Abstract
Background:
Aberrant expression of small noncoding endogenous RNA molecules known as microRNAs (miRNAs) is documented to occur in multiple cancer types including esophageal adencarcinoma (EAC) and its only known precursor, Barrett's esophagus (BE). Recent studies have linked dysregulation of specific miRNAs to histological grade, neoplastic progression and metastatic potential.
Materials and Methods:
Herein, we present a summary of previously reported dysregulated miRNAs in BE and EAC tissues as well as EAC cell lines and evaluate a cranberry proanthocyanidin rich extract's (C-PAC) ability to modulate miRNA expression patterns of three human EAC cell lines (JHEso-Ad-1, OE33 and OE19).
Results:
A review of 13 published studies revealed dysregulation of 87 miRNAs in BE and EAC tissues, whereas 52 miRNAs have been reported to be altered in BE or EAC cell lines, with 48% overlap with miRNA changes reported in tissues. We report for the first time C-PAC–induced modulation of five miRNAs in three EAC cell lines resulting in 26 validated gene targets and identification of key signaling pathways including p53, angiogenesis, T-cell activation and apoptosis. Additionally, mutiple cancer related networks were ideintified as modulated by C-PAC utilizing Kyoto Encyclopedia of Genes and Genomes (KEGG), Protein Analysis Through Evolutionary Relationships (PANTHER), and MetaCore analysis tools.
Conclusions:
Study results support the cancer inhibitory potential of C-PAC is in part attributable to C-PAC's ability to modify miRNA profiles within EAC cells. A number of C-PAC–modulated miRNAs have been been identified as dysregulated in BE and EAC. Further insights into miRNA dysregulation and modulation by select cancer preventive agents will support improved targeted interventions in high-risk cohorts.
Keywords: Barrett's esophagus, chemoprevention, cranberry, JHAD1, microRNA, OE19, OE33, esophageal adenocarcinoma, polyphenols, proanthocyanidins
BACKGROUND
Esophageal cancer is the eighth most commonly diagnosed cancer worldwide, with squamous cell carcinoma and adenocarcinoma comprising the two main esophageal cancer histologies.[1–3] Esophageal adenocarcinoma (EAC) is the major histological type of esophageal cancer diagnosed in the western world today. Risk factors associated with EAC and the only known precursor lesion, Barrett's esophagus (BE), are still being unraveled; however, persistent, symptomatic reflux of gastric and duodenal contents, known as gastro-esophageal reflux disease (GERD), have long been known to correlate with the development of BE and EAC.[1,4,5] Obesity has also been reported to impart increased risk for EAC and a 1.5-2-fold increased risk for GERD.[6,7] Rates of EAC have increased at an alarming pace in the US and Western Europe in recent years.[8–11] Esophageal cancer is the fourth leading cause of cancer-related mortality in UK males and the sixth leading cause of cancer-related deaths in UK females.[10] In the US, 16,980 new incident cases of esophageal cancer are estimated to occur in 2011 and 14,710 deaths, representing the 7th leading cause of cancer-related deaths among US males.[11] Thus, there is a large population at increased risk for the development of Barrett's and EAC, illustrating the potential global health significance of this growing problem. Moreover, mortality figures closely parallel the incidence data, reflecting the poor 5-year survival rate of only 17%[11] for esophageal cancer in the US. The latter is due to late stage of diagnosis coupled with ineffective screening, preventive and treatment options. Chemoprevention with efficacious bioactive constituents derived from various food stuffs is an active area of investigation supported by the fact that plant-based diets rich in fruits and vegetables have generally been associated with reduced risk for EAC and BE.[12–14] Cranberries, for example, have been reported to have a multitude of positive health effects ranging from improved immune function and decreased infections to cardiovascular benefits, and more recently, cancer inhibition.[15–17] Our laboratory has specifically been investigating the ability of a proanthocyanidin-rich cranberry extract (C-PAC) to inhibit cancers of the aerodigestive tract.[18–20]
In brief, in this report, we sought first to summarize the current findings on microRNA (miRNA or miR) expression patterns in BE through EAC pathologies and second, to identify C-PAC–induced alterations of miRNAs following treatment of a panel of three validated EAC human cell lines. The results summarize our knowledge of esophageal miR targets across varying histopathological categories and provide new insight regarding miR modulation following C-PAC exposure and identify cancer-related pathways linked to miR modulation by the chemopreventive agent under evaluation in the context of EAC. Recent studies have linked dysregulation of specific miRNAs to histological grade, neoplastic progression, metastatic potential, treatment responiveness and patient prognosis.[21] Thus, improving of knowledge of discriminate miRNA expression profiles holds valuable clinical utility.
MATERIALS AND METHODS
Cranberry proanthocyanidin preparation
Briefly, cranberries were homogenized in 70% aqueous acetone, filtered and the pulp discarded. Collected C-PAC was concentrated under reduced pressure and the extract isolated using bioassay-directed fractionation as previously reported.[22–24] Methods including 13C nuclear magnetic resonance (NMR), electrospray mass spectrometry, matrix-assisted laser desorption/ionization time-of-flight mass spectrometry, and acid-catalyzed degradation with phloroglucinol have all been utilized to confirm the presence of A-type linkages and the concentration of PACs present in the C-PAC extract.[22–24] Purified proanthocyanidin extract was freeze-dried and stored at –80°C until dissolved in media for individual experiments.
Cell-lines and cultures conditions
A panel of three authenticated human EAC cell lines was utilized in this series of experiments.[25] Specifically, JH-ESOAd1, referred to as JHAD1, isolated in 1997 from a distal EAC, stage III, N0 (Johns Hopkins University, Baltimore, MD, USA), OE33 cells isolated in 1993 from a distal EAC, stage II, N0, (ECACC, Wiltshire, UK), and OE19 cells isolated in 1993 from an adenocarcinoma at the gastro-esophageal junction, stage III, N1 (ECACC, Wiltshire, UK) were utilized. Cells were grown in Roswell Park Memorial Institute (RPMI) 1640 medium containing L-glutamine (2.0 mM), penicillin (104 units/ml), streptomycin (104 μg/ml), sodium pyruvate (1 mM), and 0–10% fetal bovine serum (FBS) depending on the experiment. Cells were maintained as monolayers at 37°C with 95% air and 5% CO2 throughout all studies.
Cell viability assays
C-PAC–induced inhibition of cell viability was determined utilizing the 4-[3-(4-iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1,3-benzene disulfonate (WST-1) colorimetric assay (Roche Applied Science, Indianapolis, IN, USA) as we previously reported.[19] Briefly, the viability assay is based on the ability of metabolically active cells to cleave the tetrazolium salt, WST-1, into a formazen dye. JHAD1 and OE19 cells were plated in sterile 96-well plates at 12E3 and 24E3 cells/well, respectively, and allowed to adhere for 35 hours (70% confluency) prior to treatment with C-PAC at concentrations of 25–100 μg/ml for 24, 48, 72 and 96 hours. Complete phenol red-free RPMI medium with 5% FBS was utilized for viability assays. Plates were processed according to the manufacturer's instructions with spectrophotometer readings at 450 nm (Biotek Synergy HT). A minimum of six wells were analyzed for each test condition and time-point.
