Abstract
Clinical research has linked post-traumatic stress disorder (PTSD) with deficits in fear extinction. However, it is not clear whether these deficits result from stress-related changes in the acquisition or retention of extinction or in the regulation of extinction memories by context, for example. In this study, we used the single prolonged stress (SPS) animal model of PTSD and fear conditioning procedures to examine the effects of prior traumatic stress on the acquisition, retention, and context-specificity of extinction. SPS administered one week prior to fear conditioning had no effect on the acquisition of fear conditioning or extinction but disrupted the retention of extinction memories for both contextual and cued fear. This SPS effect required a post-stress incubation period to manifest. The results demonstrate that SPS disrupts extinction retention, leading to enhanced fear renewal; further research is needed to identify the neurobiological processes through which SPS induces these effects.
Fear conditioning and extinction have been extensively used in recent years to study the neurobiology of psychiatric disorders characterized by excessive fear responses like phobia and post-traumatic stress disorder (PTSD) (Hofmann 2007; Hamm 2009; Koenigs and Grafman 2009; Yamamoto et al. 2009; Norrholm et al. 2010). Fear extinction refers to a form of learning that occurs when a conditioned fear stimulus (CS) no longer predicts the occurrence of an aversive event (Bouton et al. 2006; Quirk et al. 2006). It is commonly measured as a reduction in the magnitude of conditioned fear responses, including freezing behavior (Bouton et al. 2006; Quirk et al. 2006). Extinction memories are context-specific insofar as extinction retention is optimal in the context in which extinction was acquired (Bouton et al. 2006). If an extinguished CS is presented in a context that is inconsistent with the extinction context, conditioned fear returns; a phenomenon referred to as fear renewal (Corcoran and Maren 2001, 2004; Rothbaum and Davis 2003; Bouton et al. 2006).
The persistence of traumatic fear memories in PTSD suggests this disorder might be associated with extinction deficits. Previous clinical research supports this assertion (Rothbaum and Davis 2003; Anderson et al. 2004; Ressler et al. 2004; Quirk et al. 2006; Milad et al. 2008) and suggests these deficits are induced by trauma (Milad et al. 2008). However, inconsistent findings among clinical studies make it difficult to determine specific aspects of extinction that are disrupted in PTSD. Some studies report that PTSD patients have deficits in acquiring extinction as a result of enhanced fear conditioning (Peri et al. 2000; Norrholm et al. 2010), while other studies report select deficits in extinction retention with no change in fear conditioning or acquisition of extinction (Milad et al. 2008, 2009). Surprisingly, even though conditioned fear is renewed when extinction is tested outside of the extinction context (Corcoran and Maren 2001, 2004; Rothbaum and Davis 2003; Bouton et al. 2006), fear renewal has never been evaluated in PTSD patients. Thus, it is currently unclear what aspects of extinction are affected in PTSD.
Ethical constraints make it difficult to evaluate the effects of traumatic stress on fear extinction in humans, but this relationship can be readily studied using animal models of PTSD (Armario et al. 2008). These models use stressors that induce changes in hypothalamic-pituitary-adrenal (HPA) axis function and/or anxiety behavior that mimic specific PTSD symptoms (Armario et al. 2008). Previous studies have examined the effects of trauma-like stress on certain aspects of extinction. For example, studies reported that exposing rats to predator odor (Adamec et al. 2006; Cohen et al. 2006) disrupts acquisition and retention of cued extinction in subsets of these rats (Goswami et al. 2010). The results of other work suggest that exposing rats to a single prolonged stress (SPS) (Liberzon et al. 1997, 1999; Yamamoto et al. 2009) disrupts retention of context extinction (Yamamoto et al. 2007). However, in this study, contextual fear conditioning and acquisition of contextual fear extinction were not differentiated. As a result, it is unknown if the observed contextual extinction retention deficit in stressed rats was caused by enhanced contextual fear conditioning and/or deficits in acquisition of contextual extinction. It is also possible that extinction deficits were related to the contextual regulation of extinction, including enhanced fear renewal. Therefore, we conducted the present study to evaluate the effects of trauma-like stress using the SPS animal model on multiple aspects of fear conditioning and extinction, including acquisition and retention of contextual and cued fear extinction, and fear renewal.
While several animal PTSD models (e.g., Adamec et al. 2006; Cohen et al. 2006; Armario et al. 2008), are available, we used the SPS model, because it enhances arousal (Khan and Liberzon 2004; Kohda et al. 2007) and induces changes in HPA axis function similar to those observed in PTSD patients (Yehuda et al. 1993; Liberzon et al. 1997, 1999). SPS comprises two components: a single prolonged stress episode involving the serial application of three stressors (restraint, forced swim, ether) and a quiescent period of 7 d (Liberzon et al. 1997, 1999; Yamamoto et al. 2009). The effects of the single prolonged stress episode on HPA axis function are not observed shortly after stress exposure but develop during the quiescent period (Liberzon et al. 1997, 1999). Thus, we conducted an additional experiment to determine whether the quiescent period was also required for the development of extinction deficits.
Results
Experiment 1: SPS disrupts retention of context extinction
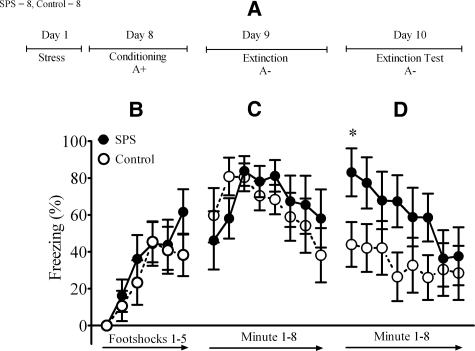
The design for Experiment 1 is illustrated in Figure 1A. In this experiment, we examined the effect of SPS on extinction of fear to a context that had been paired with aversive footshock. SPS was administered 1 week prior to contextual fear conditioning, which consisted of five footshock presentations in a distinct context (Context A). An analysis of variance (ANOVA) of post-shock freezing during the conditioning session revealed a significant main effect of time (F(5,55) = 19.41, P < 0.001) but no main effect of stress (F(1,13) = 0.33, P = 0.57) or interaction between stress and time (F(5,65) = 1.08, P = 0.38). These results indicated that SPS did not affect freezing during contextual fear conditioning (Fig. 1B).
