Abstract
Severe neonatal hypernatremia is an important electrolyte disorder that has serious effects. Cerebral venous thrombosis and aortic thrombosis are relatively rare in severe neonatal hypernatremic dehydration. The authors report a case of cerebral venous thrombosis, associated with aortic thrombosis revealed by dehydration in a 9-day-old boy. Diagnostic was performed using Doppler ultrasound and imaging techniques. Thrombosis was resolved after anticoagulation treatment with low-molecular weight heparin for 1 month, and then was substituted by oral anticoagulant. The case report is followed by a review of the literature dealing with clinical, aetiological and therapeutic aspects of neonatal thrombosis.
Background
Thrombotic diseases, either venous or arterial, are uncommon events in neonates. However, this disorder can cause mortality or result in serious morbidity and disability. The pathophysiology of these events, in the context of the neonatal haemostatic system and the importance of both inherited and acquired prothrombotic disorders, remains poorly defined. In our case the most important risk of the development of thrombosis is dehydration. The management of neonatal thrombosis is extrapolated largely from data in adults. Little information is available on management strategies or the efficacy and safety of therapeutic agents, and there is a clear need to investigate these issues.
Case presentation
A 9-day-old male baby was admitted to our neonatal intensive care unit because of lethargy, breast refusal and hypotonia. His mother did not have any medical care during her pregnancy. He was delivered with delayed cry but no cyanosis. There was no history of umbilical artery catheterisation. The child was the fourth born of non-consanguineous parents and there was no family history of thrombosis.
On the 5th day postnatally, the baby developed a lethargy and refused the breast. He was brought to our hospital because of dehydration. Physical examination revealed a hypotonic infant, weighing 3800 g, with signs of severe dehydration. His eyes were sunken, his lips and skin were remarkably dry. Furthermore, the pertinent features on examination were that the right femoral pulses were poorly palpable and a systolic blood pressure gradient of 30 mm Hg was measured between his upper and lower right limbs. There was no hypertension. No purpuric skin rash was present. The rest of the clinical examination was within normal limits.
Investigations
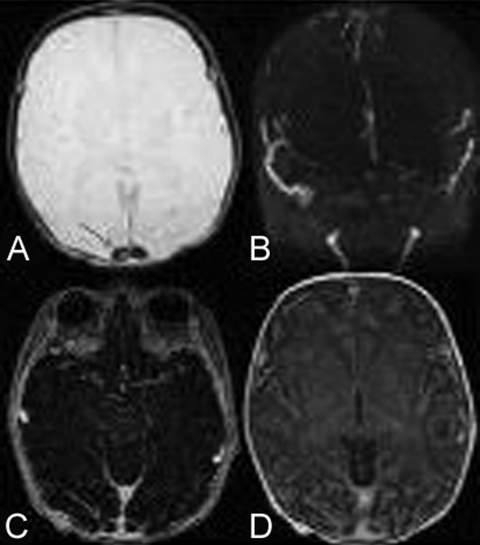
Serum sodium level was 158 meq/l, potassium 3.8 meq/l, calcium 2.95 µmol/l, creatinine 212 µmol/l, urea 95.5mmol/l, haemoglobin 16.8 g/dl, white count 23670/mm3 with 65% neutrophils, platelets 56000/mm3. Diagnosis of cerebral sinovenous thrombosis was suspected after using cranial ultrasound through the anterior fontanel, and confirmed with MRI (figure 1).
Figure 1.
MRI axial sections showing superior sagittal sinus thrombosis in different sequences T2 (A), 2D time-of-flight (B), spoiled gradient recalled (C) and T1 injected (D).
On echocardiography, the aorta was normal in size with no evidence of thoracic coarctation. Abdominal Doppler ultrasound showed bilateral increased renal parenchymal echogenicity without renal venous thrombosis, but a large thrombus was noted in the abdominal aorta extending from below the origin of ostium renal artery to the right common iliac artery. The left aortic branches were not involved.
The specific investigations of thrombophilic state (AT III, protein C, protein S) were normal for the baby and his mother.
Differential diagnosis
In this case, the most differential diagnosis is the thrombophilic disorders (AT III, protein C and protein S).
Treatment
The child was treated for severe hypernatremic dehydration and acute renal failure with appropriate intravenous fluid and electrolyte therapy.
The low molecular weight heparin was started for thrombosis with a dose of 1.5 mg/kg/dose administered subcutaneously. He began to pass urine and, by the end of third day, electrolytes disorders and creatinine concentrations returned to normal.
For the difficulty in administering heparin at home, the treatment was substituted with oral anticoagulant (acenocoumarol) at 0.12 mg/kg/d.
Outcome and follow-up
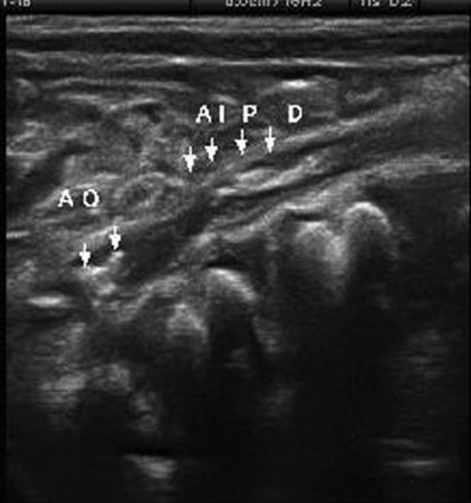
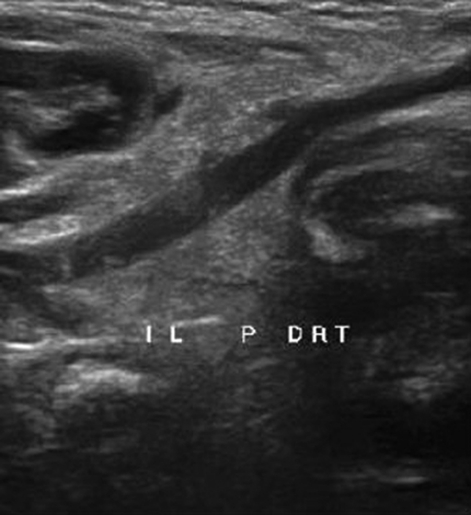
On 32nd day, the child was discharged with oral anticoagulant. After 1 week, a control MRI revealed recanalisation of superior sagittal sinuses. For aortic thrombosis, we noted on Doppler ultrasound a persistence of small thrombus in aorta and partial thrombus in the right common iliac artery (figure 2). One month later, no evidence of thrombus existence was noted, but the small thrombus in aorta was calcified (figure 3).
