cells (TREM)-2 and DAP12. J Immunol. 2006;177:2051–2055. doi: 10.4049/jimmunol.177.4.2051. [DOI] [PubMed] [Google Scholar]
- 10.Turnbull IR, Gilfillan S, Cella M, Aoshi T, Miller M, Piccio L, Hernandez M, Colonna M. TREM-2 attenuates macrophage activation. J Immunol. 2006;177:3520–3524. doi: 10.4049/jimmunol.177.6.3520. [DOI] [PubMed] [Google Scholar]
- 11.Paloneva J, Kestila M, Wu J, Salminen A, Bohling T, Ruotsalainen V, Hakola P, Bakker AB, Phillips JH, Pekkarinen P, Lanier LL, Timonen T, Peltonen L. Loss-of-function mutations in TYROBP (DAP12) result in a presenile dementia with bone cysts. Nat Genet. 2000;25:357–361. doi: 10.1038/77153. [DOI] [PubMed] [Google Scholar]
- 12.Paloneva J, Manninen T, Christman G, Hovanes K, Mandelin J, Adolfsson R, Bianchin M, Bird T, Miranda R, Salmaggi A, Tranebjaerg L, Konttinen Y, Peltonen L. Mutations in two genes encoding different subunits of a receptor signaling complex result in an identical disease phenotype. Am J Hum Genet. 2002;71:656–662. doi: 10.1086/342259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonifaz LC, Bonnyay DP, Charalambous A, Darguste DI, Fujii S, Soares H, Brimnes MK, Moltedo B, Moran TM, Steinman RM. In vivo targeting of antigens to maturing dendritic cells via the DEC-205 receptor improves T cell vaccination. J Exp Med. 2004;199:815–824. doi: 10.1084/jem.20032220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trumpfheller C, Finke JS, Lopez CB, Moran TM, Moltedo B, Soares H, Huang Y, Schlesinger SJ, Park CG, Nussenzweig MC, Granelli-Piperno A, Steinman RM. Intensified and protective CD4+ T cell immunity in mice with anti-dendritic cell HIV gag fusion antibody vaccine. J Exp Med. 2006;203:607–617. doi: 10.1084/jem.20052005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Idoyaga J, Lubkin A, Fiorese C, Lahoud MH, Caminschi I, Huang Y, Rodriguez A, Clausen BE, Park CG, Trumpfheller C, Steinman RM. Comparable T helper 1 (Th1) and CD8 T-cell immnity by targeting HIV gag p24 to CD8 dendritic cells within antibodies to Langerin, DEC205, and Clec9A. Proc Natl Acad Sci USA. 2011;108:2384–2389. doi: 10.1073/pnas.1019547108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bunting M, Bernstein KE, Greer JM, Capecchi MR, Thomas KR. Targeting genes for self-excision in the germ line. Genes Dev. 1999;13:1524–1528. doi: 10.1101/gad.13.12.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park SH, Cheong C, Idoyaga J, Kim JY, Choi JH, Do Y, Lee H, Jo JH, Oh YS, Im W, Steinman RM, Park CG. Generation and application of new rat monoclonal antibodies against synthetic FLAG and OLLAS tags for improved immunodetection. J Immunol Methods. 2008;331:27–38. doi: 10.1016/j.jim.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hawiger D, Inaba K, Dorsett Y, Guo M, Mahnke K, Rivera M, Ravetch JV, Steinman RM, Nussenzweig MC. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med. 2001;194:769–780. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reilly RT, Gottlieb MB, Ercolini AM, Machiels JP, Kane CE, Okoye FI, Muller WJ, Dixon KH, Jaffee EM. HER-2/neu is a tumor rejection target in tolerized HER-2/neu transgenic mice. Cancer Research. 2000;60:3569–3576. [PubMed] [Google Scholar]
- 20.Idoyaga J, Cheong C, Suda K, Suda N, Kim JY, Lee H, Park CG, Steinman RM. Langerin/CD207 receptor on dendritic cells mediates efficient antigen presentation on MHC I and II products in vivo. J Immunol. 2008;180:3647–3650. doi: 10.4049/jimmunol.180.6.3647. [DOI] [PubMed] [Google Scholar]
