Abstract
Compared to level walking, additional muscle actions are required to raise and lower the center of mass during uphill and downhill walking, respectively. However, it remains unclear which muscle recruitment strategies are employed at typical grades when walking over a range of speeds. Based on previous reports, we hypothesized that, across a range of walking speeds, hip, knee, and ankle extensor muscle activations increase with steeper uphill grade, but only knee extensor muscle activations increase with steeper downhill grade. We also hypothesized that these changes in muscle activations with grade become more pronounced with faster walking speed. To test these hypotheses, ten young adult subjects (5M/5F) walked on a standard treadmill at seven grades (0, ±3, ±6, and ±9°) and three speeds (0.75, 1.25, and 1.75 m·s−1). We quantified the stance phase electromyographic activities of the gluteus maximus (GMAX), biceps femoris (BF), rectus femoris (RF), vastus medialis (VM), medial gastrocnemius (MG), and soleus (SOL) muscles. On average, compared to level walking, hip (BF: 635%, GMAX: 345%), knee (RF: 165%, VM: 366%), and ankle (MG: 175%, SOL: 136%) extensor muscle activities increased to walk up 9°, but only knee (RF: 310%, VM: 246%) extensor muscle activities increased to walk down 9°. Further, these changes in muscle activations with grade became greater with faster walking speed. We conclude that people employ distinct uphill (hip, knee, and ankle extensors) and downhill (knee extensors) muscle recruitment strategies generally across walking speeds and progressively with steeper grade.
Keywords: Uphill, Downhill, EMG, Electromyography, Muscle Activity
1. Introduction
The ability to appropriately recruit leg muscles in response to changes in the environment is fundamental to the control of human locomotion. For example, many of our daily activities routinely include walking up and down hills which involve very different biomechanics [1, 2]. Electromyographic (EMG) recordings can provide insight into how the neuromuscular system makes these adjustments. A handful of studies have investigated muscle activations during uphill or downhill walking and have provided important insight into the muscle recruitment strategies employed [3–7]. However, most have done so at a single, self-selected walking speed, and recent evidence also suggests that faster walking speeds elicit greater increases in thigh muscle activations with steeper uphill grade [8]. We sought to build upon these studies to obtain a more comprehensive understanding of how leg muscle activations change when walking up and downhill over a range of walking speeds.
The major leg muscles are most active during the stance phase of walking [9], when they perform work on the center of mass and support body weight. Compared to level walking, additional muscle actions are required to raise and lower the center of mass during uphill and downhill walking, respectively. Lay and colleagues [4] recorded leg muscle EMG signals for walking at extreme uphill and downhill grades (21.3°). They surmised that the leg extensor muscles in particular meet these demands presumably through greater concentric activity to walk uphill and greater eccentric activity to walk downhill (though see [10]). More specifically, Lay et al. [4] reported that when walking at the same speed, hip, knee, and ankle (plantarflexor) extensor muscle activations increased during uphill walking, but only the knee extensor muscle activations increased during downhill walking. However, it remains unclear whether such muscle recruitment strategies occur generally over a range of walking speeds, and/or at more moderate and typical grades.
When walking faster over level ground, the normalized temporal patterns of leg muscle activity remain fairly stable but with increasing amplitudes [11–15]. Is this also the case when walking up or downhill? Recently, Wall-Scheffler et al. [8] found that stance phase quadriceps muscle activity increased during uphill walking to a greater extent when walking faster. This finding demonstrates an interaction between grade and walking speed for muscles of the thigh when walking uphill, but it is not known if the same trend generally occurs in other leg muscles during both uphill and downhill walking. A more comprehensive understanding of how leg muscle activations change with grade and speed could help guide the development of therapies aimed at improving the quality of life and maintaining the independence of people with walking ability limitations due to injury, disease, or aging (e.g. [16]). A first step in this direction is to establish these muscle activation patterns for healthy, young adults.
