Abstract
Continuous production of red blood cells (RBCs) in an automated closed culture system using hematopoietic stem cell (HSC) progenitor cell populations is of interest for clinical application because of the high demand for blood transfusions. Previously, we introduced a four-compartment bioreactor that consisted of two bundles of hollow fiber microfiltration membranes for transport of culture medium (forming two medium compartments), interwoven with one bundle of hollow fiber membranes for transport of oxygen (O2), carbon dioxide (CO2), and other gases (forming one gas compartment). Small-scale prototypes were developed of the three-dimensional (3D) perfusion cell culture systems, which enable convection-based mass transfer and integral oxygenation in the cell compartment. CD34+ HSC were isolated from human cord blood units using a magnetic separation procedure. Cells were inoculated into 2- or 8-mL scaled-down versions of the previously designed 800-mL cell compartment devices and perfused with erythrocyte proliferation and differentiation medium. First, using the small-scale 2-mL analytical scale bioreactor, with an initial seeding density of 800,000 cells/mL, we demonstrated approximately 100-fold cell expansion and differentiation after 7 days of culture. An 8-mL laboratory-scale bioreactor was then used to show pseudocontinuous production by intermediately harvesting cells. Subsequently, we were able to use a model to demonstrate semicontinuous production with up to 14,288-fold expansion using seeding densities of 800,000 cells/mL. The down-scaled culture technology allows for expansion of CD34+ cells and stimulating these progenitors towards RBC lineage, expressing approximately 40% CD235+ and enucleation. The 3D perfusion technology provides an innovative tool for studies on RBC production, which is scalable.
Introduction
Restrictions in donor blood availability, especially with specific or rare blood groups, can limit the availability of red blood cells (RBC) for medical therapy. In addition, current voluntarily donated blood cannot always be used in emergency medicine because of changes in blood properties during processing and extended storage1,2. In general blood donations have been insufficient to keep up with the high demand, which is a main reason that efforts to produce RBC have received attention. In the treatment of people during civilian or military emergency traumas, a ready supply of safe and effective RBC is required to sustain life.
In healthy adult individuals, hematopoietic stem cells (HSC) in the bone marrow provide a continuous supply of blood cells in the human body. Despite extensive research conducted over the past 20 or more years, HSC in vitro culture expansion and differentiation toward blood cell lineages is not sufficiently optimized for industrial production3. In general, there are many difficulties and process challenges relating to HSC cultivation for the production of type O, Rh-negative RBCs in standard Petri dish cultures and in bioreactor systems4,5. Such challenges can be attributed in part to difficulties encountered during culture, which include but are not limited to donor variability6, recreation of a complex microenvironment7, and the interaction between various culture parameters8. In addition to the common ABO and Rh RBC antigens, there are hundreds of other RBC antigens whose corresponding antibodies need to be considered in certain circumstances when developing a universal blood source9. CD34+ HSC derived from human placentas are thought to be a candidate for in vitro blood cell production, and functional RBC have been successfully generated, yielding up to five RBC units from a single cord blood donation using two-dimensional (2D) culture10–13. Conventional in vitro models such as 2D culture in Petri dishes are valuable systems for studying RBC proliferation and differentiation, but their ability to mimic bone marrow environments is limited for a number of reasons; they provide simple static conditions, and larger-scale production is costly and problematic. Recreating the HSC niche in vitro is difficult because of the various cell types involved and the complex geometrical architecture. A dynamic culture device that reflects the three-dimensional (3D) architecture of the bone marrow and enables more physiological gradients in a perfused environment would be a significant step forward toward the investigation of CD34+ proliferation and differentiation and the potential to develop large-scale systems to produce blood products outside the body.
Despite many obstacles, transforming HSC into mature RBC has continued to receive considerable attention from many research institutes around the world because finding a true blood substitute would be medicine's Holy Grail. Various types of bioreactors, including stirred tank and perfusion, have been evaluated for the maintenance, expansion, and differentiation of HSC14–16. It is difficult to draw direct outcome parameter comparisons (e.g., total fold expansion and levels of differentiation) between the numerous bioreactor platforms given the inherent differences in design. Furthermore, medium formulations can vary greatly in composition, and other cell types, including osteoblasts and mesenchymal cells, are often used in co-culture with HSCs. Taking into account all the various bioreactors that have been designed and studied, hollow-fiber membrane technology bioreactors typically allow greater tissue densities to be reached17, consistent with the natural bone marrow in which effective cell differentiation occurs at high densities and low O2 concentrations (near 8% at the edge of the blood vessels). In one particular commercially available two-compartment bioreactor (FiberCell Duet, FiberCell systems, Inc., www.fibercellsystems.com), cells are distributed around a bundle of hollow fibers, nutrition is delivered using diffusion, and external oxygenation is limited using nonuniform mass exchange with large substrate gradients. We suggest that bioreactors with at least three compartments and physiological matrices are required to address integral oxygenation and decentralized mass exchange for practical large-scale culture systems.
Our group previously introduced multicompartment bioreactor technology for dynamic 3D perfusion culture of human liver cells in a capillary scaffold, providing decentralized mass exchange with low gradients and integral oxygenation18. The device was initially developed as a clinical bioartificial liver support bioreactor with a cell compartment accommodating up to 800 g of cells. Based on the use of three interwoven hollow-fiber capillary membrane systems, the bioreactor provides independent “arteriovenous” medium perfusion, O2 and gas supply, and CO2gas removal for cells located in the cell compartment between the capillaries19,20. Previous studies have shown that parenchymal and nonparenchymal liver cells cocultured in this bioreactor technology form tissue-like structures21,22. This technology has also been used for successful clinical application as a liver support bioreactor23. Because our culture results from primary human liver cells cultured in the clinical-scale system showed stable metabolic cell activity in vitro, support of differentiation, and sufficient performance in clinical applications on patients with liver failure, we believed that it could be further developed by producing down-scaled versions of the technology for in vitro investigations on RBC lineage direction of CD34+ HSC. Parameters such as medium perfusion, feed rates, O2 gas supply, and cell numbers used were adjusted for the 2- and 8-mL scale variants according to the cell compartment size and the circulating medium volume.
