Abstract
Purpose
Lymphatic invasion (LI) in primary cutaneous melanomas was recently found to be common. In this study, we evaluated LI as an independent prognostic factor.
Experimental Design
This study included 251 patients with vertical growth phase (VGP) primary cutaneous melanomas who had paraffin-fixed lesional tissue and were in a prospective cohort seen between 1972 and 1991, had no clinical evidence of regional nodal disease at diagnosis and had at least ten years of follow-up. Dual immunohistochemistry (IHC) staining was used to detect lymphatic endothelium (podoplanin) and melanoma cells (S-100). Multivariate logistic regression for ten-year metastasis was used to define independent prognostic factors and a prognostic tree was developed to characterize and discriminate risk groups. Kaplan-Meier disese-free survival curves for those with and without LI within current AJCC stages were compared using the log-rank statistic.
Results
LI was observed in 43% (108 of 251) of the study melanomas. The multivariate model for ten-year metastasis identified 4 independent prognostic factors: tumor thickness, mitotic rate (MR), LI, and anatomic site. The prognostic tree identified a group of patients with thin (≤1 mm thick) melanomas and poor prognosis: stage IB melanomas with LI. Survival curves for time to first metastasis demonstrated significantly poorer prognosis for patients with LI compared to those without it for both stages IB and IIA.
Conclusions
LI is common across the range of tumor thicknesses in primary VGP melanomas. It is an independent prognostic factor and significantly increases the risk of metastasis in patients in clinical stages IB and IIA.
INTRODUCTION
Clinically apparent distant metastasis is the cause of most deaths in patients with primary cutaneous melanoma and melanoma has a propensity for lymph node metastasis (1,2). Both the currently available staging system (3) and identified prognostic factors are limited in their ability to discriminate reliably between melanoma patients in clinical Stages I–II (and Stage III with “microscopic lymph node involvement”) who will manifest subsequent disease recurrence and death and those who will remain disease free. Therefore, it is important to develop prognostic biomarkers to identify, on the one hand, high-risk patients who are candidates for clinical trials of more aggressive staging, treatment and follow up and, on the other, low risk patients who can avoid more invasive procedures and who can be followed more infrequently.
Lymphangiogenesis and lymphatic invasion (LI, defined as melanoma cells in lymphatic vessels) have been under increasing investigation in the lesions of primary melanoma because of the recent availability of antibodies specific for lymphatic endothelial cells (4, 5). It has been shown that patients whose primary lesions had high densities of lymphatic vessels had shorter disease-free and overall survival rates (5,6). We and others have demonstrated that LI detected by immunohistochemistry (IHC) in primary melanomas is common, ranging from 16% to 47% (7–9), whereas blood vascular invasion is uncommon, ranging from 1–3% (7, 8). We recently presented evidence of lymphangiogenesis in areas with regression in the radial growth phase adjacent to VGP lesions, and LI appeared in part to explain the association of regression with poorer prognosis (10). LI’s association with the status of regional nodes or with distant metastasis remains controversial (7, 11–13). The contradictory findings are likely due to the small sample sizes and short follow up that characterize these studies. Nevertheless, these results suggest that evidence of lymphangiogenesis and of LI in the primary lesion underlie melanoma’s propensity for lymph node metastasis and indicate that the prognostic value of LI needs to be more fully examined.
In the present Phase II biomarker study, we examined the prognostic role of LI using double immunostaining for the melanocytic marker S-100 and the lymphatic vessel marker podoplanin in a large cohort of patients with long follow up (10–35 years) who were diagnosed and treated prior to the routine use of sentinel lymph node (SLN) biopsies for staging. We addressed the following four hypotheses: 1) LI is associated with metastasis; 2) LI is an independent prognostic factor for metastasis after adjustment for commonly ascertained prognostic factors; 3) A prognostic tree would identify subgroups of patients defined by LI that are at high risk for metastasis; and 4) Groups of patients with LI would have significantly higher metastasis rates within each of the AJCC stages for patients without evident regional node enlargement.
MATERIALS AND METHODS
Study patients and design
The 287 patients eligible for this nested, retrospective study of a prospective cohort had VGP primary melanomas and no regional nodal procedure or apparent metastases at the time of definitive treatment between 1972 and 1991 at the University of Pennsylvania’s Pigmented Lesion Clinic. They had at least ten years of protocol-driven, prospective follow-up and had paraffin blocks available for IHC staining. Thirty-six patients were excluded because their tissue sections lacked VGP or for other technical reasons related to IHC, resulting in a study cohort of 251 patients. The protocol was approved by Institutional Review Board at University of Pennsylvania.
IHC Staining
We detailed our methodology for detecting LI in our Phase I feasibility study that used a separate and smaller set of patients (8). Briefly, antibody to lymphatic endothelium was visualized with the brown chromogen DAB (3,3' diaminobenzidine, DakoCytomation, Carpinteria, CA) and antibodies to melanoma cells with the red chromogen Nova Red (Vector Laboratories, Burlingame, CA). The D2-40 antibody (mouse monoclonal, 1:25 dilution, Signet Laboratories, Dedham, MA) that specifically detects a fixation resistant epitope on podoplanin was used to decorate lymphatic endothelium. Melanoma cells were identified using S-100 antibody (rabbit polyclonal, 1:50, DakoCytomation). IHC assays were done on 5 µm-thick formalin-fixed, paraffin-embedded tissue sections and staining was done on a DakoCytomation Autostainer using the EnVision+ HRP DAB system (DakoCytomation) according to manufacturer’s recommendations.