RNA isolation and miRNA assay
JHAD1, OE33 and OE19 AC cells were seeded at 5E6, 14E6 and 14E6 cells (60% confluency), respectively, in T75 flasks and permitted to adhere for 30 hours prior to C-PAC (50 μg/ml) treatment. Cells were harvested 6 hours after C-PAC treatment using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) as per the manufacturer's recommendations and RNA was isolated utilizing standard phenol–chloroform extraction procedures. RNA quality was determined by Nanodrop using the 8000 Spectrophotometer (Thermo Scientific, Wilmington, NC, USA), and RNA integrity and presence of the small RNA fraction was determined using the Bioanalyzer 2100 capillary electrophoresis system (Agilent, Santa Clara, CA, USA). Sixty nanograms of total RNA was reverse transcribed using the human Megaplex Primer Pools A and B and the TaqMan miRNA reverse transcription kit (Applied Biosystems, Foster City, CA, USA) according to the manufacturer's instructions. Each sample was pre-amplified for 12 cycles using human pool A and B Taqman® Megaplex™ PreAmp Primers and PreAmp Master Mix (Applied Biosystems) according to the manufacturer's instructions. For each sample, the preamplification reactions A and B were diluted and each reaction was combined with Taqman® Gene-Expression Master Mix (Applied Biosystems) divided into eight aliquots and each aliquot was added to one of the eight sample ports of the TaqMan® Array A or B (v2.0), respectively. The TaqMan® Array Human miRNA Card Set v2.0 enables detection of 667 human miRNAs, 3 miRNA endogenous reference controls and 1 miRNA assay not related to human as a negative control. The real-time polymerase chain reaction (PCR) reactions were run according to the manufacturer's instructions. RealTime Statminer Software (Integromics, Philadelphia, PA, USA) was used to analyze the data. The global geometric mean of all expressed miRNA assays was used to normalize the data.[26]
MiRNA targets and pathway analysis
MiRNA targets were determined using miRWalk (http://www.ma.uni-heidelberg.de/apps/zmf/mirwalk/), a comprehensive database of human, mouse and rat miRNAs on predicted and validated targets associated with specific genes, pathways, diseases or inherited disorders, organs, cell lines and transcription factors.[27] The validated targets module of miRwalk was utilized to derive gene target information for miRs previously reported to be altered in BE and EAC compared to normal esophageal tissues and to determine gene targets altered in EAC cell lines following C-PAC treatment [Tables 1–4]. In addition, miRNA predicted targets in mRNA selected regions were determined and analyzed for the five common miRs altered in all three EAC cell lines following C-PAC treatment [Supplemental Table 2]. Next, gene targets were analyzed utilizing the Database for Annotation, Visualization and Integrated Discovery (DAVID, v6.7)[28] and signaling pathways were further investigated through use of the PANTHER(http://www.pantherdb.org)[29,30] software, a system for inferring gene function based on published scientific experimental evidence and evolutionary relationships to predict function. PANTHER utilizes gene family and subfamily information, gene ontology classes (molecular function, biological process, and cellular component), evolutionary relationships, PANTHER protein classes and pathway diagrams to classify genes based on their function. Gene target lists were further subjected to both enrichment analysis and network analysis using GeneGo's MetaCore software (http://www.genego.com) which is an integrated knowledge base and pathway analysis tool based on a proprietary manually curated database of human protein–protein, protein–DNA and protein compound interactions, metabolic and signaling pathways, all supported by proprietary ontologies. The enrichment analysis provided lists of biological pathways and functional ontologies (GO and GeneGo) statistically over-represented in each target list (false discovery rate or FDR < 0.05). We also used the GeneGo Analyze Network algorithm with canonical pathways to build statistically significant biological networks from each target list (at FDR < 0.05). The results of this analysis serve to further investigate potential pathways altered by C-PAC and to compare pathway results generated from PANTHER and KEGG, as well as provide additional information which may only be available through GeneGo's proprietary database.[31,32]
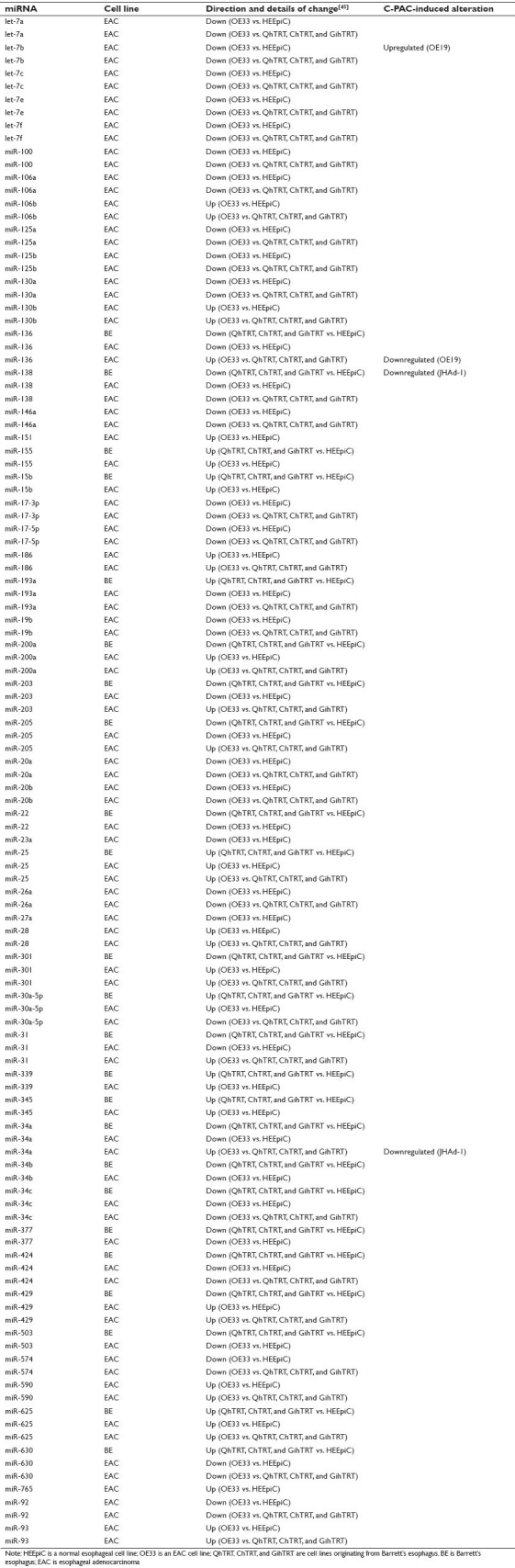
Table 1.