- 21.Liu K, Iyoda T, Saternus M, Kimura Y, Inaba K, Steinman RM. Immune tolerance after delivery of dying cells to dendritic cells in situ. J Exp Med. 2002;196:1091–1097. doi: 10.1084/jem.20021215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu K, Idoyaga J, Charalambous A, Fujii S, Bonito A, Mordoh J, Wainstok R, Bai XF, Liu Y, Steinman RM. Innate NKT lymphocytes confer superior adaptive immunity via tumor-capturing dendritic cells. J Exp Med. 2005;202:1507–1516. doi: 10.1084/jem.20050956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fujii S, Liu K, Smith C, Bonito AJ, Steinman RM. The linkage of innate to adaptive immunity via maturing dendritic cells in vivo requires CD40 ligation in addition to antigen presentation and CD80/86 costimulation. J Exp Med. 2004;199:1607–1618. doi: 10.1084/jem.20040317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Idoyaga J, Suda N, Suda K, Park CG, Steinman RM. Antibody to Langerin/CD207 localizes large numbers of CD8α+ dendritic cells to the marginal zone of mouse spleen. Proc Natl Acad Sci USA. 2009;106:1524–1529. doi: 10.1073/pnas.0812247106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ingersoll MA, Spanbroek R, Lottaz C, Gautier EL, Frankenberger M, Hoffmann R, Lang R, Haniffa M, Collin M, Tacke F, Habenicht AJ, Ziegler-Heitbrock L, Randolph GJ. Comparison of gene expression profiles between human and mouse monocyte subsets. Blood. 2010;115:e10–19. doi: 10.1182/blood-2009-07-235028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Belz GT, Behrens GMN, Smith CM, Miller JFAP, Jones C, Lejon K, Fathman CG, Mueller SN, Shortman K, Carbone FR, Heath WR. The CD8α+ dendritic cell is responsible for inducing peripheral self-tolerance to tissue-associated antigens. J Exp Med. 2002;196:1099–1104. doi: 10.1084/jem.20020861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Albert ML, Pearce SFA, Francisco LM, Sauter B, Roy P, Silverstein RL, Bhardwaj N. Immature dendritic cells phagocytose apoptotic cells via αVβ5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J Exp Med. 1998;188:1359–1368. doi: 10.1084/jem.188.7.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rubartelli A, Poggi A, Zocchi MR. The selective engulfment of apoptotic bodies by dendritic cells is mediated by the αVβ3 integrin and requires intracellular and extracellular calcium. Eur J Immunol. 1997;27:1893–1900. doi: 10.1002/eji.1830270812. [DOI] [PubMed] [Google Scholar]
- 29.Sancho D, Joffre OP, Keller AM, Rogers NC, Martinez D, Hernanz-Falcon P, Rosewell I, Reis e Sousa C. Identification of a dendritic cell receptor that couples sensing of necrosis to immunity. Nature. 2009;458:899–903. doi: 10.1038/nature07750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iyoda T, Shimoyama S, Liu K, Omatsu Y, Akiyama Y, Maeda Y, Takahara K, Steinman RM, Inaba K. The CD8+ dendritic cell subset selectively endocytoses dying cells in culture and in vivo. J Exp Med. 2002;195:1289–1302. doi: 10.1084/jem.20020161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schulz O, Pennington DJ, Hodivala-Dilke K, Febbraio M, Reis E Sousa C. CD36 or αVβ3 and αVβ5 integrins are not essential for MHC class I cross-presentation of cell-associated antigen by CD8α+ murine dendritic cells. J Immunol. 2002;168:6057–6065. doi: 10.4049/jimmunol.168.12.6057. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.