Abstract
The authors report the case of a previously well 34-year-old woman presenting with a hypertensive crises and a grand-mal seizure following elective caesarean section. Initial treatment of extreme hypertension, of a presumed eclamptic aetiology, with magnesium and labetalol was complicated by intermittent profound hypotensive episodes. This was accompanied by severe biventricular failure and fluctuating systemic vascular resistance. Abdominal ultrasound revealed a left suprarenal mass. A diagnosis of phaeochromocytoma was confirmed on abdominal CT and urinary assays. The patient was stabilised with α and β blockade, was successfully extubated and subsequently had the tumour surgically excised. The cardiac function returned to normal on echocardiography and she has made a complete recovery.
Background
Phaeochromocytoma are rare catecholamine-secreting enterochromaffin tumours, remembered historically by the axiom ‘tumours of 10’s’ (10% bilateral, 10% extra-adrenal, 10% above the diaphragm, 10% malignant and 10% familial). The abrupt and life-threatening presentation of this case, following a caesarean section in a previously fully active and fit young woman, required considerable cross-specialty expertise in her management. The case is unusual and provides several lessons in the management of this critical condition with a high risk of death to the mother and potentially her child.
Case presentation
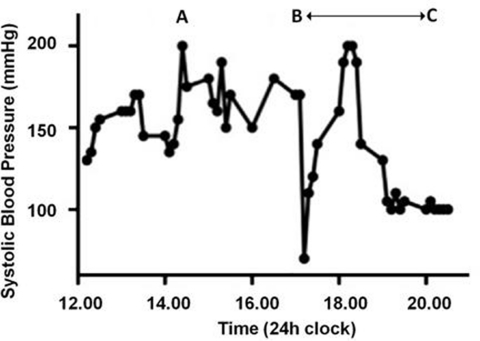
A 34-year-old woman presented for a term elective caesarean section having had a previous emergency caesarean section for fetal distress. She had no co-morbidities and the antenatal course was unremarkable with normal blood pressure and urinalysis throughout. She underwent an uneventful combined spinal-epidural using 2.2 ml of 0.5% heavy bupivacaine and 20 mcg fentanyl, delivered intrathecally. Systolic blood pressure (SBP) prior to neuraxial blockade was 130 mm Hg and remained stable without vasopressors. Following delivery of a healthy female infant, a rapid 1.5 l placental haemorrhage occurred followed by a further 1 l haemorrhage over 3 h from superficial uterine vessels. Although initially asymptomatic and cardiovascularly stable, she had severe headache accompanied by a SBP surge to 180 mm Hg, following ergometrine (figure 1A). This initially settled with 10 mg of labetalol. Supplementary analgesia during the 3 h procedure totalled 30 mls of 0.5% bupivacaine, 10 ml 2% lignocaine and 6 mg diamorphine via an extradural catheter and 30 mg ketamine intravenously.
Figure 1.
(A–C) Graph illustrating the patients cardiac instability in the first 8 h following surgery.
In recovery she was persistently hypertensive (170 mm Hg systolic) and had a witnessed 1 min tonic-clonic seizure, treated with a 4 g magnesium infusion. She remained obtunded (Glascow Coma Scale 3/15) and became hypotensive (SBP 60 mm Hg) with an arterial blood gas revealing a lactic acidosis (base deficit-21). She was resuscitated with 2 l of crystalloid, 6 mg of ephedrine and an airway was secured using alfentanil 1 mg (to obtund a pressor response) and suxamethonium 100 mg. While the blood pressure remained stable on intubation she became markedly hypertensive (SBP 220) and tachycardic at 160 beats/min. The blood pressure settled with labetalol and magnesium infusions.
She was transferred to intensive care unit and managed as severe pre-eclampsia with labetalol, magnesium and glycerine trinitrate infusions. Her blood pressure continued to be labile with intermittent severe hypertension and profound hypotension (figure 1B,C).
