Abstract
A biodegradable elastomeric scaffold was created by electrospinning a mixed solution of poly(ester urethane)urea (PEUU) and porcine dermal extracellular matrix (dECM) digest, with PEUU included to provide elasticity, flexibility, and mechanical support and dECM used to enhance bioactivity and biocompatibility. Micrographs and differential scanning calorimetry demonstrated partial miscibility between PEUU and dECM. With greater dECM content, scaffolds were found to possess lower breaking strains and suture retention strength, although initial modulus was greater with higher dECM concentrations. The hybrid scaffolds containing 0% to 50% dECM had tensile strengths of 5 to 7 MPa, breaking strains of 138% to 611%, initial moduli of 3 to 11 Mpa, and suture retention strengths of 35 to 59 MPa. When hydrated, scaffolds were found to contract markedly with 50% dECM content. When used in a rat full-thickness abdominal wall replacement model, no herniation, infection, or tissue adhesion was observed after 4 and 8 weeks with a scaffold containing 25% dECM or a control 100% PEUU scaffold. Scaffolds incorporating dECM were significantly thicker at the time of explant, with greater numbers of associated smooth muscle actin–positive staining cells than in the control, but minimal cellular infiltration and remodeling of the scaffold were detected regardless of dECM addition. The processing of dECM and PEUU from a mixed solution thus provided a scaffold with evidence of better bioactivity and with mechanical properties not achievable with digested dECM alone.
Introduction
The approach of forming blended materials containing synthetic and biologically derived components is attractive in that one might potentially preserve bioactivities associated with the biological components while preserving the mechanical properties and parameter control associated with synthetic materials. Digested extracellular matrix (ECM) is of greater interest than purified proteins because it contains multiple growth factors and unknown bioactive agents that appear to function in situ to recruit progenitor cells and promote tissue remodeling,1,2 but poor processability and often nonideal mechanical properties act to limit its application in tissue repair and regeneration.
Combining ECM digests and synthetic materials to form processable blends has previously been reported. This materials development approach is in contrast to other composite techniques wherein the synthetic and biological components are kept distinct in the processing, and molecular level blending is not likely.3,4 Specific reports investigating a processable blend approach include the mixing of porcine urinary bladder matrix (UBM) digest and a biodegradable elastomeric poly(ester urethane) urea (PEUU) in hexafluoroisopropanol (HFIP) solvent followed by electrospinning to form an elastic composite material that had better cellular infiltration with greater ECM content in subcutaneous implants.5 In addition, two reports have examined the combination of small intestinal submucosa (SIS) powder and polycaprolactone (PCL) into a solution that was electrospun to form composites for potential application in neural tissue engineering,6,7 although to our knowledge, no reports exist regarding the application of such blended materials in a functional setting.
Worldwide, approximately 20 million operations are performed annually for abdominal wall hernia repair with patch placement.8 Clinically, nonbiodegradable synthetic patches, such as polypropylene9, polyester,10 and expanded poly(tetrafluoroethylene) (ePTFE)11, are associated with complications including seromas and fistula12,13, patient discomfort14, surgical site infections12,15, and limited physical activity of the patient due to poorer abdominal wall compliance16, whereas the weak mechanical properties, size limitation, and high cost of biological materials, such as acellular dermal matrix17,18 and SIS19,20, limited their use.21 A tissue engineering approach to hernia repair would be a promising alternative to meet current clinical needs through placing a biodegradable synthetic polymer–biological material blend patch at the abdominal wall site to provide temporary mechanical support and regenerate new abdominal muscles.
The objective of the current report was to develop a biodegradable elastomeric scaffold from a blended solution of synthetic polyurethane and porcine dermal ECM (dECM) digest and evaluate such a construct in an abdominal wall replacement model. Specifically, a fibrous scaffold of PEUU and dECM digest was fabricated by electrospinning from a single solution of the blended components. The morphology, thermal properties, shrinkage with hydration, and mechanical properties of formed scaffolds were investigated in vitro. A comparative study with blended PEUU and dECM and PEUU scaffolds was performed in a full-thickness abdominal wall defect model in rats. Histological and biomechanical evaluations of explanted constructs were performed to assess the host tissue response.
Materials and Methods
Materials
PEUU was synthesized from soft segment PCL diol (number average molecular weight=2000, Sigma), hard-segment 1,4-butanediisocyanate (BDI, Sigma) with a chain extender putrescine (BDA, Sigma) at a feeding molar ratio of PCL:BDI:BDA=1:2:122. HFIP (Oakwood, Inc.) was used as received.
dECM digest preparation
Fresh porcine skin was obtained from a local abattoir from pigs weighing approximately 100 to 120 kg. Skin samples were cleaned of connective tissue and the epithelium and placed in deionized water at 4°C for 24 hours. The tissue was then incubated in 0.02% trypsin/0.05% ethylenediaminetetraacetic acid at 37°C for 5 hours, followed by 3% (v/v) Triton-X at 4°C for 48 hours on a rocker and then 4% (w/v) deoxycholic acid at 4°C for 48 hours. The resultant dermal matrix was treated with 0.1% peracetic acid/4% ethanol for 2 hours and rinsed with deionized water and phosphate buffered saline (PBS). The dermal matrix sheets were then frozen and lyophilized. Lyophilized sheets were frozen again and pulverized into particulate form using a Waring commercial blender and Wiley Mill. A dECM solution was prepared by adding particulate lyophilized matrix to 1 mg/mL pepsin in hydrogen chloride for a final concentration of 10 mg/mL matrix/mL suspension. The suspension was mixed on a stir plate at room temperature for 48 hours, at which time no visible pieces of dermal matrix could be seen by the naked eye. The solution was homogenous and cloudy, with high viscosity. The dermal matrix digest without clarification was then neutralized and lyophilized for mixing with the polymer.