Cell proliferation over time was assessed by determination of glucose use and lactate production. To evaluate the suitability of the miniaturized bioreactors for in vitro CD34+ differentiation and RBC lineage direction studies, surface marker expression of proliferating and differentiating CD34+-derived cells was analyzed using flow cytometry. The metabolic performance of primary human placenta–derived CD34+ cells maintained in the laboratory-scale prototypes were investigated with respect to the cell expansion factors during growth, long-term stability of the down-scaled systems, cell harvesting capabilities, fractionated harvesting for cell production, and the ability to passage cells from one bioreactor system to others to continuously increase the overall scale of the culture.
Materials and Methods
Size-scaling of the bioreactor technology
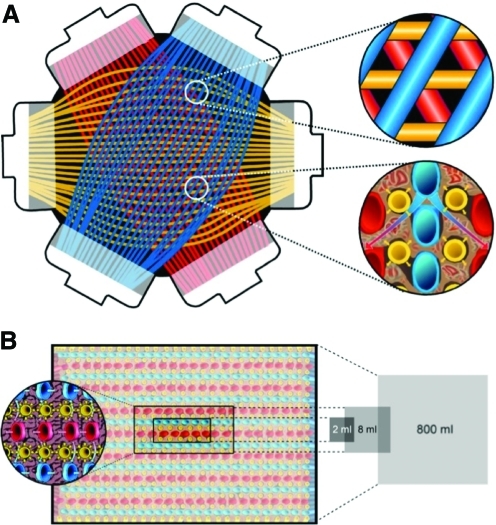
Down-scaling of the clinical-scale bioreactor prototype (cell compartment volume 800 mL) was realized by reducing the length and the number of capillary layers in two axes of the 3D space within the bioreactor, resulting in a laboratory-scale bioreactor with a cell compartment volume of 8 mL and a further miniaturized prototype with a cell compartment volume of 2 mL (Figs. 1 and 2). Each bioreactor consists of three independent interwoven capillary systems that facilitate counter-current medium perfusion and decentralized oxygenation with low gradients (Fig. 1). Two sets of the hydrophilic media perfusion capillary membranes were made of microporous polyethersulphone with a molecular weight cut-off of approximately 500,000 Daltons (Da) (mPES, Membrana, Wuppertal, Germany; inner diameter 300 μm±40 μm, wall thickness 100 μm±25 μm, pore size 0.5 μm±0.1 μm) for the transport of culture medium in a decentralized pattern in the cell compartment, mimicking arteriovenous perfusion. The third was made of hydrophobic multilaminate hollow-fiber membrane capillaries (MHF, Mitsubishi, Tokyo, Japan; inner diameter 200±10 μm, wall thickness 42±3 μm, oxygen (O2) permeability > 0.8E 5 cm3/cm2 * sec * cmHg) for the decentralized transport of O2 and CO2 in the cell compartment. The structure of the capillary network is described in detail elsewhere18–20. Bioreactors were integrated into a perfusion device (Fig. 2), which was designed to include all equipment required for long-term cell maintenance: separate pump units for medium recirculation and medium substitution with electronic flow and pressure control, a heating chamber for the bioreactor with automated temperature control, and a gas mixing unit for regulation of air and CO2flow rates. Specific operational parameters for each bioreactor scale is given in Table 1, and Fig. 3 depicts the medium circuit tube system configuration used to operate the bioreactor. The perfusion tubing with bubble traps was made of standard medical-grade dialysis Tygon (2275 formulation; Saint-Gobin Performance Plastics). Sterilization was performed using ethylene oxide according to clinical standards.
FIG. 1.
Internal structure of hollow-fiber bioreactor. (A) Shown is the arrangement of capillary layers in the bioreactor and a closer look at the smallest capillary unit with medium capillaries that are counter-currently perfused (red and blue) with integrated gas capillaries (yellow). (B) Scale-down of the technology from clinical scale (800 mL) to laboratory scales (8 or 2 mL) by varying the number and length of capillary layers. Color images available online at www.liebertonline.com/tec
FIG. 2.
Bioreactor and perfusion unit prototypes. Bioreactor perfusion system with pump heads for medium recirculation (left) and medium substitution (right); a heating chamber for bioreactor maintenance; rotameters for air, oxygen, CO2, and total gas flow; and a display for digital monitoring and regulation of perfusion parameters. Bioreactor prototypes with different cell compartment volumes (from the left to the right: 800, 8 and 2 mL) are shown in the front. Color images available online at www.liebertonline.com/tec
Table 1.
Bioreactor Operating Parameters
| 800 mL bioreactor | 8 mL bioreactor | 2 mL bioreactor | |
|---|---|---|---|
| Cell number inoculated CD34+ HSCs | 8×107 (theoretical) | 8.0×105 1.8×106 (actual) | 8×105 (actual) |
| Circulating volume (mL) | 1200 | 50 | 25 |
| Medium recirculation rate (mL/min) | 250 | 20 | 15 |
| Medium feed rate (mL/h) | 150 | 1–4 | 1 |
| Air flow rate (mL/min) | 550–600 | 30–50 | 30–50 |
| CO2 flow rate (mL/min) | 10–12 | 3–5 | 1–2 |
| Average pressure in the cell compartment (mmHg) | 40 | 10 | 5 |
| Medium pH value | 7.35–7.45 | 7.35–7.45 | 7.35–7.45 |
| Temperature (°C) | 38 | 38 | 38 |
Bioreactor perfusion conditions in 800, 8 or 2 mL hollow-fiber scale bioreactors using HSCs.
HSCs, hematopoietic stem cells.
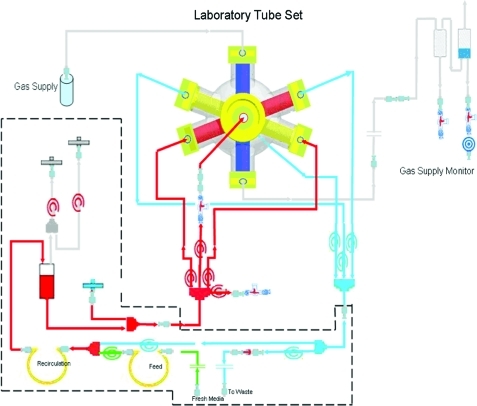
FIG. 3.
