Abstract
Objective
This study tested whether IL-17A is involved in the pathogenesis of mouse myocardial ischemia-reperfusion (I/R) injury and investigated the mechanisms.
Background
Inflammatory processes play a major role in myocardial I/R injury. We recently identified interleukin (IL)-17A as an important cytokine in inflammatory cardiovascular diseases such as atherosclerosis and viral myocarditis. However, its role in myocardial I/R injury remains unknown.
Methods
The involvement of IL-17A was assessed in functional assays in mouse myocardial I/R injury by neutralization/repletion or genetically deficiency of IL-17A, and its mechanism on cardiomyocyte apoptosis and neutrophil infiltration were further studied in vivo and in vitro.
Results
IL-17A was elevated following murine left coronary artery ligation and reperfusion. Intracellular cytokine staining revealed that γδT lymphocytes, but not CD4+ helper T cells, were a major source of IL-17A. Anti-IL-17A mAb treatment or IL-17A knockout markedly ameliorated I/R injury, as demonstrated by reduced infarct size, reduced cardiac troponin T levels and improved cardiac function. This improvement was associated with a reduction in cardiomyocyte apoptosis and neutrophil infiltration. On the contrary, repletion of exogenous IL-17A induced the opposite effect. In vitro study showed that IL-17A mediated cardiomyocyte apoptosis through regulating the Bax/Bcl-2 ratio, induced CXC chemokine-mediated neutrophil migration and promoted neutrophil-endothelial cell adherence through induction of endothelial cell E-selectin and inter-cellular adhesion molecule (ICAM)-1 expression.
Conclusions
IL-17A mainly produced by γδT cells plays a pathogenic role in myocardial I/R injury by inducing cardiomyocyte apoptosis and neutrophil infiltration.
Keywords: interleukin-17, inflammation, ischemia/reperfusion, γδT cell
Introduction
The rapid restoration of blood flow through the occluded coronary artery by mechanical or pharmacological intervention is the most effective therapy to limit infarct size and improve the clinical outcome after acute myocardial infarction (MI) (1). However, reperfusion after ischemia itself causes additional cardiomyocyte death and increases infarct size in a process called myocardial ischemia/reperfusion (I/R) injury. Potential mediators of reperfusion injury include oxidative stress and inflammation (2).
IL-17A is a member of the IL-17 family, which includes six structurally related isoforms: IL-17A, IL-17B, IL-17C, IL-17D, IL-17E, and IL-17F (3). Multiple cell types, including CD4+ αβ T (Th17) cells, γδ T cells, natural killer (NK) cells, and neutrophils, produce IL-17A (4). IL-17A acts on a variety of cells through its ubiquitous receptors, IL-17RA and IL-17RC, and is a critical mediator of neutrophil recruitment and migration through the induction of granulopoiesis and the production of neutrophil chemokines, including lipopolysaccharide-induced CXC chemokine (LIX), cytokine-induced neutrophil chemoattractant (KC), and macrophage inflammatory protein-2 (MIP-2) (5).
IL-17A plays an important role in host defense and mediates autoimmune diseases (6). We and others have reported that IL-17A is linked to the pathogenesis of several cardiovascular diseases, including atherosclerosis (7–9), hypertension, viral myocarditis, and dilated cardiomyopathy (7–12). Recently, IL-17A has also been found to contribute to brain, kidney and intestine I/R injury (13–15), but the mechanisms involved are still largely unknown.
In this study, we investigated the expression of IL-17A in reperfused ischemic myocardium and the subsets of IL-17A-releasing cells and characterized the functional involvement of IL-17A in myocardial I/R injury. We show that IL-17A is largely produced by γδT cells and plays a pathogenic role in myocardial I/R injury by inducing cardiomyocyte apoptosis and neutrophil infiltration.
Methods
Methods section is available at http://content.onlinejacc.org/
Results
IL-17A increases following myocardial I/R injury
To test the involvement of IL-17A in myocardial I/R injury, we first investigated IL-17A levels in the myocardium at different reperfusion time points following I/R. IL-17A mRNA and protein expression increased significantly as early as 1 hour post reperfusion, rose progressively until 24 hours and then began to decrease, although it remained at a high level compared with the sham group until 72 hours after I/R (Supplementary Figure 1A and 1B).
In addition to Th17 cells, γδT cells, NK cells, and neutrophils were reported to secret IL-17A (4). To identify which leukocytes were responsible for the elevation of IL-17A in myocardial I/R, we used intracellular cytokine staining combined with staining for various surface markers. Over 95% of the IL-17A-secreting leukocytes were CD3+ T cells, including about 70% γδTCR+ cells and 13% CD4+ cells, indicating that γδT cells, rather than Th17 cells, were the major source of IL-17A (Supplementary Figure 1C). NK cells and neutrophils comprised only a minor proportion of IL-17A-secreting leukocytes. Following 24 hours of reperfusion, there was a marked increase in the myocardial content of γδT cells. Simultaneously, the percentage of IL-17+ γδTCR+ cells relative to γδT cells also increased (Supplementary Tables 1 and 2). These results suggest that myocardial I/R led to an increase not only in the number of infiltrated γδT cells, but also in the IL-17A production of these cells, which accounted for a large part of the elevated myocardial IL-17A.
The IL-17 family contains of 5 members including IL-17A. Among these, IL-17F has the highest degree of homology with IL-17A and exerts similar effects, but to a lesser extent (8). We therefore also examined mRNA expression of IL-17B, C, D, E, and F in hearts following I/R. The level of IL-17A mRNA was much higher than that of the other IL-17 family members, approximately 5-fold greater than that of IL-17F (Supplementary Figure 2).
