Abstract
Telecytology is the interpretation of cytology material at a distance using digital images. For more than a decade, pioneering efforts to introduce telecytology into clinical practice have been reported. A Medline search for “telecytology” and “cytology” reveals a voluminous literature, though much of what has been published to date is based on technologies that are rapidly becoming obsolete. The technological limitations of previous techniques, including the transmission of static digital images and dynamic streaming images, have limited telecytology to minor niches. The primary problem with these technologies is that the remote viewer can only see a small fraction of the material on the original slides, introducing the possibility of diagnostic error based not only on image quality but also on image selection. Remote robotic microscopy offers one possible solution to this problem, but to date has found limited acceptance, principally attributable to slow operating times. Whole slide imaging seems to be a much more promising solution, though cytology-specific literature regarding its use is still scant. The advent of whole slide imaging opens up new possibilities for telecytology by enabling high-quality images of entire cytology specimens to be available to anyone, anywhere via the Internet. Although challenges remain, especially with regard to capturing the full microscopy experience including multiple planes of focus and sharp high-powered images, rapidly advancing technology promises to overcome these limitations. Increasing application of whole slide imaging technology in surgical pathology will undoubtedly also increase its application to cytology due to the increasing affordability and practicality of the equipment as it serves a larger number of useful roles within a pathology department. The current and expanding applications of telecytology for clinical practice, education, quality assurance, and testing will be reviewed.
Keywords: Digital images, remote microscopy, telecytology, whole-slide imaging
INTRODUCTION
Telecytology is the interpretation of cytology material at a distance using digital images. Although there is a long history of attempts to implement telecytology, it still has only limited applications and acceptance. This stems largely from the challenge involved in making imaging systems of sufficient quality to consistently and accurately reproduce cytology material present on glass slides. Cytology material is difficult to image, relative to histology, for a number of reasons. Paraffin-embedded tissue is cut to create a single uniform plane of tissue for staining and examination. Cytology material, on the other hand, frequently contains a significant element of three-dimensionality resulting from cell clustering or as an artifact of preparation techniques. Furthermore, diagnostic cytology material, unlike histology, is more frequently widely and randomly distributed across glass slides, especially in smeared preparations, requiring more intensive screening of a larger area in order to find the best areas for diagnostic interpretation. The greater reliance of cytologists on subtle features of individual cells and background material is another problem, as this requires greater magnification and higher quality images. Finally, the frequent need for complimentary stains, usually Papanicolaou and Romanowsky stains in tandem, increases the number of slides that must be examined to render a confident diagnosis.
Counterbalancing the problematic aspects of telecytology, however, are powerful incentives to develop imaging technology for use in cytology material. Unlike histology, where a tissue block remains behind for potential recuts if a slide is lost or broken, many cytology slides are unique and irreplaceable. It is not uncommon for life-altering patient diagnoses to rest entirely on the appearance of a few cells present on a single cytology slide. For this reason, developing telecytology capabilities that could reduce the need to physically send precious glass slides over long distances, or even to create a permanent digital copy that could be stored long term, is highly desirable. Along the same lines, cytology education and testing suffer because of the difficulty of acquiring a sufficient number of comparable and high-quality slides for wide distribution and easy access. Telecytology offers the possibility of providing users with access to digitized teaching sets comprised of typical and unique cases. Ideally, outstanding examples from glass slide teaching sets scattered in laboratories around the world could be compiled into a universally accessible database for the benefit of cytologists everywhere, akin to the effort involved in creating the online static digital Bethesda System for cervical cytology.[1]
The evolution of telecytology is approaching a critical stage in which it is becoming more reasonable to expect much broader application and acceptance of this technology. Increasing numbers of cytology procedures requiring immediate evaluation are being performed in settings where expert cytopathologists are a limiting factor, for instance fine needle aspiration of thyroid nodules. Most telecytology studies in the literature focus on the review of static digital microphotographs or video microscopy that only allow the remote viewer to assess a tiny fraction of the total case material selected by the host reviewing the actual glass slides. Advances in whole-slide imaging (WSI), however, offer the possibility of creating higher quality reproductions of entire glass slides that can be stored and accessed in real time. Moreover, current applications that accompany WSI viewing permit teleconferencing whereby several individuals can remotely log in to simultaneously view a case. This opens up entirely new possibilities for telecytology, including the prospect of providing final diagnoses based on virtual images alone.
TELECYTOLOGY METHODS
Telecytology depends, fundamentally, on the ability to convert optical information presented in a microscope eyepiece into a digital image that can be remotely transmitted. Digital imaging devices (e.g., digital camera, WSI scanner), computers, and networks (i.e. the Internet) make this task ever-easier. A digital image is represented in a computer by a two-dimensional array of numbers, each element of which represents a pixel (short for picture element). The imaging process involves capturing, saving, editing (if necessary), and sharing digital images.[2] There are multiple types of devices that can be used to acquire digital images [Figure 1]. Many of these devices, particularly microscope-mounted cameras, are already widely installed in many pathology laboratories and used for several purposes including teaching and tumor board presentations. Commercial software is available for a few thousand dollars that allows for remote live viewing of the images produced by such cameras. Other systems, such as remotely controlled robotic microscopes and whole-slide scanners are being used, but to a lesser degree, largely for telepathology of surgical pathology material (teleconsultation) and frozen sections (including brain smears).
Figure 1.

