Abstract
Self-reactive natural antibodies initiate injury following ischemia and reperfusion of certain tissues, but their role in ischemic stroke is unknown. We investigated neoepitope expression in the post-ischemic brain, and the role of natural antibodies in recognizing these epitopes and mediating complement-dependent injury. A novel IgM mAb recognizing a subset of phospholipids (C2) and a previously characterized anti-annexin IV mAb (B4) were used to reconstitute and characterize injury in antibody deficient Rag1−/− mice after 60 minutes of middle cerebral artery occlusion and reperfusion. Reconstitution with C2 or B4 mAb in otherwise protected Rag1−/− mice restored injury to that seen in wild-type mice, as demonstrated by infarct volume, demyelination and neurological scoring. IgM deposition was demonstrated in both wild-type mice and reconstituted Rag1−/− mice, and IgM co-localized with the complement activation fragment, C3d, following B4 mAb reconstitution. Further, recombinant annexin IV significantly reduced infarct volumes in wild-type mice and in Rag1−/− mice administered normal mouse serum, demonstrating that a single antibody reactivity is sufficient to develop cerebral ischemia reperfusion injury in the context of an entire natural antibody repertoire. Finally, C2 and B4 mAbs bound to hypoxic, but not normoxic, human endothelial cells in vitro. Thus, the binding of pathogenic natural IgM to post-ischemic neoepitopes initiates complement-dependent injury following murine cerebral ischemia and reperfusion and, based also on previous data investigating IgM reactivity in human serum, there appears to be a similar recognition system in both mouse and man.
Introduction
A number of events occur during tissue ischemia, and the pathophysiology of ischemia reperfusion injury (IRI) 4 is complex. Clinical and animal studies have established a causal role for complement in IRI of various organs and tissues(1), including the brain following ischemic stroke (2). Cleavage of complement component C3 is a central event in complement activation, and studies with C3 deficient (3) and inhibited (4) mice have revealed a key role for complement in murine ischemic stroke. Complement can be activated via the classical, lectin and alternative pathways, and recent data indicate a central role for the lectin pathway in ischemic stroke (5, 6).
In models of intestinal (7, 8), myocardial (9) and skeletal muscle (10) IRI, complement activation is triggered by natural circulating IgM that recognizes cellular neoepitopes that become exposed as a result of ischemia. These pathogenic natural antibodies recognize a restricted antigen repertoire and are mainly the product of B-1 lymphocytes in mice and humans (11), and are constitutively expressed throughout life. Although IgM bound to reperfused tissue recruits both C1q and mannose binding lectin (MBL) (classical and lectin pathway recognition molecules, respectively) (12, 13), IRI has been shown to be dependent on MBL binding to IgM, at least with regard to intestinal and myocardial IRI (14–16). Seminal studies by Zhang et al (7) showed that antibody-deficient Rag1−/− mice were resistant to intestinal IRI, and that natural self-reactive IgM restored IRI. Subsequently, the target of a clonally specific monoclonal Ab that reconstituted intestinal IRI in Rag1−/− mice was identified as non-muscle myosin (17), and the same antibody has since been shown to also restore myocardial and skeletal muscle IRI in Rag1−/− mice (9, 18). However, even though a peptide mimic of non-muscle myosin can block IRI in wild type (wt) mice (9, 19), it is clear that other targets for self-reactive Abs exist, at least in the post-ischemic intestine. In this context, intestinal IRI in Rag1−/− mice can also be restored by administration of an anti-annexin IV IgM mAb (8), or the combined administration of anti-phospholipid and anti-β-2-glycoprotein 1 mAbs (20).
Thus, whereas current evidence indicate that multiple cellular neoepitopes are exposed post-ischemia, the predominance of their expression in different tissues, as well as the relative contributions of different targets and self-reactive Abs in driving IRI in different tissues, is not known. Here we identify a novel IgM mAb that recognizes a subset of phospholipids, and show that this mAb, as well as a previously characterized anti-annexin IV mAb, recognizes post-ischemic neoepitopes in the brain, activates complement, and propagates cerebral IRI.
Materials and Methods
C2 mAb isolation and purification
The C2 mAb hybridoma was isolated following the fusion of spleen cells from unmanipulated wt C57BL/6 mice as described previously (8). Briefly, splenocytes from healthy C57BL/6 mice were fused with the SP2/0-AG14 myeloma cell line by standard protocol to establish hybridomas. The hybridomas were then screened by both Western blot analysis using intestine epithelial cell lysates, and by flow cytometric analysis of isolated intestine epithelial cells. Positive wells were further sub-cloned until a monoclonal population was obtained. To purify mAbs, Ab from the exhausted supernatants of cultured hybridomas was affinity purified on a column of agarose beads with goat anti-human IgM (Sigma-Aldrich, St. Louis, MO). Bound mAb was eluted with a buffer containing 0.1M glycine, pH 2.3, and collected into a buffer containing 1.5M Tris, pH 8.8. Eluted mAb was dialyzed against PBS, pH 7.4, for 48 h and concentrated using centrifugal filtration on Centricon Plus-20 (Millipore, Billerica, MA). Ab concentration was determined by measuring the A280 of the sample and purity was confirmed by analysis on a 10% SDS-PAGE gel.
Characterization of C2 mAb and anti-phospholipid antibodies in mouse serum
ELISAs to determine reactivity of Abs to various phospholipids were performed using microtiter plates (Immulon 1B, Dynatech Laboratories, Chatilly, VA) coated with 100μl/well of 50μg/ml phospholipid in methanol. The plates were dried under blowing air to allow the organic solvent to evaporate, and the wells then washed with PBS and blocked with 1% BSA. Supernatant from the mAb hybridoma cell lines was added to wells and bound antibody detected by alkaline phosphatase conjugated goat anti-mouse IgM (Jackson ImmunoResearch Labs, West Grove, PA). For detection of Abs in serum, serial dilutions of mouse serum samples prepared in RPMI containing 10% FBS were added to wells coated with phospholipids, and bound antibody detected with AP-conjugated anti-mouse IgG or anti-mouse IgM Ab, followed by p-nitrophenylphosphate (Sigma-Aldrich) at 1 mg/ml. Relative units (R.U.) of antibody were calculated by comparing OD at 405 nm for individual titrated serum with standard curve of OD measurements of titrated standard high titer polyclonal anti-phospholipid hybridoma supernatants. High titer polyclonal supernatants were generated during the isolation of the C2 mAb hybridoma; they represent polyclonal wells that screened positive for binding to synthetic phospholipids after fusion, and contained IgG and IgM Abs. Phospholipids assayed: (PS)-1,2-Distearoyl-sn-Glycerol-3-[Phospho-L-Serine] (Avanti Polar-lipids, Inc., Alabaster, AL), Cardiolipin from bovine heart, (PE)-1,2-diacyl-sn-glycero-3-posphoethanolamine, (PG)-1,2-Diacyl-sn-glycero-3-phospho-(1-rac-glycerol) from yolk lecithin, and PC(10)-BSA (Biosearch Technologies, Inc., Novato, CA).
Mice
Adult male C57BL/6 and Rag1−/− mice on the C57BL/6 background were obtained from the Jackson Laboratory (Ben Harbor, ME) and allowed to acclimate 7 days before use. Animal studies were approved by the Medical University of South Carolina.
Middle cerebral artery occlusion
Eight-week-old male mice were anesthetized with chloral hydrate (350mg/kg) and xylazine (4mg/kg) i.p. prior to performing middle cerebral artery occlusion (MCAO) as previously described (4). Briefly, a blunted 4-0 nylonsuture was passed through the internal carotid artery to occlude the middle cerebral artery. After 60 minutes ischemia, the suture was removed for a 24h period of reperfusion before sacrifice, as consistent with previous literature (3, 4, 21, 22). Blood pressure, temperature, and ipsilateral cerebral blood flow(measured by laser Doppler), were measured before, during and after ischemia as previously described (23, 24). Mice with ischemic blood flow less than 25% were included in our studies. Mice receiving normal mouse serum were administered 300μl freshly isolated pooled C57BL/6 serum by tail vein injection at the time of reperfusion. Rag1−/− mice for mAb reconstitution experiments were randomized into different mAb treatment groups and were administered mAb or control i.v. by tail vein injection at the time of reperfusion. Operators were blinded to experimental groups.