The purpose of our study was to quantify leg extensor muscle activations during the stance phase of level, uphill, and downhill walking at various speeds. We hypothesized that over a range of walking speeds, compared to level walking, 1. hip, knee, and ankle extensor muscle activations would increase with steeper uphill grade, but 2. only knee extensor muscle activations would increase with steeper downhill grade. We also hypothesized that, 3. the changes in muscle activations with grade would become more pronounced with faster walking speed. To test these hypotheses, we recorded leg extensor muscle activations while subjects walked on a treadmill on the level, at a range of uphill and downhill grades, and at various speeds.
2. Methods
2.1 Subjects
Ten young adults (5F/5M) volunteered for this study (mean ± standard deviation, age: 25.3 ± 3.9 years; height: 1.73 ± 0.10 m; mass: 69.2 ± 13.1 kg). All subjects were experienced treadmill users and had no known neuromuscular, cardiovascular, and orthopedic diseases.. Each subject gave written informed consent before participating as per the University of Colorado Institutional Review Board.
2.2 Experimental Protocol
Subjects walked on a classic motorized treadmill (model 18–60, Quinton Instruments, Seattle, WA) set to 1.25 m·s−1 and level for 5 min prior to testing. We modified this treadmill to provide calibrated, digital electronic readouts for velocity and grade. Subjects then completed 21, one-minute walking trials at seven grades (0, ±3, ±6, and ±9°) and three speeds (0.75, 1.25, and 1.75 m·s−1). Grade-speed combinations were conducted in an individualized random order for each subject. We recorded EMG and temporal stride kinematics during the final 15 s of each trial.
2.3 Measurement and Data Analysis
We recorded surface electromyographic (EMG) signals at 2000 Hz from the following muscles of the right leg: gluteus maximus (GMAX), biceps femoris (BF), rectus femoris (RF), vastus medialis (VM), medial gastrocnemius (MG), and soleus (SOL). After preparing the shaved skin with fine sandpaper and alcohol, we placed pre-amplified single differential electrodes (Trigno, Delsys, Inc., Boston MA) over the muscle bellies according to recommendations of Cram and Kasman [17]. We verified electrode position and signal quality by visually inspecting the EMG signals while subjects contracted each instrumented muscle. To synchronize EMG signals to the timing of gait cycle events, we detected heelstrikes and toe-offs with pressure sensitive insoles worn in the subject’s right shoe, sampled at 1000Hz (B&L Engineering, Tustin, CA). We employed a threshold of 5% of the peak insole voltage during stance to identify each heelstrike and toe-off.
We used a custom script written in MATLAB (MathWorks, Inc., Natick, MA) to process the EMG data. The Delsys hardware automatically bandpass filtered all EMG signals (20 – 450 Hz). We defined baseline muscle activity as two standard deviations above the activity observed while the subjects relaxed in a supine position. The MATLAB script full-wave rectified and normalized the EMG data to the mean amplitudes over a complete stride of level walking at 1.25 m·s−1. This script interpolated the normalized and rectified EMG data to obtain 1001 data points corresponding to the gait cycle (right heel-strike to right heel-strike). For each subject, we then computed the mean normalized EMG activity during specific portions of the stance phase for each experimental condition. Corresponding to the primary EMG bursts during stance, we calculated these means from heelstrike to midstance for GMAX, BF, RF, and VM and from midstance to toe-off for MG and SOL. We visually confirmed that these temporal boundaries included the primary stance phase EMG bursts for each muscle across all walking speeds and grades. The timing of gait cycle events obtained from the pressure sensitive insole provided the average stride frequency (SF) and stance time (tstance).
2.4 Statistical Analysis
We calculated mean values of stride frequency, stance time, and all EMG measures over eight consecutive strides. A two-factor (grade × speed) analysis of variance (ANOVA) for repeated measures tested for significant effects of grade and speed (p<0.05). When a significant main effect or grade-speed interaction was found, we performed post-hoc comparisons with a Bonferroni adjusted level of significance for the six dependent EMG variables and two dependent kinematic variables (0.05/8, p<0.0063). Post-hoc comparisons were focused between level and all uphill and downhill walking conditions and between walking speeds.