Bioreactor perfusion schematic. A detailed schematic of the perfusion periphery connected to the laboratory-scale bioreactor. The bioreactor was integrated into a processor-controlled perfusion device with electronic flow/pressure-controlled perfusion, media, and waste pump operation. A heating unit provided a constant temperature of 37°C within the perfusion circuit. Flow rates of air, O2, CO2, and N2 were controlled by a gas-mixing unit on the perfusion device. Color images available online at www.liebertonline.com/tec
Hematopoietic stem cell isolation and 2D 2-day preculture
Postpartum placentas were procured under informed consent, with donor eligibility documentation, and underwent a series of quality control tests, including serology, bacteriology, and human leukocyte antigen typing. Total nucleated cells as the source of HSC were generated by treating the donor-matched umbilical cord blood and placenta-derived stem cells with ammonium chloride (StemCell Technologies, Vancouver, BC, Canada). The subsequent purification of HSC was performed using RoboSep automated cell isolation system and EasySep Human Progenitor Cell Enrichment Kit (StemCell Technologies). The purity of isolated cell populations was measured using CD34+ cell surface marker expression as determined using flow cytometry analysis. After isolation, cells were shipped overnight from New Jersey to Pittsburgh. Upon receipt, a viable cell count was determined, and cells were then placed in 25-cm2 static t-flask culture (BD Biosciences) at a density of 100,000 cells/mL using expansion and differentiation culture medium and cultured in an incubator with a 5% CO2 atmosphere for 2 days before the start of the experimental culture period. After the preculture period, final cell count and viability assessment was performed before further 2D plate or 3D bioreactor perfusion culture. Expansion and differentiation of HSC into RBC were performed as previously described10,24.
2D control cultures
HSCs were cultured using sixwell plates (BD Biosciences) at a density of 20,000 cells/mL in a medium volume of 2 mL in parallel with bioreactor cultures to check for proper cell growth and to identify potential bacterial or fungal contamination easily. On day 4, one volume of cell culture was diluted in three volumes of fresh medium. On day 7, one volume of cell culture was diluted with four volumes of fresh medium. Afterward, media were replenished at days 11 (as of day 4), 14 (as of day 7), 18, and 21 after the procedure as of day s4 and 7, respectively.
Measurement of metabolic parameters in bioreactors
The partial pressures of O2 and CO2 and the pH values in the recirculation medium were regularly measured using a blood gas analyzer (ABL 5 Blood Gas Analyzer, Copenhagen Radiometer, Copenhagen, Denmark), and the culture pH was maintained between 7.35 and 7.45 by adjusting the air:CO2 gas ratio on the perfusion system while maintaining the same overall gas flow rate.
Concentrations of glucose and lactate in samples from the culture perfusate were analyzed (YSI 2300 STAT Plus Glucose & Lactate Analyzer), and the medium flow rate was adjusted, if necessary, by increasing from an initial 1 mL/h to a maximum feed rate of 4 mL/h. In parallel, lactate dehydrogenase (LDH) levels were monitored daily using a bioassay (QuantiChrom LDH Kit, BioAssay Systems) as an indicator of cell death. A culture showing decreasing metabolic activity, defined as a decrease in lactate production or an increase in LDH concentration, was used as a criterion to determine the harvest day for bioreactor cultures unless specific time points for harvest or passaging were required in the experimental plan. The daily parameter assessments were calculated as mg/dL or IU/L, if not otherwise indicated.
Statistical analysis
Values are given as means±standard deviations (SD) in the text and in figures, if not otherwise indicated.
Cultivation of cells in bioreactors
An initial study was conducted to establish successful growth and differentiation conditions for human CD34+ progenitor populations in the 2-mL-scale 3D bioreactor system. Successive studies were then conducted in the 8-mL-scale bioreactor system to demonstrate cell harvesting capabilities, sequential cell removal, and passaging of cells.
High-density 2-mL bioreactor study
An initial density of approximately 800,000 cells/mL was inoculated into bioreactors with 2-mL cell compartments (n=3). Culture management was performed according to observed trends in lactate production, glucose usage, and medium pH, as detailed previously. After approximately 7 days of culture, cells were harvested from a single initial bioreactor by completely rinsing 400 mL of Iscove's modified Dulbecco's medium (IMDM) (Invitrogen, Gibco) supplemented with penicillin-streptomycin (1% v/v) at a flow rate of 20 mL/min. During rinsing, the bioreactor was vigorously shaken to enhance cell liberation from the capillary network. The harvested cell suspension was centrifuged (475 g, 8 min) and analyzed with respect to cell yield, viability (using an Improved Neubauer hemacytometer with Trypan blue staining), and surface marker expression. Cell surface marker expression was determined using flow cytometry using a variety of fluorescently labeled antibodies; CD34 fluorescein isothiocyanate- (present on hematopoietic stem cells), CD71 allophycocyanin- (proliferating marker during RBC development), CD45 (common leukocyte antigen expressed on all nucleated HSC), and CD235a phycoerythrin-conjugated (present on RBC) antibodies. Cells were also co-stained with CD235a phycoerythrin and a monomeric cyanine nucleic acid stain, TO-PRO-3 iodine, to determine enucleation (all flow cytometry reagents were obtained from BD Biosciences). Quantification of surface marker expression was conducted using a FACSVantage SE DiVa (Becton Dickinson, Franklin Lakes, NJ) flow cytometer running DiVa v 4.1.2 software.