Neutralization of endogenous IL-17A protects against, while repletion of exogenous IL-17A exacerbates, myocardial I/R injury
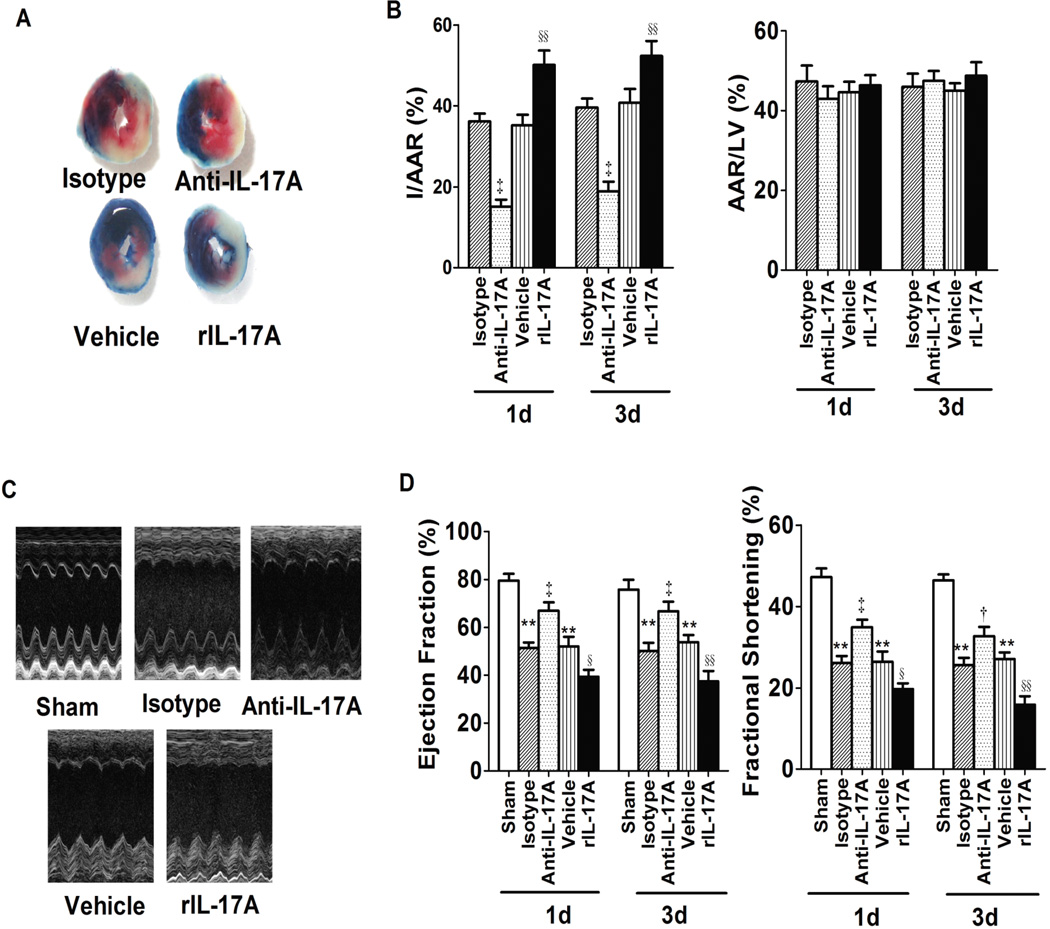
To determine the causative role of IL-17A in the development of myocardial I/R injury, we treated the myocardial I/R mice systemically with neutralizing anti-IL-17A monoclonal antibody (mAb) or recombinant IL-17A before reperfusion. IL-17A expression reached a peak at 24 hours after myocardial I/R and remained elevated for 72 hours; therefore we measured infarct size at 1 day or 3 days following reperfusion. The ratio of area at risk (AAR) to left ventricle (LV) area was the same in experimental and control groups at both time points, indicating that ligature was reproducibly performed at the same level of the left anterior coronary artery. However, the ratio of infarct area to AAR was significantly lower in mice treated with anti-IL-17A mAb relative to those treated with isotype and greater in mice treated with recombinant IL-17A relative to vehicle alone at both time points (Figure 1A and 1B). Serum cardiac troponin T (cTnT) level, an index of myocyte injury, was also significantly lower in the anti-IL-17A mAb group and higher in the rIL-17A group (Supplementary Figure 3A).
Fig. 1.
IL17A neutralization and repletion affected mouse myocardial I/R injury. A, Representative images of LV slices from different groups at 1 day after reperfusion. The nonischemic area is indicated in blue, the AAR in red and the infarct area in white. B, Quantification of infarct size of myocardial tissues 1 day or 3 days after reperfusion (n=6–8). C, Representative M-mode echo-cardiography images of the left ventricular 1 day after reperfusion. D, Ejection fraction and LV fractional shortening (n=8). **P<0.01 versus sham; †P<0.05, ‡P<0.01 versus isotype; §P<0.05, §§P<0.01 versus vehicle.
To determine the effect of IL-17A on cardiac function after I/R and to demonstrate the clinical relevance of our findings, we measured EF and FS by echocardiography. EF and FS were both remarkably reduced at 1 day and 3 days after myocardial I/R compared with the sham group. Furthermore, treatment with anti-IL-17A mAb increased EF and FS after 1 day and 3 days reperfusion, indicating improved cardiac function. Consistent with infarct size, a marked deterioration of cardiac function, as shown by a decrease of EF and FS, was observed in the rIL-17A group (Figure 1C and 1D). To test whether IL-17A affects hemodynamic properties, we measured left ventricular end-diastolic pressure (LVEDP) and the derivative of left ventricular pressure (dP/dt) using a millar catheter (SPR-671, Millar Instruments, Houston, TX). LV hemodynamics were augmented in the anti-IL-17A mAb-treated group compared with the isotype group. Specifically, LVEDP was lower, while maximum and minimum dP/dt, the indices of contractility, were increased in the anti-IL-17A mAb-treated group. Conversely, treatment with rIL-17A led to a reduction of dP/dt and an elevation of LVEDP (Table 1). Taken together, changes in the level of IL-17A can have profound reciprocal effects on myocardial I/R injury, in terms of both infarct area and cardiac function.
Table 1.
Hemodynamics measurements of myocardial I/R mice.
| Reperfusion time day) |
LVEDP (mmHg) |
dP/dtmax (mmHg/s) |
−dP/dtmin (mmHg/s) |
|
|---|---|---|---|---|
| Sham | 1 | 2.7±0.6 | 5786±289 | −5471±238 |
| Isotype | 1 | 8.8±0.8* | 3760±257* | −3575±218* |
| Anti-IL-17A | 1 | 4.4±0.6‡ | 4846±245† | −4412±263† |
| Vehicle | 1 | 8.4±0.8* | 3945±298* | −3743±304* |
| rIL-17A | 1 | 14.6±1.3§§ | 2447±165§§ | −2727±345§ |
| Sham | 3 | 3.3±0.3 | 5488±247 | −5659±336 |
| Isotype | 3 | 9.2±0.9* | 3662±254* | −3320±192* |
| Anti-IL-17A | 3 | 4.8±0.4‡ | 4763±325† | ‡4617±166‡ |
| Vehicle | 3 | 9.0±1.3* | 3759±201* | −3627±166* |
| rIL-17A | 3 | 15.7±1.2§§ | 2450±244§§ | −2683±275§ |
n=6–8, values are mean ± SEM;
P < 0.01 vs. sham;
P < 0.05,
P < 0.01 vs. isotype;
P < 0.05,
P < 0.01 vs. vehicle.