Pathology Definitions
All original H&E slides were read at the time of definitive treatment by pathologists associated with the Pigmented Lesion Clinic (chiefly D.E.E. and W.H. Clark Jr.). Attributes included tumor thickness and anatomical level; dermal MR, expressed in terms of mitoses per square millimeter; VGP tumor infiltrating lymphocytes (TIL), classified as either brisk, nonbrisk, or absent; regression, if present in the radial growth phase that often accompanies the VGP; microscopic satellites; ulceration; and vascular (blood or lymphatic) invasion. When describing the patients’ tumor characteristics in Table 1 and Figure 1, thickness was categorized as in AJCC staging using the TNM T classes (≤1mm, 1–2 mm, 2–4 mm, and >4 mm) and MR was trichotomized (<1, 1–6, and >6 mitoses per mm2) with the lower cutpoint as in AJCC staging and upper cutpoint as previously reported (14).
Table 1.
Patient and Tumor Characteristics, by Lymphatic Invasion Status
| LI Absent (n=143) | LI Present (n=108) | All Patients (n=251) | |||||
|---|---|---|---|---|---|---|---|
| Characteristic | n | Percent | n | Percent | p-value | n | Percent |
| Thickness | |||||||
| ≤ 1.00 mm | 85 | 59.4 | 40 | 37.0 | 0.0005 | 125 | 49.8 |
| 1.01 – 2.00 mm | 35 | 24.5 | 29 | 26.9 | 64 | 25.5 | |
| 2.01 – 4.00 mm | 20 | 14.0 | 29 | 26.9 | 49 | 19.5 | |
| > 4.00 mm | 3 | 2.1 | 10 | 9.3 | 13 | 5.2 | |
| Mitotic Rate | |||||||
| < 1.0 | 74 | 51.7 | 35 | 32.4 | <0.0001 | 109 | 43.4 |
| 1.00 – 6.0 | 52 | 36.4 | 38 | 35.2 | 90 | 35.9 | |
| > 6.00 | 17 | 11.9 | 35 | 32.4 | 52 | 20.7 | |
| Ulceration | |||||||
| Present | 10 | 7.0 | 25 | 23.1 | 0.0003 | 35 | 13.9 |
| Absent | 133 | 93.0 | 83 | 76.9 | 216 | 86.1 | |
| VGP TIL | |||||||
| Absent | 49 | 34.3 | 25 | 23.2 | 0.0558 | 74 | 29.5 |
| Present | 94 | 65.7 | 83 | 76.8 | 177 | 70.5 | |
| Gender | |||||||
| Male | 61 | 42.7 | 67 | 62.0 | 0.0024 | 128 | 51.0 |
| Female | 82 | 57.3 | 41 | 38.0 | 123 | 49.0 | |
| Anatomic Site | |||||||
| Axial | 88 | 61.5 | 75 | 69.4 | 0.1937 | 163 | 64.9 |
| Extremity | 55 | 38.5 | 33 | 30.6 | 88 | 35.1 | |
| Age at Diagnosis | |||||||
| < 60 Years | 107 | 74.8 | 71 | 66.7 | 0.1166 | 178 | 70.9 |
| ≥ 60 Years | 36 | 25.2 | 37 | 34.3 | 73 | 29.1 | |
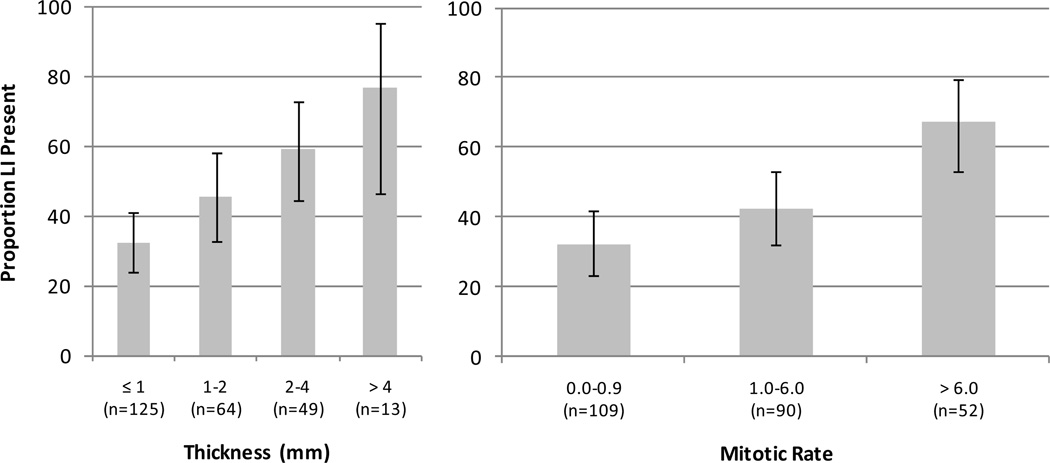
Figure 1.