Differential expression of miRNAs in BE and EAC tissues as identified in 13 studies and C-PAC–induced alterations in EAC cell lines
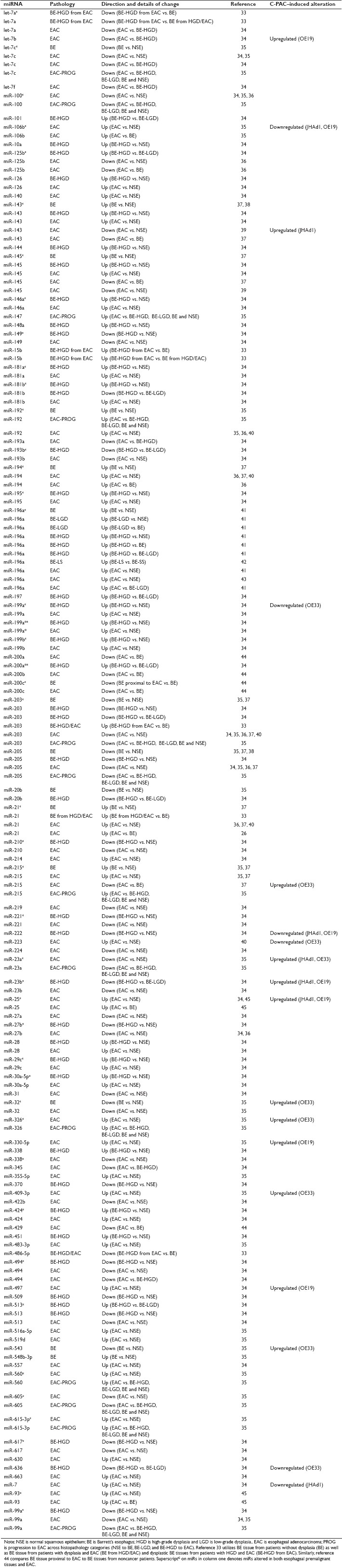
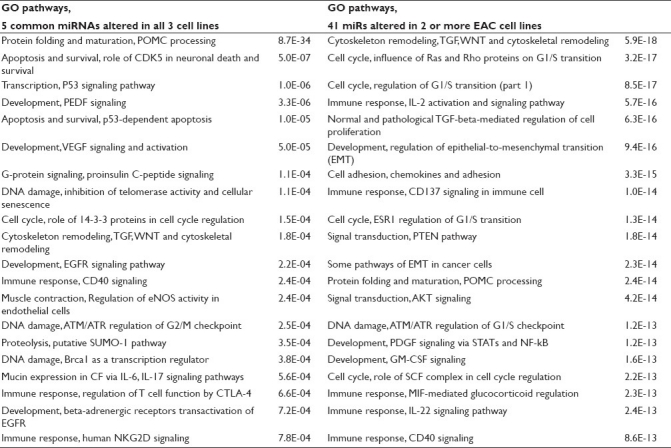
Table 4.
PANTHER pathways resulting from micoRNAs altered in BE and EAC tissues or EAC cell lines following C-PAC treatment
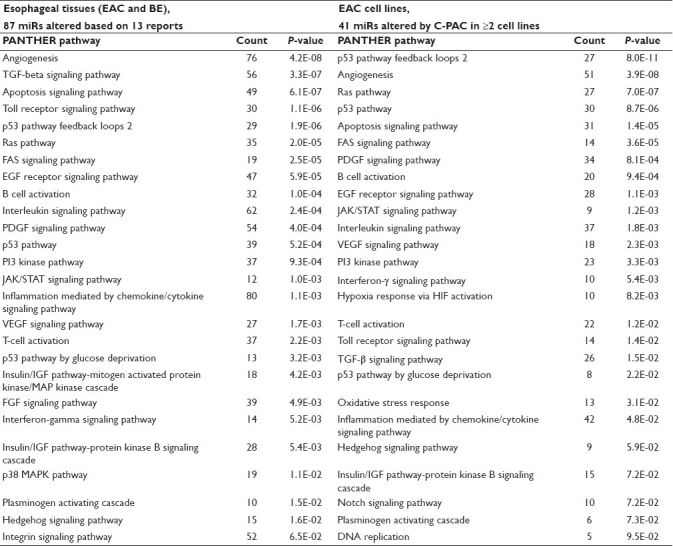
Supplemental Table 2.
Pathways resulting from C-PAC altered predicted miR targets based on the five common miRs modulated in JHAD1, OE19 and OE33 EAC cell lines
Statistical analysis
Results are presented as the mean value ± SE for cell viability experiments. Viability data were evaluated for statistical significance comparing media-treated versus C-PAC-treated EAC cells using the Student's t test (two-sided, P < 0.05). The miRNA data were analyzed as described above with only common miRNAs which were altered ≥2-fold in all three EAC cell lines (with P < 0.05) included in the analysis to derive validated targets and evaluate pathways impacted by C-PAC treatment.
Literature review
A comprehensive review of the literature through July 1, 2011 identified 13 original research studies evaluating dysregulation of miRNAs in BE or EAC tissues relative to normal squamous epithelium (NSE),[33–45] whereas only one study characterizing changes in EAC or BE cell lines relative to normal utilizing authenticated esophageal cell lines was identified.[45] Studies characterizing miRNA alterations in esophageal squamous cell carcinoma were excluded, as were reports primarily focused on miRNAs linked to prognosis, therapeutic response or other clinical outcomes. We regret if any studies meeting the inclusion criteria were omitted. Results of the 13 published studies reporting dysregulated miRNAs in BE, EAC, or cell lines of BE or EAC origin are summarized in Table 1 and Supplemental Table 1, respectively. Generally, we have included what the previous authors described as significantly modulated miRs based upon the methodology and criteria of the individual studies. However, miRs were excluded based upon a lack of significant difference in addition to a fold change value less than two-fold compared to normal or a lower grade of histopathological change. In addition, for all miRs altered in esophageal tissues, the miRWalk database was utilized to determine “validated” and “predicted” gene targets for further pathway analysis across the three databases as described above.
Supplemental Table 1.
Differential expression of miRs in cell lines of BE and EAC origin as previously reported[45] and C-PAC–induced miR alterations in EAC cell lines
RESULTS AND DISCUSSION
Inhibition of EAC cellular viability by C-PAC
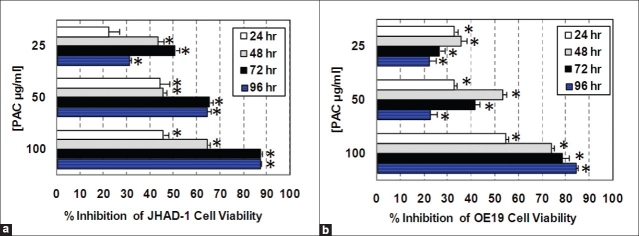
Pre-treatment of JHAD-1 and OE19 EAC cells with C-PAC at 25, 50, and 100 μg/ml resulted in a concentration- and time-dependent significant inhibition of cellular viability. The data presented in Figures 1a and 1b support an IC50 of 50 μg/ml for C-PAC. Similar concentrations of C-PAC have previously been found to inhibit the viability and proliferation of lung (NCI-H460) and colon cancer (SW460) cell lines, increase the percentage of cells accumulating at the G1 checkpoint, induce apoptosis, modulate global gene expression profiles and alter select proteins linked to cell cycle regulation and apoptosis.[18–20] In addition, C-PACs’ inhibitory effects are greater in cancer cells compared to normal HET1A esophageal cells (data not shown) and C-PAC shows superior inhibition of EAC cell viability (85–90% inhibition) compared to black raspberry extract (<40%).[20] C-PAC has unique A-type chemical linkages found only in a limited number of fruits to date including cranberry, chokeberry, plums and avocado,[17,46,47] which may account for some of the improved inhibitory capacity and other unique mechanisms by which C-PAC inhibits cancer-related processes in EAC cells. This unique A-type linkage is important for C-PACs’ anti-adhesion effects in the bladder as previously reported by Dr. Howell and colleagues.[23,24,46] Moreover, similar in vitro concentrations reported to block bacterial adhesion to the bladder wall inhibit cancer cell growth in vitro and these levels appear to be behaviorally achievable and efficacious in human cohorts for inhibiting urinary tract infections.
