Abstract
Protein glycosylation is a common and complex posttranslational modification of proteins, which expands functional diversity while boosting structural heterogeneity. Glycoproteins, the end products of such a modification, are typically produced as mixtures of glycoforms possessing the same polypeptide backbone but differ in the site of glycosylation and/or in the structures of pendant glycans, from which single glycoforms are difficult to isolate. The urgent need for glycan-defined glycoproteins in both detailed structure-function relationship studies and therapeutic applications has stimulated an extensive interest in developing various methods for manipulating protein glycosylation. This review highlights emerging technologies that hold great promise in making a variety of glycan-defined glycoproteins, with a particular emphasis in the following three areas: specific glycoengineering of host biosynthetic pathways, in vitro chemoenzymatic glycosylation remodeling, and chemo-selective and site-specific glycosylation of proteins.
INTRODUCTION
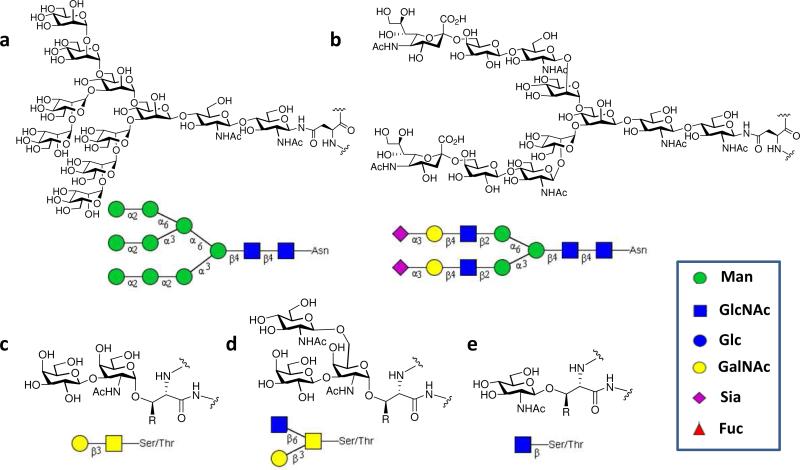
Recent advances in glycobiology and functional glycomics revealed diverse roles of glycans and glycoconjugates in biological systems (1). The glycoprotein is an important class of glycoconjugates involved in a wide variety of biological recognition processes: cell adhesion, cell differentiation, host-pathogen interaction, and immune response (2-7). Intra-molecularly, glycosylation plays an important role in modulating a protein's intrinsic properties such as folding, intracellular trafficking, stability, and pharmacokinetics (8). Protein glycosylation can be very diverse and dynamic. A survey suggests that there are at least 41 different types of sugar-amino acid linkages, with N-glycosylation (at the side chain of Asn), O-GalNAc glycosylation (at the Ser/Thr residues), and O-GlcNAc glycosylation (at the Ser/Thr residues) as the major forms (9). While the common N- and O-glycans function mainly at the cell surface, the dynamic O-GlcNAc glycosylation of nuclear, mitochondrial, and cytosolic proteins plays important roles in signal transduction by interplay with protein phosphorylation (10, 11). An important feature of protein glycosylation is the structural complexity of glycans. Representative N- and O-glycan structures are shown in Figure 1. The number of glycan variants can grow very rapidly when the glycan core is further branched and decorated with various terminal sugars, e.g., sialic acids, and non-carbohydrate functional groups such as sulfate, phosphate, and acetate. Another common feature of glycosylation is structural heterogeneity. In contrast to nucleic acids and proteins that are biosynthetically assembled on templates and under direct transcriptional control, the biosynthesis of glycans on glycoproteins have no known template, and glycosylation patterns are dictated by many factors (amino acid sequences, local peptide conformations at the glycosylation sites, and the accessibility and localization of activated substrates, enzymes, and co-factors). As a result, glycoproteins are usually produced as mixtures of glycosylation variants, i.e., glycoforms that share the same polypeptide backbone but differ in the sites of glycosylation and/or in the structures of the pendant glycans.
Figure 1.
Structures of representative N- and O-linked glycans on glycoproteins. (a) high-mannose type N-glycan; (b) bi-antennary complex type N-glycan; (c) core 1 O-GalNAc glycan; (d) core 2 O-GalNAc glycan; (e) O-GlcNAc.
Compelling evidence has shown that appropriate glycosylation is important for pharmacokinetics, cellular distributions, and biological activities of therapeutic glycoproteins (6, 7, 12-16). Nevertheless, the challenge in controlling glycosylation to a desired, homogeneous glycoform is well reflected by the fact that most of glycoprotein-based drugs are still produced as mixtures of glycoforms. Thus, when making therapeutic glycoproteins, the manufacturer is required to deliver the products with strictly consistent ratio and identity of glycoforms to ensure a reproducible clinical performance. In principle, changes in quality attributes are acceptable only if they do not alter safety and clinical efficacy (17). Even so, a recent study on three commercial glycoprotein drugs (darbepoetin alfa, rituximab, and etanercept) on the market from different batches has revealed significant changes in the identity of their glycoforms, implicating possible alterations of their clinical efficacy (18). This study once again raises a serious regulatory question and re-emphasizes the importance in controlling glycosylation when manufacturing glycoprotein-based therapeutics.
The last decade has witnessed tremendous progress in this field, and many chemical, enzymatic, and cell-based glycoengineering methods were explored in order to overcome a series of technical hurdles on the road toward homogeneous glycoproteins, which are the topics of a series of excellent recent reviews (19-34). This review highlights selected emerging technologies that hold great promise in generating a variety of glycan-defined glycoproteins. Emphasis is placed on recent developments in three areas: Engineering of host glycan biosynthetic pathways, in vitro chemoenzymatic glycosylation remodeling, and chemo-selective site-specific glycosylation of proteins. What was not covered in the present review is the chemical synthesis of natural glycoproteins, which has also progressed to a new level through the exploration and elegant application of various ligation methods such as the native chemical ligation, expressed protein ligation, and sugar-assisted ligation (35-41). Interested readers are referred to those recent reviews that had excellent coverage of the general aspects of this topic (20, 22, 23, 25, 29, 31, 33, 42). Taken together, these emerging technologies provide important new tools for deciphering the biological functions of glycoproteins and for facilitating the development of glycoprotein-based therapeutics.
GLYCOENGINEERING IN MAMMALIAN CELLS
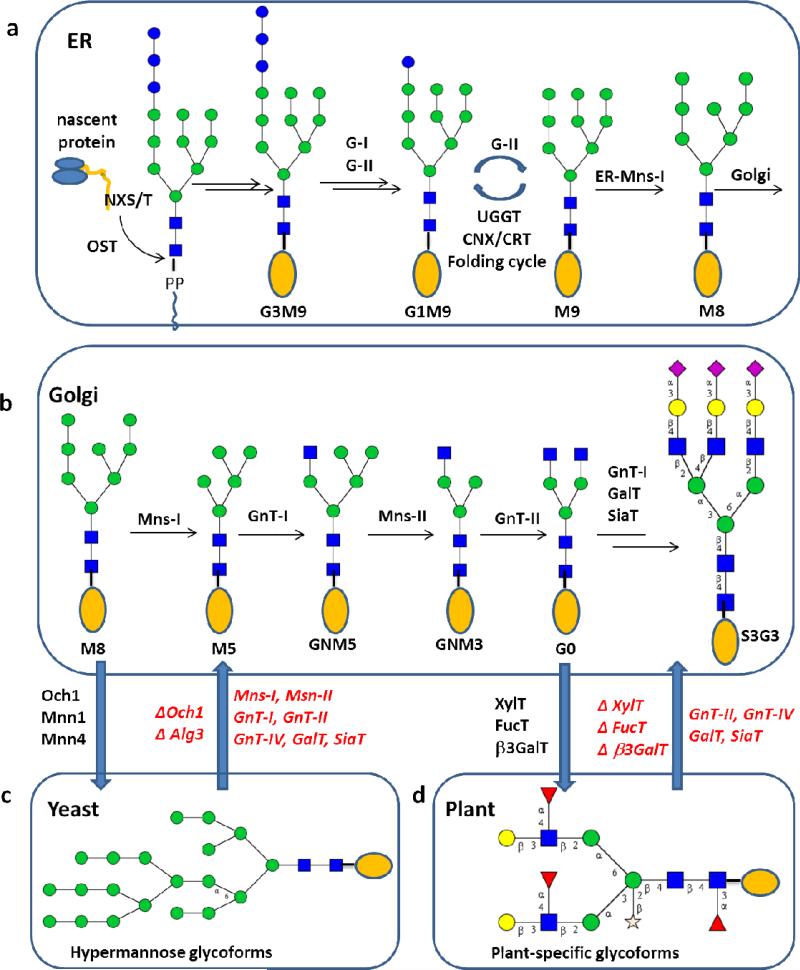
Most recombinant therapeutic glycoproteins currently used in clinical treatment, including monoclonal antibodies (mAbs) and erythropoietin (EPO), are produced in Chinese hamster ovary (CHO) cell lines (12, 43). The biosynthesis of mammalian glycoproteins involves a highly complex glycosylation network. In the case of N-glycosylation, a common oligosaccharide precursor (Glc3Man9GlcNAc2) is transferred by an oligosaccharyltransferase (OST) from the dolichol pyrophosphate-linked glycolipid to the amide nitrogen of an asparagine (Asn) side chain in a consensus sequence Asn-X-Ser/Thr of a nascent polypeptide (where X is any amino acid but proline). The precursor is then processed by ER α-glucosidases I and II (G-I and G-II) to the mono-glucosylated glycoform (Glc1Man9GlcNAc2), which is the key intermediate in the calnexin/calreticulin-mediated protein folding cycle in protein quality control. Once correctly folded, the precursor is trimmed by G-II and the ER α-mannosidase (ER Mns-I) to Man8GlcNAc2-protein (Figure 2a). The Man8GlcNAc2 glycoform is then translocated to the Golgi apparatus, where the glycoprotein is further trimmed by Golgi α-mannosidases (Mns-I and Mns-II) and is then remodeled by a set of glycosyltransferases (e.g., GnT-III for bisecting; GnT-IV and GnT-V for branching; and SiaT for sialylation) to build various glycoforms (Figure 2b). The mature glycoforms are the outcome of a very complex spatiotemporal glycosylation network. As a result, mammalian glycoproteins are often produced as mixtures of glycoforms. The goal of glycoengineering is to make glycoproteins carrying more defined glycans by controlling and altering the glycan biosynthetic pathways.
Figure 2.
N-glycan biosynthetic pathways in eukaryotes and glycoengineering. (a) the shared early steps in the ER leading to the Man8GlcNAc2 (M8) glycoform, which is translocated to Golgi for further processing; (b) processing and branching in mammalian host leading to mature glycoproteins; (c) glycan processing in yeast leading to hypermannosylation and its engineering being directed to the mammalian glycosylation pathway; (d) glycan processing in plants leading to plant-specific glycoform and its engineering being directed to the mammalian glycosylation pathway.