Proportion of melanomas with LI by thickness and MR. Error bars represent exact 95% confidence intervals.
For this study, IHC stained slides cut from archived tissue blocks were reviewed independently by at least two pathologists (XX, CL, PJZ, or DEE), who were again blinded to clinical outcome. LI (present or absent) was defined as the presence anywhere within the primary tumor of S-100 positive cell(s) with morphological features of melanoma cells in lumens highlighted by podoplanin staining. Questionable instances were confirmed or refuted by use of multispectral imaging (MSI, see below). Disagreements were resolved by consensus reading.
MSI Analysis
Slides were examined using a Leica DMRA2 microscope (Leica Microsystems Inc., Bannockburn, IL) equipped with planapochromatic lenses (8). Potential foci of LI were imaged at 200x through a liquid crystal filter using the Nuance Multispectral Imaging System (Cambridge Research and Instrumentation Inc., Woburn, MA). Spectral data were acquired from 420–720 nm and spectral unmixing was accomplished by Nuance software v1.42 and pure spectral libraries of individual chromogens (slides stained with only DAB, Nova red or hematoxylin). Nonspecific background staining was subtracted from each image individually. To visualize several spectral markers simultaneously, images were then evaluated using unmixed images generated by the Nuance system.
Clinical Definitions
All patients in this study were followed per protocol for 10–35 years. The primary, binary, clinical endpoint for this study was “first metastasis” within ten years of definitive treatment wherein metastasis was defined to be metastasis to regional soft tissue (including lymph nodes), disseminated disease or, if there was no information on preceding metastases, melanoma-specific death. In the survival analyses time to first metastasis was defined as the time from definitive surgery to the first metastasis, as defined above. Seventy-two patients had events within ten-years of definitive therapy: regional skin (n=8), regional lymph nodes (n=35), disseminated disease (n=28) or melanoma-specific death (n=1). Patients were censored when they died from causes unrelated to melanoma or were alive at the time of last follow up. The median follow-up time for those alive at last follow up was 20.3 years (n=171, range was 10.1 to 34.6 years). The median time to first metastasis was 2.42 years (n=80). The definitions for AJCC sub-stages were based on the definitions for clinical staging of the revised version of the melanoma AJCC staging system (2), since only clinical information about the status of the regional lymph nodes was known for this cohort that pre-dated the use of SLN biopsies and did not include patients who underwent prophylactic regional node dissections.
Statistical analysis
The cohort of 287 study-eligible patients was a second random sample from 430 patients with available paraffin blocks from a consecutive cohort of 1128 patients treated between 1972 and 1991. The first random sample of 143 patients (106 had analyzable tissue sections) were included in our published feasibility study (8). In that study ten-year metastasis rates were 46% and 28% for patients with and without LI. These estimates were used to evaluate the power of the chi-square test (alpha=0.05, two-sided) for this Phase II study. With samples sizes of 100 and 200 in the two groups, respectively, this statistical test was determined to have a power of 84%.
Chi-squared tests were used to evaluate differences between the study cohort (n=251) and those excluded (n=36). Univariate and multivariate logistic regression analyses were used to estimate unadjusted and adjusted odds ratios (OR) for ten-year metastasis and their standard errors. Thickness and MR were used as continuous variables in the univariate and multivariate logistic regression models. The Hosmer-Lemeshow statistic was used to assess lack-of-fit. The reduced model was obtained by stepwise elimination of non-significant factors from the full logistic regression model that included 7 factors using the likelihood ratio test. The accuracy of the predicted values was assessed with the area under the receiver operating curve (ROC). The prognostic tree was produced using recursive partitioning that selected optimal variables and associated cut-points producing two patient groups minimizing misclassification at each step (15). Here, thickness and MR were categorized as described in “Clinical Definitions” (above). Ten-fold cross validation was used to select the final tree. Kaplan-Meier (KM) survival curves were computed from which ten-year metastasis rates were derived and the log-rank statistic was used to evaluate differences between final subgroups.
RESULTS
Clinical and tumor characteristics for the 251 patients included in this study are presented in Table 1. The characteristics of the study patients were not significantly different from the source cohort of 1128 patients seen at the Pigmented Lesion Clinic (1972–1991) with the exception of MR (the proportion of study patients with a MR <1 was 35% compared to 39% for non-study patients). The 36 eligible patients excluded from analysis because of insufficient tissue had characteristics similar to the 251 study patients except for thickness; the proportions of patients with thin melanomas were 66% and 43% in the two groups, respectively (data not shown).