Measurement of infarct volume
After 24h of reperfusion, mice were sacrificed by isofluorane overdose and cervical dislocation. Brains were perfused transcardially with PBS before their removal, and then placed in a Rodent Brain Matrix (EMS, Hatfield, PA). Brains were cut into 2mm coronal sections and stained with 2% triphenyltetrazolium chloride (TTC)(25). Percent infarct for each section was determined using NIH Image Analysis Software, and total infarct volume was calculated by summation of infarct areas of all brain slices for each hemisphere. Mice not surviving to 24h were not analyzed for infarct volume.
Neurological Deficit
Neurological deficit was determined, independent and blinded, as described (26). Scoring was assigned as follows: 0, normal motor function; 1, torso and contralateral forelimb flexion when lifted by tail; 2, contralateral circling when held by tail on flat surface, though normal at rest; 3, contralateral leaning when at rest; 4, no spontaneous motor activity.
Histopathology
Brains were sectioned using a Rodent Brain Matrix and placed in 4% paraformaldehyde for 48h at 4°C. Brains were then either processed to paraffin or immersed in 20% sucrose in paraformaldehyde and embedded in OCT medium for cryosectioning. Paraffin sections were stained with Luxol Fast Blue/Nissl stain for morphological analysis, as previously described (4).
Immunohistochemistry
Paraffin sections were cut at 8μm and deparaffinized. Sections were exposed to heated citrate buffer, pH 6.0, for two 10-minute cycles for antigen retrieval and blocked with normal horse serum (Vector Labs, Burlingame, CA). C3d deposition was detected using a goat anti-mouse C3d (1:20, R&D Systems, Minneapolis, MN), and IgM binding was detected using a goat anti-mouse IgM (1:50, Sigma-Aldrich). Primary antibodies were detected using the goat-IMMpress (Vector Labs), and negative controls omitted primary antibodies. Slides were coversliped with Cytoseal-60 (Richard-Allan Scientific, Kalamazoo, MI) and imaged by light microscopy.
Immunofluorescence
Cryosections were cut at 8μm, fixed in cold acetone, washed in running water and equilibrated in PBS. Double staining for C3d and IgM deposition was performed. Goat anti-mouse C3d (1:20, R&D Systems) was applied and detected with rat anti-goat IgG AlexaFluor-555 conjugate (1:200, Invitrogen, Carlsbad, CA). After washing with PBS, anti-mouse IgM FITC conjugate (1:50, Sigma-Aldrich) was applied, followed by ToPro-3 (1:5000, Invitrogen) as a nuclear marker. Slides were coversliped with Vecta fluorescent hard mount (Vector Labs) and imaged on a Leica TCS-SP2 confocal microscope.
Annexin IV purification and treatment
Recombinant annexin IV was generated and purified as previously described (8). In one experiment, annexin IV was administered i.v. to C57BL/6 mice (100μg in 100μl PBS) 5 min prior to reperfusion. In a second experiment, annexin IV (100μg in 100μl PBS) was administered i.v. to Rag1−/− mice 5 min prior to infusion of 300μl of freshly isolated normal mouse serum, which was administered just prior to reperfusion. Briefly, recombinant protein was expressed in transformed E. coli by 0.3mM isopropyl β-D-thiogalactoside. Bacteria were collected and lysed, and following centrifugation, the supernatant was adjusted to pH 7.6 and run on a TALON resin column (BD Clontech, Mountain View, CA). A discontinuous urea gradient was used to refold the protein, and an imidazole gradient was used to eluted the protein. Coomassie staining confirmed recombinant protein purity.
In vitro binding of IgM
Mouse brain endothelial cells (bEnd.3, ATCC, Manassas, VA) and human endothelial cells (HUVEC, Lonza, Walkersville, MD) were grown to at least 80% confluence in Endothelial Growth Media(Lonza) on chambered microscope slides (Nunc, Rochester, NY). Slides were washed with PBS and incubated in DMEM serum-free medium for 3h in a Coy Anaerobic Chamber with O2 monitoring, in no more than 0.1% oxygen. The medium was then supplemented with IgM mAb (15μg/ml) and incubation continued under normoxic cell culture conditions for 3h. The slides were fixed in cold acetone, and IgM was detected with an IgG anti-mouse IgM-FITC (1:100, Sigma-Aldrich). Slides were coversliped with Vecta fluorescent hard mount (Vector Labs) and imaged on Leica TCS-SP2 confocal microscope. Difficulties in detaching viable cells after hypoxic culture precluded analysis by flow cytometry.
Statistical Analysis
Statistical analysis was done using Prism 4 (Graphpad Software Inc). Infarct volumes were compared using ANOVA or Student’s t test and neurological deficits were compared using Kruskal-Wallis or Mann-Whitney test, as applicable. Value of p< 0.05 was considered significant.
Results
C2 mAb recognizes a subset of phospholipids
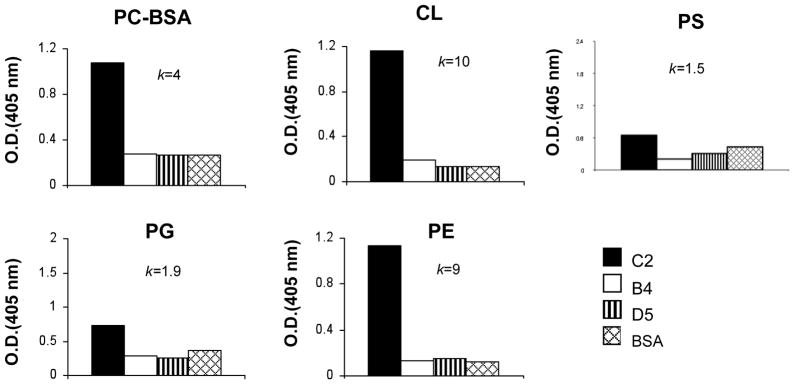
Kulik et al (8) previously reported a strategy to isolate and identify mAbs that bind to neoepitopes exposed on ischemic cells. The strategy involved the use of isolated intestinal epithelial cells expressing ischemia or apoptosis associated neoepitopes to screen and identify hybridomas created from B cells of wt C57BL/6 mice (8). Here we characterize 3 of the IgM mAbs isolated by this technique (D5, B4 and C2) in a model of murine ischemic stroke. The specificity of two of these mAbs has been previously determined (8); B4 mAb recognizes annexin IV, and D5 mAb, used in this study as an isotype control Ab, recognizes cytokeratin 19. C2 mAb did not react in Western blots of intestinal epithelial cells (not shown), and since altered phospholipid exposure on apoptotic/ischemic cells has been suggested as a target for pathogenic natural Abs (20, 27), we investigated C2 mAb specificity in anti-phospholipid ELISAs. C2 was shown to recognize a subset of phospholipids that included phosphatidylcholine, phosphatidylethanonlamine and cardiolipin, but not phosphatidylglycerol or phosphatidylserine (Figure 1). B4 mAb and D5 mAb did not recognize any phospholipid tested (Figure 1).
Figure 1.
Binding of IgM mAbs to phospholipids. Antibody binding was detected by ELISA as described in the text. Phospholipids analyzed: phosphorylcholine-BSA (PC-BSA), cardiolipin (CL), phosphatidylethanolamine (PE), phosphotidylglycerol (PG) and phosphatidylserine (PS). Negative control was BSA. K is proportion of OD405(C2) to OD405(BSA) and data shown is representative of two independent experiments.