Investigations
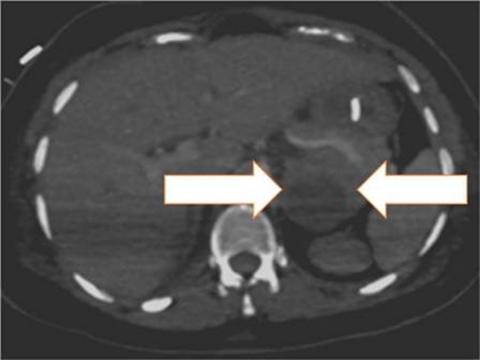
CT brain demonstrated no intracranial pathology. Trans-oesophageal Doppler revealed a low cardiac index (1–1.5 l/min.m−2) and a high but variable systemic vascular resistance index (from 1200–3500 dyne.s.cm−5.m−2). Urgent trans-thoracic echocardiography demonstrated severe biventricular failure (left ventricular ejection fraction 10–15%). Electrocardiography revealed no ischaemia but a 12 h troponin I was elevated (21.4 µg/l). An abdominal ultrasound scan revealed a 5.3 cm suprarenal mass. A contrast-enhanced abdominal CT scan (figure 2) revealed a necrotic suprarenal tumour and phaeochromocytoma was confirmed by 24 h urinary metanephrine assay (table 1). No proteinuria was detected in the antenatal period or within the 48 h following her C-section and seizure event.
Figure 2.
Contrast-enhanced CT scan showing a supra-renal tumour (broad arrows).
Table 1.
Twenty four h urine collection showing significantly elevated urinary metanephrines
| Result (nmol/dl) | Normal range (nmol/dl) | |
|---|---|---|
| Metadrenaline | 80641 | 0–1250 |
| Normetadrenaline | 50333 | 0–3350 |
| 3-methoxytyramine | 7235 | 0–2750 |
Twenty four h later the echocardiogram had returned almost to normal with a left ventricular ejection fraction of 50%.
Differential diagnosis
The differential, and our initial consideration, lay between an abrupt onset eclampsia type syndrome or an alternative explanation for the severe cardiovascular instability.
Treatment
Following the postpartum haemorrhage, she received 1 l of crystalloid, 500 ml colloid and two units of blood. Post transfusion her haemoglobin was 11.2 g/dl (preop 12.9 g/dl). Syntocinon (5 units intravenously followed by a 40 unit infusion), ergometrine (500 mcg intramuscularly) and carboprost (250 mcg intramuscularly, 250 mcg intrauterine) were also administered. The blood pressure was fluid responsive and (contrary to a pre-eclampsia protocol) she received 6 litres of fluid over 24 h). Haemodynamic stability was established with α (phenoxybenzamine) and β-blockade (bisoprolol and metoprolol).
On confirmation of a phaeochromocytoma she was fully fluid resuscitated, α-blocked with phentolamine and transferred to a tertiary centre.
Outcome and follow-up
She was extubated 9 days after her caesarean section and underwent successful surgery 6 weeks later. She has made a complete recovery.
Discussion
Intraoperative presentation of undiagnosed phaeochromocytoma is rare and mortality can be as high as 85%.1 Anaesthesia and surgery (including caesarean section)2 can potentially trigger a phaeochromocytoma crisis with profound haemodynamic disturbance.3 The trigger for this crisis may have been the intraoperative stress and blood loss, although ergotamine has previously been described as a cause.4 Although the diagnostic dilemma between pre-eclampsia and phaeochromocytoma in pregnancy is well documented,3 5 to our knowledge this is the first case report involving a seizure in the context of peripartum hypertension. Such a presentation is strongly indicative of eclampsia (and was treated as such) until proven otherwise. Although eclampsia is defined as a seizure in association with signs and symptoms of pre-eclampsia, the absence of antenatal hypertension and proteinuria does not preclude the diagnosis. In up to 38% of cases of eclampsia patients have no evidence of preseizure hypertension and proteinuria but subsequently develop these within 24 h.6 The exact aetiology of the seizure in this patient remains unknown, but may result from effects of the phaeochromocytoma on reducing seizure threshold via its actions on metabolic or hypertensive parameters.7 8 Furthermore, the administered of ergometrine may have induced neurological sequelae, as previously reported with an ergotamine analogue prescribed in a case with phaeochromocytoma.9
Magnesium exerts antihypertensive actions directly on the vasculature as a calcium-antagonist and indirectly by reducing catecholamine release. It also increases seizure threshold. Such properties have led to its use first-line in eclampsia and severe pre-eclampsia. In phaeochromocytoma it usually constitutes supplementary therapy, however James et al10 report it’s successful use in three phaeochromocytoma cases resistant to phentolamine and nitroprusside. Phentolamine, doxazosin and phenoxybenzamine (α blockers) are recommended for first-line management of phaeochromocytoma (although sodium nitroprusside was historically used). Side effects of α-blockade include hypotension, particularly postural. These patients commonly have a contracted intravascular volume (as evidenced in our case by preserved Hb 11.2 g/dl despite 2.5 l haemorrhage) and vasodilatation mediated by α-blockade can cause profound hypotension and has proven fatal.11 Adequate fluid resuscitation prior to α blockade is essential. This is somewhat different to severe pre-eclampsia where patients are fluid restricted (85 ml/h) to avoid pulmonary oedema. In this patient due to the fluid-responsive nature of the life-threatening hypotensive episodes the pre-eclampsia protocol was ignored and the patient received 6 litres of fluid over the first 24 h, which may have been important in her survival.