Figure 1.
SPS induced deficits in contextual extinction retention. The numbers of SPS and control rats are given in the top left corner. (A) Diagram illustrates experimental design used in this study. The two character (e.g., A+) symbol describes conditioning and extinction parameters. First letter denotes context and second character denotes the presence or absence of footshocks. (B) SPS had no effect on freezing during conditioning or (C) extinction, (D) but disrupted contextual extinction retention. One rat was removed from the control group because it did not display a conditioned response. (*) Significant post hoc comparison between SPS and control groups at P < 0.05.
One day after fear conditioning, rats were placed into Context A for an 8-min extinction session. An ANOVA of minute by minute freezing revealed a significant main effect of time (F(1,13) = 13.32, P < 0.001). There was no main effect of treatment (F(1,13) = 0.07, P = 0.80) or treatment × time interaction (F(7,91) = 1.38, P = 0.24). These results indicated that conditioned freezing decreased over the course of the extinction session, and there was no significant difference between SPS or control rats (Fig. 1C).
Two days after fear conditioning (and 1 d after extinction), rats were returned to Context A for an 8-min extinction test to assess the retention of extinction. An ANOVA with the factors treatment (SPS vs. control), session (extinction vs. extinction test), and time (minute 1–8) revealed a significant three-way interaction (F(7,910) = 2.55, P = 0.04). This interaction was driven by significant differences in the levels of freezing across the first minute of the two extinction sessions (t(13) = 2.21, P < 0.05) (Fig. 1D). This reflected the finding that freezing in SPS rats was greater at the start of the extinction test when compared to controls. These results demonstrate that SPS disrupts retention of contextual extinction.
Experiment 2: SPS disrupts retention of extinction of cued fear
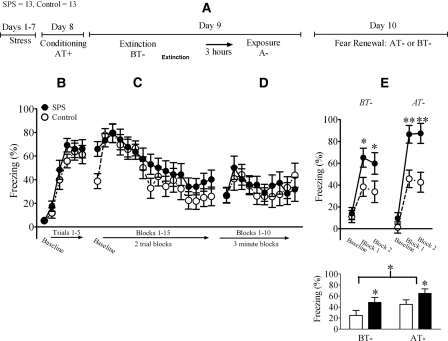
The design for Experiment 2 is illustrated in Figure 2A. In this experiment, we evaluated the effects of SPS on both the extinction and renewal of fear to an auditory CS using an ABA fear renewal paradigm (Chang et al. 2009). As in Experiment 1, SPS was administered 1 week prior to fear conditioning. On the conditioning day, rats were placed into Context A (the fear conditioning context) and subjected to five tone-shock trials. An ANOVA of cued freezing during the fear conditioning session revealed a significant main effect of trial (F(5,120) = 49.89, P < 0.001). There was no significant main effect of treatment (F(1,24) = 1.07, P = 0.31) or treatment × trial interaction (F(5,120) = 0.39, P = 0.85), which indicated that SPS had no effect on freezing during cued conditioning (Fig. 2B).
Figure 2.
SPS disrupted cued extinction retention and enhanced fear renewal. (A) Illustrates the experimental design used in this study. “T” denotes tone presentation. (B) SPS had no effect on freezing during conditioning, (C) extinction, or (D) re-exposure to the conditioning context. (E) SPS disrupted cued extinction retention and enhanced cued freezing during fear renewal. In the top panel, baseline and cued freezing (analyzed in blocks of five trials) were analyzed using ANOVA. In the bottom panel, cued freezing was subtracted from baseline freezing and these difference scores analyzed using ANOVA. One rat was removed from the control group because it did not display a conditioned response. (*) Main effect of stress; (**) main effect of context.
One day after fear conditioning, rats were placed into Context B (the extinction context) for a 30-tone extinction session. An ANOVA of freezing to the auditory CS during this session revealed a significant main effect of trial (analyzed in two trial blocks) (F(1,24) = 33.85, P < 0.001). Although baseline freezing in SPS rats was increased when compared to controls, there was no significant main effect of treatment (F(1,24) = 1.08, P = 0.31) or a treatment × trial interaction (F(15,360) = 1.19, P = 0.30) on this measure. These results indicated that conditioned fear to the CS and the extinction of that fear were not affected by SPS (Fig. 2C).
Three hours after extinction, rats were re-exposed to the fear conditioning context without tone presentations. Re-exposure to the conditioning context represents a context extinction procedure that lessens the potential confounding effect contextual conditioned freezing can have on fear renewal and is a standard procedure in ABA fear renewal paradigms (Chang et al. 2009). An ANOVA of freezing induced by re-exposure to the fear conditioning context did not reveal a significant main effect of time (F(9,216) = 0.76, P = 0.14), main effect of treatment (F(1,24) = 0.07, P = 0.8), or treatment × time interaction (F(9,216) = 0.65, P = 0.76) (Fig. 2D).