Monoclonal Abs B4 and C2 restore injury in Rag1−/− mice following ischemic stroke
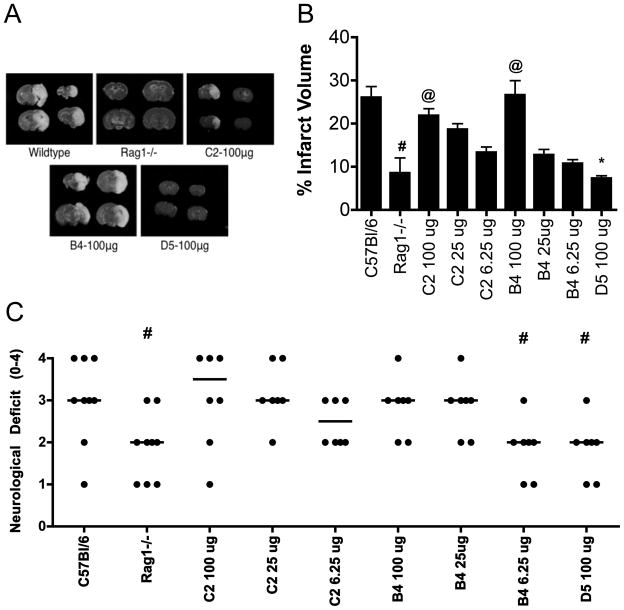
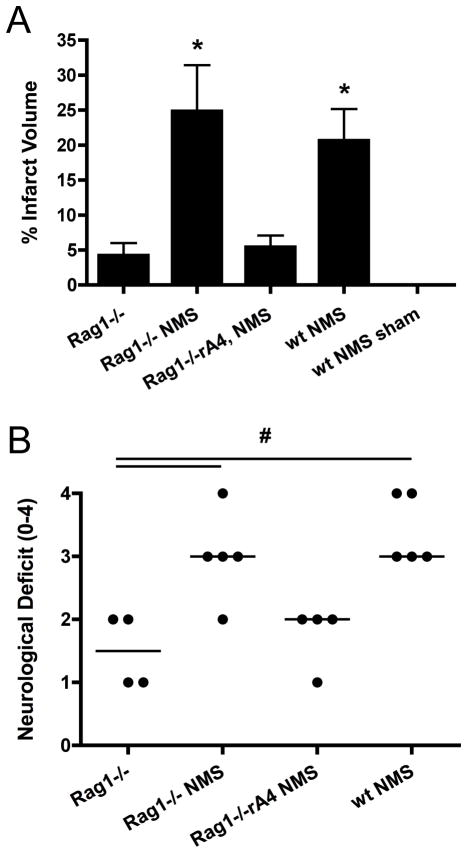
C57BL/6 wt and antibody-deficient Rag1−/− mice were subjected to 1h MCAO-induced cerebral ischemia followed by 24h reperfusion. Rag1−/− mice showed significantly improved survival at 24h compared to wt controls, with 100% (18/18) and 59% (10/16) of Rag1−/− and wt mice surviving, respectively (p=0.006). Infarct size was also significantly reduced 24h post-reperfusion in Rag1−/− mice compared to surviving wt mice (Figure 2b), and this is in agreement with previous data (21, 22). Rag1−/− mice also displayed an improved neurological function (Figure 2c), correlating with the reduction of infarct volume.
Figure 2.
Effect of Rag1 deficiency and IgM mAb reconstitution on post-ischemic infarct size and neurological deficit. (A) Representative TTC staining of gross brains 24h following ischemic stroke. (B) Rag1−/− mice had significantly reduced infarct volume compared to wt C57BL/6 mice following 60-minute MCAO and 24 hours reperfusion. Reconstitution with C2 or B4 mAb restored infarct size in a dose dependent manner. D5 mAb did not restore injury in Rag1−/− mice. #p< 0.0012 versus C57BL/6, @p< 0.001 versus Rag1−/−, *p< 0.0011 versus B4 100μg; n= 16 (C57BL/6 and Rag1−/−) and n= 8 (mAb groups). Results are expressed as Mean ± SD. (C) Neurological scoring at 24h post-reperfusion was significantly improved in Rag1−/− mice compared to C57BL/6 wt mice and Rag1−/− mice reconstituted with B4 or C2 mAb. #p< 0.05 versus C57BL/6; n= 10 (C57BL/6 and Rag1−/−) and n= 8 (mAb groups). Horizontal line represents median score.
It was shown previously that B4 mAb restores intestine IRI in otherwise protected Rag1−/− mice, identifying annexin IV as a post-ischemic neoepitope expressed in the intestine (8). B4 mAb, but not D5 mAb, also restored cerebral IRI in Rag1−/− mice in terms of infarct size and neurological outcome, demonstrating that annexin IV is also expressed post-ischemically in the brain (Figure 2). C2 mAb also restored cerebral IRI in Rag1−/− mice. To investigate whether there may be a quantitative difference in the post-ischemic exposure of annexin IV and a subset of phospholipids in terms of IgM-dependent cerebral IRI, we performed mAb dose response reconstitution experiments. There was no significant difference between the ability of B4 mAb or C2 mAb to restore post-IR infarct volume or neurological deficit in Rag1−/− mice (Figure 2). In a separate experiment, we also demonstrated that C2 mAb restored intestinal IRI in Rag1−/− mice (data not shown).
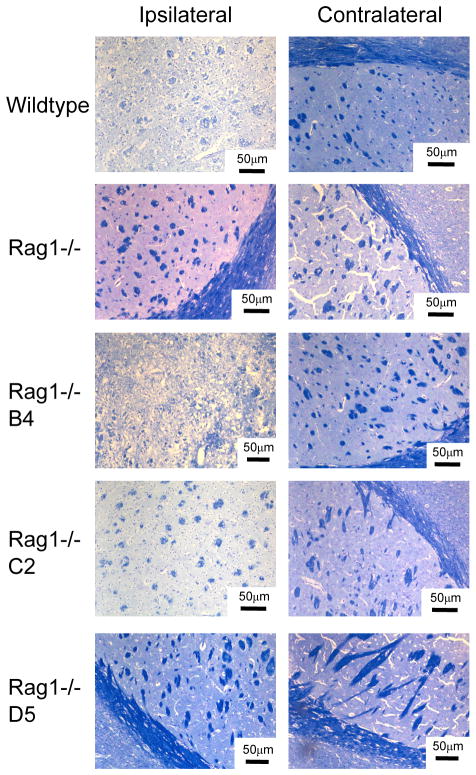
The effect of Rag1 deficiency and Ab reconstitution on cerebral IRI was further investigated by analysis of demyelination at 24h post reperfusion. Luxol Fast Blue and Nissl staining of brain sections revealed less myelin loss in the ipsilateral brain of Rag1−/− mice compared to wt mice, with restoration of myelin loss in Rag1−/− mice reconstituted with B4 and C2 mAbs (Figure 3). The contralateral brain of all groups was unaffected following MCAO or mAb administration (Figure 3).
Figure 3.
Effect of Rag1 deficiency and IgM mAb reconstitution on integrity of neuronal tissue. Mice were subjected to 60-minute MCAO and 24 hours reperfusion. Wildtype or Rag1−/− mice that were reconstituted with 100μg C2 or B4 mAb had loss of myelin and tissue integrity as indicated by Luxol Fast Blue/Nissl staining. Demyelination was not observed in Rag1−/− mice or Rag1−/− mice reconstituted with 100μg control D5 mAb. Representative images from 3 separate experiments. Scale bar represents 50μm.
As expected, there was no damage observed in the contralateral hemisphere in any group (not shown). Also, to confirm that cerebral blood flow was interrupted by the MCAO procedure, blood flow was measured by laser Doppler before ischemia, during ischemia and 10 minutes post-ischemia (4). There were no significant differences in cerebral blood flow between any of the groups (not shown). Changes in blood pressure and body temperature can significantly influence the outcome post stroke, and we therefore measured blood pressure, heart rate and temperature before, during and after ischemia in Rag1−/− and wt mice. There were no differences between the groups (supplemental table 1).
