Abstract
Many synthetic drugs reported to be used for the treatment of inflammatory disorders are of least interest now a days due to their potential side effects and serious adverse effects and as they are found to be highly unsafe for human assistance. Since the last few decades, herbal drugs have regained their popularity in treatment against several human ailments. Herbals containing anti-inflammatory activity (AIA) are topics of immense interest due to the absence of several problems in them, which are associated with synthetic preparations. The primary objective of this review is to provide a deep overview of the recently explored anti-inflammatory agents belonging to various classes of phytoconstituents like alkaloids, glycosides, terpenoids, steroids, polyphenolic compounds, and also the compounds isolated from plants of marine origin, algae and fungi. Also, it enlists a distended view on potential interactions between herbals and synthetic preparations, related adverse effects and clinical trials done on herbals for exploring their AIA. The basic aim of this review is to give updated knowledge regarding plants which will be valuable for the scientists working in the field of anti-inflammatory natural chemistry.
Keywords: Alkaloids, anti-inflammatory agents, cannabinoids, clinical trials, glycosides, herbals, inflammation
INTRODUCTION
The word “inflammation”, derived from the latin word “inflammare”, (to set on fire), is a complex biological process including several chemical mediators which are induced by vascular tissue of the body, when it comes in contact with several harmful stimuli like pollens, irritants, pathogens, and damaged cells. It provides a protective comeback that helps in healing of tissues. Sometimes, inflammation seems to produce events that are quite serious and become chronic like occurrence of rheumatoid arthritis and hay fever which may be life threatening.[1,2] Hence, proper representative measures are to be taken against it. In brief, inflammations are generally of two types: acute inflammation and chronic inflammation. The pathological changes, causes, mediators and threat observed in acute and chronic inflammations are enlisted in Table 1.
Table 1.
Brief account on types of inflammation

Inflammations are generally characterized by certain regular events such as redness, swelling, heat, pain, and at certain times lead to exudation and loss of function. The process of inflammation involves several events and mediators which are potent chemical substances found in the body tissues, such as prostaglnadins, leukotrienes, prostacyclins, lymphokines, and chemokines like interferon-α (IFN-α), γ, interleukin (IL)-1, IL-8, histamine, 5-hydroxytryptamine (5-HT), and tissue necrosis factor-α [Figure 1].[3] These mediators produce several chemical pathways and events to evoke a complementary response against external stimuli. Studies show that in a very few cases, inflammations are tolerable; but in almost 99% of cases, inflammations seem to be severe and intolerable, and if not treated properly with initial first aid along with proper diagnosis and drug therapy, they may lead to loss of life. Examples of some diseases where inflammations are quite harmful include asthma, rheumatoid arthritis, vasculitis, and glomerulonephritis.[4,5] Hence, drug therapy used against inflammation must be satisfactory enough to decrease its severity. Till date, synthetic drugs have been used widely to treat inflammations and related diseases in a fastrack way. But according to various clinical studies, these synthetic molecules are no longer safer. Reports suggest almost 90% of the drugs used against inflammation produce drug related toxicities, iatrogenic reactions, and adverse effects complicating the treatment process.[6–8] Hence, a shift in the area of anti-inflammatory treatment has been observed from the use of synthetics to natural therapy. Various disorders born out of inflammation are enlisted briefly in Table 2.
Figure 1.
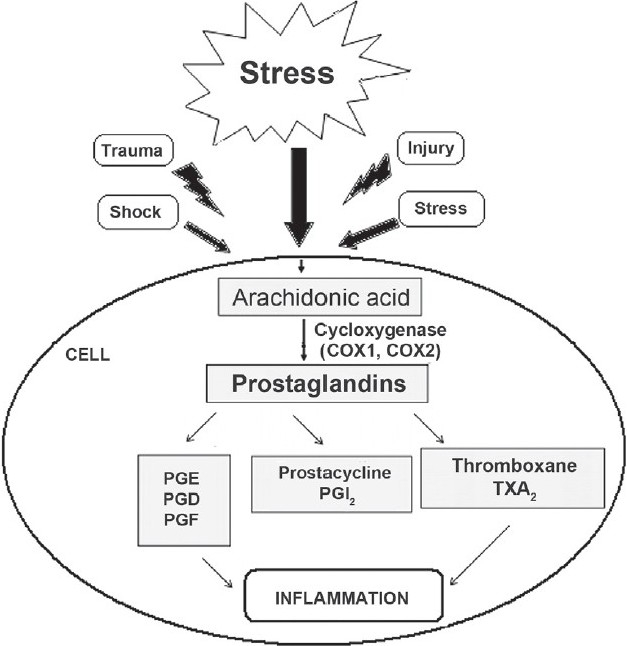
Overview of inflammation process and synthesis of mediators of inflammation like prostaglandins, prostacyclins and leukotrienes
Table 2.
Brief account on anti-inflammatory agents having potential drug related toxic effects
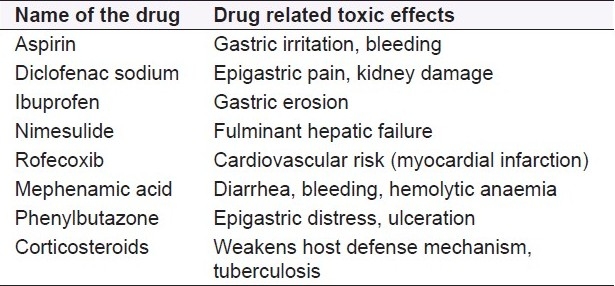
Anti-inflammatory drugs of synthetic origin are classified as steroidal and nonsteroidal anti-inflammatory agents. The origin of these chemical compounds started when salicylates were isolated from leaf extract of willow bark Salix alba and were potentially used by the people of North America in 200 BC and regarded as first generation anti-inflammatory agents.[9] Following this first anti-inflammatory agent, acetyl salicylic acid was synthesized and, likewise, other synthetic compounds came into existence thereof. Examples of such agents include propionic acid derivatives like ibuprofen, flurbiprofen, naproxen; anthranalic acid derivatives like mephenamic acid, oxicam derivative piroxicam, pyrrole, indole; and pyrrazolone derivatives like ketorolac, indomethacin, and phenylbutazone.[10] The second- and third-generation compounds with preferential and selective cyclooxygenase (COX2) inhibitory activities, like nimesulide, nabumetone, celocoxib, rofecoxib, valdecoxib, etoricoxib, were discovered. Apart from the nonsteroidal drugs, various corticosteroids such as hydrocortisone, betamethasone, and beclomethasone which are primarily used as anti-inflammatory agents. Like nonsteroidal anti-inflammatory drugs (NSAIDs), the steroidal agents are also found to have several adverse effects that include hirsutism, Cushing's habitus, hypersensitivity reactions, peptic ulceration, hyperglycemia, osteoporosis, and immunodeficiency related problems.
In a study conducted by World Health Organization (WHO), none of these compounds were found to be safer, as they are associated with a series of unacceptable findings like drug-related or drug-induced toxic effects, and cause harmful adverse effects and secondary effects on long-term use.[11–13] Some common side effects of these synthetic drugs include gastric irritation, ulceration, bleeding, renal failure, interstitial nephritis, hepatic failure, headache, thrombocytopenia, hemolytic anaemia, asthma exacerbation, skin rashes, angioedema, and pruritis. Hence, this approach for treatment of inflammatory diseases by herbal drugs has keen interest to the researchers. From the study made globally, it has been known that the market for use of herbal drugs in the treatment of inflammatory diseases constitutes 83% worldwide and is expected to reach a value of around more than 95% in the forthcoming years due to increased acceptability of these preparations.[14–17]
The field in which plant-based anti-inflamatory agents are being explored as a potential alternative tool in this era of 21st century has given rise to several varieties of beneficial compounds isolated from plants. These include the substances belonging to various classes of phytopharmaceuticals like alkaloids, glycosides, terpenoids, polysaccharides, flavonoids, phenolic compounds, cannabinoids, steroids, fatty acids, plant extracts and agents derived from marine organisms, and terrestrial plants.
PHYTOCONSTITUENTS RESPONSIBLE FOR ANTI-INFLAMMATORY ACTIVITY
Alkaloids
Alkaloids are the basic nitrogenous compounds shown to have marked physiological action when given in low concentration. The important plant families containg alkaloids responsible for anti-inflammatory activities (AIAs) include Solanaceae, Leguminosae, Apocynaceae, Liliaceae, Papaveraceae, Rutaceae, and Ranunculaceae.
One of the most promising compounds is tetrandine, which is chemically a bisbenzylisoquinoline alkaloid natural analogue of berbamine [Figure 2], and is extracted from the tuberous roots of creeper Stephania tetrandra S. Moore (Menispermaceae). It is used for the treatment of rheumatic diseases and is also efficacious in silicosis associated with inhibition of collagen synthesis, inhibits neutrophil and monocyte locomotion and lymphocyte transformation. Its AIAs are due to inhibition of both COX and lipooxygenase (LOX) pathways of inflammation. Like tetradine, berbamine is also found to have significantly greater inhibitory activity on prostaglandin E2 (PGE2) generation, with stronger inhibitory effects on natural-killer cell population. Tetrandine also suppresses the release and activity of inflammatory cytokines, lipid mediators, histamine as well as has an inhibitory capacity on monocytes to produce tumor necrosis factor (TNF)-α. Thus, tetrandrine is found as a prototypic tool compound for the development of new class of anti-inflammatory agents.[18,19]
Figure 2.
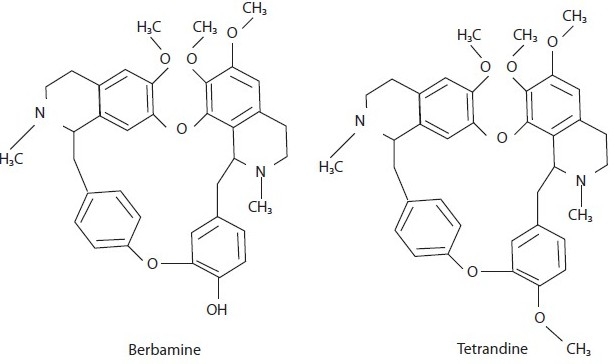
Bisbenzylisoquinoline alkaloid derivatives with antiinflammatory activity
Extract of Buxus papillosa (Buxaceae) is specially used in rheumatism and skin diseases. Likewise, the extracts of Buxus senzpervirens, used as a folk medicine in Turkey, have now regained potential interest for their anti-inflammatory action.[20,21] The ethanolic extract of Adhatoda vasica has prominent activity against inflammations in cotton pellet induced granuloma model of inflammation.[22] Similarly, a report by Rajput et al, on the alcoholic and aqueous extracts of the same plant also shows the extracts to have a considerable dose-dependent AIA against several animal models of inflammation.[23]
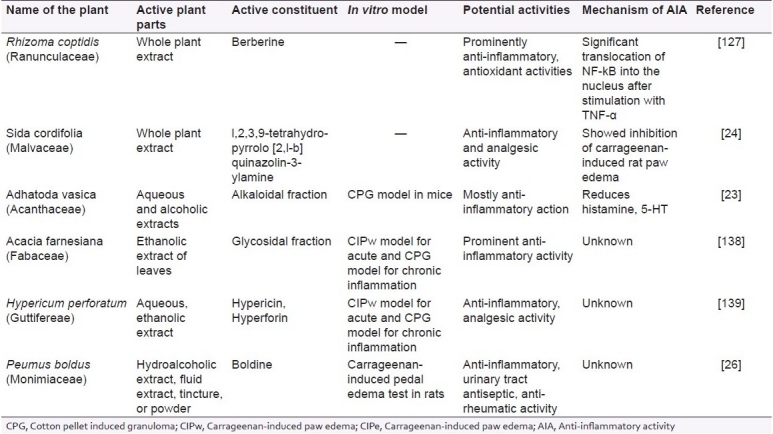
Newer alkaloids isolated from the extract of Sida cordifolia (Malvaceae) have shown high value of AIA when tested in several animal models like acetic acid induced writhing model, tail-flick latency in radiant heat tail-flick method in mice, and carrageenan-induced rat paw edema.[24] In Table 3, recent updates of several other alkaloids having prominent AIA are enlisted.
Table 3.
List of alkaloidal drugs used for treatment of inflammation
Glycosides
Like alkaloids, glycosides also possess potential AIA. These are found indigenously in the following families: Leguminosae, Scrophulariaceae, Polygonaceae, Solanaceae, and Myrsinaceae.