Input devices for creating digital images: (far left) digital camera attached via adapter to a light microscope, (middle left) whole-slide scanners showing (upper) the Aperio Scanscope XT CS Scanner, scans up to 120 slides; and (lower) the Omnyx VL4 whole-slide scanner that scans up to four slides at a time and (middle right) robotic microscopes including (upper) the Nikon CoolScope II, one glass slide scanner and (lower) the Trestle 5L50, 50 slide loaders (far right) Cambridge Research and Instrumentation (CRi) Nuance multispectral imaging (MSI) camera
Microscopic digital images can be static (still images), viewed live (real-time video or robotic microscopy), or viewed after scanning of the glass slides (WSI or virtual microscopy).[2–5] Efforts are underway to standardize the process of acquiring, storing, and displaying digital images in pathology in a manner similar to radiology.[6–9] WSI is a digital imaging modality that uses computerized technology to scan and convert (digitize) glass slides into digital images so that they can be viewed on a computer using viewing software.[4,10,11] This software allows a user to scan from field to field and increase or decrease the magnification, simulating panning around and zooming in or out with a conventional microscope. Tables 1 and 2 list the advantages and disadvantages of WSI. Current WSI technology provides rapid, high-quality image capture and storage, integrated with image viewer software to allow virtual microscopy anywhere in the world over the Internet.
Table 1.
Advantages of whole slide imaging in cytopathology practice

Table 2.
Disadvantages of whole-slide imaging in cytopathology practice

WSI technology has started to have a significant impact on cytopathology practice in various aspects including telecytology, performing cytology quality assurance, cytology education, competency assessment, image analysis, and research [Figure 2].[12–16] Recent advanced capabilities include a decrease in the time to scan slides (only a-few-minute range) and the ability to handle a large number of slides with automated feeders holding up to 300 slides.[8,13,15,17,18] WSI is also becoming increasingly robust with regard to scanning three-dimensional structures.[17,19] The Z-stack function (so called because the planes of focus are along the z-axis) offers manual-focusing capability of selected areas on virtual slides.[1] This is often necessary for cytological specimens with thick preparations or cell clustering.[4,13,20] Unfortunately, WSI has some drawbacks, some of which are exacerbated in cytology. The scanners and software are expensive and require training. An individual WSI device currently costs approximately $135,000 with basic viewing software,[21] with some systems being considerably more. In addition to the devices themselves, substantial costs are incurred for labor needed to operate and troubleshoot the scanner, long-term image storage and secure transmission, and other less obvious items such as upgraded monitors for pathologists, image analysis software, and integration of the scanner software with the LIS.[21] Depending on the volume of scanning and the number of sites that need to be served, WSI systems routinely cost hundreds of thousands or even millions of dollars. Scan times have improved considerably but still can be quite lengthy, especially for slides that are required to be digitized at high magnification (e.g. ×40), have a large area to be scanned, and need to be scanned in using multiple planes of focus, as is often the case in cytology. Longer scan times correspond to larger image files, increasing the challenges involved in their storage and transmission. Furthermore, not all WSI devices are capable of scanning multiple planes of focus, while others only allow Z-stack functionality to be used for delimited portions of the virtual slide. Traditionally, scanner manufacturers have focused on histology applications because of these issues. However, as scanning technology improves and servers become capable of handling larger files at lower cost, cytology applications are becoming ever-more feasible. In the future, decreasing barriers and increasing demand should bring about the introduction of more scanners with advanced capabilities suitable for telecytology applications.
Figure 2.
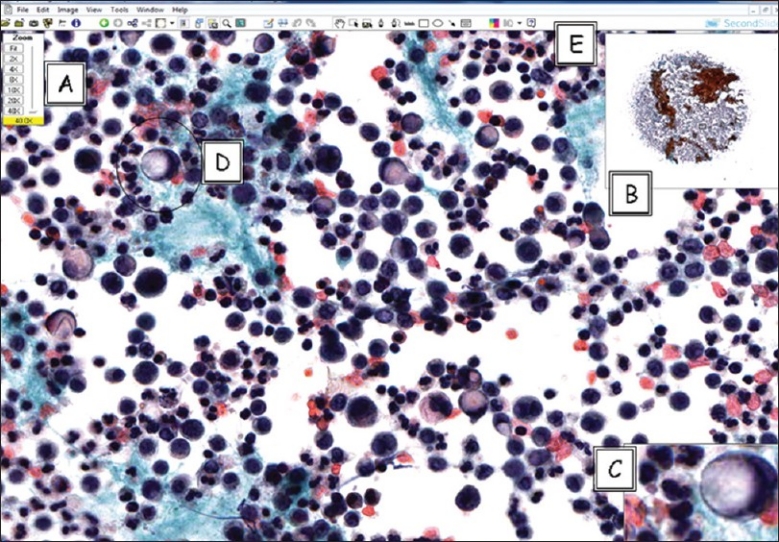
A whole-slide image (digitized slide) of signet-ring carcinoma in pleural effusion illustrating the viewer software provided by the vendor to allow for remote viewing and manipulation of images by the cytopathologist. (A) Zoom slider, (B) thumbnail image, (C) magnified field, (D) circled area is the annotation layer information used to mark up areas of interest, (E) drawing tool bar
CLINICAL APPLICATIONS
Telecytology has been used in niche applications as a part of clinical practices for a number of years. Most of the literature reporting applications of telecytology for patient care revolves around either static images for consultation[22–32] or streaming of live video images for remote immediate assessment.[33–35] Use of remotely controlled (dynamic) robotic systems for telecytology has also been reported.[36–38] Only a few applications of WSI for clinical use have been published to date,[11,38,39] but this technology seems to be spurring renewed interest in research in the area of telepathology.[40]
Studies reporting on the use of static images are typically somewhat older and often are the result of attempts at making cytopathology expertise more readily available either by enabling the transmission of cytopathology materials from areas with limited cytology resources[24,28,29] or by speeding up more routine consult processes.[22] The use of static images has the advantage of being easily implemented. All that is required is a microscope-mounted camera of the type widely used by many pathologists for other functions, and hence is readily available without the need to purchase new equipment. The static images also require only a small amount of computer memory; hence they can be readily transmitted and viewed over existing networks. However, the images produced by these cameras can only capture a tiny fraction of the information contained in a glass slide. This makes the remote viewer entirely dependent on the judgment of the person who captures the images. Unrepresentative images can easily lead to misdiagnosis. Static images are also subject to quality issues, with seemingly minor variations in image parameters (e.g., color, contrast) potentially leading to markedly different interpretations.[41]