Analysis of IgM binding and C3 deposition following ischemic stroke
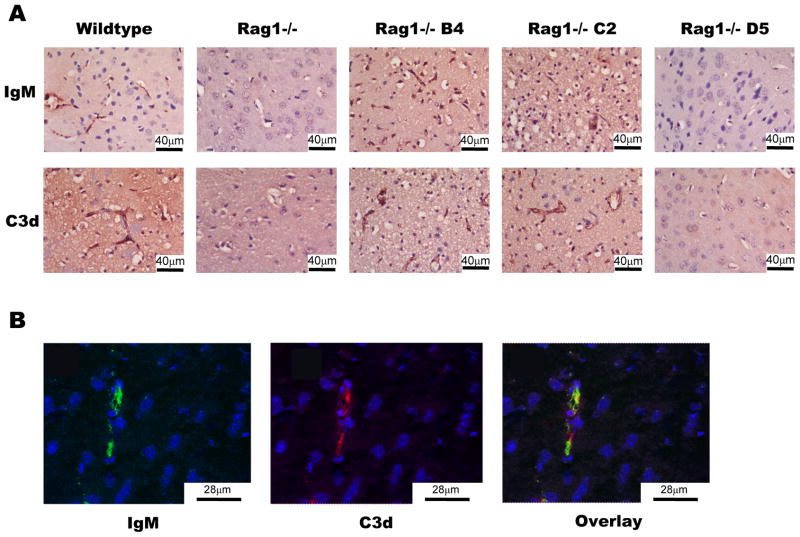
The deposition of C3 in post-ischemic mouse brains has been shown previously, and C3 deficiency or inhibition protects against ischemic stroke (3, 4). To investigate the relationship between post-ischemic IgM binding and complement activation, we investigated IgM and C3d deposition in infarcted areas of the brain at 24h post-reperfusion (C3d is a cleaved activation fragment of C3). As would be expected, there was no detectable IgM in post-ischemic brain sections from Rag1−/− mice, and only very low levels of C3 deposition were detected (Figure 4a). In contrast, post-ischemic brain sections from wt mice and Rag1−/− mice reconstituted with either B4 or C2 mAb showed high levels of deposited IgM and C3. IgM deposition was not detected in brains of Rag1−/− mice reconstituted with mAb D5 (Figure 4a), and no IgM or C3 deposition was detected in contralateral brain tissue (not shown). Additional confocal studies revealed colocalization of IgM and C3 on vessel endothelium of post-ischemic brains from Rag1−/− mice reconstituted with B4 (Figure 4b). Together, these results indicate specific binding of B4 and C2 mAbs to neoepitopes in the post-ischemic brain with subsequent IgM-mediated activation of complement.
Figure 4.
Deposition of IgM and complement following IgM mAb reconstitution of Rag1−/− mice. Mice were subjected to 60-minute MCAO and 24 hours reperfusion. (A) Wildtype and Rag1−/− mice reconstituted with 100μg C2 or B4 mAb had increased IgM and C3d deposition. Rag1−/− mice and Rag1−/− mice reconstituted with 100μg D5 mAb had little C3d and no IgM deposition. Scale bar represents 40μm. (B) Rag1−/− mice reconstituted with 100μg B4 mAb demonstrate IgM and C3d binding on the vessel endothelium in the parenchyma of the ipsilateral brain, with B4 and C3d colocalized on the vessel endothelium in overlayed images. Representative images from 3 separate experiments. Scale bar represents 28μm.
Recombinant annexin IV blocks cerebral IRI in wt mice
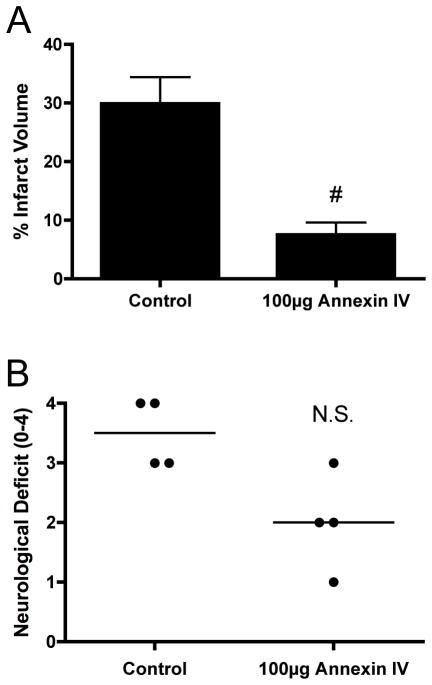
Reconstitution of cerebral IRI in Rag1−/− mice with either B4 or C2, but not D5, shows that specific IgM is sufficient to mediate cerebral injury. Previous data from Carroll’s group has shown that an IgM mAb recognizing non-muscle myosin on post-ischemic tissue is also capable of reconstituting IRI, at least in the intestine, heart and hind limb (9, 17). Thus, multiple neoepitopes are expressed on post-ischemic tissue, and to investigate whether a single Ab reactivity is sufficient to develop cerebral IRI in the context of an entire natural Ab repertoire, we investigated the effect of recombinant annexin IV on cerebral IRI in wt mice. Recombinant annexin IV (100μg) or vehicle control (PBS) was injected into wt mice 5 min before reperfusion. Recombinant annexin IV significantly reduced infarct volumes, with percentage infarct similar to that seen in Ab-deficient Rag1−/− mice (Figure 5a). There was also a strong trend toward reduced neurological deficit in annexin IV treated mice (Figure 5b), although this did not reach significance.
Figure 5.
Effect of recombinant annexin IV on post-ischemic infarct size and neurological deficit in C57BL/6 wild type mice. Mice were subjected to 60-minute MCAO and 24 hours reperfusion with injection of 100μg recombinant annexin IV immediately after reperfusion. (A) Infarct volume was significantly reduced in mice treated with 100μg Annexin IV (#p< 0.003 versus control, n=4). Results are expressed as Mean ± SD. (B) Neurological deficit was not significantly different between groups at 24 hours (#p = 0.058, n= 4). Horizontal line represents median score.
Normal mouse serum restores injury in Rag1−/− mice following ischemic stroke
To address the physiological relevance of the above mAb reconstitution experiments in the context of cerebral IRI, we determined whether normal mouse serum was capable of restoring injury in Rag1−/− mice. After 1h of MCAO and upon reperfusion, Rag1−/− mice were administered 300μl of freshly isolated pooled C57BL/6 mouse serum via tail vein injection. At 24h following ischemia, Rag1−/− mice that were reconstituted with mouse serum had significantly larger infarct volumes (Figure 6a) compared to Rag1−/− controls (injected with 300μl saline). Infarct data was functionally relevant in that the increased infarct resulted in significantly worsened neurological deficits (Figure 6b). Normal mouse serum restored injury levels to those seen in wt mice (refer to Figure 2). Data above showing that recombinant annexin IV protected wt mice from cerebral IRI (Figure 5)indicates that a single Ab reactivity is sufficient to drive injury in the context of an entire natural Ab repertoire. To further strengthen this conclusion, we also administered recombinant annexin IV together with normal mouse serum to Rag1−/− mice. The cerebral injury seen in Rag1−/− mice after administration of normal mouse serum was reversed with the co-administration of recombinant annexin IV in terms of infract volume (Figure 6a). There was also a strong trend toward improved neurological deficit (p = 0.058, Figure 6b). In control experiments, we also infused wt mouse serum into wt mice prior to either MCAO or a sham procedure; there was no deleterious effect of the serum infusion itself (Figure 6a).
Figure 6. Post-ischemic infarct size and neurological deficit in mice administered normal mouse serum and annexin IV.
Rag1−/− or wt mice were subjected to 60-minute MCAO and 24 hours reperfusion, with injection of 300 ul normal mouse serum, with or without co-administration of 100 ug annexin IV, prior to reperfusion. (A) Infarct volume was significantly increased in Rag1−/− mice treated with normal mouse serum, but the increase in infarct volume was reversed with co-administration of annexin IV. Infarct volumes in wt mice treated with normal mouse serum were similar to Rag1−/− mice treated with normal mouse serum, indicating that serum infusion alone does not contribute to injury. There was no infarct in sham operated wt mice that were treated with normal mouse serum. *p< 0.001 (Rag1−/−, Rag1−/− + annexin IV/normal mouse serum, sham +normal mouse serum, n = 4) and (Rag1−/− + normal mouse serum, wt + normal mouse serum, n = 5). Results are expressed as mean ± SD. (B) Neurological deficit at 24h post-reperfusion was significantly impaired in Rag1−/− mice treated with normal mouse serum, but not in Rag1−/− mice treated with both mouse serum and annexin IV. Neurological deficit in wt mice treated with normal mouse serum were similar to Rag1−/− mice treated with normal mouse serum, indicating that serum infusion alone does not contribute to neurological deficit, in accordance with data for infarct volume. #p<0.05 (Rag1−/− and Rag1−/− + annexin IV/normal mouse serum, n = 4) and (Rag1−/− + normal mouse serum, wt + normal mouse serum, n = 5). Horizontal line represents median score. (NMS = normal mouse serum).