Cell production studies
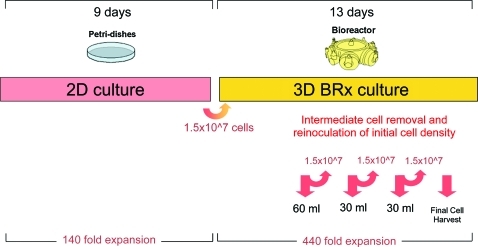
Pseudo-continuous 8-mL bioreactor production study
Subsequently, we used a bioreactor with an 8-mL cell compartment to show pseudo-continuous production by inoculating an initial density of approximately 1.8 million cells/mL and then intermediately harvesting cells from the cell compartment. Figure 4 shows the overall outline of the experiment. For intermediate harvesting of cells from the 8-mL bioreactor, 30 to 60 mL of medium was aspirated from the cell compartment using a 16-G needle and 60-mL syringe. The harvested cell suspensions were centrifuged and analyzed with respect to cell yield, viability, and fluorescence-activated cell sorting (FACS) marker expressions, as previously described. Afterward, the original density of approximately 1.8 million cells/mL was reinoculated into the bioreactor to investigate cell recovery and further cell growth. Final fractional harvesting was performed by aspirating three consecutive 30-mL samples from the cell compartment and then rinsing the internal cell compartment with 400 mL of supplemented IMDM. The harvested cell suspensions were centrifuged (475×g, 8 min) and analyzed with respect to cell yield, viability, and surface marker expression to determine the degree of homogeneity between the different harvest fractions.
FIG. 4.
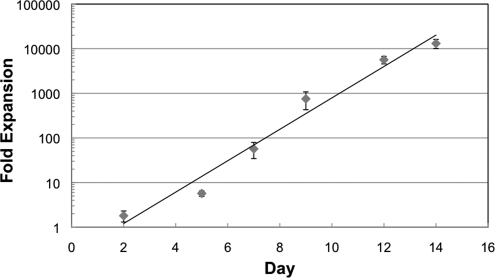
Pseudo-continuous production study time course. CD34+ HSCs were cultured for 9 days in 2D Petri dish cultures and then 1.5×107 total cells (1.8 million cells/mL) were inoculated into an 8-mL 3D bioreactor culture. Culture maintenance was performed according to lactate production, glucose consumption, and LDH levels as previously described. Intermediate cell harvests were performed on days 5, 7, 9, 11, and 13. After each harvest, the original density of cells was reinoculated into the bioreactor. HSCs, hematopoietic stem cells; 2D, two-dimensional; 3D, three-dimensional; LDH, lactate dehydrogenase. Color images available online at www.liebertonline.com/tec
Semicontinuous 8-mL bioreactor production study
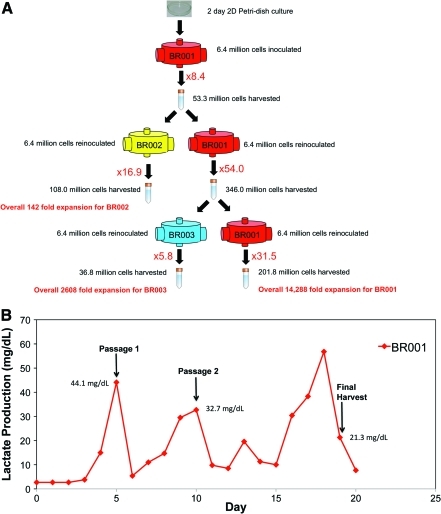
Finally, we demonstrated semicontinuous production by intermediately harvesting and reseeding into two bioreactors, as outlined in Fig. 5A. An initial density of 800,000 cells/mL was inoculated into an 8-mL bioreactor. After approximately 5 days of culture, when lactate production reached a peak, cells were harvested from a single initial bioreactor by completely rinsing 400 mL of supplemented IMDM through the cell compartment. During rinsing, the bioreactor was vigorously shaken to enhance cell liberation from the capillary network. The harvested cell suspension was centrifuged and analyzed with respect to cell yield, viability, and surface marker expression, as previously described. After harvest, the initial seeding density of approximately 800,000 cells/mL was then re-inoculated back into the same bioreactor and also into a newly established bioreactor. This passaging technique was repeated with follow-up bioreactors when lactate reached a maximum level, establishing additional bioreactors over the course of 3 weeks.
FIG. 5.
(A) Schematic for semi-continuous 8-mL bioreactor study. After 2 days in 2D Petri dish culture cells were inoculated into an 8-mL bioreactor (BR001) at a density of 800,000 cells/mL and cultured for 5 days until lactate production reached a maximum level. Cells harvested from BR001 were passaged into a new bioreactor (BR002) at a density of 800,000 cells/mL with a separate fraction of the cells being reinoculated into BR001 at the original density. The production process was carried out for another passage, splitting cells into BR003, before final harvest on day 19. (B) Lactate production levels for BR001 during the semi-continuous production study. Points at which the reactor was passaged and harvested are indicated along with the corresponding lactate levels. The reactor was passaged according to observed peaks in lactate production and harvested when the lactate level dropped, according to the culture management strategy outlined in the Materials and Methods section. Color images available online at www.liebertonline.com/tec
Results
After isolation and before shipment, the CD34+ purity of the cells was 79.0±8.1% as determined using flow cytometry. After shipment, the viability of cells used was 95.3±6.2% as determined using Trypan blue staining. Primary human placenta–derived CD34+ cells could be cultured in the two laboratory-scale prototypes, providing a 2- or 8-mL cell compartment. The CD34+ HSC used showed stable functions, with continuous increase of glucose usage and lactate production, whereas the cell number increased in the 2- and 8-mL cell compartment prototypes over 7 to 19 days. Glucose usage and lactate production were comparable between the 2- and 8-mL scaled-down bioreactors (data not shown). The results for 2D cultures are specified in Fig. 6. Comparison with the characterized 3D culture results indicates that the overall cell expansion values are similar if not greater than the control cultures but on a different scale with regard to cell number per culture vessel. In both laboratory bioreactor scales, removal of viable cells from the bioreactors was possible without affecting subsequent cell growth. The following results describe sequential cell removal, cell harvesting capabilities, and passaging of cells from one bioreactor system to another.
FIG. 6.
Control cultures in 2D. Fold expansion of 2D Petri-dish control cultures maintained at a density of 20,000 cells/mL was used to check for infection and proper cell growth. Results are presented as a mean of n = 5 cultures ± standard deviation.
