Abstract
The genus Alnus has been reviewed for its chemical constituents and biological activities including traditional importance of some common species. The plants of this genus contain terpenoids, flavonoids, diarylheptanoids, phenols, steroids, and tannins. Diarylheptanoids are the dominant constituents within the genus Alnus, few of them exhibited antioxidant effects and inhibitory activity against nuclear factor kappaB activation, nitric oxide and tumor necrosis factor-α production, human umbilical vein endothelial cells, farnesyl protein transferase, cell-mediated low-density lipoprotein oxidation, HIF-1 in AGS cells, and the HIV-1-induced cytopathic effect in MT-4 cells. Some ellagitannines showed hepatoprotective activity even in a dose of 1 mg/kg which is ten-fold smaller compared with the dose of traditional flavonoid-based drugs. The members of genus Alnus are well known for their traditional uses in the treatment of various diseases like cancer, hepatitis, inflammation of uterus, uterine cancer, rheumatism, dysentery, stomachache, diarrhea, fever, etc. The aim of the present review is to summarize the various researches related to the chemistry and pharmacology of genus Alnus.
Keywords: Alnus, antioxidant effects, diarylheptanoids, HIV-I, inhibitory activity
INTRODUCTION
Betulaceae or the Birch family includes six genera of deciduous nut-bearing trees and shrubs, including the birches, alders, hazels, hornbeams, and hop-hornbeams, numbering about 130 species. These are mostly natives of the temperate Northern Hemisphere, with a few species reaching the Southern Hemisphere in the Andes in South America. Alnus (alders) is an important genus belonging to Betulaceae which comprises 30 species worldwide.[1,2] Almost all plants of this genus have been traditionally used as folk medicine in Ayurveda, Unani, and Chinese medical systems.
OBJECTIVES OF THE REVIEW
Alnus is one of the genera having potential medicinal values. The plants of this genus have been found active against many live-threatening disorders like hepatitis, HIV-1 viral replication, and cancer. The aim of the present review is to delineate the various plants with their chemical constituents and biological activities. Various traditional uses of some common species have also been summarized. These informations can create a center of attention for scientists and herbologists for this genus, and consequently this database might play a major role in future research.
TRADITIONAL USES OF ALNUS SPECIES
The members of genus Alnus are well known for their traditional medicinal values. These have been used for the treatment of various diseases including cancer and as an alterative, astringent, cathartic, emetic, febrifuge, galactogogue, hemostatic, parasiticide, skin tonic, vermifuge, etc. Alnus japonica is a popular folk medicine in Korea for cancer and hepatitis.[3] The bark of Alnus glutinosa is alterative, astringent, cathartic, febrifuge, tonic, and useful in mouth and throat inflammations, the vinegar extract of inner bark of plant produces a useful wash to treat lice and a range of skin problems such as scabies and scabs.[4–6] The leaf, roots, and bark of A. nepalensis are used in dysentery, stomach ache, and diarrhea in Indian system of medicine (Ayurveda).[7] A decoction of the root of A. nepalensis is prescribed to treat diarrhea and paste from the leaves is applied on cuts and wounds as a hemostatic.[8] The mixture of leaves of Alnus jorullensis and branches of Polylepis racemosa R. et P is used to treat inflammation of uterus, uterine cancer, and rheumatism.[9] The bark of Alnus hirsuta is used in Korean and Chinese traditional medicine as remedies for fever, hemorrhage, alcoholism, and diarrhea.[10,11] The decoction of A. glutinosa barks is used to treat swelling, inflammation, and rheumatism.[12] It has also been used as an astringent, bitter, emetic, and hemostatic, and for the treatment of sore throat and pharyngitis.[13,14] Contemporary indigenous healers used the bark of Alnus rubra for various medicinal teas.[15,16]
CHEMICAL CONSTITUENTS OF GENUS ALNUS
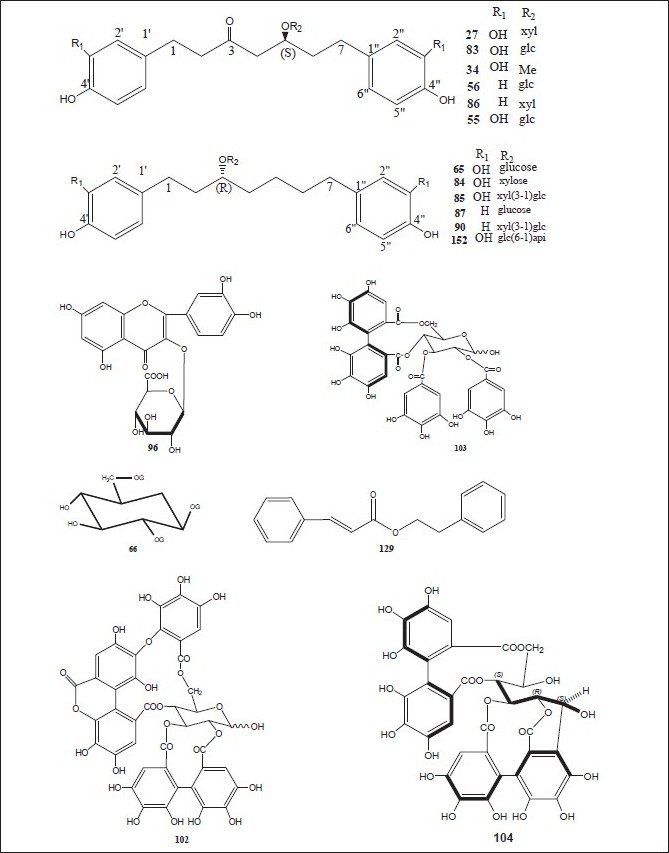
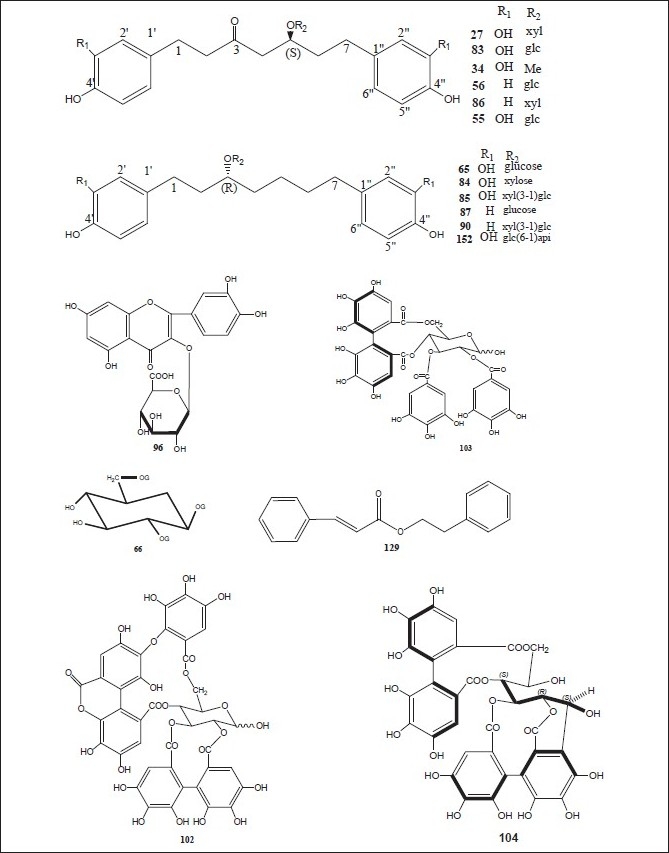
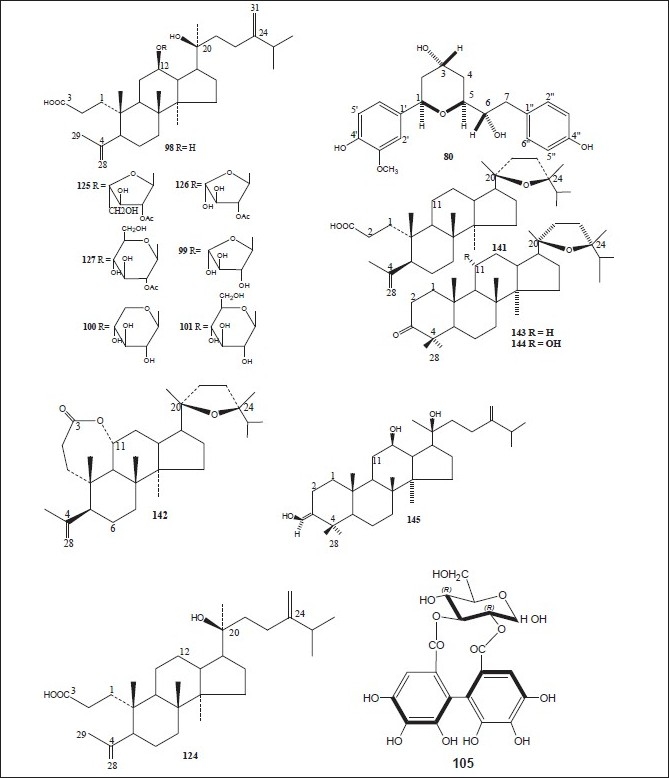
The plants of the genus Alnus contain various types of plant secondary metabolites including terpenoids, flavonoids, diarylheptanoids, phenols, steroids, tannins, and many others. The plants and their chemical constituents have been summarized below, whereas the chemical structures of various compounds isolated from different parts of genus Alnus are drawn in Figures 1–8.