Natural Abs recognizing similar phospholipids as C2 mAb are present in wt mice
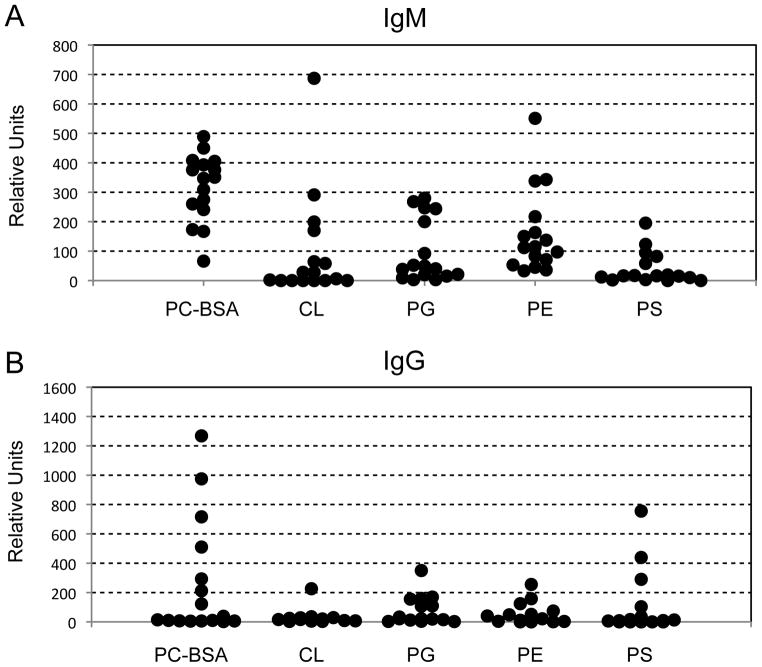
It has been shown previously that natural IgM Abs to annexin IV are present in wt C57BL/6 mice (8). We used an ELISA to investigate specificities and relative levels of anti-phospholipid Abs in wt C57BL/6 mouse serum. IgM reactivity to phosphatidyl choline and phosphatidyl ethanolamine, albeit variable, was found in all sera analyzed (Figure 7a), and these two phospholipids are recognized by C2 mAb (refer to Figure 1). IgG reactivity to certain phospholipids was also seen in some sera (Figure 7b), although the overall proportion of sera with negative reactivity was much higher than for IgM.
Figure 7.
Wildtype sera from C57BL/6 mice show presence of antibody binding to phospholipids. Antibody binding was detected by ELISA as described in the text. Normal serum samples were tested for levels of IgM antibody (A) and IgG antibody (B) recognizing the following panel of phospholipids: phosphorylcholine-BSA (PC-BSA), cardiolipin (CL), phosphotidylglycerol (PG), phosphatidylethanolamine (PE) and phosphatidylserine (PS). Each dot represents a single animal.
B4 and C2 mAbs bind to hypoxic but not normoxic human endothelial cells
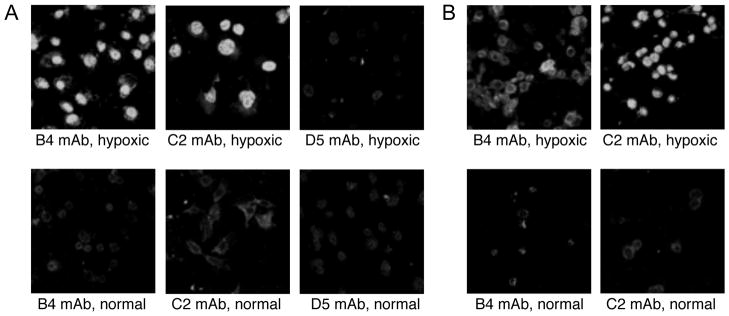
Previous work has demonstrated the presence of anti-annexin IV and anti-phospholipid IgM in the natural human Ab repertoire (8, 28), and post-ischemic blockade of annexin IV or phospholipid neoepitopes represent a potential therapeutic strategy to inhibit IRI. Also, direct translation development of B4 and C2 mAb derivatives may be feasible. We therefore determined whether human endothelial cells express B4 and C2 epitopes following exposure to hypoxia. Human umbilical vein endothelial cells were exposed to a period of hypoxia followed by a period of reoxygenation, and were then incubated with either B4, C2 or control D5 mAb. Immunofluorescence detection showed strong binding of both B4 and C2 mAbs to hypoxia-stressed, but not to normoxic, HUVEC. Control D5 mAb bound only weakly to both hypoxic and normoxic HUVEC (Figure 8a). To validate these results, we also investigated in vitro binding of B4 and C2 mAb to hypoxic and normoxic mouse brain endothelial cells (bEnd.3). As with HUVEC, both mAbs bound strongly to hypoxic, but not normoxic cells (Figure 8b).
Figure 8.
Recognition of hypoxic mouse and human endothelial cells by monoclonal antibodies in vitro. HUVEC (A) and mouse brain endothelial cells, bend.3 (B), were subjected to 3 hours hypoxia followed by 3 hours reoxygenation, and C2, B4 and D5 mAb binding determined by immunofluorescence (D5 mAb shown only for HUVEC). Normal culture conditions were used as normoxic control. C2 and B4 mAbs bound to hypoxic, but not normoxic human and mouse endothelial cells. D5 control mAb did not bind to either normal or hypoxic HUVEC. Representative images from 3 experiments.
Discussion
In this study we identified a novel IgM mAb (C2) that recognizes a subset of phospholipids and that reconstitutes cerebral IRI in Rag1−/− mice. The mAb was identified from a panel of mAbs recognizing intestine epithelial cells, a major cellular target of intestine IRI, using the same approach for the previous identification of anti-annexin IV B4 mAb (8). Previous studies have shown that anti-phospholipid and anti-annexin IV Abs are present in normal mouse and human serum (8, 20), and since both C2 and B4 mAbs restored cerebral IRI in Rag1−/− mice, the data indicate that distinct neoepitopes and their recognition by natural Abs are involved in the propagation of acute cerebral IRI. To put these findings in a more physiological context, we demonstrated that IgM Abs that recognize a similar phospholipid subset as C2 mAb are present in normal mouse serum, and that normal mouse serum restored cerebral IRI in Rag1−/− mice. We further showed that IgM is deposited in infarcted areas of wt mouse brains following ischemic stroke, and recombinant annexin IV significantly reduced infarct volumes in wt mice and in Rag1−/− mice reconstituted with normal mouse serum, demonstrating that a single Ab reactivity is sufficient to develop injury in the context of an entire natural Ab repertoire. Also, an IgM mAb isolated from the same panel that contained C2 and B4 mAbs (D5 mAb, anti-cytokeratin 19 (8)), did not restore cerebral IRI in Rag1−/− mice, demonstrating that injury was dependent on specific recognition of self-antigen and not passive deposition of IgM. Post-ischemic binding of IgM activates complement as indicated by the co-localization of IgM with C3d in wt mice and Rag1−/− mice reconstituted with B4 mAb, and previous data has shown that complement activation following cerebral IR results in a pro-inflammatory and pro-thrombogenic phenotype within the cerebral microvasculature (4).
Previous studies have shown that T cells traffic to the post-ischemic brain within 24h of reperfusion and play a role in the pathophysiology of ischemic stroke. Indeed, reconstitution of Rag1−/− mice with either CD4+ or CD8+ T cells restores injury after cerebral IRI in the same model of transient ischemic stroke used here (22). The mechanism of T cell-dependent injury is not known, but it is not dependent on antigen recognition, TCR co-stimulation or a pro-thrombotic effect (21). These and other studies on the role of T cells in cerebral IRI in Rag1−/− and SCID mice (21, 22, 29) have demonstrated Ab-independent injury. In the current study we demonstrate Ab-dependent and T cell independent injury in Rag1−/− mice, indicating compensatory mechanisms can contribute to cerebral IRI. There are similar findings in other organs where T cells have been shown to play a pathogenic role in IRI. For example, T and B cell deficient (Rag1−/− and/or SCID) mice are also protected from intestinal and myocardial IRI, and injury can be restored by independent reconstitution of either T cells or IgM (reviewed in (30)).