Figure 1.
Inhibition of cell viability by cranberry proanthocyanidins (C-PAC). (a) C-PAC–induced inhibition of JHAD1 cellular viability over time. (b) C-PAC–induced inhibition of OE19 cellular viability over time. Reported inhibition is relative to vehicle or media-treated esophageal adenocarcinoma cells. Evaluations were in replicates of six per cell line, per experimental time point (*P < 0.05, significantly different from media-treated controls by t-test)
MiRNA alterations in BE, EAC and esophageal cell lines
Table 1 summarizes aberrant miRNA expression in BE and EAC tissues as identified in 13 original research studies.[33–45] A total of 87 miRNAs were identified from the previously published reports based on a thorough literature search through July 1, 2011. A total of 44 miRNAs were altered in both EAC and premalignant tissues as indicated by a superscript on the common miRNA in Table 1. Ten miRNAs were uniquely altered in BE tissues compared to NSE with the upregulated including miR-10a, miR-144, miR-148a, miR-451 and miR-548b-3p, and the downregulated including miR-222, miR-370, miR-509, miR-543, and miR-636. References 33–35 include analysis of patient tissues with varying degrees of histopathological alteration providing some insight into the potential miRNAs linked to EAC progression. The latter may prove particularly useful for patient stratification and for assessing select miRNAs as biomarkers of early efficacy in chemopreventive intervention trials.
The last column of Table 1 includes miRNAs altered in EAC cell lines following a 6-hour treatment with C-PAC. A total of 10 miRNAs (let-7b, miR-106b, miR-143, miR-199a, miR-215, miR-223, miR-23b, miR-32, miR-543, and miR-7) were altered by C-PAC inversely of at least one previously reported miRNA aberration associated with the BE or EAC, supporting that C-PAC may in part normalize miRNA expression in EAC. In addition, six of C-PAC–modulated miRs identified have been reported to be differentially expressed between EAC compared to NSE and also in premalignant pathologies, supporting that C-PAC may hold cancer inhibitory potential at late stages of neoplastic transformation, as well as early during the development of esophageal premalignancy. Importantly, let-7 family members and miR-143 are associated with tumor suppressor activity[48,49] and both are downregulated in BE and EAC,[33–35,37,39] yet upregulated following C-PAC treatment. MiR-223 was recently linked to gastric cancer invasion[50] and is documented to be upregulated in EAC compared to normal esophageal tissues.[40] C-PAC treatment results in downregulation of miR-223 in OE33 EAC cells, again supporting the potential benefits of C-PAC against EAC and precursor lesions. C-PAC also inversely impacted let-7b, miR-136, and miR-34a based upon BE and EAC cell line findings summarized in Supplemental Table 1. A total of 52 miRNAs were dysregulated in BE or EAC cells when compared to normal esophageal cells or a more normal histopathological\ state as previously reported by Dr. Meltzer and colleagues.[45] Additionally, 25 of the 52 (48.1%) miRNA alterations detected in esophageal cell lines were in common with those reported in tissue-based studies. Overall, there are a relatively small number of validated BE and EAC cell lines[25] and few studies characterizing miRNAs alterations in those lines compared to normal cells.
miRNA modulation in JHAD1, OE19 and OE33 cell lines following C-PAC treatment
A 6-hour C-PAC (50 μg/ml) treatment of JHAD1, OE19 and OE33 EAC cells resulted in significant modulation of five common miRNAs in all three cell lines. Common miRNAs significantly upregulated ≥2.0-fold included miR-410 and miR-520d-5p, whereas common downregulated ≤2.0-fold included miR-202, miR-516a-3p, and miR-586, as detailed in Figure 2a. In addition, the Venn diagrams in Figures 2b and 2c show additional miR overlap between cell lines, with specific miRs listed in Figure 2a. Overall, the greatest number of C-PAC–induced miR alterations occurred in the JHAD1 cell line (n = 98), followed by the OE33 (n = 85) and OE19 (n = 81) cell lines. We have found the OE19 cells to be the most tumorigenic in a mouse xenograft model and generally this cell line has been more resistant to treatment with cancer inhibitory agents compared to the OE33 and JHAD1 cell lines. The five common miRs altered by C-PAC treatment across all three cell lines resulted in identification of 26 validated gene targets utilizing the miRWalk database as detailed in Table 2. A number of gene targets have been implicated in cancer, including tumor suppressor genes (p53 and p16), oncogenes (Rb and ErbB) and inflammatory linked transcription factors (NFkB). Pathway analysis utilizing the target genes resulting from the five miRs altered by C-PAC treatment in all three EAC cell lines resulted in 23 KEGG pathways detected as modulated. Identified KEGG pathways included pathways in cancer, a number of specific cancers (bladder, nonsmall cell lung cancer, chronic myeloid leukemia, prostate cancer, glioma, melanoma, small cell lung cancer, endometrial cancer, basal cell carcinoma, renal cell cancer, colorectal cancer), cell cycle, p53 signaling, B-cell receptor signaling, focal adhesion, ErbB signaling, apoptosis, Toll-like receptor, T-cell receptor and Wnt signaling. We have previously reported that C-PAC has inhibitory effects in esophageal, colon and lung cancer cell lines;[18–20] however, this is the first report evaluating the ability of C-PAC to modulate miRNA expression profiles.
Figure 2.
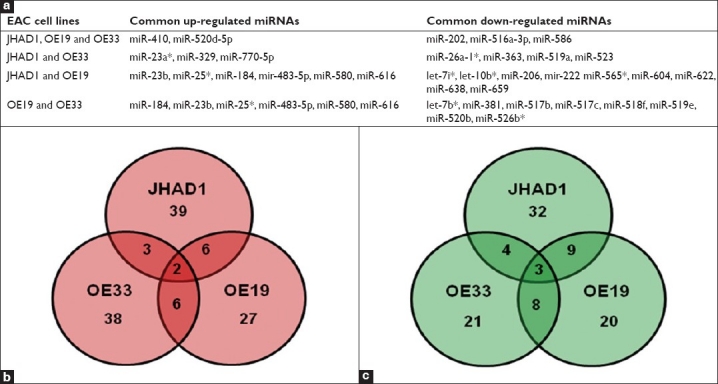
Effects of C-PAC treatment on miRNA expression patterns in JHAD1, OE19 and OE33 esophageal adenocarcinoma cell lines. (a) Details of commonly up and downregulated miRNAs following a 6-hour C-PAC treatment by cell line; (b) Venn diagram illustrating the number of C-PAC upregulated miRNAs in each cell line; (c) Venn diagram illustrating the number of downregulated miRNAs associated with C-PAC treatment
Table 2.