One way to achieve more defined glycosylation is to perform mutagenesis and to select mutants capable of producing specific glycoforms. Mutagenized CHO cells had specific genes knocked out along the glycosylation pathways, and clones were screened against an array of cytotoxic plant lectins to select toxic lectin-resistance (LecR) mutants that produce glycoforms with altered or simplified glycans (44, 45). This approach led to the discovery of a series of valuable LecR CHO cell lines capable of producing specific glycoforms with more defined N- and O-glycans than the parent CHO cells (44, 45). For example, the Lec1 cell line produces predominantly high-mannose type glycoforms (46), the Lec2 cell line produces asialylated glycoproteins (47), and the Lec13 cell line is capable of making monoclonal antibodies with low fucose content that demonstrate enhanced antibody-dependent cellular cytotoxicity (ADCC) (48). Interestingly, a recent glycomics analysis of the N-glycan profiles from 9 LecR CHO mutants revealed that some CHO mutants could make glycoforms carrying novel N-glycans of unexpected size and complexity, including those with long poly LacNAc chains and terminal Lewis(x) and sialyl-Lewis(x) determinants (49). These gain-of-function mutants suggest that simultaneous mutations on several seemingly “unrelated” genes could result in the production of unusual N-glycans that might not be predicted by targeted mutations, implicating the complexity of the glycosylation network. This approach was also extended to the human embryonic kidney (HEK) cell lines to generate HEK Lec mutants that restrict glycosylation predominantly to Man5GlcNAc2 or hybrid types (50, 51). Most of the Lec cell lines are commercially available from ATCC and are valuable for a wide application in glycobiology.
A complementary technology to mutagenesis is the use of specific small-molecule inhibitors to block selected enzymes in the biosynthesis pathway, which can lead to the generation of simplified and/or more uniformed glycoforms. For example, N-butyl deoxynojirimycin inhibits the trimming of the Glc3Man9GlcNAc2-protein by ER α-glucosidases I and II, thus leading to the glycoprotein carrying the full-length N-glycan precursor; kifunensine inhibits the ER α-mannosidase-I (ER Mns-I) activity resulting in formation of the Man9GlcNAc2 glycoform; and swainsonine inhibits the Golgi α-mannosidase II (Mns-II), leading to the generation of Man5GlcNAc2 and/or hybrid type glycoforms. This technology has been successfully used in facilitating X-ray crystallographic studies on glycoproteins by simplifying the glycosylation patterns (52); for producing mAbs with enhanced ADCC function (53); and for probing structure-function relationships of HIV-1 envelope glycoproteins by controlling the glycosylation at the high-mannose status (54-56).
While the knockout mutagenesis and inhibitor interference can simplify or re-direct glycosylation, overexpression of certain glycoprocessing enzymes in the host system can also change glycosylation profiles and enrich the production of desired glycoforms. Notable examples in this category include: overexpression of α-2,3-SiaT and β-1,4-GalT in the host cells to increase terminal sialylation, an important modification for prolonging the serum's half-life of therapeutic glycoproteins (57); overexpression of GnT-I, GnT-IV and GnT-V to increase the branching structures (58, 59); and overexpression of GnT-III to enhance the bisecting GlcNAc containing glycoforms (60, 61). Since the presence of a bisecting GlcNAc moiety blocks the biosynthetic attachment of a core fucose (detrimental to ADCC), this GnT-III overexpressed CHO cell line has been successfully applied to produce monoclonal antibodies with enhanced ADCC activity (60, 61).
GLYCOENGINEERING IN NON-MAMMALIAN EUKARYOTIC CELLS
Glycoengineering in yeast
N-glycosylation in yeast shares the conserved early steps with mammalian cells, yielding the common N-glycan, Man8GlcNAc2. Yeast glycan processing diverges from humans at this point, when a crucial mannosyltransferase, Och1, adds an α-(1-6)-linked mannose to the α-(1-3)-branching mannose of the Man3GlcNAc2 core. This newly added α-(1-6)-linked mannose serves as the key starting point for an iterative chain elongation leading to hypermannosylation of the glycan (62). Yeast, with its genetics being well characterized, is a cost-effective and high-yielding system for expressing recombinant proteins. Nevertheless, the hypermannose moieties are immunogenic in humans. Thus, abolishing or avoiding hypermannosylation activity is the first step in generating recombinant humanized glycoproteins from yeast. Several methods have been used to prevent hypermannosylation, including: deletion of Och1 in S. cerevisiae and P. pastoris (63, 64); modification of the Glc3Man9GlcNAc2 to a Man5GlcNAc2 lipid precursor before block transfer (65); and addition of α-(1-2)-mannosidase to the ER-cis Golgi boundary using a C-terminal HDEL peptide. Deletion or inhibition of the och1 gene is the most efficient means of preventing hyper-mannosylation, but presents sickly phenotypes in S. cerevisiae. The methylotrophic yeast, P. pastoris, is an alternative to S. cerevisiae, since disruption of the och1 gene has little effect on its growth (66).
Once the biosynthesis in yeast is arrested at the Man8 or Man5 intermediates by knockout of och1 or agl3 genes in P. pastoris, the high-mannose intermediates could be directed to the mammalian biosynthetic pathways by functional transfer of the mammalian glycan processing enzymes into yeast (Figure 2c). The advantage of glycoengineering yeast (e.g., P. pastoris) to produce humanized glycoproteins is homologous secretory pathways to those in mammalian system. A combinatorial genetic approach was used to introduce mammalian Mns-I and GnT-I enzymes. Evaluation of the combinatorial libraries led to the identification of an engineered strain capable of producing the key glycoform, GNM5 (64). A similar approach was used for introducing Mns-II and GnT-II that catalyze the removal of the terminal two mannose residues in GNM5 and the addition of a GlcNAc at the exposed α-1,6-branch mannose, respectively. Screening of the libraries led to the discovery of a strain that produces the key homogeneous complex type (G0) N-glycan (67) (Figure 2c). Alternatively, the combinatorial genetic approach was also applied to engineering P. pastoris in which alg3 was deleted arresting the biosynthesis at the Man5 stage. Introduction and localization of Mns-I, GnT-I, Mns-II and GnT-II, together with the mammalian β-1,4-galactosyltransferase, led to the production of the biantennary, galactosylated complex type N-glycan (68). The addition of sialic acid to the terminus of complex type N-glycan involved the transfer of genes responsible for biosynthesis and transfer of sialic acid moiety along the secretary pathways into the yeast host. Evaluation of the library identified an engineered strain that was able to produce recombinant human erythropoietin (EPO) carrying remarkably uniformly disialylated N-glycan (69). The glycoengineered yeast system was also applied for producing rituximab, a monoclonal antibody used for the cancer treatment (70). The yeast recombinant rituximab carries a homogeneous complex N-glycan Gal2GlcNAc2Man3GlcNAc2, which has the same antigen binding property as the CHO produced commercial one, but demonstrated much higher affinity to FcγIIIa receptor and much more potent ADCC activity than the CHO-produced, commercial rituximab. This is mainly due to the absence of core fucose in the N-glycan from the yeast-expressed rituximab. Application of similar glycoengineered strains to the production of human lactoferrin (hLF) led to a high yield of recombinant hLF, but the recombinant glycoprotein was still heterogeneous in glycosylation, with the desired S2G2 and G2 glycoforms as the major components (71). This result suggests that homogeneity of glycosylation in the engineered yeast also depends on specific sequence of the glycoproteins. In addition to the combinatorial genetic approach, an alternative method for glycoengineering of P. pastoris is the use of GlycoSwitch technology (72). This approach consists of the disruption of och1 gene and the stepwise introduction of mammalian enzymes. Each engineering step results in introduction and localization of one enzyme along the secretory pathway, but may consist of multiple cycles of screening, analysis, and optimizations. Valuable engineered strains were identified and successfully used for production of glycoproteins carrying human-like complex type N-glycans (72). These remarkable accomplishments showcase the power of glycoengineering yeast to produce defined protein glycosylation. Further work may be directed to the optimization of the engineered strains for their stability and efficiency, as well as evolving new strains capable of producing bisecting and branched mammalian N-glycans.
Progress has also been made in engineering yeast cells to produce human-like O-linked glycoproteins (73). Yeast does not have the glycosylation machinery to build GalNAc-Ser/Thr linkage found in humans. In this study, genes encoding ppGalNAcT, β-(1,3)-GalT and other enzymes essential for assembling the substrates were introduced into Saccharomyces cerevisiae. Then yeast strains capable of making mucin type O-glycopeptide and O-glycoprotein were selected. Meanwhile, the common yeast O-mannosylation pathway was suppressed by incorporating a small-molecule inhibitor in the medium. This method was successfully applied to produce human glycoprotein podoplanin carrying the O-linked Galβ-1,3-GalNAc glycan. Upon in vitro sialylation, the resulting glycosylated podoplanin could induce platelet aggregation, indicating the restoration of biological activity for which the mucin-type glycosylation is required. It is to be tested whether the engineered strains are equally efficient to produce other O-glycosylated proteins.
Glycoengineering in plant cells
While engineered CHO cells can generate glycosylation patterns similar to those found in humans, there are several disadvantages of using mammalian expression system, including instability, long incubation time, high cost of maintenance, and possible pathogenic contamination from the serum in cell media. Plant cells share essentially the same initial steps as that in mammalian system, until it reaches the GlcNAcMan3GlcNAc2 core in Golgi. Then the core is decorated by additions of plant-specific bisecting β-1,2-xylose and core α-1,3-fucose that are not found in mammalian N-glycoproteins (Figure 2d). The N-glycans are often capped with α-1,4-fuocose and β-1, 3-galactose residues to form Lea structural motifs, but plant cells lack the machinery to make highly branched and sialylated N-glycans. Thus, the goal of making humanized glycoprotein in plant cells requires the elimination of the plant-specific β-1, 2-xylose and core α-1, 3-fucose structural motifs that are highly immunogenic in humans and meanwhile the addition of the enzymes and auxiliary proteins that are needed to undertake humanized N-glycosylation. To achieve this goal, one approach is to apply RNA interference (RNAi) technology to shut down expression of the plant-specific endogenous α-1,3- fucosyltransferase (α-1,3-FucT) and β-1,2-xylosyltransferase (β-1,2-XylT) genes. An impressive example is the production of human anti-CD30 monoclonal antibody in cell culture of the aquatic plant Lemna minor with an RNAi construct targeting the expression of α-1,3-FucT and β-1,2-XylT genes (74). The resultant recombinant mAb was shown to contain a single human bi-antennary (nongalactosylated) N-glycan without attachment of plant-specific Xyl and Fuc-containing motifs. The RNA interference method was also used for production of an HIV-neutralizing monoclonal antibody 2G12 in Nicotiana benthamiana (a tobacco-related species) (75). The plant-produced recombinant mAb carries a major humanized N-glycan and shows antigen-binding and HIV neutralization activity similar to the mammalian cell-derived mAb.
To make more complex humanized glycoforms, an alternative approach is to transfer the human N-glycan branching machinery into the plant system together with the deletion of the plant-specific glyco-genes. This was recently achieved by glycoengineering of the N. benthamiana (76). Modification included the deletion of plant-specific XylT and FucT genes and functional transfer of the modified genes encoding human GnT-III, GnT-IV, and GnT-V enzymes. The engineered plants were used to express human erythropoietin (EPO) and human serum transferrin, leading to the production of glycoforms carrying tri- and tetra-antennary complex N-glycans with or without bisecting GlcNAc moieties. A key technical point is that the genes encoding mammalian glycosyltransferases GnT-III, -IV, and -V were modified by replacing the human cytoplasmic tail, transmembrane domain, and stem region (CTS) with the plant-specific Golgi targeting sequences, so that they were appropriately localized for the biosynthesis of the N-glycans. This remarkable study showcases the power of plant glycoengineering to produce humanized therapeutic glycoproteins carrying complex type N-glycans with great glycosylation uniformity.
