Abstract
Type I interferons (IFN-I) are normally produced during antiviral responses, yet high levels of chronic IFN-I expression correlate with autoimmune disease. A variety of viral sensors generate IFN-I in their response, but other than TLRs it is not fully known which pathways are directly involved in the development of spontaneous immune pathologies. To further explore the link between IFN-I induced by viral pathways and autoimmunity, we generated a new transgenic (Tg) mouse line containing multiple copies of Ifih1, a gene encoding the cytoplasmic dsRNA sensor MDA5 with proven linkage to diabetes and lupus. We show that MDA5 overexpression led to a chronic IFN-I state characterized by resistance to a lethal viral infection through rapid clearance of virus in the absence of a CD8+ or antibody response. Spontaneous MDA5 activation was not sufficient to initiate autoimmune or inflammatory pathology by itself even though every immune cell population had signs of interferon activation. When combined with the lupus-susceptible background of the FcγR2B deficiency, MDA5 overexpression did accelerate the production of switched autoantibodies, the incidence of glomerulonephritis and early lethality. Thus, MDA5 Tg mice provide evidence that chronic elevated levels of IFN-I are not sufficient to initiate autoimmunity or inflammation although they might exacerbate an ongoing autoimmune pathology.
Keywords: MDA5, Ifih1, type one IFN, virus, autoimmunity
Introduction
Several families of germ line-encoded innate immune sensors, including TLRs, RIG-I-like and NOD-like receptors, recognize pathogens within the endosome, cytosol and extracellular regions of the cell (1). TLRs located in endosomes primarily activate phagocytic and antigen presenting cells, while cytoplasmic nucleic acid sensors are designed to recognize RNA from viral replication intermediates in infected cells (2). The RIG-I-like receptor family, consisting of RIG-I, MDA5 and LGP2, are localized in the cytosol of cells throughout the body and recognize small RNA products derived from picorna-, flavi-, and paramixoviruses (2). Activation of MDA5 and RIG-I leads to induction of the type I-IFNs, which have a wide range of effects such as inducing the expression of anti-viral genes, upregulating antigen presentation, and shaping the lymphocyte response against the pathogen (3, 4).
Due to the range of effects IFN-I can have on the immune system, it is not surprising that it has been implicated in systemic autoimmunity (5, 6). For instance, patients with systemic lupus erythematosus (SLE) display elevated levels of transcripts for IFN-I target genes in their circulation (7). IFN-I-containing serum from lupus patients was shown to induce dendritic cell maturation of human monocytes (8). Furthermore, high levels of IFN-I in patients are associated with increased IgG autoantibody titers, whereas lower IFN-I levels are associated with the IgM isotype, suggesting type I IFN may play a role in class switching in SLE (9). A variety of mouse models have demonstrated a positive role for IFN-I in lupus (10–12). However, IFN-I has also been shown to be protective in another model of SLE (13, 14).
The link between IFN-I and spontaneous autoimmune disease in human and mouse experimental settings implies that there are IFN-I producing pathways capable of spontaneous and chronic activation, which may be active even in the absence of pathogenic triggers. TLR7 is an example of an IFN-I producing pathway that contributes to autoimmune disease through spontaneous activation (15–17). TLR7 was shown to be necessary and sufficient for the development of autoimmune inflammatory disease in mice. While it is assumed that the pathogenic effect of TLR7 activation is mainly due to increased production of IFN-I, this claim has not been formally proven. Furthermore, IFN-αβ receptor deficiency in the lupus prone FcγR2B yaa mice, which harbor a TLR7 duplication, show reduced but not eliminated pathology (11). Thus, it is uncertain whether IFN-I alone can initiate autoimmune inflammatory disease. It is also unclear whether pathways other than TLRs play a role in spontaneous immune activation, and whether this activation can lead to the chronic elevated IFN-I gene expression that is detected in autoimmune pathologies.
We have explored the possibility of spontaneous activation of MDA5 as a trigger of inflammatory disease because Ifih1, the gene that codes for MDA5, has been linked to type I diabetes and lupus in humans (21–25). Specifically, a loss-of-function allele is associated with resistance to disease and susceptibility genotypes are associated with elevated expression, raising the possibility that increased expression or activity of MDA5 could contribute to autoimmune development (21, 23). Levels of Ifih1 are often highly elevated during viral infections and inflammatory diseases (18–20), making overexpression a probable scenario that would mimic infection or self-upregulation of the pathway. Therefore, we generated mice overexpressing Ifih1, and have begun characterizing them. We have observed that they develop chronic IFN-I responses in the absence of autoimmune pathology or inflammation. These results suggest that natural ligands can spontaneously activate the MDA5 pathway in the absence of viral infections and that IFN-I, not inflammatory cytokines, are the product of this activation. Thus, MDA5 overexpressing mice provide an excellent system in which to assess the role for chronic IFN-I in a variety of different settings, and an example of uncoupling of IFN-I production and inflammatory conditions.
Materials and Methods
Mice
MDA5 transgenic mice were made using a Bacterial Artificial Chromosome (BAC) transgenic approach. The BAC clone RP23-34J8 containing MDA5 genomic DNA (Ifih1) was modified using a BAC recombineering kit (Gene Bridges, Dresden, Germany). The upstream genes Gca and Kcnh7 were replaced with a neomycin resistance cassette so that only Ifih1 genomic DNA remained in the BAC. The resulting BAC was used to inject C57Bl/6 embryos in our transgenic facility (NCI, Frederick, MD). Founders were screened for the presence of the transgene using the following PCR primers: Forward 5’-AGTTTACAAGTGGACAGCAAGCG-3’ and reverse 5’-GCATATACACTCAGAAGAACTCGTC-3’. All other genotyping for the transgene was conducted with these primers or real-time PCR primers (see below). All experiments were conducted using a single founder. Type one Interferon alpha/beta receptor 1 knockout (IFNRαβ1−/−) and FcγR2B−/− mice were obtained from the Taconic NIAID colony. All animal experiments were approved by the National Institute of Allergy and Infectious Diseases Animal Care and Use Committee.
Antibodies/Flow Cytometry
Antibodies against the following antigens were used for flow cytometric analysis of splenocytes: B220, GL7, FAS, IgG2a, CD138, CD4, CD69, CD45rb, CD44, CD11b, Ly6G, Ly6C, GR1, Ly6A/E, ICOS, CD3ε, TNF, CXCR5 from BD Biosciences (San Jose, CA) and CD62L, CD8α, CD11c, CD122, IFN-gamma, IL2, KLRG1, PD1 and Fc block (CD16/32) from ebioscience (San Diego, CA). Flow cytometry was conducted using a FACS caliber or LSR2 (BD Biosciences). Flow data was analyzed using FlowJo (Tree Star, Ashland, OR). For detection of VSV antigen-specific CD8+ T cells, splenocytes were stained with a H-2Kb pentamer with the peptide epitope from the nucleoprotein (n52-59) (Proimmune, Oxford, UK) (26).
Genotyping and real-time PCR primers
The following primers were used for real-time PCR analysis: IRF7 forward 5’-CAGCGAGTGCTGTTTGGAGAC-3’ and reverse 5’-AAGTTCGTACACCTTATGCGG-3’, Mx1 forward 5’-GATCCGACTTCACTTCCAGATGG-3’ and reverse 5’-CATCTCAGTGGTAGTCAACCC-3’, Isg15 forward 5’-AGCAAGCAGCCAGAAGCAGACTC-3’ and reverse 5’-GGAAAGCCGGCACACCAATC-3’, Oas2 forward 5’-CCGGGCCAGTGCACAAGTTAG-3’ and reverse 5’-CGATGGCACCGAGGACACC-3’, MDA5 forward 5’-GTGATGACGAGGCCAGCAGTTG-3’ and reverse 5’-ATTCATCCGTTTCGTCCAGTTTCA-3’, Tlr7(taqman) forward 5’-GTACCAAGAGGCTGCAGATTAGAC-3’ probe 5’-TGGAGATACCACAGGATCTGCCAT-3’ and reverse 5’-TAGCCTCAAGGCTCAGAAGATG-3’ and beta-actin forward 5’-GGCGCTTTTGACTCAGGATT-3’ and reverse 5’-GGGATGTTTGCTCCAACCAA-3’. For genotyping IFNRαβ1−/− mice, 4 primers were used: IFNRαβ1 upper 5’-CATACGCTTGATCCGGCTACCTG-3’ and IFNRαβ1 lower 5’-TCCCTTCCTCTGCTCTGACACGA-3’ generate a 487bp fragment from the KO allele. UM4 upper 5’-AAGATGTGCTGTTCCCTTCCTCTGCTCTGA-3’ and UM5 lower 5’-ATTATTAAAAGAAAAGACGAGGCGAAGTGG-3’ generate a 150bp fragment from the WT allele and a 1300bp fragment from the KO allele. For genotyping FcγR2B−/− mice, 3 primers were used: FcREC1 forward 5’-AAGGCTGTGGTCAAACTCGAGCC-3’, OL4080 5’-TTGACTGTGGCCTTAAACGTGTAG-3’ and OL4143 5’-CTCGTGCTTTACGGTATCGCC-3’ generates a ~190bp WT fragment and a ~300bp KO fragment. For measurement of VSV by real-time PCR, see below. For measurement of interferon signature genes by real-time PCR, whole splenocytes, purified B cells or CD4-depleted splenocytes were used from multiple mice.