Common miRNAs altered by C-PAC treatment of JHAD1, OE19, and OE33 EAC cell lines, validated gene targets and resultant pathways
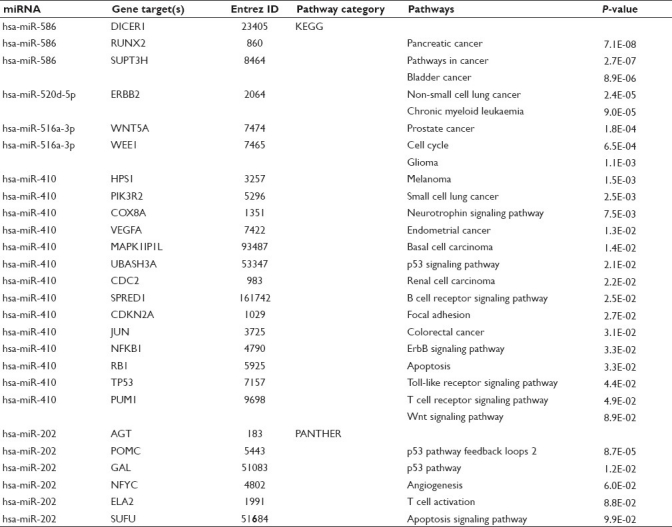
Next, PANTHER pathway analysis was conducted to gain additional insight into metabolic and signaling pathways impacted by C-PAC treatment. PANTHER analysis resulted in the identification of five C-PAC altered pathways in EAC cells, with angiogenesis being the only unique addition beyond the KEGG identified pathways as documented in Table 2. MetaCore pathway analysis was also applied utilizing the same 26 validated gene targets and resulted not only in a number of pathways previously identified by KEGG and PANTHER, but also in some unique pathways such as G-protein signaling, anti-angiogenic pigment epithelium-derived factor (PEDF) signaling, DNA damage, vascular endothelial growth factor (VEGF), mucin and immune response, among others. A number of the C-PAC–modulated pathways are logical targets for chemoprevention of EAC, given the documented molecular alterations occurring on the continuum from BE to EAC.[51–53] Similarly, the top 20 canonical pathways identified via GeneGo/MetaCore analysis of the targets resulting from the five miRs altered by C-PAC in all three EAC cell lines include not only P53 signaling, apoptosis, cell cycle, immune response, but also DNA damage-related pathways as displayed in Table 3. MetaCore pathway analysis was also conducted utilizing validated targets derived from all 41 miRs altered in two or more EAC cell lines. The top 20 canonical pathways included more gene ontology categories linked to immune response, cell cycle and epithelial-to-mesenchymal transition, but less involvement of DNA damage pathways when compared to the targets derived form the five common miRs altered in all three cell lines. Other unique categories resulting from the five common miR targets included proteolysis and mucin expression, whereas the 41 miRs dysregulated in two or more cell lines resulted in alterations in AKT and PTEN signaling. These data support that C-PAC modulated some common cancer-linked pathways across all three EAC cell lines; however, unique pathways were also detected between the EAC cell lines in terms of C-PAC–induced changes likely reflecting differences in the molecular profiles of the individual cell lines.
Table 3.
Canonical pathways enriched with targets of miRNAs altered by C-PAC treatment of EAC cell lines
Predicted targets derived from the five commonly altered miRs were also assessed and resulted in identification of 395 gene targets, 12 KEGG and three PANTHER pathways which are summarized in Supplemental Table 2. Interestingly, the Toll-like receptor signaling pathway was the only one in common with pathways identified utilizing only validated targets. Our data analysis supports that the choice of the database impacts outcomes. We found that utilizing multiple databases to assess miR targets provided additional information regarding potential mechanisms by which C-PAC inhibits cancer cell growth; however, additional validation of findings is required to better understand the true overlap between pathways identified via the various databases and actual molecular alterations. A recent paper by Shmelkov et al., evaluated multiple pathway databases for identifying transcriptional regulatory targets and reported little overlap between experimentally obtained target genes compared to targets identified in transcriptional regulatory pathway databases.[32] The authors did report that the MetaCore pathway database results intersected with experimental results in 84% of the cases compared to 24% utilizing the KEGG database, for example; PANTHER was not included in the analysis comparing the various databases. In addition to the results varying by database choice, we detected little overlap between pathways identified as altered utilizing “validated” versus “predicted” gene targets. Thus, both the types of targets and database choice are important considerations which stand to significantly impact interpretation of study results.
Pathways resulting from miRNAs altered in BE and EAC and pathways altered in EAC cell lines by C-PAC treatment
Based on the published literature, a total of 87 miRs have been reported to be dysregulated in EAC and precursor lesions, as detailed in Table 1. Utilizing the miRWALK database, 4335 validated gene targets were derived from the 87 dysregulated miRs. Depositing the gene ENTREZ IDs for the targets in DAVID resulted in 1786 recognized annotations for conducting pathway analysis utilizing the PANTHER database. Next, to assess whether C-PAC impacted PANTHER pathways altered in EAC tissues, 1665 validated gene targets were derived from the 41 miRs (altered in two or more EAC cell lines post–C-PAC treatment); 981 of the 1665 targets were recognized annotations utilized for PANTHER pathway analysis. Results are summarized in Table 4. Based on the 87 dysregulated miRs reported in the published literature [Table 1], a total of 26 PANTHER pathways were identified as significantly altered, with angiogenesis, transforming growth factor (TGF)-β-signaling, apoptosis, Toll receptor signaling and p53 pathway feedback loops 2 representing the top five pathways. C-PAC altered miRs (≥2 EAC cell lines) also resulted in the identification of 26 modulated pathways with 21 of the 26 overlapping with those identified as altered in EAC or BE tissues, supporting that C-PAC may normalize altered miRNA profiles in BE and EAC. This is also consistent with the data in Table 1 showing that C-PAC inversely modulated 10 miRs dysregulated in BE or EAC. Pathways altered in EAC or BE which were not modulated by C-PAC treatment included the Ras pathway, insulin/insulin-like growth factor (IGF) pathway-mitogen activated protein kinase/MAP kinase cascade, fibroblast growth factor (FGF) signaling, p38 pathway and integrin signaling. Similarly, C-PAC altered a small number of PANTHER pathways not reported in EAC tissues, including oxidative stress response, hypoxia response via hypoxia-inducible factor activation, DNA replication and Notch signaling pathways. These results and those detailed in Tables 2 and 3 support that C-PAC may hold promise as an inhibitor against other cancers with abberations in genes and pathways identified. As an example, mutations in P53, P16, PTEN, PIK3CA, RAS, and more recently, NOTCH have all been linked to cancers of the head and neck[54] and based on the results presented are targets potentially modulated by C-PAC treatment.