Finally, as a way to produce sialylated glycoproteins in plants that do not have the sialylation machinery, an entire mammalian sialylated N-glycan biosynthetic pathway was introduced into N. benthamiana plants (77). It was shown that the coordinated expression of the genes for the biosynthesis, activation, transport, and transfer of Neu5Ac to terminal galactose in N. benthamiana plants deficient in endogenous xylosylation and fucosylation was able to efficiently produce monoclonal antibody 2G12 carrying a biantennary complex type N-glycan at the Fc domain. Surprisingly, the glycoengineered plant expression was able to produce the mAb with a high level of Fc sialylation, which is in contrast to the mammalian expression system (e.g. CHO cell line) that usually produces mAbs with a very low level of Fc sialylation.
CHEMOENZYMATIC GLYCOSYLATION REMODELING
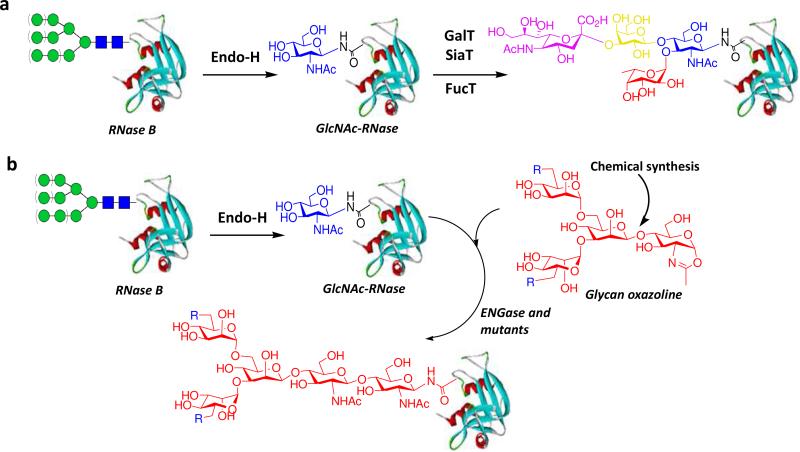
While glycoengineering of host expression system has achieved tremendous progresses, complete control of expression to produce truly homogeneous glycoproteins remains difficult. In vitro chemoenzymatic glycosylation remodeling of natural and recombinant glycoproteins provides an attractive approach toward glycan-defined glycoforms. Most of work in this category was done on N-glycoproteins. In this approach, the heterogeneous N-glycans are enzymatically trimmed down to the innermost N-acetylglucosamine (GlcNAc), giving a homogeneous GlcNAc-containing protein. The sugar chains are then extended by enzymes such as glycosyltransferases and endoglycosidases to provide a mature, glycan-defined glycoprotein. A classic example for this approach is the glycosylation remodeling of ribonuclease (RNase) B (a heterogeneous glycoprotein containing Man5GlcNAc2 to Man9GlcNAc2 glycoforms) to a homogeneous glycoform carrying an N-linked sialyl Lewis X moiety (78) (Figure 3a). Briefly, the high-mannose N-glycan in RNase B was removed by Endo-H to give GlcNAc-RNase B. The sugar chain was then elongated by sequence additions of galactose, sialic acid and fucose under the catalysis of β-1,4-galactosyltransferase, α-2,3-sialyltransferase, and α-1,3- fucosyltransferase, respectively, to give a novel ribonuclease glycoform. But a potential drawback of this strategy is that sequential sugar chain extension does not guarantee the homogeneity of end product, as when one or more enzymatic steps do not go to completion, it will end up with mixtures of glycoforms.
Figure 3.
Chemoenzymatic approaches to glycosylation remodeling of glycoproteins. (a) sugar chain extension by sequential glycosyltransferase-catalyzed reactions; (b) sugar chain extension by endoglycosidase-catalyzed transglycosylation.
An alternative to sequential sugar chain extension is the en bloc transfer of a pre-assembled large oligosaccharide to the protein in a single step under the catalysis of an endo-β-N-acetylglucosaminidase (ENGase) (Figure 3b). ENGases are a class of endoglycosidases that cleave N-glycans from glycoproteins by hydrolyzing the glycosidic bond in the chitobiose core of N-glycans. A few ENGases were found to have transglycosylation activity capable of transferring the released N-glycan to a GlcNAc acceptor to form a new glycosidic linkage. Two enzymes of the glycoside hydrolase family 85 (GH85), the Endo-A from Arthrobacter protophormiae that is specific for high-mannose type N-glycans and the Endo-M from Mucor hiemalis that can hydrolyze both high-mannose type and bi-antennary complex type N-glycans, are particularly useful for synthetic purpose (79). While a block transfer of a large oligosaccharide is a unique advantage of this strategy, use of this enzymatic transglycosylation for synthesis had encountered significant limitations including: the use of large excess of natural N-glycopeptide/N-glycan as donor substrate, low transglycosylation yield, and product hydrolysis. Two important recent developments, the exploration of synthetic sugar oxazolines as donor substrates and the generation of novel glycosynthase mutants, have provided a timely solution to the major problems encountered in the ENGase-catalyzed synthesis (27, 34, 79). The idea to explore sugar oxazolines as donor substrates was originated from the assumption that ENGase-catalyzed reaction proceeds by a substrate-assisted mechanism, in which the 2-acetamido group in the substrate serves as a nucleophile to attack the anomeric center when the glycosidic oxygen is protonated by the enzyme, forming a presumed sugar oxazolinium ion intermediate. The oxazolinium intermediate should go either for transglycosylation or for hydrolysis. It was hypothesized that the activated sugar oxazoline might serve as a good substrate for transglycosylation. The hypothesis was proved correct and indeed synthetic sugar oxazolines corresponding to the N-glycan core turned out to be excellent substrates for transglycosylation for glycopeptide synthesis (80-83). Subsequent studies indicated that Endo-A could efficiently take a series of truncated and selectively modified N-glycan oxazolines for transglycosylation but had low hydrolytic activity on the glycopeptide product carrying truncated N-glycans. The high transglycosylation activity of the activated sugar oxazoline coupled with the low hydrolytic activity of the modified “ground-state” glycopeptide product by Endo-A accounts for the highly efficient synthesis of various glycopeptides carrying a truncated or selectively modified N-glycan (81, 82, 84-86). In contrast, the Endo-M has more significant hydrolytic activity toward the truncated glycopeptides, resulting in less efficient synthesis. It was found that Endo-A was also very flexible for the structures of the acceptors and the chemoenzymatic approach was successfully extended to glycosylation remodeling of ribonuclease B to provide various homogeneous glycoforms carrying core N-glycans, azido-tagged N-glycans, and other large oligosaccharide ligands (87, 88).
When the wild type enzymes (Endo-A and Endo-M) were applied to the synthesis of glycoproteins carrying natural N-glycans, quick enzymatic hydrolysis of the product could not be avoided, as the products are the natural substrates of these hydrolases. To address this problem, novel glycosynthase mutants were created. Site-directed mutagenesis and subsequent screening of a small mutant library led to the discovery of an Endo-M mutant, N175A, which was able to take Man9GlcNAc oxazoline corresponding to the full-size natural high-mannose type N-glycan for transglycosylation to form a large N-glycopeptide, but lacked the hydrolytic activity on the natural glycopeptide product (89). An equivalent mutant of Endo-A, the EndoA-N171A, was also a glycosynthase that could use Man9GlcNAc oxazoline for transglycosylation with diminished product hydrolysis activity (90). Added to the list was another Endo-A mutant, EndoA-E173Q, which also acted as a glycosynthase for transglycosylation without product hydrolysis (91). The N171 of Endo-A (equivalent to N175 in Endo- M) was predicted to be a residue essential for the orientation of the 2-acetamido group and for promoting oxazoline formation during the hydrolysis. Mutation at this critical residue thus aborted its function for promoting oxazoline formation, resulting in the elimination of hydrolysis activity. But when external sugar oxazoline was supplied, the mutant could still proceed with it at the catalytic site for transglycosylation. The E173 of Endo-A (E177 of Endo-M) was assumed to be the general acid/base in the catalysis. These assumptions were confirmed by the recently solved crystal structures of Endo-A in complexes with GlcNAc-Asn and a non-hydrolyzable oxazoline analog, Man3GlcNAc-thiazoline (92, 93).
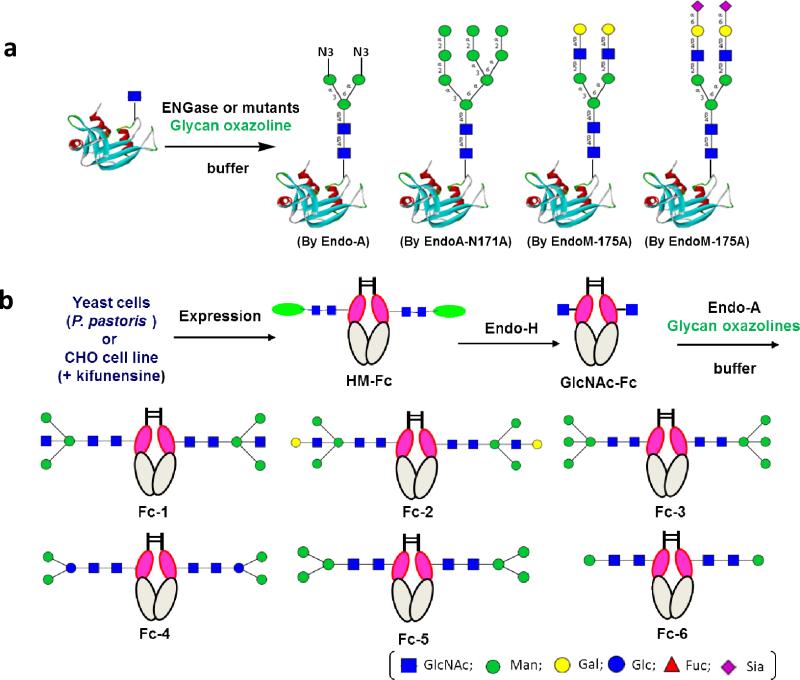
The discovery of the glycosynthase mutants permitted the synthesis of homogeneous glycoproteins carrying intact natural N-glycans as well as selectively modified N-glycans (Figure 4a). One important feature for the enzymatic method is preservation of the natural pentasaccharide core, Man3GlcNAc2, in the glycoprotein product. Recently it was found that EndoM-N175A and EndoMN175Q mutants were able to use both non-sialylated and sialylated glycan oxazolines for transglycosylation, allowing the synthesis of sialylated glycoproteins (90, 94-96). The combined use of these enzymes enabled the construction of a class of novel N-glycan clusters, which showed unusual lectin recognition properties (97). As another application, homogeneous Glc1Man9GlcNAc2 glycoforms were synthesized by a combined chemical and enzymatic approach (98) . The mono-glucosylated high-mannose type glycoform and a selectively modified (Galβ−1,4-G1Man9GlcNAc2) glycoform could be specifically recognized by lectin calreticulin. The synthetic homogeneous glycoforms should be valuable for deciphering the molecular mechanism of the calnexin/calreticulin-mediated protein folding process, the study of which has hitherto been hampered by the difficulties in obtaining glycan-defined glycoprotein intermediates.