Two days after fear conditioning and 1 d after extinction, rats were either tested for extinction retention in the extinction context or tested for fear renewal in the fear conditioning context. Freezing during this extinction retention test was analyzed using two different statistical analyses. In the first analysis, cued freezing was analyzed in two five-trial blocks, and baseline and cued freezing were analyzed using ANOVA. There was a main effect of trial blocks (F(2,44) = 83.37, P < 0.001) and a significant trial blocks × context interaction (F(2,44) = 4.99, P = 0.01). These results indicated that cued freezing was enhanced in the fear conditioning context when compared to the extinction context (i.e., resulting in fear renewal). There was also a main effect of treatment (F(1,22) = 16.27, P = 0.001), which demonstrated that SPS enhanced freezing in both the extinction and fear conditioning contexts (Fig. 2E, top panel). However, there were no trial × treatment × testing context (F(2,44) = 0.3, P = 0.75) or treatment × testing context (F(1,22) = 1.09, P = 0.31) interactions. These results suggest that SPS disrupts cued extinction retention and enhances freezing during fear renewal.
In the second analysis, baseline freezing was subtracted from cued freezing, and these freezing difference scores were analyzed using ANOVA. This method has been previously used to selectively analyze the effects of experimental treatments on fear renewal (Corcoran and Maren 2004; Ji and Maren 2005). There was a main effect of testing context (F(1,22) = 4.40, P < 0.05), demonstrating that cued freezing was enhanced in the fear conditioning context when compared to the extinction context (i.e., resulting in fear renewal). There was also a main effect of treatment (F(1,22) = 6.34, P = 0.02), which demonstrated that SPS enhanced cued freezing in both the extinction and fear conditioning contexts (Fig. 2E, bottom panel), but no treatment × testing context interaction (F(1,22) = 0.05, P = 0.82). Taken together, these results also suggest that SPS disrupts cued extinction retention and enhances freezing during fear renewal.
Experiment 3: Extinction retention deficits induced by SPS are not observed shortly after stress exposure but develop over time
Previous research has demonstrated that the 7-d period after application of single prolonged stressors (i.e., restraint, forced swim, ether exposure) is needed to observe SPS-induced changes in HPA axis function (Liberzon et al. 1997, 1999). The aim of this experiment was to determine if a similar time interval is necessary to observe the effect of the single prolonged stressors on extinction retention.
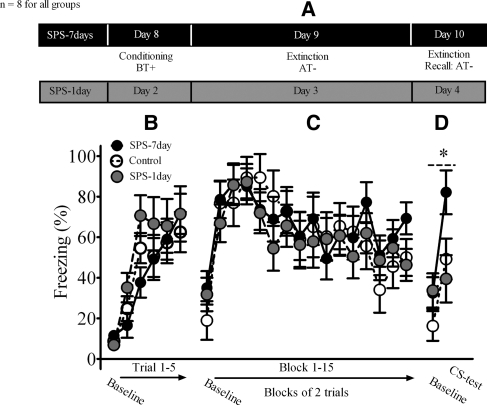
The design for this experiment is illustrated in Figure 3A. Rats were placed into Context B (the fear conditioning context) and subjected to five tone-shock trials. An ANOVA of cued freezing during the fear conditioning session revealed a significant main effect of trial (F(5,95) = 28.78, P < 0.001). There was no significant stress (F(1,9) = 0.72, P = 0.50) or stress × trial interaction (F(10,95) = 0.95, P = 0.50). These results demonstrated that freezing during fear conditioning was not different among the stress groups (i.e., control, SPS-1 d, SPS-7 d) (Fig. 3B).
Figure 3.
The effect of stress on extinction retention required a post-stress incubation period. (A) Illustrates the experimental design used in this experiment. (B) Neither the SPS-1 d nor SPS-7 d rats displayed different freezing levels during conditioning or (C) extinction. (D) Extinction retention was impaired in the SPS-7 d group, but not in the SPS-1 d group. One rat was removed from the SPS-7 d group, because its mean for the extinction test was greater than two standard deviations below the group mean. (*) Main effect of stress.
One day after fear conditioning, a 30-tone extinction session commenced in Context A (the extinction context). An ANOVA of cued freezing during this session revealed a significant main effect of trial (F(1,19) = 7.64, P = 0.01). There was no significant main effect of stress (F(1,19) = 0.19, P = 0.83) or stress × trial interaction (F(30,285) = 0.83, P = 0.71). These results indicated that expression of cued fear and acquisition of extinction were not different among the stress groups (Fig. 3C).
Two days after fear conditioning (and 1 d after extinction), an extinction retention test was conducted in the extinction context. An ANOVA of cued freezing during this test revealed a significant main effect of trial (F(5,95) = 28.78, P < 0.001) and a treatment × trial interaction that approached significance (F(2,18) = 2.95, P = 0.08). Given this potentially significant finding, we separately analyzed cued freezing during the extinction test between SPS-7 d and control rats and SPS-1 d and control rats. There was a significant main effect of treatment for SPS-7 d compared to control rats (F(1,13) = 6.25, P = 0.02), but no main effect of treatment for SPS-1 d compared to control rats (F(1,12) = 0.157, P = 0.7). These findings demonstrated that during the extinction test, cued freezing was enhanced in the SPS-7 d group when compared to controls, which demonstrated an extinction retention deficit in the SPS-7 d group but not in the SPS-1 d group (Fig. 3D). These findings suggest a post-stress incubation period is necessary in order to observe SPS effects on extinction retention.