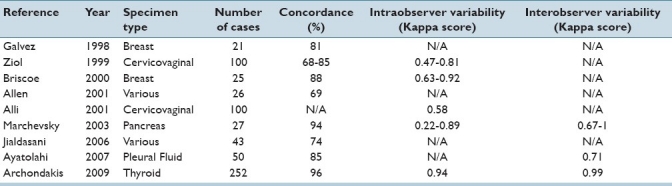
Reported uses of static images for telecytology include gynecologic material (Pap tests)[23,30,42,43] as well as nongynecological specimen types including aspirates of breast,[25,26,31] thyroid,[27,44] and pancreas,[32] as well as pleural fluid specimens.[24] In general, past studies demonstrated good concordance between diagnoses made using still photomicrographs presented on a computer screen when compared to those made following review of the corresponding glass slides [Table 3]. However, several problems were apparent in these studies. Most important was the inability to reliably depict the entire case material with only a few representative photographs. This drawback of static telecytology inevitably led to diagnostic difficulties, since overall cellularity is an important factor in many cases, especially for nongynecologic specimens. Indeed, different studies of breast aspirates showed a tendency toward both false-positive diagnoses[31] and excessive hedging resulting in a tendency to call true positive cases only suspicious.[26] A lack of focus planes also contributed to diagnostic uncertainty. Two separate studies of Pap tests found that small cells, including HSIL and carcinoma, were especially difficult to accurately classify using only a single focus plane.[30,42] A system of Z-stack videos showing areas of interest has been proposed as an alternate way to overcome this difficulty,[20] but so far this has had only very limited application. Another issue is that the most challenging cases, those most in need of expert consultation, tend to perform the most poorly. One study of telecytology consultation found that 9% of telecytology consults cases had a major discrepancy between the initial diagnosis and the final glass slide diagnosis, despite the fact that 11% of the telecytology consult attempts were deferred.[28] Another study found only 48% diagnostic concordance between the telecytology consultant opinion and the opinion of the referring pathologist, with a 31% discrepancy rate between the diagnosis based on static images and the final glass slide diagnosis in those cases where the slides were reviewed.[22] A pilot study marrying static telecytology with automated Pap test slide scanning has also been published.[45] The authors demonstrated that remote interpretation of the pre-selected fields of view selected by the scanning algorithm yielded sensitivity levels comparable to those seen for the same scanning device in prospective trials that did not employ the telecytology component. The goal of the study was to demonstrate the feasibility of using such scanners to provide access to cytology screening in parts of the world that lack cytotechnologists and pathologists. Studies of this kind raise the exciting possibility of exporting cytopathology expertise to the developing world.[46]
Table 3.
Summary of studies using static images for telecytology
A few studies have reported the use of remote robotic microscopy as a means of overcoming the shortcomings of telecytology based on static photographs.[36–38] Remotely controlled slides offer the possibility of real-time (dynamic) simulated microscopy enabling thorough examination of potentially all the material present on slides. One study found that remote robotic examination of pancreas Diff--Quik adequacy slides had an interobserver kappa of 0.61 when compared with the final diagnosis, compared to a kappa of 0.79 for the same slides viewed on site with a conventional microscope.[37] The difference was not statistically significant and most discrepancies occurred early in the study, leading the authors to conclude that with sufficient training and experience this technology could be a viable alternative to live attendance at remote sites.[37] Another study also found promising results with diagnostic concordance rates above 90%.[36] However, this technology is slow and awkward due to the delays involved in sending instructions to the robotic controls from the remote location, then having to wait for the image to refresh before making additional adjustments. This problem is especially acute for specimens that are thick or sparse and require extensive scanning.[36] The equipment is also expensive, often no longer supported by vendors, and has no other uses, unlike the competing technology of WSI.
Another technology that has proven to be popular for telecytology is remote live video microscopy [Figure 3]. Many standard microscope-mounted cameras have the capacity to stream images, making the technology relatively affordable. Studies to date have applied this technology to remote locations within the same medical center,[33–35] allowing cytopathologists to view cases for immediate adequacy purposes collected in suites inconveniently distant from the main laboratory. The findings support the use of the technology, with adequacy interpretation results that are comparable for video telecytology and conventional microscopy, reporting concordance rates in excess of 95%.[33–35] The main drawback of this technology is that the quality of the interpretation depends heavily on the ability of the person who moves the slide and selects the fields of view. Although feedback by telephone can help the consultant guide the remote microscope operator, the system works better if that individual has enough training to locate and recognize critical cells independently.[33,35] Typically, a senior pathology resident, cytopathology fellow, or cytotechnologist is employed for this purpose. Surprisingly, to the best of our knowledge, there are no published reports of the use of video microscopy across institutions for remote consultations. Undoubtedly the technology has been used in this way, but its utility in this setting may be limited by the need to have the requesting and consulting pathologists simultaneously involved in case review, creating cumbersome scheduling problems. Newer technology using high-definition (HD) cameras and secure networking telecommunication solutions offers promising improvements.
Figure 3.