Figure 1.
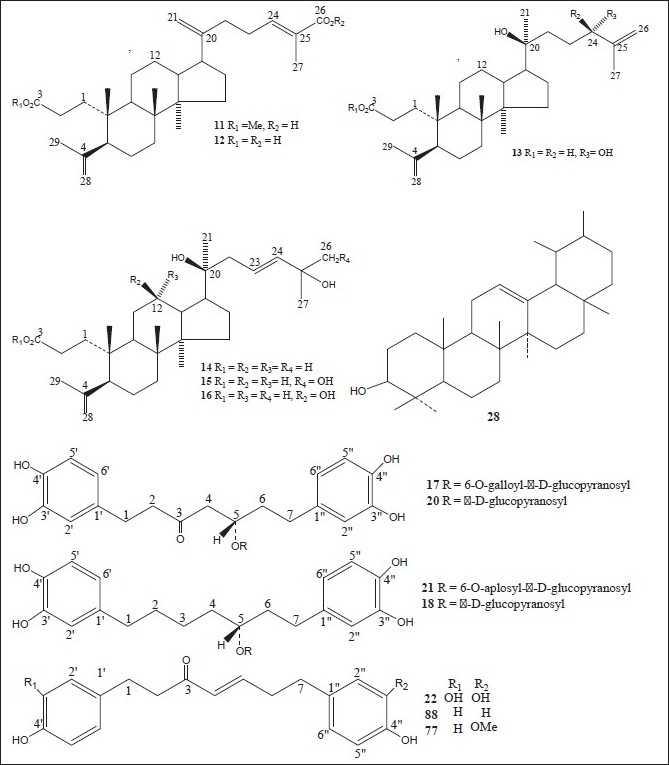
Chemical structures of compounds isolated from genus Alnus
Figure 8.

Chemical structures of compounds isolated from genus Alnus
Figure 2.
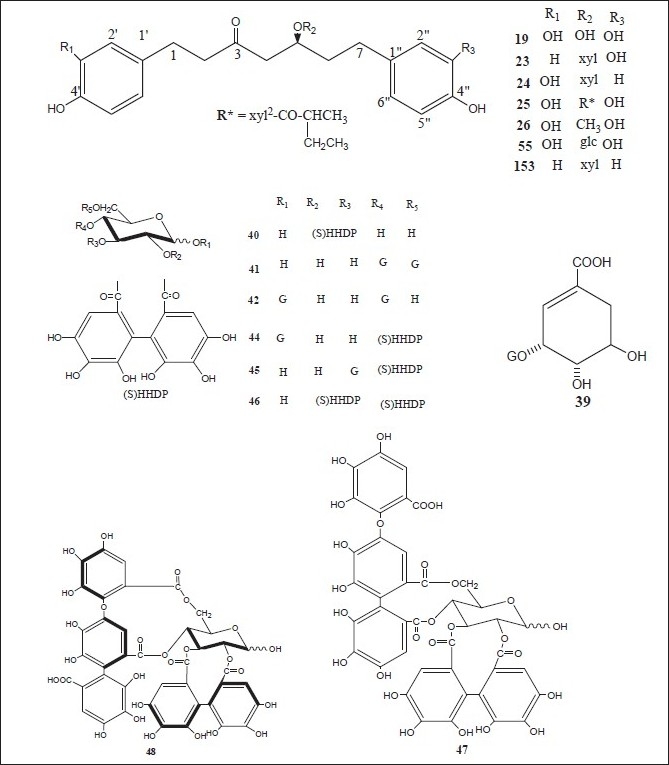
Chemical structures of compounds isolated from genus Alnus
Figure 3.
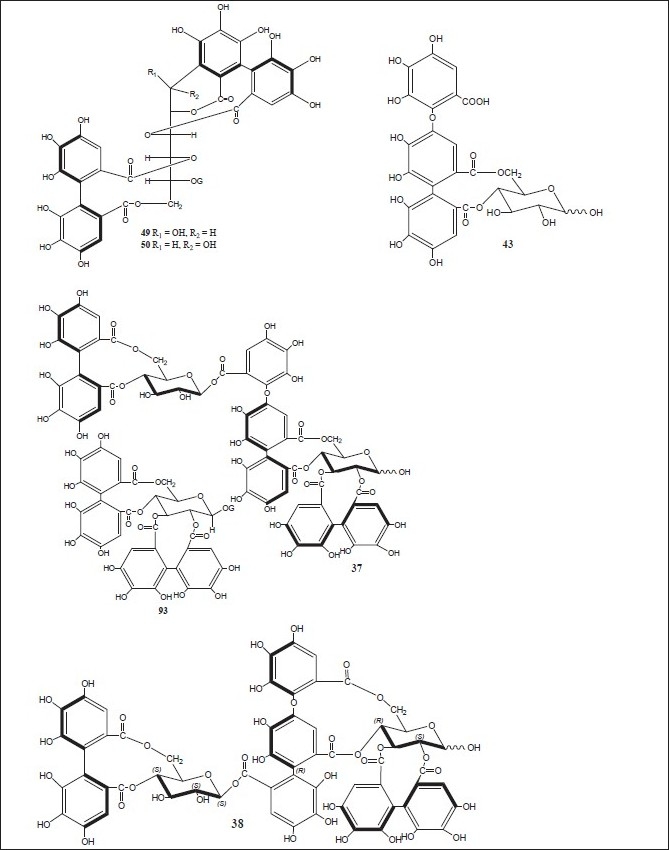
Chemical structures of compounds isolated from genus Alnus
Figure 4.
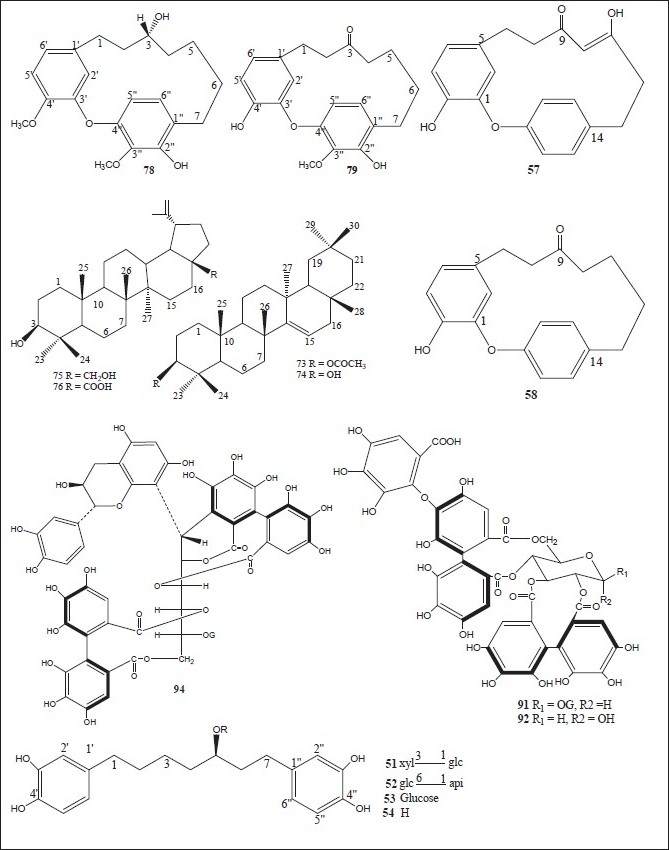
Chemical structures of compounds isolated from genus Alnus
Figure 5.
Chemical structures of compounds isolated from genus Alnus
Figure 6.
Chemical structures of compounds isolated from genus Alnus
Figure 7.