Discussion
We have demonstrated deficits in contextual and cued extinction retention and enhanced fear renewal in animals exposed to SPS; an animal model of PTSD associated with enhanced arousal (Khan and Liberzon 2004; Kohda et al. 2007), altered HPA axis function (Liberzon et al. 1997, 1999), and hippocampal and medial prefrontal cortex (mPFC) abnormalities (Liberzon et al. 1999; Kohda et al. 2007; Knox et al. 2010). In contrast to these deficits, SPS exposure had no effect on acquisition or expression of conditioned fear or acquisition of conditioned extinction. Cued freezing during fear renewal was higher in SPS animals, and, insofar as extinction might have influenced renewal freezing, enhanced renewal freezing in SPS rats might have been caused by extinction retention deficits induced by SPS. However, it is also possible that a SPS-induced deficit in context processing contributed to enhanced renewal freezing in SPS rats. This is especially so because extinction retention and fear renewal were tested in two different contexts and expression of extinction during fear renewal is modulated by contextual processing (Bouton et al. 2006). Indeed, some suggestions that contextual processing abnormalities might be present both in PTSD (Liberzon and Sripada 2008; Rougemont-Bucking et al. 2011) and in SPS (Kohda et al. 2007; Yamamoto et al. 2007, 2009) have been reported in the literature. Similarly, there are a number of possible mechanisms that could contribute to the SPS extinction retention deficits we observed, such as deficits in consolidation and/or retrieval of extinction memory. A deficit in one or both memory processes could lead to a deficit in extinction retention because deficits in either of these memory processes would enhance freezing upon presentation of the extinguished CS, irrespective of the context in which it is presented. Thus, while the results of the study clearly demonstrate that SPS disrupts extinction retention, further research will be needed to determine if extinction consolidation and/or retrieval are affected by SPS or if context processing deficits are involved in SPS enhancement of cued freezing during fear renewal.
Exposure to other kinds of stressors also induces deficits in extinction retention. These include brief uncontrollable stress (Izquierdo et al. 2006), chronic stress (Miracle et al. 2006; Garcia et al. 2008; Baran et al. 2009; Wilber et al. 2011), and footshock stress (Rau et al. 2005; Maren and Chang 2006). Also, animals that are vulnerable to the effects of stress show extinction retention deficits (Goswami et al. 2010). A comparison of SPS-induced extinction retention deficits with other types of stress-induced extinction retention deficits reveals certain similarities. For example, both SPS and chronic stress exposure alter contextual processing (Kohda et al. 2007; Yamamoto et al. 2007, 2009; Baran et al. 2009), and these changes in contextual processing might contribute to chronic stress-induced extinction retention deficits (Baran et al. 2009) and SPS-enhanced fear renewal (see above). However, there are also notable differences. Exposing animals to brief uncontrollable and chronic stress prior to fear conditioning, or conducting fear conditioning in animals that are vulnerable to stress, enhances cued conditioned responding during fear conditioning and/or fear extinction (Izquierdo et al. 2006; Miracle et al. 2006; Goswami et al. 2010; Wilber et al. 2011), which suggests that stress-induced changes in fear memory may contribute to changes in extinction retention. This interpretation is also supported by the observation that, when the cue and the footshock presentations are not explicitly paired during fear conditioning (i.e., pseudoconditioning), chronic stress pre-exposure has no effect on extinction retention (Baran et al. 2009; Wilber et al. 2011). SPS exposure prior to fear conditioning disrupted extinction retention without having any effects on acquisition or expression of conditioned fear. While this suggests that SPS exposure disrupts extinction retention without affecting fear memory expression, further research is needed to explicitly test this.
Neurobiology of extinction retention deficits
Exposure to SPS induced deficits in the retention of both cued and contextual extinction in our animals, and there are number of specific neurobiological mechanisms that could mediate these effects. With respect to specific brain regions involved in extinction retention, previous research has demonstrated that the infralimbic (IL) region of the medial prefrontal cortex (mPFC) is critical for this. Temporary inactivation of the IL cortex disrupts acquisition of extinction (Sierra-Mercado et al. 2006); N-Methyl-D-aspartic receptor blockade in the IL cortex disrupts acquisition and consolidation of extinction (Burgos-Robles et al. 2007; Sotres-Bayon et al. 2009); stimulation of the IL enhances extinction retention (Vidal-Gonzalez et al. 2006); and permanent IL cortical lesions disrupt extinction retrieval (Lebron et al. 2004). It is currently believed that the IL cortex modulates extinction retention by modulating neural activity in brain regions critical for the expression of conditioned fear, such as the intercalated region, basolateral complex, and central nucleus of the amygdala (Rosenkranz and Grace 2002; Rosenkranz et al. 2003; Pare et al. 2004; Quirk and Mueller 2008; Li et al. 2011).
Studies that have specifically focused on the neurobiology of stress-induced extinction retention deficits also suggested that stress-induced changes in IL cortical function may underlie stress-induced extinction retention deficits (Izquierdo et al. 2006; Baran et al. 2009; Wilber et al. 2011). For example, stress-induced retraction of apical dendrites in the IL is associated with extinction retention deficits (Izquierdo et al. 2006; Miracle et al. 2006), and rats exposed to chronic stress show deficits in extinction retention and fail to show an enhancement in single unit activity in the IL cortex during extinction retention testing (Wilber et al. 2011). On the molecular level, repeated stress exposure enhances corticosterone-glucocorticoid receptor (GR) binding (Meaney et al. 1985; Xu et al. 1998; Liu and Aghajanian 2008; Gourley et al. 2009) and excitatory neurotransmitter release (Moghaddam 1993; Martin and Wellman 2011), which then can disrupt IL function (McEwen 2001; Miracle et al. 2006; Liu and Aghajanian 2008; Wilber et al. 2011). While there are clearly differences between repeated stress exposure within chronic stress procedures and SPS, there may yet be intermediate outcomes (e.g., enhanced GR signaling), by which chronic stress and SPS induce extinction retention deficits. For example, SPS does not alter baseline or stress-enhanced corticosterone levels (Liberzon et al. 1997; laboratory observation) but enhances GR expression in emotional circuits in the brain (Liberzon et al. 1999; Stout et al. 2010), including the PFC (Knox et al. 2011). SPS-enhanced GR expression in the PFC may serve to enhance corticosterone-GR binding in the IL cortex, which may disrupt IL cortical function, thereby inducing extinction retention deficits. This interpretation is indirectly supported by the finding that both SPS extinction retention deficits and GR enhancement require a similar post-stress incubation period to manifest (Liberzon et al. 1999; Experiment 3). Thus, SPS extinction retention deficits may not be observed one day after stress exposure (Experiment 3), because enhanced GR expression in the mPFC has not occurred at this point in time.