CONCLUSIONS
Pathway results across three database platforms utilizing gene targets derived from miRs altered in BE and EAC tissues or by C-PAC treatment of EAC cell lines detected multiple common pathways, but also uncovered a limited number of unique pathways per database illustrating the effect that the database choice has on study results. Moreover, divergent pathways were detected when comparing “validated” miR targets to “predicted” miR targets, raising another important consideration for the interprtation of research results. Overall, the data support that C-PAC, a proanthocyanidin-rich cranberry extract, has potent inhibitory effects on the viability of EAC cells, which is in part attributable to modulation of select miRNAs, some of which are known to be altered in EAC and precursor lesions. Still, mechanistic information regarding the cancer inhibitory potential of C-PAC and other cranberry constituents is limited, particularly in terms of in vivo research, compared to other berry types like black raspberries.[55–59] Our results support that future targeted interventions utilizing C-PAC in cohorts at increased risk for EAC progression or other cancers as idenitified by pathway analysis may prove promising. It is encouraging that several miRs modulated by C-PAC in EAC cell lines have also been reported to be inversely dysregulated in premalignant esophageal pathologies as well as EAC [Table 1], supporting that C-PAC may hold chemopreventive potential at early stages during the development of esophageal premalignancy as well as later stages characterized by neoplastic transformation and progression to EAC. The fact that C-PAC also modulates angiogenesis and PEDF signaling based on pathway analysis further supports this finding. Interestingly, black raspberries have recently been reported to inhibit late stage carcinogenesis in a preclinical model for esophageal squamous cell carcinoma through modulation of pathways linked to proliferation, apoptosis, inflammation, angiogenesis, cyclooxygenase and lipoxygenase. Thus, although the findings reported herein are limited by their descriptive nature, they are still informative for the identification of pathways linked to EAC progression or pathways modulated by C-PAC treatment, in turn serving to guide more mechanistically driven investigations. Further in vitro, in vivo and clinical investigations are warranted to assess the true chemopreventive efficacy of C-PAC toward reducing BE and EAC risk. Improving our knowledge of dysregulated miRNAs in specific cancers (EAC) and premalignant conditions (BE) will permit identification of potential miRNA-based therapeutic or chemopreventive targets and support improved mechanistically informed interventions with the overarching goal of reducing cancer risk in high-risk cohorts. Efficacious cancer risk reducing interventions are especially needed for esophageal and other cancers plagued with ineffective screening methods, late stage diagnosis, cancers with limited treatment options and those with poor prognosis. Improving our understanding of specific miRNAs altered by natural or dietary sources of cancer inhibitory agents, such as C-PAC, is a relatively new area of investigation,[60,61] but one that may lead to promising mutitargeted chemopreventive strategies utilizing single agents that modulate mutiple cancer-associated pathways or combinations of agents targeting complementary pathways as a risk-reducing strategy.
AUTHOR'S PROFILE
Dr. Laura Ann Kresty, Department of Epidemiology and Public Health, University of Miami Miller School of Medicine and Sylvester Cancer Center, Miami, Florida 33136; USA
Dr. Jennifer Clarke, Department of Epidemiology and Public Health, University of Miami Miller School of Medicine and Sylvester Cancer Center, Miami, Florida 33136; USA
Ms. Kristin Ezell, Department of Epidemiology and Public Health, University of Miami Miller School of Medicine and Sylvester Cancer Center, Miami, Florida 33136; USA
Ms. Amy Exum, Department of Epidemiology and Public Health, University of Miami Miller School of Medicine and Sylvester Cancer Center, Miami, Florida 33136; USA
Dr. Amy B Howell, Marucci Center for Blueberry Cranberry Research, Rutgers University, Chatsworth, New Jersey 08019; USA
Dr. Toumy Guettouche, Institute for Human Genomics, University of Miami Miller School of Medicine, Miami, Florida 33136; USA
REFERENCES
- 1.Lambert R, Hainaut P. The multidisciplinary management of gastrointestinal cancer.Epidemiology of oesophagogastric cancer. Best Pract Res Clin Gastroenterol. 2007;21:921–45. doi: 10.1016/j.bpg.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Devesa SS, Blot WJ, Fraumeni JF., Jr Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer. 1998;83:2049–53. [PubMed] [Google Scholar]
- 3.Brown LM, Devesa SS, Chow WH. Incidence of adenocarcinoma of the esophagus among white Americans by sex, stage, and age. J Natl Cancer Inst. 2008;100:1184–7. doi: 10.1093/jnci/djn211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Locke GR, 3rd, Talley NJ, Fett SL, Zinsmeister AR, Melton LJ., 3rd Prevalence and clinical spectrum of gastroesophageal reflux: a population-based study in Olmsted County, Minnesota. Gastroenterology. 1997;112:1448–56. doi: 10.1016/s0016-5085(97)70025-8. [DOI] [PubMed] [Google Scholar]
- 5.Holmes RS, Vaughan TL. Epidemiology and pathogenesis of esophageal cancer. Semin Radiat Oncol. 2007;17:2–9. doi: 10.1016/j.semradonc.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 6.El-Serag H. The association between obesity and GERD: a review of the epidemiological evidence. Dig Dis Sci. 2008;53:2307–12. doi: 10.1007/s10620-008-0413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ryan AM, Healy LA, Power DG, Byrne M, Murphy S, Byrne PJ, et al. Barrett esophagus: prevalence of central adiposity, metabolic syndrome, and a proinflammatory state. Ann Surg. 2008;247:909–15. doi: 10.1097/SLA.0b013e3181612cac. [DOI] [PubMed] [Google Scholar]
- 8.Corley DA, Kubo A, Levin TR, Block G, Habel L, Rumore G, et al. Race, ethnicity, sex and temporal differences in Barrett's oesophagus diagnosis: A large community-based study, 1994-2006. Gut. 2009;58:182–8. doi: 10.1136/gut.2008.163360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Botterwreck AA, Schouten LI, Volovics A, Dorant E, van Den Brandt PA. Trends in incidence of adenocarcinoma of the oesophagus and gastric cardia in ten European countries. Int J Epidemiol. 2000;29:645–54. doi: 10.1093/ije/29.4.645. [DOI] [PubMed] [Google Scholar]