Interferon α, β response PCR Gene Expression Array
Type One IFN response genes were measured using a real-time QPCR array (PAMM-016) from SABiosciences (Frederick, MD). RNA was isolated from CD43− splenic B cells from WT, MDA5 Tg and B6.Yaa mice. cDNA was synthesized and subjected to QPCR as described in the kit. Raw Ct values were analyzed using the RT2 Profiler PCR Array Data Analysis (http://pcrdataanalysis.sabiosciences.com/pcr/arrayanalysis.php?target=upload).
Measurement of blood cell populations
Differential blood counts and blood chemistry were measured at the NIH Department of Laboratory Medicine using standard procedures.
VSV infections
The Indiana strain of Vesicular Stomatitis Virus (VSV-IND) was kindly provided by Jonathan Yewdell (NIAID/NIH). VSV was injected i.v. at 2×106 PFUs/mouse in 200 µL of Hanks Balanced Salt Solution. For lethal dose experiments, 2×108 PFUs/mouse was used.
VSV neutralization assay
In order to determine the level of VSV neutralizing antibodies in VSV-infected mouse serum, a Vero cell neutralizing assay was performed. Vero Cells were plated the night before in 96-well flat bottom dishes at 3.3×104 cells/well. The next day, 160 PFUs of VSV (in 100µL volume) was incubated 1:1 with various dilutions of serum from infected animals (serum dilutions 1:1280, 1:2560, 1:5120, 1:10240, and 1:50960) for 1 hr at 37°C. This neutralized VSV was then plated on the Vero cells at 100µL/well and left for 1 hr at 37°C. Supernatants were then replaced with 1% methycellulose diluted in MEM and left overnight at 37°C. The next morning, the cells were fixed in 50% ethanol, 5%PFA and 4.25% NaCL for 30 minutes at RT and stained with 0.5% crystal violet for 1 hr at RT. Plaques were then counted from dried plates and anti-VSV serum titers were determined by the serum dilution required to inhibit 50% of plaque formation compared to VSV without antibodies.
ELISAs/Cytokine Bead arrays
Total IgG, IgG1 and IgG2a were measured in the sera of mice using an Enzyme-linked immunosorbent assay (ELISA) (Southern Biotech, Birmingham, Alabama). Anti-nuclear antibodies were detected in sera using an ELISA (Alpha Diagnostic International, San Antonio, TX). IgG anti-dsDNA antibodies were detected using a commercially available anti-dsDNA ELISA kit (Calbiotech, San Diego, CA) and secondary goat anti-mouse IgG-Alkaline Phosphatase antibody (Southern Biotech, Birmingham, AL). Circulating IFNγ, TNFα, IL12-p70, and MCP-1 were detected using the Mouse Inflammation kit cytometric bead array (BD Biosciences).
Measurement of VSV by Real-Time PCR
VSV-IND mRNA was measured in the spleen and liver of infected mice using fluorescent probe-based Real-Time PCR (27). The following PCR primers used were: Forward 5’-TGATACAGTACAATTATTTTGGGAC-3’ Reverse 5’-GAGACTTTCTGTTACGGGATCTGG-3’ and probe (6-FAM) 5’-ATGATGCATGATCC+A+G+C-3’(Iowa Black© FQ) where “+” indicates a locked nucleic acid (LNA) (Integrated DNA Technologies, Coralville, IA). Mice were euthanized and ~20mg of liver or spleen samples were harvested and immediately homogenized in Trizol® (Invitrogen, Carlsbad, CA). RNA was purified according to the manufacturers protocol and cDNA was synthesized from 1µg of total RNA using the iscript cDNA synthesis kit (Biorad, Hercules, CA). As a positive control for the PCR, RNA was isolated from in vitro-infected Vero cells and cDNA was synthesized. Five-fold dilution standard curves were generated from this cDNA and normalized relative values were calculated for tissue samples from these curves.
Glomerulonephritis scoring
For Glomerulonephritis scoring, kidney sections from WT (n=3) FcγR2B−/− (n=6) and MDA5 Tg FcγR2B−/− (n=8) mice were stained with H&E. The diameter was measured for multiple Glomeruli and a threshold for “affected” was determined to be greater than or equal to 78µm (this diameter was the greatest diameter for a healthy WT mouse). A score was then generated for each kidney based on the % affected glomeruli (0: 0–19% affected, 1: 20–30% affected, 2: 31–50% affected, 3: 51–70% affected and 4: 100% affected).
Statistics
In all experiments, unless otherwise specified in the figure legend, a one-way ANOVA with Bonferroni post-hoc test was performed for multiple groups.
Results
MDA5 Transgenic mice display a chronic IFN-I signature with minor pathological consequences
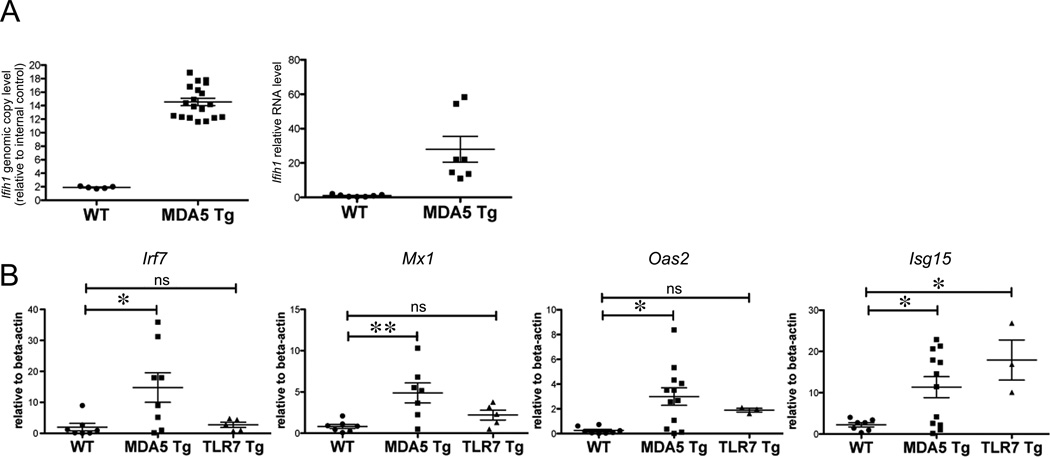
We first characterized transgenic mice with increased expression of MDA5 (MDA5 Tg), which we generated by insertion of 12 copies of a BAC clone encoding the endogenous murine Ifih1 gene (Fig. 1A). Ifih1 mRNA levels in MDA5 Tg splenocytes were ~25 fold increased over WT controls (Fig. 1A).
Figure 1. MDA5 Transgenic mice display a chronic IFN-I signature with minor pathological consequences.
(A) Ifih1 genomic and mRNA levels were measured by real-time PCR on ear DNA or splenic RNA from mice of the indicated genotype. β-actin was used as standard. (B) IFN-I-target gene expression was measured by real-time PCR on RNA from spleens (CD4-depleted or CD43-negative) from mice of the indicated genotype. TLR7 Tg mice contain between 8 and 16 copies of TLR7 genomic DNA (16). Each dot represents an individual mouse. Data are pooled from multiple experiments. For B, a statistical T-test was performed. *P<0.05, **P<0.01.