Alternatively, SPS effects on extinction retention can be mediated through SPS-induced changes in excitatory neurotransmitter levels, as SPS exposure attenuates glutamate levels in the mPFC (Knox et al. 2010). If SPS effects on glutamate levels in the IL cortex reflect the physiological status of glutamatergic neurotransmission in these animals, then this could result in decreased excitatory tone in the IL cortex, which, in turn, could directly affect extinction retention by disrupting IL cortical modulation of downstream targets, such as the intercalated region of the amygdala (Rosenkranz and Grace 2002; Rosenkranz et al. 2003; Pare et al. 2004; Quirk et al. 2006; Li et al. 2011). It has been proposed also that an “amygdala kindling mechanism” may mediate footshock-induced extinction retention deficits (Rau et al. 2005). We find no evidence of amygdala involvement in SPS effects on extinction retention (Knox et al. 2010). If amygdala kindling is associated with footshock-induced extinction retention deficits, SPS and footshock stress induce extinction retention deficits via different neurobiological mechanisms. Thus, SPS may induce extinction retention deficits by enhancing GR expression and/or decreasing glutamate levels in the IL region of the mPFC, but further research is needed to explore these possibilities.
Unexpected findings and potential limitations
We found no freezing difference between SPS and control rats during the baseline period of the fear renewal test. This might seem inconsistent with the contextual extinction retention deficit we have observed in Experiment 1, because the baseline period during renewal also reflects contextual extinction retention. However, the duration of the contextual extinction sessions differed greatly between the two experiments (8 min in Experiment 1, 30 min in Experiment 2). This procedural difference may explain the apparently contradictory findings and may also suggest that increasing extinction training for SPS animals might overcome the observed contextual extinction deficits. This hypothesis and the additional possibility that increasing cued extinction training may also attenuate cued extinction retention deficits induced by SPS should be explicitly addressed by future research.
In this study, animals exposed to SPS developed extinction retention deficits as a group, but only a proportion of individuals that experience trauma develop PTSD (Kessler et al. 1995; Yehuda and LeDoux 2007). Indeed, there is also variability in animal responses to SPS exposure (see Standard Errors). However, additional studies will be required to directly test this hypothesis. Combining SPS with other experimental manipulations (e.g., exposing genetically susceptible strains of animals to SPS) or increasing the number of rats exposed to SPS and developing a criteria for selecting rats that are most affected by SPS (Cohen et al. 2005) might be used to address these important questions.
Summary
Previous clinical studies suggested that trauma exposure induced selective deficits in extinction retention in PTSD patients (Milad et al. 2008, 2009). The results of our study using the PTSD animal model SPS further supports this hypothesis, as we have found similar, newly acquired extinction retention deficits in animals exposed to SPS treatment. Detailed examination of fear conditioning and extinction also revealed evidence of enhanced fear renewal in SPS exposed animals, a finding that can be directly caused by SPS extinction retention deficit, or, alternatively, suggests context processing deficits in SPS animals. Our time line experiments further suggest that trauma-induced deficits in extinction retention may require a post-trauma incubation period to manifest. Previous SPS studies suggest possible mechanisms that could mediate SPS extinction retention deficits and fear renewal enhancement, such as increased GR expression and/or decreased glutamatergic signaling in the IL. However, additional research is required to address these questions empirically.
Materials and Methods
Subjects
The subjects were 68 adult male Sprague Dawley rats (42–45-d-old; 150 g), obtained from Charles River (Wilmington, MA). Upon arrival, all rats were pair-housed for a minimum of 3 d and were then individually housed after exposure to stress or a control procedure. All rats had ad libitum access to water and standard rat chow. All experimental procedures were approved by the Veteran Affairs Institutional Animal Care Usage Committee.
SPS
Prior to conditioning, the rats were assigned to a stress or control procedure. Rats in the stress group were exposed to restraint for 2 h, followed immediately by 20 min of forced swimming. Forced swimming occurred in a plastic tub (55.6-cm diameter, 45.4-cm height), filled two-thirds from the bottom with water (20–24°C). Fifteen minutes after the forced swim, rats were exposed to ether (75 mL) in a glass dessicator until they were fully anesthetized displaying no toe or tail pinch response (<5 min of ether exposure). Immediately after the induction of general anesthesia, rats were removed from the dessicator, housed singly, and left undisturbed for either 1 d (SPS-1 d) or 7 d (SPS-7 d). Rats assigned to the control group were housed singly, left undisturbed, and remained in the housing colony until experimental procedures commenced.
Behavioral apparatus
All sessions were conducted in eight identical rodent observation chambers constructed of aluminum and Plexiglas (30 × 24 × 21 cm; MED Associates), situated in sound-attenuating chambers and located in an isolated room. The floor of each chamber consisted of 19 stainless steel rods (4 mm in diameter) spaced 1.5 cm apart (center to center). The grid floor was connected to a shock source and a solid-state grid scrambler (MED Associates) which delivered the footshock unconditioned stimulus (UCS). Mounted on one wall of the chamber was a speaker to provide a distinct auditory CS; on the opposite wall was a 15-W house light and a fan, which provided background noise (65 dB). Cameras mounted to the ceiling of the sound-attenuating chambers were used to record behavior, which was scored offline.