Chemical structures of compounds isolated from genus Alnus
Chemical constituents of Alnus japonica
Luteolin 7,4’-dimethyl ether (pillion) (1); scutellarein-6,7,4’-trimethyl ether (salvigenin) (2); kaempferide (3); isohamnetin (4); rhamnazin (5); quercetin-7,3’,4’-trimethyl ether (6); the 3,6,4’-trimethyl ether of 6-hydroxy-kaempferol (7); acacetin (8); apigenin-7,4’-dimethyl ether (9); scutellarein-6,4’-dimethyl ether (10); methyl (24E)-3,4-secodammara-4(28),20,24-trien-26-oic acid-3-oate (11); (24E)-3,4-secodammara-4(28),20,24-trien-3,26-dioic acid (12); (20S,24S)-20,24-dihydroxy-3,4-secodammara-4(28),23-dien-3-oic acid (13); (23E)-(20S)-20,25-dihydroxy-3,4-secodammara-4(28),23-dien-3-oic acid (14); (23E)-(20S)-20,25,26-trihydroxy-3,4-secodammara-4(28),23-dien-3-oic acid (15); (23E)-(12R,20S)-12,20,25-trihydroxy-3,4-secodammara-4(28),23-dien-3-oic acid (16); hirsutanonol 5-O-(6-O-galloyl)-β-D-glucopyranoside (17); 3-deoxo-hisutanonol 5-O-β-D-gucopyranoside (18); hirsutanonol (19); hirsutanonol 5-O-β-D-glucopyranoside (20); 3-deoxo-hisutanonol 5-O-(6-O- β-D-apiosyl)- β-D- glucopyranoside (21); hirsutenone (22); 7-(3,4-dihydroxyphenyl)-5-hydroxy-1-(4-hydroxyphenyl)-3-heptanone-5-O-β-D-xylopyranoside (23); 1-(3,4-dihydroxyphenyl)-5-hydroxy-7-(4-hydroxyphenyl)-3-heptanone-5-O-β-D-xylopyranoside (24); 1,7-bis-(3,4-dihydroxyphenyl)-5-hydroxy-3-heptanone-5-O-[2-(2-methylbutenoyl)]-β-D-xylopyranoside (25); 1,7-bis-(3,4-dihydroxyphenyl)-5-methoxy-3-heptanone (26); oregonin (27); β-amyrin (28); 3-O-acetyl- β-amyrin (29); 3-O-acetyltaraxerol (30); glutinone (31), lupenone (32); quercitin (33); 5-O-methylhirsutanonol (34); glutinol (35); taraxerone (36); alnusjaponins A (37); alnusjaponins B (38); 5-O-galloyl-(-)-shikimic acid (39); 2,3-(S)-hexahydroxydiphenoyl-D-glucose (40); 4,6-di-O-galloyl-D-glucose (41); 1,4-di-O-galloyl- β -D-glucose (42); 4,6-(S)-valoneoyl-D-glucose (43); strictinin (44); gemin D (45); pedunculagin (46); praecoxin A (47); flosin A (48); stachyurin (49); casuarinin (50); 1,7-bis-(3,4-dihydroxyphenyl)-heptane-3-O-β-D-glucopyranosyl(1→3)-β-D-xylopyranoside (51); 1,7-bis-(3,4-dihydroxyphenyl)-heptane-3-O-β-D-apiofuranosyl(1→6)-β-D-glucopyranoside (52); 1,7-bis-(3,4-dihydroxyphenyl)-heptane-5-O-β-D-glucopyranoside (53); 1,7-bis-(3,4-dihydroxyphenyl)-5-hydroxyheptane (54); 1,7-bis-(3,4-dihydroxyphenyl)-heptane-3-one-5-O-β-D-glucopyranoside (55); platyphylloside (56); garugamblin-3 (57); acerogenin L (58); oregonoyl A (59); oregonoyl B (60); platyphyllone (61) and platyphyllonol-5- xylopyranoside (62).[17–30]
Chemical constituents of Alnus hirsuta
(5S)1,7-Bis-(3,4-dihydroxyphenyl)-heptane-5-hydroxy-3-one {hirsutanonol} (19); hirsutanone (22); (5S)1,7-bis-(3,4-dihydroxyphenyl)-heptane-3-one-5-O-β-D-xylopyranoside{oregonin} (27); (5S)-O-methylhirsutanonol (34); pedunculagin (46); praecoxin A (47); (5R)1,7-bis-(3,4-dihydroxyphenyl)-heptane-5-O-β-D- glucopyranoside (53); (5S)1,7-bis-(3,4-dihydroxyphenyl)-heptane-3-one-5-O-β-D-glucopyranoside (55); platyphylloside (56); gentisic acid 5-O-β-D-(6’-O-galloyl)glucopyranoside (63); tellimagrandin I (64); rubranoside A (65); 1,2,6-tri-O-galloyl-β-D-glucose (66); 1,4,6-tri-O-galloyl-β-D-glucose (67); 1-desgalloyleugeniin (68); 1-desgalloylrugosin F (69); rugosin F (70); hirsunin (71); (5R)-1,7-bis (3,4-dihydroxyphenyl)-heptane-5-ol (72); taraxeryl acetate (73); taraxerol (74); betulin (75); betulinic acid (76); 1-(4-hydroxyphenyl)-7-(4-hydroxy-3-methoxyphenyl)-4-hept-3-one (77); 2-oxytrycyclo[13.2.2.13,7]eicosa-3,5,7(20),15,17,18-hexaen-10-16-diol (78); 2-oxytrycyclo[13.2.2.13,7]eicosa-3,5,7-(20),15,17,18-hexaen-10-one (79); rhoiptelol B (80); (5R)1,7-bis-(3,4-dihydroxyphenyl)-heptane-5-O-β-D- xylopyranoside (81); hirsutoside (82); hirsutanonol 5-O-beta-D-glucopyranoside (83); rubranoside B (84); rubranoside C (85); platyphyllonol 5-O-beta-D-xylopyranoside (86); aceroside VII (87); platyphyllenone (88); (5R)-1,7-bis(3,4-dihydroxyphenyl)-heptane-5-O--β-D-glucoside (89) and (3R)-1,7-bis-(4-dihydroxyphenyl)-3-heptanol-3-O-β-D-glucopyranosyl(1→3)-β-D xylopyranoside (90).[11,30–44]
Chemical constituents of Alnus sieboldiana
Strictinin (44); pedunculagin (46); stachyurin (49); casuarinin (50); 1,2,6-tri-O-galloyl-β-D-glucose (66); 1-O-galloyl-2,3-(S)hexahydroxydiphenoyl-4,6-(s)tergalloyl-β-D-glucose (91); 2,3-(s)hexahydroxydiphenoyl-4,6-(s)tergalloyl-β-D-glucose (92); 1(β)-O-galloylpendunculagin (93); stenophyllanin A (94); pinocembrin (95); quercetin-3-O-β-D-glucuronide (96); alnuserrudiolone (97); alnustic acid (98); alnustic acid 12-O-α-L-arabinofuranoside (99); alnustic acid 12-O-β-D-xylopyranoside (100); alnustic acid 12-O-β-D-glucopyranoside (101); alnusiin (102); tellimagrandin I (103); casuariin (104); 2,3-O-(S)-hexahydroxydiphenoyl-D-glucose (105); 3,5,7-trihydroxy-6-methoxyflavone (106); 5-hydroxy-6,7,8-tritmethoxyflavone (107); 5-hydroxy-3,6,7-trimethoxyflavone (108); 3,5,7-trihydroxy-6-methoxyflavanone (109); chrysin (110); izalpinin (111), tectochrysin (112); pinobanksin (113); strobopinin (114); naringenin (115); pinosylvin (116); pinosylvin monomethyl ether (117); pinosylvin dimethyl ether (118); yashabushidiol A (119); yashabushidiol B (120); yashabushiketodiol A (121); yashabushiketodiol B (122); yashabushitriol (123).[29,45–54]
Chemical constituents of Alnus pendula
Pinocembrin (95); alnustic acid (98); 12-O-α-L- arabinofuranoside of alnustic acid (99); 12-O-β-D- xylopyranoside of alnustic acid (100); 12-O-β-D- glucopyranoside of alnustic acid (101); pinosylvin (116); monomethyl ether of pinosylvin (117); 12-deoxy alnustic acid {(20S)-20-hydroxy-24-methylene-3,4-secodammar-4-(28)-en-3-oic acid} (124); alnustic acid 12-O-(2’-O-acetyl)-α-L-arabinofuranoside (125); (12R, 20S)-12-O-(2’-O-acetyl-β-D-xylopyranosyl)-20-hydroxy-24-methylene-3,4-secodammae-4(28)-en-3-oic acid (126); (12R, 20S)-12-O-(2’-O-acetyl-β-D-glucopyranosyl)-20-hydroxy-24-methylene-3,4-secodammae-4(28)-en-3-oic acid (127); alnustone (1,7-diphenyl-1,3-heptadien-5-one) (128); β-phenylethyl cinnamate (129); cinnamaldehyde (130); benzylacetone (131); β-phenylethyl alcohol (132); pinostrobin (133); alpinetin (134); galangin (135); eugenol (136); chavicol (137); cinnamic acid (138); β-phenylpropionic acid (139) and benzoic acid (140).[55–58]
Chemical constituents of Alnus serrulatoides (Call.)