Since activation of MDA5 leads to production of IFN-I (2), we next tested for spontaneous IFN-I-induction in MDA5 Tg mice. Indeed, expression of the IFN-I stimulated genes (ISGs) Irf7, Mx-1, Oas2 and Isg15 was 5–15 fold increased compared to controls. ISG expression in MDA5 Tgs was comparable or even greater than in the highly inflammatory and pathogenic environment of mice with a similar extra copy number of Tlr7 (~8–16 copies) (Fig. 1B) (16).
Despite elevated levels of IFN-I target genes in MDA5 Tg mice, there were surprisingly no pathological abnormalities in the liver, kidney, gut, lymph nodes, pancreas and thymus as determined by histological analysis. Circulating inflammatory cytokine levels were mostly comparable between MDA5 Tg and controls; only MCP-1 was significantly elevated in MDA5 Tgs (Supplemental Fig. 1A). There was also a small but significant increase in spleen size and blood analysis revealed mild anemia in the form of reduced mean corpuscular volume (MCV) of erythrocytes in MDA5 Tgs (Supplemental Fig. 1B & data not shown). In contrast to TLR7 overexpressing mice, which die starting at 2 months of age, MDA5 Tg mice exhibited identical morbidity to transgenic-negative littermate controls, up to 10 months of age, despite having a spontaneous IFN signature (16).
Evidence of cellular IFN-I activation, yet normal immune cell homeostasis in MDA5 Tgs
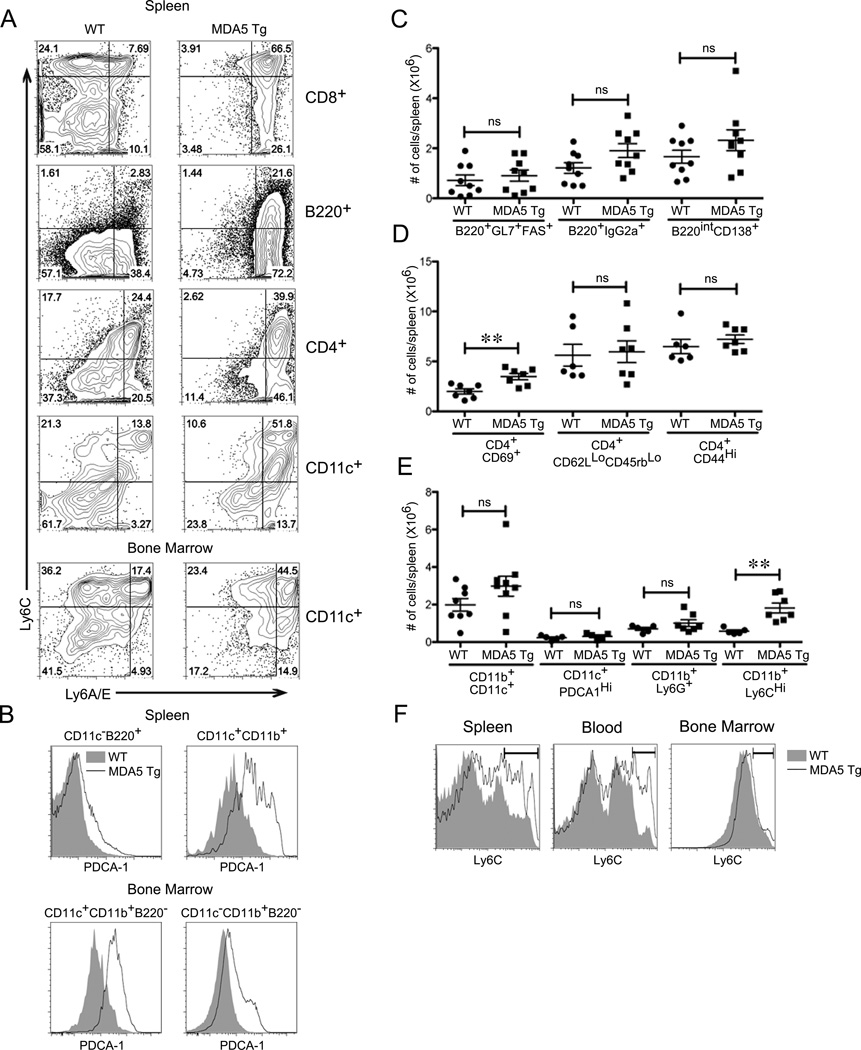
Given that IFN-I produced by antiviral pathways has been associated with a number of changes in immune cells (28, 29), we next analyzed the ex vivo phenotype of splenic cell populations in MDA5 Tgs using flow cytometry. The cell surface markers Ly6C and Ly6A/E (or Sca-1) are commonly upregulated in response to IFN-I (30). Indeed, both markers were highly upregulated on most splenic populations, including B cells, CD8+ T cells and DCs, while only Ly6A/E was enriched on CD4+ T cells (Fig. 2A). Populations in the bone marrow (BM) were comparable in numbers but also showed signs of IFN-I stimulation in MDA5 Tgs. Ly6C and Ly6A/E were enriched on DCs and Ly6A/E on CD11b+ cells in the BM (Fig. 2A and Supplemental Fig. 1C: left panel). The IFN-I-inducible pDC marker PDCA-1 was also upregulated in multiple myeloid and lymphoid populations in the spleen and BM, albeit at intermediate levels compared to the normally high expression on pDCs (Fig. 2B)(31).
Figure 2. Evidence of cellular IFN-I activation, yet normal lymphocyte homeostasis in MDA5 Tgs.
(A) Ly6C and Ly6A/E surface expression was evaluated by flow cytometry on CD8+, B220+ CD4+ and CD11c+ cells from WT and MDA5 Tg spleens or bone marrow. (B) PDCA-1 surface expression levels were evaluated on B cells and cDCs in the spleen as well as cDCs and monocytes in the bone marrow of WT and MDA5 Tg mice. (C–E) Splenocytes from MDA5 Tg and WT littermate controls were gated by flow cytometry using the indicated markers. Each dot represents absolute numbers of cells from an individual mouse. Mice were between 2 and 6 months of age with little age-dependent differences observed. A statistical T-test was performed comparing WT to MDA5 Tg. **P<0.01. (F) Representative dot plots of Ly6C staining on monocytes with a gate showing the frequency of CD11b+Ly6CHi cells. In A, B and F, representative cytometric plots from at least three mice in each group are shown.
MDA5 Tg spleens had normal numbers of B220+ B Cells, CD4+ T cells and CD8+ T cells. In the B cell compartment, there was a slight, yet non-significant increase in IgG+ class-switched MDA5 Tg B cells (Fig. 2C). There was no additional evidence of spontaneous B cell activation: numbers of germinal center B cells and plasma cells were comparable in WT and MDA5 Tg animals. Likewise, CD4+ T cells did not display signs of an activated phenotype in MDA5 Tgs, with the exception of increased CD69 expression (Fig. 2D). Additionally, FOXP3+ T regulatory cell frequency was similar between WT and MDA5 Tg mice (data not shown). Numbers of conventional dendritic cells and granulocytes were comparable between MDA5 Tg and WT (Fig. 2E). Despite the obvious signs of IFN-I production, which is thought to promote cDC maturation, we did not find evidence of cDC activation, as measured by CD40, MHC-II and CD86 expression on CD11c+CD11b+ cells from MDA5 Tg mice (Supplemental Fig. 1C: right panel). Additionally, we did not observe an increase in pDC frequency, defined by CD11c+PDCA1Hi cells (Fig. 2E). One splenic cell population that expanded significantly in MDA5 Tg mice was that of monocytes expressing high levels of Ly6C (Fig. 2E). This expansion was observed in the spleen and blood, but not in the BM of MDA5 Tgs (Fig. 2F). This type of cellular activation is characterized by IFN-I-inducible surface markers, but is fundamentally different than what we previously observed in the highly inflammatory TLR7 Tg environment (16).