The suitability of the miniaturized bioreactors in the 2- and 8-mL scaled versions for studies on RBC lineage direction was demonstrated by three studies:
High-density 2-mL bioreactor study
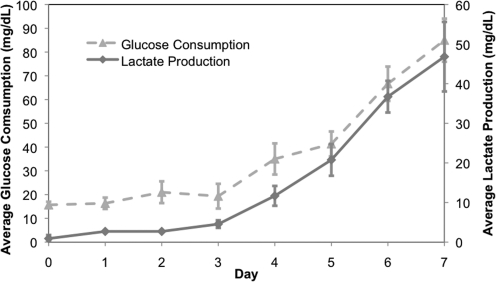
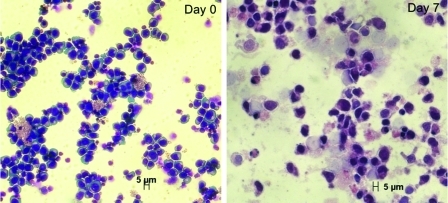
Using the smallest scale 2-mL cell compartment bioreactor (n=3), with an initial seeding density of 800,000 cells/mL, we demonstrated 105±33-fold expansion of inoculated cells and differentiation toward RBC lineage after 2 days of Petri dish culture followed by 7 days of bioreactor culture. The trends in glucose consumption and lactate production during bioreactor cultures are shown in Fig. 7. Cells harvested expressed the CD71 transferrin receptor (93.6±4.75%), which indicates that the cells were still in a proliferative stage of erythropoiesis, although harvested cells also expressed the surface marker CD235a (45.5±16.8%), which is present on mature RBC. Moreover, Wright-Giemsa images (Fig. 8) reveal late progenitor cells after 7 days of bioreactor culture.
FIG. 7.
Glucose consumption and lactate production trends. Glucose consumption (mg/dL) and lactate production (mg/dL) for high-density 2-mL-scale bioreactors (n = 3) inoculated at a density of 800,000 cells/mL and cultured for 7 days after 2 days of Petri dish preculture. Cultures were harvested when lactate production and glucose consumption levels reached a peak and started to plateau. In addition to the measurements of glucose and lactate, the culture pH was monitored daily and maintained between 7.35 and 7.45 by adjusting the air/CO2 gas ratio while maintaining the same overall gas flow rate.
FIG. 8.
Wright-Giemsa staining images. Cell samples were stained before inoculation (day 0) and after recovery (day 7) for a bioreactor from the high-density 2-mL bioreactor study. CD34-positive hematopoietic stems cells are shown at day 0, while late progenitor hematopoietic cells can be seen at day 7. Color images available online at www.liebertonline.com/tec
Cell harvesting studies
Pseudocontinuous production study in an 8-mL bioreactor
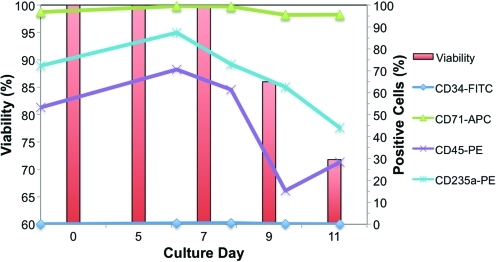
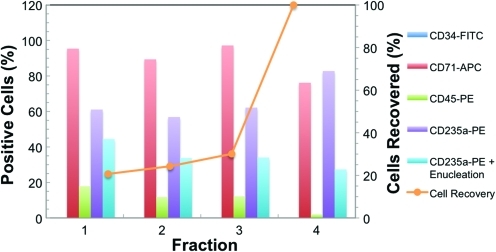
During culture, removal and reinoculation of viable cells from one bioreactor back into the same bioreactor was possible without affecting cell proliferation. Figure 4 shows that an overall 440 cumulative fold expansion was achieved in the 8-mL laboratory bioreactor for such a cell production study. The complete contents within the cell compartment were removed using a fractional harvest method. Postharvest visual and microscopic inspection of the inner structures of the bioreactor, including all capillaries, yielded no cells and confirmed that we were able to harvest efficiently from the bioreactor cell compartment. Figs. 9 and 10 show results of cell surface marker expression during the culture period and at the final harvest, respectively. FACS analysis of cells from intermediate harvests on days 5, 7, 9, and 11 indicated that the cells rapidly lost CD34 expression while concomitantly displaying expression of CD71, indicating that the cultures were in a proliferative state. Furthermore, the fractions obtained during the final cell harvest gave similar surface marker expression, indicating that the individual fractions were representative of the cell compartment, regardless of the harvest rinsing volume. Such results suggest that samples can be drawn on a regular basis for culture analysis or, more importantly, for inoculation into another bioreactor, which is critical for semicontinuous RBC production. Cells from the various fractions of the final harvest also expressed approximately 30% to 45% CD235a+ and enucleation, which are characteristic markers of mature RBC.
FIG. 9.
Cell surface antigen profiles from FACS and viabilities for intermediate harvests from the pseudo-continuous production study. After each intermediate cell harvest (days 5, 7, 9, and 11) FACS analysis was performed for CD34-FITC, CD71-APC, CD235a-PE, and CD45-PE markers. The inoculated cells at day 0 were pre-cultured over 2 days in 2D Petri dishes, which explains why the CD34+ cells were already differentiated. Cells rapidly lost CD34 expression and expressed high levels of CD71 (proliferative marker) while showing some expression of a characteristic RBC marker (CD235a). FACS, fluorescence-activated cell sorting. Color images available online at www.liebertonline.com/tec
FIG. 10.