LI is common in primary melanomas and is associated with poor prognostic factors
LI was present in 43% (95% CI = 36.9% to 49.2%) of patients’ lesions, significantly higher than the 4.6% (95% CI = 2.2% – 6.9%) observed when the H&E stained slides were reviewed at the time of definitive therapy (McNemar’s Test, p<0.0001). Four characteristics differed significantly between patients with LI and those without (Table 1): thickness (p=0.0005), MR (<0.0001), ulceration (0.0003) and gender (0.0024). The proportion of patients with LI increased as both thickness and MR increased (Cochran-Armitage test for trend, both p<0.0001; see Figure 1). Of note, LI was common in the thin VGP tumors: 32% had LI.
LI is an independent prognostic marker for metastasis
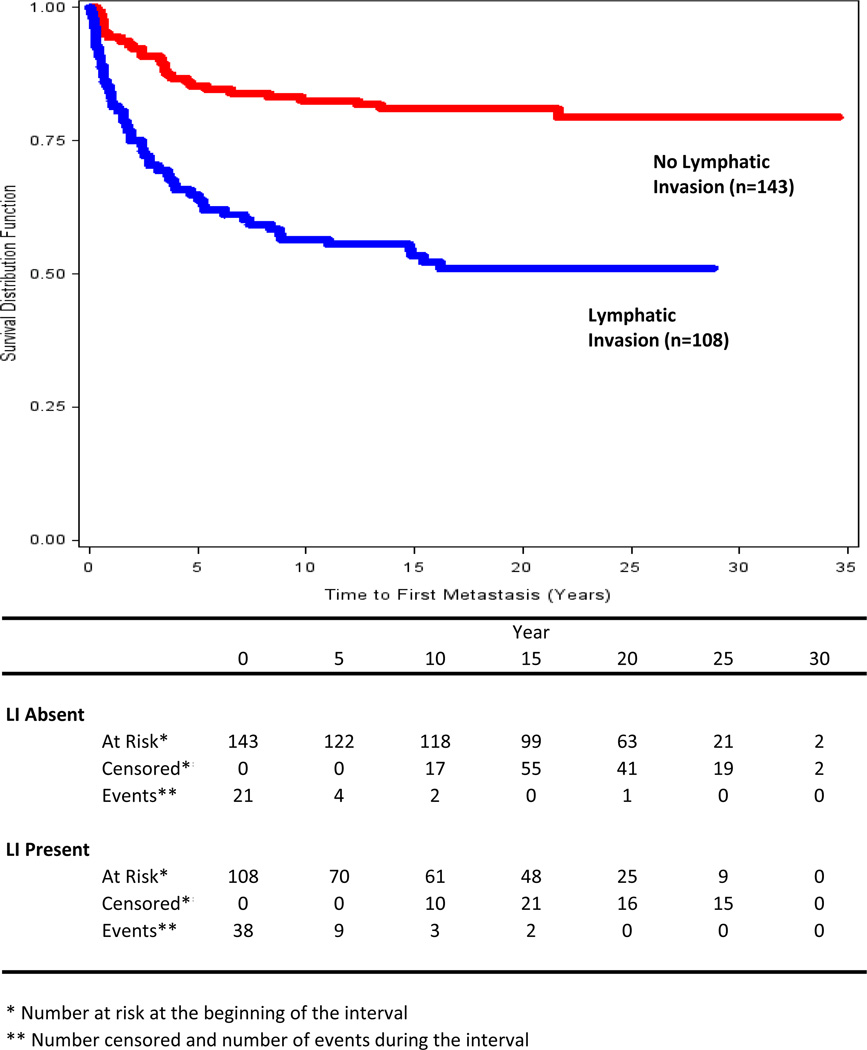
The overall ten-year metastasis rate was 27.8% (95% CI = 20.8% – 37.1%) based on the KM curve for all patients. There was a significant difference between the KM curves (Figure 2) for those with and without LI (p<0.001). The events in the first ten years included 90% of all known metastases (n=80). Of those who had a first metastasis within ten years of treatment (n=72), 65.2% had LI (95% CI = 54.3% – 76.3%); of those without a ten-year metastasis (n=179), only 34.0% had LI (95% CI = 27.2% – 41.0%). More than half (n=43, 59.7%) of the 72 first metastatic events that occurred within ten years involved the regional skin (n=8, 11%) or lymph nodes (n=35, 48.6%).
Figure 2.
Kaplan-Meier survival curves for time to first metastasis in patients whose melanomas had (blue) and did not have (red) LI.
LI was significantly associated with ten-year metastasis (unadjusted OR=3.6, p<0.001). In the reduced multivariate logistic regression analyses of ten-year metastasis that included four of the seven prognostic variables (Table 2), LI was a significant independent prognostic factor (adjusted OR=2.2, p=0.026). The area under the ROC curve for the predicted probabilities from the reduced multivariable model was 0.86.
Table 2.