Gomes and coworkers reported that the glycosidal fraction isolated from the leaves of Muesa chisia (Myrsinaceae) have profound pharmacological properties for curing inflammation. The activity was found to be mainly due to the aglycone tetrahydroxy triterpene of the oleanene series present in its glycosidal fraction. Similarly, M. chisiu is shown to have AIA resembling that of synthetic compounds like aspirin, phenylbutazone, indomethacin, in several animal models like carrageenan-induced pedal edema in rat, cotton pellet granuloma, formaldehyde-induced arthritis, and Freund's complete adjuvant induced polyarthritis.[25] Akin to this, glycosides isolated from Harpagophytum procumbens (Pedaliaceae), a perennial herb indigenous to South Africa, were evaluated for their prominent AIA in carrageenan-induced pedal edema.[26]
Organic extract isolated from the aerial parts of Hypericum perforatum and Hypericum reflexum (Hypericaceae) using solvents like chloroform, methanol with subsequent fractionation, when examined for AIA showed significant restrain of acetic acid induced writhing in mice and formalin-induced pain, tail-flick test in rats.[27]
In addition to this, the phenolic glycosides isolated from the bark of Hydnocarpus annamensis were found to contain significant inflammation restraining components in its extract under spectroscopic studies. They were likely to be phenolic glycosides I and II.[28]
Similarly, profound research done on hexane and ethyl acetate fractions of the methanol extract of Dystaenia takeshimana (Umbelliferae) showed significant COX2, 5-lipoxygenase (5-LOX), prostaglandin D2 (PGD2) and leukotriene C4 (LTC4) inhibitory activity when examined in mouse bone marrow-derived mast cells. The spectroscopic and nuclear magnetic resonance (NMR) studies concluded that the probable constituents responsible for such activity may be coumarins, phenethyl alcohol derivatives, and two major steroidal principles, beta-sitosterol and dacusterol.[29]
Likewise, methanolic extract of leaf extracts isolated from the chromatographic fractionation of plant Andrographis stenophylla (Acanthaceae) suggested promising AIA against acute pedal paw edema in rats induced by carrageenan and Freund's adjuvant. Other uses of the extract is for treatment of snake bite caused by Naja naja venom, which causes cardiotoxicity, neurotoxicity and hemorrhagic events.[30]
Aesculushippo castanum (Hippocastanaceae), when assessed for its AIA on CoCl2-induced inflammation and hypoxia in human vascular endothelial cells, was found to give hopeful results due to the presence of a triterpenoid saponin glycoside, aescin.[31]
Xu et al, reported six different novel triterpenoid saponins which were found to have significant inflammation retarding activity when tested in xylene-induced mouse ear edema. These existed indigenously in roots of Codonopsis lanceolata (Campanulaceae). Among these, codonolaside I-III are of recent interest.[32]
Terpenoids
Terpenoids are spread in several families of the plant world. The major plant families with terpenoids showing AIA include Umbelifereae, Lamiaceae, Taxodiaceae, Capparidaceae, Cucurbitaceae, Burseraceae, and Asteraceae, which have significance in the area of the research.
Sesquiterpenes derived from the leaves and barks of Artemisia species like Artemisia annua and Artemisia sinica (Compositae) are of considerable interest nowadays due to several uses such as anti-inflammatory and antimalarial activity. Major constituent responsible for AIA includes diterpenoidal saponins like artemisin and artemisinin [Figure 3]. Similarly, the extract isolated from the bark of Artemisia asiatica contains abundant amount of sesquiterpene lactones such as artemisolide, which exhibited inhibitory action against nuclear factor kB (NF-kB) cells in LPS-induced inflammation in macrophage RAW 264.7 cells, on lipopolysaccharide (LPS)-induced PGE2 and on nitric oxide (NO) production effectively.[33]
Figure 3.

Sesquiterpeniods isolated from Artemisiaspecies
Dried rhizomes of Curcuma longa, Curcuma ambada and several other species of this family have been found to contain potential chemical constituents responsible for several useful pharmacological actions like antioxidant, antiallergic, AIA, and anticancer activities. Constituents like curcumin and zingiberene are found to be responsible for their prominent activities. Recently, research reported that Curcuma xanthorrhiza, Curcuma domestica and Zingiber cassumunar are of vital importance due to the presence of highly active constituent.[34]
Similarly, ethanolic extract of flowers of Arnica montana (Asteraceae) was found to contain newer anti-inflammatory principles like 1,5-trans-guaianolide on NF-kB EMSA cells and in the IL-8 ELISA cells in vitro as well as in vivo [Table 4].[35]
Table 4.
List of terpenoids as potential anti-inflammatory agents
Aqueous ethanolic extracts of Lavandula multifida (Lamiaceae), a Moroccan traditional plant investigated for its activity in croton oil induced ear edema in mice followed by bioassay of the extract, was found to inhibit the inflammatory response positively in comparison to that of indomethacin.[36]
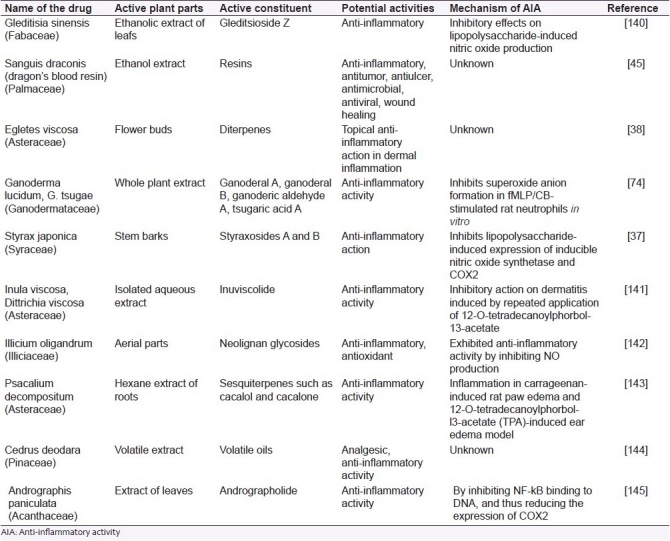
Constituents like styraxosides A and B [Figure 4], and lignans like eugenol and masutakeside I present abundantly in the stem barks of Styrax japonica (Styraceae), when subjected to preliminary screening for AIA in several models, showed potential AIA in LPS-induced NO and PGE2 production by the RAW 264.7 macrophage cell line. The activity of the plant may be probably due to its styraxoside A content, which is a potent inhibitor of NO and PGE2 production and responsible for release of TNF-α and IL-1β.[37]
Figure 4.
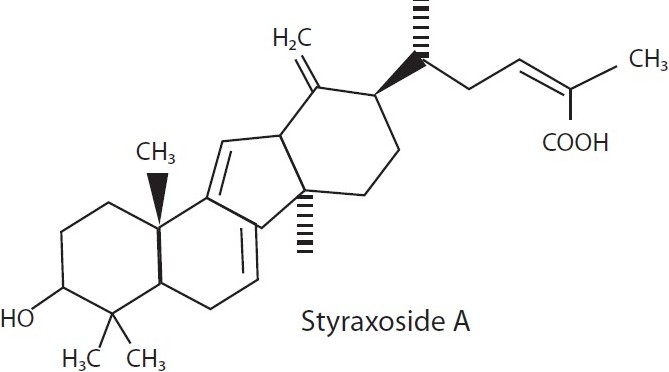
Styraxoside from Styrax japonica
Diterpenes present in the floral buds of Egletes viscosa (Asteraceae) have a topical AIA in dermal inflammation induced in mouse ear model due to the presence of centipedic acid and tanabalin.[38]
Sesquiterpenoids isolated from the whole plant extracts of Youngia japonica belonging to the family Asteraceae are now reported to exhibit a wide spectrum of AIA at doses of 50 mg/kg (i.p.) in mice.[39]
Resins
Boswellia serrata (salai guggal) belonging to the family Burseraceae was found to possess significant AIA. When the ethanol extract of resin was injected in vivo against carrageenan-induced pedal edema in rats, mice and in adrenalectomized rat model, it showed prominent AIA, along with anti-arthritic activity against formaldehyde- and adjuvant-induced arthritis in rats. The probable mechanism may be inhibition of leukotriene production.[40] In support to this, Ammon et al, reported AIA due to inhibition of leukotriene production in calcium-induced stimulation of 5-LOX in rat peritoneal polymorphonuclear (PMN) leukocytes in vitro.[41]
Recently, research done on several other species of Boswellia sp. like Boswellia carterii, commonly known as “Ruxiang Gummi olibanum”, revealed it to have a wider range of AIA in randomized, double-blinded clinical trials conducted by injecting oleoresin extract along with Complete Freund's adjuvant (CFA).[42]
Plants belonging to the Burseraceae family are reported to contain AIA due to presence of oleogum resin, boswellic acid. The action may be probably due to the inhibition of 5-LOX, leukocyte elastase enzyme and oxygen radicals competitively. Several uses of these plants include in autoimmune inflammatory diseases like Crohn's disease, ulcerative colitis, rheumatoid arthritis and bronchial asthma.[43]
Orhan et al, worked on the aqueous and ethanolic extracts of oleo gum resin obtained from Pistacia vera (Anacardiaceae). The extract evoked promising AIA on carrageenan-induced hind paw edema model in mice due to α-pinene content.[44]
Resin obtained from dragon's blood (Sanguis draconis) and from Daemonorops draco (Palmae) showed significant results for AIA and are used as a traditional medicine. Choy et al, reported that the ethanol extract of resin when tested against inflammation induced by LPS on RAW 264.7 cells showed positive AIA. The suggested mechanism may involve inhibition of NO and PGE2 by selective down-regulation of intrinsic nitric oxide synthetase (iNOS) and inhibition of COX2 gene expression via the suppression of NF-kB activation.[45]
In a study conducted by Seo and co-workers, where they worked on a resinous exudate obtained from the plant Pinus densiflora (Pinaceae), it was found that the resin is highly efficacious in treatment against inflammation along with several inflammatory diseases like periodontitis and gingivitis. Upon in vitro evaluation, the extract exhibited strong AIA against human gingival fibroblasts, with the strongest scavenging activity on superoxide anion radicals and on 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radicals, and inhibition of hyaluronidase. When evaluated in animal models like arachidonic acid induced ear edema in mice and acetic acid induced writhing response test in mice, it exhibited 100% therapeutic value for counteracting inflammation, compared to that of the test drug, aminopyrine, whose activity was upto 76.9%.[46]
Essential oils
Essential oils obtained from the roots of Carlina acanthifolia showed promising anti-inflammatory and antimicrobial activities against gram-positive bacteria. In vivo studies showed prominent AIA in a dose-dependent manner, which was comparable to that of indomethacin on carrageenan-induced rat paw edema model.[47]
Similarly, the essential oils of Cordia verbenacea (Boraginaceae) were found to reduce the carrageenan-induced paw edema in rats induced by bradykinin, substance-P, histamine, and platelet-activating factor (PAF) in mice. The major constituents of oil include sesquiterpene compounds like α-humulene and trans-caryophyllene.[48]
Gaultheria yunnanensis (Ericaceae) contains analgesic and anti-inflammatory principles due to the presence of active constituent, gaultherin [Figure 5] (methyl salicylate diglycoside), and exhibited promising AIA in rats and against inflammation induced by carrageenan and croton oil in mice ear.[49]
Figure 5.
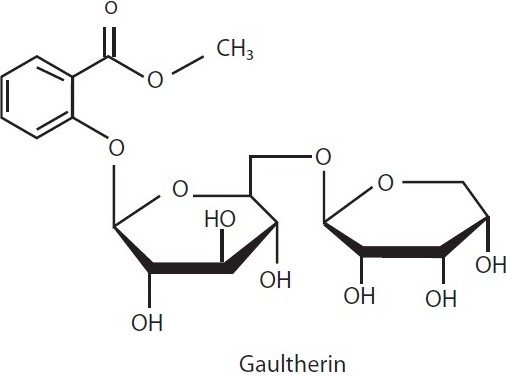
Anti-inflammatory principle isolated from extract of Gaultheria yunnanensis
Caryophyllene, thujopsene, α-humulene, β-acoradiene, germacrene-D, bicyclogermacrene, calamenene, germacrene-B, spathulenol and globulol are the major essential oils found abundantly in the leaf extract of [Figure 6] Casearia sylvestris, and were found to be responsible for AIA. When tested in vitro, it showed a significant 36% reduction in paw size volume elicited by carrageenan in rats; when evaluated for gastric ulcers, it was found to reduce 90% of stress-induced gastric ulcers, as compared to cimetidine which inhibits only upto 70%.[50]
Figure 6.