Figure 4.
Chemoenzymatic synthesis of different glycoforms by the ENGase-catalyzed transglycosylation. (a) examples of synthetic RNase glycoforms produced by using ENGase and related glycosynthase mutants; (b) glycoengineering of human IgG-Fc.
Interestingly, wild type Endo-A was found to have a low but clearly detectable activity on complex type N-glycan oxazoline for transglycosylation, but it did not hydrolyze the “ground state” complex type glycoprotein (94). The promiscuity of Endo-A on the highly activated sugar oxazolines implicates an exciting opportunity to improve its transglycosylation activity on complex glycan oxazoline by directed evolution. In another study, a systematic mutagenesis at the N175 site of Endo-M was performed, which generated several mutants such as N175Q that showed much improved transglycosylation activity (99). Kinetic studies indicated that most of the mutants had a large Km value (in the mM range), implicating a low affinity to the substrates. Future studies should be directed to improve the enzymatic efficiency, e.g., by site-directed mutagenesis or directed evolution. Another family GH85 enzyme, Endo-D from Streptococcus pneumoniae, was also able to perform transglycosylation with sugar oxazoline, but the wild type enzyme had low transglycosylation efficiency, mainly due to quick hydrolysis of the substrate by the enzyme (94, 100). It would be interesting to see how specific Endo-D mutants would work. Recently, a class of glycoside hydrolase family 18 (GH18) enzymes, including Endo-F1, Endo-F2, and Endo-F3 from Flavobacterium meningosepticum, was also found to have transglycosylation activity (101). Specifically, the Endo-F3 recognized a core-fucosylated GlcNAc-peptide acceptor for transglycosylation, permitting an efficient synthesis of core-fucosylated complex N-glycopeptides. Endo-F3 represents the first endoglycosidase found to be capable of taking core-fucosylated GlcNAc-peptide acceptor for transglycosylation. It remains to be tested whether Endo-F3 is equally efficient to work with core-fucosylated GlcNAc-protein acceptor.
The chemoenzymatic method uses a GlcNAc-containing protein as the key intermediate for sugar chain extension, which can be obtained by de-glycosylation of natural or recombinant glycoproteins produced in eukaryotic cells. E. coli could not make glycoproteins as it lacks the protein glycosylation machinery. However, recent discovery of a protein N-glycosylation machinery in Campylobacter jejuni (102, 103) and its successful functional transfer into E. coli have raised an exciting opportunity to produce recombinant N-glycoproteins in bacteria (104, 105). Nevertheless, the attached bacterial N-glycan, a unique heptasaccharide GalNAc-α1,4-GalNAc-α1,4-[Glc-β1,3-]GalNAc-α1,4-GalNAc-α1,4-GalNAc-α1,3-Bac-β1,N-Asn, is completely different from eukaryotic N-glycans, and the glycan is linked to the asparagine (Asn) in an extended consensus sequence (D/EZNXS/T, where Z and X can be other amino acids) through an unusual deoxysugar, bacillosamine (Bac). Recently, this bacterial expression system was explored to produce homogeneous glycoproteins with eukaryotic N-glycosylation (106). The method involves the engineering and functional transfer of the C. jejuni glycosylation machinery in E. coli to express glycosylated proteins in which the bacterial Bac-Asn linkage was replaced with the key GlcNAc-Asn linkage found in human N-glycoproteins. The external bacterial glycans were then trimmed by α-N-acetylgalactosaminidase to the innermost GlcNAc, and then the GlcNAc was extended by the ENGase-catalyzed transglycosylation to fulfill a eukaryotic N-glycosylation. This method combines the power of protein expression in E. coli, biotechnology's work horse, and the flexibility of the in vitro glycosylation remodeling system, providing a potentially general platform for producing eukaryotic N-glycoproteins. While this work provides proof-of-concept data, several problems remain, including the low efficiency of glycosylating heterologous proteins and the requirement of an extended consensus sequence at the glycosylation sites by PglB, the bacterial oligosaccharyltransferase. Future studies should be directed to addressing these problems including engineering PglB to expand its specificity and mechanistic investigations of the glycoprotein secretary pathways.
As a notable example for its application, the chemoenzymatic method was successfully applied to glycoengineering of human IgG-Fc (107, 108) . In an initial study, human IgG-Fc was expressed in yeast Pichia pastoris, and the heterogeneous yeast N-glycans were removed by Endo-H treatment to leave only the innermost GlcNAc attached at the glycosylation sites. It was found that Endo-A could catalyze the transfer of Man3GlcNAc oxazoline to the seemingly hindered GlcNAc residues of the Fc homodimer (GlcNAc-Fc) under very mild conditions (pH 7.0, 23 °C), without the need of denaturing the Fc domain. Thus the native structure of IgG-Fc homodimer was kept intact during glycosylation remodeling processes. Complete glycosylation was achieved at the two glycosylation sites of the homodimer, generating a homogeneous glycoform of IgG-Fc when excess sugar oxazoline was used. On the basis of this initial success, an extended study aiming to elucidate the structure-activity relationships related to the effects of Fc glycosylation on Fcγ receptor binding was reported recently (108). The human IgG-Fc was expressed in CHO cells in the presence of an α-mannosidase inhibitor, kifunensine, to confer the Endo-H sensitive high-mannose glycoform, which was deglycosylated by Endo-H to provide the key aceptor, GlcNAc-Fc as a homodimer. A series of sugar oxazolines were chemically synthesized and transferred to the GlcNAc-Fc acceptor by Endo-A to give an array of homogeneous Fc glycoforms with altered glycan structures (Figure 4b). The Endo-A was found to be remarkably efficient to take various modified N-glycan core oxazolines, including the bisecting sugar-containing derivatives, for Fc glycosylation remodeling. SPR binding studies unambiguously proved that the presence of a bisecting sugar moiety could enhance the binding of Fc to the activating receptor FcγRIIIa, independent of Fc core-fucosylation, but this modification had little effect on the affinity of Fc to the inhibitory Fcγ receptor, FcγRIIb. It was also shown that the α-linked mannose residues in the pentasaccharide Man3GlcNAc2 core was essential to maintain a high-affinity of Fc to both FcγRIIIa and FcγRIIb. Further studies along this line should provide additional pure glycoforms for more detailed structural and functional studies of human IgG-Fc glycosylation.
CHEMO-SELECTIVE AND SITE-SPECIFIC GLYCOSYLATION OF PROTEINS
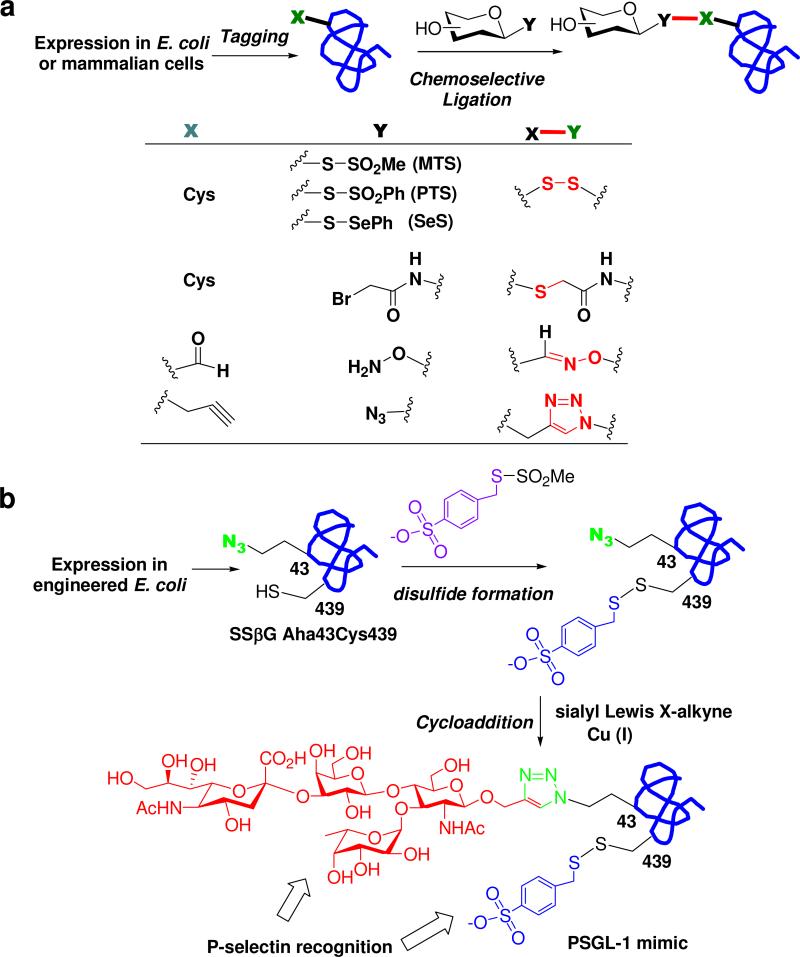
Site specific glycosylation of recombinant proteins can be achieved by chemo-selective ligation between bio-orthogonally tagged proteins and glycans. In this approach, specific tags are introduced at pre-determined glycosylation sites by site-directed mutagenesis. The tags are then reacted with a modified glycan via bio-orthogonal chemo-selective ligation. This topic was the focus of two excellent recent reviews (29, 32). Therefore, we provide here only a brief highlight of this strategy. Figure 5a shows the general approach of this protein glycosylation strategy. Of the natural amino acid residues, cysteine (Cys) is the most widely used as a tag, which can be introduced by site-directed mutagenesis. The free cysteine residue in the expressed protein can be selectively modified with a thiol-reactive functional group that is pre-installed in a sugar moiety to fulfill a site-specific glyco-conjugation. A series of cysteine-reactive functionality, including glycosyl iodo-/bromo-acetamide, glycosyl methanethiosulfonate (GlycoMTS), and glycosyl phenylthiosulfonate (GlycoPTS), were installed in a sugar moiety and was ready for conjugation through a disulfide or a thioether linkage. This strategy was applied to the production of artificially glycosylated erythropoietin (EPO) by introducing cysteines at the conserved N-glycosylation sites, followed by chemo-selective reaction with glycosyl iodoacetamide (109, 110). This method was also successfully used to selectively introduce sugar chains at the conserved N-glycosylation sites (Asn-297) of human IgG-Fc, probing the effects of the glycosylation on antibody's effector functions (111). Another remarkable example was the use of this “tag and modify” strategy to make novel anti-bacterial glycodendriproteins that contain branched sugar chains at predetermined sites in the protein (112).
Figure 5.
Chemo-selective and site-specific glycosylation of proteins. (a) a general chemo-selective and site-specific strategy; (b) a dual tagging approach to generating a functional PSGL-1 mimic.