Alnuserrudiolone (97); alnustic acid (98); alnustic acid-12-O- α-L-arabinofuranoside (99); alnustic acid-12-O- β-D-xylopyranoside (100); alnustic acid-12-O-β-D-glucopyranoside (101); alnustic acid-12-O-(2’-O-acetyl)-α-L-arabinofuranoside (125); alnustic acid-12-O-(2’-O-acetyl)- β-D-xylopyranoside (126); alnustic acid-12-O-(2’-O-acetyl)- β-D-glucopyranoside (127); alnuseric acid {(20S,24R)-20,24-epoxy-24-methyl-3,4-secodammar-4(28)-en-3-oic acid} (141); alnuselide {(11R,20S,24R)-20,24- epoxy-24-methyl-3,4-secodammar-4(28)-en-3-oic acid} (142); alnincanone (143); alnuserol (144) and alnuserrutriol (145).[59–66]
Chemical constituents of Alnus rubra
Salvigenin (2); acacetin (8); hirsutanonol (19); oregonin (27); glutinone (31); lupenone (32); 7,4’-dimethoxy-5-hydroxyflavone (34); taraxerone (36); 1,7-bis-(3,4-dihydroxyphenyl)-heptan-3-one-5-O-β-D-glucopyranoside (55); platyphylloside (56); rubranoside A (65); (5R)-1,7-bis-(3,4-dihydroxyphenyl)-heptane-5-ol (72); taraxeryl acetate (73); taraxerol (74); betulin (75); rubranoside B (84); rubranoside C (85); (5R)-1,7-bis-(3,4-dihydroxyphenyl)-heptane-5-O-β-D-glucoside (89); pectolinaringenin (146); lupeol (147); β-sitosterol (148); diarylheptenone-1-(3’,4’-dihydroxyphenyl)-7-(4”-hydroxyphenyl)-4-hepten-3-one (149); 1,7-bis(p-hydroxyphenyl)-4-hepten-3-one (150); apigenin (151); rubranoside D (152) and (s)-1,7-bis-(4-hydroxyphenyl)-heptane-3-on-5-O-β-D-xylopyranoside (153).[67–70]
Chemical constituents of Alnus crispa
Kaempferol 3, 7-dimethyl ether (kumatakenin) (154); quercetin 3,7-dimethyl ether (155) and quercetin-3,7,4’trimethyl ether (156).[29]
Chemical constituents of Alnus koehnei
Salvigenin (2); kaempferide (3); quercetin-7,3’,4’-trimethyl ether (6); 3,6,4’-trimethyl ether of 6-hydroxykaempferol (7); acacetin (8); quercetin-3,7-dimethyl ether (155); kaempferol (157); rhamnetin (158); isorhamnetin (159); quercetin-3,3’-dimethyl ether (160); the 3,6-dimethyl ether of 6-hydroxykaempferol (161), 6,4’-dimethyl ether of 6-hydroxykaempferol (162) and quercetagetin-3,6,4’-trimethyl ether (163).[29]
Chemical constituents of Alnus sinuate
Kumatakenin (154); quercetin-3,7-dimethyl ether (155) and genkwanin (164).[29]
Chemical constituents of Alnus nepalensis
(5S)1,7-Bis-(3,4-dihydroxyphenyl)-heptane-3-one-5-O-β-D-xylopyranoside{oregonin}(27); taraxerone (36); taraxerol (74); betulin (75); betulinic acid (76); lupeol (147) and β-sitosterol (148).[71–72]
Chemical constituents of Alnus fruticosa
β-Amyrin (28); lupenone (32); β-sitosterol (148); glutin-5-en-3-ol (165) and α-amyrin (166).[73]
Chemical constituents of Alnus kamtschatica
β-Amyrin (28); lupenone (32); β-sitosterol (148); glutin-5-en-3-ol (165) and α-amyrin (166).[73]
Chemical constituents of Alnus glutinosa
Hirsutanonol (19); oregonin (27); genkwanin (164); rhododendrin {3-(4-hydroxyphenyl)-l-methylpropyl-β-D-glucopyranoside} (167) and glutinic acid (2,3-pentadienedioic acid) (168).[74–77]
Chemical constituents of Alnus maximowiczii
Pinocembrin (95); pinosylvin monomethyl ether (117); pinosylvin dimethyl ether (118); 1,7-diphenylhept-3-en-5-one (169); 2,3,4-trimethoxyphenatheren (170); 1,7-diphenyl-hept-1-ene-3,5-dione (171); 1,7-diphenylheptane-3,5-dione (172); alnustinol (173); cryptomeridiol 11-o-monoacetate (174); β-eudesmol acetate (175) and elemol acetate (176).[78]
Chemical constituents of Alnus firma
β-phenylethyl cinnamate (129); pinostrobin (133); alpinetin (134); cinnamic acid (138); yashabushiketol (177); dihydroyashabushiketol (178); trans-stilbene (179); triglycerides (180); 4-hydroxy-2,6-dimethoxyphenyl-6’-O-syringoyl-β-D-glucopyranoside (181); 4-hydroxy-2,6-dimethoxyphenyl-6’-O-vanilloyl-β-D-glucopyranoside (182); myricetin-3-O- β-D-galactopyranoside (183) alnustic acid methyl ester (184); β-amyrin acetate (29); β-amyrin (28); β-sitosterol (148); β-sitosterol 3-O-β-D-glucoside (185); pinocembrin (95); alnustinol (173); quercetin (33); quercetin-3-O-α-L-arabinofuranoside (186); isoquercitrin (187); (+)-catechin (188); (–)-epicatechin (189) and quercetrin (190).[79–83]
Chemical constituents of Alnus cordata (Desf.)
Quercetol-3-sophoroside (191).[84]
BIOLOGICAL ACTIVITIES OF GENUS ALNUS
Inhibitory activity against HIF-1 in AGS cells
Jin et al. (2007) investigated the triterpenoids and diarylheptanoids isolated from EtOAc-soluble fraction from the MeOH extract of the stem bark of A. hirsuta (Betulaceae), for their effects on the hypoxia-induced HIF-1 activation using an HIF-1a-mediated reporter gene assay in AGS cells. Among the isolated compounds, two diarylheptanoids, 2-oxatrycyclo[13.2.2.13,7]eicosa-3,5,7(20),15,17,18-hexaen-10-16-diol (78) and 2-oxatrycyclo [13.2.2.13,7]eicosa-3,5,7-(20),15,17,18-hexaen-10-one (79), inhibited HIF-1 activation dose-dependently with 50% inhibitory concentrations (IC50) values of 11.2 and 12.3 microM, respectively. These two compounds had no significant cytotoxicity to the AGS cells at the effective concentration for the inhibition of HIF-1 activation.[10]
Antimicrobial activities
Middleton et al. (2005) tested antibacterial activity of A. glutinosa. The MeOH extract was found to be active against the following eight bacterial species: Citrobacter freundii NCTC 9750, Escherichia coli NCIMB 8110, Escherichia coli NCIMB 4174, Klebsiella aerogenes NCTC 9528, Lactobacillus plantarum NCIMB 6376, Pseudomonas aeruginosa NCTC 6750, Staphylococcus aureus NCTC 10788, and Staphylococcus aureus NCTC 11940 (MRSA), the most potent activity was against E. coli (NCIMB 8110) with an MIC value of 1.25 × 10-1 mg/ml. Despite the high MIC value against MRSA (1.00 mg/ml), this finding could be considered significant, at least qualitatively, because this activity was not due to a purified compound, but due to crude extract.[14] In 1995, Saxena et al. tested the antimicrobial activity of methanol extract of the bark of A. rubra against Gram-positive and Gram-negative bacteria. Diarylheptanoid and its glycoside (oregonin) were identified as the two constituents responsible for this activity.[69]
General toxicity
n-Hexane, DCM, and MeOH extracts of A. glutinosa were tested for general toxicity using the brine shrimp lethality assay by Middleton et al. in 2005. All three extracts of A. glutinosa showed low levels of toxicity toward brine shrimps (LD50 values were in the range of 1.29 × 10-1 to 8.30 × 10-1 mg/ml).[14]
Inhibitory effects in human umbilical vein endothelial cells
Han et al. (2007) reported the inhibitory effects of two diarylheptanoids, 5-O-methylhirsutanonol (34) and oregonin (27), isolated from the methanolic extracts of Alnus japonica leaves, on the expression of adhesion molecules in human umbilical vein endothelial cells (HUVECs). 5-O-methylhirsutanonol and oregonin inhibited tumor necrosis factor (TNF)-alpha-induced upregulation of vascular cell adhesion molecule-1 and intercellular adhesion molecule-1, which also prevented adhesion of monocytes to HUVECs, and slightly suppressed the mRNA expression of the inflammation-associated gene interleukin-1beta. A further study demonstrated the inhibitory effect of compound 5-O-methylhirsutanonol on DNA-binding of nuclear factor kappaB (NF-κB) and on the phosphorylation and degradation of inhibitory factor κBα in TNF-alpha-stimulated HUVECs. These results indicate that these two compounds may be useful in the prevention and treatment of atherosclerosis through attenuation of adhesion molecule expression by inhibition of NF-κB activation.[28]
Inhibition of cyclooxygenase-2 expression
Lee et al. (2000) examined the inhibitory effect of oregonin (27) and hirsutanonol (19) isolated from the bark of A. hirsuta on 12-O-tetradecanoylphorbol-13-acetate-induced cyclooxygenase-2 expression in immortalized human breast epithelial MCF10A cells.[38]
Nitric oxide synthesis inhibitory activity
Lee et al. (2000) assessed the leaves extract of A. hirsuta for nitric oxide (NO) production inhibitory effects in vitro. Oregonin (27) and hirsutanonol (19) were found to be potent inducible nitric oxide synthase (iNOS) inhibitors. These compounds showed inhibition of NO synthesis in dose-dependent manners by murine macrophage-like RAW 264.7 cells stimulated with interferon-gamma (IFN-gamma) plus lipopolysaccharide (LPS). Their IC50 were 3.8 and 14.3 microM, respectively. The inhibitory effects of these compounds on NO synthesis were due to suppression of iNOS mRNA expression, as determined by Northern blotting.[39]
Protective effects in human HepG2 cells
Park et al. (2010) assessed the diarylheptanoid derivatives isolated from the ethyl acetate-soluble fraction of the stem bark of A. hirsuta for their hepatoprotective effects against tert-butyl hydroperoxide (t-BHP)-induced toxicity in HepG2 cells. Of these isolates, compounds (27, 34, 57, and 79-84) sificant hepatoprotective effects on t-BHP-induced damage to HepG2 cells, with (5S)-O-methylhirsutanonol (34) exhibiting the greatest protective effect (50.7+/-3.7% at a concentration of 10 μM).[43]