Cellular phenotype in MDA5 Tgs is dependent on type I IFN and is initiated in radio-resistant cells
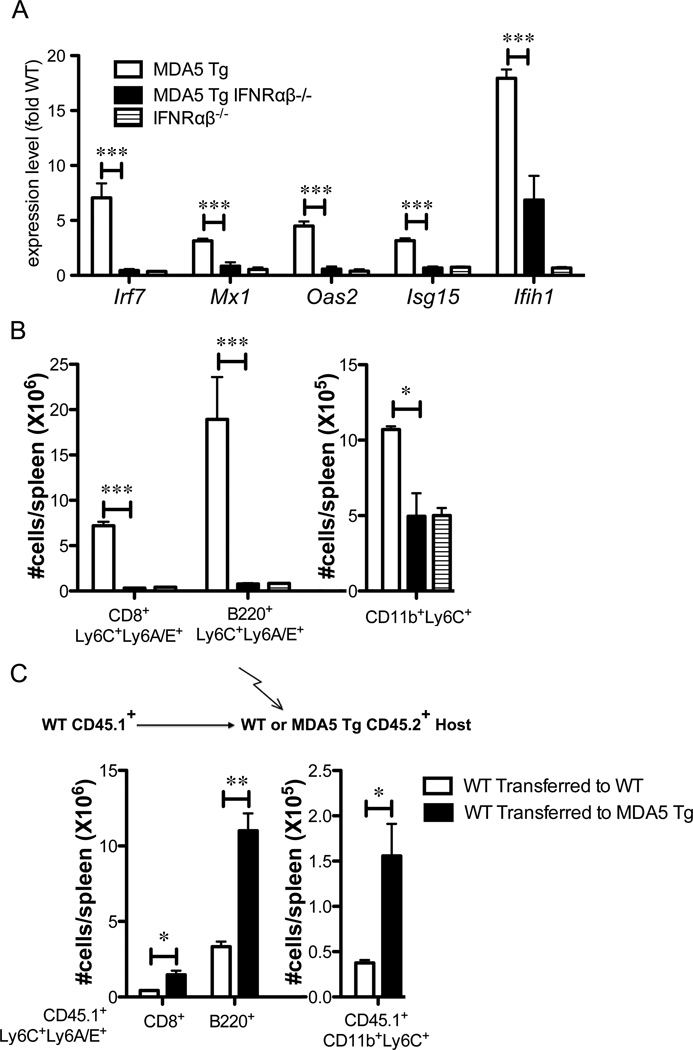
To test whether the phenotype observed in MDA5 Tg was driven by chronic IFN-I, we crossed them to mice deficient for the IFN-I receptor (IFNRαβ1), which eliminates all IFN-I signaling (32, 33). As expected, the upregulation of Irf7, Mx1, Oas2 and Isg15 mRNA in MDA5 Tg splenocytes was completely reversed to WT levels in MDA5 Tg mice deficient for the IFN-I receptor (Fig. 3A). Additionally, mRNA levels for Ifih1 were reduced by 60% in MDA5 Tg IFNRαβ−/− mice, suggesting that an IFN-I-dependent feed-forward pathway drives higher expression of Ifih1. We next looked at the cellular phenotype of MDA5 Tg mice deficient in the IFN-I receptor. High expression of Ly6C and Ly6A/E on CD8+ and B220+ cells in MDA5 Tg mice, as well as the expansion of Ly6Chi monocytes, were completely dependent on the IFN-I response (Fig. 3B).
Figure 3. Cellular phenotype in MDA5 Tgs is dependent on type I IFN and is initiated in radio-resistant cells.
(A) mRNA levels of the gene indicated were measured by real time PCR. Values are plotted as fold average WT. n=3–5 mice in each group. (B) Surface expression of Ly6C and Ly6A/E measured by flow cytometry on splenic cells gated as indicated below each graph. N=3–5 mice in each group. (C) T cell-depleted congenically marked bone marrow (WT CD45.1+) was transferred into lethally irradiated WT or MDA5 Tg CD45.2+ recipients (see schematic). Spleen cells from reconstituted mice were analyzed three months post-transfer, gated CD45.1 positive (donor-derived cells) and stained with the indicated markers for flow cytometry. Absolute numbers of cells per gate for individual mice are displayed. A statistical T-test was performed. Bone marrow transfer experiment was conducted twice with similar results. One experiment is depicted. *P<0.05, **P<0.01, ***P<0.001.
To determine the cellular source of IFN-I in MDA5 Tgs, we performed BM reconstitution experiments. Lethally irradiated WT or MDA5 Tg mice were reconstituted with T cell-depleted WT BM expressing the CD45.1 allotype so that donor and recipient cells could be distinguished. As shown in figure 3C, WT CD8+ T cells and B220+ B cells of donor origin (CD45.1+) displayed high levels of Ly6C and Ly6A/E when they developed in an MDA5 Tg environment but not when transferred to a WT mouse. Furthermore, Ly6CHi monocytes derived from WT BM were expanded when they developed in the MDA5 Tg environment. In mixed BM chimera studies, where MDA5 Tg BM was allowed to develop with WT BM, WT CD8+ T cells did not acquire the Ly6C+Ly6A/E+ phenotype, nor did CD11b+Ly6C+ cells expand (Supplemental Fig. 2). Thus, we conclude that the major cellular source for IFN-I in MDA5 Tg mice is a radiation-resistant population.
MDA5 Tg mice are resistant to lethal VSV infection
Since the chronic levels of IFN-I in the MDA5 Tg mice had surprisingly little effect on B cell activation, T cell activation and spontaneous disease development, we questioned whether or not the IFN-I levels induced by the transgene were physiologically important. Hence we tested the MDA5 Tgs ability to respond to a virus that is sensitive to the IFN-I response (32). We chose Vesicular Stomatitis Virus (VSV) due to its ability to induce IFN-I, a B cell response and a cytotoxic T cell response (34).
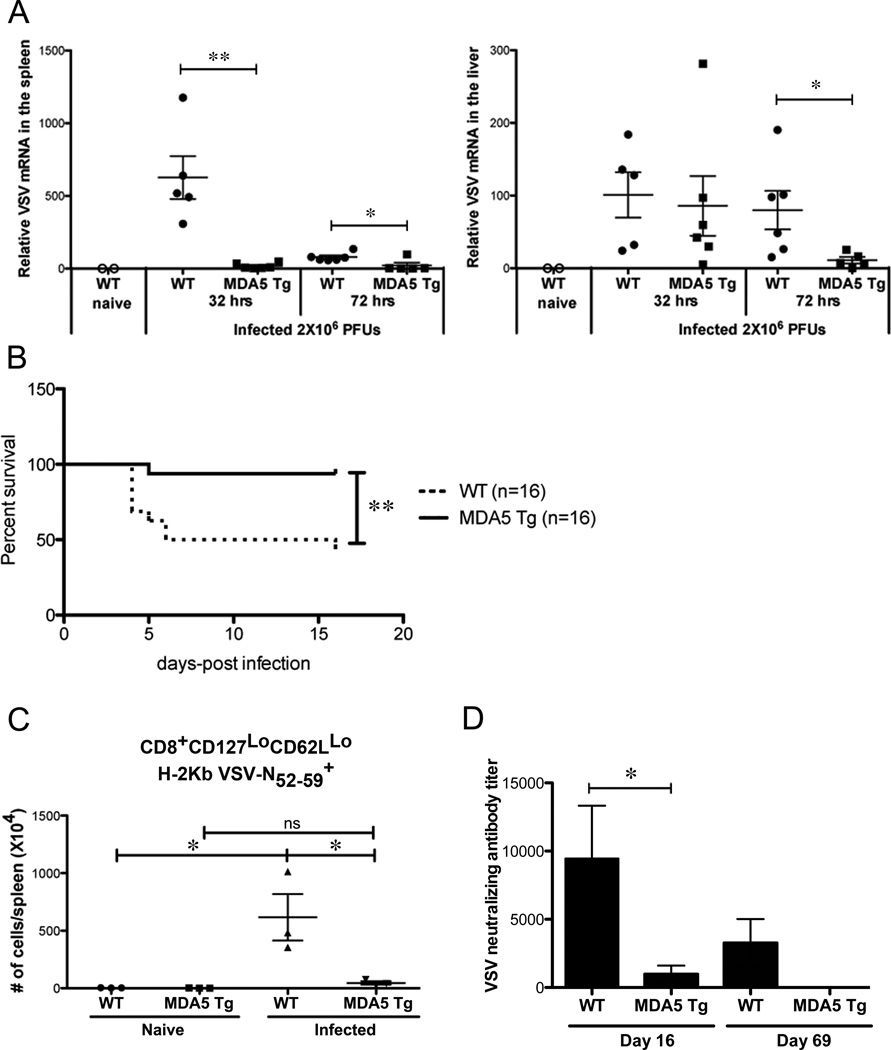
We measured virus titers in the spleens and livers of mice infected with VSV early after infection before there is an appreciable adaptive immune response (34). MDA5 Tgs had significantly lower levels of VSV mRNA, measured at 32 or 72 hours in the spleen, or at 72 hours after infection in the liver. (Fig. 4A). Thus, the MDA5 Tg environment is not permissive to VSV survival early during the course of infection.
