Abstract
The repair and management of full-thickness skin defects resulting from burns and chronic wounds remain a significant unmet clinical challenge. For those skin defects exceeding 50%–60% of total body surface area, it is impractical to treat with autologous skin transplants because of the shortage of donor sites. The possibility of using tissue-engineered skin grafts for full-thickness wound repair is a promising approach. The primary goal of tissue-engineered skin grafts is to restore lost barrier function, but regeneration of appendages, such as hair follicles, has to be yet achieved. The successful regeneration of hair follicles in immunodeficient mice suggests that creating human hair follicles in tissue-engineered skin grafts is feasible. However, many limitations still need to be explored, particularly enriching isolated cells with trichogenic capacity, maintaining this ability during processing, and providing the cells with proper environmental cues. Current advances in hair follicle regeneration, in vitro and in vivo, are concisely summarized in this report, and key requirements to bioengineer a hair follicle are proposed, with emphasis on a three-dimensional approach.
Introduction
The repair and management of full-thickness wounds, such as extensive burns and chronic ulcers, have been a long-standing clinical challenge.1,2 In the treatment of such wounds, autologous grafts are considered the gold standard; however, two major challenges associated with these grafts limit their wide use, including the limited availability of intact skin for transplantation when skin defects exceed 50%–60% of total body surface area1 and the creation of additional morbidity at donor sites.2 Harvesting split-thickness autologous skin grafts can minimize some morbidity, but the lack of full dermis in such grafts often results in increased scarring and keloid formation, especially in children.3,4 Tissue-engineered skin grafts have been recognized as a promising alternative to autografts,3,4 because of their ability to rapidly close wounds for preventing dehydration and infection, without the limits of tissue availability.5,6 However, many skin appendages such as hair follicles, sebaceous glands, and sweat glands cannot be restored in the healed wounds by current tissue-engineered skin grafts7 mainly because of their limited self-regeneration capacity in adults8,9 and the lack of such appendage structures in the skin grafts.
The lack of hair in grafted sites has both psychosocial and physiological impacts on the patient.10 Hair follicles cannot regenerate on their own after damage.8 In this regard, new and healthy hair follicles or trichogenic cells need to be introduced to the affected area for regeneration of hairs. Several models have been established to address the reconstitution of hair follicles; however, most are based on the intact, healthy skin of immunodeficient mice,7,11 overlooking the complex effects of the wound microenvironment. In contrast to the extensive efforts in developing tissue-engineered skin grafts from hair follicle-derived stem cells,12 attempts to regenerate hair follicles in skin grafts have been limited. This is partially due to the lack of an effective approach to incorporate follicular cells into skin grafts. This review briefly discusses the fundamental components of hair follicles, current advances in follicular regeneration, and key requirements to engineer follicles.
Anatomy and Biology of Hair Follicles
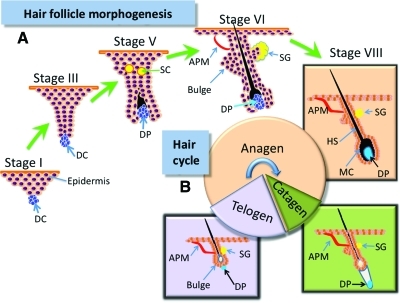
The mature hair consists of the hair shaft and the oval hair follicle with a multicylindrical stem. A human has around 5 million hair follicles with two types of hairs—terminal hair (long, thick pigmented) and vellus hair (thin, unpigmented).2,13 Hair follicles are complex, highly regenerative, ectodermal–mesodermal structures enriched with stem cells. They have lifelong cycles of growth following three distinct stages: anagen (rapid growth), catagen (regression), and telogen (resting period) (Fig. 1B). Intrinsic control of each stage involves various factors (e.g., cytokines, hormones, neurotransmitters, and their cognate receptors),14,15 even though the coordination of these factors remains to be elucidated.
FIG. 1.
(A) Schematic depiction of neofolliculogenesis in embryonic development. (B) Mature hair growth cycle with three distinct phases: anagen (growth phase), catagen (regression phase), and telogen (resting phase). APM, arrector pili muscle (red); DC, dermal condensate (blue); DP, dermal papilla (blue); HS, hair shaft (black); MC, melanocytes; ORS, outer root sheath; SC, sebocytes (yellow); SG, sebaceous gland. Color images available online at www.liebertonline.com/teb
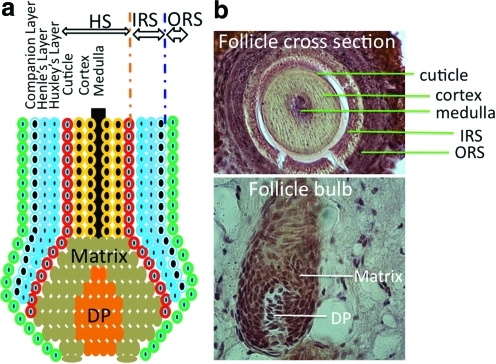
In the hair follicle, dermal papilla (DP), also called follicular papilla, an onion-like structure surrounded by hair bulb matrix (Fig. 2), is considered the commander of the hair follicle. It determines the hair thickness, length, and life cycle by adjusting the volume of DP cell (DPC) aggregate and secretory activity.10,16 The hair bulb not only produces the shaft, but also provides melanin granules to trichocytes for pigmentation.
FIG. 2.
(a) Schematic illustration of the concentric layers of a hair follicle bulb with ORS, IRS, and shaft. The IRS is composed of four layers: companion layer, Henle's layer, Huxley's layer, and the IRS cuticle. (b) Optical images of rat vibrissa hair follicle structures. Thin sections of the fixed vibrissa tissue specimen were stained with hematoxylin and eosin. DP, dermal papilla; HS, hair shaft; IRS, inner root sheath. Color images available online at www.liebertonline.com/teb
Hair matrix keratinocytes, located within the hair bulb, are the point of origin for trichocytes and cells of the inner root sheath (IRS) (Fig. 2). These cells have a high proliferative capacity, important for the growth and regeneration of the hair follicle and epidermis. The pluripotent epithelial stem cells located within the bulge can differentiate into outer root sheath (ORS), hair matrix, and hair shaft17 and can generate new hair by forming a secondary hair germ and help the wound healing process by reconstituting keratinocytes in response to damages to the bulge area.10
The dermal sheath (DS) surrounding the hair follicle contains progenitor cells that can differentiate into DP upon the removal of papilla.18 Beneath the DS, lies the ORS, and around the IRS is the basal layer of interfollicular epidermis.14 The IRS contains four layers: companion layer (CL), Henle's layer, Huxley's layer, and the IRS cuticle (Fig. 2).19 The interaction between mesenchymal (mainly DPCs) and epithelial (mainly follicular keratinocytes) portions of the hair follicle plays an essential role in normal hair growth.14 Other elements of the hair follicle, such as neurons and blood vessels, are derived from mast cell precursors.20 Neuronal stem cells are also found in hair follicles.21 As a center for several populations of stem cells, hair follicles have gained increasing attention for use as a cell source in regenerative medicine.