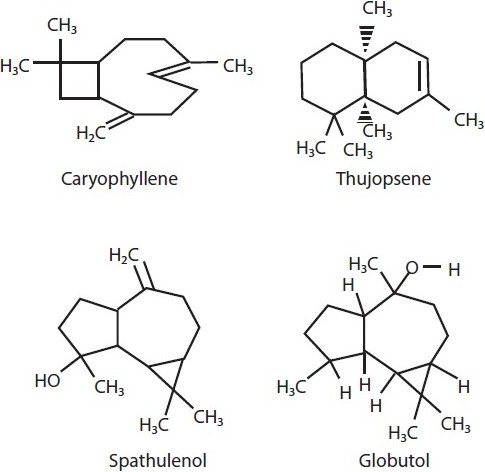
Compounds isolated from the volatile oil extract of Casearia sylvestris
Essentials oils isolated from the n-hexane, chloroform, and methanol extracts of leaves of Rosmarinus officinalis (Labiatae) showed promising AIA when evaluated against croton oil induced inflammation in mice, topically in a dose-dependent manner which is comparable to that of indomethacin. Upon bioassay, it was demonstrated that the activity may be probably due to the presence of major active constituents like ursolic acid, oleanolic acid and micromeric acid.[51]
Polysaccharides
The plants Echinacea purpurea and Echinacea angustifolia (Asteraceae) have been known since earlier days due to their immunostimulating and skin-repairing properties. Aqueous fractions obtained from the roots of these were found to have promising AIA in the skin of mice subjected to croton oil induced inflammation, due to the presence of echinacin, a polysaccharide found in high molecular weight fraction of the extract.[52]
Comaruman, a pectic polysaccharide extracted from the aerial parts of Comarum palustre (Rosaceae), has shown promising AIA when given orally. Comaruman was found to reduce the edema observed for 24 h after injection of 2% formalin in mice paw. In addition to this, the polysaccharide also activates adhesion of peritoneal leukocytes in vitro for AIA.[53]
Artemisia tripartita (Asteraceae) showed AIA due to the presence of polysaccharides which alter macrophage function, neutrophil count, and complement fixation function due to the presence of sulfated polysaccharides like xylose, glucose, arabinose, galactose and galactosamine.[54]
Flavonoids
Kaempferol-di-coumaroyl-glucoside is the flavonol glycoside found abundantly in the extract of Quercus itex (Fagaceae). It showed prominent inhibitory activity against inflammation when applied topically. Prior evaluation for its in vitro activity in several animal models by inducing inflammatory response and dermatitis by phorbol ester (croton oil) gave satisfactory results.[55]
El-Ghazaly et al, studied that salicin is the major constituent of Populus tremula (Salicaceae) responsible for AIA using chorion allantoic membrane of hen's egg.[56]
Flavonoids like quercetin, morin, oleanolic acid, ursolic acid, glycyrrhetic acid, and caffeine were found to suppress ear edema in the inflammation induced using 12-O-tetradecanoylphorbol-13-acetate (TPA) in rabbits, predominantly due to inhibition of lipoxygenase. Biflavonoids including sumantoflavone and robustaflavone isolated from the extracts of Selaginella tamariscina were shown to inhibit the progression of inflammatory reactions dose dependently due to inhibition of NO production, while robustaflavone marginally affected iNOS gene expression responsible for NO synthesis.[57]
Caesalpinia pulcherrima showed prominent AIA due to the presence of flavonoids isolated from the leaf extract, and upon in vitro study, these exhibited strong AIA against LPS and IFN-γ induced inflammatory response in murine peritoneal macrophage cell lines.[58]
Eupalitin-3-O-β-D-glucoside, a flavonol glycoside isolated from the ethanolic extract of Tephrosia spinosa (Leguminosae), elicited significant AIA against carrageenan-induced paw edema, which was comparable to that of indomethacin.[59]
Similarly, phenolic flavonoids isolated from plants of indigenous origin were reported to have strong AIA by reducing the expression of adhesion molecules exhibited on endothelial cells along with selectins, VECAM-1, PECAM-1 and were shown to have prominent use in atherosclerosis. These include methoxyflavone and hydroxyflavone which were found to inhibit monocyte adhesions to TNF-α.[60]
Flavonoid isolated from the extract of leaves and roots of Scutellaria baicalensis (Lamiaceae), Ginkgo biloba (Ginkgoaceae) and Gentiana scabra (Gentianaceae) were reported to have topical AIA against chronic skin inflammation like atopic dermatitis. The preparation is being available in market as “SK Ato Formula” and is meant for topical application and contains flavonoids like gingkolide A and B (biflavonoids) [Figure 7]. It shows satisfactory effects in animal models with chronic type of skin inflammation induced by TPA treatment to mouse ear. The probable mechanisms behind its AIA are by inhibition of PGE2 synthesis, COX2 and consequent suppression of proinflammatory gene expression.[61]
Figure 7.
Flavonoids from leaf extracts of Ginkgo biloba
Park et al. (2006) evaluated the extract of Ginkgo biloba and found that it exhibited inhibitory action against LPS-induced NO synthesis and PGE2 production in RAW 264.7 macrophage cell line. The major active principles found include terpenes and bi-flavonoids responsible for potent inhibition of NO and PGE2 production.[62]
Flavonoids like 5-O-demethylnobiletin [Figure 8] isolated from several plant extracts are found to inhibit the inflammatory response elicited by TPA in mouse ear and acute mouse paw edema induced by carrageenan. The probable mechanism of action responsible for AIA may be inhibition of 5-LOX and elastase, respectively. The flavone was also reported to inhibit leukotriene B4 (LTB4) formation in rat neutrophils and elastase release in human neutrophils.[63]
Figure 8.
Flavonoids from leaf extracts of Sideretis tragoriganum
Flavonoidal principles isolated from dichloromethane and ethanol extracts from the aerials part of Artemesia copa, such as spinacetin, jaceosidin, axillarin, penduletin, tricin and chrysoeriol, exhibited promising AIA against inflammatory mediators in mouse macrophage (RAW 264.7) cell line stimulated by LPS.[64]
Similarly, Achillea millefolium, a traditional herb used for the treatment of gastrointestinal and hepato-biliary disorders, has been found to inhibit the production of proteases, human neutrophil elastase and matrix metalloproteinase like enzymes responsible for initiation of inflammatory process, due to the presence of flavonoids and dicaffeoylquinic acids.[65]
Phenolic compounds
Breu et al, investigated several phenolic compounds with saturated alkyl side chains and sulfur-containing components isolated from Allium spp. (Alliaceae) and they were found to inhibit 5-LOX against porcine leukocytes. The AIA was mainly due to the catechol content, cepaenes and unsaturated thiosulfinates.[66]
Similarly, Almeida et al, reported on lapachol [Figure 9], a phenolic compound isolated from the species belonging to the family Bignoniaceae. It exhibited a significant anti-inflammatory–antiedematogenic action on carrageenan-induced pedal edema by upto 57%.[67]
Figure 9.

A phenolic compound isolated from plants of Bignoniaceae family
Sambucus ebulus (Caprifoliaceae) showed promising in vitro and in vivo AIA due to the presence of polyphenolic compound, caffeic acid, isolated from the methanolic extracts, in carrageenan-, serotonin-induced pedal edema and adjuvant-induced arthritis in rats.[68]
Wu and co-workers reported that the plant extract obtained from the aerial parts of Laggera alata (Asteraceae) had strong action against inflammation, due to the presence of phenolic principles, when evaluated on acute and chronic inflammations in vivo in carrageenan-induced rat paw edema, xylene-induced mouse ear edema and acetic acid induced vascular permeability in mouse. The probable mechanism may be the physiological inhibition of leukocyte migration, reduction of serum lysozyme levels, nitric oxide, PGE2 and malondialdehyde levels dose dependently.[69]
Cannabinoids
Cannabinoids are now explored as a novel category of compounds occuring indigenously in fruits of Cannabis sativa (Cannabinaceae). The biological activity of the extract may be due to the Δ-tetrahydro cannabinol (Δ-THC) content. Formukong et al, showed that the presence of cannabinoids and olivetol inhibited the inflammation caused by tetradecanoyl phorbol-acetate induced erythema in mouse ear and phenylbenzoquinone-induced writhing response satisfactorily. The probable mechanism behind its AIA may involve its inhibitory activity against prostaglandin synthesis and mobilization.[70]
Recently, Zurier et al, isolated a dihydrostilbene containing compound, canniprene, from Cannabis sativa, and showed it to be most active against human neutrophils due to inhibition of 5-LOX. Similarly, olivetolic acid with a cannabinoid nucleus having free C-5 hydroxyl group is the novel anti-inflammatory principle that shows peripheral effects by inhibiting COX and LOX.[71]
Steroids
Several plants and extracts from 3.6Velutinol A.[73]
Steroidal and triterpenoidal saponins isolated from Ganoderma lucidum and Ganoderma tsugae exhibit AIA by inhibiting the release of β-glucuronidase from rat neutrophils stimulated by formyl-Met-Leu-Phe (fMLP)/cytochalasin B.[74]
Fatty acids
Fatty acids are known from earlier days because of their several therapeutic activities like anti-inflammatory, antioxidant, free radical scavenging, antihyperlipidemic activities.
Oil obtained from the leaves of Oenothera biennis (Onagraceae), called “evening primrose oil”, has gained popularity in orthodox system of medicine against inflammation seen in rheumatoid arthritis, due to the presence of fatty acids like cis-linoleic acid (γ-linoleic acid) abundantly (upto 9%).
Fish oils obtained from marine organisms are of promising therapeutic value in several inflammatory disorders like psoriasis, eczema, allergy lipid lowering activity under various clinical studies. The proposed mechanism of action of oil includes reduction of lipid level which may be due to 5-LOX, 15-LOX, 15-HEPE inhibitory activity when examined on epidermal enzymes and basophilic leukemia cells of rat. The major constituents responsible include eicosapentaenoic acid and docosahexaenoic acid.[75]
Fixed oils obtained from the seeds of linseed Linum usitatissimum, soyabean, and Ocimum sanctum contain α-linolenic acid as the major constituent found to be responsible for prominent AIA against rat paw edema induced by arachidonic acid. The oil produced inhibition in leukotriene-induced paw edema; L. usitatissimum oil produced maximum percentage inhibition in carrageenan and arachidonic acid induced paw edema models.[76]
Plant glycoproteins
Glycoproteins are of natural origin and found indigenously in animal body abundantly. Recent investigation made by Oho et al, reveals that Rhus verniciflua (Anacardiaceae) showed the presence of glycoproteins abundantly in its fruits. The glycoproteins showed promising AIA due to their inhibitory effect on proteins inducing inflammation and on NO production in LPS-induced inflammation on RAW264.7 cell lines. Also, results reveal that the glycoprotein present in plant showed strong antioxidant activity against lipid peroxyl radicals in cell-free system.[77]
Similarly, the hydroalcoholic extract obtained from the most commonly available plant “Madder” Rubia cordifolia (Rubiaceae) was found to ameliorate the series of toxic inflammatory events produced by an anticancer drug, cisplatin, in Swiss Albino mice. It was found to inhibit the toxic responses like nephrotoxicity due to oxidative stress, lipid peroxidation, glutathione depletion, superoxide dismutase, and catalase.[78]
SPECIFIC PHYTOCONSTITUENTS WITH PROMISING VALUE OF ANTI-INFLAMMATORY ACTIVITY
Lignans are the potential phytoconstituents obtained from hexane extracts of Phyllanthus amarus (Euphorbiaceae), which are found to inhibit carrageenan-induced paw edema and neutrophil influx in rats. These lignans include a group of phytoconstituents such as phyltetralin, nirtetralin, niranthin, phyllanthin. Among these, only nirtetralin was found to inhibit the inflammation induced by carrageenan, due to increase in IL1-β level in tissues, while the whole extract was found to reduce the paw edema elicited by bradykinins, platelet activating factors (PAF) and endothelin-1 prominently.[79]
Like lignans, the “neolignans”, isomeric structural analogues isolated from stem barks of several species of Piper kadsura (Piperaceae), are of promising value in the field of pharmacognosy for treatment of several inflammatory disorders of human ailments and in the inhibition of polymethacrylic acid induced production of reactive oxygen species (ROS) in human PMN neutrophils.[80]
Sosa and co-workers (2001) isolated a novel class of compounds with potent topical anti-inflammatory principle from the extract of Achillea pannonica (Asteraceae) by fractionation. These are derivatives of germacrane and were found to exhibit AIA against inflammatory events initiated by croton oil induced dermatitis in the mouse ear in a dose-dependent manner, by upto 61%.[81]
Verminoside (an iridoid glycoside) is found abundantly in the dichloromethane extract of Kigelia africana (Bignoniaceae) occurring in northern zones of Africa. When evaluated for its in vitro anti-inflammatory activity, this constituent showed higher potential of inhibiting the expression of iNOS and NO release in mouse J774.A1 macrophage cell line.[82]
Ethanolic extract of seeds obtained from the chromatographic fractionation of Nyctanthes arbortristis (Oleaceae) was found to contain analgesic–anti-inflammatory and antinociceptive activities due to the presence of arbortristoside-A. In an animal study, the extract exhibited significant action against carrageenan-, serotonin-, and histamine-induced inflammatory reactions, due to inhibition of arachidonic acid synthesis.[83]
Phenylpropanoids are the active antinflammatory principles isolated from the species of Illicium like Illicium tashiroi, Illicium anisotum, Illicium arborescens and are potentially found to have promising AIA due to inhibition of histamine release, when tested on rat basophilic RBL-2H3 leukemia cells stimulated by A23187.[84]
Plants belonging to the family of Iridaceae like Eleutherine americana contain a novel class of [Figure 10] plant constituents such as naphthoquinones used for their AIA. When evaluated in vitro, they showed satisfactory action against inflammation induced by LPS in mouse macrophage RAW 264.7 cell lines.[85] Similarly, luteolin is a newer flavone which occurs indigenously in several plants and helps a lot for its antioxidant, antinflammatory and antiallergic functions as compared to other flavonoids.[86]
Figure 10.