Inhibitory activity on lipopolysaccharide-induced nitric oxide production in BV2 microglia
Methanolic extract of Alnus firma barks significantly attenuated NO production in LPS-stimulated BV2 microglia. Lee et al. (2010) found that 4-hydroxy-2,6-dimethoxyphenyl-6’-O-syringoyl-β-Dglucopyranoside, phenolic glycosides, and diarylheptanoids, isolated from the bark of A. firma showed significant inhibitory effect on LPS-induced NO production in BV2 microglial cells at concentration ranging from 10 to 100 microM.[80]
Inhibition against the HIV-1
Yu et al. (2007) observed that triterpenoids and flavonoids isolated from Alnus firma S. Z. inhibit HIV-1 virus replication and controlled its essential enzymes. In this study, the inhibition of HIV-1 viral replication and its essential enzymes, such as reverse transcriptase, protease, and alpha-glucosidase, were observed using 18 Korean plant extracts. Among the extracts, the methanol extract of Alnus firma leaves showed potent inhibition against the HIV-1-induced cytopathic effect (CPE) in MT-4 cells on microscopic observation (the minimum concentration for complete inhibition of HIV-1-induced CPE, IC = 50 μg/ml). The alnustic acid methyl ester (183) exhibited inhibition against HIV-1 protease, with an IC50 of 15.8 microM, quercetin (33) and myricetin 3-O-beta-D-galactopyranoside (182) displayed inhibition against HIV-1 reverse transcriptase, with IC50 values of 60 microM. Based on these results, however, inhibition of viral replication by methanol extract of Alnus firma leaves was adjudged to be acutely related to the protease inhibition activation of the alnustic acid methyl ester as well as the reverse transcriptase inhibition activation of flavonoids.[83]
Inhibitory activity against NF-kB activation and NO and TNF-a production
Jin et al. (2007) investigated diarylheptanoids from the stem bark of A. hirsuta for their inhibitory activity against LPS-induced NF-kB activation and NO and TNF-alpha production. Among them, compounds 2-oxytrycyclo[13.2.2.13,7]eicosa-3,5,7(20),15,17,18-hexaen-10-16-diol (78), 2-oxytrycyclo[13.2.2.13,7]eicosa-3,5,7-(20),15,17,18-hexaen-10-one (79), and (5S)1,7-bis-(3,4-dihydroxyphenyl)-heptane-5-hydroxy-3-one {hirsutanonol} (19) displayed inhibitory activity against NF-kB activation and NO and TNF-alpha production with IC50 values of 9.2-9.9 microM, 18.2-19.3 microM, and 22.3-23.7 microM, respectively, in RAW264.7 cells. These active compounds had no significant cytotoxicity in RAW264.7 cells at their effective concentrations. This supports the pharmacological use of A. hirsuta, which has been employed as a herbal medicine for the treatment of inflammatory diseases.[85]
Inhibitory activity on farnesyl protein transferase
Kang et al. (2004) reported that diarylheptanoid isolated from the fruits of Alnus japonica very mildly inhibited FPTase and the inhibitory activity very much depends on the structure of diarylheptanoids.[86]
Hepatoprotective activity
Buniatian et al. studied hepatoprotective properties of altan (obtained on the basis of ellagitannines from the cones of black alder A. glutinosa) on the model of acute liver damage induced with tetrachloromethane. It was found that altan exhibits the hepatoprotective activity even in a dose of 1 mg/kg which is ten-fold smaller than the dose of traditional flavonoid-based drugs. Altan limits choleopoiesis disorder, has an anti-inflammatory and membrane stabilizing effect, and recovers physiological antioxidant system.[87]
Inhibitory activity against cell-mediated low-density lipoprotein oxidation
Kang et al. (2006) evaluated the inhibitory activity of two cyclic diarylheptanoids, garugamblin-3 (57) and acerogenin L (58), isolated from the MeOH extract of the fruits of Alnus japonica Steud., against human low-density lipoprotein (LDL) oxidation in the thiobarbituric acid-reactive substance assay with IC (50) values of 2.9 and 1.7 microM, respectively, and they also inhibited cell-mediated LDL oxidation more than five times more strongly than that of a well-known antioxidant, probucol, at a concentration of 10 microM. Compound (57) had no effect on the anti-atherogenesis in LDL receptor-deficient mice.[27] Lee et al. (2005) assessed the antioxidant effects of diarylheptanoid derivatives from Alnus japonica on human LDL oxidation.[88]
Inhibition of diacylglycerol acyltransferase
During the screening for diacylglycerol acyltransferase (DGAT) inhibitors from natural products, Chung et al. found that the lupine-type triterpenoid betulinic acid (76) isolated from the methanol extract of A. hirsuta potently inhibited DGAT in the rat liver microsomes with an IC50 value of 9.6 and 8.1 microM using [(14)C]oleoyl-CoA as a substrate. A decrease in the apparent Vmax was observed with betulinic acid (76), whereas the apparent Km remained constant. Therefore, a Lineweaver-Burk plot of DGAT inhibition by betulinic acid (76) showed a noncompetitive type of inhibition. In the cell-based assay, betulinic acid (76) inhibited triglyceride (TG) formation by human HepG2 cells. These findings suggest that betulinic acid (76) may be a potential lead compound in the treatment of obesity.[89]
Cytotoxic activities
Choi et al. (2008) analyzed the cytotoxic activities of compounds [(19), (22), (27), and (51) to (57)] isolated from the bark of Alnus japonica against murine B16 melanoma, human SNU-1 gastric cancer, human SNU-354 hepatoma cancer, and human SNU-C4 colorectal cell lines. The diarylheptanoids showed potent cytotoxic activities against murine B16 melanoma cells and human SNU-C1 gastric cancer cell when the cell viability was analyzed by MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide) assay.[90]
Melanogenesis inhibitory activities
Cho et al. (2003) examined the inhibitory effect of diarylheptanoid isolated from the bark of A. hirsuta Turcz. on melanogenesis by measuring the melanin level and tyrosinase activity in B16 melanoma cell. Melanin level and tyrosinase activity were reduced by 75 to 85% on addition of diarylheptanoids to incubation medium of the melanoma cell. On the other hand, melanin level and tyrosinase activity were reduced by 13 to 43% on the addition of diarylheptanoids to incubation medium of the melanoma cell treated with melanogenesis stimulator, a-MSH and forskolin. These melanogenesis inhibitory effects were significantly different compared with control.[91]
Antitumor activity
Sheth et al. (1973) reported that betulin (75) isolated from Alnus oregona showed antitumor activity for the Walker 256 (5WA16) tumor systems.[92]
Immunological properties
Joo et al. (2009) demonstrated that hirsutenone (22) may have ideal properties as a new calcineurin inhibitor. It is small in molecular weight and possesses a potential comparable with that of cyclosporine A for the inhibition of allergen-induced T-cell and mast cell activation. Moreover, due to its lipophilic properties, hirsutenone (22) has a high affinity for the skin compartment and a low potential for absorption into the systemic circulation. Thus, hirsutenone (22) is an attractive source for developing a topical drug for T cell-based anti-atopic dermatitis by its actions as a calcineurin inhibitor.[93–95]
Antiviral activity
Tung et al. investigated anti-influenza components from the bark of Alnus japonica. Antiviral testing of isolated compounds were performed against KBNP-0028 (H9N2) avian influenza virus. Result of study showed that platyphyllone (61) was strongly active (EC50 = 29.9 lM), and platyphyllonol-5-xylopyranoside (EC50 = 105.0 lM) (62) was moderately active against KBNP-0028 as compared with the positive control, zanamivir (EC50 = 16.9 lM), respectively.[96]
CONCLUSION
The genus Alnus is widespread all over the world, and many species of this genus have been used as traditional herbal medicines. The chemical investigation of Alnus species has revealed many components from this genus with significant bioactivities. On the basis of the above discussion, it may be concluded that all the members of genus Alnus have different biological activities. The genus is well known for its traditional medicinal uses including cancer, hepatitis, inflammation of uterus, uterine cancer, rheumatism, dysentery, stomachache, diarrhea, fever, etc. The plants contain variety of bioactive constituents, i.e., triterpenoids, flavonoids, tannins, phenols, steroids, diarylheptanoids, etc. Among them, diarylheptanoids (hirsutenone, oregonin, (5S)-O-methylhirsutanonol, 2-oxatrycyclo[13.2.2.13,7]eicosa-3,5,7(20),15,17,18-hexaen-10-16-diol, 2-oxatrycyclo [13.2.2.13,7]eicosa-3,5,7-(20),15,17,18-hexaen-10-one, garugamblin-3, acerogenin L, etc.) are highly potent bioactives.