Functions of Hair Follicles
Although the precise role of hair cycling is unclear, it is generally accepted that this process removes debris and parasites from skin surface and encapsulates harmful chemicals within trichocytes.15 Hair cycling also serves as a self-protecting system, by removing rapidly proliferating keratinocytes in the catagen phase and preventing oxidative stress and malignant degeneration.10 It is speculated that the hair follicle may also have paracrine and endocrine functions on various cells and structures within the skin.10,22,23 Many factors secreted by follicles are known to be involved in wound healing, and mouse models have shown a relationship between the presence of follicles and the rate of wound healing, reepithelialization, and tissue expansion.23
Trichogenesis and Trichogenic Cells
The term trichogenesis refers to the cells' ability to form a new hair follicle. It is easy to postulate that cells at different stages of folliculoneogenesis have different trichogenic potentials and the most trichogenic cells should be those in the earliest stages of hair follicle development. During embryogenesis, mesenchymal stem cells aggregate into dermal condensations beneath the epidermis. With the participation of various factors, these aggregates induce the proliferation and differentiation of epidermal cells into follicular cells (Fig. 1A).7 Zheng et al.24 found that most trichogenic dermal cells were in a brief window between stages 1 and 3 of follicle development (Fig. 1A) and they showed that hypodermal injection of trichogenic mouse cells from stages 1 to 3 into mouse skin led to rapid formation of hair follicles. Although cells in early folliculoneogenesis stages have the most trichogenic capacity, adult hair follicles still retain their ability to regenerate.25
Regeneration of hair follicles during the normal hair cycle is mainly controlled by stem cells located in the epithelial bulge. Recent studies have shown that follicular stem cells can be isolated and used to regenerate hair follicles upon injection into immunodeficient mice when combined with follicular dermal cells.26
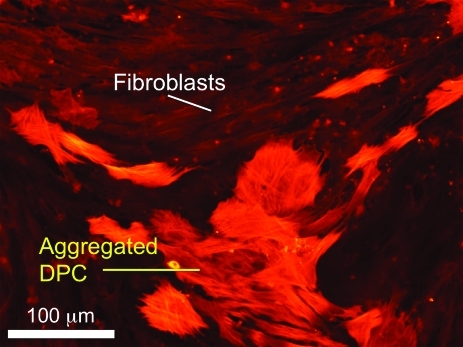
Follicular dermal cells play an essential role in hair morphogenesis and cycling. They can be divided into two distinct groups: DPCs and DS cells (DSCs), based on their specific markers, morphology, and function.27 Both cells are thought to be from the same mesenchymal origin, a dermal condensation,28 although some consider the DPCs to be of neural crest origin because of their expression of neural markers.29,30 DPCs and DSCs share several properties, but also have notable distinctions. For example, DSCs have a low alkaline phosphatase (ALP) activity and a high expression of alpha-smooth muscle actin (α-SMA) both in vivo and in vitro. In contrast, DPCs have a high ALP activity and an undetectable expression of α-SMA in vivo. Only upon in vitro culture, they show strong α-SMA expression (Fig. 3).27 DSCs are thought to play an important role in maintaining the DP, acting as a reservoir for DPCs.31,32 DSCs are able to differentiate into DPCs upon DPC loss,30 and vice versa,13 suggesting functional similarity and comparable hair formation potential between DPCs and DSCs.33 Actually, both DPCs and DSCs can substitute for dermal condensations to produce hair follicles as shown in mouse ears and footpads.30 However, without epidermal cells, neither DPCs nor DSCs can form hair follicles,34 demonstrating the necessity of mesenchymal–epidermal interactions.
FIG. 3.
Coculture of rat vibrissa DPCs with DFBs. DPCs expressed alpha-smooth muscle actin upon in vitro culture tended to aggregate, whereas DFBs did not. Scale bar=100 μm. DPCs, DP cells; DFBs, dermal fibroblasts. Color images available online at www.liebertonline.com/teb
Isolation and Culture of Trichogenic Dermal Cells
Both DSCs and DPCs can be expanded in vitro and used to generate hair follicles in vivo. The feasibility of this approach has been demonstrated by Reynolds and Jahoda using adult rat skin.19,34 To obtain DPCs or DSCs, various protocols have been explored, including explant outgrowth and enzymatic dissociation. In both approaches, microdissection is required to extract the hair follicles.7,33 In contrast to the low yield from explant outgrowth,35 which is thought to be mainly due to poor explant adhesion, enzymatic dissociation using type IV collagenase,36 dispase,37 or a combination of type I collagenase and dispase38 has proven to be more effective in obtaining a large quantity of viable DPCs within a short time frame. However, enzymatic damage to isolated cells and cell surface proteins may still be a problem. Li et al.39 have tried to facilitate the attachment and outgrowth of DPCs from follicular explants by a brief digestion of the hair follicles with collagenase I. This approach still requires approximately 1 week for the cells to migrate out. Further improvement of cell isolation efficiency is still needed.
Approaches to hair follicle formation with isolated cells are largely similar, but work is still needed to find ways of maintaining trichogenic potential. It has been found that the capacity of DPCs to aggregate closely correlates with their ability to form hair follicles.40 Freshly isolated DPCs retain a high tendency to aggregate, but this characteristic gradually disappears with extended culture. Horne and coworkers41 observed that DPCs cultured beyond 6 passages could no longer form cell aggregates or induce hair follicles upon implantation. Supplementation of culture media with 10 ng/mL fibroblast growth factor 2 (FGF-2) could eliminate this restriction by rescuing the lost inductive capacity of DPCs, even at passages as late as 26.42 This has been demonstrated with both rat and human cells.41,42 Coculture with keratinocytes or culture in keratinocyte-conditioned media also helps to retain the follicle-inducing capacity of passaged DPCs up to passage 90.43 These findings allow the generation of large numbers of inductive dermal cells from a small number of isolated hair follicles.
