Abstract
Background:
Simultaneous presentation of multiple primary central nervous system (CNS) malignancies is extremely rare. There have been only eight cases of meningiomas co-existing with primary cerebral lymphoma, reported in the literature.
Case Description:
We present a case of a patient who underwent surgical resection of an olfactory grove meningioma that was interdigitated with a primary CNS B-cell lymphoma. Following surgery, the patient was treated with high-dose methotrexate, and has no evidence of recurrence after 18 months.
Conclusion:
Because of the early recognition of these two distinct pathologies, the patient received directed adjuvant therapies, and has exceeded the survival of all other cases reported in the literature.
Keywords: central nervous system lymphoma, meningioma, synchronous central nervous system tumors
INTRODUCTION
Simultaneous presentation of multiple primary central nervous system (CNS) malignancies is rare, especially in the absence of a genetic syndrome or prior intracranial radiotherapy. The annual incidence of simultaneous primary CNS malignancies has been quoted to be less than one in a million.[6] When they do occur, these synchronous lesions can pose multiple obstacles including radiographic and histological diagnosis, surgical planning, and adjuvant therapy. Here, we present a patient who underwent surgical resection of an olfactory grove meningioma that was interdigitated with a B-cell lymphoma. Because of the early detection and diagnosis of this rare simultaneous tumor, the patient has exceeded the survival of other cases reported in the literature.
Of all of the primary intracranial tumors, meningiomas are most commonly found occurring in conjunction with tumors of different histology. Meningiomas have been reported to occur in conjunction with brain metastasis, gliomas, pituitary adenomas, craniopharyngiomas, and B-cell lymphoma.[3,7,11] It is hypothesized that meningiomas have a higher likelihood of simultaneously occurring with another primary intracranial tumor due to the high frequency of incidental intracranial meningiomas and their long clinical evolution before diagnosis.[7,10]
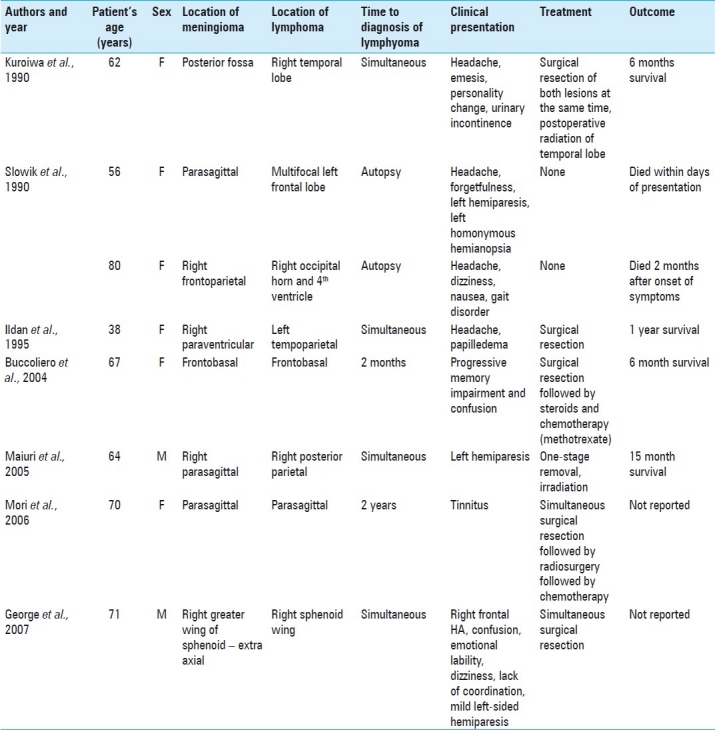
A review of the literature revealed only eight cases of meningiomas co-existing with primary cerebral lymphoma[2,4,5,7,8,10] [Table 1]. In the eight cases of reported synchronous meningioma–lymphomas, two were discovered at autopsy,[1,7,10] two had delayed presentation of lymphoma after a meningioma diagnosis,[1,8] and three were simultaneous presentations.[2,5] Of the three reports of simultaneous lesions, only one of the cases was a truly simultaneous, contiguous meningioma–lymphoma.[2,7]
Table 1.
Reported cases of synchronous meningioma and lymphoma
Here, we report a case of a meningioma coinciding in space and time with a primary CNS lymphoma.