Electrospun PEUU/dECM hybrid sheets
PEUU and dECM digest were dissolved in HFIP to obtain a 10% (w/v) solution containing 0, 15, 25, and 50 wt% dECM to PEUU. The mixed solutions were cloudy, in contrast to PEUU alone in HFIP, which was clear. Neither sediment nor particles were visible in the cloudy mixed solutions. These solutions were used for electrospinning directly, without any further processing. A 10 mL syringe loaded with the mixed solution was mounted on a syringe pump, and the solution was fed at 1 mL/h through a stainless steel capillary (1.2 mm inner diameter) suspended 15 cm over a stainless steel mandrel (19-mm diameter) rotating at 250 rpm. The mandrel was mounted on a stage that reciprocally translated 8 cm along the direction of the mandrel axis at a rate of 5 cm/s. Two high-voltage generators were used to charge the steel capillary to 15 kV and the mandrel to –10 kV. After 4 hours, the fibrous sheet was removed from the mandrel and dried in a vacuum oven at room temperature overnight. PEUU, PEUU/dECM 85/15, PEUU/dECM 75/25, and PEUU/dECM 50/50 refer to fibrous hybrid sheets from PEUU blended with 0, 15, 25 and 50 wt% dECM, respectively.
Sheet characterization
The morphology of electrospun sheets was observed using scanning electronic microscopy (SEM, JEOL 6330F) after gold coating. The fiber diameter was measured using ImageJ software (National Institutes of Health). Thermal properties of the blended sheets and PEUU and dECM controls were detected uisng differential scanning calorimetry (DSC, Shimazu, DSC60) at a heating rate of 20°C/min over a range of –100°C to 200°C with nitrogen flow.
A 2.5×20 mm strip cut from an electrospun sheet was used to measure uniaxial tensile strength and breaking strain with a MTS Tytron 250 MicroForce Testing Workstation at a 1-inch/min crosshead speed, according to ASTM D638-98. Five samples were tested for each group. Suture retention strength was measured using a BIOSYN UM-214 4-0 suture (Syneture) under the same conditions. A single suture loop was created 5 mm from the short edge of a 5×20 mm strip and fixed on the upper clamp. Suture retention strength was calculated as suture load/(suture diameter×sample thickness) at the point of tearing.
To measure sample contraction under aqueous conditions, a 10-mm-diameter sample (D0) was punched from sheets and immersed in PBS at 37°C. After 24 hours, the sample diameter (D1) was recorded using a sliding caliper. The shrinkage ratio was calculated as (D0-D1)/D0×100%. Four samples were used for each group.
In vivo abdominal wall placement
Adult female syngeneic Lewis rats (28 rats, 10–12 weeks old, 200–250 g, Harlan Sprague Dawley, Inc.) were used for the abdominal wall reconstruction procedure. The research protocol followed the National Institutes of Health guidelines for animal care and was approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh. Research was conducted in compliance with the Animal Welfare Act Regulations and other federal statutes relating to animals and experiments involving animals and adhered to the principles set forth in the Guide for Care and Use of Laboratory Animals, National Research Council, 1996. The PEUU/dECM 75/25 scaffold was selected for in vivo evaluation based on a desire to maximize dECM content while also avoiding a high degree of shrinkage and a loss of mechanical properties. A PEUU scaffold was used as a control.
Rats were anesthetized using 1.25% to 2.5% isoflurane inhalation with 100% oxygen and their abdomens shaved and prepared with povidone–iodine solution. Surgeries were performed on a heating blanket in a sterile environment using a procedure based on that reported by Lai et al.23 A skin incision was made 2 cm caudally of the xiphoid process in the midline of the abdomen, 3.5 cm in length. A full-thickness rectangular defect (1×2.5 cm) involving all layers of the abdominal wall, including the fascia, rectus abdominis muscle, and peritoneum (with the exception of the skin and subcutaneous tissue), was then created using surgical scissors. This anatomic defect was then reconstructed using size-matched PEUU patches (n=14) or with PEUU/dECM 75/25 patches (n=14) selected randomly. The patches (1×2.5 cm, 400-μm thick) were sutured to the residual muscle (usually to the rectus abdominis muscle on the cranial and caudal side and the external and internal oblique muscles on the lateral side) with a continuous suture using 7-0 polypropylene thread without overlap between muscles and patches, ensuring direct contact with the subcutaneous tissue and the peritoneal viscera. Skin closure was performed over the patch by double-layer suturing. The animals were allowed to recover from anesthesia and returned to their cages. For postoperative analgesic treatment, 0.1 mg/kg of buprenorphine was administered subcutaneously two times per day for 3 days after surgery.
To control for differences in mechanical loading due to the anisotropic nature of the patches, all patches were oriented so that the circumferential direction of the mandrel was aligned with the circumferential direction of the abdomen and the axial direction of the mandrel was aligned with the longitudinal axis upon implantation. For each group, the implanted samples were surgically retrieved 4 and 8 weeks after implantation (n=7 per group per time point). At retrieval, animals were euthanized with the inhalation of isoflurane (5%), and the sutured line was reopened to expose the site of patch placement. Representative specimens were photographed in situ, and the samples were removed by dissecting the surrounding muscle along an apron border approximately 5 mm from the original suture line of materials and muscles. Subsequently, a 1×1 cm piece was cut from each retrieved sample (not including the suture line). The abdominal wall thickness was consistently measured in the middle of the half of the explanted construct used for mechanical testing. The size of the caliper tips covered a major portion of this explanted section. Biaxial mechanical properties were measured for all samples. Histological staining was performed using hematoxylin and eosin (H&E) and Masson's trichrome, and immunolabeling with anti-alpha smooth muscle actin (αSMA, 1:200, Abcam) and anti-CD 68 (1:100, AbD Serotec) was performed.
Histological image analysis
Histological images were analyzed using ImageJ software to determine cellular migration, cellular density in the surrounding tissue, and percentage of αSMA positively stained area (cells) to the total tissue area, as described below. A trained observer analyzed all images in a standardized, blinded fashion. For cellular migration, each cross-section was evaluated by assessing three regions defined by dividing the newly formed tissue area by the total cross-sectional area including the scaffold and surrounding new tissue and reported as a mean percentage. For cell density, nucleated cells in five regions surrounding the scaffolds were counted and reported as mean cells per mm2. Cells were defined as intact round structures with Hoechst 33342 (Invitrogen) staining. Finally, αSMA-positive cells were quantified in five regions, and the positive area occupied by these cells was divided by the total tissue area and reported as a mean percentage.