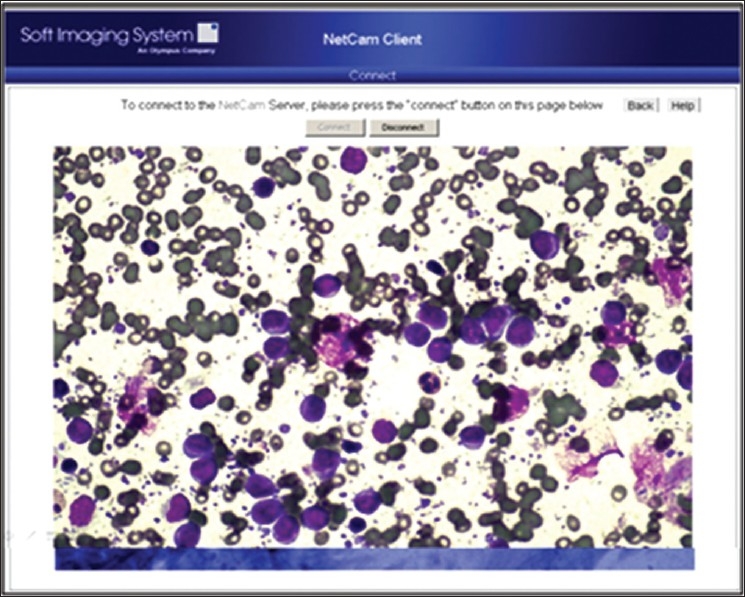
Telecytology using NetCam for on-site evaluation and triaging of a cytology specimen. Case of a lymph node FNA that was suspicious for lymphoma requiring submission of the fresh sample for flow cytometric study
WSI offers the possibility of overcoming many of the aforementioned problems associated with other methods. Since the whole slide is available for review by the remote viewer, issues of field selection and lack of low-power impression are largely nullified. Commercially available scanners capable of producing Z-stack images can also overcome the plane of focus problem. Software for manipulating the virtual image on the computer screen of the viewer eliminates the need for a skilled person on site to move the slide or for the user to struggle with a cumbersome remote robotics application [Figure 4]. Although WSI scanners are presently more expensive relative to standard microscope-mounted digital cameras, they have numerous other potential uses to help justify their cost (e.g. image analysis). The digital cameras incorporated within these WSI scanners are automated and provide consistent images, reducing concerns about image quality. There are very few publications in the literature regarding the use of WSI for clinical telecytology. One study has been published where the investigators report that WSI performed using Pap tests demonstrated excellent performance of the virtual images, with diagnostic accuracy comparable to that glass slides.[39] However, issues of speed in this study were of concern, causing users to prefer the glass slide over the WSI equivalent. A survey of cytotechnologists given WSI virtual slides to examine also found that the slowness of the system was the predominant complaint.[47] WSI manufacturers are currently working on alternative means of displaying and manipulating the images in order to make diagnosis faster and easier, looking to create the pathologist “cockpit” with optimized monitors and control devices.
Figure 4.
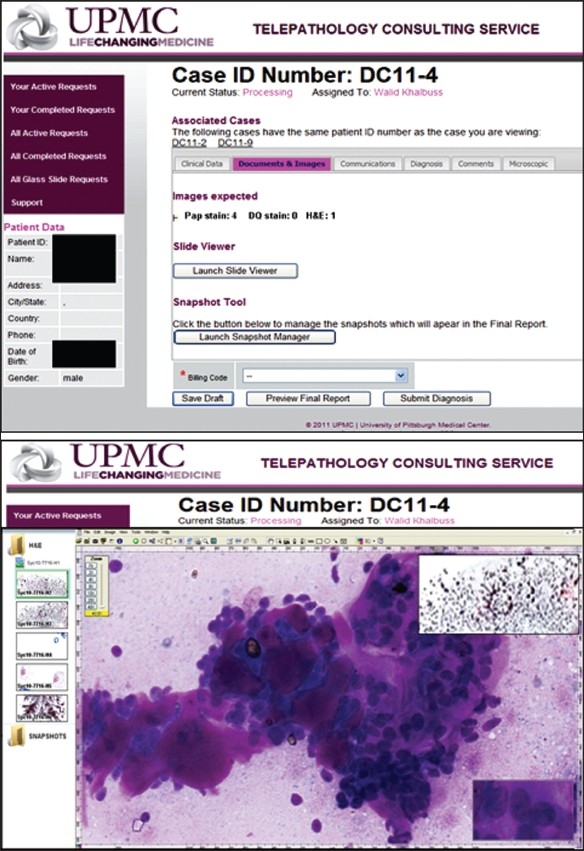
Telepathology portal used by the University of Pittsburgh Medical Center. Upper: Documents and images (static or whole slide) uploaded via the portal are available for review. Lower: Launching ImageScope viewer allows virtual slides to be viewed online
Clearly, a key problem for WSI is the scanning time required for digitizing cytology slides, which at present takes approximately 10 minutes for a conventionally prepared smeared slide at 20× with one focus plane, and more than an hour for multi-plane scanning necessary to generate Z-stack images.[18] This makes immediate consultation difficult due to the delays introduced by the extra scanning step. Although frozen section diagnosis using WSI has been successfully demonstrated,[48,49] the application of WSI for immediate cytology adequacy interpretation on a routine basis will most likely have to wait for significant technological improvements in scanners. Another barrier is the file sizes needed to adequately capture large three-dimensional cytology slides. Such digital image files may be in the hundreds of megabytes to gigabyte range, making their storage and transmission challenging,[18] although this issue seems to be less imposing with the passage of time as compression algorithms continually improve and data storage options become ever cheaper. Information technology restrictions of institutional firewalls and other security issues, in addition to secure routing of private patient metadata information along with standardized DICOM images, will also need to be overcome. Finally, issues related to standardization and validation are extremely important for WSI because of the potential for this technology to completely replace the conventional optical microscope. This has caused the US Food and Drug Administration to consider requiring WSI manufacturers to get approval for their devices before they can be used for clinical purposes. Partly in response to this possibility, the College of American Pathologists (CAP) has developed recommendations for validating WSI systems for routine clinical diagnostic use, including cytology specimens that will require cytology-specific validation.