3. Results
As expected, stance phase muscle activity amplitudes generally increased with faster walking speed for all grades tested (Figures 1–3, Table S1). Also, as walking speed increased, subjects took progressively faster strides, and spent less time in stance (Table 1, p<0.0063). Figures 1–3 display the mean EMG values and example EMG profiles across all conditions for muscles acting to extend the hip, knee, and ankle, respectively. Table S1 summarizes these values. All muscles showed significant grade and speed effects, and all muscles except GMAX showed significant grade-speed interactions (p<0.05).
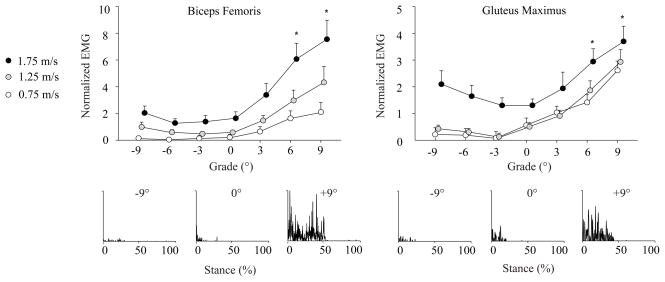
Figure 1.
Mean (SE) EMG signals for muscles acting to extend the hip (GMAX and BF) during the first half of stance normalized to the mean activity during level walking at 1.25 m·s−1 (Mean ± SE). Representative rectified EMG data are also provided for a single stride of one subject walking at 1.25 m·s−1 on the level, uphill (+9°), and downhill (−9°), each on the same scale. Compared to level walking, GMAX and BF activities increased at uphill grades steeper than 3° across the walking speeds tested, but were not different when walking downhill (p<0.0063). Note: 1.25 and 1.75 m·s−1 points are shifted slightly to the right for clarity. * Significantly different from level walking across speeds according to post-hoc comparisons with Bonferroni adjusted level of significance (p < 0.0063).
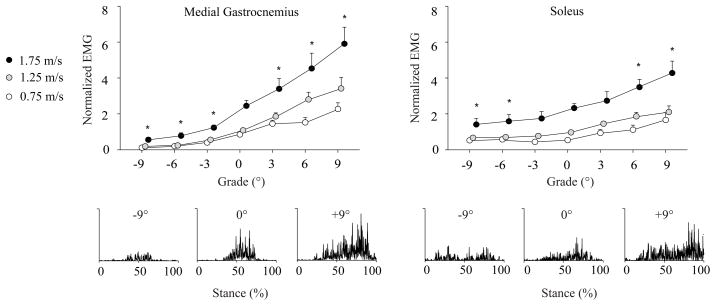
Figure 3.
Mean (SE) EMG signals for muscles acting to extend the ankle (MG and SOL) during the second half of stance normalized to the mean activity during level walking at 1.25 m·s−1. Representative rectified EMG data are also provided for a single stride of one subject walking at 1.25 m·s−1 on the level, uphill (+9°), and downhill (−9°), each on the same scale. Compared to level walking, muscle activity increased at all uphill grades for MG and steeper than 6° for SOL (p<0.0063). When walking downhill, muscle activity decreased at all grades for MG, and for grades steeper than 6° for SOL. Note: 1.25 and 1.75 m·s−1 points are shifted slightly to the right for clarity. * Significantly different from level walking across speeds according to post-hoc comparisons with Bonferroni adjusted level of significance (p < 0.0063).
Table 1.