Biaxial mechanical testing
Biaxial mechanical testing was performed for patches before implantation and for retrieved samples at each time point (4 and 8 weeks) using a previously described method.24 Preimplant patches were immersed in liquid for a minimum of 5 minutes before sample preparation and testing. Historical data from our group were used to provide mechanical properties for the native rat abdominal wall.25
Statistical analysis
All results are presented as means±standard deviations, except for biaxial mechanical curves, for which means (standard errors) were used. Statistical analyses for fiber diameter, mechanical properties, shrinkage ratio, and histological parameters were performed using one-way ANOVA followed by post hoc least significant differences testing of specific differences. Differences were considered significant at p<0.05.
Results
Electrospun sheet characteristics
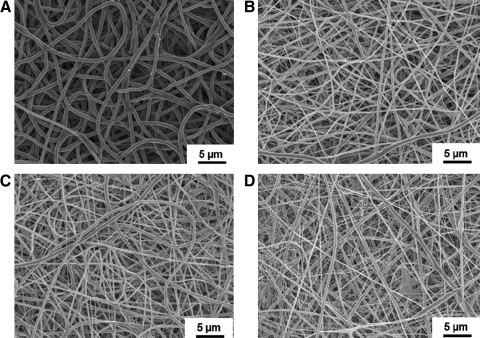
The electrospun scaffold morphology as visualized using SEM is seen in Fig. 1. Continuous fibers without beads were observed for PEUU and PEUU/dECM scaffolds with mass ratios of 85/15, 75/25, and 50/50. Pure dECM in HFIP could not be electrospun to achieve fibers (data not shown). Qualitatively, greater distribution in fiber diameters was observed with grater dECM content, including the appearance of fine web-like fiber populations between larger fibers. In contrast, a highly homogenous fiber population was observed in the PEUU scaffold. The mean fiber diameter for the PEUU scaffold was 743±269 nm, which was significantly higher than for all PEUU/dECM scaffolds (p<0.05). Fiber diameters of PEUU/dECM 85/15 (492±162 nm) and PEUU/dECM 75/25 (446±147 nm) scaffolds were not significantly different (p>0.05) and PEUU/dECM 50/50 had the smallest mean fiber diameter (327±182 nm; p<0.05). The fine web-like fibers observed were not used in determining fiber mean diameters (Table 1).
FIG. 1.
Scaffold fiber morphologies of poly(ester urethane)urea (PEUU) (A), PEUU/dermal extracellular matrix (dECM) 85/15 (B), PEUU/dECM 75/25 (C), and PEUU/dECM 50/50 (D) under scanning electron microscopy.
Table 1.
Scaffold Morphological and Mechanical Properties
| Sample | Fiber diameter (nm) | Tensile strength (MPa) | Breaking strain (%) | Initial modulus (MPa) | Suture retention (N/mm2) |
|---|---|---|---|---|---|
| Mean±Standard Deviation | |||||
| PEUU | 743±269 a | 5±1 a | 611±81 a | 3±1 a | 59±2 a |
| PEUU/dECM 85/15 | 492±162 b | 7±1 b | 229±37 b | 6±1 b | 53±6 a |
| PEUU/dECM 75/25 | 446±147 b | 6±1 a | 199±30 b | 8±1 c | 39±1 b |
| PEUU/dECM 50/50 | 327±182 c | 5±1 a | 138±13 c | 11±2 d | 35±7 b |
Values with the same superscript for any one measured parameter were not found to be significantly different from one another but were statistically different from values with a different superscript.
PEUU, poly(ester urethane)urea; dECM, dermal extracellular matrix.
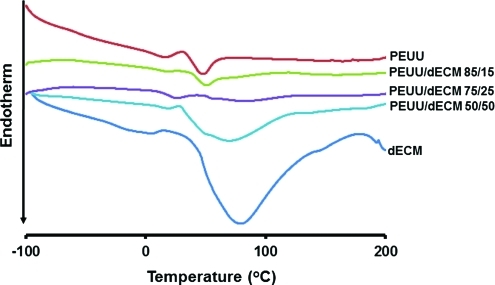
Thermal properties of electrospun PEUU/dECM were determined using DSC with dECM digest and PEUU controls (Fig. 2). The DSC spectrum of the electrospun PEUU material demonstrated a glass transition temperature (Tg) of –55°C and a melting temperature (Tm) of 39°C, whereas dECM demonstrated a broad transition ranging from 30°C to 180°C with a peak at 80°C, no Tg was obvious in the spectra of PEUU/dECM sheets. With PEUU/dECM 85/15, the DSC spectrum showed a Tm of 43°C, which was higher than for PEUU alone, whereas PEUU/dECM 50/50 showed a broad transition, with a peak at 62°C, lower than that of dECM. A weak and broad ECM melting transition along with a low Tm (21°C) from the PCL soft segment in PEUU was observed in the DSC spectrum of PEUU/dECM 75/25.
FIG. 2.
Differential scanning calorimetry curves for PEUU, PEUU/dECM scaffolds, and dECM. Color images available online at www.liebertonline.com/tec
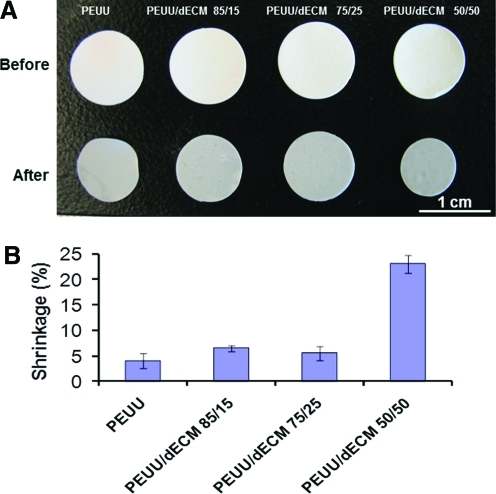
Sample contraction was investigated after immersion in PBS at 37°C for 24 hours (Fig. 3). The PEUU/dECM 50/50 sheet experienced significantly greater shrinkage (22%) than sheets with less dECM blended with PEUU (∼6% shrinkage; Fig. 3).
FIG. 3.