QUALITY ASSURANCE
Although error is difficult to define in anatomical pathology because much of the interpretation is subjective with high interobserver variability, laboratories are still required to participate in quality assurance (QA) measures, such as the independent review of cases in an effort to identify significant diagnostic errors and take corrective action. For gynecologic cytology, 10% review of Pap tests is required in the USA by CLIA ’88 regulations. Although the CAP publishes a series of checklists to guide cytopathology QA, the specific implementation of such a program is intentionally left flexible to accommodate the wide variety of pathology practices that exist. Typical approaches to cytology QA include a review of cases in a variety of settings such as intradepartmental, interdepartmental, or extradepartmental review and second opinions requested by patients or clinicians.[50,51] Discrepancies should be noted and resolved between pathologists and, if necessary, amended reports or addenda issued. There is good reason behind extensive QA programs in anatomical pathology and cytopathology as it has been estimated that the actual error rate likely ranges from 1% to 5%, and in a study of self-reported discrepancies among 72 institutions, 6.7% of anatomical pathology diagnoses were found to be discrepant at second review.[52] In 1% of these cases, a significant clinical event occurred as a result of these discrepancies.[52] Statistics based upon second review in the same institution are likely to reflect an underrepresentation of errors because traditional case reviews are associated with a number of potential biases, including reviewer knowledge of the original diagnosis and/or the identity of the sign-out pathologist.[15,52]
Therefore, establishing mechanisms for QA case review with truly independent outside pathologists has great appeal as a means of achieving maximal quality improvement impact. A major hindrance to establishing a multifacility QA program, however, is the expense and difficulty of moving and managing slides between facilities, especially if the QA is to be done before or soon after to the sign-out date when the detection of potential errors would be of most benefit to the patient. Automated WSI, in which all the slides in a case are imaged in their entirety at high resolution and made available to cytopathologists on a network, is a modality that may prove useful in cytopathology case review.[44] A digitized case could allow a QA system to hide the identity of the original sign-out pathologist and, if desired, the original diagnosis. More importantly, however, digital slides available on a network can mitigate the problems of glass slide logistics and, by so doing, enable routine multifacility cytopathology QA.[43,51,53]
EDUCATIONAL AND TESTING APPLICATIONS
Digital imaging is beginning to replace the traditional classroom with microscopes in medical education, including cytopathology.[54–56] Digital imaging undoubtedly offers significant advantages over the traditional light microscope in education and training. Cytology glass slides are often irreplaceable and therefore often withheld from teaching sets. Also, the use of glass slides is limited by the fact the colors of stains fade over time, glass slides can be easily broken or lost, the slides can be used only by one person at a time, and a microscope is necessary for viewing. The main advantage of WSI for education is that the images are easily accessible. A web-based virtual slide library can be permanently stored, enabling users to review cytological educational material “anytime, anywhere” without microscopes or glass slides.[57] In contrast, the traditional microscopy classroom is costly to set up and maintain, and high-quality cytology glass slides are impossible to duplicate or replace.[58] WSI image quality has proven to be sufficient for educational purposes.[56,59,60] Virtual slides may be annotated and shared by participants, such as pathology residents before conferences.[55] With digital slide conferences, advantages include improved access to large teaching sets, enhanced annotations, and instant access to linked related clinical data such as radiology images. Due to the increase in usage of WSI in pathology and cytology education, demands for adequate training in digital imaging is becoming an increasingly necessary component of training in pathology residency and cytotechnology training programs. Digitzed slides in cytology can even be employed to support a "virtual" rotation for trainees.[58,61,62]
WSI technology also offers the ability to introduce effective online cytology educational programs and online cytology atlases such as the USCAP Virtual Slide Box, which offers unknown cases in anatomical pathology and cytopathology. The International Academy of Cytology (IAC) recently provided several digital educational materials on their web site, including cases with virtual slides and static images and online lectures, seminars, and workshops. The American Society of Cytology (ASC) has also begun to incorporate WSI into its case studies for continuing education and is building a Virtual Atlas that will be available to members online.[63] It is conceivable that in the near future cytopathlogy testing will be greatly altered by the use of WSI. Virtual slides solve many of the thorniest issues involved in testing diagnostic proficiency. Having a single digital copy of a slide that can be viewed by all test takers not only eliminates the logistical nightmare of maintaining and distributing large sets of glass slides, but also ensures that every test participant is viewing identical images. This greatly reduces the burden of validation and increases fairness and reproducibility, as long as participants are adequately trained to view such images. The American Board of Pathology has already been using virtual slides for a subset of its pathologist certification examination questions for a number of years. Another obvious area for application of this technology would be Pap test proficiency testing mandated in the USA by CLIA ’88.[64] Educational testing, such as the popular Interlaboratory Comparison Program offered by the CAP, would be another logical venue for WSI for the same reasons. Supplemental materials available online for these CAP tests already include additional still images.
CONCLUSIONS
The practice of cytology is evolving rapidly, and cytologists must prepare now for the “digital” tomorrow. In the coming years, several changes such as the advancement of personalized medicine, adoption of standards like DICOM, and the emergence of technological advances like digital pathology will greatly impact how a cytologist performs his/her job. Early efforts to use digital images and the Internet to render diagnoses via telecytology have shown promise despite suboptimal older technology that initially was restricted to reproducing only a tiny fraction of the material on a glass slide. WSI offers the prospect of true virtual microscopy, and may in time even replace glass slides in routine practice. We are rapidly approaching this reality as vendors continue to build newer, faster, and cheaper scanners with sophisticated software to improve digital pathology workflow. The potential for telecytology is only just beginning to be realized. Cytologists can look forward to accessing, reviewing, sharing, and even analyzing the digital data in their “digitized” slides.
Footnotes
Available FREE in open access from: http://www.jpathinformatics.org/text.asp?2011/2/1/51/91129.