IL-17A knockout ameliorates myocardial I/R injury
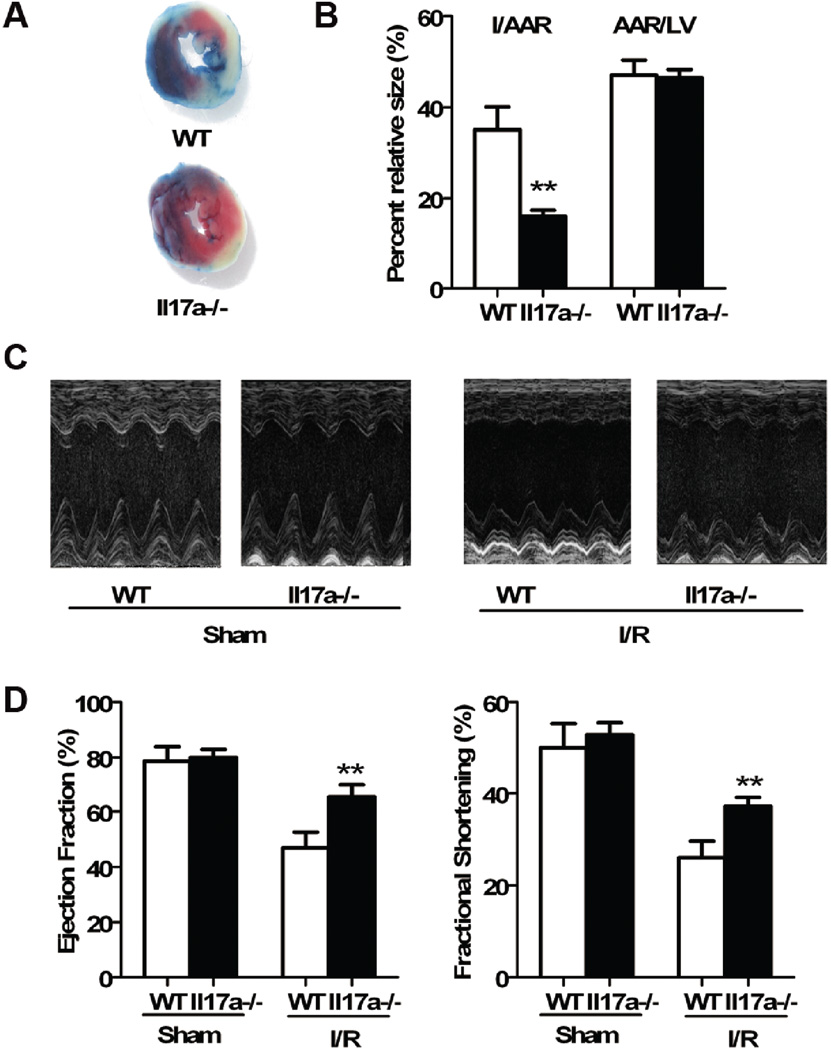
To elucidate the impact of IL-17A on myocardial I/R injury in the genetically IL-17A free situation, we compared myocardial I/R injury levels between Il17a−/− and wild-type C57BL/6 mice. Prior to surgery, we excluded the possibility of cardiac abnormality in Il17a−/− mice by echocardiography. Echocardiographic parameters of Il17a−/− mice were comparable to those of wild-type mice (Supplementary Table 3). Il17a−/− and wild-type mice were then subjected to myocardial I/R. The extent of injury and heart functions were assayed 1 day after initiation of reperfusion. With similar AAR, the infarct size/AAR was significantly smaller in Il17a−/− than in wild-type mice (Figure 2A and 2B). Less serious injury was also manifested by lower levels of serum cTnT in Il17a−/− mice (Supplementary Figure 3B). In parallel, we observed improved cardiac function, as indicated by elevated EF and FS, in Il17a−/− mice after myocardial I/R compared with wild-type (Figure 2C and 2D). This protection was also demonstrated by hemodynamic measurements. LVEDP was lower, while LV dP/dt was higher in Il17a−/− mice at 1 day after reperfusion (Table 2). These results confirm that IL-17A plays a pathogenic role in myocardial I/R injury.
Fig. 2.
IL-17A knockout ameliorated myocardial I/R injury. A, Representative images of LV slices from wild-type and Il17a−/− mice at 1 day after I/R. B, Quantification of infarct size of myocardium 1 day after reperfusion (n=8). C, Representative M-mode images of the LV after sham and myocardial I/R from wild-type and Il17a−/− mice. D, Ejection fraction and LV fractional shortening (n=8). ** P<0.01 versus wild-type.
Table 2.
Hemodynamics measurements of Il17a−/− mice after I/R.
| Reperfusion time(day) |
LVEDP (mmHg) |
dP/dtmax (mmHg/s) |
−dP/dtmin (mmHg/s) |
|
|---|---|---|---|---|
| WT Sham | 1 | 3.0±0.5 | 5898±282 | −5564±172 |
| WT I/R | 1 | 8.36±1.1 | 3832±155 | −3534±157 |
| Il17a−/− Sham | 1 | 2.8±0.4 | 5784±291 | −5369±148 |
| Il17a−/− I/R | 1 | 4.3±0.6* | 4903±265** | −4717±172* |
n=8, values are mean ± SEM;
P < 0.01,
P < 0.05 vs. wild-type.
IL-17a facilitates cardiomyocyte apoptosis following I/R in vivo or oxidative stress in vitro
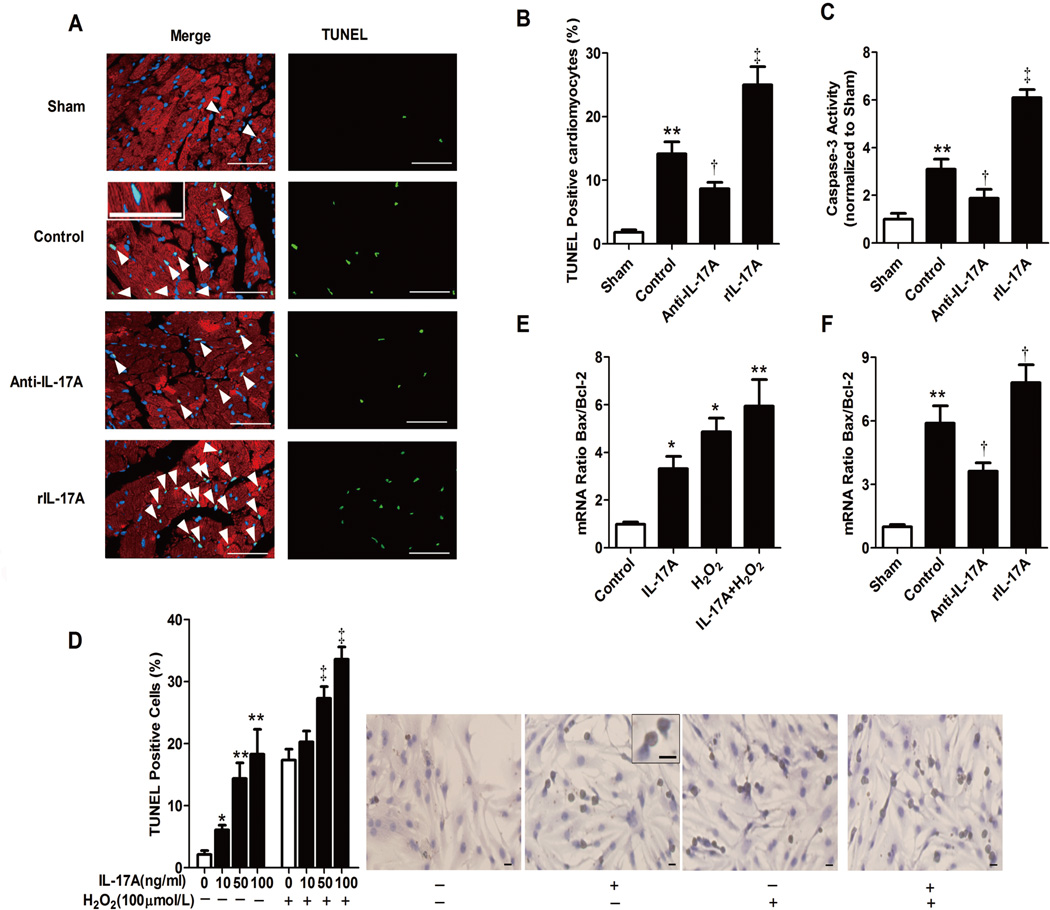
Apoptosis contributes significantly to myocardial I/R injury (16). We hypothesized that the role of IL-17A on myocardial I/R is associated with effects on cardiomyocyte apoptosis. To test this hypothesis, we carried out TUNEL labeling of LV sections from different experiment groups at 3 hours after myocardial I/R. As shown in Figure 3A and 3B, anti-IL-17A mAb treatment remarkably decreased the number of TUNEL-positive cardiomyocytes compared with control, suggesting that IL17A neutralization inhibited the extent of cardiomyocyte apoptosis. In contrast, repletion of IL-17A conferred the opposite effect. Concomitantly, caspase 3 activity determined by a caspase colorimetric assay from ischemic myocardium was down-regulated by anti-IL-17A mAb and up-regulated by rIL-17A (Figure 3C). These data indicate that IL-17A has a pro-apoptotic effect on cardiomyocytes following myocardial I/R in vivo.