Two unique contexts were created by manipulating auditory, visual, and olfactory cues: Context A comprised a 1% acetic acid solution placed in trays at the bottom of the chambers, the house light on, chamber doors closed, and fans on in the chambers; Context B comprised a 1% ammonium hydroxide solution in chambers, red light on, chamber doors open, and fans off.
Experiment 1: Contextual fear conditioning, extinction, and extinction retention
On Day 1, 16 rats (SPS = 8; control = 8) were transported from their home cages in squads of eight and placed in the conditioning context (Context A). Rats received five unsignaled footshocks (1.0 mA, 1 sec) beginning 210 sec after being placed in the chambers. There was a 60-sec inter-trial interval (ITI), and the rats remained in the chambers for 60 sec after the last footshock presentation. One day after conditioning, all rats were placed back into Context A for 8 min without any presentations of the US in order to extinguish fear responding to the context. Two days after conditioning, all rats were placed into Context A for 8 min to test extinction.
Experiment 2: Cued fear conditioning, extinction, and fear renewal
A separate group of 28 rats (SPS = 14, control = 14) were placed in Context A and received five paired presentations of a tone (10 sec, 2 kHz, 80 dB) that coterminated with a footshock (1.0 mA, 1 sec) beginning 180 sec after being placed in Context A. There was a 60-sec ITI, and the rats remained in the chambers for 60 sec after the last footshock presentation. One day after conditioning, all rats were placed into a novel context (Context B) and were presented with 30 tone presentations (10 sec, 2 kHz, 80 dB, 60-sec ITI), in the absence of footshock, beginning 180 sec after being placed into the chambers in order to extinguish fear responding to the tone (i.e., extinction training). Three hours following extinction training, all rats were re-exposed to Context A for 30 min without any stimuli presentations. Two days after conditioning, rats were placed into the extinction context (Context B; SPS = 6, control = 6) or the conditioning context (Context A; SPS = 8, control = 8) and were presented with 10 tones beginning 180 sec after being placed into the chambers in order to assess extinction retention in these contexts.
Experiment 3: Cued fear conditioning, extinction, and extinction retention
Prior to fear conditioning, 16 rats were exposed to SPS and left undisturbed for either 7 d (SPS-7 d, n = 8), as in the previous experiments, or 1 d (SPS-1 d, n = 8). Another group of eight rats were assigned to the control condition. Rats were placed in Context B and fear conditioned to a tone cue as described above. One day after conditioning, all rats were placed into a novel context (Context A) and were presented with 30 tones (10 sec, 2 kHz, 80 dB, 60-sec ITI) beginning 180 sec after being placed into the chambers in order to extinguish fear responding to the tone. Two days after conditioning, all rats were placed back into the extinction context (Context A) and were presented with two tones beginning 180 sec after being placed into Context A in order to assess cued extinction retention.
Data analysis and statistical analysis
Freezing was defined as the absence of movement, except that necessary for breathing, for >2 sec and quantified as a percentage of the total time recorded. These values were analyzed using ANOVA, and post hoc comparisons using t-tests, with a Bonferroni correction, were performed when significant overall F ratios were obtained. The criterion for significance was set at P < 0.05. Rats that did not show a conditioned freezing response >30% at the start of a fear extinction session were excluded from final analyses. In addition, rats exhibiting freezing levels ±2 standard deviations from a group mean were removed from the analyses. All data are represented as means ± SEM.
Acknowledgments
The research in this report was funded by a VA Merit Award and Department of Defense grant (W81XWH-08-1-0661) to I.L. and an NIH grant (R01MH065961) to S.M. We thank Tori Nault and Curtis Henderson for their help in conducting this study.
References
- Adamec R, Head D, Blundell J, Burton P, Berton O 2006. Lasting anxiogenic effects of feline predator stress in mice: Sex differences in vulnerability to stress and predicting severity of anxiogenic response from the stress experience. Physiol Behav 88: 12–29 [DOI] [PubMed] [Google Scholar]
- Anderson P, Jacobs C, Rothbaum BO 2004. Computer-supported cognitive behavioral treatment of anxiety disorders. J Clin Psychol 60: 253–267 [DOI] [PubMed] [Google Scholar]
- Armario A, Escorihuela RM, Nadal R 2008. Long-term neuroendocrine and behavioural effects of a single exposure to stress in adult animals. Neurosci Biobehav Rev 32: 1121–1135 [DOI] [PubMed] [Google Scholar]
- Baran SE, Armstrong CE, Niren DC, Hanna JJ, Conrad CD 2009. Chronic stress and sex differences on the recall of fear conditioning and extinction. Neurobiol Learn Mem 91: 323–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME, Westbrook RF, Corcoran KA, Maren S 2006. Contextual and temporal modulation of extinction: Behavioral and biological mechanisms. Biol Psychiatry 60: 352–360 [DOI] [PubMed] [Google Scholar]
- Burgos-Robles A, Vidal-Gonzalez I, Santini E, Quirk GJ 2007. Consolidation of fear extinction requires NMDA receptor-dependent bursting in the ventromedial prefrontal cortex. Neuron 53: 871–880 [DOI] [PubMed] [Google Scholar]