- 10.Cancer in the UK Summary. [Last cited on 2011 Aug 15]. Available from: http://info.cancerresearchuk.org/cancerstats/
- 11.Atlanta, GA: American Cancer Society; 2011. American Cancer Society: Cancer Facts and Figures, 2011. [Google Scholar]
- 12.Washington DC: American Institute for Cancer Research; m2007. World Cancer Research Fund/American Institute for Cancer Research: Food, nutrition, physical activity, and prevention of cancer: A global perspective. [Google Scholar]
- 13.Kubo A, Levin TR, Block G, Rumore GJ, Quesenberry CP, Jr, Buffler P, et al. Dietary antioxidants, fruits, and vegetables and the risk of Barrett's esophagus. Am J Gastroenterol. 2008;103:1614–23. doi: 10.1111/j.1572-0241.2008.01838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kubo A, Levin TR, Block G, Rumore GJ, Quesenberry CP, Jr, Buffler P, et al. Dietary patterns and the risk of Barrett's esophagus. Am J Epidemiol. 2008;167:839–46. doi: 10.1093/aje/kwm381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Howell AB. BiEACtive compounds in cranberries and their role in prevention of urinary tract infections. Mol Nutr Food Res. 2007;51:732–7. doi: 10.1002/mnfr.200700038. [DOI] [PubMed] [Google Scholar]
- 16.Neto CC, Amoroso JW, Liberty AM. Anticancer activities of cranberry phytochemicals: an update. Mol Nutr Food Res. 2008;52(Suppl):S18–27. doi: 10.1002/mnfr.200700433. [DOI] [PubMed] [Google Scholar]
- 17.Pappas E, Schaich KM. Phytochemicals of cranberries and cranberry products: Characterization, potential health effects, and processing stability. Crit Rev Food Sci Nutr. 2009;49:741–81. doi: 10.1080/10408390802145377. [DOI] [PubMed] [Google Scholar]
- 18.Kresty LA, Howell AB, Baird M. Cranberry proanthocyanidins mediate growth arrest of lung cancer cells through modulation of gene expression and rapid induction of apoptosis. Molecules. 2011;16:2375–90. doi: 10.3390/molecules16032375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kresty LA, Howell AB, Baird M. Cranberry proanthocyanidins induce apoptosis and inhibit acid-induced proliferation of human esophageal adenocarcinoma cells. J Agric Food Chem. 2008;56:676–80. doi: 10.1021/jf071997t. [DOI] [PubMed] [Google Scholar]
- 20.Kresty LA, Exum A, Zeyzus-Johns B. New York: Springer Press; 2010. Berries in the Prevention of Esophageal Adenocarcinoma.In Berries and Cancer Prevention. Chapter 5; pp. 101–14. [Google Scholar]
- 21.Albulescu R, Neagu M, Albulescu L, Tanase C. Tissular and soluble miRNAs for diagnostic and therapy improvement in digestive tract cancers. Expert Rev Mol Diagn. 2011;11:101–20. doi: 10.1586/erm.10.106. [DOI] [PubMed] [Google Scholar]
- 22.Foo LY, Lu Y, Howell AB, Vorsa N. The structure of cranberry proanthocyanidins which inhibit adherence of uropathogenic P-fimbriated Escherichia coli in vitro. Phytochemistry. 2000;54:173–81. doi: 10.1016/s0031-9422(99)00573-7. [DOI] [PubMed] [Google Scholar]
- 23.Foo LY, Lu Y, Howell AB, Vorsa N. A-Type proanthocyanidin trimers from cranberry that inhibit adherence of uropathogenic P-fimbriated Escherichia coli. J Nat Prod. 2000;63:1225–8. doi: 10.1021/np000128u. [DOI] [PubMed] [Google Scholar]
- 24.Howell AB, Reed JD, Krueger CG, Winterbottom R, Cunningham DG, Leahy M. A-type proanthocyanidins and uropathogenic bacterial anti-adhesion activity. Phytochemistry. 2005;66:2281–91. doi: 10.1016/j.phytochem.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 25.Boonstra JJ, van Marion R, Beer DG, Lin L, Chaves P, Ribeiro C, et al. Verification and unmasking of widely used human esophageal adenocarcinoma cell lines. J Natl Cancer Inst. 2010;102:271–4. doi: 10.1093/jnci/djp499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Becker C, Hammerle-Fickinger A, Riedmaier I, Pfaffl MW. mRNA and microRNA quality control for RT-qPCR analysis. Methods. 2010;50:237–43. doi: 10.1016/j.ymeth.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 27.Dweep H, Sticht C, Pandey P, Gretz N. miRWalk - Database: Prediction of possible miRNA binding sites by “walking” the genes of 3 genomes. J Biomed Inform. 2011;44:839–47. doi: 10.1016/j.jbi.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 28.Dennis G, Jr, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, et al. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol. 2003;4:P3. [PubMed] [Google Scholar]
- 29.Thomas PD, Campbell MJ, Kejariwal A, Mi H, Karlak B, Daverman R, et al. PANTHER: a library of protein families and subfamilies indexed by function. Genome Res. 2003;13:2129–41. doi: 10.1101/gr.772403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mi H, Dong Q, Muruganujan A, Gaudet P, Lewis S, Thomas PD. PANTHER version 7: improved phylogenetic trees, orthologs and collaboration with the Gene Ontology Consortium. Nucleic Acids Res. 2010;38:D204–10. doi: 10.1093/nar/gkp1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen HC, Chen GH, Chen YH, Liao WL, Liu CY, Chang KP, et al. MicroRNA deregulation and pathway alterations in nasopharyngeal carcinoma. Br J Cancer. 2009;100:1002–11. doi: 10.1038/sj.bjc.6604948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shmelkov E, Tang Z, Aifantis I, Statnikov A. Assessing quality and completeness of human transcriptional regulatory pathways on a genome-wide scale. Biol Direct. 2011;6:15. doi: 10.1186/1745-6150-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bansal A, Lee IH, Hong X, Anand V, Mathur SC, Gaddam S, et al. Feasibility of mcroRNAs as biomarkers for Barrett's Esophagus progression: a pilot cross-sectional, phase 2 biomarker study. Am J Gastroenterol. 2011;106:1055–63. doi: 10.1038/ajg.2011.37. [DOI] [PubMed] [Google Scholar]
- 34.Yang H, Gu J, Wang KK, Zhang W, Xing J, Chen Z, et al. MicroRNA expression signatures in Barrett's esophagus and esophageal adenocarcinoma. Clin Cancer Res. 2009;15:5744–52. doi: 10.1158/1078-0432.CCR-09-0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fassan M, Volinia S, Palatini J, Pizzi M, Baffa R, De Bernard M, et al. MicroRNA expression profiling in human Barrett's carcinogenesis. Int J Cancer. 2011;129:1661–70. doi: 10.1002/ijc.25823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feber A, Xi L, Luketich JD, Pennathur A, Landreneau RJ, Wu M, et al. MicroRNA expression profiles of esophageal cancer. J Thorac Cardiovasc Surg. 2008;135:255–60. doi: 10.1016/j.jtcvs.2007.08.055. discussion 260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wijnhoven BP, Hussey DJ, Watson DI, Tsykin A, Smith CM, Michael MZ South Australian Oesophageal Research Group. MicroRNA profiling of Barrett's oesophagus and oesophageal adenocarcinoma. Br J Surg. 2010;97:853–61. doi: 10.1002/bjs.7000. [DOI] [PubMed] [Google Scholar]