Fig. 3.
IL-17A mediated cardiomyocyte apoptosis in vivo and in vitro. A, Representative photographs of TUNEL-stained heart sections from different groups after 3 hours reperfusion. Apoptotic nuclei were identified by TUNEL staining (green), cardiomyocyte by anti-sarcomeric actin antibody (red) and total nuclei by DAPI staining (blue). Arrowheads indicate apoptotic cardiomyocytes. Scale bar: 50 mm. B, Percentages of TUNEL-positive nuclei over total number of nuclei (n=4–5). C, Caspase 3 activity in myocardium was assessed after 3 hours of reperfusion and the values were normalized to sham (n=5), **P<0.01 versus sham; †P<0.05, ‡P<0.01 versus control. D, Mouse neonatal cardiomyocyte apoptosis under H2O2 and different doses of IL-17A. Brown staining indicates TUNEL positive cells. Results are representative of three independent assays. Scale bar: 100 mm. *P<0.05, **P<0.01 vs. H2O2(−)IL-17A(−), ‡P<0.01 versus H2O2(+)IL-17A(−). E, Real-time PCR determined mouse neonatal cardiomyocyte Bcl-2 and Bax mRNA levels. The results were expressed as Bax/Bcl-2 ratio. *P<0.05, **P<0.01 versus control. F, Real-time PCR determined mRNA level of Bcl-2 and Bax in ischemia myocardium. The results were expressed as ratio of Bax/Bcl-2. **P<0.01 versus sham, †P<0.05 versus control.
To test whether IL-17A directly induces cardiomyocyte apoptosis, we used primary cultures of neonatal mice ventricular myocytes that co-expressed IL-17RA and IL-17RC, as detected by real-time PCR and western blot (Supplementary Figure 4A and 4B). Cardiomyocytes were cultured with or without H2O2, a potent inducer of oxidative stress (17), in the presence or absence of recombinant IL-17A. As expected, H2O2 treatment increased the frequency of apoptotic cardiomyocytes, whereas IL-17A incubation further augmented apoptosis. Interestingly, incubation with IL-17A alone also induced apoptosis in a dose-dependent manner (Figure 3D). Taken together, these data indicate that IL-17A has a direct pro-apoptotic effect on cardiomyocytes.
To understand mechanisms through which IL-17A induces cardiomyocyte apoptosis, we examined expression of the Bcl-2 family by real-time PCR. The pro- to anti-apoptotic protein (Bax/Bcl-2) ratio was significantly increased in cardiomyocytes exposed to H2O2. IL-17A alone also increased the Bax/Bcl-2 ratio, which was further elevated under oxidative stress situation. Similarly, administration of rIL-17 in vivo increased the Bax-to-Bcl-2 ratio following myocardial I/R, while that of anti-IL-17A mAb decreased the ratio (Fig. 3E and 3F, Supplementary Figure 5). These results show that IL-17A induces cardiomyocyte apoptosis, at least in part, through the regulation of pro- to anti-apoptotic protein ratio of Bcl-2 family.
IL-17A mediates neutrophil recruitment through regulation of CXC ELR+ chemokine expression following I/R in vivo and oxidative stress in vitro
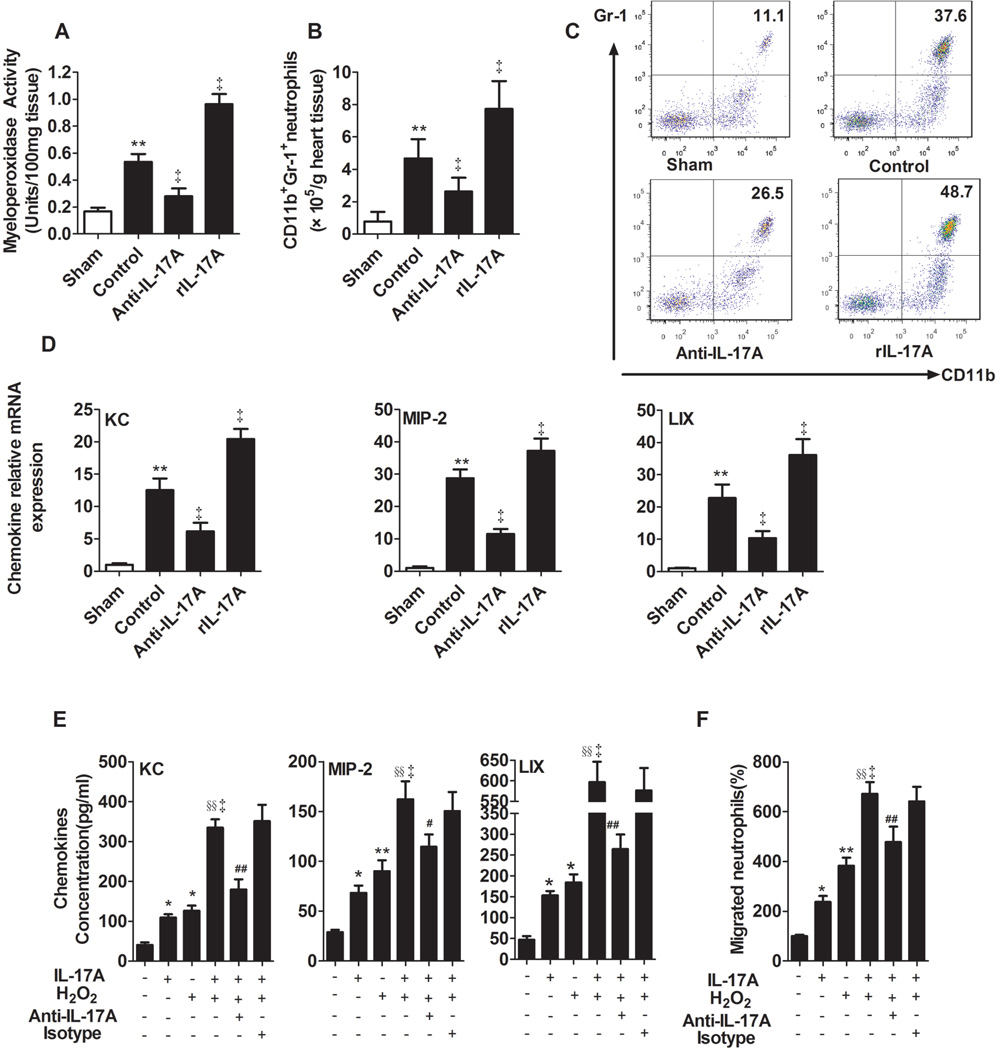
Neutrophil infiltration is a hallmark of inflammatory injury following myocardial I/R (17), and one of the main functions of IL-17A is neutrophil recruitment (19). As determined by MPO activity and FACS analysis of CD11b+Gr-1+ neutrophils, myocardial I/R induced a surge in neutrophil recruitment to myocardium. Anti-IL-17A mAb treatment reduced neutrophil recruitment, while treatment with rIL-17A promoted this effect (Figure 4A–C).