Figure 4. MDA5 Tg mice are resistant to lethal VSV infection.
(A) WT or MDA5 Tgs mice were infected with 2×106 PFU VSV and at 32 and 72 hrs post-infection, spleen and liver RNA was isolated. VSV L-gene mRNA was detected using real-time PCR. Results are expressed as values from a standard curve generated from in vitro infected vero cells (see methods). Each dot represents a single mouse. Data are pooled from 2 independent experiments. (B) WT or MDA5 Tgs were challenged with 200×106 PFU VSV i.v. (the LD50) and monitored for survival. (C) WT or MDA5 Tg mice were infected i.v. with 2×106 PFU VSV and splenic antigen-specific CD8 T cells were measured 8 days later using fluorochrome-labeled H-2Kb/peptide pentamers. Each dot represents absolute number of cells from the indicated gate. Three independent experiments were performed with similar results. A representative experiment is shown. (D) WT or MDA5 Tg mice were infected with VSV, sera were collected 16 and 69 days later and tested for neutralizing activity to VSV in vitro. Neutralizing antibody titer is determined by the dilution required to inhibit 50% of plaque colonies. One of two similar experiments shown. n=3 mice in each group. For A and D, a statistical T-test was performed. For B, Log-rank (Mantel-Cox) and Gehan-Breslow-Wilcoxon tests were performed with similar P values. *P<0.05, **P<0.01.
Since MDA5 Tg mice rapidly cleared VSV compared to WT mice, we next asked whether they were resistant to the LD50 of VSV. When we challenged the mice with 2×108 PFUs of VSV, a dose high enough to kill 50% of WT mice, only 1 out of 16 MDA5 Tg mice succumbed to the infection (Fig. 4B).
The possibility still remained that MDA5 Tgs were mounting a stronger VSV-specific CD8 or B cell response against the virus and this accounted for the increased survival. Therefore, we measured the antigen-specific CD8 T cell response against the virus using a pentamer specific for a VSV immunodominant epitope (H-2Kb VSV N52–59 peptide). Although there was a robust expansion of antigen-specific CD8 T cells from WT mice at day 8-post infection, the MDA5 Tg response was significantly muted (Fig. 4C). To rule out the possibility of a stronger humoral response against the virus in MDA5 Tgs, we measured neutralizing antibody titers. MDA5 Tgs had a significantly lower titer of VSV neutralizing antibodies 16 days after infection compared to WT controls (Fig. 4D). Importantly, although there was still detectable viral neutralizing activity in WT sera 69 days after infection, there was no detectable neutralizing activity in MDA5 Tg sera, indicating that the B cell response against the virus was not delayed in these animals. Therefore, MDA5 overexpression appears to poise animals to be protected from viral infection, in the absence of priming of B and T lymphocytes normally associated with long-term memory.
The MDA5 Transgene enhances B cell responses in FcγR2B−/− mice
Since type one IFN has been associated with SLE and Single Nucleotide Polymorphisms (SNPs) in Ifih1 are associated with SLE, we next tested whether transgenic expression of MDA5 could exacerbate disease in a lupus-prone mouse strain (5, 22). We crossed the MDA5 Tgs to FcγR2B deficient mice, known to develop spontaneous disease characterized by the presence of anti-nuclear antibodies, splenomegaly and glomerulonephritis (35). Because FcγR2B deficient mice develop lethal disease between 6–9 months of age, we analyzed mice between the ages of 3–6 months old for signs of accelerated disease.
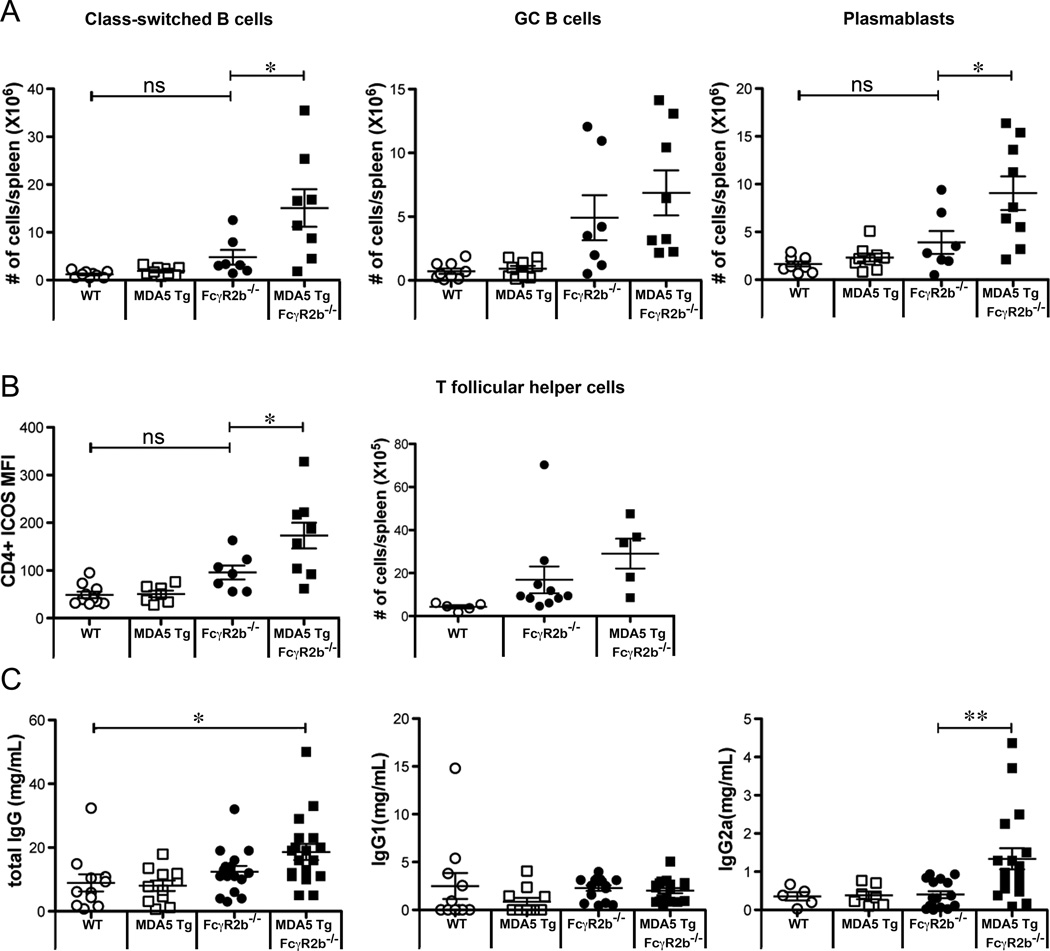
We analyzed B cell subsets in FcγR2B deficient mice with or without the MDA5 transgene. As shown earlier, the MDA5 Tg alone was not sufficient to drive B cell activation or isotype switching (Fig. 2D and 5A). In mice homozygous for the FcγR2B deletion, however, MDA5 Tg expression significantly enhances the number of class-switched B cells (B220+ IgG+) and plasmablasts (B220intCD138+) (Fig. 5A). Germinal center B cell and follicular helper CD4+ T cell numbers were comparable in MDA5 Tg FcγR2B−/− compared to FcγR2B−/− mice (Fig. 5A and 5B). We did, however, detect significantly higher ICOS expression on CD4+ cells from these mice (Fig. 5B). Additionally, we observed higher levels of Ly6C and Ly6/E on B cells in MDA5 Tg FcγR2B−/− mice (data not shown). The observed higher number of antibody producing and T helper cells correlated with elevated concentration of serum IgG2a in MDA5 Tg FcγR2B−/− mice (Fig. 5C).
Figure 5. The MDA5 Transgene enhances B cell responses in FcγR2B−/− mice.