In Vivo Induction of Hair Follicles
Formation of new hair follicles does not occur in the adult; however, follicle formation can be experimentally induced using appropriate hair follicle structures or cells.25,44 In this endeavor, early efforts were made by transplanting either entire intact follicles or partial follicles. For instance, Cohen has shown the formation of new whisker by implanting isolated rat and guinea pig vibrissa trichogenic papilla to the rat ear.45 Following a similar approach but with only different portions of whisker follicles, a series of studies were performed by Oliver in an attempt to regenerate partial or complete hair follicles. It was found that the regeneration of DP was a prerequisite step to hair follicle formation, and root sheath cells surrounding hair follicles could regenerate the DP.18,46 It has been reported that intact DP, with epidermis formed from the keratinocytes of a rat footpad, can reconstruct the hair follicle and also sebaceous gland-like structures upon transplantation into the dorsal skin of a rat.47 Despite the success of hair follicle transplantation, its potential application is strictly constrained by the availability of intact follicles.
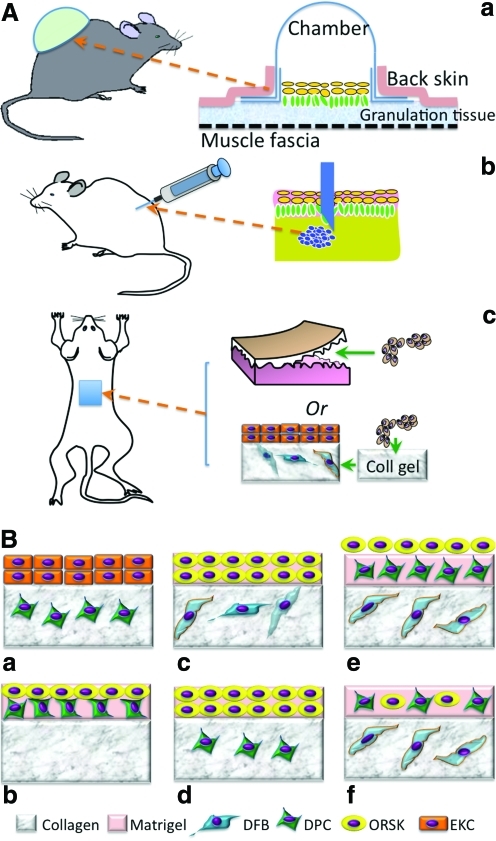
In recognition of the imperative contribution of DP to in vivo neofollicle formation, it is reasonable to assume that the cells isolated from DP alone may be sufficient to induce new hairs. To better evaluate the regeneration of hair follicles by dissociated single cells or aggregates, several suitable experimental models have been established. Three major models, that is, the silicon chamber model,48 the patch implantation model,24 and the subcutaneous cell injection model,49 have been developed for in vivo use in immunodeficient mice (Fig. 4A). In the first model, a bell-shaped silicon chamber is used to cover the murine full-thickness wound and thus protect cells in the wound bed. Using this model, it has been shown that a mixture of neonatal mouse epidermal and dermal cells can lead to hair formation after 3 weeks.48,50 With the same model, Kishimoto and Ehama assessed the trichogenic efficiency of dissociated DPCs using an elegant green fluorescent protein tag to determine the involvement of versican-positive DPCs in folliculogenesis.51 The result clearly confirmed that only versican-positive DP-derived cells could result in new hair follicle formation but not negative ones. Compared with the chamber model, the subcutaneous injection model [Fig. 4A(b)] is more convenient and rapid for a wide application. Using this model, Jahoda et al.41 have also shown that cultured papilla cells can lead to hair growth after injection into the follicles of a nude mouse ear. However, this subcutaneous injection of DPCs mainly restores preexisting injured hair follicles rather than completely regenerating a new one probably because of the lack of mesenchymal–epidermal interaction. To this end, an effort was made to subcutaneously inject a “hair bud,” composed of epidermal cell aggregates and DPCs, and indeed new hair formation was observed,25 suggesting the need of epidermal component in DPC-induced folliculogenesis. Interestingly, in this study the hair follicles grew outward from the aggregates, with the hair shaft projecting inward to the apoptotic cavity in the center of the epidermal aggregate.25 The subcutaneous implantation of a mixture of DS and ORS from human hair follicles within a customized cabin (1 cm in diameter and 0.5 cm in height) or as a “cyst” induced new follicle-like structures, but only the “cyst” showed hair shaft formation,28 indicating the need of cell aggregation. Potential challenges with this model are the difficulties of controlling the injection location and cell confinement and guiding hair to grow outward. In this regard, patch implantation can better overcome these issues. Jahoda and coworker34,52 demonstrated that both pelage (i.e., small hairs of animals) and vibrissa DPCs retained their trichogenic capacity by introducing either pelage or vibrissa DPCs in between the enzymatically separated epidermis and dermis of embryonic rat foot pads and then transplanting the combination to the rat dorsum. Similarly, the subcutaneous implantation of mouse foot pad tissues with DPC aggregates (1×104 cells/aggregate) inserted in the epidermal–dermal pockets formed follicular structures after 2 weeks in nude mice.42 So far all these in vivo models have proved the competency of DPCs in inducing hair follicle formation; however, this capacity requires the synergy of epidermal cells from either the epidermis, ORS keratinocytes (ORSKs), or bulge keratinocyte stem cells. Notably, the obtained in vivo folliculogenesis is mainly evidenced in mice or rats, which do not completely replicate the human environment. Further studies are needed to translate the results in mice to those in humans.
FIG. 4.
(A) Three typical in vivo models for evaluating the trichogenesis of isolated hair follicle cells, including the silicon chamber (a), subcutaneous injection (b), and patch implantation (c). (B) Typical combinations of various isolated hair follicle cells for in vitro trichogenesis in organotypic cultures using collagen, matrigel, or sandwiched gels. ORSKs, ORS keratinocytes; EKCs, epidermal keratinocytes. Color images available online at www.liebertonline.com/teb
In Vitro Generation of Hair Follicles
Unpredictable in vivo hair formation has inspired efforts to find a more controllable and reliable in vitro system. Several potential advantages can be identified from the in vitro system as summarized in Table 1 along with some foreseeable challenges.
Table 1.