In addition to the natural Cys residue, the ability to introduce a series of novel functionalized unnatural amino acid residues through genetic manipulation has significantly expanded the scope and diversity of the “tag and modify” strategy. Common bio-orthogonal tags include azide-, aldehyde-, alkyne, and alkene-containing residues, which can be selectively reacted with an appropriate functional group installed in a sugar moiety under mild, bio-compatible conditions. As an early example, unnatural amino acids containing a ketone “handles” were introduced in protein by the amber codon suppression technology (113). Chemo-selective reaction with a glyco-acylhydrazide allowed site-specific attachment of the sugar moiety through an oxime linkage. Additional unnatural amino acids such as azido-homoalanine (Aha) and homopropargylglycine (Hpg) can be incorporated into proteins by employing a Met (-) auxotrophic strain, Escherichia coli B834 (DE3), to express the target protein in the presence of the corresponding unnatural amino acids instead of methionine (114-116). As an elegant application, the azide group served as a tag to introduce a GlcNAc moiety via a triazole linkage through the Cu (I)-catalyzed alkyne-azide cycloaddition. The sugar chain was then efficiently extended by an endoglycosidase-catalyzed transglycosylation to provide a glycoprotein with a more complex sugar moiety (117). Another method to introduce an aldehyde tag consists of two steps: the insertion of a five-residue consensus sequence (CXPXR, where X can be any other amino acids) at the glycosylation sites during recombinant expression and subsequent in situ oxidation of the Cys in the consensus CXPXR sequence to a formylglycine (fGly) residue by a formylglycine generating enzyme (FGE), which is co-expressed in the system (118, 119). FGE recognizes specifically the CXPXR sequence, permitting site-specific modification of the Cys residue. This approach was recently applied to site-specific glycosylation of human growth hormone (hGH) (120). Briefly, the consensus CXPXR sequence was introduced into hGH and oxidized in situ by the co-expressed FGE. The resulting aldehyde-bearing hGH was then reacted with synthetic aminooxy-glycans under an acidic condition (pH 3.5-3.8) to give a moderate yield of the glycosylated hGH. An important feature of such a ligation is that the oxime linkage could closely mimic the sugar-amino acid linkages found in natural N- and O-glycoproteins. The “tag and modify” technology allows a quick access to homogeneous glycoproteins and it should find wide applications for both fundamental research and probably biomedical applications. A drawback of this strategy is that unnatural sugar-amino acid linkages are introduced into the conjugate, which might not perfectly mimic the natural counterparts and could be potentially immunogenic if used in humans.
Since different tags could be selectively introduced at different sites in a protein by the abovementioned genetic approach, it becomes possible to introduce multiple distinct glycans and/or other functional groups in a given protein, through orthogonal chemo-selective ligations. A remarkable example was recently reported for the construction of a synthetic glycoprotein that functionally mimics the P-selectin glycoprotein ligand-1 (PSGL-1) (121). Two posttranslational modifications, including a sulfate group at Tyr-48 and a sialylated glycan attached at the Ser-57 of PSGL-1, are essential for the binding of PSGL-1 to P-selectin in the primary rolling/adhesion phases of the inflammatory response. The LacZ-type reporter enzyme, Sulfolobus solfataricus β-galactosidase (SSβG), was used as a bacterial scaffold protein to introduce a sulfotyrosine mimic group at position 439 and a sialylated glycan at position 43. This was achieved by expression of a ten-point (Met)10(Cys)1 to (Met43)1(Ile)9(Ser)1 SSβG mutant, which also contains an additional mutation at position 439 to introduce a Cys residue, in the Met-auxotrophic E. coli strain B834(DE3) in the presence of a Met analog. The expression provided a tagged TIM-barrel protein SSβG-Aha43-Cys439, which contains a thiol tag at site 439 and an azide tag at site 43. The tagged protein was selectively reacted with a novel Cys-modifying reagent Tyr-MTS to introduce the sulfotyrosine mimic at position 439, followed by a second orthogonal click chemistry with the alkyne-containing sialyl Lewis X to attach a sialylated glycan at position 43 (Figure 5b). Binding studies demonstrated a clear synergistic effect between the sulfotyrosine mimic and the sialoglycan for P-selectin recognition. Interestingly, the modified SSβG still maintains a LacZ type enzyme activity. This property was successfully used to detect in vivo inflammatory brain lesions by its specific recognition of P-selectin and subsequent enzymatic reactions for X-Gal tissue staining (121). This remarkable achievement will stimulate further interests in applying the “tag and modify” strategy for functional studies of posttranslational modifications.
CONCLUSIONS
A major challenge in functional glycomics studies and development of carbohydrate-based therapeutics is the structural micro-heterogeneity of glycoconjugates. Recent advances in host glycoengineering and in vitro chemoenzymatic glycosylation remodeling have made it possible to obtain a series of homogeneous, glycan-defined glycoproteins. In addition, bio-orthogonal site-specific glycosylation is emerging as an attractive strategy permitting a quick access to various glycosylated proteins for functional studies, although the unnatural linkages introduced might not always perfectly mimic the functions of the natural counterparts. These emerging technologies complement each other and can be combined to further expand our synthetic repertoire. Despite enormous progress in this field, many technical problems remain. In contrast to site-directed mutagenesis that permits site-directed alteration of amino acid residues in proteins at will, there is no practical chemical and biological means to discriminate different sites for introducing distinct glycans with natural linkages; the preparation of homogeneous O-glycoproteins is lagging behind; extensive mechanistic and genetic studies on the glycosylation network (particularly the secretory pathways) are needed for glycoengineering in non-mammalian host system in order to have a perfect control of the outcome; new enzymes and mutants with improved efficiency and more flexibility in taking various glycans are needed for protein glycosylation remodeling; and, finally, inventing new concepts to permit site-specific chemical glycosylation of proteins through native sugar-amino acid linkages should be chemists’ next challenge. With synergetic efforts from both chemists and biologists, we can expect another wave of new advances in this exciting field in the next few years.
KEYWORDS.
Glycoprotein the covalent conjugate of a protein and a mono- or oligosaccharide
Glycoconjugate the covalent conjugate of a mono-/oligosaccharide with a non-sugar moiety such as a lipid, a peptide, and a protein.
Glycoform glycoprotein variants that possess the same polypeptide backbone but differ in the nature and site of glycosylation
Glycosylation the covalent attachment of a carbohydrate (usually through the reducing end) to a hydroxyl group or other functional groups in another molecule to form a glycosidic linkage.
Glycoengineering specific alteration of the glycan structures in a glycoconjugate by chemical and biological means.
Chemoenzymatic a combined chemical and enzymatic approach to the synthesis of natural and unnatural compounds.
Transglycosylation a glycohydrolase-catalyzed reaction in which the released sugar is transferred to an acceptor other than water to form a new glycoside.
ACKNOWLEDGEMENTS
This work was financially supported by the National Institutes of Health (NIH grants GM080374 and GM096973).
REFERENCES
- 1.Hart GW, Copeland RJ. Glycomics hits the big time. Cell. 2010;143:672–676. doi: 10.1016/j.cell.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Varki A. Biological roles of oligosaccharides: all of the theories are correct. Glycobiology. 1993;3:97–130. doi: 10.1093/glycob/3.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dwek RA. Glycobiology: Toward understanding the function of sugars. Chem. Rev. 1996;96:683–720. doi: 10.1021/cr940283b. [DOI] [PubMed] [Google Scholar]
- 4.Haltiwanger RS, Lowe JB. Role of glycosylation in development. Annu. Rev. Biochem. 2004;73:491–537. doi: 10.1146/annurev.biochem.73.011303.074043. [DOI] [PubMed] [Google Scholar]
- 5.Dube DH, Bertozzi CR. Glycans in cancer and inflammation--potential for therapeutics and diagnostics. Nat. Rev. Drug Discov. 2005;4:477–488. doi: 10.1038/nrd1751. [DOI] [PubMed] [Google Scholar]
- 6.Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat. Rev. Immunol. 2008;8:34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- 7.Jefferis R. Glycosylation as a strategy to improve antibody-based therapeutics. Nat. Rev. Drug Discov. 2009;8:226–234. doi: 10.1038/nrd2804. [DOI] [PubMed] [Google Scholar]
- 8.Helenius A, Aebi M. Intracellular functions of N-linked glycans. Science. 2001;291:2364–2369. doi: 10.1126/science.291.5512.2364. [DOI] [PubMed] [Google Scholar]
- 9.Spiro RG. Protein glycosylation: nature, distribution, enzymatic formation, and disease implications of glycopeptide bonds. Glycobiology. 2002;12:43R–56R. doi: 10.1093/glycob/12.4.43r. [DOI] [PubMed] [Google Scholar]
- 10.Zachara NE, Hart GW. The emerging significance of O-GlcNAc in cellular regulation. Chem. Rev. 2002;102:431–438. doi: 10.1021/cr000406u. [DOI] [PubMed] [Google Scholar]