(A) 3–6 month old WT, MDA5 Tg, FcγR2B−/− and MDA5 Tg FcγR2B−/− mice were analyzed for the presence of class-switched (B220+IgG2a+), GC (B220+FAS+GL7+) and plasma blast (B220intCD138+) B cells using flow cytometry. Additionally, data points for WT and MDA5 Tg animals from Figure 2 are plotted here for comparison. Absolute numbers for individual mice are displayed. (B) Mean fluorescence intensity of ICOS and numbers for CD4+CD44HiCXCR5+ T follicular helper cells were measured on splenocytes purified from mice of the indicated genotype. Each dot represents an individual mouse. (C) Total IgG, IgG1 and IgG2a was measured in the sera of 2–4 month old WT, MDA5 Tg, FcγR2B−/− and MDA5 Tg FcγR2B−/− mice by ELISA. Each dot represents a single mouse. *P<0.05, **P<0.01, ***P<0.001.
The MDA5 Transgene accelerates autoimmunity in FcγR2B−/− mice
We tracked the survival of a cohort of mice heterozygous or homozygous for the Fc receptor mutation that either lacked or contained the MDA5 transgene.
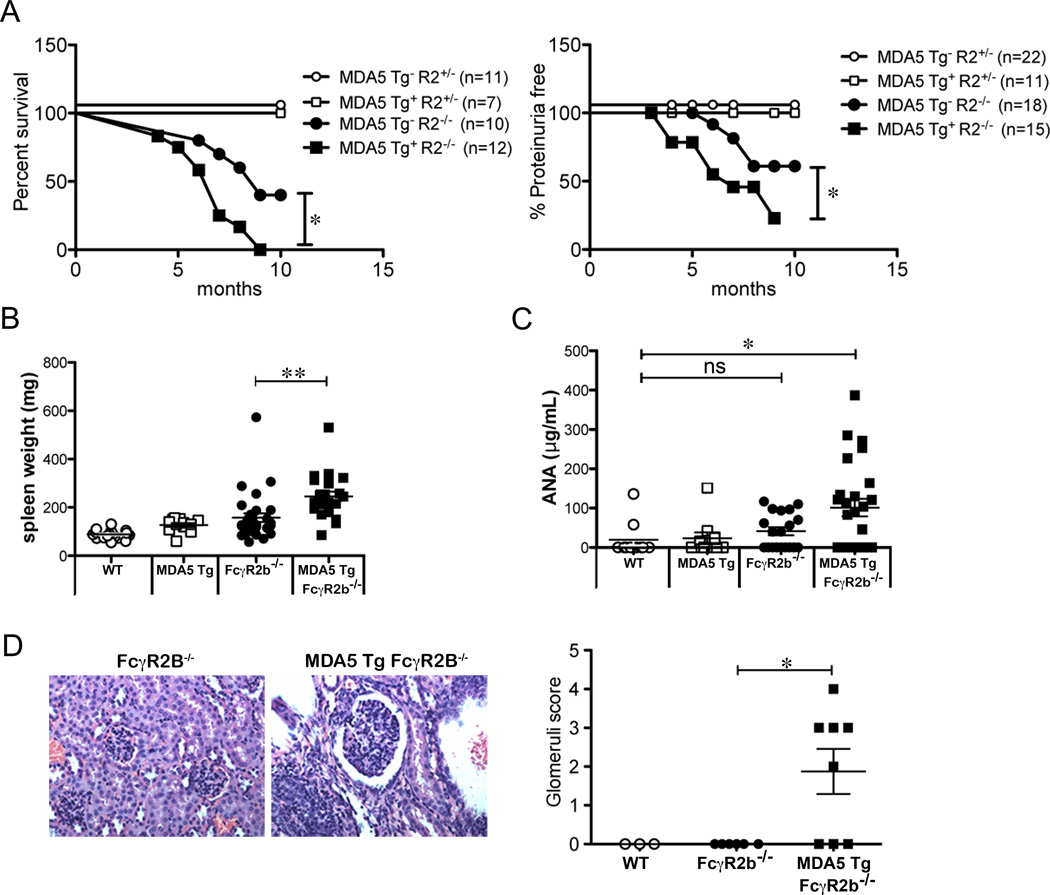
In a 10-month period, 40% of FcγR2B−/− mice survived while 100% of MDA5 Tg FcγR2B−/− mice succumbed to autoimmunity by 9 months of age (Fig. 6A left panel). We did not observe any difference in survival of MDA5 Tg mice that were heterozygous for FcγR2B. A higher fraction of MDA5 Tg FcγR2B−/− mice contained detectable amounts of protein in their urine, indicative of kidney disease (Fig. 6A right panel). Additionally, the MDA5 Tg accelerated the splenomegaly seen in FcγR2B-deficient mice (Fig. 6B). Serum ANA in MDA5 Tg FcγR2B−/− was significantly elevated compared to WT, FcγR2B−/− or MDA5 Tg littermates (Fig. 6C). The same trend was observed in antibodies specific for dsDNA (Supplemental Fig. 3). Glomerulonephritis was apparent in MDA5 Tg FcγR2B−/− mice at an age when FcγR2B−/− mice show no signs of inflammation (Fig. 6D). These data indicate that MDA5 overexpression does not drive autoimmune phenotypes, but can serve as a modifier of autoimmunity.
Figure 6. The MDA5 Transgene accelerates autoimmunity in FcγR2B−/− mice.
(A) Cumulative survival was monitored for FcγR2B+/−, MDA5 Tg FcγR2B+/−, FcγR2B−/− and MDA5 Tg FcγR2B−/− mice for 10 months (left panel). The level of proteinuria was measured in the urine using a dip stick (Right Panel). Data is represented as the percentage of mice that are proteinuria free throughout the 10-month period. (B) Spleen weights from 2–4 month FcγR2B−/− and MDA5 Tg FcγR2B−/− mice were quantified. Spleen weights for WT and MDA5 Tgs from Figure 1D are plotted for comparison. (C) Total IgG + IgM anti-nuclear antibody levels were quantified using an ANA ELISA. (D) Kidneys from 2.5–4 month old mice of the indicated genotype were stained with H&E and kidney sections were analyzed for glomerulonephritis. Glomeruli score was determined by measuring individual glomeruli from multiple fields of view. WT n=3 mice, FcγR2B−/− n=6 mice and MDA5 Tg FcγR2B−/− n=8 mice. For A–D each dot represents a single mouse. Data are pooled from multiple experiments. For A, Log-rank (Mantel-Cox) and Gehan-Breslow-Wilcoxon tests were performed with similar P values. *P<0.05, **P<0.01, ***P<0.001.
Discussion
MDA5 is a well-established ubiquitous viral dsRNA sensor that induces IFN-I through activation of IPS-I and IRF3 (2). The gene that encodes MDA5, Ifih1, has been linked to autoimmune diseases such as lupus and diabetes (21–25) and it seems plausible that aberrant expression or function of the MDA5 pathway could have pathological consequences. Our characterization of mice bearing multiple copies of the endogenous murine Ifih1 gene shows that augmented expression of MDA5 can lead to spontaneous generation of IFN-I with concomitant systemic upregulation of ISGs, but it does not induce detectable inflammatory cytokines or overt pathology. This result contrasts with the prevailing view that high levels of IFN-I are the cause of pathology both in patients and in a number of mouse models of disease (6, 10–12). Instead, our data implies that high levels of IFN-I could aggravate an ongoing inflammatory condition but that factors other than IFN-I are necessary for disease initiation. Given the difference in terms of which IFN signature genes are modulated between the TLR7 and MDA5 Tg animals, it is also possible that the qualitative differences in IFN signature genes determine the disease outcome.
The spontaneous production of IFN-I in MDA5 Tg mice is likely an amplification of a normally occurring low-level activation of this pathway, possibly in a minor cell population that is habitually exposed to small RNAs that could act as MDA5 ligands. The presence of IFN-I in the MDA5 Tg is readily detectable through the upregulation of ISGs in every immune cell population that we have tested. In fact, ISG upregulation must be widely spread in MDA5 Tg mice, as it is effective in preventing VSV replication when infected with lethal levels of the virus. The observed high expression of the Mx1 and Isg15 genes spontaneously induced by MDA5 seems sufficient to explain this preventive effect because VSV has been reported to be particularly sensitive to high expression of these genes (36, 37).