Reconstitution of Rag1−/− mice with B cells prior to ischemia in the same model we use here does not restore cerebral IRI (21), and while this finding appears to rule out a direct role for B cells in acute murine ischemic stroke, it does not exclude a role for Abs (which must be synthesized by transferred B cells), and in particular natural Abs since they are mainly the product of peritoneal B-1 cells, and which were not specifically reconstituted in the previous studies. Nevertheless, it has also been shown that B cell deficient mice are not protected from ischemic stroke (22), which does bring into question the role of both B cells and Abs in cerebral IRI, and appears to be in contradiction to the current findings. Although the T cell population is not affected in B cell deficient mice, the apparent discrepancy may be due to the fact that B cells can have both protective immune suppressive function as well as pathogenic pro-inflammatory functions. Indeed, a previous study showed that specific depletion of peritoneal B-1 cells did not alter overall circulating levels of IgM, but did reduce renal IgM binding and protected kidney function following renal IR (31), whereas mice completely deficient in mature B cells sustained more severe renal IRI compared to wt mice. The dual role of B cells was ascribed to the production of pathogenic natural IgM by B-1 cells that bound to post-ischemic mesangium on the one hand, and the production of protective IL-10 by mature B cells on the other (31). Also of interest, B cells and pathogenic Abs have been shown to play an important role in post-traumatic spinal cord injury, a condition involving ischemia and reperfusion of the spinal cord (32). Although it was shown that pathogenic antibodies mediated spinal cord pathology, the primary source and specificity of Abs was not determined, and natural Abs produced by B-1 cells are a potential source.
Natural Abs contribute to host defense against infection and serve homeostatic functions in the immune system, as well as contribute to pathogenic processes, as described here. Anti-phospholipid Abs have long been recognized as a significant component of the natural Ab repertoire, and antibodies to phosphorylcholine (a component of phosphatidylcholine) appear to be a dominant specificity (33). Further, natural Abs for Annexin IV, a soluble cytosolic Ca2+-dependent membrane binding protein family that has been identified in extracellular fluids and bound to cellular surfaces (34), represent a potential ischemic injury catalyst. Annexin IV is expressed on early apoptotic cells (35) and elevated in the brain in ethanol-induced injury (36, 37) and ischemic injury (36), and as previously shown in an intestinal murine IRI model (8)can be bound by specific natural Abs to elicit injury. We demonstrated that the C2 mAb binds to PC, as well as certain other phospholipids, such as CL and PE. Thus, the C2 mAb binding epitope is presented on various phospholipids, and accessibility of the epitope depends on the polar head since negatively charged phospholipids are not bound by C2 mAb. Our data indicate that the epitope recognized by C2 mAb is only exposed on injured or stressed cells. Further, of the anti-phospholipid IgM specificities analyzed, anti-PC IgM was present in C57BL/6 sera at the highest relative levels. It is important to note that anti-PC natural Abs do not bind nonoxidized phosphatidylcholine, which explains why they do not interact with healthy cells. The phosphorylcholine (PC) headgroup of phosphatidylcholine is exposed following oxidative damage to the polyunsaturated fatty acid side chain in position 2 of the glycerol backbone(38). In addition, apoptotic cell death can also lead to caspase-3 activation of the calcium-independent phospholipase A2 that can remove the fatty acid at the sn-2 position of phosphatidylcholine to generate lyso-phosphatidylcholine, which is also recognized by PC-specific Abs(27, 39). Thus, the maintenance of a high level of anti-PC antibody can be expected to promote the efficient clearance of injured and apoptotic cells. Significantly, it has been shown that reduction in the level of anti-PC antibody is observed in many pathological conditions, such as Alzheimer disease, rheumatoid arthritis and multiple sclerosis and others (40–44).
IgM can activate both the classical and lectin pathways, and previous studies indicate that IgM-mediated activation of the lectin pathway drives injury after intestinal and myocardial ischemia and reperfusion (14, 16) With regard to ischemic stroke, it has been shown that MBL deficient mice have reduced infarct following cerebral IRI (5, 6), and that genetically defined MBL deficiency is associated with improved outcome after acute stroke in humans (5, 38). Additionally, a recent study by Ducruet et al found that IgM and C3 localized together in the ischemic cerebral vasculature immediately following reprfusion, and that MBL is the first complement protein involved in this ischemic complex (45). The classical pathway does not appear to play a role in murine ischemic stroke (3), and studies using C1-inhibitor (39), which also inhibits the lectin pathway (40), implicate the lectin pathway. Interestingly, Ducruet et al also observed that the protective effect afforded by genetic MBL deficiency was lost in the subacute phase of stroke(45). Although further work is needed, this data supports a putative role for complement in neurogenesis subsequent to ischemic stroke(46, 47). Thus, together with the data presented here, it is likely that complement-dependent injury after cerebral ischemia and reperfusion is mediated by natural Ab-mediated activation of the lectin pathway, but the role of IgM and the lectin pathway in neurogenesis and long-term outcomes after stroke remains unclear.
It is not clear how phospholipid and annexin IV recognition by natural Abs relate to each other in terms of propagating cerebral IRI. It is also not clear how Abs to phospholipid and annexin IV relate to natural Abs that recognize non-muscle myosin and that have also been shown to be important in driving IRI in some tissues (7, 9, 18). While it is apparent that multiple Ab specificities are involved in post-ischemic neoepitope recognition and the development of tissue injury, it is puzzling that blockade of a single Ab specificity in wt mice can provide the same level of protection from IRI as complete Ab deficiency in Rag1−/− mice. This has been shown for non-muscle myosin in intestine and myocardial IRI models using either a peptide mimic or an anti-non-muscle myosin F(ab)2 fragment (9, 17, 19), and for annexin IV using recombinant protein in an intestinal IRI model (8). We demonstrated herein that recombinant annexin IV is also protective against cerebral IRI in wt mice and, further, that recombinant annexin IV protects Rag1−/− mice from cerebral IRI after the infusion of normal mouse serum that contains a full natural Ab repertoire. Possible explanations for these findings have been discussed previously (8), and to briefly reiterate, it is possible that: 1. There is a sequential expression of neoepitopes following IR and that serial recognition of these neoepitopes is required for complement-dependent injury; 2. Neoepitopes are expressed differently on different cell populations, and recognition of multiple cell types is necessary for full expression on injury; 3. Protein and phospholipid complexes are formed/exposed after IR, and binding to multiple epitopes is necessary for effective complement activation and injury. And although an IgM mAb of a single specificity can restore IRI in Rag1−/− mice, it is not clear how the dose of injected mAb required to induce IRI relates to corresponding Ab levels in wt mice. Regardless, the finding that blockade of a single Ab reactivity protects against cerebral IRI, and the fact that similar neoepitope recognition processes appear to occur in mouse and man, has potential therapeutic implications for the treatment of ischemic stroke, although further work is needed. In the current study, we evaluated outcome at 24 hours post reperfusion, and it is interesting that Ducruet et al (45) recently demonstrated that the protective effect afforded by genetic MBL deficiency at 24 hours (see above) was lost in the subacute phase of stroke. This finding supports a putative role for complement in neurogenesis subsequent to ischemic stroke(46, 47). Thus, together the data indicate that complement-dependent injury after cerebral ischemia and reperfusion is mediated by natural Ab-mediated activation of the lectin pathway, but the role of IgM and the lectin pathway (and indeed complement) in neurogenesis and long-term outcome after stroke remains unclear.
In summary, natural IgM has been shown to play an important role in the pathogenesis of IRI in certain organs via complement activation, but its role in cerebral IRI was unknown. Here we show that pathogenic IgM plays an important role in activating complement and driving cerebral injury after ischemic stroke, and we identify the involvement of two distinct self-reactive Ab specificities. Previous studies have shown that normal human sera contain IgM reactivity to phospholipids recognized by C2 mAb (28) and to annexin IV (8). Here we show that hypoxic, but not normoxic, human endothelial cells also bind C2 and B4 mAb, indicating that similar recognition processes occur in mouse and man.