REFERENCES
- 1.Yamashiro K, Shinohara T, Mitsuhashi T, Sugimura T, Taira K, Azuma M, et al. Z-axis video for cytology database is a useful tool for the case presentation prior to the cytology training workshop. Diagn Cytopathol. 2011 doi: 10.1002/dc.21760. [In Press] [DOI] [PubMed] [Google Scholar]
- 2.Hedvat CV. Digital microscopy: past, present, and future. Arch Pathol Lab Med. 2010;134:1666–70. doi: 10.5858/2009-0579-RAR1.1. [DOI] [PubMed] [Google Scholar]
- 3.Jara-Lazaro AR, Thamboo TP, Teh M, Tan PH. Digital pathology: exploring its applications in diagnostic surgical pathology practice. Pathology. 2010;42:512–8. doi: 10.3109/00313025.2010.508787. [DOI] [PubMed] [Google Scholar]
- 4.Pantanowitz L. Digital images and the future of digital pathology. J Pathol Inform. 2010;1:pii–15. doi: 10.4103/2153-3539.68332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilbur DC. Digital cytology: current state of the art and prospects for the future. Acta Cytol. 2011;55:227–38. doi: 10.1159/000324734. [DOI] [PubMed] [Google Scholar]
- 6.Daniel C, Rojo MG, Klossa J, Della Mea V, Booker D, Beckwith BA, et al. Standardizing the use of whole slide images in digital pathology. Comput Med Imaging Graph. 2011;35:496–505. doi: 10.1016/j.compmedimag.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 7.Duncan LD, Gray K, Lewis JM, Bell JL, Bigge J, McKinney JM. Clinical integration of picture archiving and communication systems with pathology and hospital information system in oncology. Am Surg. 2010;76:982–6. [PubMed] [Google Scholar]
- 8.Montalto MC. Pathology RE-imagined: the history of digital radiology and the future of anatomic pathology. Arch Pathol Lab Med. 2008;132:764–5. doi: 10.5858/2008-132-764-PRTHOD. [DOI] [PubMed] [Google Scholar]
- 9.Singh R, Chubb L, Pantanowitz L, Parwani A. Standardization in digital pathology: Supplement 145 of the DICOM standards. J Pathol Inform. 2011;2:23. doi: 10.4103/2153-3539.80719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilbertson J, Yagi Y. Histology, imaging and new diagnostic work-flows in pathology. Diagn Pathol. 2008;3(Suppl 1):S14. doi: 10.1186/1746-1596-3-S1-S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steinberg DM, Ali SZ. Application of virtual microscopy in clinical cytopathology. Diagn Cytopathol. 2001;25:389–96. doi: 10.1002/dc.10021. [DOI] [PubMed] [Google Scholar]
- 12.Prayaga AK, Loya AC, Rao IS. Telecytology-are we ready? J Telemed Telecare. 2006;12:319–20. doi: 10.1258/135763306778558141. [DOI] [PubMed] [Google Scholar]
- 13.Wilbur DC, Madi K, Colvin RB, Duncan LM, Faquin WC, Ferry JA, et al. Whole-slide imaging digital pathology as a platform for teleconsultation: a pilot study using paired subspecialist correlations. Arch Pathol Lab Med. 2009;133:1949–53. doi: 10.1043/1543-2165-133.12.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marchevsky AM, Wan Y, Thomas P, Krishnan L, Evans-Simon H, Haber H. Virtual microscopy as a tool for proficiency testing in cytopathology: a model using multiple digital images of Papanicolaou tests. Arch Pathol Lab Med. 2003;127:1320–4. doi: 10.5858/2003-127-1320-VMAATF. [DOI] [PubMed] [Google Scholar]
- 15.Stewart J, 3rd, Miyazaki K, Bevans-Wilkins K, Ye C, Kurtycz DF, Selvaggi SM. Virtual microscopy for cytology proficiency testing: Are we there yet? Cancer. 2007;111:203–9. doi: 10.1002/cncr.22766. [DOI] [PubMed] [Google Scholar]
- 16.Weinstein RS, Graham AR, Richter LC, Barker GP, Krupinski EA, Lopez AM, et al. Overview of telepathology, virtual microscopy, and whole slide imaging: prospects for the future. Hum Pathol. 2009;40:1057–69. doi: 10.1016/j.humpath.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 17.Dee FR, Donnelly A, Radio S, Leaven T, Zaleski MS, Kreiter C. Utility of 2-D and 3-D virtual microscopy in cervical cytology education and testing. Acta Cytol. 2007;51:523–9. doi: 10.1159/000325788. [DOI] [PubMed] [Google Scholar]
- 18.Rojo MG, Garcia GB, Mateos CP, Garcia JG, Vicente MC. Critical comparison of 31 commercially available digital slide systems in pathology. Int J Surg Pathol. 2006;14:285–305. doi: 10.1177/1066896906292274. [DOI] [PubMed] [Google Scholar]
- 19.Kalinski T, Zwönitzer R, Sel S, Evert M, Guenther T, Hofmann H, et al. Virtual 3D microscopy using multiplane whole slide images in diagnostic pathology. Am J Clin Pathol. 2008;130:259–64. doi: 10.1309/QAM22Y85QCV5JM47. [DOI] [PubMed] [Google Scholar]
- 20.Yamashiro K, Taira K, Matsubayashi S, Azuma M, Okuyama D, Nakajima M, et al. Comparison between a traditional single still image and a multiframe video image along the z-axis of the same microscopic field of interest in cytology: Which does contribute to telecytology? Diagn Cytopathol. 2009;37:727–31. doi: 10.1002/dc.21078. [DOI] [PubMed] [Google Scholar]
- 21.Isaacs M, Lennerz JK, Yates S, Clermont W, Rossi J, Pfeifer JD. Implementation of whole slide imaging in surgical pathology: A value added approach. J Pathol Inform. 2011;2:39. doi: 10.4103/2153-3539.84232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allen EA, Ollayos CW, Tellado MV, Butler DR, Buckner SB, Williams BH, et al. Characteristics of a telecytology consultation service. Hum Pathol. 2001;32:1323–6. doi: 10.1053/hupa.2001.29652. [DOI] [PubMed] [Google Scholar]