Advantages and Possible Challenges of In Vitro Hair Follicle Regeneration
| Advantages |
|---|
| Controlled growth conditions with defined culture media and growth substrate (e.g., collagen gel) |
| Better manipulation of the heterogenic cell–cell and cell–matrix interactions necessary for trichogenesis by precise spatial arrangement (e.g., sandwiched gel culture) |
| Identification of specific exogenous stimuli relevant to folliculogenesis (e.g., hormones, growth factors, and vitamins) |
| Possible control of the size and density of neofollicles |
| Challenges |
|---|
| Restoration of the required epithelial–mesenchymal interactions for trichogenesis |
| Preservation of the proper responses of follicular cells to signals independent of native skin milieu and surrounding stroma |
| Maintenance of the trichogenic capacity in isolated follicle cells without juxtaposition to other cells and extracellular matrix |
| Creation of the concentric structure of hair follicles |
| Regeneration of complete hair follicles with a full capacity to form new hair shaft |
| Reestablishment of the normal hair growth cycle |
The development of various three-dimensional (3D) culture methods has dramatically enhanced the possibility of in vitro approaches toward follicle formation. Clearly, effective in vitro culture needs to maximally mimic the elements of an in vivo environment, for example, creating a 3D culture where follicular cells can form concentric aggregates, maintaining the interaction between ORSKs and DPCs, incorporating basement membrane proteins, and maintaining DPCs at a low level of proliferation and apoptosis. The replication of DPC–epithelial cell interaction is considered a crucial step leading to follicle formation. To do this a most popular organotypic culture model has been developed and widely used, which combines different cell populations such as DPCs, keratinocytes, and follicle ORS cells in either collagen gel or matrigel, or a sandwiched mixture of both.28,53 The model can be as simple as just encapsulation of the cells in the hydrogel, for example, coculture of keratinocytes and ORS cells from human hair follicles in collagen gel or matrigel produced epidermoid cyst-like spheroids and spike-like structures.54 The culture of epithelial cells of the inferior portion of human hair follicles on DSC-populated gel showed the formation of hair follicle-like concentric structures.28 Compared with single gel cultures, the sandwiched collagen and matrigel system better mimic the spatial organization of skin extracellular matrix (ECM). Several representative combinations as shown in Figure 4B have been proposed for studying potential trichogenesis. Among them the most promising configurations are those with the presence of ORSKs, DPCs, and dermal fibroblasts (DFBs) [Fig. 4B(e), (f)].53 In this model, a pseudo-dermis composed of collagen I mixed with interfollicular DFBs was created, and then on top of it either stacked layers of matrigel/DPCs and matrigel/ORSKs [“layered” system; Fig. 4B(e)] or single layer of mixed DPCs and ORSKs in the matrigel [“mixed” system; Fig. 4B(f)] was placed. In both systems, the ORSKs formed spheroid aggregates and retained their characteristic keratin expression patterns, but with better ORSK proliferation in the “mixed” culture. The kinetics of proliferation and apoptosis of DPCs were similar in both systems, as was the expression of their characteristic markers including versican.
Qiao et al.55 have also tried to explore the formation of follicles using stem cells, in which cell aggregates from a mixture of embryonic mouse follicular dermal and epidermal cells were prepared using a hanging-drop method with the assistance of methyl cellulose. The extended culture of such cell aggregates led to the development of a “proto-hair,” further confirming the necessity of mesenchymal–epidermal interaction in folliculogenesis.
Compared with overly simple two-dimensional monolayer cultures, 3D culture models could more accurately replicate the in vivo environment favorable for follicle development. However, significant progress is still required to achieve the delicate hair follicle architecture. In any in vitro model, the obtained structures must be grafted into a host in order to demonstrate in vivo utility.55
Creation of Hair Follicles in Skin Grafts
Preferably, tissue-engineered skin grafts should contain both epidermal and dermal layers.56 The epidermal layer protects the wound from dehydration and microorganism invasion, and the dermal layer integrates with the wound bed upon grafting. Although no hair follicles have been regenerated in current skin grafts, the mesenchymal–epidermal interaction between epidermal and dermal layers would facilitate hair follicle regeneration. During graft fabrication, a 3D interconnected porous scaffold is often used to provide the initial surface for skin cells to adhere and grow and then define the final tissue shape.9 Either made from natural polymers such as collagen, glycosaminoglycans, or chitosan or from synthetic polymers such as poly(d,l)-lactic acid-co-glycolic acid and poly(ethylene-glycol)-b-poly(butylene terephthalate),9,57 3D skin graft scaffolds always define a 3D microenvironment for the residing cells.
Temporary 3D scaffolds will be gradually replaced by newly formed skin tissue; however, the initial cell–scaffold interactions are crucial to functional tissue development. In recognition, a great deal of ongoing efforts focus on improving the scaffold design in order to better regulate the cells for desired phenotypes. The superiority of nanofibrous matrices in supporting tissue formation has been constantly highlighted mainly because of their size and morphologic similarity to native tissue ECM fibers. The flexibility of incorporating bioactive molecules such as collagen, elastin, and growth factors into the nanofibers adds another factor in recapitulating ECM composition.58 The utilization of such nanofibers for skin regeneration has been steadily increasing. For instance, the use of polycaprolactone nanofibers containing type I collagen or gelatin can facilitate the growth of skin fibroblasts and keratinocytes and subsequently formation of skin grafts,59–61 even though current reports mainly focus on in vitro studies, and nanofiber-based skin grafts are not yet available for clinical application. A foreseeable need for hair follicle regeneration is to spatially arrange hair-forming cells to mirror the in vivo structure,57 particularly to restore the trichogenic mesenchymal–epithelial interaction; however, the small pore size of these nanofibrous matrices (<5 μm) does not allow a complete infiltration of skin cells, resulting in nonuniform spatial distribution of seeded cells. Thus, diverse attempts have been made to seek a solution to this challenge. The layer-by-layer cell assembly method, established by our group, has proven to be effective in spatially organizing cells to achieve the uniformity.62 In this method, a thin layer (5–10 μm) of nanofibers is first collected on the media surface, and then cells are evenly seeded onto the thin nanofiber layer. After cell seeding, another thin layer of nanofibers is electrospun onto the seeded cells. By repeating this process, 3D multilayered cell/nanofiber constructs can be created with great similarity to in vivo tissues where cells distribute in between ECM fibers. During this cell layering process, the flexibility to vary the cell density and type allows the spatial assembly of follicular cells together with interfollicular DFBs and epidermal keratinocytes into 3D constructs to form follicle-containing skin grafts.