Odds Ratios for Metastasis Within Ten Years (n=251)
| Prognostic Factor |
Number of Events |
Univariate Unadjusted |
Reduced Model* Adjusted |
||
|---|---|---|---|---|---|
| OR | p-value | OR | p-value | ||
| Lymphatic invasion | |||||
| Present | 47 | 3.6 | <0.001 | 2.2 | 0.026 |
| Absent | 25 | (ref) | (ref) | ||
| Thickness | 72 | 3.0 | <0.001 | 2.2 | <0.001 |
| Mitotic Rate | 72 | 1.26 | <0.001 | 1.11 | 0.022 |
| Ulceration | |||||
| Present | 23 | 6.5 | <0.001 | ||
| Not Present | 49 | (ref) | |||
| VGP TIL | |||||
| Absent | 17 | (ref) | |||
| Present | 55 | 1.5 | 0.198 | ||
| Gender | |||||
| Male | 47 | 2.3 | 0.005 | ||
| Female | 25 | (ref) | |||
| Anatomic Site | |||||
| Axial | 57 | 2.6 | 0.003 | 2.6 | 0.016 |
| Extremity | 15 | (ref) | (ref) | ||
| Age | |||||
| < 60 Years | 39 | (ref) | |||
| >= 60 Years | 33 | 2.9 | <0.001 | ||
Hosmer-Lemeshow p-value was 0.168
Among those patients with thin melanomas (n=125), LI was significantly associated with ten-year metastasis (unadjusted OR=4.3, chi-square test, p=0.027).
LI is a classifier for patients with VGP melanoma
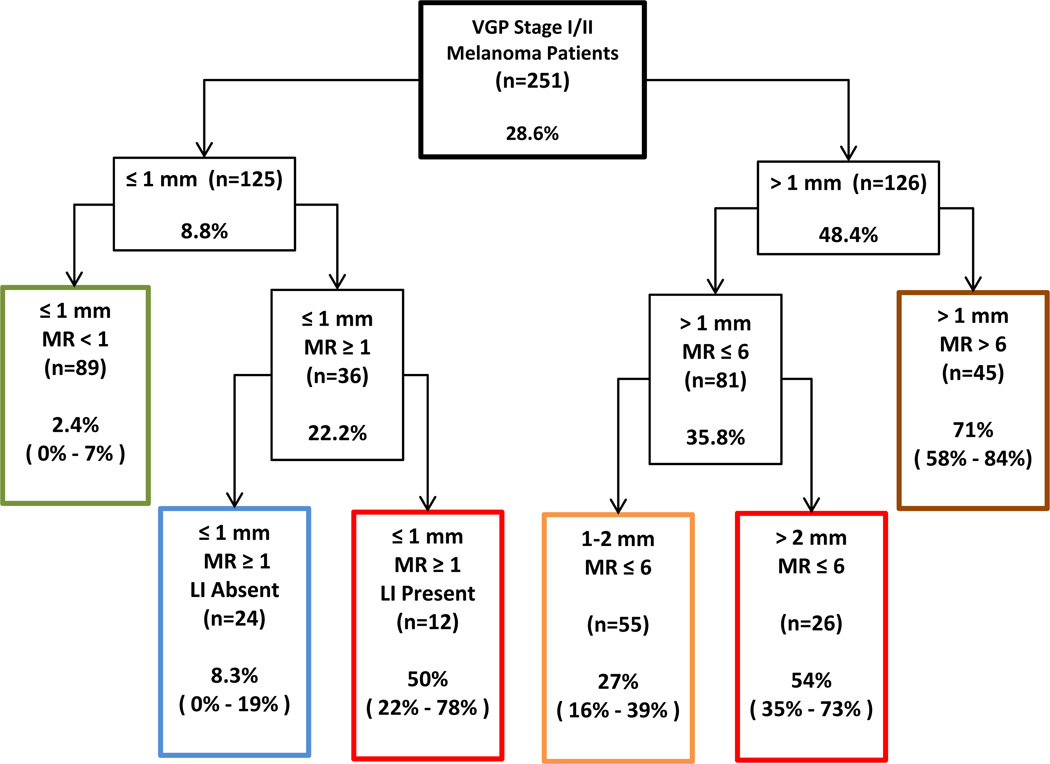
A prognostic tree was developed to investigate heterogeneity in ten-year metastasis among patients in this study and to identify subgroups with very high or very low ten-year metastasis rates (Figure 3). LI (2 categories), thickness (4 categories), MR (3 categories) and site (2 categories) were used in the algorithm to develop the tree. The first variable and split selected by the algorithm to define the two groups most different in terms of ten-year metastasis was tumor thickness ≤1 mm versus >1mm. These thin lesions were next divided into two groups, melanomas wherein MR was <1 and those wherein MR was ≥ 1. Only 1 of the 125 thin melanomas was ulcerated and it had a MR ≥1. Consequently, the two groups defined by the algorithm were equivalent to stages IA (thin lesions with MR <1 and without ulceration) and thin IB lesions, respectively. For patients with thin IB melanomas the algorithm then selected LI to distinguish two groups with different metastasis rates; the ten-year metastasis rate for those with LI was 50% (n=12, 95% CI = 22% – 78%) and it was 6-fold higher than the rate of 8.3% (n=24, 95% CI = 0% – 19%) for those without LI. Of note in Figure 3 is that the ten-year metastasis rates are for patients with VGP melanomas and that these rates are higher than would be expected for a cohort of patients that also included those with invasive radial growth phase lesions without VGP (invasive radial growth phase is characterized by melanoma cells in the dermis that have a MR of zero and that form no dermal nests larger than the largest epidermal tumor nest; it is a step in tumor progression that has little or no capacity for metastasis) (16).