Mean (SE) stride frequency and stance time during uphill and downhill walking over a range of speeds.
| Speed (m·s−1) | Downhill
|
0° | Uphill
|
|||||
|---|---|---|---|---|---|---|---|---|
| −9° | −6° | −3° | 3° | 6° | 9° | |||
| * | * | * | ||||||
| SF (Hz)† | 0.75 | 0.85 (0.01) | 0.83 (0.01) | 0.79 (0.01) | 0.74 (0.01) | 0.71 (0.02) | 0.70 (0.02) | 0.73 (0.02) |
| 1.25 | 0.99 (0.01) | 0.98 (0.01) | 0.95 (0.01) | 0.93 (0.01) | 0.91 (0.01) | 0.91 (0.02) | 0.92 (0.01) | |
| 1.75 | 1.10 (0.02) | 1.08 (0.02) | 1.06 (0.02) | 1.05 (0.01) | 1.05 (0.01) | 1.06 (0.02) | 1.07 (0.02) | |
| * | * | |||||||
| tstance (s)† | 0.75 | 0.72 (0.04) | 0.73 (0.05) | 0.78 (0.06) | 0.82 (0.04) | 0.86 (0.07) | 0.89 (0.09) | 0.83 (0.08) |
| 1.25 | 0.60 (0.04) | 0.62 (0.04) | 0.64 (0.04) | 0.64 (0.03) | 0.66 (0.04) | 0.65 (0.05) | 0.65 (0.04) | |
| 1.75 | 0.52 (0.02) | 0.55 (0.04) | 0.56 (0.02) | 0.56 (0.02) | 0.55 (0.03) | 0.57 (0.05) | 0.55 (0.05) | |
SF = Stride frequency; tstance = Stance time. Compared to level walking, subjects took progressively faster strides at all downhill grades, and spent less time in stance at downhill grades steeper than 3° (p<0.0063). These stride parameters were not significantly different when walking uphill. Asterisks (*) indicate significantly different from level walking.
Significant main effect of speed.
3.1 Uphill walking
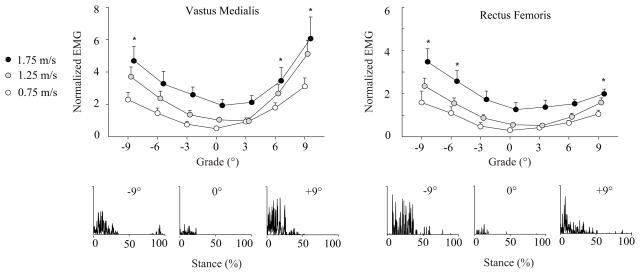
Stance phase hip, knee, and ankle extensor muscle activities progressively increased with steeper uphill grade for all the walking speeds tested (p<0.05, Figures 1–3, Table S1). Post-hoc comparisons revealed that, compared to level walking, these increases were statistically significant at all uphill grades for MG, at grades steeper than 3° for GMAX, BF, VM, and SOL, and steeper than 6° for RF (p<0.0063). All significant grade-speed interactions except that for RF showed that the increases in muscle activities with steeper uphill grade became progressively greater with faster walking speed. Compared to level walking, stride frequency (p = 0.03, 0.08, 0.83) and tstance (p = 0.15, 0.08, 0.82 for 3, 6, 9°, respectively) were not significantly different for uphill walking.
3.2 Downhill walking
For all the walking speeds tested, only the stance phase knee extensor muscle activities (RF and VM) increased with steeper downhill grade (p<0.05, Figure 2, Table S1). Compared to level walking, activity increased significantly at downhill grades steeper than 3° for RF and steeper than 6° for VM (p < 0.0063). Significant grade-speed interactions revealed that the increases in RF and VM activities with steeper downhill grade were progressively greater at faster walking speeds. Across the walking speeds tested, activity was significantly reduced at all downhill grades for MG and grades steeper than 3° for SOL (p < 0.0063). GMAX and BF activities for downhill walking were not different from level walking. Compared to level walking, subjects took progressively faster strides at all downhill grades, and spent less time in stance at grades steeper than 3° (p<0.0063).
Figure 2.
Mean (SE) EMG signals for muscles acting to extend the knee (VM and RF) during the first half of stance normalized to the mean activity during level walking at 1.25 m·s−1. Representative rectified EMG data are also provided for a single stride of one subject walking at 1.25 m·s−1 on the level, uphill (+9°), and downhill (−9°), each on the same scale. Compared to level walking, muscle activity increased at uphill grades steeper than 3° for VM and steeper than 6° for RF (p<0.0063). When walking downhill, muscle activity increased at grades steeper than 3° for RF and steeper than 6° for VM. Note: 1.25 and 1.75 m·s−1 points are shifted slightly to the right for clarity. * Significantly different from level walking across speeds according to post-hoc comparisons with Bonferroni adjusted level of significance (p < 0.0063).