- Chang CH, Knapska E, Orsini CA, Rabinak CA, Zimmerman JM, Maren S 2009. Fear extinction in rodents. Curr Protoc Neurosci Chapter 8: Unit 8.23 10.1002/0471142301.ns0823s47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen H, Zohar J, Matar MA, Kaplan Z, Geva AB 2005. Unsupervised fuzzy clustering analysis supports behavioral cutoff criteria in an animal model of posttraumatic stress disorder. Biol Psychiatry 58: 640–650 [DOI] [PubMed] [Google Scholar]
- Cohen H, Kaplan Z, Matar MA, Loewenthal U, Kozlovsky N, Zohar J 2006. Anisomycin, a protein synthesis inhibitor, disrupts traumatic memory consolidation and attenuates posttraumatic stress response in rats. Biol Psychiatry 60: 767–776 [DOI] [PubMed] [Google Scholar]
- Corcoran KA, Maren S 2001. Hippocampal inactivation disrupts contextual retrieval of fear memory after extinction. J Neurosci 21: 1720–1726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Maren S 2004. Factors regulating the effects of hippocampal inactivation on renewal of conditional fear after extinction. Learn Mem 11: 598–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia R, Spennato G, Nilsson-Todd L, Moreau JL, Deschaux O 2008. Hippocampal low-frequency stimulation and chronic mild stress similarly disrupt fear extinction memory in rats. Neurobiol Learn Mem 89: 560–566 [DOI] [PubMed] [Google Scholar]
- Goswami S, Cascardi M, Rodriguez-Sierra OE, Duvarci S, Pare D 2010. Impact of predatory threat on fear extinction in Lewis rats. Learn Mem 17: 494–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley SL, Kedves AT, Olausson P, Taylor JR 2009. A history of corticosterone exposure regulates fear extinction and cortical NR2B, GluR2/3, and BDNF. Neuropsychopharmacology 34: 707–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm AO 2009. Specific phobias. Psychiatr Clin North Am 32: 577–591 [DOI] [PubMed] [Google Scholar]
- Hofmann SG 2007. Enhancing exposure-based therapy from a translational research perspective. Behav Res Ther 45: 1987–2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Wellman CL, Holmes A 2006. Brief uncontrollable stress causes dendritic retraction in infralimbic cortex and resistance to fear extinction in mice. J Neurosci 26: 5733–5738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji J, Maren S 2005. Electrolytic lesions of the dorsal hippocampus disrupt renewal of conditional fear after extinction. Learn Mem 12: 270–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB 1995. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry 52: 1048–1060 [DOI] [PubMed] [Google Scholar]
- Khan S, Liberzon I 2004. Topiramate attenuates exaggerated acoustic startle in an animal model of PTSD. Psychopharmacology (Berl) 172: 225–229 [DOI] [PubMed] [Google Scholar]
- Knox D, Perrine SA, George SA, Galloway MP, Liberzon I 2010. Single prolonged stress decreases glutamate, glutamine, and creatine concentrations in the rat medial prefrontal cortex. Neurosci Lett 480: 16–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox D, Nault T, Henderson C, Liberzon I 2011. Linking single prolonged stress-induced extinction deficits to single prolonged stress enhanced glucocorticoid receptor expression in limbic regions. In Neuroscience Meeting Planner, 284.02. Society for Neuroscience (online), Washington, DC [Google Scholar]
- Koenigs M, Grafman J 2009. Posttraumatic stress disorder: The role of medial prefrontal cortex and amygdala. Neuroscientist 15: 540–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohda K, Harada K, Kato K, Hoshino A, Motohashi J, Yamaji T, Morinobu S, Matsuoka N, Kato N 2007. Glucocorticoid receptor activation is involved in producing abnormal phenotypes of single-prolonged stress rats: A putative post-traumatic stress disorder model. Neuroscience 148: 22–33 [DOI] [PubMed] [Google Scholar]
- Lebron K, Milad MR, Quirk GJ 2004. Delayed recall of fear extinction in rats with lesions of ventral medial prefrontal cortex. Learn Mem 11: 544–548 [DOI] [PubMed] [Google Scholar]
- Li G, Amano T, Pare D, Nair SS 2011. Impact of infralimbic inputs on intercalated amygdala neurons: A biophysical modeling study. Learn Mem 18: 226–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberzon I, Sripada CS 2008. The functional neuroanatomy of PTSD: A critical review. Prog Brain Res 167: 151–169 [DOI] [PubMed] [Google Scholar]
- Liberzon I, Krstov M, Young EA 1997. Stress-restress: Effects on ACTH and fast feedback. Psychoneuroendocrinology 22: 443–453 [DOI] [PubMed] [Google Scholar]
- Liberzon I, Lopez JF, Flagel SB, Vazquez DM, Young EA 1999. Differential regulation of hippocampal glucocorticoid receptors mRNA and fast feedback: Relevance to post-traumatic stress disorder. J Neuroendocrinol 11: 11–17 [DOI] [PubMed] [Google Scholar]
- Liu RJ, Aghajanian GK 2008. Stress blunts serotonin- and hypocretin-evoked EPSCs in prefrontal cortex: Role of corticosterone-mediated apical dendritic atrophy. Proc Natl Acad Sci 105: 359–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Chang CH 2006. Recent fear is resistant to extinction. Proc Natl Acad Sci 103: 18020–18025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin KP, Wellman CL 2011. NMDA receptor blockade alters stress-induced dendritic remodeling in medial prefrontal cortex. Cereb Cortex 21: 2366–2373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS 2001. Plasticity of the hippocampus: Adaptation to chronic stress and allostatic load. Ann N Y Acad Sci 933: 265–277 [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Aitken DH, Bodnoff SR, Iny LJ, Tatarewicz JE, Sapolsky RM 1985. Early post-natal handling alters glucocorticoid receptor concentrations in selected brain regions. Behav Neurosci 99: 765–770 [DOI] [PubMed] [Google Scholar]