In designing the in vitro hair follicle-inducing environment, it is critical to provide the cells with necessary chemical cues for both growth and differentiation. These factors can be either incorporated into the scaffold during fabrication63 or supplemented into the culture media. Several major signaling pathways have been identified for their involvement in hair follicle formation. Work has shown that Wnt signaling, responsible for the formation of hair placodes, is necessary for maintaining the hair follicle-inducing ability of cultured DPCs.23,64 Although sonic hedgehog (Shh) signaling is not required for initiating hair follicle development, Shh signaling is essential for controlling growth and morphogenesis of the hair follicle.65 It is reported that Shh expressed by hair follicle epithelium controls DP development, formation, and maturation via influencing both mesenchyme and epithelium beyond the hair germ stage of development.66 On the other hand, overexpression of Shh suppressed the morphogenesis of hair follicles in embryos.67 Platelet-derived growth factor-A has been found to be responsible for the formation of DP and DS in a synergic fashion with Shh.68 Hepatocyte growth factor (HGF), a mitogen,69 motogen,70 and morphogen71 for a number of different organs,69 is also expressed by isolated and cultured human hair follicles72,73 and involved in hair growth. HGF has been shown to stimulate follicle growth and DNA synthesis in human hair as well as mouse vibrissae,74 upregulate DNA synthesis in hair bulb-derived keratinocytes,75 and modulate cyclic hair growth in mice.76 Another factor involved in hair growth is insulin growth factor I (IGF-I). The use of both IGF-I (10 ng/mL) in conjunction with IGF-II (100 ng/mL) showed an increased ability over insulin in preventing the catagen stage in hair follicles.36 Ascorbic acid-2-phosphate has been shown to have a stimulatory effect on DPC growth at a concentration of 0.25 mM, and this effect, however, was not shared by ORS cells or keratinocytes.77 The drug of minoxidil was also found to prolong the anagen stage through proliferative and antiapoptotic effects on the cells.78 The FGF and bone morphogenetic protein inhibitor, noggin, is among the other important signals involved in hair morphogenesis.79 With increasing understanding of molecular contribution of various factors to hair follicle development, more efforts are required to synergistically incorporate these factors during the creation of a follicle-friendly environment, especially with nanofibers, in tissue-engineered skin grafts.
Perspectives and Challenges
The innate regeneration ability of hair follicles has motivated our attempts to explore the possibility of producing new hair follicles through tissue engineering and stem cell technology. Among several critical hair-inducing factors identified, in vitro emulation of the in vivo epithelial–mesenchymal interaction has been shown to be important. Therefore, more effective 3D approaches that recapture epithelial–mesenchymal interactions are needed.80 The development of folliculoid organotypic systems has helped us to move along this path.53 Layering of ORS cells on top of DPC-containing matrigel in a layered sandwich approach or the mixing of DPCs and ORS cells in matrigel in a mixed sandwich improves on previous 3D models (Fig. 4B). However, several challenges have emerged during in vitro culture. These include replicating neofollicles so that they produce hair in the same way as natural hair follicles, ensuring a high efficiency of hair follicle formation with appropriately chosen trichogenic cell populations, attracting other skin cells such as melanocytes and Merkel cells into the engineered hair follicles, guiding the migration of stem cells and progenitor cells to the proper areas, and regulating their differentiation.
Conclusions
The addition of hair and hair follicles to skin grafts provides a number of benefits. In addition to psychosocial effects, follicles can influence wound healing especially reepithelialization by generating short-lived “transient amplifying” cells from bulge stem cells and contribute to the ultimate success of skin grafts.81,82 To create hair follicles in tissue-engineered skin grafts, a 3D skin scaffold that incorporates the necessary conditions is needed. Although current 3D skin scaffolds meet the need for 3D architecture and environment, they still do not provide other conditions necessary to induce hair follicles, such as the presence of certain growth factors and hormones as well as the mesenchymal–epithelial and neuroepithelial interactions.14,16,83 As such, further work must be done to incorporate these factors into an in vitro 3D system for inducing the formation of follicle-like structures in skin grafts.
Acknowledgments
The authors apologize for not including many other colleagues' wonderful work in this manuscript because of page limitations. The authors are grateful to Mr. Lawrence Chan, Dr. Jason Fong, and Mr. Thomas Cattabiani for their editorial assistance. The authors acknowledge the financial support partially from NIAMS (Grant No. 1R21 AR056416) and by Innovation and Entrepreneurship Doctoral Fellowship from Stevens Institute of Technology.
Disclosure Statement
No competing financial interests exist.
References
- 1.Loss M. Wedler V. Künzi W. Meuli-Simmen C. Meyer V.E. Artificial skin, split-thickness autograft and cultured autologous keratinocytes combined to treat a severe burn injury of 93% of TBSA. Burns. 2000;26:644. doi: 10.1016/s0305-4179(00)00045-0. [DOI] [PubMed] [Google Scholar]
- 2.Böttcher-Haberzeth S. Biedermann T. Reichmann E. Tissue engineering of skin. Burns. 2010;36:450. doi: 10.1016/j.burns.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 3.Aarabi S. Longaker M.T. Gurtner G.C. Hypertrophic scar formation following burns and trauma: new approaches to treatment. PLoS Med. 2007;4:234. doi: 10.1371/journal.pmed.0040234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berman B. Viera M.H. Amini S. Huo R. Jones I.S. Prevention and management of hypertrophic scars and keloids after burns in children. J Craniofac Surg. 2008;19:989. doi: 10.1097/SCS.0b013e318175f3a7. [DOI] [PubMed] [Google Scholar]