- 11.Hart GW, Slawson C, Ramirez-Correa G, Lagerlof O. Cross talk between O-GlcNAcylation and phosphorylation: roles in signaling, transcription, and chronic disease. Annu. Rev. Biochem. 2011;80:825–858. doi: 10.1146/annurev-biochem-060608-102511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sethuraman N, Stadheim TA. Challenges in therapeutic glycoprotein production. Curr. Opin. Biotechnol. 2006;17:341–346. doi: 10.1016/j.copbio.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 13.Aggarwal S. What's fueling the biotech engine-2009-2010. Nat. Biotechnol. 2010;28:1165–1171. doi: 10.1038/nbt1110-1165. [DOI] [PubMed] [Google Scholar]
- 14.Kaneko Y, Nimmerjahn F, Ravetch JV. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science. 2006;313:670–673. doi: 10.1126/science.1129594. [DOI] [PubMed] [Google Scholar]
- 15.Anthony RM, Nimmerjahn F, Ashline DJ, Reinhold VN, Paulson JC, Ravetch JV. Recapitulation of IVIG anti-inflammatory activity with a recombinant IgG Fc. Science. 2008;320:373–376. doi: 10.1126/science.1154315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anthony RM, Wermeling F, Karlsson MC, Ravetch JV. Identification of a receptor required for the anti-inflammatory activity of IVIG. Proc. Natl. Acad. Sci. USA. 2008;105:19571–19578. doi: 10.1073/pnas.0810163105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chirino AJ, Mire-Sluis A. Characterizing biological products and assessing comparability following manufacturing changes. Nat. Biotechnol. 2004;22:1383–1391. doi: 10.1038/nbt1030. [DOI] [PubMed] [Google Scholar]
- 18.Schiestl M, Stangler T, Torella C, Cepeljnik T, Toll H, Grau R. Acceptable changes in quality attributes of glycosylated biopharmaceuticals. Nat. Biotechnol. 2011;29:310–312. doi: 10.1038/nbt.1839. [DOI] [PubMed] [Google Scholar]
- 19.Koeller KM, Wong CH. Emerging themes in medicinal glycoscience. Nat. Biotechnol. 2000;18:835–841. doi: 10.1038/78435. [DOI] [PubMed] [Google Scholar]
- 20.Herzner H, Reipen T, Schultz M, Kunz H. Synthesis of glycopeptides containing carbohydrate and Peptide recognition motifs. Chem. Rev. 2000;100:4495–4538. doi: 10.1021/cr990308c. [DOI] [PubMed] [Google Scholar]
- 21.Hang HC, Bertozzi CR. Chemoselective approaches to glycoprotein assembly. Acc. Chem. Res. 2001;34:727–736. doi: 10.1021/ar9901570. [DOI] [PubMed] [Google Scholar]
- 22.Davis BG. Synthesis of glycoproteins. Chem. Rev. 2002;102:579–601. doi: 10.1021/cr0004310. [DOI] [PubMed] [Google Scholar]
- 23.Grogan MJ, Pratt MR, Marcaurelle LA, Bertozzi CR. Homogeneous glycopeptides and glycoproteins for biological investigation. Annu. Rev. Biochem. 2002;71:593–634. doi: 10.1146/annurev.biochem.71.110601.135334. [DOI] [PubMed] [Google Scholar]
- 24.Wildt S, Gerngross TU. The humanization of N-glycosylation pathways in yeast. Nat. Rev. Microbiol. 2005;3:119–128. doi: 10.1038/nrmicro1087. [DOI] [PubMed] [Google Scholar]
- 25.Buskas T, Ingale S, Boons GJ. Glycopeptides as Versatile Tools for Glycobiology. Glycobiology. 2006;16:113R–136R. doi: 10.1093/glycob/cwj125. [DOI] [PubMed] [Google Scholar]
- 26.Bennett CS, Wong CH. Chemoenzymatic approaches to glycoprotein synthesis. Chem. Soc. Rev. 2007;36:1227–1238. doi: 10.1039/b617709c. [DOI] [PubMed] [Google Scholar]
- 27.Wang LX, Huang W. Enzymatic transglycosylation for glycoconjugate synthesis. Curr. Opin. Chem. Biol. 2009;13:592–600. doi: 10.1016/j.cbpa.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bernardes GJ, Castagner B, Seeberger PH. Combined approaches to the synthesis and study of glycoproteins. ACS Chem. Biol. 2009;4:703–713. doi: 10.1021/cb900014n. [DOI] [PubMed] [Google Scholar]
- 29.Gamblin DP, Scanlan EM, Davis BG. Glycoprotein synthesis: an update. Chem. Rev. 2009;109:131–163. doi: 10.1021/cr078291i. [DOI] [PubMed] [Google Scholar]
- 30.Rich JR, Withers SG. Emerging methods for the production of homogeneous human glycoproteins. Nat. Chem. Biol. 2009;5:206–215. doi: 10.1038/nchembio.148. [DOI] [PubMed] [Google Scholar]
- 31.Yuan Y, Chen J, Wan Q, Wilson RM, Danishefsky SJ. Toward fully synthetic, homogeneous glycoproteins: advances in chemical ligation. Biopolymers. 2010;94:373–384. doi: 10.1002/bip.21374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chalker JM, Bernardes GJ, Davis BG. A “Tag-and-Modify” Approach to Site-Selective Protein Modification. Acc. Chem. Res. 2011;44:730–741. doi: 10.1021/ar200056q. [DOI] [PubMed] [Google Scholar]
- 33.Schmaltz RM, Hanson SR, Wong CH. Enzymes in the synthesis of glycoconjugates. Chem. Rev. 2011;111:4259–4307. doi: 10.1021/cr200113w. [DOI] [PubMed] [Google Scholar]
- 34.Wang LX. The amazing transglycosylation activity of endo-beta-N-acetylglucosaminidases. Trends Glycosci. Glycotechnol. 2011;23:33–52. doi: 10.4052/tigg.23.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Macmillan D, Bertozzi CR. Modular assembly of glycoproteins: towards the synthesis of GlyCAM-1 by using expressed protein ligation. Angew. Chem. Int. Ed. 2004;43:1355–1359. doi: 10.1002/anie.200352673. [DOI] [PubMed] [Google Scholar]
- 36.Warren JD, Miller JS, Keding SJ, Danishefsky SJ. Toward fully synthetic glycoproteins by ultimately convergent routes: a solution to a long-standing problem. J. Am. Chem. Soc. 2004;126:6576–6578. doi: 10.1021/ja0491836. [DOI] [PubMed] [Google Scholar]
- 37.Yamamoto N, Tanabe Y, Okamoto R, Dawson PE, Kajihara Y. Chemical synthesis of a glycoprotein having an intact human complex-type sialyloligosaccharide under the Boc and Fmoc synthetic strategies. J. Am. Chem. Soc. 2008;130:501–510. doi: 10.1021/ja072543f. [DOI] [PubMed] [Google Scholar]
- 38.Piontek C, Ring P, Harjes O, Heinlein C, Mezzato S, Lombana N, Pohner C, Puttner M, Varon Silva D, Martin A, Schmid FX, Unverzagt C. Semisynthesis of a homogeneous glycoprotein enzyme: ribonuclease C: part 1. Angew. Chem. Int. Ed. 2009;48:1936–1940. doi: 10.1002/anie.200804734. [DOI] [PubMed] [Google Scholar]
- 39.Piontek C, Varon Silva D, Heinlein C, Pohner C, Mezzato S, Ring P, Martin A, Schmid FX, Unverzagt C. Semisynthesis of a homogeneous glycoprotein enzyme: ribonuclease C: part 2. Angew. Chem. Int. Ed. 2009;48:1941–1945. doi: 10.1002/anie.200804735. [DOI] [PubMed] [Google Scholar]
- 40.Nagorny P, Fasching B, Li X, Chen G, Aussedat B, Danishefsky SJ. Toward fully synthetic homogeneous beta-human follicle-stimulating hormone (beta-hFSH) with a biantennary N-linked dodecasaccharide. synthesis of beta-hFSH with chitobiose units at the natural linkage sites. J. Am. Chem. Soc. 2009;131:5792–5799. doi: 10.1021/ja809554x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tan Z, Shang S, Halkina T, Yuan Y, Danishefsky SJ. Toward homogeneous erythropoietin: non-NCL-based chemical synthesis of the Gln78-Arg166 glycopeptide domain. J. Am. Chem. Soc. 2009;131:5424–5431. doi: 10.1021/ja808704m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu CY, Wong CH. Chemistry and glycobiology. Chem. Commun. (Camb) 2011;47:6201–6207. doi: 10.1039/c0cc04359a. [DOI] [PubMed] [Google Scholar]
- 43.Hossler P, Khattak SF, Li ZJ. Optimal and consistent protein glycosylation in mammalian cell culture. Glycobiology. 2009;19:936–949. doi: 10.1093/glycob/cwp079. [DOI] [PubMed] [Google Scholar]
- 44.Patnaik SK, Stanley P. Lectin-resistant CHO glycosylation mutants. Methods Enzymol. 2006;416:159–182. doi: 10.1016/S0076-6879(06)16011-5. [DOI] [PubMed] [Google Scholar]
- 45.Esko JD, Stanley P. Glycosylation Mutants of Cultured Cells. In: Varki A, Cummings R, Esko J, Freeze H, Stanley P, Bertozzi C, Hart G, Etzler M, editors. Essentials of Glycobiology. Cold Spring Harbor Laboratory Press; Cold Spring Harbor (NY): 2009. pp. 649–660. 2010/03/20. [Google Scholar]
- 46.Chen W, Stanley P. Five Lec1 CHO cell mutants have distinct Mgat1 gene mutations that encode truncated N-acetylglucosaminyltransferase I. Glycobiology. 2003;13:43–50. doi: 10.1093/glycob/cwg003. [DOI] [PubMed] [Google Scholar]
- 47.Stefanich EG, Ren S, Danilenko DM, Lim A, Song A, Iyer S, Fielder PJ. Evidence for an asialoglycoprotein receptor on nonparenchymal cells for O-linked glycoproteins. J. Pharmacol. Exp. Ther. 2008;327:308–315. doi: 10.1124/jpet.108.142232. [DOI] [PubMed] [Google Scholar]
- 48.Shields RL, Lai J, Keck R, O'Connell LY, Hong K, Meng YG, Weikert SH, Presta LG. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. J. Biol. Chem. 2002;277:26733–26740. doi: 10.1074/jbc.M202069200. [DOI] [PubMed] [Google Scholar]
- 49.North SJ, Huang HH, Sundaram S, Jang-Lee J, Etienne AT, Trollope A, Chalabi S, Dell A, Stanley P, Haslam SM. Glycomics profiling of Chinese hamster ovary cell glycosylation mutants reveals N-glycans of a novel size and complexity. J. Biol. Chem. 2010;285:5759–5775. doi: 10.1074/jbc.M109.068353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reeves PJ, Callewaert N, Contreras R, Khorana HG. Structure and function in rhodopsin: high-level expression of rhodopsin with restricted and homogeneous N-glycosylation by a tetracycline-inducible N-acetylglucosaminyltransferase I-negative HEK293S stable mammalian cell line. Proc. Natl. Acad. Sci. USA. 2002;99:13419–13424. doi: 10.1073/pnas.212519299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Crispin M, Chang VT, Harvey DJ, Dwek RA, Evans EJ, Stuart DI, Jones EY, Lord JM, Spooner RA, Davis SJ. A human embryonic kidney 293T cell line mutated at the Golgi alpha-mannosidase II locus. J. Biol. Chem. 2009;284:21684–21695. doi: 10.1074/jbc.M109.006254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chang VT, Crispin M, Aricescu AR, Harvey DJ, Nettleship JE, Fennelly JA, Yu C, Boles KS, Evans EJ, Stuart DI, Dwek RA, Jones EY, Owens RJ, Davis SJ. Glycoprotein structural genomics: solving the glycosylation problem. Structure. 2007;15:267–273. doi: 10.1016/j.str.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou Q, Shankara S, Roy A, Qiu H, Estes S, McVie-Wylie A, Culm-Merdek K, Park A, Pan C, Edmunds T. Development of a simple and rapid method for producing non-fucosylated oligomannose containing antibodies with increased effector function. Biotechnol. Bioeng. 2008;99:652–665. doi: 10.1002/bit.21598. [DOI] [PubMed] [Google Scholar]
- 54.Scanlan CN, Ritchie GE, Baruah K, Crispin M, Harvey DJ, Singer BB, Lucka L, Wormald MR, Wentworth P, Jr., Zitzmann N, Rudd PM, Burton DR, Dwek RA. Inhibition of mammalian glycan biosynthesis produces non-self antigens for a broadly neutralising, HIV-1 specific antibody. J. Mol. Biol. 2007;372:16–22. doi: 10.1016/j.jmb.2007.06.027. [DOI] [PubMed] [Google Scholar]