Cell surface antigen profiles from FACS for final cell harvest from the pseudo-continuous production study. On the final day of harvest (day 13), 30 mL of medium was aspirated from the cell compartment three consecutive times (fractions 1, 2, and 3) and then finally 400 mL of fresh IMDM medium (fraction 4) was rinsed through the cell compartment. During each fractional harvest, cell samples were collected and FACS analysis was performed for CD34-FITC, CD71-APC, CD235a-PE, CD45-PE, and CD235a plus enucleation markers. Surface marker expression was similar among all fractions and gave a minimum of 30% mature RBCs (CD235a+ enculeation). The complete contents with the bioreactor cell compartment were removed using a fractional harvest method (orange x–y scatter line). Color images available online at www.liebertonline.com/tec
Semicontinuous 8-mL bioreactor study results
The semicontinuous production process (Fig. 5A) was used to produce total cell expansion of approximately 15,000 fold over 19 days. By using FACS and immunofluorescence, as shown in Fig. 11, we demonstrated that a variety of differentiated blood cell lineages could be maintained in the bioreactor. The method of culturing cells in one bioreactor, harvesting, and establishing a new bioreactor culture while reusing the original bioreactor by reinoculation demonstrates an opportunity for continuous production of cells. Bioreactors were harvested according to peak lactate production levels, as shown and described in Fig. 5B. Current results indicate that such a methodology can support significant expansion of HSC and cells with RBC lineage characteristics, although some variability can be seen between bioreactors. Conditioned bioreactor cultures resulted in higher fold expansion than the newly established bioreactor cultures.
FIG. 11.
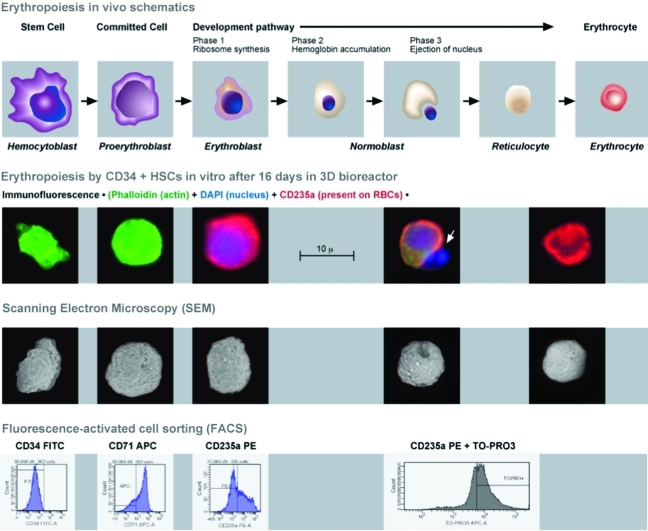
Cells harvested from an 8-mL bioreactor after 16 days in culture were analyzed using immunofluoroesence, scanning electron microscopy (SEM), and FACS. For immunofluorescence the following colors were used for staining: Phalloidin (green) for the presence of actin, CD235a (red) for the characteristic marker present on RBCs, and DAPI for the presence of a nucleus. We were able to capture a nucleus being expelled from a cell (white arrow). Interestingly, the SEM image below also shows a cell that is invaginating in preparation for ejection of the nucleus. FACS results indicate that these cells have lost the CD34+ marker, are in a proliferative stage of growth, and possess markers (CD235a and enucleation) characteristic of mature RBCs. The above image was adapted from Benjamin Cummings, an imprint of Addison Wesley Longman, Inc. Color images available online at www.liebertonline.com/tec
Discussion
A major challenge in developing RBC production systems is the availability of in vitro cell models that reflect the growth of HSC in bone marrow, allow longer-term culture for in vitro studies on RBC lineage direction of HSCs, and can be adapted to scales compatible with clinical-scale RBC production. To overcome the limitations of conventional 2D culture models, various bioreactor technologies were developed to address better cell maintenance in vitro. Bioreactors that are currently used for in vitro 3D perfusion tissue density culture in regenerative medicine research—mostly developed for liver cells—are hollow fiber based and exhibit two functional compartments25,26. Xu et al. pointed out that four bioreactor compartments are necessary to enable oxygenation and distributed mass exchange with low gradients27. Integral oxygenation allows mass exchange limitations to be addressed using separate oxygenators in a circuit28, but most of these models were used only for clinical applications and are not available or cost effective for laboratory scale research applications. In this study, we show the feasibility of scaling down a 3D multicompartment bioreactor perfusion technology previously used for extracorporeal liver support from a cell compartment volume of 600- to 800-mL scale to a miniaturized laboratory scale with cell compartment volumes of 2 and 8 mL for in vitro studies on CD34+ HSC differentiation towards RBC.
Conventional 2D Petri dish culture systems typically allow CD34+ HSC to grow to a maximum density of 2x106 cells/mL. Using the 2-mL bioreactor scale with an initial seeding density of 800,000 cells/mL, we were able to demonstrate successful growth of CD34+ HSC reaching approximate densities of 8x107 cells/mL (n=3). Collins et al. were able to achieve densities of 6.0x106 cells/mL in stir tank bioreactors with HSC,29 whereas other two-compartment perfusion bioreactors have reached densities of 1.0x108 cells/mL (www.fibercellsystems.com), but with other mammalian cells. In addition, Koller et al. achieved a density of 1x107 cells/mL when culturing CD34 enriched cells from human bone marrow in a perfusion bioreactor30. Our 8-mL laboratory bioreactor supported densities of approximately 2.0x108 cells/mL (data not shown), and with optimal medium formulations, we believe a further twofold increase can be achieved. The additional studies conducted in the 8-mL bioreactor system demonstrate cell harvesting capabilities, sequential cell removal, and passaging of cells.
The bioreactor passaging methodology used in the semicontinuous 8-mL bioreactor study generated a 15,000-fold expansion in 19 days. In comparison, Koller et al. were able to achieve approximately 1,300-fold expansion of CD34-enriched human bone marrow cells over 14 days,30 and Liu et al. achieved 430-fold expansion of HSC from umbilical cord blood over 8 days in a rotating wall bioreactor31. Although such comparisons can be instructive, they are limited by the differences in the bioreactor systems and the various differences in culture methodologies.