The study was approved by the Institutional Review Board. The clinical and radiological findings, operative note, and pathology report were reviewed.
CASE REPORT
A 65-year-old right-handed woman was referred for evaluation of daily headaches occurring for a 3-week period. Her headaches were associated with nausea and vomiting. Magnetic resonance imaging (MRI) demonstrated a very large intracranial frontal fossa mass with significant bifrontal edema [Figure 1]. She was placed on steroids and neurosurgical follow-up was arranged.
Figure 1.
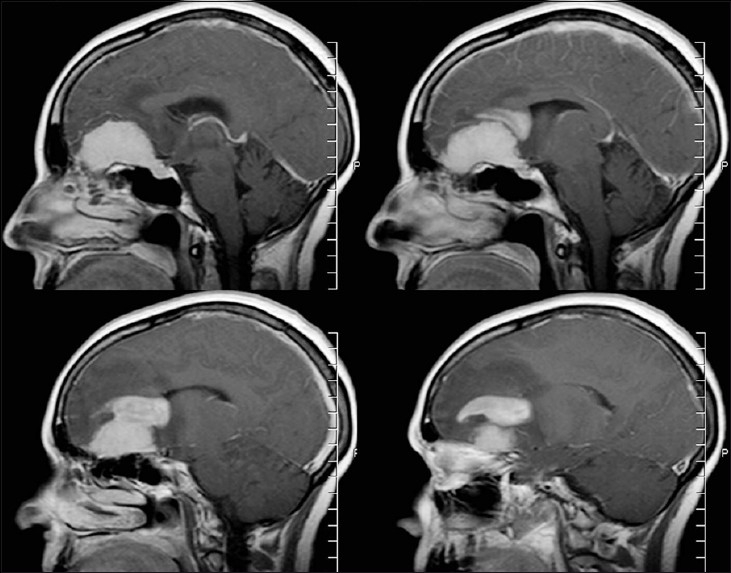
Sagittal MRI with contrast shows midline dural-based frontal fossa mass with a separate “cap-like” mass and surrounding vasogenic edema
Upon presenting to the neurosurgical clinic, her headaches, nausea, and vomiting had all resolved. She denied any seizure history, balance difficulty, or speech difficulty. Her family did note that her motivation had decreased over the past few years. Her past medical history includes uterine fibroids, arthritis, and fluid retention. Her past surgical history includes a hysterectomy, tonsillectomy, and lumbar laminectomy. She has no allergies.
Physical exam
On physical exam, the patient was found to be neurologically intact, except a decreased sense of smell. A review of her MRI demonstrated a dural-based mass that followed the olfactory groove which was associated with an infiltrative cap-like mass that was not typical for a meningioma.
Surgical resection
A bilateral subfrontal approach was utilized. The tumor was initially noted to be of soft consistency and to have well-defined margins. However, as the resection progressed to the superior surface of the tumor, the macroscopic characteristics changed. In this region, the tumor was noted to appear infiltrative without a clear border. A specimen from this area was sent for pathologic review. Frozen section showed numerous “small blue cells” concerning for lymphoma. At this point, the surgical resection was stopped because it was felt that the meningioma component of the tumor had been completely removed. The patient awoke from anesthesia and was noted to be neurologically intact.
Histopathology
Pathologic review of surgical specimens revealed two different neoplasms. The first one was a WHO grade I meningioma that comprised cells exhibiting numerous whorl formation and occasional psammoma bodies. Also, foci of dense aggregates of cells with marked nuclear enlargement and hyperchromasia were visualized. The Wright-stained cytospin showed a monoclonal population of lymphocytes with many large cells consistent with lymphoma. This specimen was further studied using flow cytometry which revealed a monoclonal population of B-cell lymphocytes. Figure 2 illustrates the malignant lymphoma interdigitating with the WHO grade I meningioma.
Figure 2.