- 55.Doores KJ, Burton DR. Variable loop glycan dependency of the broad and potent HIV-1-neutralizing antibodies PG9 and PG16. J. Virol. 2010;84:10510–10521. doi: 10.1128/JVI.00552-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang Q, Li C, Wei Y, Huang W, Wang LX. Expression, glycoform characterization, and antibody-binding of HIV-1 V3 glycopeptide domain fused with human IgG1-Fc. Bioconjugate Chem. 2010;21:875–883. doi: 10.1021/bc9004238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weikert S, Papac D, Briggs J, Cowfer D, Tom S, Gawlitzek M, Lofgren J, Mehta S, Chisholm V, Modi N, Eppler S, Carroll K, Chamow S, Peers D, Berman P, Krummen L. Engineering Chinese hamster ovary cells to maximize sialic acid content of recombinant glycoproteins. Nat. Biotechnol. 1999;17:1116–1121. doi: 10.1038/15104. [DOI] [PubMed] [Google Scholar]
- 58.Fukuta K, Abe R, Yokomatsu T, Kono N, Asanagi M, Omae F, Minowa MT, Takeuchi M, Makino T. Remodeling of sugar chain structures of human interferon-gamma. Glycobiology. 2000;10:421–430. doi: 10.1093/glycob/10.4.421. [DOI] [PubMed] [Google Scholar]
- 59.Goh JS, Zhang P, Chan KF, Lee MM, Lim SF, Song Z. RCA-I-resistant CHO mutant cells have dysfunctional GnT I and expression of normal GnT I in these mutants enhances sialylation of recombinant erythropoietin. Metab. Eng. 2010;12:360–368. doi: 10.1016/j.ymben.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 60.Davies J, Jiang L, Pan LZ, LaBarre MJ, Anderson D, Reff M. Expression of GnTIII in a recombinant anti-CD20 CHO production cell line: Expression of antibodies with altered glycoforms leads to an increase in ADCC through higher affinity for FC gamma RIII. Biotechnol. Bioeng. 2001;74:288–294. [PubMed] [Google Scholar]
- 61.Ferrara C, Brunker P, Suter T, Moser S, Puntener U, Umana P. Modulation of therapeutic antibody effector functions by glycosylation engineering: influence of Golgi enzyme localization domain and co-expression of heterologous beta1, 4-N-acetylglucosaminyltransferase III and Golgi alpha-mannosidase II. Biotechnol. Bioeng. 2006;93:851–861. doi: 10.1002/bit.20777. [DOI] [PubMed] [Google Scholar]
- 62.Gemmill TR, Trimble RB. Overview of N- and O-linked oligosaccharide structures found in various yeast species. Biochim. Biophys. Acta. 1999;1426:227–237. doi: 10.1016/s0304-4165(98)00126-3. [DOI] [PubMed] [Google Scholar]
- 63.Chiba Y, Jigami Y. Production of humanized glycoproteins in bacteria and yeasts. Curr. Opin. Chem. Biol. 2007;11:670–676. doi: 10.1016/j.cbpa.2007.08.037. [DOI] [PubMed] [Google Scholar]
- 64.Choi BK, Bobrowicz P, Davidson RC, Hamilton SR, Kung DH, Li H, Miele RG, Nett JH, Wildt S, Gerngross TU. Use of combinatorial genetic libraries to humanize N-linked glycosylation in the yeast Pichia pastoris. Proc. Natl. Acad. Sci. USA. 2003;100:5022–5027. doi: 10.1073/pnas.0931263100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Davidson RC, Nett JH, Renfer E, Li H, Stadheim TA, Miller BJ, Miele RG, Hamilton SR, Choi BK, Mitchell TI, Wildt S. Functional analysis of the ALG3 gene encoding the Dol-P-Man: Man(5)GlcNAc(2)-PP-Dol mannosyltransferase enzyme of P-pastoris. Glycobiology. 2004;14:399–407. doi: 10.1093/glycob/cwh023. [DOI] [PubMed] [Google Scholar]
- 66.Vervecken W, Kaigorodov V, Callewaert N, Geysens S, De Vusser K, Contreras R. In vivo synthesis of mammalian-like, hybrid-type N-glycans in Pichia pastoris. Appl. Environ. Microbiol. 2004;70:2639–2646. doi: 10.1128/AEM.70.5.2639-2646.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hamilton SR, Bobrowicz P, Bobrowicz B, Davidson RC, Li H, Mitchell T, Nett JH, Rausch S, Stadheim TA, Wischnewski H, Wildt S, Gerngross TU. Production of complex human glycoproteins in yeast. Science. 2003;301:1244–1246. doi: 10.1126/science.1088166. [DOI] [PubMed] [Google Scholar]
- 68.Bobrowicz P, Davidson RC, Li H, Potgieter TI, Nett JH, Hamilton SR, Stadheim TA, Miele RG, Bobrowicz B, Mitchell T, Rausch S, Renfer E, Wildt S. Engineering of an artificial glycosylation pathway blocked in core oligosaccharide assembly in the yeast Pichia pastoris: production of complex humanized glycoproteins with terminal galactose. Glycobiology. 2004;14:757–766. doi: 10.1093/glycob/cwh104. [DOI] [PubMed] [Google Scholar]
- 69.Hamilton SR, Davidson RC, Sethuraman N, Nett JH, Jiang Y, Rios S, Bobrowicz P, Stadheim TA, Li H, Choi BK, Hopkins D, Wischnewski H, Roser J, Mitchell T, Strawbridge RR, Hoopes J, Wildt S, Gerngross TU. Humanization of yeast to produce complex terminally sialylated glycoproteins. Science. 2006;313:1441–1443. doi: 10.1126/science.1130256. [DOI] [PubMed] [Google Scholar]
- 70.Li H, Sethuraman N, Stadheim TA, Zha D, Prinz B, Ballew N, Bobrowicz P, Choi BK, Cook WJ, Cukan M, Houston-Cummings NR, Davidson R, Gong B, Hamilton SR, Hoopes JP, Jiang Y, Kim N, Mansfield R, Nett JH, Rios S, Strawbridge R, Wildt S, Gerngross TU. Optimization of humanized IgGs in glycoengineered Pichia pastoris. Nat. Biotechnol. 2006;24:210–215. doi: 10.1038/nbt1178. [DOI] [PubMed] [Google Scholar]
- 71.Choi BK, Actor JK, Rios S, d'Anjou M, Stadheim TA, Warburton S, Giaccone E, Cukan M, Li H, Kull A, Sharkey N, Gollnick P, Kocieba M, Artym J, Zimecki M, Kruzel ML, Wildt S. Recombinant human lactoferrin expressed in glycoengineered Pichia pastoris: effect of terminal N-acetylneuraminic acid on in vitro secondary humoral immune response. Glycoconj. J. 2008;25:581–593. doi: 10.1007/s10719-008-9123-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jacobs PP, Geysens S, Vervecken W, Contreras R, Callewaert N. Engineering complex-type N-glycosylation in Pichia pastoris using GlycoSwitch technology. Nat. Protoc. 2009;4:58–70. doi: 10.1038/nprot.2008.213. [DOI] [PubMed] [Google Scholar]
- 73.Amano K, Chiba Y, Kasahara Y, Kato Y, Kaneko MK, Kuno A, Ito H, Kobayashi K, Hirabayashi J, Jigami Y, Narimatsu H. Engineering of mucin-type human glycoproteins in yeast cells. Proc. Natl. Acad. Sci. USA. 2008;105:3232–3237. doi: 10.1073/pnas.0710412105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cox KM, Sterling JD, Regan JT, Gasdaska JR, Frantz KK, Peele CG, Black A, Passmore D, Moldovan-Loomis C, Srinivasan M, Cuison S, Cardarelli PM, Dickey LF. Glycan optimization of a human monoclonal antibody in the aquatic plant Lemna minor. Nat. Biotechnol. 2006;24:1591–1597. doi: 10.1038/nbt1260. [DOI] [PubMed] [Google Scholar]
- 75.Strasser R, Stadlmann J, Schahs M, Stiegler G, Quendler H, Mach L, Glossl J, Weterings K, Pabst M, Steinkellner H. Generation of glyco-engineered Nicotiana benthamiana for the production of monoclonal antibodies with a homogeneous human-like N-glycan structure. Plant Biotechnol. J. 2008;6:392–402. doi: 10.1111/j.1467-7652.2008.00330.x. [DOI] [PubMed] [Google Scholar]
- 76.Castilho A, Gattinger P, Grass J, Jez J, Pabst M, Altmann F, Gorfer M, Strasser R, Steinkellner H. N-glycosylation engineering of plants for the biosynthesis of glycoproteins with bisected and branched complex N-glycans. Glycobiology. 2011;21:813–823. doi: 10.1093/glycob/cwr009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Castilho A, Strasser R, Stadlmann J, Grass J, Jez J, Gattinger P, Kunert R, Quendler H, Pabst M, Leonard R, Altmann F, Steinkellner H. In planta protein sialylation through overexpression of the respective mammalian pathway. J. Biol. Chem. 2010;285:15923–15930. doi: 10.1074/jbc.M109.088401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Witte K, Sears P, Martin R, Wong CH. Enzymatic glycoprotein synthesis: Preparation of ribonuclease glycoforms via enzymatic glycopeptide condensation and glycosylation. J. Am. Chem. Soc. 1997;119:2114–2118. [Google Scholar]
- 79.Wang LX. Chemoenzymatic synthesis of glycopeptides and glycoproteins through endoglycosidase-catalyzed transglycosylation. Carbohydr. Res. 2008;343:1509–1522. doi: 10.1016/j.carres.2008.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fujita M, Shoda S, Haneda K, Inazu T, Takegawa K, Yamamoto K. A novel disaccharide substrate having 1,2-oxazoline moiety for detection of transglycosylating activity of endoglycosidases. Biochim. Biophys. Acta. 2001;1528:9–14. doi: 10.1016/s0304-4165(01)00164-7. [DOI] [PubMed] [Google Scholar]
- 81.Li B, Zeng Y, Hauser S, Song H, Wang LX. Highly efficient endoglycosidase-catalyzed synthesis of glycopeptides using oligosaccharide oxazolines as donor substrates. J. Am. Chem. Soc. 2005;127:9692–9693. doi: 10.1021/ja051715a. [DOI] [PubMed] [Google Scholar]
- 82.Li H, Li B, Song H, Breydo L, Baskakov IV, Wang LX. Chemoenzymatic Synthesis of HIV-1 V3 Glycopeptides Carrying Two N-Glycans and Effects of Glycosylation on the Peptide Domain. J. Org. Chem. 2005;70:9990–9996. doi: 10.1021/jo051729z. [DOI] [PubMed] [Google Scholar]
- 83.Rising TW, Claridge TD, Davies N, Gamblin DP, Moir JW, Fairbanks AJ. Synthesis of N-glycan oxazolines: donors for endohexosaminidase catalysed glycosylation. Carbohydr. Res. 2006;341:1574–1596. doi: 10.1016/j.carres.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 84.Zeng Y, Wang J, Li B, Hauser S, Li H, Wang LX. Glycopeptide synthesis through endo-glycosidase-catalyzed oligosaccharide transfer of sugar oxazolines: probing substrate structural requirement. Chem. Eur. J. 2006;12:3355–3364. doi: 10.1002/chem.200501196. [DOI] [PubMed] [Google Scholar]
- 85.Rising TW, Heidecke CD, Moir JW, Ling Z, Fairbanks AJ. Endohexosaminidase-catalysed glycosylation with oxazoline donors: fine tuning of catalytic efficiency and reversibility. Chem. Eur. J. 2008;14:6444–6464. doi: 10.1002/chem.200800365. [DOI] [PubMed] [Google Scholar]
- 86.Rising TW, Claridge TD, Moir JW, Fairbanks AJ. Endohexosaminidase M: exploring and exploiting enzyme substrate specificity. ChemBioChem. 2006;7:1177–1180. doi: 10.1002/cbic.200600183. [DOI] [PubMed] [Google Scholar]