Our results confirm the biocompatibility of the 3D perfusion bioreactor for nonadherent CD34+ HSC, the feasibility of cell expansion, and erythrocyte lineage support in this unique hollow-fiber system. We have also demonstrated the ability to reuse the remaining progenitor cell fraction for further expansion and differentiation. Furthermore, the experiments on fractional cell removal show that vital cells could be harvested from the bioreactor during cultivation without affecting cell growth and metabolic activity. One possible reason that the conditioned bioreactors produced more cells than newly manufactured bioreactors, as demonstrated in the semicontinuous 8-mL bioreactor study (Fig. 5A), could be because of the extracellular matrix deposited on the hollow fiber membranes. The 2- and 8-mL bioreactor scales showed similar total fold expansion and maturation of the cells based on FACS results. Such results indicate the potential for continuous growth of proliferating cells in a closed system for cell production, from which cells can be regularly retrieved for further growth or additional experiments. The miniaturized bioreactor technology in combination with a well-controlled perfusion environment offers suitable conditions for optimization of the performance of longer-term studies on RBC production under constant conditions. In addition, the structure of the perfusion circuit allows for application of different growth factor regimens, including repeated-dose application and continuous infusion. We suggest that the 2-mL scaled bioreactor is most suitable when the number of available cells is limited, whereas the 8-mL scale is more suitable if sufficient sample material is required for combined analysis using polymerase chain reaction, gene arrays, flow cytometry, and additional histology.
This study shows successful scale-down of the proposed bioreactor technology from large clinical-scale devices to smaller prototypes of the same technology. The main advantages of the model can be seen in a 3D perfused cell environment provided by the physically active scaffold formed by the nutrition and oxygenation capillaries. because the currently described systems were scaled down versions of clinical-scale devices, we believe information obtained using laboratory-scale systems can be applied to optimizing clinical RBC production.
Acknowledgments
This study was partially supported by grants from the National Institutes of Health (R21 EB005739-01, J.G.), the European Commission (EU STREP-CT-2005-018940, J.G.), and the Federal Ministry of Education and Research, Germany (BMBF, 01GN0526 and 01GG0731/ 0313911, K.Z.). We are grateful for the discussions, excellent technical support, and photographs from Patrick Over, Matthew Young, and Daniel McKeel (McGowan Institute); SEM and immunofluorescence from Simon Watkins and Donna Beer Stolz (Center for Biological Imaging); and graphics from Wolfgang Mudra (Charité). We would also like to acknowledge the subcontract to Defense Advanced Research Projects Agency award FA9550-08-1-0392 P00004.
Disclosure Statement
The bioreactor prototypes were purchased from StemCell Systems GmbH, Berlin, Germany, a university spin-off of the Charité, Berlin. J.G. licensed intellectual property to the company. No author has shares or financial interests in the company.
References
- 1.Bersch C. The give and take of blood banking. Medical Laboratory Observer [on-line] Mar, 2010. http://www.mlo-online.com/ http://www.mlo-online.com/ [PubMed]
- 2.Yap C. Lau L. Krishnaswamy M. Gaskell M. Yii M. Age of transfused red cells and early outcomes after cardiac surgery. Ann Thorac Surg. 2008;86:554. doi: 10.1016/j.athoracsur.2008.04.040. [DOI] [PubMed] [Google Scholar]
- 3.Kirouac D.C. Zandstra P.W. The systematic production of cells for cell therapies. Cell Stem Cell. 2008;3:369. doi: 10.1016/j.stem.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 4.Noll T. Jelonek N. Schmidt S. Biselli M. Wandrey C. Cultivation of hematopoietic stem and progenitor cells: biochemical engineering aspects. Adv Biochem Eng Biotechnol. 2002;74:111. doi: 10.1007/3-540-45736-4_6. [DOI] [PubMed] [Google Scholar]
- 5.Kowalczyk M. Waldron K. Kresnowati P. Danquah M.K. Review: process challenges relating to hematopoietic stem cell culture in bioreactors. J. Ind Microbiol Biotechnol. 2011;38:761. doi: 10.1007/s10295-011-0951-6. [DOI] [PubMed] [Google Scholar]
- 6.Dahlberg A. Delaney C. Bernstein I.D. Ex vivo expansion of human hematopoietic stem and progenitor cells. Blood. 2011;117:6083. doi: 10.1182/blood-2011-01-283606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Panoskaltsis N. Mantalaris A. Wu J.H.D. Engineering a mimicry of bone marrow tissue ex vivo. J Biosc Bioeng. 2005;100:28. doi: 10.1263/jbb.100.28. [DOI] [PubMed] [Google Scholar]
- 8.Koller M.R. Manchel I. Palsson M.A. Maher R.J. Palsson B.O. Different measures of human hematopoietic cell culture performance are optimized under vastly different conditions. Biotechnol Bioeng. 1996;50:505. doi: 10.1002/(SICI)1097-0290(19960605)50:5<505::AID-BIT4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 9.Zimring J.C. Spitalnik S.L. Roback J.D. Hillyer C.D. Transfusion-induced autoantibodies and differential immunogenicity of blood group antigens: a novel hypothesis. Tranfusion. 2007;47:2189. doi: 10.1111/j.1537-2995.2007.01468.x. [DOI] [PubMed] [Google Scholar]
- 10.Douay L. Andreu G. Ex vivo production of Human red blood cells from hematopoietic stem cells: what is the future in transfusion. Transfus Med Rev. 2007;21 doi: 10.1016/j.tmrv.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Giarratana M.C. Kobari L. Lapillonne H. Chalmers D. Kiger L. Cynober T. Marden M.C. Wajcman H. Douay L. Ex vivo generation of fully mature human red blood cells from hematopoietic stem cells. Nat Biotechnol. 2005;23:69. doi: 10.1038/nbt1047. [DOI] [PubMed] [Google Scholar]