Fig. 4.
IL-17A increased cardiac neutrophil recruitment and migration. Mice were assessed for chemokine expression and neutrophil infiltration at 3 hours after myocardial I/R. A, Cardiac MPO activity in tissue samples. B, The number of CD11b+Gr-1+ neutrophils infiltrated in myocardium were analyzed by flow cytometry. C, The representative flowcytometry dot plots of CD11b+Gr-1+ neutrophils infiltrated in myocardium. D, LIX, KC and MIP-2 mRNA levels were analyzed by real-time PCR. **P<0.01 versus sham; ‡P<0.01 versus control. E, ELISA determined mouse cardiomyocytes KC, MIP-2 and LIX levels in the supernatants. F, Neutrophil migration, means±SEM. The value was normalized relative to medium alone. *P<0.05, **P<0.01 versus medium; ‡P<0.01, versus H2O2; §§P<0.01 versus IL-17A; #P<0.05, ##P<0.01 versus IL-17A/ H2O2. All experiments were repeated three times. n=4–5.
The CXC ELR+ (glutamic acid -leucine -arginine) chemokines KC, MIP-2 and LIX are not only potent neutrophil chemoattractants, but also IL-17A target genes (20). Consistent with increased neutrophil infiltration, myocardial I/R caused a significant induction of mRNA and protein levels of all three chemokines. Moreover, neutralization or repletion of IL-17A had the opposite effect on expression of these chemokines following myocardial I/R (Figure 4D and Supplementary Figure 6A). IL-17A also increased these three chemokines expression of cardiomyocyte in vitro as determined by ELISA. Stimulation with IL-17A in addition to H2O2 led to a dose-dependent potentiation of the effect of H2O2, markedly up-regulating these chemokines expression. Pre-incubation with anti-IL-17A Ab could completely abolish IL-17A activity (Figure 4E, Supplementary Figure 6B). Furthermore, in vitro neutrophil migration assay showed a marked enhancement of neutrophil migration to media from cells treated with IL-17A or H2O2 alone and was synergistically enhanced by conditioned media from cells treated with both agents (Figure 4F). The activity of IL-17A could be completely abolished by anti-IL-17A mAb, but not isotype mAb, indicating an IL17A-specific effect.
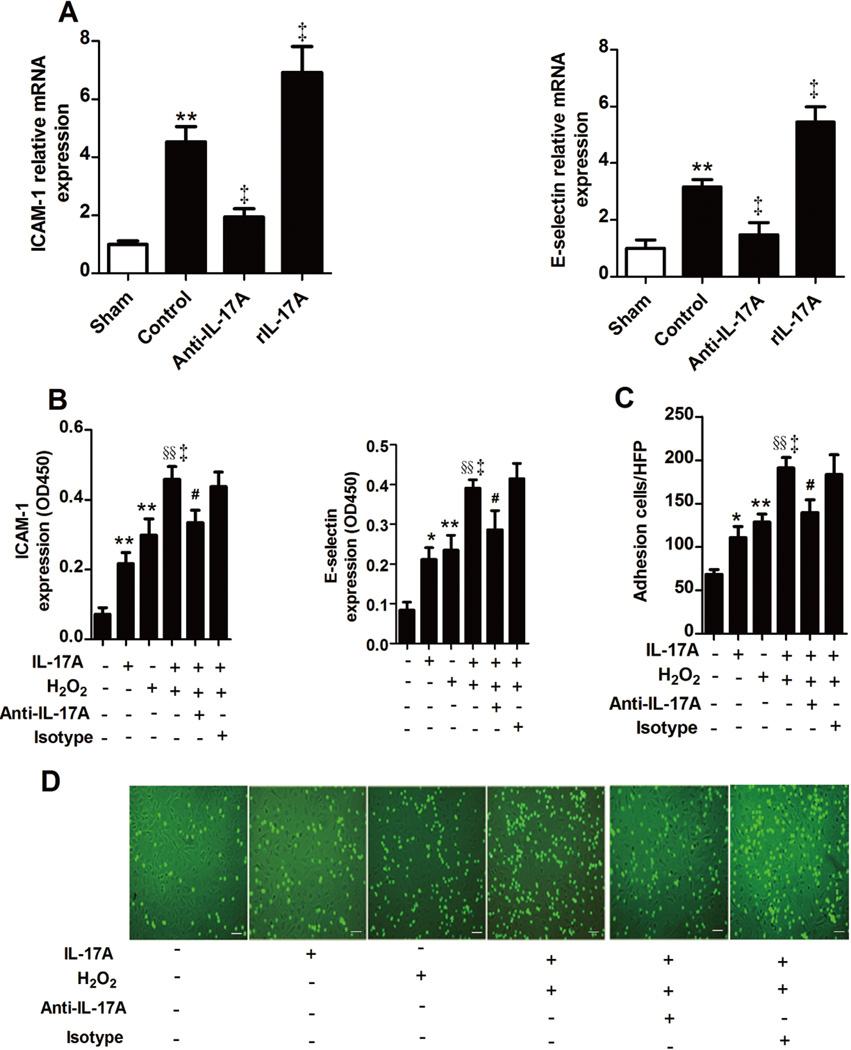
IL-17A regulates ICAM-1 and E-selectin expression and stimulates endothelial activation and neutrophil-endothelial cell adhesion
Adhesion molecule expression, an indicator of endothelium activation, is a prerequisite for neutrophil-endothelium adherence, which in turn contributes to myocardial I/R injury (18). To further explore the mechanisms by which IL-17A affects myocardial I/R injury, we focused on ICAM-1 and E-selectin expression. After 30 minutes of ischemia and 3 hours of reperfusion, we detected a substantial increase of ICAM-1 mRNA level and a moderate but significant increase of E-selectin mRNA levels within I/R myocardium. Treatment with anti-IL-17A mAb attenuated this induction, whereas rIL-17A treatment had the opposite effect (Figure 5A). This effect of IL-17A on the expression of ICAM-1 and E-selectin was further confirmed by Western blotting (Supplementary Figure 6C). As to the cellular level, stimulation with IL-17A led to a higher surface expression of ICAM-1 and E-selectin on endothelial cells (ECs) as detected by cell-based ELISA and an increase adherence of neutrophils to ECs as detected by neutrophil-EC adhesion assays. Moreover, addition of IL-17A to H2O2 led to a potentiation of the increased ICAM-1 and E-selectin surface expression and neutrophil adherence caused by H2O2 alone. When anti-IL-17A mAb was added, this augmentation could be absolutely blocked (Figure 5B–D and Supplementary Figure 6D).