Discussion
This study quantified leg extensor muscle activations during the stance phase of level, uphill, and downhill walking over a range of speeds. Compared to level walking, hip, knee, and ankle extensor muscle activations increased to walk uphill but only knee extensor muscle activations increased to walk downhill. This study expands upon our understanding of muscle activities during uphill and downhill walking to show that these muscle recruitment strategies occur generally over a range of walking speeds and progressively with steeper grades representative of those routinely encountered in daily activities. For those grades and speeds which coincide with previous studies, we found general agreement between our EMG data and published findings [3, 4, 8, 12–15].
First, we accept our hypothesis that hip, knee, and ankle extensor muscle activations would increase with steeper uphill grade across a range of walking speeds. Consistent with prior reports for extremely steep uphill grades [4], muscles acting to extend the hip, knee, and ankle joints all contribute to raising the body’s center of mass when walking uphill. In the present study, compared to level walking, hip extensor muscle activities increased considerably more (GMAX: 345%, BF: 635%) to walk up 9° than those of the ankle extensors (SOL: 136%, MG: 175%). This finding suggests a pronounced role of proximal extensor muscles to walk uphill, and is consistent with biomechanical studies that demonstrate the greatest increases in extensor moments and power generation when walking uphill occur at the hip [1, 2]. Our results may have important implications for elderly adults, who preferentially recruit hip extensor muscles even during level walking [18, 19]. Monaco et al. [19] have proposed that this age-related change in muscle recruitment is a compensation for decreased ankle extension strength. Such a hip-reliant recruitment strategy may be inadequate to meet the demands of uphill walking.
Second, we accept our hypothesis that only knee extensor muscle activations would increase with steeper downhill grade across a range of walking speeds. Thus, even at a fast walking speed, only the muscles acting to control flexion of the knee contribute to lowering the body’s CoM when walking downhill. On average, compared to level walking, the 310% increase in RF activity to walk down 9° was comparable to the 246% increase of VM. This recruitment strategy, which differs substantially from that adopted to walking uphill, occurs consistently across speed and, as shown by Lay et al. [4], even at extreme downhill grades. These results suggest that downhill walking may be particularly challenging for individuals suffering from quadriceps muscle atrophy and knee extension weakness (e.g. [20]).
Many researchers have shown that leg extensor muscle activations increase when walking faster over level ground [11–15]. Our study demonstrates that a similar trend generally occurs when walking faster at various uphill and downhill grades. Moreover, our results support our third hypothesis, that the changes in muscle recruitment that occur during uphill or downhill walking would become more pronounced at faster speeds. Consistent with previous studies of level walking [11–13], these findings suggest that rather than altering the muscle recruitment strategies adopted to walk uphill or downhill, faster walking speeds act to increase the “gains” of these strategies.
In general, the pairs of extensor muscles investigated at each leg joint responded similarly to changes in grade and walking speed. However, we did observe several notable differences. At the ankle, MG activity increased considerably more with steeper uphill grade than SOL activity except when walking very slowly (1.25 m·s−1: 217% vs. 116%, 1.75 m·s−1: 141% vs. 84%). Thus, at typical walking speeds, MG may play a more important role than SOL to raise the CoM when walking uphill. It is also possible that the biarticular MG is used to stabilize the knee during uphill walking. Although the two knee extensors responded similarly when walking downhill, we observed on average a much more pronounced increase in VM activity (366%) compared to RF activity (165%) with steeper uphill grade. Compared to the biarticular RF, people may recruit the VM, a uniarticular knee extensor, to a greater extent to more selectively extend the knee during the first half of stance when walking uphill. Finally, compared to slower walking speeds, walking at 1.75 m·s−1 appeared to elicit greater GMAX activity when walking downhill (figure 1). A secondary statistical comparison revealed that at each downhill grade, GMAX activity was significantly greater at 1.75 m·s−1 than at 0.75 m·s−1 or 1.25 m·s−1 (p<0.004). This greater activity may be necessary when walking quickly downhill to control hip flexing during initial stance.