- 12.Douay L. From stem cells to red blood cells in vitro: “the 12 labors of Hercules.”. Clin Lab Med. 2010;30:391. doi: 10.1016/j.cll.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 13.Douay L. Lapillonne H. Turhan A.G. Stem cells—a source of adult red blood cells for transfusion purposes: present, future. Crit Care Clin. 2009;25:383. doi: 10.1016/j.ccc.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 14.Peng C. Palsson B. Determination of specific oxygen uptake rates in human hematopoietic culture and implication for bioreactor design. Ann Biomed Eng. 1996;24:373. doi: 10.1007/BF02660886. [DOI] [PubMed] [Google Scholar]
- 15.Cabrita G. Ferreira B.S. Lobato da Silva C. Goncalves R. Almeida-Porada G. Cabral J. Hematopoietic stem cells: from the bone to the bioreactor. Trends Biotechnol. 2003;21:233. doi: 10.1016/S0167-7799(03)00076-3. [DOI] [PubMed] [Google Scholar]
- 16.Koller M.R. Bender J.G. Papoutsakis E.T. Miller W.M. Beneficial effects of reduced oxygen tension and perfusion in long-term hematopoietic cultures. Ann N Y Acad Sc. 1992;665:105. doi: 10.1111/j.1749-6632.1992.tb42578.x. [DOI] [PubMed] [Google Scholar]
- 17.Abbot S. The three “R”s of Blood transfusion in 2020: routine, reliable and robust. Clin Lab Med. 2010;30:405. doi: 10.1016/j.cll.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 18.Gerlach J.C. Encke J. Hole O. Müller C. Ryan C.J. Neuhaus P. Bioreactor for a larger scale hepatocyte in vitro perfusion. Transplantation. 1994;58:984. doi: 10.1097/00007890-199411150-00002. [DOI] [PubMed] [Google Scholar]
- 19.Gerlach J.C. Mutig K. Sauer I.M. Schrade P. Efimova E. Mieder T. Naumann G. Grunwald A. Pless G. Mas A. Bachmann S. Neuhaus P. Zeilinger K. Use of primary human liver cells originating from discarded grafts in a bioreactor for liver support therapy and the prospects of culturing adult liver stem cells in bioreactors: a morphologic study. Transplantation. 2003;76:781. doi: 10.1097/01.TP.0000083319.36931.32. [DOI] [PubMed] [Google Scholar]
- 20.Schmelzer E. Mutig K. Schrade P. Bachmann S. Gerlach J.C. Zeilinger K. Effect of human patient plasma ex vivo treatment on gene expression and progenitor cell activation of primary human liver cells in multi-compartment 3d perfusion bioreactors for extracorporeal liver support. Biotech Bioeng. 2009;103:817. doi: 10.1002/bit.22283. [DOI] [PubMed] [Google Scholar]
- 21.Zeilinger K. Holland G. Sauer I.M. Efimova E. Kardassis D. Obermayer N. Liu M. Neuhaus P. Gerlach J.C. Time course of primary liver cell reorganization in three-dimensional high-density bioreactors for extracorporeal liver support: an immunohistochemical and ultrastructural study. Tissue Eng. 2004;10:1113. doi: 10.1089/ten.2004.10.1113. [DOI] [PubMed] [Google Scholar]
- 22.Gerlach J.C. Zeilinger K. Grebe A. Puhl G. Pless G. Sauer I. Grunwald A. Schnoy N. Muller C. Neuhaus P. Recovery of preservation-injured primary human hepatocytes and nonparenchymal cells to tissue like structures in large-scale bioreactors for liver support: an initial transmission electron microscopy study. J Invest Surg. 2003;16:83. doi: 10.1080/08941930390194370. [DOI] [PubMed] [Google Scholar]
- 23.Pless G. Steffen I. Zeilinger K. Sauer I.M. Katenz E. Kehr D.C. Roth S. Mieder T. Schwartlander R. Müller C. Wegner B. Hout M.S. Gerlach J.C. Evaluation of primary human liver cells in bioreactor cultures for extracorporeal liver support on the basis of urea production. Artif Organs. 2006;9:686. doi: 10.1111/j.1525-1594.2006.00285.x. [DOI] [PubMed] [Google Scholar]
- 24.Neildez-Nguyen TM. Wajcman H. Marden MC. Bensidhoum M. Moncollin V. Giarratana MC. Kobari L. Thierry D. Douay L. Human erythroid cells produced ex vivo at large scale differentiate into red blood cells in vivo. Nat Biotechnol. 2002;20:467. doi: 10.1038/nbt0502-467. [DOI] [PubMed] [Google Scholar]
- 25.Sussman N.L. Kelly J.H. Improved liver function following treatment with an extracorporeal liver assist device. Artif Organs. 1993;17:27. doi: 10.1111/j.1525-1594.1993.tb00381.x. [DOI] [PubMed] [Google Scholar]
- 26.Watanabe F.D. Mullon C.J. Hewitt W.R. Arkadopoulos N. Kahaku E. Eguchi S. Khalili T. Arnaout W. Shackleton C.R. Rozga J. Solomon B. Demetriou A. A clinical experience with a bioartificial liver in the treatment of severe liver failure. a phase i clinical trial. Ann Surg. 1997;225:484. doi: 10.1097/00000658-199705000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu A.S.L. Luntz T.L. Macdonalds J.M. Kubota H. Hsu E. London R.E. Reid L.M. Lineage and biology and liver. In: Lanza RP, editor; Langer R, editor; Vacanti J, editor. Principles of Tissue Engineering. San Diego CA: Academic Press; 1999. pp. 559–598. [Google Scholar]
- 28.Hay P.D. Veitich A.R. Smith M.D. Cousins R.B. Gaylor J.D. Oxygen transfer in a diffusion-limited hollow fiber bioartificial liver. Artif Organs. 2000;24:278. doi: 10.1046/j.1525-1594.2000.06499.x. [DOI] [PubMed] [Google Scholar]
- 29.Collins P.C. Nielsen L.K. Patel P.E. Papoutsakis T. Miller W.M. Characterization of hematopoietic cell expansion, oxygen uptake, and glycolysis in a controlled, stirred-tank bioreactor system. Biotechnol Prog. 1998;14:466. doi: 10.1021/bp980032e. [DOI] [PubMed] [Google Scholar]
- 30.Koller M.R. Manchel I. Newsom B.S. Palsson M.A. Palsson B.O. Bioreactor expansion of human bone marrow: comparison of unprocessed, density-separated, and cd34-enriched cells. J Hematother. 1995;4:159. doi: 10.1089/scd.1.1995.4.159. [DOI] [PubMed] [Google Scholar]
- 31.Liu Y. Liu T. Fan X. Ma X. Cui Z. Ex vivo expansion of hematopoietic stem cells derived from umbilical cord blood in rotating wall vessel. J Biotechnol. 2006;124:592. doi: 10.1016/j.jbiotec.2006.01.020. [DOI] [PubMed] [Google Scholar]