Fig. 5.
IL-17A mediated neutrophil-EC adhesion. A, Mice were assessed for myocardium ICAM-1 and E-selectin expression by real-time PCR at 3 hours after I/R (n=4–5). **P<0.01 versus sham; †P<0.05, ‡P<0.01 versus control. B, Mouse myocardial EC expression of ICAM-1 and E-selectin was measured by a cell-based ELISA, as indicated by the OD450nm. C, Neutrophil adhesion on ECs was determined by fluorescence microscopy. D, Representative photomicrograph of neutrophils adhering to ECs. Neutrophils are identified by green fluorescence. Scale bar: 50 mm. Values are means±SEM; *P<0.05, **P<0.01 versus medium; ‡P<0.01 versus H2O2; §§P<0.01 versus IL-17A; # P<0.05 versus IL-17A/H2O2. All experiments were repeated three times.
Discussion
The present study reveals a critical role for IL-17A in mediating mouse myocardial I/R injury. Myocardial infiltrated γδ T lymphocytes, but not Th17 cells, were a major source of IL-17A. Anti-IL-17A mAb treatment or IL-17A knockout markedly ameliorated I/R injury, which was associated with a reduction in cardiomyocyte apoptosis and neutrophil infiltration. Repletion of exogenous IL-17A induced the opposite effect. In vitro experiments demonstrated that IL-17A could induce cardiomyocyte apoptosis through regulation of the Bax/Bcl-2 ratio. Moreover, IL-17A enhanced neutrophil infiltration by promoting EC E-selectin and ICAM-1 expression and inducing CXC chemokine-mediated neutrophil migration.
I/R triggers a vigorous inflammatory response, augmented by the generation and release of various cytokines, chemokines and adhesion molecules, which ultimately exacerbates tissue injury (21). Increasing evidence indicates that the elements of both adaptive immunity and innate immunity participate in I/R injury (22). Notably, IL-17A acts as a bridge between adaptive and innate immunity through the potent induction of a gene expression program typical of the inflammatory response, presenting a unique position in the immune response process (3). This finding has prompted new insight into the role of IL-17A in I/R injury. A growing body of evidence demonstrates that IL-17A is involved in the immune response during tissue I/R injury in brain, kidney and intestine (13–15). However, little was previously known about myocardial I/R, although IL-17A is involved in the pathogenesis of diverse cardiovascular diseases (7–12). Most importantly, we have found elevated circulating levels of IL-17A in patients with acute MI (23). Therefore, we focused on IL-17A in myocardial I/R using a mouse model. Here, we observed elevated IL-17A in the myocardium beginning at 1 hour after I/R, peaking at 24 hours and decreasing thereafter, which suggests that IL17A acts in the early stages of myocardial I/R.
Although prior studies of IL-17A have focused largely on CD4+ Th17 cells, several reports indicate that γδT cells are also a major source of this cytokine (4). In our study, most of the IL-17A+ cells were CD3+ T lymphocytes. To our surprise, about 70% of the IL-17A-producing T lymphocytes were CD4− but TCRγδ+. The differentiation of Th17 cells from naïve-CD4 cells usually takes several days in vivo and is currently thought to depend on TCR engagement. However, IL-17-producing γδT cells can immediately respond to stimuli, such as pathogens or IL-1/IL-23, with no need for prior stimulation via the TCR, and produce high amounts of IL-17A within hours (24). Considering the nearly instantaneous elevation of IL-17A in myocardium after I/R, it is no wonder that γδT lymphocytes, but not Th17 cells, were a major source of IL-17A, which is consistent with cerebral I/R (13). However, IL-17A producing cells detected in kidneys after 3 hours of reperfusion were CD11b+GR-1+ neutrophils (14), which differs from our results that little of IL-17A producing cells are neutrophils. In intestinal I/R (15), although Edgerton et al. focused on CD4+ T cells, they only examined the co-localization of CD3 and IL-17A, but performed no further examination of T cell subsets. This discrepancy may also be attributed to differences between tissues of I/R model.
Il17a−/− mice showed a significant reduction in infarct volume after cerebral I/R (13). Il17a−/− and Il17r−/− mice also showed protective effects on kidney function and morphology after renal I/R. Such protection was also observed by treatment with neutralizing anti-IL-17A mAb (14). In the present study, both neutralization of IL-17A just before induction of reperfusion and IL-17A genetically deficiency markedly ameliorated I/R injury, as demonstrated by reduced infarct size, cTnT levels, and improved cardiac function, whereas exogenous IL-17A significantly exacerbated I/R injury, demonstrating a critical role for IL-17A in myocardial I/R injury.
Apoptosis has been proposed to be an important mechanism for a significant amount of cell death in reperfused ischemic myocardium (16). It could be regulated by oxygen free radicals, cytokines such as TNF-α and IL-6, and neutrophil accumulation (25). The Bcl-2 family consists of pro- and anti-apoptotic members. The balance between pro-apoptotic and anti-apoptotic proteins determines the possibility of cells to either survive or undergo apoptosis after a certain stimulus or injury. IL-17A has been shown to induce vascular smooth muscle cell and airway epithelial cell apoptosis, but it has no effect on keratinocyte and osteoblast cell apoptosis (9, 26–28). Our in vivo study showed that neutrolization or repletion of IL-17A could regulate cardiomyocyte apoptosis, as confirmed by the change of TUNEL positive cardiomyocytes, caspase-3 activity and the ratio of pro-apoptotic (Bax) and anti-apoptotic (Bcl-2) proteins. IL-17A may trigger apoptosis directly in cardiomyocytes or indirectly as a mediator that orchestrates other factors. Our in vitro study further confirmed that IL-17A had direct pro-apoptosis effect on cardiomyocyte. When the cardiomyocytes were exposed to oxidative stress, extrinsic and intrinsic signal pathways are activated, Fas messenger RNA and Bcl-2 family proteins are up-regulated, and the redox state is changed, which can ultimately drive cardiomyocyte into apoptosis (25, 29, 30). The regulation of caspase-3 activity and ratio of Bax/Bcl-2 demonstrated that IL-17A activated intrinsic signal pathways. Remarkably, myocardial IL-17R expression was not affected by I/R in vivo or oxidative stress in vitro (data not shown), therefore the mechanisms through which IL-17A alone or interaction with oxidative stress modifies the apoptosis signal pathway in myocardial I/R remain to be elucidated.