Newer flavonoid from Eleutherine Americana: Phyllanthin, Niranthin, Pinobatol, Luteolin
Chen et al, isolated the phytoconstituents having benzoic acid skeleton from the fruits of Melicope semecarpifolia (Rutaceae) and these were found to have AIA on human neutrophils, due to inhibitory action on superoxide anion generation and elastase release.[87]
Luteolin diglucuronide, apigenin diglucuronide, and semi-pure luteolin diglucuronide are potentially a newer class of carotenoid compounds isolated from the leaves of Perilla nankinensis and were reported to inhibit the carrageenan-induced inflammatory events in rat paw edema model.[88] Luteolin elicited mild acute and chronic AIA in mice, and upon oral administration, it suppressed the paw edema induced by carrageenan at anti-inflammatory doses and cotton pellet induced granuloma in rats, by temporary blockage of leukocyte infiltration and elevated level of 6-keto-PGF1α in the inflammatory exudate due to down-regulation of the mRNA expression of COX2 in inflammatory responses.[89]
Like luteolin, luteins are compounds having basic carotenoid skeleton [Figure 11] and are derived from the extract of a plant Tagetes erecta, known widely as “marigold”, belonging to the family Compositae. They have shown satisfactory results with their anti-inflammatory as well as antioxidant properties when evaluated in vitro on carrageenan- and dextran-induced acute paw edema in mice. The mechanism behind this activity may be its superoxide radical scavenging action which leads to inhibition of photolysis of vitamin B2 (riboflavin) present in the body and hence decreases formation of hydroxyl free radicals.[90]
Figure 11.
Carotenoid with AIA from Tagetes erecta
Hinkitiol is a compound that belongs to the class of tropolone derivatives, commonly known as β-thujaplicin, present abundantly in the heartwood of plants belonging to the family Cupressaceae. It showed promising AIA in LPS-induced macrophage like RAW264.7 cell line by inhibition of production of TNF-α to stop the inflammation of skin and also reduced hair follicle apoptosis.[91]
Sesquiterpenes isolated from the leaves of Yacon tree, Smalianthus sonchifolia, (Asteraceae) are reported to contain a newer group of compounds called melampolides which are likely to have AIA on murine macrophage RAW264.7 cells. The probable mechanism may be the inhibition of NO production induced by LPS.[92]
Evodiamine, rutaecarpine and goshuyuamide II are now identified as a novel class of anti-inflammatory agents isolated from the fruits of Evodia rutaecarpa (Rutaceae). These compounds are found to elicit outstanding AIA when treated against LPS-induced inflammation on RAW 264.7cell lines. The mechanism responsible for their action may involve stronger inhibitory action on PGE2 generation from COX2, as evodiamine has been found to restrain COX2 induction only.[93]
Yao and co-scientists reported in recent years the presence of a series of phytoconstituents belonging to a major therapeutic chemical class of compounds in plants known as stilbenolignans, isolated from lignans of Gnetum cleistostachyum (Gnetaceae). They reported that these compounds are quite efficacious in reducing the signs and symptoms of inflammation when examined on inflammation models observed to have TNF-α inhibitory activity. These compounds are gnetofuran A, gnetumontanin C, lehmbachol D, gnetifolin F, gnetucleistol F.[94] Similarly, lignans isolated from chromatographic fractionation of the extract of Saussurea conica (Asteraceae), such as conicaoside, conicaols A and B, and in addition to this, 2,3-dibenzylbutyrolactone-type lignans such as arctigenin and matairesinol, were found to have higher AIAs in vitro on LPS-induced inflammation in rat macrophages.[95]
Polyzellin and polysylvin [Figure 12] are the stilbene skeleton containing compounds isolated from the fruiting bodies and leaves of Polyozellus multiplex (Thelephoraceae) and Pinus densiflora (Pinaceae), respectively, and have been recently investigated for their AIA. They were found to significantly inhibit the LPS-induced NO and NF-kB production in a dose-dependent manner in murine macrophagial RAW264.7 cell line.[96,97]
Figure 12.

Stilbene containing compounds isolated from Polyozellus multiplex
Euonymus laxiflorus (Celastraceae) is a plant with AIA. When RAW264.7 macrophagial cell line was screened, it was found to counteract the inflammation in a concentration-dependent manner. The major constituent responsible for such activity is laxifolone A.[98]
Geniposide and genipin are the two major anti-inflammatory principles isolated from the ethanolic extract of Gardenia jasminoides (Rubiaceae) fruits and have been instituted nowadays in the treatment of several diseases related to causes of inflammation like jaundice, headache, edema, fever, and hepatic disorders. When analyzed for their antinflammatory activity, these produced acute responses in carrageenan-induced rat paw edema with inhibition of vascular permeability of the bed dose dependently.[99]
Patel et al, accounted for a newer class of anti-inflammatory agents which are basically prenylated derivatives of resveratrol obtained from the chromatographic isolated part of fungal infected parts. The probable mechanism behind its activity might be inhibition of COX2 enzyme induction and subsequent inhibition of the expression of mRNA thereof.[100]
Trichloromethane extract of dried rhizomes of Zingiber species like Zingiber cassumunar (Zingiberaceae) were found to contain phenylbutenoids which exhibited promising AIA by inhibiting the COX2 generation and thus reduced the level of PGE2.[101]
Marsik and co-workers experimented on the extract obtained from seeds of Nigella sativa (Ranunculaceae) and evolved a newer group of plant secondary metabolites such as dithymoquinone, thymo hydroquinone, and thymoquinone which are reported to have promising AIA due to inhibition of COX1 and COX2 enzymes significantly in comparison to indomethacin. Among these constituents, thymoquinone and thymohydroquinone exhibited a stronger value of antinflammatory effect due to more tight binding with the COX2 enzyme prominently.[102]
Marrubiin is a potent plant constituent isolated from Marrubium vulgare. When subjected to screening models of inflammation like microvascular leakage in mice ears, it exhibited promising AIA in a dose-dependent fashion.[103]
Gigantol [Figure 13], a chemical constituent isolated from the whole plant extract of Cymbidium goeringii (Orchidaceae), was found to have significant AIA when examined in LPS-, NO-, and PGE2-induced edema in RAW 264.7 cells. The basic mechanism behind its AIA may be due to the presence of active constituent, gigantol, which was found to suppress the expression of iNOS and COX2 along with inhibition of mRNA level in RAW 264.7 cell line.[104]
Figure 13.
Chemical constituent isolated from Cymbidium goeringii
MISCELLANEOUS ANTI-INFLAMMATORY AGENTS
Algae
Spirulina fusiformis (Oscillateriaceae), known as “blue green algae”, are important due to their AIA and allied therapeutic uses like analgesic and antiarthritic actions. When studied for its AIA in rats by measuring the paw volumes, body weights as measures of inflammation showed promising results in terms of reduction in body weight as compared to adjuvants.[105]
Methanolic extract of Cheilanthes farinosa (Adianthaceae), a fern grown indigenously in southeast Africa, showed stronger value of AIAs due to the presence of rutin, cinnamic acids, caffeic acid and its quinic acid derivative, chlorogenic acid.[106]
Research work in the recent years has led to the discovery of “marine red algae” obtained from Neorhodomela aculeate. When investigated for their anti-inflammatory and antioxidant properties, they showed promising value with neuronal and microglial cells. Similarly, other actions of the extract include potent neuroprotective effect elicited by glutamate-induced neurotoxicity and inhibition of ROS expression in murine hippocampal HT22 cell line, and inhibition of H2O2-induced lipid peroxidation in rat brain homogenates.[107]
Marine plants
Pseudopterosins are newer class of natural products isolated from Pseudopterogorgia elisabe, which have been characterized as diterpene pentose glycosides. The pseudopterosins possess considerable analgesic activity and AIA.[108]
In search for new biologically active natural products, a number of isolates derived from algae and sponges were evaluated. Of these, palisol and dictyol C demonstrated the most potent inhibition of COX2.[109]
Methanolic extract of the sea grass Zostera japonica has shown AIA. Its hexane, dichloromethane, acetate and water extracts showed the highest capacity to inhibit expression in LPS stimulated J774A cell line due to the presence of palmitic acid, palmitic acid methyl ester, linoleic acid methyl ester, oleic acid methyl ester and linoleic acid.[110]
Kang et al, showed that dichloromethane and ethanol extracts of the brown seaweeds, Sargassum fulvellum and Sargassum Thunbergii, when examined for analgesic, anti-inflammatory and other allied activities showed reduced signs of inflammation in mice.[111]
Traditional plants and plant preparations with anti-inflammatory activity
Some of the plants traditionally belonging to particular geographical regions are equipped with constituents having highly selective anti-inflammatory activity. These include plants from China, Korea and Japan.
The aqueous extract of the tuber of Smilax china, popularly known in China as “Jin Gang Ten”, was tested for its AIAs in rats with egg-albumin–induced edema and its anti-nociceptive effects in mice, using hot-plate test and acetic acid induced abdominal constriction test, respectively.[112]
Ethanolic extract of roots of Clematis mandshurica (Ranunculaceae) is used in Korea as a traditional medicine in the treatment of several inflammatory diseases. The major constituents concanavalin A is responsible for AIA by inhibiting NO, PGE2 and other pro-inflammatory mediators in LPS/IFN-γ induced inflammation.[113]
Similarly, the extracts obtained from traditional Chinese medicines, such as Changtai granules, are reported to have better AIA due to their inhibitory action on myeloperoxidase enzymes. When given orally once daily, it has been found to exhibit AIA which helps in the inhibition of 2,4,6-trinitrobenzene sulfonic acid induced ulcerative colitis and colonic injuries thereof in a trial done in vitro. This makes it a drug of choice in several inflammatory disorders, especially in colitis and bowel diseases.[114]
Wen-Pi-Tang-Hab-Wu-Ling-San (WHN), a preparation widely used in the traditional medicine of Korea, is found to have satisfactory AIA. When the extract was subjected to prior evaluation of its AIA in trials done in vitro, it showed positive response against inflammation by strongly inhibiting the excessive production of mediators like NO, TNF-α, IL-1β and IL-6, respectively.[115,116]
Likewise, a promising Chinese herb, Seungma-galgeun-tang, has been used widely in China as a folk medicine recipe for broad-spectrum treatment of acute and chronic inflammatory disorders. It has been found to inhibit the generation of NO, PGE2, COX2, TNF-α, IL-12, IL-1β, and activation of NF-kB competitively and to inhibit the secretion of NO in BV-2 microglia without affecting cell viability.[117]
In a finding made by Tseng et al, where they described a Chinese traditional herbal decoction type formulation known as Cheng-Chi-Tang, it was reported to have promising AIA in several inflammation and related disorders like pain, and inflammation produced due to regular use of purgatives, painful abdomen, hard stools and fever.[118]
Similarly, Lo et al, reported a formulation called San Huang-Xie-Xin-Tang, which is widely used in traditional oriental medicine. It was shown to have prominent AIA due to the presence of baicalin in a preclinical study that was conducted on LPS-induced inflammation.[119]
Panax notoginseng is a Chinese herbal used as a dietary supplement. It was found to exhibit several therapeutic activities like immunomodulatory, anti-inflammatory, and antiviral. Among the several species of ginseng, P. notoginseng inhibited the LPS-induced synthesis of TNF-α and IL-6 in a concentration-dependent manner in RAW264.7 macrophages.[120] Similarly, the research work conducted on the roots of Panax ginseng (Araliaceae), “red ginseng”, instituted several newer groups of chemical constituents such as ginsenosides Rg3 and Rh2 which have been shown to have promising AIA in LPS and INF-γ induced inflammation in murine BC-2 microglial cells. Among these, Rh2 is found to have greater value of activity due to its inhibitory action on NO synthesis and expression of mRNA of NO and hence reduces the severity of inflammatory response.[121]
Phthalide lactones like Z-ligustilide and senkyunolide A isolated from the dried rhizome of Ligusticum chuanxiong (Apiaceae), a traditional Chinese herb used for the treatment of inflammatory and cardiovascular diseases. In vitro study showed AIA by inhibiting LPS and TNF-α induced inflammatory reaction along with inhibitory action on mRNA gene expression. Also, the Z-ligustilide was found to be more potent as compared to that of senkyunolide A.[122]
“Bolengguazi”, a formulation quite widely used in Tibetan medicine, was found to have a stronger AIA. It was reported to contain the seed extract of Herpetospermum pedunculosum, Momordica cochinchinensis and Momordica charantia, by Fang and co-workers. It was found to have a significant analgesic activity and AIA in several models of inflammation like egg-albumin–induced paw edema and cotton pellet granuloma tests, and the activity is comparable to that of indomethacin.[123,124]
Aqueous and butanolic extracts obtained from the rhizomes, leaves and inflorescence of Solidago chilensis exhibited profound inhibitory activity against leukocytes, neutrophils and exudation of inflammation induced by carrageenan due to inhibition of leukocytes, neutrophils, myeloperoxidase, adenosine-deaminase, and TNF-α of the inflammation.[125]
Rhizoma coptidis, a herbal originated from Chinese medicine, has been well established for the treatment of common dermatological disorders. The major constituents like berberine, tannins, and terpenes are responsible for AIA in human keratinocytes by inhibiting TNF-α.[126]
Lycopodium clavatum (Lycopodiaceae), a plant that originated from Turkish region, showed promising AIA when used as different extracts like petroleum ether, chloroform, ethyl acetate and methanol along with the alkaloidal fraction obtained from the aerial parts models of inflammations like acetic acid induced increased capillary permeability in mice.[127]
Pluchea quitoc (Asteraceae), a Brazilian plant of traditional medicine, has now widely gained acceptance for treatment of inflammation as well as of digestive and respiratory diseases. For confirming its AIA, it was tested in carrageenan-induced paw edema and pain in mice. It also inhibited neurogenic pain in rats, induced by formalin.[128]
Kalanchoe brasiliensis (Crassulaceae) was recently reported to contain high AIA from its salt preparation named as kalanchosine dimalate prepared from the fresh juice obtained from aerial parts of the plant. The major components of preparation include a mixture of kalanchosine and malic acid in a 1:2 stoichiometric proportion where kalanchosine is chemically 3,6-diamino-4,5-dihydroxyoctanedioic acid. Clinical study of the preparation against several inflammatory disorders reveals that the major principle responsible for AIA of salt may be the kalanchosine.[129]
Kampo medicines are the traditional medicines that originated in Japan based on the silent concept of treatment of diseases. In a study conducted by Ara et al, they evaluated the efficacy of this system of treatment through clinical trials in vitro against periodontal diseases, where inflammation was induced by LPS. The possible mechanism behind this may involve the inhibition of production of inflammatory mediators like PGE2, IL-6, IL-8 and COX2 in a dose-dependent manner. Trials showed that therapy is quite efficacious in reducing the disease progression upto 24 h duration without any viable growth of human gingival fibroblasts by Porphyromonas gingivalis.[130]
SAFETY ASPECTS OF HERBAL PREPARATIONS
Herbal medicine generally uses various parts of plants or mixtures of plant extracts to treat illness, to promote health, to restore the body's ability to protect, regulate and heal by itself. Several preparations are now used and marketed successfully worldwide as over-the-counter products. But as far as the safety aspects are concerned, these do not mean that they are safe to use. According to a study, about 40% of the anticancer drugs made from plants have been shown to successfully treat cancer, but they still have serious side effects.[13] Similar to these, many herbal remedies are reported to have serious side effects, adverse effects and some of them have a tendency to interact with the synthetic preparations. For example, some herbs can make skin more sensitive to light and should not be given during radiotherapy. Similarly, St. John's Wort is used in the treatment of depression, but it interacts with iron to reduce its therapeutic efficacy.[131,132] A brief review of some of the herbal anti-inflammatory preparations representing the potential interactions and incompatibilities is presented in Table 5. As per Therapeutic Goods Administration (TGA), Australia, the adverse effects of herbals are classified into two different groups as intrinsic events and extrinsic events. Intrinsic events are again subclassified into two types as Type-A and Type-B reactions. The adverse effects of herbals are presented in Figure 14.