- 5.Rheinwald J.G. Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinising colonies from single cells. Cell. 1975;6:331. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- 6.Metcalfe A.D. Ferguson M.W. Tissue engineering of replacement skin: the crossroads of biomaterials, wound healing, embryonic development, stem cells and regeneration. J R Soc Interface. 2007;4:413. doi: 10.1098/rsif.2006.0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang C.C. Cotsarelis G. Review of hair follicle dermal cells. J Dermatol Sci. 2010;57:2. doi: 10.1016/j.jdermsci.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin P. Wound healing-Aiming for perfect skin regeneration. Science. 1997;276:75. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 9.Liu C. Xia Z. Czernuszka J.T. Design and development of three-dimensional scaffolds for tissue engineering. Chem Eng Res Des. 2007;85:1051. [Google Scholar]
- 10.Paus R. Foitzik K. In search of the “hair cycle clock”: A guided tour. Differentiation. 2004;72:489. doi: 10.1111/j.1432-0436.2004.07209004.x. [DOI] [PubMed] [Google Scholar]
- 11.Chuong C.M. Cotsarelis G. Stenn K. Defining hair follicles in the age of stem cell bioengineering. J Invest Dermatol. 2007;127:2098. doi: 10.1038/sj.jid.5700947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verma V. Verma P. Ray P. Ray A.R. Preparation of scaffold from human hair proteins for tissue-engineering applications. Biomed Mater. 2008;2:025007. doi: 10.1088/1748-6041/3/2/025007. [DOI] [PubMed] [Google Scholar]
- 13.Otberg N. Richter H. Variations of hair follicle size and distribution in different body sites. J Invest Dermatol. 2004;122:14. doi: 10.1046/j.0022-202X.2003.22110.x. [DOI] [PubMed] [Google Scholar]
- 14.Krause K. Foitzik K. Biology of the hair follicle: the basic. Semin Cutan Med Surg. 2006;25:2. doi: 10.1016/j.sder.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 15.Stenn K.S. Paus R. Controls of hair follicle cycling. Physiol Rev. 2001;81:449. doi: 10.1152/physrev.2001.81.1.449. [DOI] [PubMed] [Google Scholar]
- 16.Jahoda C.A. Reynolds A.J. Dermal-epidermal interactions. Adult follicle-derived cell populations and hair growth. Dermatol Clin. 1996;14:573. doi: 10.1016/s0733-8635(05)70385-5. [DOI] [PubMed] [Google Scholar]
- 17.Blanpain C. Lowry W.E. Geoghegan A. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004;118:635. doi: 10.1016/j.cell.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 18.Oliver R.F. Histologic study of whisker regeneration in the hooded rat. J Embryol Exp Morphol. 1966;16:231. [PubMed] [Google Scholar]
- 19.Schneider M.R. Schmidt-Ullrich R. Paus R. The hair follicle as a dynamic miniorgan. Curr Biol. 2009;19:132. doi: 10.1016/j.cub.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 20.Jahoda C.A. Cell movement in the hair follicle dermis—more than a two-way street? J Invest Dermatol. 2003;121:1267. doi: 10.1111/j.1523-1747.2003.12585.x. [DOI] [PubMed] [Google Scholar]
- 21.Amoh Y. Li L. Katsuoka K. Hoffman R.M. Multipotent nestin-positive keratinnegative hair follicle bulge stem cells can form neurons. Proc Natl Acad Sci U S A. 2005;102:5530. doi: 10.1073/pnas.0501263102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foitzik K. Lindner G. Mueller-Roever S. Control of murine hair follicle regression (catagen) by TGF-beta1 in vivo. FASEB J. 2000;14:752. doi: 10.1096/fasebj.14.5.752. [DOI] [PubMed] [Google Scholar]
- 23.Mayer J.A. Chuong C.M. Widelitz R. Rooster feathering, androgenetic alopecia, and hormone-dependent tumor growth: what is in common? Differentiation. 2004;72:474. doi: 10.1111/j.1432-0436.2004.07209003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng Y. Nace A. Chen W. Mature Hair Follicles Generated from Dissociated Cells: A Universal Mechanism of Folliculoneogenesis. Dev Dyn. 2010;239:2619. doi: 10.1002/dvdy.22398. [DOI] [PubMed] [Google Scholar]
- 25.Zheng Y. Du X. Wang W. Boucher M. Parimoo S. Stenn K. Organogenesis from dissociated cells: generation of mature cycling hair follicles from skin-derived cells. J Invest Dermatol. 2005;124:867. doi: 10.1111/j.0022-202X.2005.23716.x. [DOI] [PubMed] [Google Scholar]
- 26.Stenn K.S. Cotsarelis G. Bioengineering the hair follicle: fringe benefits of stem cell technology. Curr Opin Biotechnol. 2006;16:1. doi: 10.1016/j.copbio.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 27.Colin A. B. Smooth muscle α-actin is a marker for hair follicle dermis in vivo and in vitro. Cell Science. 1991;99:627. doi: 10.1242/jcs.99.3.627. [DOI] [PubMed] [Google Scholar]
- 28.Wu J.J. Zhu T.Y. Liu R.Q. Mai Y. Cheng B. Lu Z.F. Zhong B.Y. Tang S.Q. Hair follicle reformation induced by dermal papilla cells from human scalp skin. Arch Dermatol Res. 2006;298:183. doi: 10.1007/s00403-006-0686-9. [DOI] [PubMed] [Google Scholar]
- 29.Fernandes K.J. McKenzie I.A. Mill P. Smith K.M. Akhavan M. Barnabe-Heider F. A dermal niche for multipotent adult skin-derived precursor cells. Nat Cell Biol. 2004;6:1082. doi: 10.1038/ncb1181. [DOI] [PubMed] [Google Scholar]
- 30.Dry Driskell R.R. Giangreco A. Jensen K.B. Mulder K.W. Watt F.M. Sox2-positive dermal papilla cells specify hair follicle type in mammalian epidermis. Development. 2009;136:2815. doi: 10.1242/dev.038620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Supp D.M. Boyce S.T. Engineered skin substitutes: practices and potentials. Clin Dermatol. 2005;23:403. doi: 10.1016/j.clindermatol.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 32.Ito M. Liu Y. Yang Z. Nguyen J. Liang F. Morris R.J. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med. 2005;11:1351. doi: 10.1038/nm1328. [DOI] [PubMed] [Google Scholar]