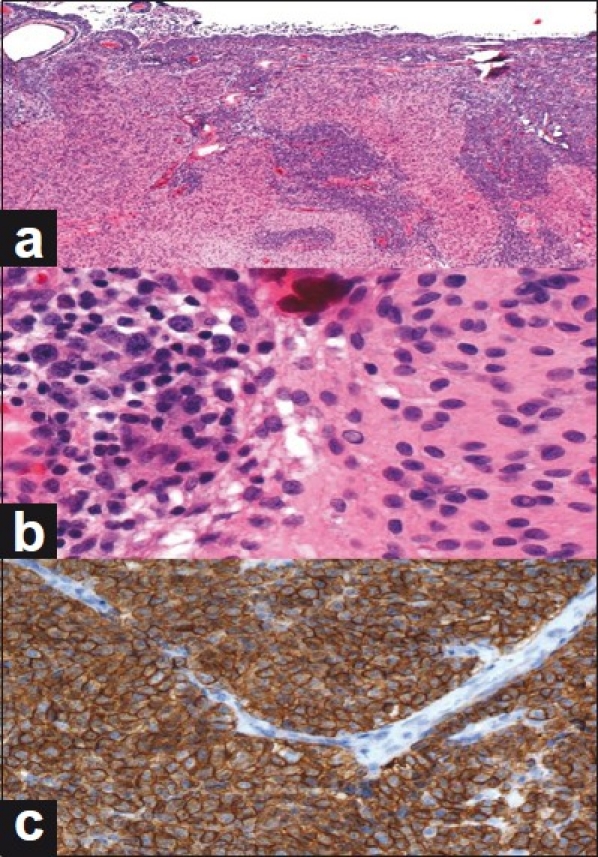
Microscopic review of dual pathologies. (a) Low-power view of meningioma containing foci of large B-cell lymphoma; the latter is characterized by dense aggregates of cells having marked nuclear enlargement and hyperchromasia. (b) Enlarged cells of B-cell lymphoma (left) and contiguous meningioma (right); an intranuclear pseudoinclusion is present in the meningothelial component. (c) Immunohistochemical staining for CD20, a B-cell marker, demonstrates diffuse positivity in the tumor cells. Non-staining, arch-shaped vascular endothelium is present in the upper half of the field
Additional work up / follow-up
After surgical resection and pathologic review, the patient underwent an extensive work up for systemic lymphoma. She was found to be immunocompetent and free of any systemic lymphoma. Therefore, the B-cell lymphoma that was interdigitated with the olfactory groove meningioma represented a primary CNS lymphoma. She was started on treatment for a primary CNS lymphoma, which consisted of high-dose methotrexate every 2 weeks. At this time, she has completed six doses of induction treatment and now has completed seven doses of monthly maintenance treatment. She remains neurologically intact, and her Karnofsky score is 90%. Her 18-month follow-up imaging is stable without any signs of new or enlarging masses.
DISCUSSION
Distinction of tumor pathology is important to appreciate early in the diagnosis and even before surgical intervention, if possible, due to the differences in both optimal treatment modalities and strategies and predicted outcomes. The gold standard of care for meningiomas and primary CNS lymphomas could not be more different: complete surgical resection versus systemic chemotherapy and radiation. The first line therapy for a primary CNS lymphoma is not surgical resection due to the multifocal and infiltrative nature of this neoplasm. Primary CNS lymphoma has been shown to be both chemosensitive and radiation sensitive. To date, methotrexate is the chemotherapy agent with the most proven activity against primary CNS lymphoma. Several studies of high-dose methotrexate have shown improved disease control and longer survival.[9]
Therefore, early recognition of these two distinct neoplasms can help direct therapeutic interventions. In the reported cases of synchronous meningioma-lymphoma found in Table 1, the survival following surgical resection was reported to be approximately 6 months; this interval has varied by only a few months since the earliest reported case in the 1990s. This lack of improvement in overall survival since the first documented case of synchronous meningioma and lymphoma suggests that there is a more complicated underlying pathogenesis that must be understood before any advances in survival will be seen.
Various mechanisms for the development of these two primary intracranial neoplasms have been proposed. The first one suggests that meningioma development incites a glial inflammatory response which results in B-cell proliferation, and therefore lymphoma generation.[1,5,7] The second one proposes a two hit genetic model in which the meningioma serves as an oncogenic factor.[8,11] This postulate has been evaluated in the glioblastoma–meningioma concurrent lesions through immunohistochemical analysis that revealed a high p53 positivity rate for both lesions.[11]
The predominance of these tumors occurring from the sixth to the eighth decade of life would lend support to the theory of mere coincidence, however. The male to female ratio of the meningioma–lymphoma association is 2:7 based on our literature findings. This female predominance would suggest that possibly estrogen or other hormonal pathways play a role in this combined pathogenesis.