Table 5.
Overview of some serious adverse events occuring by combined use of herbals with synthetic preparations[132–135]
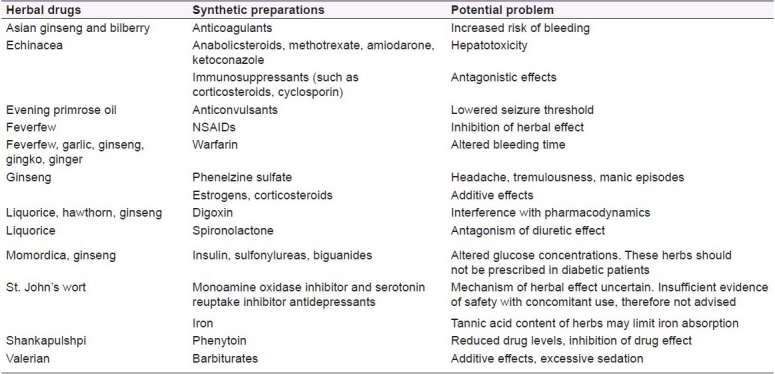
Figure 14.
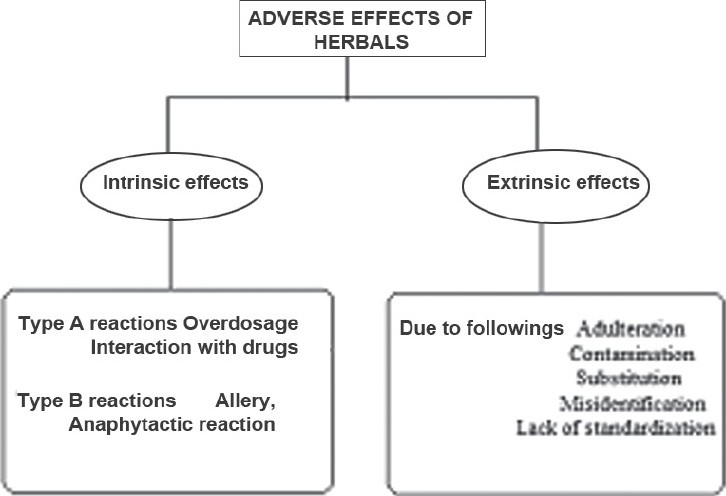
Overview of adverse events seen with herbal preparations and their cause
CLINICAL TRIALS ON HERBALS WITH AIA
Several clinical trials of herbal drugs have been done, representing them as a better aid in the treatment of anti-inflammatory disorders. Conducting clinical trials on traditional/herbal medicines is challenging due to lot of reasons, for instance, it is very difficult, impracticable or sometimes impossible to have active and control groups with identical color, smell and taste. Also, the use of placebo involves similar difficulties as the herbal drug may exhibit its strong aroma of its own or may have a specific distinguished taste, which abolishes the placebo action. Examples of clinical studies done on the list of herbals found to be efficacious against inflammation and related disorders are enlisted in Table 6.
Table 6.
Brief overview of clinical trials on herbal drugs with anti-inflammatory activity

CONCLUSION
Though several researches are being performed in the area of herbal drugs for exploring newer and safer alternatives in order to combat against several inflammatory reactions, recently an annual publication released by WHO and several health regulatory bodies worldwide reported certain key problems like safety of herbal preparation and herbal formulation being a newer area of thought for researchers. Although it is widely perceived that “natural” products are safe, evidence suggests that upon clinical use these preparations are never being seen without risk. Study says that among every 90 patients set in trials with a disease like rheumatoid arthritis, 82% were reported to have chances of slight alterations in their physical as well as mental behavior, while 31% had experienced adverse effect. Hence, expert key commentaries are required in the field of herbals regarding their production and marketing in terms of better regulatory cheks.
ACKNOWLEDGMENT
Authors want to express their gratitude to Faculty of Pharmacy, Hamdard University, New Delhi, for the scientific environment
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Denko CW. A role of neuropeptides in inflammation. In: Whicher JT, Evans SW, editors. Biochemistry of inflammation. London: Kluwer Publisher; 1992. pp. 177–81. [Google Scholar]
- 2.Singh A, Malhotra S, Subban R. Anti inflammatory and analgesic agents from Indian medicinal plants. Int J Integ Biol. 2008;3:57–72. [Google Scholar]
- 3.Serhan CN, Savill J. Resolution of inflammation: The beginning programs the end. Nat Immunol. 2005;6:1191–7. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 4.Collins CK. Robbins Pathologic Basis of Disease. Philadelphia: W.B Saunders Company; 1998. [Google Scholar]
- 5.Bottazzi B, Doni A, Garlanda C, Mantovani A. An integrated view of humoral innate immunity: Pentraxins as a paradigm. Ann Rev Immunol. 2010;28:157–83. doi: 10.1146/annurev-immunol-030409-101305. [DOI] [PubMed] [Google Scholar]
- 6.Lanas A. Nonsteroidal antiinflammatory drugs and cyclooxygenase inhibition in the gastrointestinal tract: A trip from peptic ulcer to colon cancer. Am J Med Sci. 2009;338:96–106. doi: 10.1097/MAJ.0b013e3181ad8cd3. [DOI] [PubMed] [Google Scholar]
- 7.Rossi S. Australian medicines handbook. Adelaide: Australian Medicines Handbook Pvt Ltd; 2006. [Google Scholar]
- 8.Kandulski A, Venerito M, Malfertheiner P. Non steroidal anti-inflammatory drugs (NSAIDs) - balancing gastrointestinal complications and the cardiovascular risk. Deutsche Medizinische Wochenschrift. 2009;134:1635–40. doi: 10.1055/s-0029-1233993. [DOI] [PubMed] [Google Scholar]
- 9.Rainsford KD, Whitehouse MW. Antiinflammatory/antipyretic salicylic acid esters with low gastric activity. Agents Action. 1980;10:451–5. doi: 10.1007/BF01968046. [DOI] [PubMed] [Google Scholar]
- 10.Fiebich BL, Chrubasik S. Effects of an ethanolic Salix extract on the release of selected inflammatory mediators in vitro. Phytomedicine. 2004;11:135–8. doi: 10.1078/0944-7113-00338. [DOI] [PubMed] [Google Scholar]
- 11.MacLennan AH, Wilson DH, Taylor AW. Prevalence and cost of alternative medicine in Australia. Lancet. 1996;347:569–72. doi: 10.1016/s0140-6736(96)91271-4. [DOI] [PubMed] [Google Scholar]
- 12.Ang-Lee MK, Moss J, Yuan CS. Herbal medicines and perioperative care. J Am Med Asso. 2001;286:208–16. doi: 10.1001/jama.286.2.208. [DOI] [PubMed] [Google Scholar]
- 13.Drew AK, Myers SP. Safety issues in herbal medicine:implications for the health professions. WHO Report. [cited in 1997. accessed on 2009 Oct 26]. Available from: https://www.mja.com.au/public/issues/may19/drew/drew.html . [DOI] [PubMed]
- 14.New approaches to good practice. Oxford: Oxford University Press; 1993. British Medical Association. Complementary medicine; pp. 9–36. [Google Scholar]
- 15.Anonymous. Program profile. International liaison brings global vision to OAM. Complementary and Alternative Medicine at the NIH. 1996;3:3–10. [Google Scholar]
- 16.Bent S, Ko R. Commonly used herbal medicines in the United States: A review. Am J Med. 2004;116:478–85. doi: 10.1016/j.amjmed.2003.10.036. [DOI] [PubMed] [Google Scholar]
- 17.Boullata JI, Nace AM. Safety issues with herbal medicine. Pharmacother. 2000;20:257–69. doi: 10.1592/phco.20.4.257.34886. [DOI] [PubMed] [Google Scholar]
- 18.Ferrante A, Seow WK, Rowan-Kelly B, Thong YH. Tetrandrine, a plant alkaloid, inhibits the production of tumour necrosis factor-alpha (cachectin) by human monocytes. Clin Exp Immunol. 1990;80:232–5. doi: 10.1111/j.1365-2249.1990.tb05239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teh BS, Seow WK, Li SY, Thong YH. Inhibition of prostaglandin and leukotriene generation by the plant alkaloids tetrandrine and berbamine. J Immunopharmacol. 1990;12:321–6. doi: 10.1016/0192-0561(90)90088-5. [DOI] [PubMed] [Google Scholar]
- 20.Rahman A, Ahmad D, Asif E, Ahmad S, Sener B, Turkoz S. Chemical constituents of Buxus sempervirens. J Nat Prod. 1991;54:79–82. [Google Scholar]
- 21.Rahman A, Asif E, Ali SS, Nasir H, Jamal SA, Ata A, et al. New steroidal alkaloids from the roots of Buxus papillosa. J Nat Prod. 1992;55:1063–70. [Google Scholar]
- 22.Chakraborty A, Brantner AH. Study of alkaloids from Adhatoda vasica nees on their antiinflammatory activity. Phytother Res. 2001;15:532–4. doi: 10.1002/ptr.737. [DOI] [PubMed] [Google Scholar]
- 23.Rajput N, Srivastava DN, Sahni YP, Nigam JM. Role of mediators in anti-inflammatory activity of Adhatoda vasica on carragenan induced paw oedema in rats. J Vet Pharmacol Toxicol. 2006;5:32–6. [Google Scholar]
- 24.Sutradhar RK, Rahman AM, Ahmad M, Bachar SC, Saha A, Roy TG. Antiinflammatory and analgesic alkaloid from Sidacordifolia Linn. Pak J Pharm Sci. 2007;20:185–8. [PubMed] [Google Scholar]
- 25.Gomes A, Sharma RM, Ghatak BJ. Pharmacological investigation of a glycosidal fraction isolated from Maesa chisia D. Don var. angustifolia Hook f and Th. Indian J Exp Biol. 1987;25:826–31. [PubMed] [Google Scholar]
- 26.Lanhers MC, Fleurentin J, Mortier F, Vinche A, Younos C. Anti-inflammatory and analgesic effects of an aqueous extract of Harpagophytum procumbens. Planta Med. 1992;58:117–23. doi: 10.1055/s-2006-961411. [DOI] [PubMed] [Google Scholar]
- 27.Sanchez-Mateo CC, Bonkanka CX, Hernandez-Perez M, Rabanal RM. Evaluation of the analgesic andtopical anti-inflammatory effects of Hypericum reflexum L. fil. J Ethnopharmacol. 2006;107:1–6. doi: 10.1016/j.jep.2006.01.032. [DOI] [PubMed] [Google Scholar]
- 28.Shi HM, Wen J, Jia CQ, Jin W, Zhang XF, Yao ZR, et al. Two new phenolic glycosides from the barks of Hydnocarpus annamensis and their antiinflammatory and antioxidant activities. Planta Med. 2006;72:948–50. doi: 10.1055/s-2006-946678. [DOI] [PubMed] [Google Scholar]
- 29.Kim JS, Kim JC, Shim SH, Lee EJ, Jin WY, Bae K, et al. Chemical constituents of the root of Dystaenia takeshimana and their anti-inflammatory activity. Arch Pharmacal Res. 2006;29:617–23. doi: 10.1007/BF02968244. [DOI] [PubMed] [Google Scholar]
- 30.Thangavel N, Gupta JK. Anti-inflammatory and anti-snake venom activity of Andrographis stenophylla leaf. Asian J Chem. 2007;19:1307–12. [Google Scholar]
- 31.Montopoli M, Froldi G, Comelli MC, Prosdocimi M, Caparrotta L. Aescin protection of human vascular endothelial cells exposed to cobalt chloride mimicked hypoxia and inflammatory stimuli. Planta Med. 2007;73:285–8. doi: 10.1055/s-2007-967118. [DOI] [PubMed] [Google Scholar]
- 32.Xu LP, Wang H, Yuan Z. Triterpenoid saponins with anti-inflammatory activity from Codonopsis lanceolata. Planta Med. 2008;74:1412–5. doi: 10.1055/s-2008-1081318. [DOI] [PubMed] [Google Scholar]
- 33.Juteaua F, Masotti V, Bessiere JM, Dherbomez M, Vianoa J. Antibacterial and antioxidant activities of Artemisia annua essential oil. Fitoterapia. 2002;73:532–5. doi: 10.1016/s0367-326x(02)00175-2. [DOI] [PubMed] [Google Scholar]
- 34.Kohli K, Ali J. Curcumin: A natural antiinflammatory agent. Indian J Pharmacol. 2005;37:141–7. [Google Scholar]
- 35.Lindenmeyer MT, Tubaro A, Sosa S, Merfort I. New sesquiterpene lactones from Arnica tincture prepared from fresh flower heads of Arnica montana. Planta Med. 2005;71:1044–52. doi: 10.1055/s-2005-871284. [DOI] [PubMed] [Google Scholar]