ACKNOWLEDGEMENT
The authors are thankful to UGC New Delhi, India [Grant No. F.4-3/2006(BSR)/11-84/2008(BSR)], for financial assistance.
Footnotes
Source of Support: UGC New Delhi, India [Grant No. F.4-3/2006(BSR)/11-84/2008(BSR)]
Conflict of Interest: None declared
REFERENCES
- 1.USDA, ARS, National Genetic Resources Program, Germplasm Resources Information Network (GRIN) Beltsville, Maryland, USA: National Germplasm Resources Laboratory; [Last accessed on 2003]. GRIN Database. Available from: http://www.ars-grin.gov/cgibin/npgs/html/taxon.pl?2448 . [Google Scholar]
- 2.Mitchell A, Wilkinson J. Parey's Buch der Baume (The Trees of Britain and Northern Europe, 1982) 3rd ed. Berlin Germany: Blackwell Wissenschafts-Verlag; 1997. [Google Scholar]
- 3.Kim ST, Kim JD, Ahn SH, Ahn GS, Lee YI, Jeong YS. Hepatoprotective and antioxidant effects of Alnus japonica extracts on acetaminophen-induced hepatotoxicity in rats. Phytother Res. 2005;18:971–5. doi: 10.1002/ptr.1540. [DOI] [PubMed] [Google Scholar]
- 4.Holtom J, Hylton W. Complete Guide to Herbs. Vol. 46. Emmaus, PA: Rodale Press; 1979. p. 269. ISBN 0-87857-262-7. [Google Scholar]
- 5.Launert E. Edible and Medicinal Plants. Vol. 21. Hamlyn UK: 1981. p. 254. ISBN 0-600-37216-2. [Google Scholar]
- 6.Lust J. The Herb Book. New York: Bantam books; 1983. ISBN 0-553-23827-2. [Google Scholar]
- 7.Pande PC, Tiwari L, Pande HC. Bishen Singh, Mahendra Pal Singh publishers, 23-A. New Connaught Place, Dehradun, India: 2006. Folk-Medicine and Aromatic Plants of Uttaranchal; p. 29. [Google Scholar]
- 8.Changkija S. Folk medicinal plants of the Nagas in India. Asian Folklore Studies. 1999;58:205–30. [Google Scholar]
- 9.Gerald BH, Fernandez ID, Villegas LF, Vaisberg AJ. A survey of traditional medicinal plants from the Callejon de Huaylas, Department of Ancash, Peru. J Ethnopharmacol. 1998;61:17–30. doi: 10.1016/s0378-8741(98)00009-9. [DOI] [PubMed] [Google Scholar]
- 10.Jin WY, Cai XF, Na MK, Lee JJ, Bae KH. Triterpenoids and Diarylheptanoids from Alnus hirsuta Inhibit HIF-1 in AGS Cells. Arch pharm Res. 2007;30:412–8. doi: 10.1007/BF02980213. [DOI] [PubMed] [Google Scholar]
- 11.Lee SJ. Korea folk Medicine. Seoul, Korea: Seoul National University Publishing Center; 1996. [Google Scholar]
- 12.Grieve M. A Modern Herbal Alder. 2004. Available from: http://www.botanical.com/ botanical/mgmh/a/ alder019.html .
- 13.Holistic Online Database. Herb Information. 2004. Available from: http://www.holistic-online.com/ Herbal0Med/Herbs/h90.htm .
- 14.Middleton P, Stewart F, Al-Qahtani S, Egan P, Rourke C, Abdulrahman A, et al. Antioxidant, antibacterial activities and general toxicity of Alnus glutinosa, Fraxinus excelsior and Papaver rhoeas. Iran J Pharm Res. 2005;2:81–6. [Google Scholar]
- 15.Turner JJ, Hebda R. Contemporary use of bark for medicine by two salishan native elders of Southeast Vancouver Island, Canada. J Ethnopharmacol. 1990;29:59. doi: 10.1016/0378-8741(90)90098-e. [DOI] [PubMed] [Google Scholar]
- 16.Chen J, Gonzalez-Laredo RF, Karchesy JJ. Minor diarylheptanoid glycosides of Alnus rubra bark. Phytochemistry. 2000;53:971. doi: 10.1016/s0031-9422(99)00523-3. [DOI] [PubMed] [Google Scholar]
- 17.Aoki T, Ohta S, Suga T. Six novel secodammarane-type triterpenes from male Flowers of Alnus japonica. Phytochemistry. 1988;27:2915. [Google Scholar]
- 18.Nomura M, Tokoroyama T, Kubota T. Biarylheptanoids and other constituents from wood of Alnus japonica. Phytochemistry. 1981;20:1097. [Google Scholar]
- 19.Omoto T, Nikaido T, Ikuse M. Studies on the Constituents of Pollen. III. On the Constituents of Pollen Grains of Alnus jaonica STEUD. (1) Yakugaku Zasshi. 1974;94:367–70. doi: 10.1248/yakushi1947.94.3_367. [DOI] [PubMed] [Google Scholar]
- 20.Wollenweber E, Wassum M. Salvigenin und ein neues natürliches flavon aus den knospen von alnus japonica. Tetrahedron Lett. 1972;13:797–800. [Google Scholar]
- 21.Keher JP. Free radicals as mediators of tissue injury and disease. Crit Rev Toxicol. 1993;23:21–48. doi: 10.3109/10408449309104073. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez-Lareo RF, Helm RF, Chen J, Karchesy JJ. Two acylated diarylheptanoid glycosides from red alder bark. J Nat Prod. 1998;61:1292–4. doi: 10.1021/np980083l. [DOI] [PubMed] [Google Scholar]
- 23.Wada H, Tachibana H, Fuchino H, Tanaka N. Three new diarylheptanoid glycosides from Alnus japonica. Chem Pharm Bull. 1998;46:1054–5. [Google Scholar]
- 24.Suga T, Hirata T. New C31 secodammarane-type triterpenoids, alnuseric acid and alniselide, in the male flowers of Alnus serrulatoides. Bull Chem Soc. 1979;52:1153–5. [Google Scholar]
- 25.Lee MW, Tanaka T, Nonaka GI, Nishioka I. Dimeric ellagitannins from Alnus japonica. Phytochemistry. 1992;31:2835–9. [Google Scholar]
- 26.Lee JH, Yeom SH, Kim MK, Kim HJ, Shim JG, Lee MW. Antioxidant activities of diarylheptanoids from Alnus Japonica and their structural relationship. Kor J Pharmacogn. 2003;34:190–2. [Google Scholar]
- 27.Kang HM, Ryong Kim J, Jeong TS, Choi SG, Ryu YH, Taeg Oh G, et al. Cyclic diarylheptanoids inhibit cell-mediated low-density lipoprotein oxidation. Nat Prod Res. 2006;20:139–43. doi: 10.1080/14786410500045895. [DOI] [PubMed] [Google Scholar]
- 28.Han JM, Lee WS, Kim JR, Son J, Nam KH, Choi SC, et al. Effects of diarylheptanoids on the tumor necrosis factor-α-induced expression of adhesion molecules in human umbilical vein endothelial cells. J Agric Food Chem. 2007;55:9457–64. doi: 10.1021/jf072157h. [DOI] [PubMed] [Google Scholar]
- 29.Wollenweber E. Flavonoids from Alnus crispa, A. japonica, A. koehnei and A. sinuate. Phytochemistry. 1974;13:2318–9. [Google Scholar]
- 30.Terazawa M, Miyake M, Okuyama H. Phenolic compounds in living tissue of woods. V.Reddish orange staining in keyamahannoki (Alnus hirsuta) and hannoki (A.Japonica) [Betulaceae] caused by the interaction of hirsutoside and catechol oxidase after cutting the woods. Mokuzai Gakkaishi. 1984;30:601–7. [Google Scholar]
- 31.Lee MW, Tanaka T, Nonaka GI, Nishioka I. Hirsunin, an ellagitannin with a diarylheptanoid moiety, from Alnus hirsuta var. Microphylla. Phytochemistry. 1992;31:967–70. [Google Scholar]
- 32.Suga T, Iwata N, Asakawa Y. Chemical constituents of the male flower of Alnus pendula (Betulaceae) Bull Chem Soc Jpn. 1972;45:2058. [Google Scholar]