In the present study, we used EMG techniques to obtain insight into the strategies adopted by the neuromuscular system to walk uphill and downhill over a range of speeds. However, one limitation of our study is that the mechanical behavior of muscle-tendon complexes cannot be directly inferred from EMG data alone. The actions of the biarticular BF, RF, and MG are particularly difficult to resolve. For example, intuitively we might expect that the increase in ankle extensor muscle activity when walking uphill reflects an increase in concentric action of the medial gastrocnemius (MG). However, Lichtwark and Wilson [10], using ultrasound techniques, demonstrated that the MG fascicles generate force nearly isometrically when walking at moderate uphill and downhill grades (5.7°). Further, in addition to raising or lowering the body’s CoM, individual leg extensor muscles likely perform multiple tasks when walking up or downhill which can be difficult to discern. For example, when walking over level ground, MG contributes to forward propulsion, swing initiation, and supporting body weight [21]. Future studies might better characterize muscular contributions to walking uphill and downhill by using musculoskeletal simulations (e.g. [22]). Finally, our findings are limited to healthy young adults and may not reflect the muscle recruitment strategies adopted by people with a variety of walking challenges (e.g., injury, disease, or aging).
In this study we investigated the effects of grade and speed on leg muscle activations during walking in healthy young adults. We conclude that hip, knee, and ankle extensor muscle activations increase to walk uphill but only knee extensor muscle activations increase to walk downhill. People employ these distinct strategies generally across walking speeds and progressively with steeper grade. Future studies could determine whether these muscle recruitment strategies are impaired in people with walking ability limitations due to injury, disease, or aging, for whom uphill and downhill walking can represent critical barriers to functional independence.
Supplementary Material
Acknowledgments
A grant from NIH (5T32AG000279) and a student Grant-in-Aid Award from the American Society of Biomechanics awarded to J.R.F. supported this study.
Footnotes
Conflicts of Interest
The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lay AN, Hass CJ, Gregor RJ. The effects of sloped surfaces on locomotion: A kinematic and kinetic analysis. Journal of Biomechanics. 2006;39:1621–8. doi: 10.1016/j.jbiomech.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 2.McIntosh AS, Beatty KT, Dwan LN, Vickers DR. Gait dynamics on an inclined walkway. Journal of Biomechanics. 2006;39:2491–2502. doi: 10.1016/j.jbiomech.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 3.Arendt-Nielsen L, Sinkjaer T, Nielsen J, Kallesoe K. Electromyographic patterns and knee joint kinematics during walking at various speeds. Journal of Electromyography & Kinesiology. 1991;1(2):89–95. doi: 10.1016/1050-6411(91)90002-M. [DOI] [PubMed] [Google Scholar]
- 4.Lay AN, Hass CJ, Nichols RT, Gregor RJ. The effects of sloped surfaces on locomotion: an electromyographic analysis. Journal of Biomechanics. 2007;40(6):1276–85. doi: 10.1016/j.jbiomech.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 5.Leroux A, Fung J, Barbeau H. Adaptation of the walking pattern to uphill walking in normal and spinal-cord injured subjects. Experimental Brain Research. 1999;126(3):359–68. doi: 10.1007/s002210050743. [DOI] [PubMed] [Google Scholar]
- 6.Patla AE. Effects of walking on various inclines on EMG patterns of lower limb muscles in humans. Human Movement Science. 1986;5:345–57. [Google Scholar]