Whatever the pathogenic mechanism may be, the fact remains that early detection and accurate diagnosis of each neoplasm will aid the surgeon and oncologist in pursuing the appropriate surgical and adjunctive treatment intervention, and will ultimately lead to longer survival.
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2011/2/1/181/90716
Contributor Information
Amber S. Gordon, Email: agordon@uabmc.edu.
Kenneth E. Fallon, Email: kefallon@uab.edu.
Kristen O. Riley, Email: koriley@uabmc.edu.
REFERENCES
- 1.Buccoliero AM, Taddei GL, Caldarella A, Mennonna P, Ammannati F, Taddei A, et al. Meningioma-primary brain lymphoma association. Neuropathology. 2004;24:336–40. doi: 10.1111/j.1440-1789.2004.00570.x. [DOI] [PubMed] [Google Scholar]
- 2.George B, Kumar R, Johns P, Bartlett RJ, O’Brien D. Contiguous synchronous occurrence of primary cerebral lymphoma and meningioma. Br J Neurosurg. 2007;21:35–8. doi: 10.1080/02688690701192794. [DOI] [PubMed] [Google Scholar]
- 3.Honegger J, Buchfelder M, Schrell U, Adams EF, Fahlbusch R. The coexistence of pituitary adenomas and meningiomas: Three case reports and a review of the literature. Br J Neurosurg. 1989;3:59–69. doi: 10.3109/02688698909001027. [DOI] [PubMed] [Google Scholar]
- 4.Ildan F, Bagdatoglu H, Boyar B, Haciyakupoglu S, Gonlusen G, Tunali N. Combined occurrence of primary cerebral lymphoma and meningioma. Neurosurg Rev. 1995;18:45–8. doi: 10.1007/BF00416477. [DOI] [PubMed] [Google Scholar]
- 5.Kuroiwa T, Ohta T, Kobata H, Yamamoto H, Kimura N. Coexistence of intracranial meningioma and primary malignant lymphoma--Case report. Neurol Med Chir (Tokyo) 1990;30:268–71. doi: 10.2176/nmc.30.268. [DOI] [PubMed] [Google Scholar]
- 6.Lee EJ, Chang CH, Wang LC, Hung YC, Chen HH. Two primary brain tumors, meningioma and glioblastomamultiforme, in opposite hemispheres of the same patient. J ClinNeurosci. 2002;9:589–91. doi: 10.1054/jocn.2002.1086. [DOI] [PubMed] [Google Scholar]
- 7.Maiuri F, Cappabianca P, Iaconetta G, Esposito F, Messina A. Simultaneous presentation of meningiomas with other intracranial tumours. Br J Neurosurg. 2005;19:368–75. doi: 10.1080/02688690500305548. [DOI] [PubMed] [Google Scholar]
- 8.Mori Y, Kondo T, Iwakoshi T, Kida Y, Kobayashi T, Yoshimoto M, et al. Malignant lymphoma arising in the cerebral parenchyma adjacent to a parasagittal meningioma. Neurol Med Chir (Tokyo) 2006;46:398–400. doi: 10.2176/nmc.46.398. [DOI] [PubMed] [Google Scholar]
- 9.Morris PG, Abrey LE. Therapeutic challenges in primary CNS lymphoma. Lancet Neurol. 2009;8:581–92. doi: 10.1016/S1474-4422(09)70091-2. [DOI] [PubMed] [Google Scholar]
- 10.Slowik F, Jellinger K. Association of primary cerebral lymphoma with meningioma: Report of two cases. Clin Neuropathol. 1990;9:69–73. [PubMed] [Google Scholar]
- 11.Suzuki K, Momota H, Tonooka A, Noguchi H, Yamamoto K, Wanibuchi M, et al. Glioblastoma simultaneously present with adjacent meningioma: Case report and review of the literature. J Neurooncol. 2010;99:147–53. doi: 10.1007/s11060-009-0109-9. [DOI] [PubMed] [Google Scholar]