- 23.Alli PM, Ollayos CW, Thompson LD, Kapadia I, Butler DR, Williams BH, et al. Telecytology: intraobserver and interobserver reproducibility in the diagnosis of cervical-vaginal smears. Hum Pathol. 2001;32:1318–22. doi: 10.1053/hupa.2001.29651. [DOI] [PubMed] [Google Scholar]
- 24.Ayatollahi H, Khoei A, Mohammadian N, Sadeghian MH, Azari JB, Ghaemi MR, et al. Telemedicine in diagnostic pleural cytology: a feasibility study between universities in Iran and the USA. J Telemed Telecare. 2007;13:363–8. doi: 10.1258/135763307782215343. [DOI] [PubMed] [Google Scholar]
- 25.Briscoe D, Adair CF, Thompson LD, Tellado MV, Buckner SB, Rosenthal DL, et al. Telecytologic diagnosis of breast fine needle aspiration biopsies. Intraobserver concordance. Acta Cytol. 2000;44:175–80. doi: 10.1159/000326357. [DOI] [PubMed] [Google Scholar]
- 26.Galvez J, Howell L, Costa MJ, Davis R. Diagnostic concordance of telecytology and conventional cytology for evaluating breast aspirates. Acta Cytol. 1998;42:663–7. doi: 10.1159/000331823. [DOI] [PubMed] [Google Scholar]
- 27.Georgoulakis J, Archondakis S, Panayiotides I, Anninos D, Skagias L, Stamataki M, et al. Study on the reproducibility of thyroid lesions telecytology diagnoses based upon digitized images. Diagn Cytopathol. 2011;39:495–9. doi: 10.1002/dc.21419. [DOI] [PubMed] [Google Scholar]
- 28.Jialdasani R, Desai S, Gupta M, Kothari A, Deshpande R, Shet T, et al. An analysis of 46 static telecytology cases over a period of two years. J Telemed Telecare. 2006;12:311–4. doi: 10.1258/135763306778558132. [DOI] [PubMed] [Google Scholar]
- 29.Yamashiro K, Kawamura N, Matsubayashi S, Dota K, Suzuki H, Mizushima H, et al. Telecytology in Hokkaido Island, Japan: results of primary telecytodiagnosis of routine cases. Cytopathology. 2004;15:221–7. doi: 10.1111/j.1365-2303.2004.00147.x. [DOI] [PubMed] [Google Scholar]
- 30.Ziol M, Vacher-Lavenu MC, Heudes D, Ferrand J, Mayelo V, Molinié V, et al. Expert consultation for cervical carcinoma smears. Reliability of selected-field videomicroscopy. Anal Quant Cytol Histol. 1999;21:35–41. [PubMed] [Google Scholar]
- 31.Della Mea V, Puglisi F, Bonzanini M, Forti S, Amoroso V, Visentin R, et al. Fine-needle aspiration cytology of the breast: a preliminary report on telepathology through Internet multimedia electronic mail. Mod Pathol. 1997;10:636–41. [PubMed] [Google Scholar]
- 32.Marchevsky AM, Nelson V, Martin SE, Greaves TS, Raza AS, Zeineh J, et al. Telecytology of fine-needle aspiration biopsies of the pancreas: a study of well-differentiated adenocarcinoma and chronic pancreatitis with atypical epithelial repair changes. Diagn Cytopathol. 2003;28:147–52. doi: 10.1002/dc.10247. [DOI] [PubMed] [Google Scholar]
- 33.Alsharif M, Carlo-Demovich J, Massey C, Madory JE, Lewin D, Medina AM, et al. Telecytopathology for immediate evaluation of fine-needle aspiration specimens. Cancer Cytopathol. 2010;118:119–26. doi: 10.1002/cncy.20074. [DOI] [PubMed] [Google Scholar]
- 34.Heimann A, Maini G, Hwang S, Shroyer KR, Singh M. Use of telecytology for the immediate assessment of CT guided and endoscopic FNA cytology: Diagnostic accuracy, advantages, and pitfalls. Diagn Cytopathol. 2010 doi: 10.1002/dc.21582. [In Press] [DOI] [PubMed] [Google Scholar]
- 35.Kerr SE, Bellizzi AM, Stelow EB, Frierson HF, Jr, Policarpio-Nicolas ML. Initial assessment of fine-needle aspiration specimens by telepathology: validation for use in pathology resident-faculty consultations. Am J Clin Pathol. 2008;130:409–13. doi: 10.1309/NA7Y7THPTBF112A0. [DOI] [PubMed] [Google Scholar]
- 36.Cai G, Teot LA, Khalbuss WE, Yu J, Monaco SE, Jukic DM, et al. Cytologic evaluation of image-guided fine needle aspiration biopsies via robotic microscopy: A validation study. J Pathol Inform. 2010;1:pii–4. doi: 10.4103/2153-3539.63826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim B, Chhieng DC, Crowe DR, Jhala D, Jhala N, Winokur T, et al. Dynamic telecytopathology of on site rapid cytology diagnoses for pancreatic carcinoma. Cytojournal. 2006;3:27. doi: 10.1186/1742-6413-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Slodkowska J, Pankowski J, Siemiatkowska K, Chyczewski L. Use of the virtual slide and the dynamic real-time telepathology systems for a consultation and the frozen section intra-operative diagnosis in thoracic/pulmonary pathology. Folia Histochem Cytobiol. 2009;47:679–84. doi: 10.2478/v10042-010-0009-z. [DOI] [PubMed] [Google Scholar]
- 39.Evered A, Dudding N. Accuracy and perceptions of virtual microscopy compared with glass slide microscopy in cervical cytology. Cytopathology. 2010 doi: 10.1111/j.1365-2303.2010.00758.x. [DOI] [PubMed] [Google Scholar]
- 40.Della Mea V. 25 years of telepathology research: a bibliometric analysis. Diagn Pathol. 2011;6(Suppl 1):S26. doi: 10.1186/1746-1596-6-S1-S26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pinco J, Goulart RA, Otis CN, Garb J, Pantanowitz L. Impact of digital image manipulation in cytology. Arch Pathol Lab Med. 2009;133:57–61. doi: 10.5858/133.1.57. [DOI] [PubMed] [Google Scholar]
- 42.Eichhorn JH, Brauns TA, Gelfand JA, Crothers BA, Wilbur DC. A novel automated screening and interpretation process for cervical cytology using the internet transmission of low-resolution images: a feasibility study. Cancer. 2005;105:199–206. doi: 10.1002/cncr.21098. [DOI] [PubMed] [Google Scholar]