- 36.Sosa S, Altinier G, Politi M, Braca A, Morelli I, Della Loggia R. Extracts and constituents of Lavandula multidia with topical anti-inflammatory activity. Phytomedicine. 2005;12:271–7. doi: 10.1016/j.phymed.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 37.Yun KJ, Min BS, Kim JY, Lee KT. Styraxoside A isolated from the stem bark of Styrax japonica inhibits lipopolysaccharide-induced expression of inducible nitric oxide synthase and cyclooxygenase-2 in RAW 264.7 cells by suppressing nuclear factor-kappa B activation. Biol Pharm Bull. 2007;30:139–44. doi: 10.1248/bpb.30.139. [DOI] [PubMed] [Google Scholar]
- 38.Calou IB, Sousa DI, Cunha GM, De A, Brito GA, De C, et al. Topically applied diterpenoids from Egletes viscosa (Asteraceae) attenuate the dermal inflammation in mouse ear induced by tetradecanoylphorbol 13-acetate- and oxazolone. Biol Pharm Bull. 2008;31:1511–6. doi: 10.1248/bpb.31.1511. [DOI] [PubMed] [Google Scholar]
- 39.Chen W, Liu Q, Wang J, Zou J, Meng D, Zuo J, et al. New Guaiane, Megastigmane and Eudesmane type sesquiterpenoids and anti-inflammatory constituents from Youngia japonica. Planta Med. 2006;72:143–50. doi: 10.1055/s-2005-916182. [DOI] [PubMed] [Google Scholar]
- 40.Mack T, Ammon HP, Safayhi H. Abstracts of the International Joint Symposium of Biology and Chemistry of Active Natural Substances. Bonn. 1990:177. [Google Scholar]
- 41.Ammon HP, Mack T, Singh GB, Safayhi H. Inhibition of leukotriene B4 formation in rat peritoneal neutrophils by an ethanolic extract of the gum resin exudate of Boswellia serrata. Planta Med. 1991;57:203–7. doi: 10.1055/s-2006-960074. [DOI] [PubMed] [Google Scholar]
- 42.Fan AY, Lao L, Zhang RX, Zhou AN, Wang LB, Moudgil KD, et al. Effects of an acetone extract of Boswellia carterii Birdw: Burseraceae gum resin on adjuvant-induced arthritis in Lewis rats. J Ethnopharmacol. 2005;101:104–9. doi: 10.1016/j.jep.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 43.Ammon HP. Boswellic acids in chronic inflammatory diseases. Planta Med. 2006;72:1100–16. doi: 10.1055/s-2006-947227. [DOI] [PubMed] [Google Scholar]
- 44.Orhan I, Kupeli E, Aslan M, Kartal M, Yesilada E. Bioassay-guided evaluation of anti-inflammatory and antinociceptive activities of pistachio Pistacia vera L. J Ethnopharmacol. 2006;105:235–40. doi: 10.1016/j.jep.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 45.Choy CS, Hu CM, Chiu WT, Lam CS, Ting Y, Tsai SH, et al. Suppression of lipopolysaccharide-induced of inducible nitric oxide synthase and cyclooxygenase-2 by Sanguis Draconis: A dragon's blood resin in RAW 264.7 cells. J Ethnopharmacol. 2008;115:455–62. doi: 10.1016/j.jep.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 46.Seo YA, Suk KD. Analgesic and anti-inflammatory activity of Resina Pini. Nat Prod Sci. 2007;13:347–54. [Google Scholar]
- 47.Dordevic S, Petrovic S, Dobric S, Milenkovic M, Vucicevic D, Zizic S, et al. Antimicrobial, anti-inflammatory, anti-ulcer and antioxidant activities of Carlina acanthifolia root essential oil. J Ethnopharmacol. 2007;109:458–63. doi: 10.1016/j.jep.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 48.Passos GF, Fernandes ES, da Cunha FM, Ferreira J, Pianowski LF, Campos MM, et al. Anti-inflammatory and anti-allergic properties of the essential oil and actie compounds from Cordia verbenacea. J Ethnopharmacol. 2007;110:323–33. doi: 10.1016/j.jep.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 49.Zhang B, Li JB, Zhang DM, Ding Y, Du GH. Analgesic and anti-inflammatory activities of a fraction rich in gaultherin isolated from Gaultheria yunnanensis (Franch) Rehder Biol Pharm Bull. 2007;30:465–9. doi: 10.1248/bpb.30.465. [DOI] [PubMed] [Google Scholar]
- 50.Esteves I, Souza IR, Rodrigues M, Cardoso LG, Santos LS, Sertie JA, et al. Gastric antiulcer and anti-inflammatory activities of the essential oil from Casearia sylvestris Sw. J Ethnopharmacol. 2005;101:191–6. doi: 10.1016/j.jep.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 51.Altinier G, Sosa S, Aquino RP, Mencherini T, Loggia RD, Tubaro A. Characterization of topical antiinflammatory compounds in Rosmarinus officinalis L. J Agricul Food Chem. 2007;55:1718–23. doi: 10.1021/jf062610+. [DOI] [PubMed] [Google Scholar]
- 52.Popov SV, Popova GY, Ovodova RG, Ovodov YS. Antiinflammatory activity of the pectic polysacharide from Comarum palustre. Fitoterapia. 2005;76:281–7. doi: 10.1016/j.fitote.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 53.Tragni E, Galli CL, Tubaro A, Del Negro P, Della Loggia R. Anti-inflammatory activity of Echinacea angustifolia fractions separated on the basis of molecular weight. Pharmacol Res Commun. 1988;20:87–90. doi: 10.1016/s0031-6989(88)80848-8. [DOI] [PubMed] [Google Scholar]
- 54.Xie G, Schepetkin IA, Siemsen DW, Kirpotina LN, Wiley JA, Quinn MT. Fractionation and characterization of biologically-active polysaccharides from Artemisia tripartita. Phytochem. 2008;69:1359–71. doi: 10.1016/j.phytochem.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tubaro A, Del Negro P, Bianchi P, Romussi G, Della Loggia R. Topical anti-inflammatory activity of a new acylated flavonoid. Agents Act. 1989;26:229–30. doi: 10.1007/BF02126620. [DOI] [PubMed] [Google Scholar]
- 56.El-Ghazaly M, Khayyal MT, Okpanyi SN, Arens-Corell M. Study of the anti-inflammatory activity of Populus tremula, Solidago virgaurea and Fraxinus excelsior. Arzneimforsch. 1992;42:333–6. [PubMed] [Google Scholar]
- 57.Yang JW, Pokharel YR, Kim MR, Woo ER, Choi HK, Kang KW. Inhibition of inducible nitric oxide synthase by sumaflavone isolated from Selaginella tamariscina. J Ethnopharmacol. 2006;105:107–13. doi: 10.1016/j.jep.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 58.Rao YK, Fang SH, Tzeng YM. Antiinflammatory activities of flavonoids isolated from Caesalpinia pulcherrima. J Ethnopharmacol. 2005;100:249–53. doi: 10.1016/j.jep.2005.02.039. [DOI] [PubMed] [Google Scholar]
- 59.Chakradhar V, Babu YH, Ganapaty S, Prasad YR, Rao NK. Anti-inflammatory activity of a flavonol glycoside from Tephrosia spinosa. Nat Prod Sci. 2005;11:63–6. [Google Scholar]
- 60.Kwon HM, Choi YJ, Jeong YJ, Kang SW, Kang IJ, Lim SS, et al. Anti-inflammatory inhibition of endothelial cell adhesion molecule expression by flavone derivatives. J Agr Food Chem. 2005;53:5150–7. doi: 10.1021/jf047854d. [DOI] [PubMed] [Google Scholar]
- 61.Lim H, Son KH, Chang HW, Sang SS, Kim HP. Effects of antiinflammatory biflavonoid, ginkgetin on chronic skin inflammation. Biol Pharm Bull. 2006;29:1046–9. doi: 10.1248/bpb.29.1046. [DOI] [PubMed] [Google Scholar]
- 62.Park YM, Won JH, Yun KJ, Ryu JH, Han YN, Choi SK, et al. Preventive effect of Ginkgo biloba extract (GBB) on the lipopolysaccharide-induced expressions of inducible nitric oxide synthase and cyclooxygenase-2 via suppression of nuclearfactor-keppaB in raw 264-7 cells. Biol Pharm Bull. 2006;29:985–90. doi: 10.1248/bpb.29.985. [DOI] [PubMed] [Google Scholar]
- 63.Bas E, Recio MC, Giner RM, Manez S, Nicholas MC, Rios JL. Anti-inflammatory activity of 5-O-demethylnobiletin, a polymethoxyflavone isolated from Sideretis tragoriganum. Planta Med. 2006;72:136–42. doi: 10.1055/s-2005-873191. [DOI] [PubMed] [Google Scholar]
- 64.Moscatelli V, Hnatyszyn O, Acevedo C, Javier M, Alcaraz MJ, Ferraro G. Flavonoids from Artemesia copa with antiinflammatory activity. Planta Med. 2006;72:72–4. doi: 10.1055/s-2005-873177. [DOI] [PubMed] [Google Scholar]
- 65.Benedek B, Kopp B, Melzig MF. Achillea millefolium L. s.l.: Is the anti-inflammatory activity mediated by protease inhibition. J Ethnopharmacol. 2007;113:312–7. doi: 10.1016/j.jep.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 66.Breu T, Ustunes L, Lermioglu F, Ozer A. Antiinflammatory, analgesic, and antipyretic effects of an aqueous extract of Erythraea centaurium. Planta Med. 1991;57:34–7. doi: 10.1055/s-2006-960011. [DOI] [PubMed] [Google Scholar]
- 67.de Almeida ER, da Silva Filho AA, dos Santos ER, Lopes CA. Antiinflammatory action of lapachol. J Ethnopharmacol. 1990;29:239–41. doi: 10.1016/0378-8741(90)90061-w. [DOI] [PubMed] [Google Scholar]
- 68.Sezik E, Tabata M, Yeşilada E, Honda G, Goto K, Ikeshiro Y. Traditional medicine in Turkey: I, Folk medicine in northeast Anatolia. J Ethnopharmacol. 1991;35:191–6. doi: 10.1016/0378-8741(91)90072-l. [DOI] [PubMed] [Google Scholar]
- 69.Wu Y, Zhou C, Song L, Li X, Shi S, Mo J, et al. Effect of total phenolics from Laggeraalata on acute and chronic inflammatory models. J Ethnopharmacol. 2006;108:243–50. doi: 10.1016/j.jep.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 70.Formukong EA, Evans AT, Evans FJ. Analgesic and antiinflammatory activity of constituents of Cannabis sativa L. Inflammation. 1988;12:361–71. doi: 10.1007/BF00915771. [DOI] [PubMed] [Google Scholar]
- 71.Zurier RB. Prospects for cannabinoids as anti-inflammatory agents. J Cell Biochem. 2003;88:462–6. doi: 10.1002/jcb.10291. [DOI] [PubMed] [Google Scholar]
- 72.Yesilada E, Ezer N. The antiinflammatory activity of some Sideritis species growing in Turkey. Int J Crude Drug Res. 1989;27:38–40. [Google Scholar]
- 73.Yunes RA, Pizzolatti MG, Calixto JB, Goulart S, Ana AE, Hawkes GE. Abstracts of the phytochemical potential of tropical plants: An International Symposium. 2nd Joint Meeting of the Phytochemical Societies of Europe and North America, Miami Beach. 1992:8–12. [Google Scholar]
- 74.Ko HH, Hung CF, Wang JP, Lin CN. Antiinflammatory triterpenoids and steroids from Ganoderma lucidum and G.tsugae. Phytochemistry. 2008;69:234–9. doi: 10.1016/j.phytochem.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 75.Miller C, Yamaguchi RY, Ziboh VA. Guinea pig epidermis generates putative anti-inflammatory metabolites from fish oil polyunsaturated fatty acids. Lipids. 1989;24:998–1003. doi: 10.1007/BF02544068. [DOI] [PubMed] [Google Scholar]