Supplementary Material
Footnotes
This work was supported by grants from the National Institutes of Health (HL082485, HL86576 and AI31105) and the Department of Veterans Affairs (Merit Award NURC-051010F).
Abbreviations used in this paper: IRI, ischemia reperfusion injury; MCAO, middle cerebral artery occlusion; wt, wildtype; MBL, mannose binding lectin; TTC, 2% triphenyltetrazolium chloride.
References
- 1.Diepenhorst GMP, Van Gulik TM, Hack CE. Complement-mediated ischemia-reperfusion injury. Ann Surg. 2009;249:889–899. doi: 10.1097/SLA.0b013e3181a38f45. [DOI] [PubMed] [Google Scholar]
- 2.Pedersen ED, Loberg EM, Vege E, Daha MR, Maehlen J, Mollnes TE. In situ deposition of complement in human acute brain ischaemia. Scand J Immunol. 2009;69:555–562. doi: 10.1111/j.1365-3083.2009.02253.x. [DOI] [PubMed] [Google Scholar]
- 3.Mocco J, Mack WJ, Ducruet AF, Sosunov SA, Sughrue ME, Hassid BG, Nair MN, Laufer I, Komotar RJ, Holland MCH, Pinsky DJ, Connolly JES. Complement component C3 mediates inflammatory injury following focal cerebral ischemia. Circ Res. 2006;99:209–217. doi: 10.1161/01.RES.0000232544.90675.42. [DOI] [PubMed] [Google Scholar]
- 4.Atkinson C, Zhu H, Qiao F, Varela JC, Yu J, Song H, Kindy MS, Tomlinson S. Complement-dependent P-selectin expression and injury following ischemic stroke. J Immunol. 2006;177:7266–7274. doi: 10.4049/jimmunol.177.10.7266. [DOI] [PubMed] [Google Scholar]
- 5.Cervera A, Planas AM, Justicia C, Urra X, Jensenius JC, Torres F, Lozano F, Chamorro A. Genetically-defined deficiency of mannose-binding lectin is associated with protection after experimental stroke in mice and outcome in human stroke. PLoS ONE. 2010;5:e8433. doi: 10.1371/journal.pone.0008433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morrison H, Frye J, Davis-Gorman G, Funk J, McDonagh P, Stahl GL, Ritter L. The contribution of mannose binding lectin to reperfusion injury after ischemic stroke. Curr Neurovasc Res. 2011;8:52–63. doi: 10.2174/156720211794520260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang M, Austen WG, Jr, Chiu I, Alicot EM, Hung R, Ma M, Verna N, Xu M, Hechtman HB, Moore FD, Jr, Carroll MC. Identification of a specific self-reactive IgM antibody that initiates intestinal ischemia/reperfusion injury. Proc Natl Acad Sci U S A. 2004;101:3886–3891. doi: 10.1073/pnas.0400347101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kulik L, Fleming SD, Moratz C, Reuter JW, Novikov A, Chen K, Andrews KA, Markaryan A, Quigg RJ, Silverman GJ, Tsokos GC, Holers VM. Pathogenic natural antibodies recognizing annexin IV are required to develop intestinal ischemia-reperfusion injury. J Immunol. 2009;182:5363–5373. doi: 10.4049/jimmunol.0803980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haas MS, Alicot EM, Schuerpf F, Chiu I, Li J, Moore FD, Carroll MC. Blockade of self-reactive IgM significantly reduces injury in a murine model of acute myocardial infaction. Cardiovasc Res. 2010;87:618–627. doi: 10.1093/cvr/cvq141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weiser MR, Williams JP, Moore FD, Jr, Kobzik L, Ma M, Hechtman HB, Carroll MC. Reperfusion injury of ischemic skeletal muscle is mediated by natural antibody and complement. J Exp Med. 1996;183:2343–2348. doi: 10.1084/jem.183.5.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fleming SD, Shea-Donohue T, Guthridge JM, Kulik L, Waldschmidt TJ, Gipson MG, Tsokos GC, Holers VM. Mice deficient in complement receptors 1 and 2 lack a tissue injury-inducing subset of the natural antibody repertoire. J Immunol. 2002;169:2126–2133. doi: 10.4049/jimmunol.169.4.2126. [DOI] [PubMed] [Google Scholar]
- 12.Chan RK, Ibrahim SI, Takahashi K, Kwon E, McCormack M, Ezekowitz A, Carroll MC, Moore FD, Austen J, WG The differing roles of the classical and mannose-binding lectin complement pathways in the events following skeletal muscle ischemia-reperfusion. J Immunol. 2006;177:8080–8085. doi: 10.4049/jimmunol.177.11.8080. [DOI] [PubMed] [Google Scholar]
- 13.Lee H, Green DJ, Lai L, Hou YJ, Jensenius JC, Liu D, Cheong C, Park CG, Zhang M. Early complement factors in the local tissue immunocomplex generated druing intestinal ischemia/reperfusion injury. Mol Immuno. 2010;47:972–981. doi: 10.1016/j.molimm.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang M, Takahashi K, Alicot EM, Vorup-Jensen T, Kessler B, Thiel S, Jensenius JC, Ezekowitz RA, Moore FD, Carroll MC. Activation of the lectin pathway by natural IgM in a model of ischemia/reperfusion injury. J Immunol. 2006;177:4727–4734. doi: 10.4049/jimmunol.177.7.4727. [DOI] [PubMed] [Google Scholar]
- 15.McMullen ME, Hart ML, Walsh MC, Buras J, Takahashi K, Stahl GL. Mannose-binding lectin binds IgM to activate the lectin complement pathway in vitro and in vivo. Immunobiology. 2006;211:759–766. doi: 10.1016/j.imbio.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 16.Busche MN, Pavlov V, Takahashi K, Stahl GL. Myocardial ischemia and reperfusion injury is dependent on both IgM and mannose-binding lectin. Am J Physiol Heart Circ Physiol. 2009;297:H1853–1859. doi: 10.1152/ajpheart.00049.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang M, Alicot EM, Chiu I, Li J, Verna N, Vorup-Jensen T, Kessler B, Shimaoka M, Chan R, Friend D, Mahmood U, Weissleder R, Moore FD, Carroll MC. Identification of the target self-antigens in reperfusion injury. J Exp Med. 2006;203:141–152. doi: 10.1084/jem.20050390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Austen JWG, Zhang M, Chan RK, Friend D, Hechtman HB, Carroll MC, Moore FD. Murine hindlimb reperfusion injury can be initiated by a self-reactive monoclonal IgM. Surgery. 2004;136:401–406. doi: 10.1016/j.surg.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 19.Chan RK, Verna N, Afnan J, Zhang M, Ibrahim S, Carroll MC, Moore FD., Jr Attenuation of skeletal muscle reperfusion injury with intravenous 12 amino acid peptides that bind to pathogenic IgM. Surgery. 2006;139:236–243. doi: 10.1016/j.surg.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 20.Fleming SD, Egan RP, Chai C, Girardi G, Holers VM, Salmon J, Monestier M, Tsokos GC. Anti-phospholipid antibodies restore mesenteric ischemia/reperfusion-induced injury in complement receptor 2/complement receptor 1-deficient mice. J Immunol. 2004;173:7055–7061. doi: 10.4049/jimmunol.173.11.7055. [DOI] [PubMed] [Google Scholar]
- 21.Kleinschnitz C, Schwab N, Kraft P, Hagedorn I, Dreykluft A, Schwarz T, Austinat M, Nieswandt B, Wiendl H, Stoll G. Early detrimental T-cell effects in experimental cerebral ischemia are neither related to adaptive immunity nor thrombus formation. Blood. 2010;115:3835–3842. doi: 10.1182/blood-2009-10-249078. [DOI] [PubMed] [Google Scholar]
- 22.Yilmaz G, Arumugam TV, Stokes KY, Granger DN. Role of T lymphocytes and interferon-gamma in ischemic stroke. Circulation. 2006;113:2105–2112. doi: 10.1161/CIRCULATIONAHA.105.593046. [DOI] [PubMed] [Google Scholar]