- 43.Lee ES, Kim IS, Choi JS, Yeom BW, Kim HK, Han JH, et al. Accuracy and reproducibility of telecytology diagnosis of cervical smears. A tool for quality assurance programs. Am J Clin Pathol. 2003;119:356–60. doi: 10.1309/7ytvag4xnr48t75h. [DOI] [PubMed] [Google Scholar]
- 44.Archondakis S, Georgoulakis J, Stamataki M, Anninos D, Skagias L, Panayiotides I, et al. Telecytology: a tool for quality assessment and improvement in the evaluation of thyroid fine-needle aspiration specimens. Telemed J E Health. 2009;15:713–7. doi: 10.1089/tmj.2009.0037. [DOI] [PubMed] [Google Scholar]
- 45.Eichhorn JH, Buckner L, Buckner SB, Beech DP, Harris KA, McClure DJ, et al. Internet-based gynecologic telecytology with remote automated image selection: results of a first-phase developmental trial. Am J Clin Pathol. 2008;129:686–96. doi: 10.1309/GRAV16QP8JR5XTPF. [DOI] [PubMed] [Google Scholar]
- 46.Williams S, Henricks WH, Becich MJ, Toscano M, Carter AB. Telepathology for patient care: what am I getting myself into? Adv Anat Pathol. 2010;17:130–49. doi: 10.1097/PAP.0b013e3181cfb788. [DOI] [PubMed] [Google Scholar]
- 47.Mori I, Nunobiki O, Ozaki T, Taniguchi E, Kakudo K. Issues for application of virtual microscopy to cytoscreening, perspectives based on questionnaire to Japanese cytotechnologists. Diagn Pathol. 2008;3(Suppl 1):S15. doi: 10.1186/1746-1596-3-S1-S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Evans AJ, Chetty R, Clarke BA, Croul S, Ghazarian DM, Kiehl TR, et al. Primary frozen section diagnosis by robotic microscopy and virtual slide telepathology: the University Health Network experience. Hum Pathol. 2009;40:1070–81. doi: 10.1016/j.humpath.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 49.Fallon MA, Wilbur DC, Prasad M. Ovarian frozen section diagnosis: use of whole-slide imaging shows excellent correlation between virtual slide and original interpretations in a large series of cases. Arch Pathol Lab Med. 2010;134:1020–3. doi: 10.5858/2009-0320-OA.1. [DOI] [PubMed] [Google Scholar]
- 50.Allen KA. Evaluation methods for assessing cytotechnology students’ screening skills. Diagn Cytopathol. 2000;23:66–8. doi: 10.1002/1097-0339(200007)23:1<66::aid-dc15>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 51.Kalinski T, Sel S, Hofmann H, Zwonitzer R, Bernarding J, Roessner A. Digital workflow management for quality assessment in pathology. Pathol Res Pract. 2008;204:17–21. doi: 10.1016/j.prp.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 52.Raab SS, Nakhleh RE, Ruby SG. Patient safety in anatomic pathology: measuring discrepancy frequencies and causes. Arch Pathol Lab Med. 2005;129:459–66. doi: 10.5858/2005-129-459-PSIAPM. [DOI] [PubMed] [Google Scholar]
- 53.Bondi A, Pierotti P, Crucitti P, Lega S. The virtual slide in the promotion of cytologic and hystologic quality in oncologic screenings. Ann Ist Super Sanita. 2010;46:144–50. doi: 10.4415/ANN_10_02_07. [DOI] [PubMed] [Google Scholar]
- 54.Allen KA. Implementation of new technologies in cytotechnology education. Cancer. 1998;84:324–7. doi: 10.1002/(sici)1097-0142(19981225)84:6<324::aid-cncr2>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 55.Dee FR. Virtual microscopy in pathology education. Hum Pathol. 2009;40:1112–21. doi: 10.1016/j.humpath.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 56.Romer DJ, Suster S. Use of virtual microscopy for didactic live-audience presentation in anatomic pathology. Ann Diagn Pathol. 2003;7:67–72. doi: 10.1053/adpa.2003.50021. [DOI] [PubMed] [Google Scholar]
- 57.Li L, Dangott BJ, Parwani AV. Development and use of a genitourinary pathology digital teaching set for trainee education. J Pathol Inform. 2010;1:pii–2. doi: 10.4103/2153-3539.63822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fonyad L, Gerely L, Cserneky M, Molnar B, Matolcsy A. Shifting gears higher--digital slides in graduate education--4 years experience at Semmelweis University. Diagn Pathol. 2010;5:73. doi: 10.1186/1746-1596-5-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stewart J, 3rd, Bevans-Wilkins K, Bhattacharya A, Ye C, Miyazaki K, Kurtycz DF. Virtual microscopy: An educator's tool for the enhancement of cytotechnology students’ locator skills. Diagn Cytopathol. 2008;36:363–8. doi: 10.1002/dc.20821. [DOI] [PubMed] [Google Scholar]
- 60.Zwonitzer R, Hofmann H, Roessner A, Kalinski T. Virtual 3D microscopy in pathology education. Hum Pathol. 2010;41:457–8. doi: 10.1016/j.humpath.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 61.Foster K. Medical education in the digital age: Digital whole slide imaging as an e-learning tool. J Pathol Inform. 2010;1:pii–14. doi: 10.4103/2153-3539.68331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Heidger PM, Jr, Dee F, Consoer D, Leaven T, Duncan J, Kreiter C. Integrated approach to teaching and testing in histology with real and virtual imaging. Anat Rec. 2002;269:107–12. doi: 10.1002/ar.10078. [DOI] [PubMed] [Google Scholar]
- 63.Khalbuss WE, Pantanowitz L, Thrall MJ. Whole Slide Imaging: It's Time Has Come for the ASC! ASC Bull. 2011;48:105–9. [Google Scholar]
- 64.Gagnon M, Inhorn S, Hancock J, Keller B, Carpenter D, Merlin T, et al. Comparison of cytology proficiency testing: glass slides vs.virtual slides. Acta Cytol. 2004;48:788–94. doi: 10.1159/000326447. [DOI] [PubMed] [Google Scholar]