Macrographic view (A) and quantified shrinkage (B) of PEUU and PEUU/dECM scaffolds before and after 24 hours of phosphate buffered saline immersion at 37°C. Color images available online at www.liebertonline.com/tec
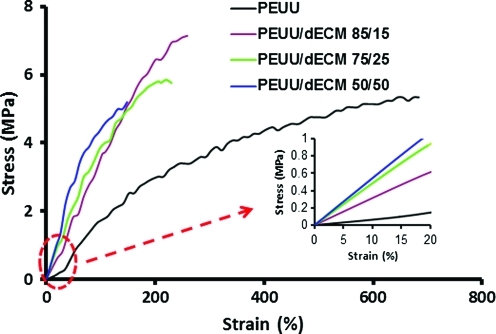
Uniaxial tensile mechanical properties of PEUU and PEUU/dECM sheets are reported in Table 1. All electrospun PEUU and PEUU/dECM sheets exhibited elastic behavior. PEUU/dECM 85/15 had the highest tensile strength (7±1 Mpa; p<0.05); to the tensile strength of the other materials were statistically equivalent. PEUU had a significantly higher breaking strain (611±81%) than PEUU/dECM sheets, and PEUU/dECM 50/50 had the lowest breaking strain (138±13%; p<0.05). There was a trend toward gretaer initial modulus with greater amount of dECM in the scaffold. Electrospun PEUU had the lowest initial modulus (3±1 Mpa), and PEUU/dECM 50/50 had the highest (11±2 Mpa; p<0.05) (Table 1; Fig. 4). Suture retention for PEUU was 59±2 MPa, which was similar to that of PEUU/dECM 85/15 (53±6 MPa; p>0.05) but significantly greater than that of PEUU/dECM 75/25 (39±1 MPa) and PEUU/dECM 50/50 (35±7 MPa). Biaxial tensile testing of these materials revealed similar trends, with the addition of dECM leading to stiffer and more isotropic behavior relative to PEUU alone (data not shown).
FIG. 4.
Typical stress–strain curves of PEUU and PEUU/dECM scaffolds, with inset showing differences in initial modulus at lower strain. Color images available online at www.liebertonline.com/tec
Postimplantation gross observations
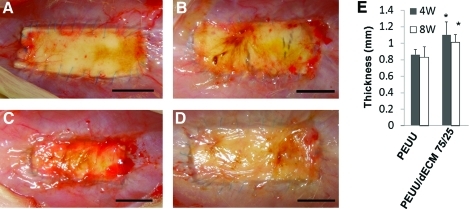
After surgery, no abnormal behavior indicating pain or distress or abnormal weight gain or loss was observed in any of the animals. No infection, herniation, or adhesion to the intra-abdominal organs was observed in either group (Fig. 5A-D) after 4 or 8 weeks. The thickness of PEUU and PEUU/dECM 75/25 patched areas was 0.8 to 1.1 mm 4 and 8 weeks after implantation, although both pre-implant sheets were 400 μm thick (Fig. 5E). The thickness of the PEUU/dECM 75/25 group was significantly greater than for the PEUU group at each time point (p<0.05), although no significant difference in thickness between time points for each group was found (p>0.05).
FIG. 5.
Representative macrographic images of PEUU (A, B) and PEUU/dECM 75/25 (C, D) after 4 (A, C) and 8 (B, D) weeks of implantation in a rat full-thickness abdominal wall defect model. Scale bar=10 mm. No herniation or tissue adhesion was observed for any samples. The wall thickness of the construct area after 4 and 8 weeks of implantation is quantified in (E). Color images available online at www.liebertonline.com/tec
Histological and immunohistochemical staining
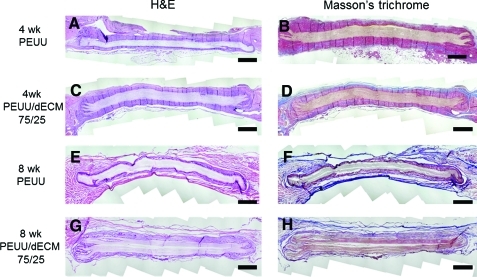
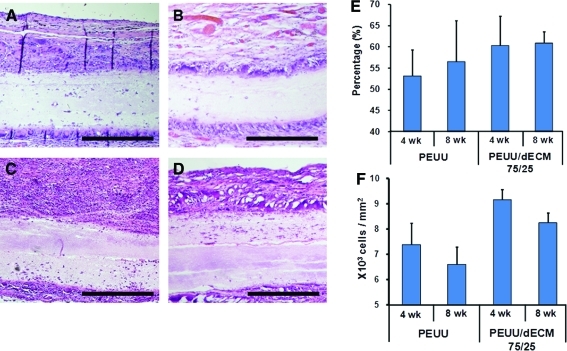
Representative cross-sections of explanted PEUU and PEUU/dECM 75/25 stained with H&E and Masson's trichrome are shown in Fig. 6. Fibrous tissue surrounded each patch at each time point. No extensive or complete cellular infiltration was observed for either patch type after 4 and 8 weeks, although an encroaching border of cell infiltration was found along the edges of the patches. Collagen fiber deposition surrounding the materials was evident from the Masson's trichrome–stained images. There appeared to be a tendency toward greater cell migration in the PEUU/dECM 75/25 explants than in the PEUU explants (Fig. 7), although no statistically significant differences were detected between groups. In contrast, the density of cells in the tissue surrounding the PEUU/dECM 75/25 patch was significantly greater than for the PEUU patch (p<0.05 at 4 and 8 weeks) (Fig. 7). Immunolabeling with anti-α-SMA and anti-CD-68 (Fig. 8) showed macrophages around the periphery of both patches at each time point, with greater relative staining for α-SMA found around the PEUU/dECM 75/25 than the PEUU patches (49.5% vs 36.0% at 4 weeks; 49.6% vs 36.2% at 8 weeks; p<0.05).
FIG. 6.
Representative cross-sections of implanted PEUU (A, B, E, F) and PEUU/dECM 75/25 scaffolds (C, D, G, H) stained with hematoxylin and eosin (H&E, left) and Masson's trichrome (right) 4 (A, B, C, D) and 8 (E, F, G, H) weeks after implantation in a rat full-thickness abdominal wall defect model. Scale bar=1 mm. Color images available online at www.liebertonline.com/tec
FIG. 7.