- 7.Tokuhiro A, Nagashima H, Takechi H. Electromyographic kinesiology of lower extremity muscles during slope walking. Archives of Physical Medicine & Rehabilitation. 1985;66(9):610–3. [PubMed] [Google Scholar]
- 8.Wall-Scheffler CM, Chumanov E, Steudel-Numbers K, Heiderscheit B. Electromyography activity across gait and incline: The impact of muscular activity on human morphology. American Journal of Physical Anthropology. 2010;143(4):601–11. doi: 10.1002/ajpa.21356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Basmajian JV, De Luca C. Muscles Alive: Their Function Revealed by Electromyography. Baltimore: Williams & Wilkins; 1985. [Google Scholar]
- 10.Lichtwark GA, Wilson AM. Interactions between the human gastrocnemius muscle and the Achilles tendon during incline, level and decline locomotion. Journal of Experimental Biology. 2006;209:4379–88. doi: 10.1242/jeb.02434. [DOI] [PubMed] [Google Scholar]
- 11.Cappellini G, Ivanenko YP, Poppele RE, Lacquaniti F. Motor patterns in human walking and running. Journal of Neurophysiology. 2006;95(6):3426–37. doi: 10.1152/jn.00081.2006. [DOI] [PubMed] [Google Scholar]
- 12.den Otter AR, Geurts AC, Mulder T, Duysens J. Speed related changes in muscle activity from normal to very slow walking speeds. Gait and Posture. 2004;19(3):270–8. doi: 10.1016/S0966-6362(03)00071-7. [DOI] [PubMed] [Google Scholar]
- 13.Hof AL, Elzinga H, Grimmius W, Halbertsma JP. Speed dependence of averaged EMG profiles in walking. Gait and Posture. 2002;16(1):78–86. doi: 10.1016/s0966-6362(01)00206-5. [DOI] [PubMed] [Google Scholar]
- 14.Murray MP, Mollinger LA, Gardner GM, Sepic SB. Kinematic and EMG patterns during slow, free, and fast walking. Journal of Orthopaedic Research. 1984;2(3):272–80. doi: 10.1002/jor.1100020309. [DOI] [PubMed] [Google Scholar]
- 15.Nilsson J, Thorstensson A, Halbertsma J. Changes in leg movements and muscle activity with speed of locomotion and mode of progression in humans. Acta Physiologica Scandinavica. 1985;123(4):457–75. doi: 10.1111/j.1748-1716.1985.tb07612.x. [DOI] [PubMed] [Google Scholar]
- 16.Liu CK, Fielding RA. Exercise as an intervention for frailty. Clinics in Geriatric Medicine. 2011;27(1):101–10. doi: 10.1016/j.cger.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cram JR, Kasman GS. Introduction to surface electromyography. Gaithersburg: Aspen Publishers; 1998. [Google Scholar]
- 18.DeVita P, Hortobagyi T. Age causes a redistribution of joint torques and powers during gait. Journal of Applied Physiology. 2000;88(5):1804–11. doi: 10.1152/jappl.2000.88.5.1804. [DOI] [PubMed] [Google Scholar]
- 19.Monaco V, Rinaldi LA, Macri G, Micera S. During walking elders increase efforts at proximal joints and keep low kinetics at the ankle. Clinical Biomechanics. 2009;24(6):493–8. doi: 10.1016/j.clinbiomech.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 20.Trappe TA, Lindquist DM, Carrithers JA. Muscle-specific atrophy of the quadriceps femoris with aging. Journal of Applied Physiology. 2001;90(6):2070–4. doi: 10.1152/jappl.2001.90.6.2070. [DOI] [PubMed] [Google Scholar]
- 21.McGowan CP, Kram R, Neptune RR. Modulation of leg muscle function in response to altered demand for body support and forward propulsion during walking. Journal of Biomechanics. 2009;42(7):850–6. doi: 10.1016/j.jbiomech.2009.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Delp SL, Anderson FC, Arnold AS, Loan P, Habib A, John CT, Guendelman E, Thelen DG. OpenSim: open-source software to create and analyze dynamic simulations of movement. IEEE Transactions on Biomedical Engineering. 2007;54(11):1940–50. doi: 10.1109/TBME.2007.901024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.