- 33.Aoki T, Ohta S, Aratani S, Hirata T, Suga T. The structures of four novel C31-secodammarane-type triterpenoid saponins from the female flowers of Alnus serrulatoides. J Chem Soc Perkin Trans 1. 1982:1399–1403. DOI: 10.1039/P19820001399. [Google Scholar]
- 34.Aoki T, Ohta S, Suga T. Triterpenoids, diarylheptanoids and their glycosides in the flowers of Alnus species. Phytochemistry. 1990;29:3611–4. [Google Scholar]
- 35.Bae CI, Gong JM, Oh JW, Kim HJ, Oh GJ, Park SK, et al. Studies on the cytotoxic constituent of Alnus hirusuta. Yakhak Hoechi. 1997;41:559–64. [Google Scholar]
- 36.Joo SS, Kim HJ, Kwon HS, Lee DI. Augmentation of macrophage antitunour activities and nitric oxide production by oregonin. Arch Pharm Res. 2002;25:457–62. doi: 10.1007/BF02976602. [DOI] [PubMed] [Google Scholar]
- 37.Joo SS, Kim MS, Oh WS, Lee DI. Enhancement of NK Cytotoxicity, antimetastasis and elongation effect of survival time in B16-F10 melanoma cells by oregonin. Arch Pharm Res. 2002;25:493–9. doi: 10.1007/BF02976608. [DOI] [PubMed] [Google Scholar]
- 38.Lee MW, Kim JH, Jeong DW, Ahn KH, Toh SH, Surth YJ. Inhibtion of cyclooxygenase-2 expression by diarylheptanoids from the bark of Alnus hirusta var. Sibirica. Biol Pharm Bull. 2000;23:517–8. doi: 10.1248/bpb.23.517. [DOI] [PubMed] [Google Scholar]
- 39.Lee MW, Kim NY, Park MS, Ahn KH, Toh SH, Hahn DR, et al. Diarylheptanoids with in vitro inducibe nitric oxide synthesis inhibitopry activity from Alnus hirusta. Planta Med. 2000;66:551–3. doi: 10.1055/s-2000-8606. [DOI] [PubMed] [Google Scholar]
- 40.Lee YA, Kim KH, Kim JS, Cho SM, Kim SW, Lee MW. Antioxidative effects of diarylheptanoids from Alnus hirusta. Yakhak Hoechi. 2000;44:193–6. [Google Scholar]
- 41.Terazawa M, Miyake M, Okuyama H. Isolation of hirsutanonol nd hirsutenone, two new diarylheptanoids from the green bark of keyamahannoki, Alnus hirsuta. Mokuzai Gakkaishi. 1973;19:45–6. [Google Scholar]
- 42.Jeong DW, Kim JS, Cho SM, Lew YA, Kim KH, Kim SW, et al. Diarylheptanoids from stem bark of Alnus hirsuta var. Sibirica. Korean J Pharmacogn. 2000;31:28–33. [Google Scholar]
- 43.Park D, Kim HJ, Jung SY, Yook CS, Jin C, Lee YS. A new diarylheptanoid glycoside from the stem bark of Alnus hirsuta and protective effects of diarylheptanoid derivatives in human HepG2 cells. Chem Pharm Bull. 2010;58:238–41. doi: 10.1248/cpb.58.238. [DOI] [PubMed] [Google Scholar]
- 44.Lee MW, Park MS, Jeong DW, Kim KH, Kim HH, Toh SH. Diarylheptanoids from the leaves of Alnus hirsuta Turcz. Arch Pharm Res. 2000;23:50–3. doi: 10.1007/BF02976466. [DOI] [PubMed] [Google Scholar]
- 45.Asakawa Y. Chemical Constituents of Alnus sieboldiana (Betulaceae). III. The synthesis and stereochemistry of yashabushiketols. Bull Chem Soc Jpn. 1972;45:1794. [Google Scholar]
- 46.Ishimatshu M, Tanaka T, Nonaka GI, Nishioka I. Alnusnins A and B from the leaves of Alnus sieboldiana. Phytochemistry. 1989;28:3179–84. [Google Scholar]
- 47.Suga T, Aoki T, Hirata T, Aoki K, Asakawa Y. Structure of alnustic acid, a new secodammarane-type triterpenic acid from Alnus sieboldiana. Bull Chem Soc Jpn. 1979;52:1698. [Google Scholar]
- 48.Suga T, Ohta S, Aoki T, Hirata T. An X-Ray crystallographic study on the absolute configuration of dihydroyashabushiketol and the solvent-dependence of its optical rotation. Bull Chem Soc Jpn. 1983;56:3353. [Google Scholar]
- 49.Sakamura F, Ohta S, Aoki T, Suga T. Triterpenoids from the female and male flowers of Alnus sieboldiana. Phytochemistry. 1985;24:2744–5. [Google Scholar]
- 50.Hashimoto T, Tori M, Asakawa Y. Five new diarylheptanoids from the male flowers of Alnus sieboldiana. Chem Pharm Bull. 1986;34:1846–9. [Google Scholar]
- 51.Ohmoto T, Nikaido T, Nozaki T, Ikuse M. Studies on the constitutuents of pollen. iv. on the constituents of Alnus sieboldiana matsum. Yakugaku Zasshi. 1977;97:176–80. doi: 10.1248/yakushi1947.97.2_176. [DOI] [PubMed] [Google Scholar]
- 52.Asakawa Y. Chemical constituents of Alnus sieboldiana (Betulaceae) ii. The isolation and structure of flavonoids and stilbenes. Bull Chem Soc Jpn. 1971;44:2761–6. [Google Scholar]
- 53.Yoshida T, Yazaki A, Memon MU, Maruyama I, Kurokawa K, Shingo T, et al. Structures of alnusiin and bicornin, new hydrolysable tannins having a monolactonized tergalloyl group. Chem Pharm Bull. 1989;37:2655–60. [Google Scholar]
- 54.Asakawa Y, Genjida F, Suga T. Four new flavonoids isolated from Alnus sieboldiana. Bull Chem Soc Jpn. 1971;44:297. [Google Scholar]
- 55.Suga T, Asakawa Y, Iwata N. 1,7-Diphenyl-1,3-heptadien-5-one. new ketone from Alnus pendula (Betulaceae) Chem Ind (London) 1971:766. [Google Scholar]
- 56.Suga T, Ohta S, Ohta E, Aoki T. A C31-secodammarane-type triterpinic acid, 12-deoxy alnustic acid, from the female flowers of Alnus pendula. Phytochemistry. 1986;25:1243–4. [Google Scholar]
- 57.Suga T, Iwata N, Asakawa Y. Chemical constituents of the male flower of Alnus pendula (Betulaceae) Bull Chem Soc Jpn. 1972;45:2058–60. [Google Scholar]
- 58.Suga T, Akoi T, Kawada Y, Ohta S, Ohta E. C31-secodammarane-type triterpenoid saponins from the male flowers of Alnus pendula. Phytochemistry. 1984;23:1297–9. [Google Scholar]
- 59.Hirata T, Suga T. Crystal and molecular structure of alnuserol, a new 11-hydroxylated C31 dammarane-type triterpene from Alnus serrulatoides. J Chem Soc Perkin Trans II. 1978:347–49. DOI: 10.1039/P29780000347. [Google Scholar]
- 60.Hirata T, Aoki T, Suga T. X-Ray crystallographic studies on the structure of alnuserrudiolone isolated from the male flowers of Alnus serrulatoides. Bull Chem Soc Jpn. 1981;54:3059. [Google Scholar]
- 61.Hirata T, Ideo R, Aoki T, Suga T. The structure of alnuserrutriol, a new C31 dammarane-type triterpenoid from the male flowers of Alnus serrulatoides. Bull Chem Soc Jpn. 1982;55:639–40. [Google Scholar]
- 62.Heslop-Harrison J. “Polln: Development and physiology”. London: London butherworths Ltd; 1971. [Google Scholar]
- 63.Suga T, Hirata T, Iwata N. Alnuserrudiolone, a new c31-dammarane-type triterpene from Alnus serrulatoides call. Chem Lett. 1974;3:971–4. [Google Scholar]
- 64.Hirata T, Murai K, Ideo R, Suga T. The reprint of the 20th symposium on the Chemistry of Natural Products. Sendai. 1976:273. [Google Scholar]