Higher magnification images of PEUU with Masson's trichrome staining for 4 (A) and 8 (B) week explanted scaffolds and PEUU/dECM 75/25 explanted scaffolds at 4 (C) and 8 (D) weeks. Scale bar=100 μm. (E) Cell migration into the cross-sectional area of the explanted specimens was quantified at 4 and 8 weeks. The cell migration percentage was defined by dividing the newly formed tissue area by the total cross-sectional area of the explanted tissue in randomly selected areas. (F) Cellularity of the tissue surrounding the scaffolds at the time of explant. The cellularity was defined as the cell numbers surrounding the patch divided by the counting area. Color images available online at www.liebertonline.com/tec
FIG. 8.
Immunolabeling for CD68 and alpha-smooth muscle actin (α-SMA) for PEUU scaffolds explanted at 4 (A) and 8 (B) weeks and PEUU/dECM 75/25 patches explanted at 4 (C) and 8 (D) weeks. Nuclear staining is blue, α-SMA staining is green, and anti-CD68 for macrophage labeling is red. α-SMA expression around PEUU/dECM 75/25 patches was greater than for PEUU patches at 4 and 8 weeks. Scale bar=200 μm. Color images available online at www.liebertonline.com/tec
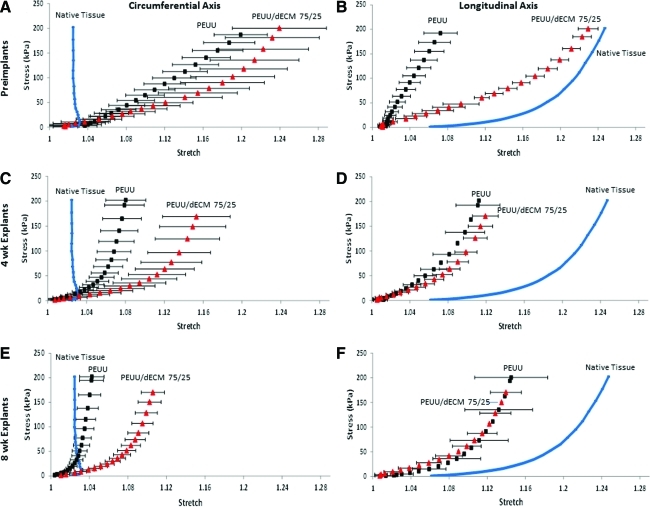
Biaxial mechanical properties of explants
Evaluation of the biaxial tensile mechanical properties of the explanted material after 4 weeks revealed that the PEUU scaffolds stiffened noticeably in both axes (p<0.001), whereas PEUU/dECM 75/25 stiffened significantly only in the longitudinal axis (Fig. 9). Eight-week explants did not display any significant change in mechanical response from what was observed in 4-week explants for either group (Fig. 9).
FIG. 9.
Mechanical behavior under equi-biaxial tension of scaffolds before implantation (A, B) and after explantation at 4 (C, D) and 8 (E, F) weeks. Native rat abdominal wall tissue behavior is presented for comparison purposes. Color images available online at www.liebertonline.com/tec
Discussion
Single stream electrospinning is commonly used to prepare synthetic/biological composite scaffolds from a blended solution of synthetic polymer and biological materials, such as purified proteins (collagen, elastin) and ECM.5–7,26–28 Such components are mixed at a molecular level to obtain an integrated material, although there may be a loss in bioactivity due to denaturation of the biological material by the organic solvent. A two-stream electrospin/electrospray technique developed for the PEUU/dECM gel biohybrid scaffold could be used to prevent exposure of the dECM to harsh solvents, maintaining bioactivity,25 although such a technique would not produce a complete mixture, which might result in less-desirable mechanical properties. In both techniques, it is important to consider the effect of interactions between the polymer and ECM components with respect to processing ability and overall construct function. In single-stream processing, ECM content may disrupt chain entanglements that are critical for the electrospinning process.29 This potential interaction is consistent with our results in that electrospinning using the reported technique with high levels of dECM (>50%) did not reliably produce fibers without beading, and pure dECM could not produce any substantial fibers. This finding effectively produces an upper limit to the quantity of dECM that a scaffold produced in this way can contain. Using a two-stream approach such as that cited above would not have such a limit, although when introducing an electrospray concurrently with polymer electrospinning, nonsolvent effects may prematurely coagulate the polymer fibers as they deposit. This effect could disrupt some of the fiber intersections within the scaffold microstructure, which can lead to softer mechanical properties.30 Such an effect might produce a functional limit on the quantity of ECM that could be incorporated within a scaffold using a two-stream approach while maintaining desirable mechanical strength. Thus, the trade-off between single-stream and two-stream electrospinning techniques should be considered for scaffold fabrication depending on the specific requirements for the application.
For scaffold processing concerns, pure dECM in HFIP could not be successfully electrospun, whereas pure UBM in HFIP was previously shown to produce fibers, albeit with the presence of beading or agglomerates.5 This difference may be due to the lower viscosity (and implied lower molecular weight) of dECM digests than of UBM digests, although the introduction of PEUU appeared to be helpful in stabilizing the electrospinning stream to achieve bead-free fibers. A web-like structure of fine fibers accompanied these bead-free fibers. This webbing phenomenon was also observed in a pure electrospun collagen scaffold31 and a carbon nanotube/silk electrospun sheet32. Thus, these fibers in the web might be produced from protein components in the dermal ECM. The web-like structure found in all PEUU/dECM hybrid sheets might indicate some degree of phase separation between dECM and PEUU,5 although the undetectable Tg and the shift of Tm suggests that some interaction exists between dECM and PEUU. Hydrogen bonding between PEUU and dECM might hinder PEUU chain segment mobility, which may have obscured the PEUU Tg. Simultaneously, the interference from hydrogen bond forces between PEUU and dECM may have decreased the degree of crystallinity for each component, shifting the Tm depending on the PEUU/dECM weight ratio. This would be occurring most obviously for PEUU/dECM 75/25. Based on this evidence, PEUU and dECM could be considered to be partially miscible.