- 33.McElwee K.J. Kissling S. Cultured peribulbar dermal sheath cells can induce hair follicle development and contribute to the dermal sheath and dermal papilla. J Invest Dermatol. 2003;121:1267. doi: 10.1111/j.1523-1747.2003.12568.x. [DOI] [PubMed] [Google Scholar]
- 34.Reynolds A.J. Jahoda C.A.B. Cultured dermal papilla cells induce follicle formation and hair growth by trans-differentiation of an adult epidermis. Development. 1992;115:587. doi: 10.1242/dev.115.2.587. [DOI] [PubMed] [Google Scholar]
- 35.Jahoda C.A.B. Oliver R.F. The growth of vibrissa dermal papilla cells in vitro. Br J Dermatol. 1981;105:623. doi: 10.1111/j.1365-2133.1981.tb00971.x. [DOI] [PubMed] [Google Scholar]
- 36.Warren R. Chestnut M.H. Wong T.K. Otte T.E. Lanners K.M. Meili M.L. Improved method for the isolation and cultivation of human scalp dermal papilla cells. J Invest Dermatol. 1992;98:693. doi: 10.1111/1523-1747.ep12499909. [DOI] [PubMed] [Google Scholar]
- 37.Chiu H.C. Chang C.H. Wu Y.C. An efficient method for isolation of hair papilla and follicle epithelium from human scalp specimens. Br J Dermatol. 1993;129:350. doi: 10.1111/j.1365-2133.1993.tb11870.x. [DOI] [PubMed] [Google Scholar]
- 38.Wu J.J. Liu R.Q. Lu Y.G. Zhu T.Y. Cheng B. Men X. Enzyme digestion to isolate and culture human scalp dermal papilla cells: a more efficient method. Arch Dermatol Res. 2005;297:60. doi: 10.1007/s00403-005-0554-z. [DOI] [PubMed] [Google Scholar]
- 39.Li Y. Li G.Q. Lin C.M. Cai X.N. One-step collagenase I treatment: an efficient way for isolation and cultivation of human scalp dermal papilla cells. J Dermatol Sci. 2005;37:58. doi: 10.1016/j.jdermsci.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 40.Osada A. Iwabuchi T. Kishimoto J. Hamazaki T.S. Okochi H. Long-term culture of mouse vibrissa dermal papilla cells and de novo hair follicle induction. Tissue Eng. 2007;13:975. doi: 10.1089/ten.2006.0304. [DOI] [PubMed] [Google Scholar]
- 41.Jahoda C.A. Horne K.A. Oliver R.F. Induction of hair growth by implantation of cultured dermal papilla cells. Nature. 1984;311:560. doi: 10.1038/311560a0. [DOI] [PubMed] [Google Scholar]
- 42.Osada A. Kobayashi K. Masui S. Hamazaki T.S. Yasuda K. Okochi H. Cloned cells from the murine dermal papilla have hair-inducing ability. J Dermatol Sci. 2009;54:129. doi: 10.1016/j.jdermsci.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 43.Inamatsu M. Matsuzaki T. Iwanari H. Yoshizato K. Establishment of rat dermal papilla cell lines that sustain the potency to induce hair follicles from afollicular skin. J Invest Dermatol. 1998;111:767. doi: 10.1046/j.1523-1747.1998.00382.x. [DOI] [PubMed] [Google Scholar]
- 44.Stenn K.S. Paus R. Controls of hair follicle cycling. Physiol Rev. 2001;81:1. doi: 10.1152/physrev.2001.81.1.449. [DOI] [PubMed] [Google Scholar]
- 45.Cohen J. The transplantation of individual rat and guinea-pig whisker papillae. J Embryol Exp Morphol. 1961;9:117. [PubMed] [Google Scholar]
- 46.Oliver R.F. Ectopic regeneration of whiskers in the hooded rat from implanted lengths of vibrissa follicle wall. J Embryol Exp Morphol. 1967;17:27. [PubMed] [Google Scholar]
- 47.Limat A. Hunzider T. Breitkreutz D. Fusenig N.E. Braathen L.R. Organotypic cocultures as model to study cell–cell and cell–matrix interactions of human hair follicle cells. Skin Pharm. 1994;7:47. doi: 10.1159/000211273. [DOI] [PubMed] [Google Scholar]
- 48.Ehama R. Ishimatsu-Tsuji Y. Iriyama S. Ideta R. Soma T. Yano K. Hair follicle regeneration using grafted rodent and human cells. J Invest Dermatol. 2007;127:2106. doi: 10.1038/sj.jid.5700823. [DOI] [PubMed] [Google Scholar]
- 49.Morris R.J. Liu Y. Marles L. Yang Z. Trempus C. Li S. Capturing and profiling adult hair follicle stem cells. Nat Biotechnol. 2004;22:411. doi: 10.1038/nbt950. [DOI] [PubMed] [Google Scholar]
- 50.Lichti U. Anders J. Yuspa S.H. Isolation and short-term culture of primary keratinocytes, hair follicle populations and dermal cells from newborn mice and keratinocytes from adult mice for in vitro analysis and for grafting to immunodeficient mice. Nat Protoc. 2008;3:799. doi: 10.1038/nprot.2008.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kishimoto J. Ehama R. Selective activation of the versican promoter by epithelial-mesenchymal interaction during hair follicle development. Proc Natl Acad Aci U S A. 1999;96:7336. doi: 10.1073/pnas.96.13.7336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jahoda C.A.B. Reynolds A.J. Dermal-epidermal interactions–follicle-derived cell populations in the study of hair growth mechanisms. J Invest Dermatol. 1993;101:33. doi: 10.1111/1523-1747.ep12362577. [DOI] [PubMed] [Google Scholar]
- 53.Havlickova B. Biro T. Mescalchin A. Arenberger P. Paus R. Towards optimization of an organotypic assay system that imitates human hair follicle-like epithelial–mesenchymal interactions. Br J Dermatol. 2004;151:753. doi: 10.1111/j.1365-2133.2004.06184.x. [DOI] [PubMed] [Google Scholar]
- 54.Limat A. Breitkreutz D. Hunziker T. Klein C.E. Braathen L.R. Outer root sheath (ORS) cells organize into epidermoid cyst-like spheroids when culture inside matrigel: a light-microscopic and immunohistological comparison between human ORS cells and interfollicular keratinocytes. Cell Tissue Res. 1994;275:169. doi: 10.1007/BF00305384. [DOI] [PubMed] [Google Scholar]
- 55.Qiao J. Turetsky A. Kemp P. Teumer J. Hair morphogenesis in vitro: formation of hair structures suitable for implantation. Regen Med. 2008;3:683. doi: 10.2217/17460751.3.5.683. [DOI] [PubMed] [Google Scholar]
- 56.MacNeil S. Biomaterials for tissue engineering of skin. Materials Today. 2008;11:5. [Google Scholar]
- 57.Uebersax L. Hagenmuller H. Hofmann S. Gruenblatt E. Muller R. Meinel L. Effect of scaffold design on bone morphology in vitro. Tissue Eng. 2006;12:3417. doi: 10.1089/ten.2006.12.3417. [DOI] [PubMed] [Google Scholar]