If high levels of ISGs induced by MDA5 overexpression are protective against a lethal viral infection, there must be a downside of this effect to explain why high expression of MDA5 and its antiviral target genes are not naturally selected. One negative effect of chronic high levels of IFN-I seems to be the inhibition of red blood cell production (38). Indeed, we observe a mild anemia in MDA5 Tg that fits the pattern of what has been described as anemia of chronic disease and that it somewhat mimics the anemia detected in patients that receive repeated IFNβ treatments (39, 40). Another issue that appears to result from this high level of ISGs is the fact that adaptive immune priming in response to a virus is impaired in MDA5 Tg animals. Because of this, long-term memory from this infection is likewise impaired.
Another downside of augmented MDA5 function and IFN-I levels is that they aggravate an ongoing autoimmune disease. While autoreactivity is not apparent in MDA5 Tg mice, this same transgene increases severity of disease in the lupus prone FcγR2B-deficient mouse. The type of autoimmune acceleration observed in the MDA5 Tg crossed to FcγR2B−/− is reminiscent of the Y-chromosome linked autoimmune accelerator (Yaa) mouse (41), which does not succumb to autoimmunity on its own. We earlier demonstrated that duplication of the Tlr7 gene was responsible for the autoimmune accelerator effect in Yaa (17). Whereas the health of TLR7 overexpressing animals is compromised when expressed greater than 2-fold, MDA5 Tg mice remain healthy even when levels are greater than ten-fold higher than WT animals. Furthermore, TLR7 activation in pDCs induces IFN-I production, and TLR7 expression itself is IFN-inducible (42, 43). We thought it possible that upregulation of TLR7 could explain why the presence of the MDA5 Tg was also accelerating lupus. However, we did not observe a significant upregulation of Tlr7 message in MDA5 Tgs and the phenotype was unaltered when crossed to Tlr7-null mice (data not shown). Additionally, when comparing IFN-I target gene expression in B cells, the MDA5 Tg had a broader and higher magnitude of induced ISGs than Yaa B cells (Table S1). These data suggest that Tlr7 gene duplication and the MDA5 Tg do not have a similar IFN-I-target gene profile and could be accelerating lupus in FcγR2B−/− mice in distinct ways. It is possible that the TLR7 Tg has B cell-intrinsic activation effects, whereas in the MDA5 Tg, B cells are mostly affected by expression of stromal cell-derived IFN-I. It would be interesting to investigate the qualitatively different contributions of other innate immune receptors to type I IFN gene induction and disease acceleration. The main effect of MDA5 Tg in autoimmune prone mice seems to be the increase in isotype-switched antibody-producing cells at early stages of disease. This is consistent with the known effects of IFN-I on B cell IgG class switching during a normal ongoing immune response as well as an autoimmune response (44, 45). These data together demonstrate the importance of regulating MDA5 expression for generating an antiviral response, and in the development of autoimmunity, in particular to prevent the IFN-enhancement of B cell activation that seems to be at the core of inflammatory disease. The MDA5 Transgenic represents an excellent system to study the effect of chronic IFN-I in the absence of inflammation.
Supplementary Material
Acknowledgements
We are grateful to Bethany Scott, Karen Hasty and Owokunile Otubusin for technical help with experiments, as well as Isharat Yusuf and Beth Walsh for critically evaluating the manuscript.
This research was supported in part by the Intramural Research Program of the NIH, NIAID.
Footnotes
The authors have no conflicting financial interests.
References
- 1.Kumar H, Kawai T, Akira S. Pathogen recognition by the innate immune system. International reviews of immunology. 2011;30:16–34. doi: 10.3109/08830185.2010.529976. [DOI] [PubMed] [Google Scholar]
- 2.Kawai T, Akira S. Toll-like receptor and RIG-I-like receptor signaling. Ann N Y Acad Sci. 2008;1143:1–20. doi: 10.1196/annals.1443.020. [DOI] [PubMed] [Google Scholar]
- 3.Liu SY, Sanchez DJ, Cheng G. New developments in the induction and antiviral effectors of type I interferon. Curr Opin Immunol. 2011;23:57–64. doi: 10.1016/j.coi.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mattei F, Schiavoni G, Tough DF. Regulation of immune cell homeostasis by type I interferons. Cytokine Growth Factor Rev. 2010;21:227–236. doi: 10.1016/j.cytogfr.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Sozzani S, Bosisio D, Scarsi M, Tincani A. Type I interferons in systemic autoimmunity. Autoimmunity. 2010;43:196–203. doi: 10.3109/08916930903510872. [DOI] [PubMed] [Google Scholar]
- 6.Crow MK. Type I interferon in systemic lupus erythematosus. Current topics in microbiology and immunology. 2007;316:359–386. doi: 10.1007/978-3-540-71329-6_17. [DOI] [PubMed] [Google Scholar]
- 7.Obermoser G, Pascual V. The interferon-alpha signature of systemic lupus erythematosus. Lupus. 2010;19:1012–1019. doi: 10.1177/0961203310371161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blanco P, Palucka AK, Gill M, Pascual V, Banchereau J. Induction of dendritic cell differentiation by IFN-alpha in systemic lupus erythematosus. Science. 2001;294:1540–1543. doi: 10.1126/science.1064890. [DOI] [PubMed] [Google Scholar]
- 9.Li QZ, Zhou J, Lian Y, Zhang B, Branch VK, Carr-Johnson F, Karp DR, Mohan C, Wakeland EK, Olsen NJ. Interferon signature gene expression is correlated with autoantibody profiles in patients with incomplete lupus syndromes. Clinical and experimental immunology. 2010;159:281–291. doi: 10.1111/j.1365-2249.2009.04057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nacionales DC, Kelly-Scumpia KM, Lee PY, Weinstein JS, Lyons R, Sobel E, Satoh M, Reeves WH. Deficiency of the type I interferon receptor protects mice from experimental lupus. Arthritis Rheum. 2007;56:3770–3783. doi: 10.1002/art.23023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richez C, Yasuda K, Bonegio RG, Watkins AA, Aprahamian T, Busto P, Richards RJ, Liu CL, Cheung R, Utz PJ, Marshak-Rothstein A, Rifkin IR. IFN regulatory factor 5 is required for disease development in the FcgammaRIIB−/−Yaa and FcgammaRIIB−/− mouse models of systemic lupus erythematosus. J Immunol. 2010;184:796–806. doi: 10.4049/jimmunol.0901748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santiago-Raber ML, Baccala R, Haraldsson KM, Choubey D, Stewart TA, Kono DH, Theofilopoulos AN. Type-I interferon receptor deficiency reduces lupus-like disease in NZB mice. J Exp Med. 2003;197:777–788. doi: 10.1084/jem.20021996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hron JD, Peng SL. Type I IFN protects against murine lupus. J Immunol. 2004;173:2134–2142. doi: 10.4049/jimmunol.173.3.2134. [DOI] [PubMed] [Google Scholar]
- 14.Schwarting A, Paul K, Tschirner S, Menke J, Hansen T, Brenner W, Kelley VR, Relle M, Galle PR. Interferon-beta: a therapeutic for autoimmune lupus in MRL-Faslpr mice. Journal of the American Society of Nephrology : JASN. 2005;16:3264–3272. doi: 10.1681/ASN.2004111014. [DOI] [PubMed] [Google Scholar]
- 15.Christensen SR, Shupe J, Nickerson K, Kashgarian M, Flavell RA, Shlomchik MJ. Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity. 2006;25:417–428. doi: 10.1016/j.immuni.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 16.Deane JA, Pisitkun P, Barrett RS, Feigenbaum L, Town T, Ward JM, Flavell RA, Bolland S. Control of toll-like receptor 7 expression is essential to restrict autoimmunity and dendritic cell proliferation. Immunity. 2007;27:801–810. doi: 10.1016/j.immuni.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pisitkun P, Deane JA, Difilippantonio MJ, Tarasenko T, Satterthwaite AB, Bolland S. Autoreactive B cell responses to RNA-related antigens due to TLR7 gene duplication. Science. 2006;312:1669–1672. doi: 10.1126/science.1124978. [DOI] [PubMed] [Google Scholar]