- 23.Endres M, Gertz K, Lindauer U, Katchanov J, Schultze J, Schrock H, Nickenig G, Kuschinsky W, Dirnagl U, Laufs U. Mechanisms of stroke protection by physical activity. Ann Neurol. 2003;54:582–590. doi: 10.1002/ana.10722. [DOI] [PubMed] [Google Scholar]
- 24.Ellsworth JL, Garcia R, Yu J, Kindy MS. Time window of fibroblast growth factor-18-mediated neuroprotection after occlusion of the middle cerebral artery in rats. J Cereb Blood Flow Metab. 2004;24:114–123. doi: 10.1097/01.WCB.0000100063.36077.CD. [DOI] [PubMed] [Google Scholar]
- 25.Bederson JB, Pitts LH, Germano SM, Nishimura MC, Davis RL, Bartkowski HM. Evaluation of 2,3,5-triphenyltetrazolium chloride as a stain for detection and quantification of experimental cerebral infarction in rats. Stroke. 1986;17:1304–1308. doi: 10.1161/01.str.17.6.1304. [DOI] [PubMed] [Google Scholar]
- 26.Hata R, Mies G, Wiessner C, Fritze K, Hesselbarth D, Brinker G, Hossmann KA. A reproducible model of middle cerebral artery occlusion in mice: hemodynamic, biochemical, and magnetic resonance imaging. J Cereb Blood Flow Metab. 1998;18:367–375. doi: 10.1097/00004647-199804000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Kim SJ, Gershov D, Ma X, Brot N, Elkon KB. I-PLA2 activation during apoptosis promotes the exposure of membrance lysophosphatidylcholine leading to binding by natural immunoglobulin M antibodies and complement activation. J Exp Med. 2002;196:655–665. doi: 10.1084/jem.20020542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cabiedes J, Cabral AR, Lopez-Mendoza AT, Cordero-Esperon HA, Huerta MT, Alarcon-Segovia D. Characterization of anti-phosphatidylcholine polyreactive natural autoantibodies from normal human subjects. J Autoimmun. 2002;18:181–190. doi: 10.1006/jaut.2001.0575. [DOI] [PubMed] [Google Scholar]
- 29.Hurn PD, Subramanian S, Parker SM, Afentoulis ME, Kaler LJ, Vandenbark AA, Offner H. T- and B-cell-deficient mice with experimental stroke have reduced lesion size and inflammation. J Cereb Blood Flow Metab. 2007;27:1798–1805. doi: 10.1038/sj.jcbfm.9600482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Linfert D, Chowdhry T, Rabb H. Lymphocytes and ischemia-reperfusion injury. Transplant Rev (Orlando) 2009;23:1–10. doi: 10.1016/j.trre.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Renner B, Strassheim D, Amura CR, Kulik L, Ljubanovic D, Glogowska MJ, Takahashi K, Carroll MC, Holers VM, Thurman JM. B cell subsets contribute to renal injury and renal protection after ischemia/reperfusion. J Immunol. 2010;185:4393–4400. doi: 10.4049/jimmunol.0903239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ankeny DP, Guan Z, Popovich PG. B cells produce pathogenic antibodies and impair recovery after spinal cord injury in mice. J Clin Invest. 2009;119:2990–2999. doi: 10.1172/JCI39780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shaw PX, Horkko S, Chang M, Curtiss LK, Palinski W, Silverman GJ, Witztum JL. Natural antibodies with the T15 idiotype may act in atherosclerosis, apoptotic clearance, and protective immunity. J Clin Invest. 2000;105:1731–1740. doi: 10.1172/JCI8472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rescher U, Gerke V. Annexins--unique membrane binding proteins with diverse functions. J Cell Sci. 2004;117:2631–2639. doi: 10.1242/jcs.01245. [DOI] [PubMed] [Google Scholar]
- 35.Sohma H, Ohkawa H, Hashimoto E, Sakai R, Saito T. Ethanol-induced augmentation of annexin IV expression in rat C6 glioma and human A549 adenocarcinoma cells. Alcohol Clin Exp Res. 2002;26:44S–48S. doi: 10.1097/01.ALC.0000026975.39372.A5. [DOI] [PubMed] [Google Scholar]
- 36.Eberhard DA, Brown MD, VandenBerg SR. Alterations of annexin expression in pathological neuronal and glial reactions. Am J Pathol. 1994;145:640–649. [PMC free article] [PubMed] [Google Scholar]
- 37.Sohma H, Ohkawa H, Hashimoto E, Toki S, Ozawa H, Kuroki Y, Saito T. Alteration of annexin IV expression in alcoholics. Alcohol Clin Exp Res. 2001;25:55S–58S. doi: 10.1097/00000374-200106001-00013. [DOI] [PubMed] [Google Scholar]
- 38.Berliner JA, Witztum JL. Apoptotic cells with oxidation-specific epitopes are immunogenic and proinflammatory. J Exp Med. 2004;200:1359–1370. doi: 10.1084/jem.20031763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen Y, Park YB, Patel E, Silverman GJ. IgM antibodies to apoptosis-associated determinants recruit C1q and enhance dendritic cell phagocytosis of apoptotic cells. J Immunol. 2009;182:6031–6043. doi: 10.4049/jimmunol.0804191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eriksson UK, Sjoberg BG, Bennet AM, de Faire U, Pedersen NL, Frostegard J. Low levels of antibodies against phosphorylcholine in Alzheimer’s disease. J Alzheimers Dis. 2010;21:577–584. doi: 10.3233/JAD-2010-091705. [DOI] [PubMed] [Google Scholar]
- 41.Gingnell M, Dahlbom I, Lindholm A, Hudecova M, Arnadottir R, Hansson T, Sundstrom-Poromaa I. Patients with polycystic ovary syndrome have lower levels of IgM anti-phosphorylcholine antibodies than healthy women. Gynecol Endocrinol. 2011;27:486–490. doi: 10.3109/09513590.2010.501880. [DOI] [PubMed] [Google Scholar]
- 42.Fusaro AE, Fahl K, Cardoso EC, de Brito CA, Jacob CM, Carneiro-Sampaio M, Duarte AJ, Sato MN. Profile of autoantibodies against phosphorylcholine and cross-reactivity to oxidation-specific neoantigens in selective IgA deficiency with or without autoimmune diseases. J Clin Immunol. 2010;30:872–880. doi: 10.1007/s10875-010-9453-y. [DOI] [PubMed] [Google Scholar]
- 43.Frostegard J. Low level natural antibodies against phosphorylcholine: a novel risk marker and potential mechanism in atherosclerosis and cardiovascular disease. J Clin Immunol. 2010;134:47–54. doi: 10.1016/j.clim.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 44.Elkan AC, Hakansson N, Frostegard J, Cederholm T, Hafstrom I. Rheumatoid cachexia is associated with dyslipidemia and low levels of atheroprotective natural antibodies against phosphorylcholine but not with dietary fat in patients with rheumatoid arthritis: a cross-sectional study. Arthritis Res Ther. 2009;11:R37. doi: 10.1186/ar2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ducruet AF, Sosunov SA, Zacharia BE, Gorski J, Yeh ML, DeRosa P, Cohen G, Gigante PR, Connolly J, ES The neuroprotective effect of genetic mannose-binding lectin deficiency is not sustained in the subacute phase of stroke. Transl Stroke Res. 2011 doi: 10.1007/s12975-011-0104-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barnum SR, Ames RS, Maycox PR, Hadingham SJ, Meakin J, Harrison D, Parsons AA. Expression of the complement C3a and C5a receptors after permanent focal ischemia: An alternative interpretation. Glia. 2002;38:169–173. doi: 10.1002/glia.10069. [DOI] [PubMed] [Google Scholar]
- 47.Rahpeymai Y, Hietala MA, Wilhelmsson U, Fotheringham A, Davies I, Nilsson AK, Zwirner J, Wetsel RA, Gerard C, Pekny M, Pekna M. Complement: a novel factor in basal and ischemia-induced neurogenesis. EMBO J. 2006;25:1364–1374. doi: 10.1038/sj.emboj.7601004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.