The mechanical properties of materials used for abdominal wall repair are of paramount importance. The tensile strength must be sufficient to prevent hernia recurrence during the entire tissue remodeling process, yet the softness and elasticity must be at a level to ensure patient comfort. The tensile strength of the PEUU/dECM hybrid scaffolds with up to 50% dECM did not decrease linearly, contrary to the effect observed with PEUU/UBM hybrid scaffolds.5 This difference may have resulted from some degree of miscibility between PEUU and dECM, as discussed above. PEUU/dECM hybrid sheets possessed a high degree of elasticity, with low initial modulus, despite the stiffness of pure dECM. A high mass fraction of dECM (50 wt%) was associated with greater stiffness and less elasticity (Table 1), as well as more contraction upon wetting (Fig. 3).
To evaluate the in vivo response, the PEUU/dECM 75/25 scaffold was selected for further evaluation, along with a control scaffold of PEUU alone. Greater quantities of ECM in a blended scaffold were expected to help improve cellular infiltration in vivo.5 As long as mechanical properties were reasonably maintained, it was hypothesized that a scaffold with the highest amount of dECM would be the most attractive for in vivo tissue remodeling, but dECM addition at high mass fractions was associated with substantial scaffold shrinkage upon wetting. When the amount of dECM in the scaffold reached 50 wt%, the shrinkage ratio increased a point where it was excluded from further study. It was believed that such a degree of shrinkage might lead to size mismatch, patient discomfort, and possibly mechanical failure (e.g., suture pull out). Therefore, the PEUU/dECM 75/25 scaffold was selected as the most appropriate scaffold to evaluate further because of its high dECM content without substantial shrinkage or mechanical property loss.
The explants from the rat abdominal wall demonstrated that dECM involvement resulted in a greater host tissue response, as evidenced by greater wall thickness (Fig. 5E) and α-SMA expressing cells (Fig. 8) in the construct area. The greater thickness might be attributed to tissue encapsulation induced by augmented cell migration and the foreign body reaction. In support of the concept that greater cellular migration was the major contributing factor to the difference in thickness between PEUU/dECM 75/25 explants and PEUU explants was the presence of a larger number of α-SMA–labeled cells (myofibroblasts) that were probably drawn to the periphery of the scaffold (Fig. 8) by chemotactic agents present in dECM. Although α-SMA staining was performed to detect myofibroblasts as important participants in the wound healing process, some putative vascular structures (vascular smooth muscle cells) were observed around the PEUU scaffolds. As noted above, more-diffuse α-SMA labeling was observed around the PEUU/dECM scaffold, which is indicative of myofibroblasts. There may also be vascular structures within the α-SMA–labeled areas of the PEUU/dECM scaffolds, but this was not clear with the labeling technique. Further labeling using an endothelial cell marker might clarify whether vascularization levels varied between the two scaffold types.
No significant differences were observed in the biomechanical response between 4 and 8 weeks because there was minimal cellular infiltration in the construct (Fig. 6 and 7). In contrast, a clinically relevant, nondegradable ePTFE patch was statistically nonsignificantly thicker and had negligible cellular infiltration, along with isotropic, stiff behavior during an 8-week implantation period in the same animal model.25 dECM alone was excluded from in vivo evaluation because of its weak mechanical properties and poor processability. In subcutaneous studies, PEUU/UBM 75/25 showed markedly greater cellular infiltration after 4 weeks of subcutaneous placement.5 Such differences in cellular infiltration may be attributed to the differences in animal models for scaffold placement. Subcutaneous locations are associated with rich capillary beds, which would provide ample body fluid for nutrient transport. Such models also surround implants on all sides with tissues that may act as sources for cell infiltration. In the full-thickness abdominal wall model, cellular infiltration is largely limited to the perimeter. The bottom of the scaffold must not adhere to the abdominal organs. Thus, such placement may experience limited angiogenesis and have less access for cell migration. Additionally, the difference in the mechanical loading between the two locations is considerable. Applied cyclic tension can lead to less fibroblast proliferation and motility in the context of implanted synthetic films than with unloaded materials.33 Cyclic loading has also been shown to alter progenitor cells from a proliferative to a more-synthetic phenotype.34 It follows that the greater mechanical loading in this study could have contributed to the lack of cell ingrowth observed in the histology. Furthermore, the greater bioactivity of dECM than of particulate UBM might be considered, although such a comparison has not been performed.
In a previous report by Valentin et al.19 in which porcine SIS ECM was used as a partial replacement for the rat abdominal wall, this material was shown to be capable of generating mechanically active abdominal wall tissue with islands and sheets of skeletal muscle. In the current report, we used a full-thickness defect model across the central abdominal area, whereas the previous report used a partial-thickness defect model next to the linea alba. The previous report thus preserved the underlying rat abdominal wall muscle, which could potentially serve as a source of myoblasts to populate the scaffold and ultimately generate functional skeletal muscle. Furthermore, the previous study used long follow-up periods, after which functional mechanical properties were assessed. Given the differences in the models used and the period of evaluation, we did not hypothesize that there would be any significant skeletal muscle generation in the patched area and instead were interested only in the passive mechanical properties as an assessment of the healing mechanical environment to maintain physical integrity and to facilitate regeneration at longer time points. This model and time scale were chosen for preliminary evaluation of the potential of this blended scaffold material. Follow-up studies that use the partial-thickness model over longer time periods would provide additional insight into the potential for this scaffolding material to support functional skeletal muscle generation.
Several additional limitations of this study should be mentioned. The small pore size and dense microstructure of the scaffolds used in this study resulted in slow cellular migration into the scaffold. It has been shown that greater quantities of ECM result in accelerated degradation and cellular infiltration, although this would require a much higher concentration than was used in the current study.5 This study confirmed that 25% dECM was insufficient to achieve extensive cellular infiltration within 8 weeks. Faster erosion associated with a higher dECM load (50%) might allow for further cell penetration but at a cost of mechanical properties. With its associated shrinkage and stiffer mechanics, greater dECM concentration within the scaffold does not appear to be an ideal method of encouraging host tissue ingrowth. A wet electrospinning technique, in which a proteinaceous fluid is electrosprayed during the electrospinning process, might also lead to better cellular infiltration,11 but it is unclear how such a process might affect the mechanical properties of a PEUU/dECM blend fiber population. Finally, an extended implantation period would be of interest to observe the ongoing host tissue response. Clinically, what happens to the resultant tissue after the resorbable components are completely removed is of great importance. At observational time points beyond 8 weeks, differences between PEUU and PEUU/dECM blend material might become more pronounced.