Learning points.
-
▶
Phaeochromocytoma is an important differential diagnosis for pre-eclampsia and eclampsia.
-
▶
Although similarities exist in treatment regimens, fluid resuscitation in phaeochromocytoma is essential
-
▶
Early recognition of a phaeochromocytoma is key.
-
▶
Phaeochromocytoma are associated with significant mortality during pregnancy to mother (12%) and fetus (17%).
-
▶
Maternal mortality is as high as 50% if phaeochromocytoma is unrecognised antenatally.12 13
Footnotes
Competing interests None.
Patient consent Obtained.
References
- 1.Sauvage MR, Tulasne PA, Arnaud JP. [Hypertensive accident in a surgical patient with unsuspected pheochromocytoma (author’s transl)]. Anesth Analg (Paris) 1979;36:155–8 [PubMed] [Google Scholar]
- 2.Zangrillo A, Valentini G, Casati A, et al. Myocardial infarction and death after caesarean section in a woman with protein S deficiency and undiagnosed phaeochromocytoma. Eur J Anaesthesiol 1999;16:268–70 [DOI] [PubMed] [Google Scholar]
- 3.Prys-Roberts C. Phaeochromocytoma–recent progress in its management. Br J Anaesth 2000;85:44–57 [DOI] [PubMed] [Google Scholar]
- 4.Del Rosso A, Fradella G, Russo L, et al. Pheochromocytoma crisis caused by contemporary ergotamine, caffeine, and nimesulide administration. Am J Med Sci 1997;314:396–8 [DOI] [PubMed] [Google Scholar]
- 5.Hudsmith JG, Thomas CE, Browne DA. Undiagnosed phaeochromocytoma mimicking severe preeclampsia in a pregnant woman at term. Int J Obstet Anesth 2006;15:240–5 [DOI] [PubMed] [Google Scholar]
- 6.Douglas KA, Redman CW. Eclampsia in the United Kingdom. BMJ 1994;309:1395–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jadoul M, Leo JR, Berends MJ, et al. Pheochromocytoma-induced hypertensive encephalopathy revealing MEN-IIa syndrome in a 13-year old boy. Implications for screening procedures and surgery. Horm Metab Res Suppl 1989;21:46–9 [PubMed] [Google Scholar]
- 8.Leiba A, Bar-Dayan Y, Leker RR, et al. Seizures as a presenting symptom of phaeochromocytoma in a young soldier. J Hum Hypertens 2003;17:73–5 [DOI] [PubMed] [Google Scholar]
- 9.Kelley BJ, Samples S, Kunkel R. PRES following administration of DHA in a patient with unsuspected phaeochromocytoma. Headache 2008;48:1237–9 [DOI] [PubMed] [Google Scholar]
- 10.James MF, Cronjé L. Pheochromocytoma crisis: the use of magnesium sulfate. Anesth Analg 2004;99:680–6, table of contents. [DOI] [PubMed] [Google Scholar]
- 11.Emanuel DA, Rowe GG, Musser MJ, et al. Prolonged hypotension with fatal termination after a phentolamine (regitine) methanesulfonate test. J Am Med Assoc 1956;161:436–9 [DOI] [PubMed] [Google Scholar]
- 12.Harper MA, Murnaghan GA, Kennedy L, et al. Phaeochromocytoma in pregnancy. Five cases and a review of the literature. Br J Obstet Gynaecol 1989;96:594–606 [DOI] [PubMed] [Google Scholar]
- 13.Sarathi V, Lila AR, Bandgar TR, et al. Pheochromocytoma and pregnancy: a rare but dangerous combination. Endocr Pract 2010;16:300–9 [DOI] [PubMed] [Google Scholar]