Neutrophil recruitment plays a major role in myocardial damage after I/R (18). Neutrophil chemotaxis and activation may be strongly regulated by CXC chemokines, particularly ELR+ chemokines with the tripeptide ELR motif (31). Interestingly, IL-17A specifically induced the expression of all ELR+CXCL chemokines (CXCL1, CXCL2, CXCL3, CXCL5, CXCL6, and CXCL8) in cultured human synoviocytes, but had no effect on the expression of non-ELR CXC chemokines (CXCL9, CXCL10 and CXCL11) (32). IL-17A has also been shown to induce KC, MIP-2 and LIX, which are rodent homologs of CXCL1, CXCL2 and CXCL5, in mouse osteoblastic cells (33) and lung fibroblasts (34). Moreover, the importance of ELR+CXC chemokines in myocardial I/R has been implicated in vivo (35). Here, we found that inhibition of IL-17A markedly decreased, whereas exogenous IL-17A increased, cardiac LIX, KC and MIP-2 expression and neutrophil infiltration in mouse I/R myocardium. In vitro experiments demonstrated that IL-17A could enhance cardiomyocyte LIX, KC and MIP-2 production and neutrophil migration to the supernatant from conditioned cardiomyocytes. Taken together, these results indicate that IL-17A contributes to myocardial I/R injury at least partially through the regulation of neutrophil infiltration to the myocardium via CXC chemokine expression.
The neutrophil-endothelium interaction is critical for neutrophil-mediated inflammation. The molecular interactions required for neutrophils to cross the endothelium are regulated via the expression of adhesion molecules on the endothelium (18). E-selectin is a key molecule for rolling, whereas ICAM-1 is important in adhesion. Deficiency in either ICAM-1 or E-selectin expression results in a marked reduction in neutrophil accumulation and myocardial injury after I/R (36). IL-17A was reported to be a potential upstream inducer of ICAM-1 and E-selectin in lung microvascular ECs (37) and human umbilical vein ECs (19). We found that anti-IL-17A mAb and rIL-17A treatments regulate the expression of ICAM-1 and E-selectin in I/R myocardium at both mRNA and protein levels. In vitro studies showed that stimulation with IL-17A resulted in endothelial activation as revealed by elevated surface expression of E-selectin and ICAM-1 as well as neutrophil-endothelial adhesion. These results, along with the neutrophil migration data, support the notion that IL-17A has a potent effect on neutrophil recruitment and adherence, which are critical processes in myocardial I/R injury.
In summary, we have presented evidence that IL-17A produced primarily by γδT cells plays a pathogenic role in myocardial I/R injury by inducing cardiomyocyte apoptosis and neutrophil infiltration. These data suggest a novel IL-17A-dependent pathway by which the immune system may influence the myocardial I/R injury. Control of IL-17A production may be of benefit for minimizing I/R-instigated myocardial damage.
Supplementary Material
Acknowledgments
This work was supported in by grants from National Natural Science Foundation of China [No. 81170303 and 30871067 to X.C.]; Program for New Century Excellent Talents in University of China [NCET-09-0380 to X.C.]; National Basic Research Program of China [973 Program: 2007CB512005 and 2012CB517805 to Y.H.L. and X.C.]; National Institutes of Health grants, USA [HL60942, HL81090 and HL88547 to G.-P.S.).
Abbreviations and acronyms
- AAR
area at risk
- cTnT
cardiac troponin T
- EF
ejection fraction
- ELR
glutamic acid–leucine-arginine
- FS
fractional shortening
- ICAM-1
inter-cellular adhesion molecule -1
- I/R
ischemia-reperfusion
- KC
cytokine-induced neutrophil chemoattractant
- LIX
lipopolysaccharide-induced CXC chemokine
- LVEDP
left ventricular end-diastolic pressure
- MI
myocardial infarction
- MIP-2
macrophage inflammatory protein-2
- TUNEL
terminal deoxynucleotidyl-transferase mediated dUTP nick-end labeling
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have reported that they have no relationship to disclose.
References
- 1.Hillis LD, Lange RA. Myocardial infarction and the open-artery hypothesis. N Engl J Med. 2006;355:2475–2477. doi: 10.1056/NEJMe068251. [DOI] [PubMed] [Google Scholar]
- 2.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 3.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 4.Roark CL, Simonian PL, Fontenot AP, Born WK, O'Brien RL. gammadelta T cells: an important source of IL-17. Curr Opin Immunol. 2008;20:353–357. doi: 10.1016/j.coi.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ley K, Smith E, Stark MA. IL-17A-producing neutrophil-regulatory Tn lymphocytes. Immunol Res. 2006;34:229–242. doi: 10.1385/IR:34:3:229. [DOI] [PubMed] [Google Scholar]
- 6.Miossec P. IL-17 and Th17 cells in human inflammatory diseases. Microbes Infect. 2009;11:625–630. doi: 10.1016/j.micinf.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Cheng X, Taleb S, Wang J, et al. Inhibition of IL-17A in atherosclerosis. Atherosclerosis. 2011;215:471–474. doi: 10.1016/j.atherosclerosis.2010.12.034. [DOI] [PubMed] [Google Scholar]
- 8.Xie JJ, Wang J, Tang TT, et al. The Th17/Treg functional imbalance during atherogenesis in ApoE(−/−) mice. Cytokine. 2010;49:185–193. doi: 10.1016/j.cyto.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 9.Erbel C, Chen L, Bea F, et al. Inhibition of IL-17A attenuates atherosclerotic lesion development in apoE-deficient mice. J Immunol. 2009;183:8167–8175. doi: 10.4049/jimmunol.0901126. [DOI] [PubMed] [Google Scholar]
- 10.Madhur MS, Lob HE, McCann LA, et al. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension. 2010;55:500–507. doi: 10.1161/HYPERTENSIONAHA.109.145094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuan J, Yu M, Lin QW, et al. Th17 cells contribute to viral replication in coxsackievirus B3-induced acute viral myocarditis. J Immunol. 2010;185:4004–4010. doi: 10.4049/jimmunol.1001718. [DOI] [PubMed] [Google Scholar]
- 12.Baldeviano GC, Barin JG, Talor MV, et al. Interleukin-17A is dispensable for myocarditis but essential for the progression to dilated cardiomyopathy. Circ Res. 2010;106:1646–1655. doi: 10.1161/CIRCRESAHA.109.213157. [DOI] [PubMed] [Google Scholar]