Figure 3.
Prognostic tree developed using recursive partitioning with ten-year metastasis rates and 95% confidence intervals for each risk group.
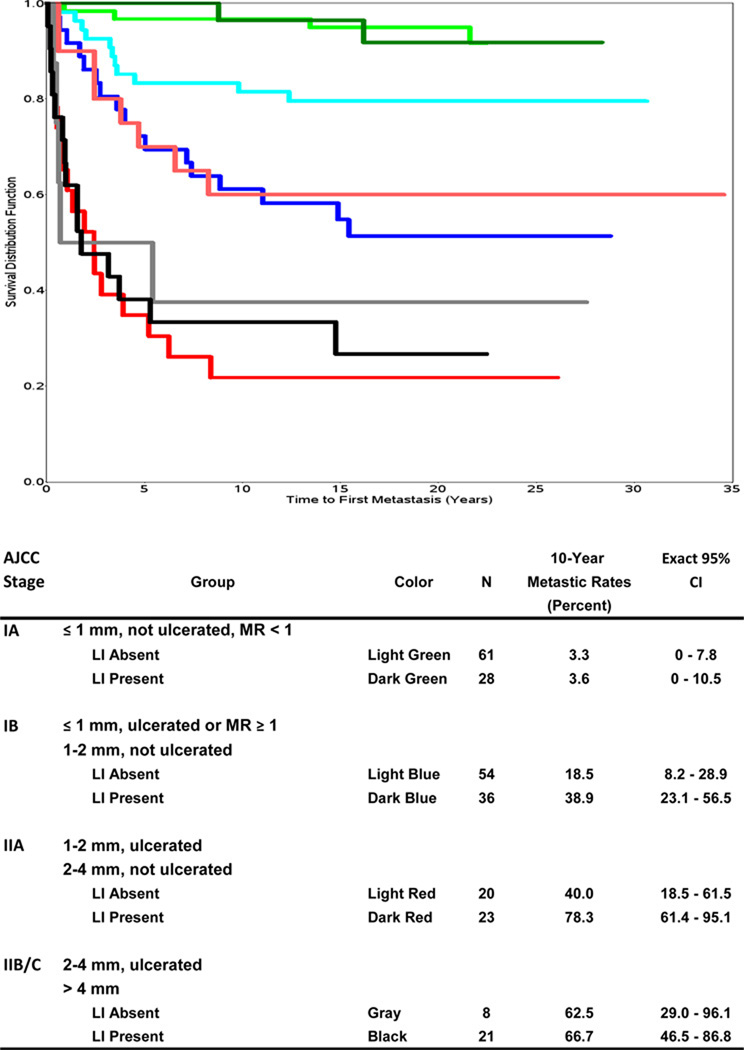
To further examine the role of LI in VGP melanomas in prognostication we examined the KM distributions for time to first metastasis for groups defined by clinical AJCC stage (Figure 4). The KM curves were not significantly different for those with and without LI in Stage IA (p=0.879). However, we observed significant differences between the KM curves for those with and without LI in Stage IB (including both thin lesions with MR ≥1 and melanomas 1–2 mm that were not ulcerated), as well as in Stage IIA (p=0.008 and 0.004, respectively). Analogous to the previously demonstrated separation of thickness-specific survival curves by ulceration (17), there was a shift to poorer prognosis with the presence of LI. Thus, Stage IB melanomas with LI had a KM curve that was similar to the KM curve for Stage IIA without LI, and Stage IIA melanomas with LI had a KM curve that was similar to the KM curve for Stage IIB and C without LI.
Figure 4.
Kaplan-Meier disease-free survival curves for time to first metastasis by AJCC stage and LI.
DISCUSSION
This study is the first to address the independent prognostic significance of LI (using a multivariate analysis) in a large cohort that has extensive patient and tumor information, as well as long-term follow up. Our feasibility study in a smaller cohort of 106 patients showed that LI was associated with worse prognosis in univariate analysis (8). With the larger cohort of patients in this Phase II study that was designed to test the hypothesis that LI is an independent prognostic factor, we demonstrated that LI has a statistically significant adjusted OR of 2.2 for ten-year metastasis in a parsimonious model that included LI, thickness, MR, and anatomic site. In addition, for the group of patients with thin melanomas, LI was also independently associated with ten-year metastasis with an unadjusted OR of 4.3. The analysis of stage-specific KM curves for time to first metastasis for those with and without LI further indicated that LI added prognostic information to AJCC staging, specifically for Stage IB and IIA melanomas. Further, the prognostic tree indicated that LI may be important in discriminating between high and low risk patients with Stage IB thin primaries.