- 58.Kanani A.G.H. Bahrami S.H. Review on electrospun nanofibers scaffold and biomedical applications. Trends Biomater Artif Organs. 2010;24:93. [Google Scholar]
- 59.Hromadka M. Collins J.B. Nanofiber applications for burn care. J Burn Care Res. 2008;29:695. doi: 10.1097/BCR.0b013e31818480c9. [DOI] [PubMed] [Google Scholar]
- 60.Zhong S.P. Zhang Y.Z. Lim C.T. Tissue scaffolds for skin wound healing and dermal reconstruction. Nanobiotechnol. 2010;2:510. doi: 10.1002/wnan.100. [DOI] [PubMed] [Google Scholar]
- 61.Powell H.M. Boyce S.T. Engineered human skin fabricated using electrospun collagen-PCL blends: morphogenesis and mechanical properties. Tissue Eng Part A. 2009;8:2177. doi: 10.1089/ten.tea.2008.0473. [DOI] [PubMed] [Google Scholar]
- 62.Yang X. Shah M.E. Wang H. Nanofiber enabled layer-by-layer approach toward three-dimensional tissue formation. Tissue Eng Part A. 2009;15:945. doi: 10.1089/ten.tea.2007.0280. [DOI] [PubMed] [Google Scholar]
- 63.Golembo M. Controlled release of novel FGF variants for bone fracture healing. Eur Cells Mater. 2003;5:64. [Google Scholar]
- 64.Beaudoin G.M., III Sisk J.M. Coulombe P.A. Thompson C.C. Hairless triggers reaction of hair growth by promoting Wnt signaling. Proc Natl Acad Sci U S A. 2005;102:14653. doi: 10.1073/pnas.0507609102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.St-Jacques B. Dassule H.R. Karavanova I. Botchkarev V.A. Li J. Danielian P.S. McMahon J.A. Lewis P.M. Paus R. McMahon A.P. Sonic hedgehog signaling is essential for hair development. Curr Biol. 1998;8:1058. doi: 10.1016/s0960-9822(98)70443-9. [DOI] [PubMed] [Google Scholar]
- 66.Chiang C. Swan R.Z. Grachtchouk M. Bolinger M. Litingtung Y. Robertson E.K. Cooper M.K. Gaffield W. Westphal H. Essential role for Sonic hedgehog during hair follicle morphogenesis. Dev Biol. 1999;205:1. doi: 10.1006/dbio.1998.9103. [DOI] [PubMed] [Google Scholar]
- 67.Ellis T. Smyth I. Riley E. Bowles J. Adolphe C. Rothnagel J.A. Wicking C. Overexpression of sonic hedgehog suppresses embryonic hair follicle morphogenesis. Dev Biol. 2003;263:203. doi: 10.1016/s0012-1606(03)00394-4. [DOI] [PubMed] [Google Scholar]
- 68.Karlsson L. Bondjers C. Betsholtz C. Roles for PDGF-A and sonic hedgehog in development of mesenchymal components of the hair follicle. Development. 1999;126:2611. doi: 10.1242/dev.126.12.2611. [DOI] [PubMed] [Google Scholar]
- 69.Rubin J.S. Chan A.M. Bottaro D.P. Cech A.C. A broad-spectrum human lung fibroblast-derived mitogen is a variant of hepatocyte growth factor. Proc Natl Acad Sci, USA. 1991;88:415. doi: 10.1073/pnas.88.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weidner K.M. Behrens J. Vandekerckhove J. Birchmeier W. Scatter factor: molecular characteristics and effect on the invasiveness of epithelial cells. J Cell Biol. 1990;111:2097. doi: 10.1083/jcb.111.5.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Montesano R. Matsumoto K. Nakamura T. Orci L. Identification of a fibroblast-derived epithelial morphogen as hepatocyte growth factor. Cell. 1991;67:901. doi: 10.1016/0092-8674(91)90363-4. [DOI] [PubMed] [Google Scholar]
- 72.Mitsui S. Ochiai A. Hotta M. Tsuboi R. Ogawa H. Genes for a range of growth factors and cyclin-dependent kinase inhibitors are expressed by isolated human hair follicles. Br J Dermatol. 1997;137:693. [PubMed] [Google Scholar]
- 73.Jindo T. Tsuboi R. Takamori K. Ogawa H. Local injection of hepatocyte growth factor/scatter factor (HGF/SF) alters cyclic growth of murine hair follicles. J Invest Dermatol. 1998;110:338. doi: 10.1046/j.1523-1747.1998.00144.x. [DOI] [PubMed] [Google Scholar]
- 74.Jindo T. Tsuboi R. Imai R. Ogawa H. The effect hepatocyte growth factor: scatter factor on human hair follicle growth. J Dermatol Sci. 1995;10:229. doi: 10.1016/0923-1811(95)00429-v. [DOI] [PubMed] [Google Scholar]
- 75.Shimaoka S. Tsuboi R. Jindo T. Imai R. Ogawa H. Hepatocyte growth factor: scatter factor expressed in follicular papilla cells stimulates human hair growth in vitro. J Cell Physiol. 1995;165:333. doi: 10.1002/jcp.1041650214. [DOI] [PubMed] [Google Scholar]
- 76.Jindo T. Tsuboi R. Takamori K. Ogawa H. Local injection of hepatocyte growth factor:scatter factor (HGF:SF) alters cyclic growth of murine hair follicles. J Invest Dermatol. 1998;110:338. doi: 10.1046/j.1523-1747.1998.00144.x. [DOI] [PubMed] [Google Scholar]
- 77.Sung Y.K. Hwang S.Y. Cha S.Y. Kim J.C. The hair growth promoting effect of ascorbic acid 2-phosphate, a long-acting Vitamin C derivative. J Dermatol Sci. 2006;41:150. doi: 10.1016/j.jdermsci.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 78.Han J.H. Kwon O.S. Chung J.H. Kim K.H. Effect of minoxidil on proliferation and apoptosis in dermal papilla cells of human hair follicle. J Dermatol Sci. 2004;34:91. doi: 10.1016/j.jdermsci.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 79.Petiot A. Conti F.J. Grose R. Revest J.M. Hodivala-Dilke K.M. Dickson C. A crucial role for Fgfr2-IIIb signalling in epidermal development and hair follicle patterning. Development. 2003;130:5493. doi: 10.1242/dev.00788. [DOI] [PubMed] [Google Scholar]
- 80.Roh C. Tao Q. Lyle S. Dermal papilla-induced hair differentiation of adult epithelial stem cells from human skin. Physiol Genomics. 2004;19:207. doi: 10.1152/physiolgenomics.00134.2004. [DOI] [PubMed] [Google Scholar]
- 81.Cotsarelis G. Epithelial stem cells: a folliculocentric view. J Invest Dermatol. 2006;126:1459. doi: 10.1038/sj.jid.5700376. [DOI] [PubMed] [Google Scholar]
- 82.Ito M. Liu Y. Yang Z. Nguyen J. Liang F. Morris R.J. Stem cells in the hair follicle bulge contribute to wound healing but not to homeostasis of the epidermis. Nat Med. 2005;11:1351. doi: 10.1038/nm1328. [DOI] [PubMed] [Google Scholar]
- 83.Botchkarev V.A. Peters E. Botchkareva N. Maurer M. Paus R. Hair cycle- dependent changes in adrenergic skin innervation, and hair growth modulation by adrenergic drugs. J Invest Dermatol. 1999;113:878. doi: 10.1046/j.1523-1747.1999.00791.x. [DOI] [PubMed] [Google Scholar]