- 13.Shichita T, Sugiyama Y, Ooboshi H, et al. Pivotal role of cerebral interleukin-17-producing gammadeltaT cells in the delayed phase of ischemic brain injury. Nat Med. 2009;15:946–950. doi: 10.1038/nm.1999. [DOI] [PubMed] [Google Scholar]
- 14.Li L, Huang L, Vergis AL, et al. IL-17 produced by neutrophils regulates IFN-gamma-mediated neutrophil migration in mouse kidney ischemia-reperfusion injury. J Clin Invest. 2010;120:331–342. doi: 10.1172/JCI38702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edgerton C, Crispin JC, Moratz CM, et al. IL-17 producing CD4+ T cells mediate accelerated ischemia/reperfusion-induced injury in autoimmunity-prone mice. Clin Immunol. 2009;130:313–321. doi: 10.1016/j.clim.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamacher-Brady A, Brady NR, Gottlieb RA. The interplay between pro-death and pro-survival signaling pathways in myocardial ischemia/reperfusion injury: apoptosis meets autophagy. Cardiovasc Drugs Ther. 2006;20:445–462. doi: 10.1007/s10557-006-0583-7. [DOI] [PubMed] [Google Scholar]
- 17.Chandrasekar B, Colston JT, de la Rosa SD, Rao PP, Freeman GL. TNF-alpha and H2O2 induce IL-18 and IL-18R beta expression in cardiomyocytes via NF-kappa B activation. Biochem Biophys Res Commun. 2003;303:1152–1158. doi: 10.1016/s0006-291x(03)00496-0. [DOI] [PubMed] [Google Scholar]
- 18.Vinten-Johansen J. Involvement of neutrophils in the pathogenesis of lethal myocardial reperfusion injury. Cardiovasc Res. 2004;61:481–497. doi: 10.1016/j.cardiores.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 19.Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of T(H)17 cells. Nature. 2008;453:1051–1057. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Onishi RM, Gaffen SL. Interleukin-17 and its target genes: mechanisms of interleukin-17 function in disease. Immunology. 2010;129:311–321. doi: 10.1111/j.1365-2567.2009.03240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steffens S, Montecucco F, Mach F. The inflammatory response as a target to reduce myocardial ischaemia and reperfusion injury. Thromb Haemost. 2009;102:240–247. doi: 10.1160/TH08-12-0837. [DOI] [PubMed] [Google Scholar]
- 22.Linfert D, Chowdhry T, Rabb H. Lymphocytes and ischemia-reperfusion injury. Transplant Rev (Orlando) 2009;23:1–10. doi: 10.1016/j.trre.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng X, Yu X, Ding YJ, et al. The Th17/Treg imbalance in patients with acute coronary syndrome. Clin Immunol. 2008;127:89–97. doi: 10.1016/j.clim.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 24.Kapsenberg ML. Gammadelta T cell receptors without a job. Immunity. 2009;31:181–183. doi: 10.1016/j.immuni.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 25.Nakamura M, Wang NP, Zhao ZQ, et al. Preconditioning decreases Bax expression, PMN accumulation and apoptosis in reperfused rat heart. Cardiovasc Res. 2000;45:661–670. doi: 10.1016/s0008-6363(99)00393-4. [DOI] [PubMed] [Google Scholar]
- 26.Sonnenberg GF, Nair MG, Kirn TJ, Zaph C, Fouser LA, Artis D. Pathological versus protective functions of IL-22 in airway inflammation are regulated by IL-17A. J Exp Med. 2010;207:1293–1305. doi: 10.1084/jem.20092054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pennino D, Eyerich K, Scarponi C, et al. IL-17 amplifies human contact hypersensitivity by licensing hapten nonspecific Th1 cells to kill autologous keratinocytes. J Immunol. 2010;184:4880–4888. doi: 10.4049/jimmunol.0901767. [DOI] [PubMed] [Google Scholar]
- 28.Shen F, Ruddy MJ, Plamondon P, Gaffen SL. Cytokines link osteoblasts and inflammation: microarray analysis of interleukin-17- and TNF-alpha-induced genes in bone cells. J Leukoc Biol. 2005;77:388–399. doi: 10.1189/jlb.0904490. [DOI] [PubMed] [Google Scholar]
- 29.Tanaka M, Ito H, Adachi S, et al. Hypoxia induces apoptosis with enhanced expression of Fas antigen messenger RNA in cultured neonatal rat cardiomyocytes. Circ Res. 1994;75:426–433. doi: 10.1161/01.res.75.3.426. [DOI] [PubMed] [Google Scholar]
- 30.Kubasiak LA, Hernandez OM, Bishopric NH, Webster KA. Hypoxia and acidosis activate cardiac myocyte death through the Bcl-2 family protein BNIP3. Proc Natl Acad Sci U S A. 2002;99:12825–12830. doi: 10.1073/pnas.202474099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frangogiannis NG. Chemokines in ischemia and reperfusion. Thromb Haemost. 2007;97:738–747. [PubMed] [Google Scholar]
- 32.Zrioual S, Toh ML, Tournadre A, et al. IL-17RA and IL-17RC receptors are essential for IL-17A-induced ELR+ CXC chemokine expression in synoviocytes and are overexpressed in rheumatoid blood. J Immunol. 2008;180:655–663. doi: 10.4049/jimmunol.180.1.655. [DOI] [PubMed] [Google Scholar]
- 33.Ruddy MJ, Shen F, Smith JB, Sharma A, Gaffen SL. Interleukin-17 regulates expression of the CXC chemokine LIX/CXCL5 in osteoblasts: implications for inflammation and neutrophil recruitment. J Leukoc Biol. 2004;76:135–144. doi: 10.1189/jlb.0204065. [DOI] [PubMed] [Google Scholar]
- 34.Zhou X, Chen Q, Moore J, Kolls JK, Halperin S, Wang J. Critical role of the interleukin-17/interleukin-17 receptor axis in regulating host susceptibility to respiratory infection with Chlamydia species. Infect Immun. 2009;77:5059–5070. doi: 10.1128/IAI.00403-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chandrasekar B, Smith JB, Freeman GL. Ischemia-reperfusion of rat myocardium activates nuclear factor-KappaB and induces neutrophil infiltration via lipopolysaccharide- induced CXC chemokine. Circulation. 2001;103:2296–2302. doi: 10.1161/01.cir.103.18.2296. [DOI] [PubMed] [Google Scholar]
- 36.Jones SP, Trocha SD, Strange MB, et al. Leukocyte and endothelial cell adhesion molecules in a chronic murine model of myocardial reperfusion injury. Am J Physiol Heart Circ Physiol. 2000;279:H2196–H2201. doi: 10.1152/ajpheart.2000.279.5.H2196. [DOI] [PubMed] [Google Scholar]
- 37.Roussel L, Houle F, Chan C, et al. IL-17 promotes p38 MAPK-dependent endothelial activation enhancing neutrophil recruitment to sites of inflammation. J Immunol. 2010;184:4531–4537. doi: 10.4049/jimmunol.0903162. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.