- 76.Singh S, Nair V, Jain S, Gupta YK. Evaluation of anti-inflammatory activity of plant lipids containing alpha-linolenic acid. Indian J Exp Biol. 2008;46:453–6. [PubMed] [Google Scholar]
- 77.Oh PS, Lee SJ, Lim KT. Glycoprotein isolated from Rhus verniciflua Stokes inhibits inflammation-related protein and nitric oxide production in LPS-stimulated RAW 264.7 cells. Biol Pharm Bull. 2007;30:111–6. doi: 10.1248/bpb.30.111. [DOI] [PubMed] [Google Scholar]
- 78.Joy J, Nair CK. Nephro protective and anti-inflammatory activities of Rubia cordifolia. Amala Res Bull. 2007;27:118–27. [Google Scholar]
- 79.Kassuya CA, Leite DF, de Melo LV, Rohder VL, Calixto JB. Anti-inflammatory properties of extracts, fractions and lignans isolated from Phyllanthus amarus. Planta Med. 2005;71:721–6. doi: 10.1055/s-2005-871258. [DOI] [PubMed] [Google Scholar]
- 80.Lin LC, Shen CC, Shen YC, Tsai TH. Antiinflammatory neolignans from Piper kadsura. J Nat Prod. 2006;69:842–4. doi: 10.1021/np0505521. [DOI] [PubMed] [Google Scholar]
- 81.Sosa S, Tubaro A, Kastner U, Glasl S, Jurenitsch J, Della Loggia R. Topical anti-inflammatory activity of a new germacrane derivative from Achillea pannonica. Planta Med. 2001;67:654–8. doi: 10.1055/s-2001-17363. [DOI] [PubMed] [Google Scholar]
- 82.Picerno P, Autore G, Marzocco S, Meloni M, Sanogo R, Aquino RP. Anti-inflammatory activity of verminoside from Kigelia africana and evaluation of cutaneous irritation in cell cultures and reconstituted human epidermis. J Nat Prod. 2005;68:1610–4. doi: 10.1021/np058046z. [DOI] [PubMed] [Google Scholar]
- 83.Das S, Samal D, Basu SP. Anti-inflammatory and antinociceptive activity of arbortristoside-A. J Ethnopharmacol. 2008;116:198–203. doi: 10.1016/j.jep.2007.11.034. [DOI] [PubMed] [Google Scholar]
- 84.Matsui T, Ito C, Itoigawa M, Okada T, Furukawa H. Anti-inflammatory activity of Phenylpropanoids and phytoquinoids from Illicium species in RBL-2H3 cells. Planta Med. 2007;73:662–5. doi: 10.1055/s-2007-981528. [DOI] [PubMed] [Google Scholar]
- 85.Han AR, Min HY, Nam JW, Lee NY, Wiryawan A, Suprapto W, et al. Identification of a new naphthalene and its derivatives from the bulb of Eleutherine Americana with inhibitory activity on lipopolysaccharide induced nitric oxide production. Chem Pharm Bull. 2008;56:1314–6. doi: 10.1248/cpb.56.1314. [DOI] [PubMed] [Google Scholar]
- 86.Seelinger G, Merfort I, Schempp CM. Antioxidant, antiinflammatory and antiallergic activities of luteolin. Planta Med. 2008;74:1667–7. doi: 10.1055/s-0028-1088314. [DOI] [PubMed] [Google Scholar]
- 87.Chen J, Cho JY, Hwang TL, Chen IS. Benzoic acid derivatives, acetophenones, and antiinflammatory constituents from Melicope semecarpifolia. J Nat Prod. 2008;71:71–5. doi: 10.1021/np0704349. [DOI] [PubMed] [Google Scholar]
- 88.Buyukokuroglu ME, Berashvili D, Gepdiremen A, Altinkeser M. Antiinflammatory and antinociceptive properties of luteolin diglucuronide and apigenin diglucuronide obtained from Perillan ankinensis. Asian J Chem. 2008;20:1900–6. [Google Scholar]
- 89.Ziyan L, Yongmei Z, Nan Z, Ning T, Baolin L. Evaluation of the anti-inflammatory activity of luteolin in experimental animal models. Planta Med. 2007;73:221–6. doi: 10.1055/s-2007-967122. [DOI] [PubMed] [Google Scholar]
- 90.Sindhu ER, Kuttan R. Antioxidant and anti-inflammatory activity of lutein and its ester. Amala Res Bull. 2007;27:261–70. [Google Scholar]
- 91.Byeon SE, Lee YG, Kim JC, Han JG, Lee HY, Cho JY. Hinokitiol: A natural tropolone derivative, inhibits TNF-alpha production in LPS-activated macrophages via suppression of NF-KB. Planta Med. 2008;74:828–33. doi: 10.1055/s-2008-1074548. [DOI] [PubMed] [Google Scholar]
- 92.Hong SS, Lee SA, Han XH, Lee MH, Hwang S, Park JS, et al. Melampolides from the leaves of Smallanthus sonchifolius and their inhibitory activity of LPS-induced nitric oxide production. Chem Pharm Bull. 2008;56:199–202. doi: 10.1248/cpb.56.199. [DOI] [PubMed] [Google Scholar]
- 93.Choi YH, Shin EM, Kim YS, Cai XF, Lee JJ, Kim HP. Anti-inflammatory principles from the fruits of Evodia rutaecarpa and their cellular action mechanisms. Arch Pharmacal Res. 2006;29:293–7. doi: 10.1007/BF02968573. [DOI] [PubMed] [Google Scholar]
- 94.Yao C, Lin M, Wang L. Isolation and biomimetic synthesis of anti-inflammatory stilbenolignans from Gnetum cleistostachyum. Chem Pharm Bull. 2006;54:1053–7. doi: 10.1248/cpb.54.1053. [DOI] [PubMed] [Google Scholar]
- 95.Fan CQ, Zhu XZ, Zhan ZJ, Ji XQ, Li H, Yue JM. Lignans from Saussurea conica and their NO production suppressing activity. Planta Med. 2006;72:590–5. doi: 10.1055/s-2006-931565. [DOI] [PubMed] [Google Scholar]
- 96.Jin XY, Lee SH, Kim JY, Zhao YZ, Park EJ, Lee BS, et al. Polyozellin inhibits nitric oxide production by down-regulating LPS-induced activity of NF-kB and SAPK/JNK in RAW 264.7 cells. Planta Med. 2006;72:857–9. doi: 10.1055/s-2006-946640. [DOI] [PubMed] [Google Scholar]
- 97.Lee J, Jung E, Lim J, Lee J, Hur S, Kim SS, et al. Involvement of nuclear factor-kB in the inhibition of pro-inflammatory mediators by pinosylvin. Planta Med. 2006;72:801–6. doi: 10.1055/s-2006-941545. [DOI] [PubMed] [Google Scholar]
- 98.Ko HC, Kuo YH, Wei BL, Chiou WF. Laxifolone A suppresses LPS/IFN-gamma-induced NO synthesis by attenuating NF-Kb translocation: Role of NF-kB p 105 level. Planta Med. 2005;71:514–9. doi: 10.1055/s-2005-864151. [DOI] [PubMed] [Google Scholar]
- 99.Koo HJ, Lim KH, Jung HJ, Park EH. Anti-inflammatory evaluation of gardenia extract, geniposide and genipin. J Ethnopharmacol. 2006;103:496–500. doi: 10.1016/j.jep.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 100.Patel B, Patel S, Hoffman R. Inhibition of cyclo-oxygenase-2 expression in mouse macrophages by 4-(3-methyl-but-1-enyl)-3,5,3’,4’-tetrahydroxystilbene: A resveratrol derivative from peanuts. Phytother Res. 2005;19:552–5. doi: 10.1002/ptr.1698. [DOI] [PubMed] [Google Scholar]
- 101.Han AR, Kim MS, Jeong YH, Lee SK, Seo EK. Cyclooxygenase-2 inhibitory phenylbutenoids from the rhizomes of Zingiber cassumunar. Chem Pharm Bull. 2005;53:1466–8. doi: 10.1248/cpb.53.1466. [DOI] [PubMed] [Google Scholar]
- 102.Marsik P, Kokoska L, Landa P, Nepovim A, Soudek P, Vanek T. In vitro inhibitory effects of thymol and quinones of Nigella sativa seeds on cyclooxygenase-1-and-2-catalyzed prostaglandin E2 biosyntheses. Planta Med. 2005;71:739–42. doi: 10.1055/s-2005-871288. [DOI] [PubMed] [Google Scholar]
- 103.Stulzer HK, Tagliari MP, Zampirolo JA, Cechinel-Filho V, Schlemper V. Antioedematogenic effect of marrubiin obtained from Marrubium vulgare. J Ethnopharmacol. 2006;108:379–84. doi: 10.1016/j.jep.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 104.Won JH, Kim JY, Yun KJ, Lee JH, Back NI, Chung HG, et al. Gigantol isolated from the whole plants of Cymbidium goeringii inhibits the LPS-induced INOS and COX-2 Expression via NF-kB inactivation in RAW 264.7 macrophages cells. Planta Med. 2006;72:1181–7. doi: 10.1055/s-2006-947201. [DOI] [PubMed] [Google Scholar]
- 105.Rasool M, Sabina EP, Lavanya B. Anti-inflammatory effect of Spirulina fusiformis on adjuvant-induced arthritis in mice. Biol Pharm Bull. 2006;29:2483–7. doi: 10.1248/bpb.29.2483. [DOI] [PubMed] [Google Scholar]
- 106.Yonathan M, Asres K, Assefa A, Bucar F. In vivo anti-inflammatory and anti-nociceptive activities of Cheilanthes farinosa. J Ethnopharmacol. 2006;108:462–70. doi: 10.1016/j.jep.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 107.Lim CS, Jin DQ, Sung JY, Lee JH, Choi HG, Ha I, et al. Antioxidant and anti-inflammatory activities of the methanolic extract of Neorhodomela aculeate in hippocampal and microglial cells. Biol Pharm Bull. 2006;29:1212–6. doi: 10.1248/bpb.29.1212. [DOI] [PubMed] [Google Scholar]
- 108.Bingol F, Sener B. A review of terrestrial plants and marine organisms having antiinflammatory activity. Pharm Biol. 1995;33:81–97. [Google Scholar]
- 109.Konig GM, Wright AD, Sticher O, Jurcic K, Offer-mann F, Redl K, et al. Abstracts of the International Research Congress on Natural Products, and 32nd Annual Meeting of American Society of Pharmacognosy. Chicago, Illinois: 1991. pp. 129–52. [Google Scholar]
- 110.Kang JY, Khan MN, Park NH, Cho JY, Lee MC, Fujii H, et al. Antipyretic, analgesic, and anti-inflammatory activities of the sea weed Sargassum fulvellum and Sargassum thunbergii in mice. J Ethnopharmacol. 2008;116:187–90. doi: 10.1016/j.jep.2007.10.032. [DOI] [PubMed] [Google Scholar]
- 111.Hua KF, Hsu HY, Su YC, Lin IF, Yang SS, Chen YM, et al. Study on the antiinflammatory activity of methanol extract from sea grass Zostera japonica. J Agr Food Chem. 2006;54:306–11. doi: 10.1021/jf0509658. [DOI] [PubMed] [Google Scholar]
- 112.Shu XS, Gao ZH, Yang XL. Anti-inflammatory and anti-nociceptive activities of Smilax china L. aqueous extract. J Ethnopharmacol. 2006;103:327–32. doi: 10.1016/j.jep.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 113.Park EK, Ryu MH, Kim YH, Lee YA, Lee SH, Woo DH, et al. Antiinflammatory effects of an ethanolic extract from Clematis mandshurica Rupr. J Ethnopharmacol. 2006;108:142–7. doi: 10.1016/j.jep.2006.04.025. [DOI] [PubMed] [Google Scholar]
- 114.Liu BG, Jia XM, Cao YY, Chen SH, Gao PH, Wang Y, et al. Changtai granule: A traditional Chinese drug, protects hapten-induced colitis by attenuating inflammatory and immune dysfunctions. J Ethnopharmacol. 2008;115:1–8. doi: 10.1016/j.jep.2007.08.045. [DOI] [PubMed] [Google Scholar]
- 115.Jung HW, Yoon CH, Kim YH, Boo YC, Park KM, Park YK. Wen-Pi-Tang-Hab-Wu-Ling-San extract inhibits the release of inflammatory mediators from LPS-stimulated mouse macrophages. J Ethnopharmacol. 2007;114:439–45. doi: 10.1016/j.jep.2007.08.035. [DOI] [PubMed] [Google Scholar]