In this study, LI was again seen to be common in VGP lesions, 43%. This rate was comparable to the rate of 33% that was observed in the independent cohort of patients included in our feasibility study (8). In contrast, at the time of definitive therapy LI was evident in the original H&E (non-IHC) slides in only 4.6% of the 251 cases. A majority of studies of LI in the literature used only IHC markers for lymphatic vessels without staining the tumor cells. We found that it was difficult to determine the nature of cells inside lymphatic channels without doing double staining, because in these vessels macrophages and other hematopoietic cells may mimic melanoma cells and melanoma cells may have smaller nuclei and mimic normal hematopoietic cells. The concordance of standard dual-labeled IHC assessment appeared to be excellent. We analyzed a subset of 97 cases and there was only 1 disagreement between the two readers (Kappa=0.98, 95% CI = 0.91 – 1.00). The high inter-rater concordance was likely due to both dual labeling and characterizing LI in binary fashion (present or absent).
Standard dual-labeled IHC appeared to be sufficient to identify LI in most of the cases. Brown DAB and nova red are chromogens commonly used for double IHC staining. We found that it was difficult to separate DAB from nova red in only about 5% of the cases, particularly when a single tumor cell is seen in a lymphatic channel. MSI analysis is useful to resolve this ambiguity. However, given the paucity of ambiguous cases, in future (Phase III) studies it is unlikely that MSI analysis with its special instrumentation and added expense will be considered necessary. Omitting MSI analysis will foster portability and generalizability of dual-labeled IHC assessment.
The present study extends the finding that LI is common in VGP primaries to demonstrate that it occurs in VGP lesions that are not advanced, at least as measured by tumor thickness: 32% of thin melanomas in our cohort had already developed LI. This is corroborated by a small prior study using double IHC staining that showed that LI was observed in 25% of thin melanomas (11). It is striking that a high proportion of thin melanomas have already developed LI.
Our previous studies have shown that MR is an important prognostic factor in thin lesions (18) and the new AJCC staging system now classifies thin melanomas with MR ≥1 as stage IB lesions. As well, LI is a biologically plausible (4) and potentially clinically useful prognostic factor. In the prognostic tree, LI stratified patients with stage IB thin melanomas into distinct groups with the LI-absent group having a ten-year disease-free rate of 92% and the LI-present group having a rate of 50%. While our sample sizes in these two groups were small, the chi-square test had moderate power to detect comparable differences; the expected power of a Fisher’s Exact test (alpha=0.05) would be 83% for a study with these same sample sizes and hypothesized rates equal to the observed ten-year metastasis rates in the two groups.
Due to the recent development of antibodies specific for lymphatic endothelial cells and suited for use in fixed, archival tissue, we were able to investigate in an adequate clinical sample with long and rigorous follow-up the role of LI as a prognostic biomarker. Despite their apparent robustness, our findings do not directly translate to clinical utility. They are limited by our Phase II design (a retrospective case-control study) and by the use of patients’ tissue samples and data from an era that predates the wide use of SLN biopsies for staging clinically uninvolved regional nodes. Nevertheless, biomarker development needs to proceed through this intermediate phase, the strength of which depends importantly on well-annotated specimens and adequate (in time and quality) patient follow-up. As well, the portability and generalizability of our techniques might be questioned. Double IHC staining is required to identify LI. Although this is not technically difficult and has been adopted by many clinical pathology laboratories, the method is not used universally in clinical practice. Despite these limitations, this study makes a compelling case for proceeding to study LI prospectively in a contemporary cohort, validating it as an independent prognostic factor in the SLN biopsy era. Also of importance will be studies that investigate whether LI is a factor that, together with other risk factors, allows for better discrimination between patients who are and are not candidates for SLN biopsy, with particular attention to patients with melanomas classified as Stage IB.
Statement of Translational Relevance.
While most patients with melanomas who are in clinical Stage I/II have good prognosis, there is considerable variability in clinical outcomes. Current, established biomarkers used in staging and prognostic models in early stage melanoma patients fail to reliably identify the significant number of patients who have a recurrence and/or die of metastatic disease. Therefore, new biomarkers are needed for better, tailored patient management. This Phase II study shows that lymphatic invasion (LI) in primary lesions is common and, controlling for established prognostic factors, is associated with ten-year metastasis. Among those with thin melanomas, the presence of LI is also associated with poor prognosis when controlling for stage. This work characterizes a biomarker novel for its practice-changing potential. Once validated in other large melanoma cohorts at other institutions, LI will provide a means to better classify those who are candidates for lymph node staging and trials of systemic adjuvant therapies.
Acknowledgments
This research was supported by the Specialized Program of Research Excellence (SPORE) on Skin Cancer (P50-CA-093372) from the National Cancer Institute (XX, DG, WH, PV, DEE, LS, PAG) and the Cancer Biostatistics Training Grant (T32-CA093283) from the National Cancer Institute (PD).