- 87.Li B, Song H, Hauser S, Wang LX. A highly efficient chemoenzymatic approach toward glycoprotein synthesis. Org. Lett. 2006;8:3081–3084. doi: 10.1021/ol061056m. [DOI] [PubMed] [Google Scholar]
- 88.Ochiai H, Huang W, Wang LX. Expeditious chemoenzymatic synthesis of homogeneous N-glycoproteins carrying defined oligosaccharide ligands. J. Am. Chem. Soc. 2008;130:13790–13803. doi: 10.1021/ja805044x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Umekawa M, Huang W, Li B, Fujita K, Ashida H, Wang LX, Yamamoto K. Mutants of Mucor hiemalis endo-beta-N-acetylglucosaminidase show enhanced transglycosylation and glycosynthase-like activities. J. Biol. Chem. 2008;283:4469–4479. doi: 10.1074/jbc.M707137200. [DOI] [PubMed] [Google Scholar]
- 90.Huang W, Li C, Li B, Umekawa M, Yamamoto K, Zhang X, Wang LX. Glycosynthases enable a highly efficient chemoenzymatic synthesis of N-glycoproteins carrying intact natural N-glycans. J. Am. Chem. Soc. 2009;131:2214–2223. doi: 10.1021/ja8074677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Heidecke CD, Ling Z, Bruce NC, Moir JW, Parsons TB, Fairbanks AJ. Enhanced glycosylation with mutants of endohexosaminidase A (endo A) ChemBioChem. 2008;9:2045–2051. doi: 10.1002/cbic.200800214. [DOI] [PubMed] [Google Scholar]
- 92.Ling Z, Suits MD, Bingham RJ, Bruce NC, Davies GJ, Fairbanks AJ, Moir JW, Taylor EJ. The X-ray crystal structure of an Arthrobacter protophormiae endo-beta-N-acetylglucosaminidase reveals a (beta/alpha)(8) catalytic domain, two ancillary domains and active site residues key for transglycosylation activity. J. Mol. Biol. 2009;389:1–9. doi: 10.1016/j.jmb.2009.03.050. [DOI] [PubMed] [Google Scholar]
- 93.Yin J, Li L, Shaw N, Li Y, Song JK, Zhang W, Xia C, Zhang R, Joachimiak A, Zhang HC, Wang LX, Liu ZJ, Wang P. Structural basis and catalytic mechanism for the dual functional endo-beta-N-acetylglucosaminidase A. PLoS One. 2009;4:e4658. doi: 10.1371/journal.pone.0004658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Huang W, Yang Q, Umekawa M, Yamamoto K, Wang LX. Arthrobacter endo-beta-N-acetylglucosaminidase shows transglycosylation activity on complex-type N-glycan oxazolines: one-pot conversion of ribonuclease B to sialylated ribonuclease C. ChemBioChem. 2010;11:1350–1355. doi: 10.1002/cbic.201000242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Umekawa M, Higashiyama T, Koga Y, Tanaka T, Noguchi M, Kobayashi A, Shoda S, Huang W, Wang LX, Ashida H, Yamamoto K. Efficient transfer of sialo-oligosaccharide onto proteins by combined use of a glycosynthase-like mutant of Mucor hiemalis endoglycosidase and synthetic sialo-complex-type sugar oxazoline. Biochim. Biophys. Acta. 2010;1800:1203–1209. doi: 10.1016/j.bbagen.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 96.Huang W, Zhang X, Ju T, Cummings RD, Wang LX. Expeditious chemoenzymatic synthesis of CD52 glycopeptide antigens. Org. Biomol. Chem. 2010;8:5224–5233. doi: 10.1039/c0ob00341g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Huang W, Wang D, Yamada M, Wang LX. Chemoenzymatic synthesis and lectin array characterization of a class of N-glycan clusters. J. Am. Chem. Soc. 2009;131:17963–17971. doi: 10.1021/ja9078539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Amin MN, Huang W, Mizanur RM, Wang LX. Convergent Synthesis of Homogeneous Glc(1)Man(9)GlcNAc(2)-Protein and Derivatives as Ligands of Molecular Chaperones in Protein Quality Control. J. Am. Chem. Soc. 2011;133:14404–14417. doi: 10.1021/ja204831z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Umekawa M, Li C, Higashiyama T, Huang W, Ashida H, Yamamoto K, Wang LX. Efficient glycosynthase mutant derived from Mucor hiemalis endo-beta-N-acetylglucosaminidase capable of transferring oligosaccharide from both sugar oxazoline and natural N-glycan. J. Biol. Chem. 2010;285:511–521. doi: 10.1074/jbc.M109.059832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Parsons TB, Patel MK, Boraston AB, Vocadlo DJ, Fairbanks AJ. Streptococcus pneumoniae endohexosaminidase D; feasibility of using N-glycan oxazoline donors for synthetic glycosylation of a GlcNAc-asparagine acceptor. Org. Biomol. Chem. 2010;8:1861–1869. doi: 10.1039/b926078a. [DOI] [PubMed] [Google Scholar]
- 101.Huang W, Li J, Wang LX. Unusual transglycosylation activity of Flavobacterium meningosepticum Endoglycosidases enables convergent chemoenzymatic synthesis of core fucosylated complex N-glycopeptides. ChemBioChem. 2011;12:932–941. doi: 10.1002/cbic.201000763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Szymanski CM, Yao R, Ewing CP, Trust TJ, Guerry P. Evidence for a system of general protein glycosylation in Campylobacter jejuni. Mol. Microbiol. 1999;32:1022–1030. doi: 10.1046/j.1365-2958.1999.01415.x. [DOI] [PubMed] [Google Scholar]
- 103.Young NM, Brisson JR, Kelly J, Watson DC, Tessier L, Lanthier PH, Jarrell HC, Cadotte N, St Michael F, Aberg E, Szymanski CM. Structure of the N-linked glycan present on multiple glycoproteins in the Gram-negative bacterium, Campylobacter jejuni. J. Biol. Chem. 2002;277:42530–42539. doi: 10.1074/jbc.M206114200. [DOI] [PubMed] [Google Scholar]
- 104.Wacker M, Linton D, Hitchen PG, Nita-Lazar M, Haslam SM, North SJ, Panico M, Morris HR, Dell A, Wren BW, Aebi M. N-linked glycosylation in Campylobacter jejuni and its functional transfer into E. coli. Science. 2002;298:1790–1793. doi: 10.1126/science.298.5599.1790. [DOI] [PubMed] [Google Scholar]
- 105.Kowarik M, Numao S, Feldman MF, Schulz BL, Callewaert N, Kiermaier E, Catrein I, Aebi M. N-linked glycosylation of folded proteins by the bacterial oligosaccharyltransferase. Science. 2006;314:1148–1150. doi: 10.1126/science.1134351. [DOI] [PubMed] [Google Scholar]
- 106.Schwarz F, Huang W, Li C, Schulz BL, Lizak C, Palumbo A, Numao S, Neri D, Aebi M, Wang LX. A combined method for producing homogeneous glycoproteins with eukaryotic N-glycosylation. Nat. Chem. Biol. 2010;6:264–266. doi: 10.1038/nchembio.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wei Y, Li C, Huang W, Li B, Strome S, Wang LX. Glycoengineering of human IgG1-Fc through combined yeast expression and in vitro chemoenzymatic glycosylation. Biochemistry. 2008;47:10294–10304. doi: 10.1021/bi800874y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zou G, Ochiai H, Huang W, Yang Q, Li C, Wang LX. Chemoenzymatic synthesis and Fcgamma receptor binding of homogeneous glycoforms of antibody Fc domain. Presence of a bisecting sugar moiety enhances the affinity of Fc to FcgammaIIIa receptor. J. Am. Chem. Soc. 2011;133:18975–18991. doi: 10.1021/ja208390n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Macmillan D, Bill RM, Sage KA, Fern D, Flitsch SL. Selective in vitro glycosylation of recombinant proteins: semi- synthesis of novel homogeneous glycoforms of human erythropoietin. Chem. Biol. 2001;8:133–145. doi: 10.1016/s1074-5521(00)90065-6. [DOI] [PubMed] [Google Scholar]
- 110.Hirano K, Macmillan D, Tezuka K, Tsuji T, Kajihara Y. Design and synthesis of a homogeneous erythropoietin analogue with two human complex-type sialyloligosaccharides: combined use of chemical and bacterial protein expression methods. Angew. Chem. Int. Ed. 2009;48:9557–9560. doi: 10.1002/anie.200904376. [DOI] [PubMed] [Google Scholar]
- 111.Watt GM, Lund J, Levens M, Kolli VS, Jefferis R, Boons GJ. Site-specific glycosylation of an aglycosylated human IgG1-Fc antibody protein generates neoglycoproteins with enhanced function. Chem. Biol. 2003;10:807–814. doi: 10.1016/j.chembiol.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 112.Rendle PM, Seger A, Rodrigues J, Oldham NJ, Bott RR, Jones JB, Cowan MM, Davis BG. Glycodendriproteins: a synthetic glycoprotein mimic enzyme with branched sugar-display potently inhibits bacterial aggregation. J. Am. Chem. Soc. 2004;126:4750–4751. doi: 10.1021/ja031698u. [DOI] [PubMed] [Google Scholar]
- 113.Liu H, Wang L, Brock A, Wong CH, Schultz PG. A method for the generation of glycoprotein mimetics. J. Am. Chem. Soc. 2003;125:1702–1703. doi: 10.1021/ja029433n. [DOI] [PubMed] [Google Scholar]
- 114.Kiick KL, Saxon E, Tirrell DA, Bertozzi CR. Incorporation of azides into recombinant proteins for chemoselective modification by the Staudinger ligation. Proc. Natl. Acad. Sci. USA. 2002;99:19–24. doi: 10.1073/pnas.012583299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang A, Winblade Nairn N, Johnson RS, Tirrell DA, Grabstein K. Processing of N-terminal unnatural amino acids in recombinant human interferon-beta in Escherichia coli. ChemBioChem. 2008;9:324–330. doi: 10.1002/cbic.200700379. [DOI] [PubMed] [Google Scholar]
- 116.Wiltschi B, Wenger W, Nehring S, Budisa N. Expanding the genetic code of Saccharomyces cerevisiae with methionine analogues. Yeast. 2008;25:775–786. doi: 10.1002/yea.1632. [DOI] [PubMed] [Google Scholar]
- 117.Fernández-González M, Boutureira O, Bernardes GJ, Chalker JM, Young MA, Errey JC, Davis BG. Site-selective chemoenzymatic construction of synthetic glycoproteins using endoglycosidases. Chem. Sci. 2010;1:709–715. [Google Scholar]
- 118.Carrico IS, Carlson BL, Bertozzi CR. Introducing genetically encoded aldehydes into proteins. Nat. Chem. Biol. 2007;3:321–322. doi: 10.1038/nchembio878. [DOI] [PubMed] [Google Scholar]
- 119.Wu P, Shui W, Carlson BL, Hu N, Rabuka D, Lee J, Bertozzi CR. Site-specific chemical modification of recombinant proteins produced in mammalian cells by using the genetically encoded aldehyde tag. Proc. Natl. Acad. Sci. USA. 2009;106:3000–3005. doi: 10.1073/pnas.0807820106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hudak JE, Yu HH, Bertozzi CR. Protein glycoengineering enabled by the versatile synthesis of aminooxy glycans and the genetically encoded aldehyde tag. J. Am. Chem. Soc. 2011;133:16127–16135. doi: 10.1021/ja206023e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.van Kasteren SI, Kramer HB, Jensen HH, Campbell SJ, Kirkpatrick J, Oldham NJ, Anthony DC, Davis BG. Expanding the diversity of chemical protein modification allows post-translational mimicry. Nature. 2007;446:1105–1109. doi: 10.1038/nature05757. [DOI] [PubMed] [Google Scholar]