- Milad MR, Orr SP, Lasko NB, Chang Y, Rauch SL, Pitman RK 2008. Presence and acquired origin of reduced recall for fear extinction in PTSD: Results of a twin study. J Psychiatr Res 42: 515–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, Zeidan MA, Handwerger K, Orr SP, Rauch SL 2009. Neurobiological basis of failure to recall extinction memory in post-traumatic stress disorder. Biol Psychiatry 66: 1075–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miracle AD, Brace MF, Huyck KD, Singler SA, Wellman CL 2006. Chronic stress impairs recall of extinction of conditioned fear. Neurobiol Learn Mem 85: 213–218 [DOI] [PubMed] [Google Scholar]
- Moghaddam B 1993. Stress preferentially increases extraneuronal levels of excitatory amino acids in the prefrontal cortex: Comparison to hippocampus and basal ganglia. J Neurochem 60: 1650–1657 [DOI] [PubMed] [Google Scholar]
- Norrholm SD, Jovanovic T, Olin IW, Sands LA, Karapanou I, Bradley B, Ressler KJ 2010. Fear extinction in traumatized civilians with post-traumatic stress disorder: Relation to symptom severity. Biol Psychiatry 69: 556–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pare D, Quirk GJ, Ledoux JE 2004. New vistas on amygdala networks in conditioned fear. J Neurophysiol 92: 1–9 [DOI] [PubMed] [Google Scholar]
- Peri T, Ben-Shakhar G, Orr SP, Shalev AY 2000. Psychophysiologic assessment of aversive conditioning in post-traumatic stress disorder. Biol Psychiatry 47: 512–519 [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D 2008. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology 33: 56–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Garcia R, Gonzalez-Lima F 2006. Prefrontal mechanisms in extinction of conditioned fear. Biol Psychiatry 60: 337–343 [DOI] [PubMed] [Google Scholar]
- Rau V, DeCola JP, Fanselow MS 2005. Stress-induced enhancement of fear learning: An animal model of post-traumatic stress disorder. Neurosci Biobehav Rev 29: 1207–1223 [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Rothbaum BO, Tannenbaum L, Anderson P, Graap K, Zimand E, Hodges L, Davis M 2004. Cognitive enhancers as adjuncts to psychotherapy: Use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry 61: 1136–1144 [DOI] [PubMed] [Google Scholar]
- Rosenkranz JA, Grace AA 2002. Cellular mechanisms of infralimbic and prelimbic prefrontal cortical inhibition and dopaminergic modulation of basolateral amygdala neurons in vivo. J Neurosci 22: 324–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz JA, Moore H, Grace AA 2003. The prefrontal cortex regulates lateral amygdala neuronal plasticity and responses to previously conditioned stimuli. J Neurosci 23: 11054–11064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbaum BO, Davis M 2003. Applying learning principles to the treatment of post-trauma reactions. Ann N Y Acad Sci 1008: 112–121 [DOI] [PubMed] [Google Scholar]
- Rougemont-Bucking A, Linnman C, Zeffiro TA, Zeidan MA, Lebron-Milad K, Rodriguez-Romaguera J, Rauch SL, Pitman RK, Milad MR 2011. Altered processing of contextual information during fear extinction in PTSD: An fMRI study. CNS Neurosci Ther 17: 227–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra-Mercado D Jr, Corcoran KA, Lebron-Milad K, Quirk GJ 2006. Inactivation of the ventromedial prefrontal cortex reduces expression of conditioned fear and impairs subsequent recall of extinction. Eur J Neurosci 24: 1751–1758 [DOI] [PubMed] [Google Scholar]
- Sotres-Bayon F, Diaz-Mataix L, Bush DE, LeDoux JE 2009. Dissociable roles for the ventromedial prefrontal cortex and amygdala in fear extinction: NR2B contribution. Cereb Cortex 19: 474–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout S, Tan M, Knox D, George SA, Liberzon I 2010. The effects of early life and adult stress on HPA-axis function and anxiety-like behavior. In Neuroscience Meeting Planner, 89.7. Society for Neuroscience (online), Washington, DC [Google Scholar]
- Vidal-Gonzalez I, Vidal-Gonzalez B, Rauch SL, Quirk GJ 2006. Microstimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear. Learn Mem 13: 728–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilber AA, Walker AG, Southwood CJ, Farrell MR, Lin GL, Rebec GV, Wellman CL 2011. Chronic stress alters neural activity in medial prefrontal cortex during retrieval of extinction. Neuroscience 174: 115–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Holscher C, Anwyl R, Rowan MJ 1998. Glucocorticoid receptor and protein/RNA synthesis-dependent mechanisms underlie the control of synaptic plasticity by stress. Proc Natl Acad Sci 95: 3204–3208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S, Morinobu S, Fuchikami M, Kurata A, Kozuru T, Yamawaki S 2007. Effects of single prolonged stress and D-cycloserine on contextual fear extinction and hippocampal NMDA receptor expression in a rat model of PTSD. Neuropsychopharmacology 33: 2108–2116 [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Morinobu S, Takei S, Fuchikami M, Matsuki A, Yamawaki S, Liberzon I 2009. Single prolonged stress: Toward an animal model of post-traumatic stress disorder. Depress Anxiety 26: 1110–1117 [DOI] [PubMed] [Google Scholar]
- Yehuda R, LeDoux J 2007. Response variation following trauma: A translational neuroscience approach to understanding PTSD. Neuron 56: 19–32 [DOI] [PubMed] [Google Scholar]
- Yehuda R, Southwick SM, Krystal JH, Bremner D, Charney DS, Mason JW 1993. Enhanced suppression of cortisol following dexamethasone administration in post-traumatic stress disorder. Am J Psychiatry 150: 83–86 [DOI] [PubMed] [Google Scholar]