- 38.Dijckmeester WA, Wijnhoven BP, Watson DI, Leong MP, Michael MZ, Mayne GC, et al. MicroRNA-143 and -205 expression in neosquamous esophageal epithelium following argon plasma ablation of Barrett's esophagus. J Gastrointest Surg. 2009;13:846–53. doi: 10.1007/s11605-009-0799-5. [DOI] [PubMed] [Google Scholar]
- 39.Feber A, Xi L, Pennathur A, Gooding WE, Bandla S, Wu M, et al. MicroRNA prognostic signature for nodal metastases and survival in esophageal adenocarcinoma. Ann Thorac Surg. 2011;91:1523–30. doi: 10.1016/j.athoracsur.2011.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mathé EA, Nguyen GH, Bowman ED, Zhao Y, Budhu A, Schetter AJ, et al. MicroRNA expression in squamous cell carcinoma and adenocarcinoma of the esophagus: Associations with survival. Clin Cancer Res. 2009;15:6192–200. doi: 10.1158/1078-0432.CCR-09-1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maru DM, Singh RR, Hannah C, Albarracin CT, Li YX, Abraham R, et al. MicroRNA-196a is a potential Marker of Progression during Barrett's metaplasia-dysplasia-invasive adenocarcinoma sequence in esophagus. Am J Pathol. 2009;174:1940–8. doi: 10.2353/ajpath.2009.080718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haiart S, Watson DI, Leong MP, Astill D, Bright T, Hussey DJ. MicroRNA-196a and microRNA-101 expression in Barrett's oesophagus in patients with medically and surgically treated gastro-oesophageal reflux. BMC Res Notes. 2011;4:41. doi: 10.1186/1756-0500-4-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luthra R, Singh RR, Luthra MG, Li YX, Hannah C, Romans AM, et al. MicroRNA-196a targets annexin A1: A microRNA-mediated mechanism of annexin A1 downregulation in cancers. Oncogene. 2008;27:6667–78. doi: 10.1038/onc.2008.256. [DOI] [PubMed] [Google Scholar]
- 44.Smith CM, Watson DI, Leong MP, Mayne GC, Michael MZ, Wijnhoven BP, et al. miR-200 family expression is downregulated upon neoplastic progression of Barrett's esophagus. World J Gastroenterol. 2011;17:1036–44. doi: 10.3748/wjg.v17.i8.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kan T, Sato F, Ito T, Matsumura N, David S, Cheng Y, et al. The miR-106b-25 polycistron, activated by genomic amplification, functions as an oncogene by suppressing p21 and Bim. Gastroenterology. 2009;136:1689–700. doi: 10.1053/j.gastro.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Howell AB. BiEACtive compounds in cranberries and their role in prevention of urinary tract infections. Mol Nutr Food Res. 2007;51:732–7. doi: 10.1002/mnfr.200700038. [DOI] [PubMed] [Google Scholar]
- 47.Gu L, Kelm MA, Hammerstone JF, Beecher G, Holden J, Haytowitz D, et al. Screening of foods containing proanthocyanidins and their structural characterization using LC-MS/MS and thiolytic degradation. J Agric Food Chem. 2003;51:7513–21. doi: 10.1021/jf034815d. [DOI] [PubMed] [Google Scholar]
- 48.Almeida MI, Reis RM, Calin GA. MicroRNA history: Discovery, recent applications, and next frontiers. Mutat Res. 2011 doi: 10.1016/j.mrfmmm.2011.03.009. [In press] [DOI] [PubMed] [Google Scholar]
- 49.Cho WC. MicroRNAs: Potential biomarkers for cancer diagnosis, prognosis and targets for therapy. Int J Biochem Cell Biol. 2010;42:1273–81. doi: 10.1016/j.biocel.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 50.Li X, Zhang Y, Zhang H, Liu X, Gong T, Li M, et al. miRNA-223 promotes gastric cancer invasion and metastasis by targeting tumor suppressor EPB41L3. Mol Cancer Res. 2011;9:824–33. doi: 10.1158/1541-7786.MCR-10-0529. [DOI] [PubMed] [Google Scholar]
- 51.Reid BJ, Li X, Galipeau PC, Vaughan TL. Barrett's oesophagus and oesophageal adenocarcinoma: time for a new synthesis. Nat Rev Cancer. 2010;10:87–101. doi: 10.1038/nrc2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Prasad GA, Bansal A, Sharma P, Wang KK. Predictors of progression in Barrett's esophagus: current knowledge and future directions. Am J Gastroenterol. 2010;105:1490–502. doi: 10.1038/ajg.2010.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kelloff GJ, Lippman SM, Dannenberg AJ, Sigman CC, Pearce HL, Reid BJ, et al. Progress in chemoprevention drug development: the promise of molecular biomarkers for prevention of intraepithelial neoplasia and cancer--a plan to move forward. Clin Cancer Res. 2006;12:3661–97. doi: 10.1158/1078-0432.CCR-06-1104. [DOI] [PubMed] [Google Scholar]
- 54.Brakenhoff RH. Cancer.Another NOTCH for cancer. Science. 2011;333:1102–3. doi: 10.1126/science.1210986. [DOI] [PubMed] [Google Scholar]
- 55.Stoner GD, Wang LS, Seguin C, Rocha C, Stoner K, Chiu S, et al. Multiple berry types prevent N-nitrosomethylbenzylamine-induced esophageal cancer in rats. Pharm Res. 2010;27:1138–45. doi: 10.1007/s11095-010-0102-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kresty LA, Frankel WL, Hammond CD, Baird ME, Mele JM, Stoner GD, et al. Transitioning from preclinical to clinical chemopreventive assessments of lyophilized black raspberries: interim results show berries modulate markers of oxidative stress in Barrett's esophagus patients. Nutr Cancer. 2006;54:148–56. doi: 10.1207/s15327914nc5401_15. [DOI] [PubMed] [Google Scholar]
- 57.Kresty LA, Morse MA, Morgan C, Carlton PS, Lu J, Gupta A, et al. Chemoprevention of esophageal tumorigenesis by dietary administration of lyophilized black raspberries. Cancer Res. 2001;61:6112–9. [PubMed] [Google Scholar]
- 58.Shumway BS, Kresty LA, Larsen PE, Zwick JC, Lu B, Fields HW, et al. Effects of a topically applied bioadhesive berry gel on loss of heterozygosity indices in premalignant oral lesions. Clin Cancer Res. 2008;14:2421–30. doi: 10.1158/1078-0432.CCR-07-4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang LS, Dombkowski AA, Seguin C, Rocha C, Cukovic D, Mukundan A, et al. Mechanistic basis for the chemopreventive effects of black raspberries at a late stage of rat esophageal carcinogenesis. Mol Carcinog. 2011;50:291–300. doi: 10.1002/mc.20634. [DOI] [PubMed] [Google Scholar]
- 60.Parasramka MA, Ho E, Williams DE, Dashwood RH. MicroRNAs, diet and cancer: New mechanistic insights on the epigenetic actions of phytochemicals. Mol Carcinog. 2011 doi: 10.1002/mc.20822. [In press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Davis CD, Ross SA. Evidence for dietary regulation of microRNA expression in cancer cells. Nutr Rev. 2008;66:477–82. doi: 10.1111/j.1753-4887.2008.00080.x. [DOI] [PubMed] [Google Scholar]