Figure 2.
Doppler ultrasound showing a persistence of small thrombus in aorta and partial thrombus in the right primitive iliac artery.
Figure 3.
Doppler ultrasound obtained a month after discharge detected recanalisation of right primitive iliac artery.
Discussion
Neonatal thrombosis is a serious event that can cause high morbidity and mortality. Neonates and infants, less than 1 year of age, account for the largest proportion of thrombotic events during the paediatric age.1
The published literature on neonatal thrombosis consists largely of case reports and small, single centre case series. Recently, however, two neonatal registries from Canada and Germany have collected prospective data on unselected cases from multiples centres.2 3 In the Canadian registry, the incidence of clinically apparent thrombosis was 2.4/1000 admissions to neonatal intensive care units; whereas, in the German study, symptomatic thrombosis was recorded in 5.1/100000 births.
The most important risk factor of the development of thrombosis in both studies was related to the use of intravascular catheters. Other risk factors were identified, including asphyxia, septicemia, dehydration and maternal diabetes. In our case, dehydration was responsible of thrombosis, and it was also secondary to maternal lactation failure, poor fluid intake and increased insensible water loss.
Severe hypernatremic dehydration, in association with cerebral venous thrombosis, has been reported infrequently in the literature. In the neonates, the main neurological manifestations of cerebral venous thrombosis are decreased level consciousness, headache and local neurological signs such as hemiparesis and cranial nerve palsies. In contrast, the primary neurological manifestations in the neonates are seizures and diffuse neurological signs such as lethargy and hypotonia like in our case. The frequency of diffuse neurological signs means that clinicians must have a high index of suspicion for cerebral venous thrombosis in neonates with severe hypernatremic dehydration and ask for imaging techniques.
Initially, abdominal ultrasound was done to search renal venous thrombosis because of its frequency in the context of dehydration, and was not detected, but we discovered a large aortic thrombosis. This event is mainly rare. Nouri et al report a major aortic thrombosis revealed by dehydration in a 10-day-old girl.4 Neonatal aortic thrombosis is most commonly precipitated by catheter placement. Other aetiologies were associated with inherited prothrombotic risk factors such as hyperhomocysteinemia caused by the abnormal methylene tetrahydrofolate reductase enzyme.5
In our patient, this association of cerebral venous and aortic thrombosis is particularly exceptional, and was not described in the literature. In this way, the thrombophilic investigations either in a child or his mother were performed, and became normal.
The management of neonatal thrombosis has no special guidelines. Different approaches vary depending on the location and extent of the thrombus. The approach to an individual infant must balance the risks and benefits. A reasonable strategy is to manage asymptomatic thrombosis by close monitoring of the size of thrombus and by providing supporting care.
Severe symptomatic thromboembolic events are typically treated with anticoagulants and/or fibrinolytic agents. Surgical thrombectomy is rarely performed in newborn. In general, this procedure is limited by the small size of blood vessels and the clinical instability of newborns with thrombosis.
Recommendations suggest that unfractionated heparin remains the most frequently used anticoagulant, although there is increasing experience with low-molecular weight heparin in this age group.1 In our patient, heparin for 32 days was sufficient for the recanalisation of the sinus, and was substituted with oral anticoagulant before discharge. The permeability of the aorta was obtained 1 month later.
Learning points.
-
▶
Neonates and infants, less than 1 year of age, account for the largest proportion of thrombotic events during the paediatric age.
-
▶
Severe hypernatremic dehydration in association with cerebral venous thrombosis has been reported infrequently in the literature.
-
▶
The diagnosis of cerebral venous thrombosis needs a high index of clinical suspicion in severe neonatal hypernatremia presenting diffuse neurological signs. Other location of the thrombus must be looked for.
Footnotes
Competing interests None.
Patient consent Obtained.
References
- 1.Seth T. Thrombosis in neonates and children. E J Med 2009;14:36–45 [Google Scholar]
- 2.Shmidt B, Andrew M. Neonatal thrombosis: report of a prospective Canadian and international registry. Paediatrics 1995;96:939–43 [PubMed] [Google Scholar]
- 3.Nowak Gottl U, Von Kries R, Gobel U. Neonatal symptomatic thromboembolism in Germany: two year survey. Arch Dis Child Fetal Neonatal 1997;76:163–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nouri S, Mahdhaoui N, Beizig S, et al. [Major neonatal aortic thrombosis: a case report]. Arch Pediatr 2007;14:1097–100 [DOI] [PubMed] [Google Scholar]
- 5.Sherman GG, Münster M, Govendrageloo K, et al. Low molecular weight heparin in the successful treatment of a spontaneous aortic thrombosis in a neonate. Pediatr Hematol Oncol 2000;17:409–13 [DOI] [PubMed] [Google Scholar]