- 116.Kaileh M, Berghe WV, Boone E, Essawi T, Haegeman G. Screening of indigenous Palestinian medicinal plants for potential anti-inflammatory and cytotoxic activity. J Ethnopharmacol. 2007;113:510–6. doi: 10.1016/j.jep.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 117.Lyu SA, Lee SY, Lee SJ, Son SW, Kim MO, Kim GY, et al. Seungma-galgeun-tang attenuates proinflammatory activities through the inhibition of NF- kB signal pathway in the BV- microglial cells. J Ethnopharmacol. 2006;107:59–66. doi: 10.1016/j.jep.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 118.Tseng SH, Lee HH, Chen LG, Wu CH, Wang CC. Effects for three purgative decoctions on inflammatory mediators. J Ethnopharmacol. 2006;105:118–24. doi: 10.1016/j.jep.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 119.Lo YC, Lin YL, Yu KL, Lai YH, Wu YC, Ann LM, et al. San-Huang-Xie-Xin-Tang attenuates inflammatory responses in lipopolysaccharide-exposed rat lungs. J Ethnopharmacol. 2005;101:68–74. doi: 10.1016/j.jep.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 120.Rhule A, Navarro S, Smith JR, Shepherd DM. Panax notoginseng attenuates LPS-induced pro-inflammatory mediators in RAW264.7 cells. J Ethnopharmacol. 2006;106:121–8. doi: 10.1016/j.jep.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 121.Bae EA, Kim EJ, Park JS, Kim HS, Ryu JH, Kim DH. Ginsenosides Rg3 and Rh2inhibit the activation of AP-1 and protein kinase A pathway in lipopolysaccharide/interferon-gamma-stimulated by BV-2 microglial cells. Planta Med. 2006;72:627–33. doi: 10.1055/s-2006-931563. [DOI] [PubMed] [Google Scholar]
- 122.Liu L, Ning ZQ, Shan S, Zhang K, Deng T, Ping Lu X, et al. Phathalide lactones from Ligusticum chuanxiong inhibit lipopolysaccharide-induced TNF-alpha production and TNF-alpha-medicated NF-kB activation. Planta Med. 2005;71:808–13. doi: 10.1055/s-2005-871231. [DOI] [PubMed] [Google Scholar]
- 123.Fang QM, Zhang H, Cao Y, Wang C. Antiinflammatory and free radical scavenging activities of ethanol extract of three seeds used as “Bolengguazi”. J Ethnopharmacol. 2007;114:61–5. doi: 10.1016/j.jep.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 124.Shreedhara CS, Vaidya VP. Screening of Momordica dioica for hepatoprotective, antioxidant, and antiinflammatory activities. Nat Prod Sci. 2006;12:157–61. [Google Scholar]
- 125.Goulart S, Moritz MI, Lang KL, Liz R, Schenkel EP, Frode TS. Anti-inflammatory evaluation of Solidago chilensis Meyen in a murinemodel of pleurisy. J Ethnopharmacol. 2007;113:346–53. doi: 10.1016/j.jep.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 126.Enk R, Ehehalt R, Graham JE, Bierhaus A, Remppis A, Greten HJ. Differential effect of Rhizoma coptidis and its main alkaloid compound berberine on TNF-alpha induced NFkB translocation in human keratinocytes. J Ethnopharmacol. 2007;109:170–5. doi: 10.1016/j.jep.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 127.Orhan I, Kupeli E, Sener B, Yesilada E. Appraisal of anti-inflammatory potential of the clubmoss, Lycopodium clavatum L. J Ethnopharmacol. 2007;109:146–50. doi: 10.1016/j.jep.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 128.Barros IM, Lopes LD, Borges MO, Borges AC, Ribeiro MN, Freire SM. Anti-inflammatory and anti-nociceptive activities of Pluchea quitoc (DC.) ethanolic extract. J Ethnopharmacol. 2006;106:317–20. doi: 10.1016/j.jep.2006.01.030. [DOI] [PubMed] [Google Scholar]
- 129.Costa SS, de Souza M, de L, Ibrahim T, de Melo GO, de Almeida AP, et al. Kalanchosine dimalate, an anti-inflammatory salt from Kalanchoe brasiliensis. J Nat Prod. 2006;69:815–8. doi: 10.1021/np050475+. [DOI] [PubMed] [Google Scholar]
- 130.Ara T, Maeda Y, Fujinami Y, Imamura Y, Hattori T, Wang PL. Preventive effects of a Kampo medicine, Shosaikoto, on inflammatory responses in LPS-treated human gingival fibroblasts. Biol Pharm Bull. 2008;31:1141–4. doi: 10.1248/bpb.31.1141. [DOI] [PubMed] [Google Scholar]
- 131.Herbal Medicine. National Center for Complementary and Alternative Medicine. [accessed on 2009 Sep 1]. Available from: http://www.nlm.nih.gov/medlineplus/herbalmedicine.html .
- 132.Herbal Medicine Safety. Herbal medications are very popular, but are they safe? From Marian Anne Eure, former About.com Guide. [accessed on 2009 Sep 24]. Available from: http://seniorhealth.about.com/cs/altmedicine1/a/herb_safety.htm .
- 133.Miller LG. Herbal medicinals: Selected clinical considerations focusing on known or potential drug-herb interactions. Arch Int Med. 1998;158:2200–11. doi: 10.1001/archinte.158.20.2200. [DOI] [PubMed] [Google Scholar]
- 134.Izzo AA, Ernst E. Interactions between herbal medicines and prescribed drugs:a systematic review. Drugs. 2001;61:2163–75. doi: 10.2165/00003495-200161150-00002. [DOI] [PubMed] [Google Scholar]
- 135.Cupp MJ. Herbal remedies: Adverse effects and drug interactions. Am Fam Phys. 1999;59:1239–45. [PubMed] [Google Scholar]
- 136.Ernst E. The risk-benefit profile of commonly used herbal therapies: Ginkgo, St. John's Wort, Ginseng, Echinacea, Saw Palmetto, and Kava. Ann Intern Med. 2002;136:42–53. doi: 10.7326/0003-4819-136-1-200201010-00010. [DOI] [PubMed] [Google Scholar]
- 137.Abebe W. Herbal medication: Potential for adverse interactions with analgesic drugs. J Clin Pharm Ther. 2002;27:391–401. doi: 10.1046/j.1365-2710.2002.00444.x. [DOI] [PubMed] [Google Scholar]
- 138.Hukkeri VI, Savadi RV, Tippimath CD, Karadi RV, Jaiprakash B. Antiinflammatory activity of leaves of Acacia farnesiana Willd. Ind Drugs. 2002;39:664–6. [Google Scholar]
- 139.Kumar V, Singh PN, Bhattacharya SK. Antiinflammatory and analgesic activity of Indian Hypericum perforatum L. Ind J Exp Biol. 2001;39:339–43. [PubMed] [Google Scholar]
- 140.Gao ZZ, Xia YF, Yao XJ, Dai Y, Wang Q. A new triterpenoids saponin from Gleditisia sinensis and structure-activity relationships of inhibitory effects on lipopolysaccharide-induced nitric oxide production. Nat Prod Res. 2008;22:320–32. doi: 10.1080/14786410701766422. [DOI] [PubMed] [Google Scholar]
- 141.Manez S, Hernandez V, Giner RM, Rios JL, del Carmen Recio M. Inhibition of pro-inflammatory enzymes by inuviscolide: A sesquiterpene lactone from Inula viscosa. Fitoterapia. 2007;78:329–31. doi: 10.1016/j.fitote.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 142.Tang WZ, Liu YB, Yu SS, Qu J, Su DM. New sesquiterpene lactone and neolignan glycosides with antioxidant and anti-inflammatory activities from the fruits of Illicium oligandrum. Planta Med. 2007;73:484–90. doi: 10.1055/s-2007-967189. [DOI] [PubMed] [Google Scholar]
- 143.Jimenez-Estrada M, Chilpa RR, Apan TR, Lledias F, Hansberg W, Arrieta D, et al. Antiinflammatory activity of cacalol and cacalone sesquiterpenes isolated from Psacalium decompositum. J Ethnopharmacol. 2006;105:34–8. doi: 10.1016/j.jep.2005.09.039. [DOI] [PubMed] [Google Scholar]
- 144.Shinde UA, Phadke AM, Nair AM, Mungantiwar AA, Dikshit VJ, Saraf MN. Studies on the antiinflammatory and analgesic activity of Cedrus deodraa (Roxb.) Loud. Wood oil. J Ethnopharmacol. 1999;65:21–7. doi: 10.1016/s0378-8741(98)00150-0. [DOI] [PubMed] [Google Scholar]
- 145.Madav SS, Tadan K, Lal J, Tripathi HC. Anti-inflammatory activity of andrographolide. Fitoterapia. 1996;67:452–8. [Google Scholar]
- 146.Herbal monograph. Himalaya herbal health care. [accessed on 2010 Apr 2]. Available from: http://www.himalayahealthcare.com/herbfinder/h_commip.html .
- 147.Deodhar SD, Sethi R, Srimal RC. Preliminary study on antirheumatic activity of curcumin (diferuloyl methane) Indian J Med Res. 1980;71:632–4. [PubMed] [Google Scholar]
- 148.Satoskar RR, Shah SJ, Shenoy SG. Evaluation of anti-inflammatory property of curcumin (diferuloyl methane) in patients with postoperative inflammation. Int J Clin Pharmacol Ther Toxicol. 1986;24:651–4. [PubMed] [Google Scholar]
- 149.Simmet T, Ammon HP. Use of boswellic acid for treating brain tumors. 2001 US6174876. [Google Scholar]
- 150.Gupta I, Gupta V, Parihar A, Gupta S, Ludtke R, Safayhi H, Ammon HP. Effects of Boswellia serrata gum resin in patients with bronchial asthma: Results of a double-blind, placebo-controlled, 6-week clinical study. Eur J Med Res. 1998;3:511–4. [PubMed] [Google Scholar]
- 151.Kimmatkar N, Thawani V, Hingorani L, Khiyani R. Efficacy and tolerability of Boswellia serrata extract in treatment of osteoarthritis of knee: A randomized double blind placebo controlled trial. Phytomedicine. 2003;10:3–7. doi: 10.1078/094471103321648593. [DOI] [PubMed] [Google Scholar]
- 152.Chrubasik S, Eisenberg E, Balan E, Weinberger T, Luzzati R, Conradt C. Treatment of low back pain exacerbations with willow bark extract: A randomized double-blind study. Am J Med. 2000;109:9–14. doi: 10.1016/s0002-9343(00)00442-3. [DOI] [PubMed] [Google Scholar]
- 153.Biegert C, Wagner I, Ludtke R, Kotter I, Lohmuller C, Gunaydin I, et al. Efficacy and safety of willow bark extract in the treatment of osteoarthritis and rheumatoid arthritis:results of 2 randomized double-blind controlled trials. J Rheumatol. 2004;31:2121–30. [PubMed] [Google Scholar]
- 154.Chrubasik S, Thanner J, Kunzel O, Conradt C, Black A, Pollak S. Comparison of outcome measures during treatment with the proprietary Harpagophytum extract doloteffin in patients with pain in the lower back, knee or hip. Phytomedicine. 2002;9:181–94. doi: 10.1078/0944-7113-00140. [DOI] [PubMed] [Google Scholar]
- 155.Chrubasik S, Kunzel O, Thanner J, Conradt C, Black A. A 1-year follow-up after a pilot study with Doloteffin for low back pain. Phytomedicine. 2005;12:1–9. doi: 10.1016/j.phymed.2004.01.005. [DOI] [PubMed] [Google Scholar]