- 18.Fink J, Gu F, Ling L, Tolfvenstam T, Olfat F, Chin KC, Aw P, George J, Kuznetsov VA, Schreiber M, Vasudevan SG, Hibberd ML. Host gene expression profiling of dengue virus infection in cell lines and patients. PLoS neglected tropical diseases. 2007;1:e86. doi: 10.1371/journal.pntd.0000086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ubol S, Masrinoul P, Chaijaruwanich J, Kalayanarooj S, Charoensirisuthikul T, Kasisith J. Differences in global gene expression in peripheral blood mononuclear cells indicate a significant role of the innate responses in progression of dengue fever but not dengue hemorrhagic fever. The Journal of infectious diseases. 2008;197:1459–1467. doi: 10.1086/587699. [DOI] [PubMed] [Google Scholar]
- 20.Walsh RJ, Kong SW, Yao Y, Jallal B, Kiener PA, Pinkus JL, Beggs AH, Amato AA, Greenberg SA. Type I interferon-inducible gene expression in blood is present and reflects disease activity in dermatomyositis and polymyositis. Arthritis Rheum. 2007;56:3784–3792. doi: 10.1002/art.22928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Downes K, Pekalski M, Angus KL, Hardy M, Nutland S, Smyth DJ, Walker NM, Wallace C, Todd JA. Reduced expression of IFIH1 is protective for type 1 diabetes. PLoS One. 2010;5 doi: 10.1371/journal.pone.0012646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gateva V, Sandling JK, Hom G, Taylor KE, Chung SA, Sun X, Ortmann W, Kosoy R, Ferreira RC, Nordmark G, Gunnarsson I, Svenungsson E, Padyukov L, Sturfelt G, Jonsen A, Bengtsson AA, Rantapaa-Dahlqvist S, Baechler EC, Brown EE, Alarcon GS, Edberg JC, Ramsey-Goldman R, McGwin G, Jr, Reveille JD, Vila LM, Kimberly RP, Manzi S, Petri MA, Lee A, Gregersen PK, Seldin MF, Ronnblom L, Criswell LA, Syvanen AC, Behrens TW, Graham RR. A large-scale replication study identifies TNIP1, PRDM1, JAZF1, UHRF1BP1 and IL10 as risk loci for systemic lupus erythematosus. Nat Genet. 2009;41:1228–1233. doi: 10.1038/ng.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu S, Wang H, Jin Y, Podolsky R, Reddy MV, Pedersen J, Bode B, Reed J, Steed D, Anderson S, Yang P, Muir A, Steed L, Hopkins D, Huang Y, Purohit S, Wang CY, Steck AK, Montemari A, Eisenbarth G, Rewers M, She JX. IFIH1 polymorphisms are significantly associated with type 1 diabetes and IFIH1 gene expression in peripheral blood mononuclear cells. Hum Mol Genet. 2009;18:358–365. doi: 10.1093/hmg/ddn342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nejentsev S, Walker N, Riches D, Egholm M, Todd JA. Rare variants of IFIH1, a gene implicated in antiviral responses, protect against type 1 diabetes. Science. 2009;324:387–389. doi: 10.1126/science.1167728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smyth DJ, Cooper JD, Bailey R, Field S, Burren O, Smink LJ, Guja C, Ionescu-Tirgoviste C, Widmer B, Dunger DB, Savage DA, Walker NM, Clayton DG, Todd JA. A genome-wide association study of nonsynonymous SNPs identifies a type 1 diabetes locus in the interferon-induced helicase (IFIH1) region. Nat Genet. 2006;38:617–619. doi: 10.1038/ng1800. [DOI] [PubMed] [Google Scholar]
- 26.Van Bleek GM, Nathenson SG. Isolation of an endogenously processed immunodominant viral peptide from the class I H-2Kb molecule. Nature. 1990;348:213–216. doi: 10.1038/348213a0. [DOI] [PubMed] [Google Scholar]
- 27.Hole K, Clavijo A, Pineda LA. Detection and serotype-specific differentiation of vesicular stomatitis virus using a multiplex, real-time, reverse transcription-polymerase chain reaction assay. J Vet Diagn Invest. 2006;18:139–146. doi: 10.1177/104063870601800201. [DOI] [PubMed] [Google Scholar]
- 28.Trinchieri G. Type I interferon: friend or foe? J Exp Med. 2010;207:2053–2063. doi: 10.1084/jem.20101664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seo YJ, Hahm B. Type I interferon modulates the battle of host immune system against viruses. Advances in applied microbiology. 2010;73:83–101. doi: 10.1016/S0065-2164(10)73004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dumont FJ, Coker LZ. Interferon-alpha/beta enhances the expression of Ly-6 antigens on T cells in vivo and in vitro. Eur J Immunol. 1986;16:735–740. doi: 10.1002/eji.1830160704. [DOI] [PubMed] [Google Scholar]
- 31.Blasius AL, Giurisato E, Cella M, Schreiber RD, Shaw AS, Colonna M. Bone marrow stromal cell antigen 2 is a specific marker of type I IFN-producing cells in the naive mouse, but a promiscuous cell surface antigen following IFN stimulation. J Immunol. 2006;177:3260–3265. doi: 10.4049/jimmunol.177.5.3260. [DOI] [PubMed] [Google Scholar]
- 32.Muller U, Steinhoff U, Reis LF, Hemmi S, Pavlovic J, Zinkernagel RM, Aguet M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 33.Novick D, Cohen B, Rubinstein M. The human interferon alpha/beta receptor: characterization and molecular cloning. Cell. 1994;77:391–400. doi: 10.1016/0092-8674(94)90154-6. [DOI] [PubMed] [Google Scholar]
- 34.Hangartner L, Zinkernagel RM, Hengartner H. Antiviral antibody responses: the two extremes of a wide spectrum. Nat Rev Immunol. 2006;6:231–243. doi: 10.1038/nri1783. [DOI] [PubMed] [Google Scholar]
- 35.Bolland S, Ravetch JV. Spontaneous autoimmune disease in Fc(gamma)RIIB-deficient mice results from strain-specific epistasis. Immunity. 2000;13:277–285. doi: 10.1016/s1074-7613(00)00027-3. [DOI] [PubMed] [Google Scholar]
- 36.Pavlovic J, Zurcher T, Haller O, Staeheli P. Resistance to influenza virus and vesicular stomatitis virus conferred by expression of human MxA protein. J Virol. 1990;64:3370–3375. doi: 10.1128/jvi.64.7.3370-3375.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harty RN, Pitha PM, Okumura A. Antiviral activity of innate immune protein ISG15. Journal of innate immunity. 2009;1:397–404. doi: 10.1159/000226245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Means RT, Jr, Krantz SB. Progress in understanding the pathogenesis of the anemia of chronic disease. Blood. 1992;80:1639–1647. [PubMed] [Google Scholar]
- 39.Weiss G, Goodnough LT. Anemia of chronic disease. The New England journal of medicine. 2005;352:1011–1023. doi: 10.1056/NEJMra041809. [DOI] [PubMed] [Google Scholar]
- 40.Alanoglu G, Kilbas S, Arslan C, Senol A, Kutluhan S. Autoimmune hemolytic anemia during interferon-beta-I b treatment for multiple sclerosis. Multiple sclerosis. 2007;13:683–685. doi: 10.1177/1352458506071333. [DOI] [PubMed] [Google Scholar]
- 41.Bolland S, Yim YS, Tus K, Wakeland EK, Ravetch JV. Genetic modifiers of systemic lupus erythematosus in FcgammaRIIB(−/−) mice. J Exp Med. 2002;195:1167–1174. doi: 10.1084/jem.20020165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bekeredjian-Ding IB, Wagner M, Hornung V, Giese T, Schnurr M, Endres S, Hartmann G. Plasmacytoid dendritic cells control TLR7 sensitivity of naive B cells via type I IFN. J Immunol. 2005;174:4043–4050. doi: 10.4049/jimmunol.174.7.4043. [DOI] [PubMed] [Google Scholar]
- 43.Thibault DL, Chu AD, Graham KL, Balboni I, Lee LY, Kohlmoos C, Landrigan A, Higgins JP, Tibshirani R, Utz PJ. IRF9 and STAT1 are required for IgG autoantibody production and B cell expression of TLR7 in mice. J Clin Invest. 2008;118:1417–1426. doi: 10.1172/JCI30065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Le Bon A, Schiavoni G, D'Agostino G, Gresser I, Belardelli F, Tough DF. Type i interferons potently enhance humoral immunity and can promote isotype switching by stimulating dendritic cells in vivo. Immunity. 2001;14:461–470. doi: 10.1016/s1074-7613(01)00126-1. [DOI] [PubMed] [Google Scholar]
- 45.Marshak-Rothstein A. Toll-like receptors in systemic autoimmune disease. Nat Rev Immunol. 2006;6:823–835. doi: 10.1038/nri1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.