REFERENCES
- 1.Morton DL, Thompson JF, Cochran AJ, Mozzillo N, Elashoff R, Essner R, et al. Sentinel-node biopsy or nodal observation in melanoma. N Engl J Med. 2006;355(13):1307–1317. doi: 10.1056/NEJMoa060992. [DOI] [PubMed] [Google Scholar]
- 2.Balch CM, Soong SJ, Gershenwald JE, Thompson JF, Reintgen DS, Cascinelli N, et al. Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol. 2001;19(16):3622–3634. doi: 10.1200/JCO.2001.19.16.3622. [DOI] [PubMed] [Google Scholar]
- 3.Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2006;27(36):6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dadras SS, Paul T, Bertoncini J, Brown LF, Muzikansky A, Jackson DG, et al. Tumor lymphangiogenesis: a novel prognostic indicator for cutaneous melanoma metastasis and survival. Am J Pathol. 2003;162(6):1951–1960. doi: 10.1016/S0002-9440(10)64328-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Massi D, Puig S, Franchi A, Malvehy J, Vidal-Sicart S, González-Cao M, et al. Tumour lymphangiogenesis is a possible predictor of sentinel lymph node status in cutaneous melanoma: a case-control study. J Clin Pathol. 2006;59(2):166–173. doi: 10.1136/jcp.2005.028431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valencak J, Heere-Ress E, Kopp T, Schoppmann SF, Kittler H, Pehamberger H. Selective immunohistochemical staining shows significant prognostic influence of lymphatic and blood vessels in patients with malignant melanoma. Eur J Cancer. 2004;40(3):358–364. doi: 10.1016/j.ejca.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Doeden K, Ma Z, Narasimhan B, Swetter SM, Detmar M, Dadras SS. Lymphatic invasion in cutaneous melanoma is associated with sentinel lymph node metastasis. J Cutan Pathol. 2009;36(7):772–780. doi: 10.1111/j.1600-0560.2008.01166.x. [DOI] [PubMed] [Google Scholar]
- 8.Xu X, Gimotty PA, Guerry D, Karakousis G, Van Belle P, Liang H, et al. Lymphatic invasion revealed by multispectral imaging is common in primary melanomas and associates with prognosis. Hum Pathol. 2008;39(6):901–909. doi: 10.1016/j.humpath.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shields JD, Borsetti M, Rigby H, Harper SJ, Mortimer PS, Levick JR, et al. Lymphatic density and metastatic spread in human malignant melanoma. Br J Cancer. 2004;90(3):693–700. doi: 10.1038/sj.bjc.6601571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yun SJ, Gimotty PA, Hwang WT, Dawson P, Van Belle P, Elder DE, et al. High lymphatic vessel density and lymphatic invasion underlie the adverse prognostic effect of radial growth phase regression in melanoma. Am J Surg Pathol. 2011;35(2):235–242. doi: 10.1097/PAS.0b013e3182036ccd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petitt M, Allison A, Shimoni T, Uchida T, Raimer S, Kelly B. Lymphatic invasion detected by D2-40/S-100 dual immunohistochemistry does not predict sentinel lymph node status in melanoma. J Am Acad Dermatol. 2009;61(5):819–828. doi: 10.1016/j.jaad.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 12.Petersson F, Diwan AH, Ivan D, Gershenwald JE, Johnson MM, Harrell R, et al. Immunohistochemical detection of lymphovascular invasion with D2-40 in melanoma correlates with sentinel lymph node status, metastasis and survival. J Cutan Pathol. 2009;36(11):1157–1163. doi: 10.1111/j.1600-0560.2008.01242.x. [DOI] [PubMed] [Google Scholar]
- 13.Niakosari F, Kahn HJ, McCready D, Ghazarian D, Rotstein LE, Marks A, et al. Lymphatic invasion identified by monoclonal antibody D2-40, younger age, and ulceration: predictors of sentinel lymph node involvement in primary cutaneous melanoma. Arch Dermatol. 2008;144(4):462–467. doi: 10.1001/archderm.144.4.462. [DOI] [PubMed] [Google Scholar]
- 14.Clark WH, Jr, Elder DE, Guerry D, 4th, Braitman LE, Trock BJ, Schultz D, et al. Model predicting survival in stage I melanoma based on tumor progression. J Natl Cancer Inst. 1989 Dec 20;81(24):1893–1904. doi: 10.1093/jnci/81.24.1893. [DOI] [PubMed] [Google Scholar]
- 15.Breiman L, Friedman J, Olshen R, Stone C. Classification and Regression Trees. New York: Chapman & Hall; 1984. [Google Scholar]
- 16.Guerry D, Synnestvedt M, Elder DE, et al. Lessons learned from tumor progression: The invasive radial growth phase of melanoma is common, incapable of metastasis, and indolent. J Invest Dermatol. 1993;100:342S–345S3. doi: 10.1111/1523-1747.ep12470248. [DOI] [PubMed] [Google Scholar]
- 17.Balch CM, Wilkerson JA, Murad TM, Soong SJ, Ingalls AL, Maddox WA. The prognostic significance of ulceration of cutaneous melanoma. Cancer. 1980;45(12):3012–3017. doi: 10.1002/1097-0142(19800615)45:12<3012::aid-cncr2820451223>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 18.Gimotty PA, Guerry D, Ming ME, Elenitsas R, Xu X, Czerniecki B, et al. Thin primary cutaneous malignant melanoma: a prognostic tree for 10-year metastasis is more accurate than American Joint Committee on Cancer staging. J Clin Oncol. 2004;22(18):3668–3676. doi: 10.1200/JCO.2004.12.015. [DOI] [PubMed] [Google Scholar]