Conclusions
A biodegradable elastomeric scaffold was created by combining a dECM digest with a biodegradable polyurethane using a single-stream electrospinning method. The resultant scaffold possessed high elasticity and flexibility and good surgical handling, with mechanical characteristics that could be manipulated by altering the PEUU/dECM ratio. Full-thickness rat abdominal wall implantation of the selected blended scaffold revealed greater thickness and α-SMA-positive expression than a PEUU control, although the blended scaffold and the control both had limited cellular infiltration and tissue remodeling. Although ECM content and identity and implant location are important considerations in the final biological response, the use of a single-stream electrospinning approach with PEUU and dECM is an easy processing option for creating a mechanically robust material from dECM digest at substantial ECM mass fractions.
Acknowledgments
Financial support provided by the Armed Forces Institute for Regenerative Medicine (W81XWH-08-2-0032) and NIH ROI HL-068816. We appreciate the assistance of Prof. Michael S. Sacks for biaxial mechanical testing and Deanna Rhoads for histological sectioning.
Disclosure Statement
No competing financial interests exist.
References
- 1.Vorotnikova E. McIntosh D. Dewilde A. Zhang J. Reing J.E. Zhang L. Cordero K. Bedelbaeva K. Gourevitch D. Heber-Katz E. Badylak S.F. Braunhut S.J. Extracellular matrix-derived products modulate endothelial and progenitor cell migration and proliferation in vitro and stimulate regenerative healing in vivo. Matrix Biol. 2010;29:690. doi: 10.1016/j.matbio.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 2.Tottey S. Corselli M. Jeffries E.M. Londono R. Peault B. Badylak S.F. Extracellular matrix degradation products and low-oxygen conditions enhance the regenerative potential of perivascular stem cells. Tissue Eng Part A. 2011;17:37. doi: 10.1089/ten.tea.2010.0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hong Y. Huber A. Takanari K. Amoroso N.J. Hashizume R. Badylak S.F. Wagner W.R. Mechanical properties and in vivo behavior of a biodegradable synthetic polymer microfiber-extracellular matrix hydrogel biohybrid scaffold. Biomaterials. 2011;32:3387. doi: 10.1016/j.biomaterials.2011.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ekaputra A.K. Prestwich G.D. Cool S.M. Hutmacher D.W. Combining electrospun scaffolds with electrosprayed hydrogels leads to three-dimensional cellularization of hybrid constructs. Biomacromolecules. 2008;9:2097. doi: 10.1021/bm800565u. [DOI] [PubMed] [Google Scholar]
- 5.Stankus J.J. Freytes D.O. Badylak S.F. Wagner W.R. Hybrid nanofibrous scaffolds from electrospinning of a synthetic biodegradable elastomer and urinary bladder matrix. J Biomater Sci Polym Ed. 2008;19:635. doi: 10.1163/156856208784089599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hong S. Kim G. Electrospun micro/nanofibrous conduits composed of poly(epsilon-caprolactone) and small intestine submucosa powder for nerve tissue regeneration. J Biomed Mater Res B Appl Biomater. 2010;94:421. doi: 10.1002/jbm.b.31670. [DOI] [PubMed] [Google Scholar]
- 7.Yoon H. Kim G. Micro/nanofibrous scaffolds electrospun from PCL and small intestinal submucosa. J Biomater Sci Polym Ed. 2010;21:553. doi: 10.1163/156856209X429166. [DOI] [PubMed] [Google Scholar]
- 8.Kingsnorth A. Treating inguinal hernias. BMJ. 2004;328:59. doi: 10.1136/bmj.328.7431.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fortelny R.H. Petter-Puchner A.H. Glaser K.S. Offner F. Benesch T. Rohr M. Adverse effects of polyvinylidene fluoride-coated polypropylene mesh used for laparoscopic intraperitoneal onlay repair of incisional hernia. Br J Surg. 2010;97:1140. doi: 10.1002/bjs.7082. [DOI] [PubMed] [Google Scholar]
- 10.Bracco P. Brunella V. Trossarelli L. Coda A. Botto-Micca F. Comparison of polypropylene and polyethylene terephthalate (Dacron) meshes for abdominal wall hernia repair: a chemical and morphological study. Hernia. 2005;9:51. doi: 10.1007/s10029-004-0281-y. [DOI] [PubMed] [Google Scholar]
- 11.Oh D.S. Manning M.M. Emmanuel J. Broyles S.E. Stone H.H. Repair of full-thickness defects in alimentary tract wall with patches of expanded polytetrafluoroethylene. Ann Surg. 2002;235:708. doi: 10.1097/00000658-200205000-00013. discussion 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Candage R. Jones K. Luchette F.A. Sinacore J.M. Vandevender D. Reed R.L. II. Use of human acellular dermal matrix for hernia repair: friend or foe? Surgery. 2008;144:703. doi: 10.1016/j.surg.2008.06.018. discussion 9. [DOI] [PubMed] [Google Scholar]
- 13.Sriussadaporn S. Kritayakirana K. Pak-art R. Operative management of small bowel fistulae associated with open abdomen. Asian J Surg. 2006;29:1. doi: 10.1016/S1015-9584(09)60284-0. [DOI] [PubMed] [Google Scholar]
- 14.O'Dwyer P.J. Kingsnorth A.N. Molloy R.G. Small P.K. Lammers B. Horeyseck G. Randomized clinical trial assessing impact of a lightweight or heavyweight mesh on chronic pain after inguinal hernia repair. Br J Surg. 2005;92:166. doi: 10.1002/bjs.4833. [DOI] [PubMed] [Google Scholar]