- 65.Hirata T, Ideo R, Suga T. Structure of alnuseric acid, the first reported naturally occurring c31-secodammarane-type triterpene from Alnus serrulatoides call. Chem Lett. 1977;6:283–6. [Google Scholar]
- 66.Hirata T, Ideo R, Suga T. Structure of alnuselide, the first reported naturally occurring c31-secodammarane-type triterpene lactone from Alnus serrulatoides. Chem Lett. 1977;6:711–4. [Google Scholar]
- 67.Jain MC, Seshadri TR. Triterpenoids from Alnus rubra. Indian J Chem. 1971;9:1026. [Google Scholar]
- 68.Chen J, Gonzalez-Laredo RF, Karchesy JJ. Minor diarylheptanoids glycosides of Alnus rubra bark. Phytochemistry. 2000;53:971–3. doi: 10.1016/s0031-9422(99)00523-3. [DOI] [PubMed] [Google Scholar]
- 69.Saxena G, Farmer S, Hancok RE, Towers GH. Antimicrobial compounds from Alnus rubra. Int J Pharmacogn. 1995;33:33–6. [Google Scholar]
- 70.Chen J, Gonzalez-Laredo RF, Karchesy JJ. Phenolic diarylheptenones from Alnus rubra bark. Planta Med. 1998;64:74–5. doi: 10.1055/s-2006-957372. [DOI] [PubMed] [Google Scholar]
- 71.Talapatra SK, Chattopadhyaya P, Talapatra B. Triterpenoid and related compounds, part-XXII: Triterpenoid constituents of Alnus nepalensis D.Don. J Indian Chem Soc. 1983;60:203. [Google Scholar]
- 72.Sati SC, Sati N, Sati OP. Oregonin: An important diarylheptanoid from the bark of Alnus nepalensis. Univ J Phytochem Ayurvedic Heights. 2010;1:16–8. ISSN 0973-3507. [Google Scholar]
- 73.Uvarova NI, Ostitokg I, Supronov NI, Elyakov GB. Triterpenoids and other constituents from the far-eastern species of alnus. Phytochemistry. 1972;11:741–3. [Google Scholar]
- 74.Klischies M, Zenk MH. Stereochemistry of C-methylation in the biosynthesis of rhododendrin in Alnus and Betula. Phytochemistry. 1978;17:1281–4. [Google Scholar]
- 75.Guz NR, Lorenz P, Metraux JP. Oregonin from the bark of European Alnus species. Biochem Syst Ecol. 2002;30:471. [Google Scholar]
- 76.O’Rourke C, Byres M, Delazar A, Kumarasamy Y, Nahar L, Stewart F, et al. Hirsutanonol, oregonin and genkwanin from the seeds of Alnus glutinosa (Betulaceae) Biochemical Systematics and Ecology. 2005;33:749–52. [Google Scholar]
- 77.Hans EA. Alcohols and resin acids in the leaf resin of Alnus glutinosa. Berich Deut Chem Ges. 1908;40:4760–4. [Google Scholar]
- 78.Tori M, Hashimoto A, Hirose K, Asakawa Y. Diarylheptanoids, flavonoids, stilbenoids, sesquiterpenoids and phenanthrene from Alnus maximowiczii. Phytochemistry. 1995;40:1263–4. [Google Scholar]
- 79.Asakawa Y. Chemical constituents of Alnus firma (Betulacaea). I. phenyl propane derivatives isolated from Alnus firma. Bull Chem Soc Jpn. 1970;43:2223–9. [Google Scholar]
- 80.Lee MA, Lee HK, Kim SH, Kim YC, Sung SH. Chemical constituents of Alnus firma and their inhibitory activity on lipopolysaccharide-induced nitric oxide production in BV2 microglia. Planta Med. 2010;76:1007–10. doi: 10.1055/s-0029-1240911. [DOI] [PubMed] [Google Scholar]
- 81.Asakawa Y, Genjida F, Hayashi S, Matsuura T. A new ketol from Alnus firma sieb. Et zucc. (Betulaceae) Tetrahedron Lett. 1969;10:3235–7. [Google Scholar]
- 82.Asakawa Y. A New ketol, 1,7-diphenyl-5-hydroxy-3-heptanone, and trans-Stilbene from Alnus firma Sieb. et Zucc. (Betulaceae) Bull Chem Soc Jpn. 1970;43:575. [Google Scholar]
- 83.Yu YB, Miyashiro H, Nakamura N, Hattori M, Park JC. Effects of triterpenoids and flavonoids isolated from Alnus firma on HIV-1 viral enzymes. Arch Pharm Res. 2007;30:820–6. doi: 10.1007/BF02978831. [DOI] [PubMed] [Google Scholar]
- 84.Sosa F, Percheron F. [Isolation and identification of a quercetol-3-sophoroside in Alnus cordata Desf. pollen] C R Acad Sci Hebd Seances Acad Sci D. 1965;261:4544–6. [PubMed] [Google Scholar]
- 85.Jin WY, Cai XF, Na MK, Lee JJ, Bae KH. Diarylheptanoids from Alnus hirsuta Inhibit the NF-kB activation and NO and TNF-α production. Biol Pharm Bull. 2007;30:810–3. doi: 10.1248/bpb.30.810. [DOI] [PubMed] [Google Scholar]
- 86.Kang HM, Son KH, Yang DC, Han DC, Kim JH, Baek NI, et al. Inhibitory activity of diarylheptanoids on farnesyl protein transferase. Nat Prod Res. 2004;18:295–9. doi: 10.1080/14786410310001620691. [DOI] [PubMed] [Google Scholar]
- 87.Buniatian ND, Chikitkina VV, Iakovleva LV. The hepatoprotective action of ellagotannins. Eksp Klin Farmakol. 1998;61:53–5. [PubMed] [Google Scholar]
- 88.Lee WS, Kim JR, Im KR, Cho KH, Sok DE, Jeong TS. Antioxidant effects of diarylheptanoid derivatives from Alnus japonica on human LDL oxidation. Planta Med. 2005;71:295–9. doi: 10.1055/s-2005-864093. [DOI] [PubMed] [Google Scholar]
- 89.Chung MY, Rho M, Lee SW, Park HR, Kim K, Lee IH, et al. Inhibition of diacylglycerol acyltransferase by betulinic acid from Alnus hirsuta. Planta Med. 2006;72:267–9. doi: 10.1055/s-2005-916178. [DOI] [PubMed] [Google Scholar]
- 90.Choi SE, Kim KH, Kwon JH, Kim SB, Kim HW, Lee MW. Cytotoxic activities of diarylheptanoids from Alnus japonica. Arch Pharm Res. 2008;31:1287–9. doi: 10.1007/s12272-001-2108-z. [DOI] [PubMed] [Google Scholar]
- 91.Cho SM, Kwon YM, Lee JH, Yon KH, Lee MW. Melanogenesis inhibitory activities of diarylheptanoids from Alnus hirsuta Turcz in B16 mouse melanoma cell. Arch Pharm Res. 2003;25:885–8. doi: 10.1007/BF02977009. [DOI] [PubMed] [Google Scholar]
- 92.Sheth K, Bianchi E, Wiedenhopf R, Cole JR. Antitumor agents from Alnus oregana (Betulaceae) J Pharm Sci. 1973;62:139–40. doi: 10.1002/jps.2600620129. [DOI] [PubMed] [Google Scholar]
- 93.Joo SS, Kim SG, Choi SE, Kim YB, Park HY, Seo SJ, et al. Suppression of T cell activation by hirsutenone, isolated from the bark of Alnus japonica, and its therapeutic advantages for atopic dermatitis. Eur J Pharmacol. 2009;614:98–105. doi: 10.1016/j.ejphar.2009.04.047. [DOI] [PubMed] [Google Scholar]
- 94.Florvaag E, Elsayed S. Comparative Studies on Tree Pollen Allergens. Int Arch Allergy Appl Immunol. 1984;75:300–8. doi: 10.1159/000233638. [DOI] [PubMed] [Google Scholar]
- 95.Meingassner JG, Aschauer H, Stuetz A, Billich A. Pimecrolimus permeates less than tacrolimus through normal, inflamed, or corticosteroid-pretreated skin. Exp Dermatol. 2005;14:752–7. doi: 10.1111/j.1600-0625.2005.00354.x. [DOI] [PubMed] [Google Scholar]
- 96.Tung NH, Kwon HJ, Kim JH, Ra JC, Ding Y, Kim JA, et al. Anti-influenza diarylheptanoids from the bark of Alnus japonica. Bioorg Med Chem Lett. 2010;20:1000–3. doi: 10.1016/j.bmcl.2009.12.057. [DOI] [PubMed] [Google Scholar]