- 15.Jernigan T.W. Fabian T.C. Croce M.A. Moore N. Pritchard F.E. Minard G. Bee T.K. Staged management of giant abdominal wall defects: acute and long-term results. Ann Surg. 2003;238:349. doi: 10.1097/01.sla.0000086544.42647.84. discussion 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weyhe D. Schmitz I. Belyaev O. Grabs R. Muller K.M. Uhl W. Zumtobel V. Experimental comparison of monofile light and heavy polypropylene meshes: less weight does not mean less biological response. World J Surg. 2006;30:1586. doi: 10.1007/s00268-005-0601-0. [DOI] [PubMed] [Google Scholar]
- 17.Eberli D. Rodriguez S. Atala A. Yoo J.J. In vivo evaluation of acellular human dermis for abdominal wall repair. J Biomed Mater Res A. 2010;93:1527. doi: 10.1002/jbm.a.32636. [DOI] [PubMed] [Google Scholar]
- 18.Burns N.K. Jaffari M.V. Rios C.N. Mathur A.B. Butler C.E. Non-cross-linked porcine acellular dermal matrices for abdominal wall reconstruction. Plast Reconstr Surg. 2010;125:167. doi: 10.1097/PRS.0b013e3181c2a6ed. [DOI] [PubMed] [Google Scholar]
- 19.Valentin J.E. Turner N.J. Gilbert T.W. Badylak S.F. Functional skeletal muscle formation with a biologic scaffold. Biomaterials. 2010;31:7475. doi: 10.1016/j.biomaterials.2010.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karpelowsky J.S. Thomas G. Shun A. Definitive abdominal wall closure using a porcine intestinal submucosa biodegradable membrane in pediatric transplantation. Pediatr Transplant. 2009;13:285. doi: 10.1111/j.1399-3046.2008.01086.x. [DOI] [PubMed] [Google Scholar]
- 21.Horan R.L. Bramono D.S. Stanley J.R. Simmons Q. Chen J. Boepple H.E. Altman G.H. Biological and biomechanical assessment of a long-term bioresorbable silk derived surgical mesh in an abdominal body wall defect model. Hernia. 2009;13:189. doi: 10.1007/s10029-008-0459-9. [DOI] [PubMed] [Google Scholar]
- 22.Guan J. Sacks M.S. Beckman E.J. Wagner W.R. Synthesis, characterization, and cytocompatibility of elastomeric, biodegradable poly(ester-urethane)ureas based on poly(caprolactone) and putrescine. J Biomed Mater Res. 2002;61:493. doi: 10.1002/jbm.10204. [DOI] [PubMed] [Google Scholar]
- 23.Lai P.H. Chang Y. Liang H.C. Chen S.C. Wei H.J. Sung H.W. Peritoneal regeneration induced by an acellular bovine pericardial patch in the repair of abdominal wall defects. J Surg Res. 2005;127:85. doi: 10.1016/j.jss.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 24.Sacks M.S. Biaxial mechanical evaluation of planar biological materials. J Elasticity. 2000;61:199. [Google Scholar]
- 25.Hashizume R. Fujimoto K.L. Hong Y. Amoroso N.J. Tobita K. Miki T. Keller B.B. Sacks M.S. Wagner W.R. Morphological and mechanical characteristics of the reconstructed rat abdominal wall following use of a wet electrospun biodegradable polyurethane elastomer scaffold. Biomaterials. 2010;31:3253. doi: 10.1016/j.biomaterials.2010.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Y.Z. Venugopal J. Huang Z.M. Lim C.T. Ramakrishna S. Characterization of the surface biocompatibility of the electrospun PCL-collagen nanofibers using fibroblasts. Biomacromolecules. 2005;6:2583. doi: 10.1021/bm050314k. [DOI] [PubMed] [Google Scholar]
- 27.Stitzel J. Liu J. Lee S.J. Komura M. Berry J. Soker S. Lim G. Van Dyke M. Czerw R. Yoo J.J. Atala A. Controlled fabrication of a biological vascular substitute. Biomaterials. 2006;27:1088. doi: 10.1016/j.biomaterials.2005.07.048. [DOI] [PubMed] [Google Scholar]
- 28.Sell S.A. McClure M.J. Barnes C.P. Knapp D.C. Walpoth B.H. Simpson D.G. Bowlin G.L. Electrospun polydioxanone-elastin blends: potential for bioresorbable vascular grafts. Biomed Mater. 2006;1:72. doi: 10.1088/1748-6041/1/2/004. [DOI] [PubMed] [Google Scholar]
- 29.Shenoya S.L. Batesa W.D. Frischb H.L. Wnek G.E. Role of chain entanglements on fiber formation during electrospinning of polymer solutions: good solvent, non-specific polymer–polymer interaction limit. Polymer. 2005;46:3372. [Google Scholar]
- 30.Amoroso N.J. D'Amore A. Hong Y. Wagner W.R. Sacks M.S. Elastomeric electrospun polyurethane scaffolds: the interrelationship between fabrication conditions, fiber topology, and mechanical properties. Adv Mater. 2011;23:106. doi: 10.1002/adma.201003210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matthews J.A. Wnek G.E. Simpson D.G. Bowlin G.L. Electrospinning of collagen nanofibers. Biomacromolecules. 2002;3:232. doi: 10.1021/bm015533u. [DOI] [PubMed] [Google Scholar]
- 32.Ayutsede J. Gandhi M. Sukigara S. Ye H. Hsu C.M. Gogotsi Y. Ko F. Carbon nanotube reinforced Bombyx mori silk nanofibers by the electrospinning process. Biomacromolecules. 2006;7:208. doi: 10.1021/bm0505888. [DOI] [PubMed] [Google Scholar]
- 33.Tanaka H. Manske P.R. Pruitt D.L. Larson B.J. Effect of cyclic tension on lacerated flexor tendons in vitro. J Hand Surg Am. 1995;20:467. doi: 10.1016/S0363-5023(05)80109-1. [DOI] [PubMed] [Google Scholar]
- 34.Estes B.T. Gimble J.M. Guilak F. Mechanical signals as regulators of stem cell fate. Curr Top Dev Biol. 2004;60:91. doi: 10.1016/S0070-2153(04)60004-4. [DOI] [PubMed] [Google Scholar]